-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaChildhood obesity: causes, consequences, and prevention
Dětská obezita: příčiny, důsledky a prevence
V důsledku dramatického nárůstu prevalence nadváhy a obezity u dětí je dětská obezita jedním z nejzávažnějších globálních problémů veřejného zdraví 21. století. K nárůstu hmotnosti dochází, když příjem energie převyšuje její výdej. Na její patogenezi se podílejí jak genetické faktory, tak faktory prostředí (např. sedavý způsob života). Dětská obezita je spojena s fyzickými, psychologickými a sociálními důsledky. U obézních dětí je vyšší riziko zvýšené glykemie nalačno, inzulinové rezistence, poruchy glukózové tolerance, diabetu 2. typu, hypertenze, syndromu polycystických ovarií (PCOS), aterosklerózy a kardiovaskulárních onemocnění (CVD), spánkové apnoe a astmatu. Psychologické a sociální důsledky zahrnují nízké sebevědomí, sociální nepohodlí a izolaci a deprese. Od doby, kdy byl COVID-19 prohlášen za celosvětovou pandemii, byly drasticky postiženy miliony dětí a dospívajících na celém světě. Na jedné straně COVID-19 zvýšil prevalenci přibývání na váze a dětské obezity, na straně druhé přestavuje COVID-19 pro obézní děti velké riziko. V rámci tohoto příspěvku uvádíme podrobnosti o endokrinních, metabolických a epidemiologických aspektech dětské obezity se stručnou diskusí o vztahu mezi COVID-19 a dětskou obezitou. Kapitola týkající se endokrinních aspektů se zaměřila na patofyziologii dětské obezity a úlohu adipocytů a inzulinu v mechanismu vzniku obezity. Kapitola o metabolických aspektech se zabývá metabolickými chorobami souvisejícími s dětskou obezitou. Naproti tomu kapitola zaměřená na epidemiologické aspekty se zabývá rizikovými faktory dětské obezity a současnými přístupy k prevenci dětské obezity.
Klíčová slova:
dětská obezita – epidemiologie – prevence – rizikové faktory – COVID-19 – endokrinní role – metabolická role
Authors: Aus Tariq Ali; Faisal Al-Ani; Osamah Al-Ani
Published in the journal: Čes. slov. Farm., 2023; 72, 21-36
Category: Přehledy a odborná sdělení
doi: https://doi.org/https://doi.org/10.5817/CSF2023-1-21Summary
As a result of the dramatic increase in the prevalence of overweight and obesity among children, childhood obesity is one of the most critical global public health challenges of the 21st century. Weight gain occurs when energy intake exceeds energy expenditure. Both genetic and environmental factors (such as a sedentary lifestyle) are implicated in its pathogenesis. Childhood obesity is associated with physical, psychological, and social consequences. Obese children are at higher risk of elevated fasting blood glucose, insulin resistance, impaired glucose tolerance, type 2 diabetes, hypertension, polycystic ovarian syndrome (PCOS), atherosclerosis and cardiovascular disease (CVD), sleep apnea, and asthma. Psychological and social consequences include low self-esteem, social discomfort and isolation, and depression. Since COVID-19 was declared a global pandemic, millions of children and adolescents worldwide have been affected drastically. While COVID-19 has increased the prevalence of weight gain and childhood obesity, obese children, on the other hand, have suffered excessively from COVID-19. Here, we provide details on the endocrine, metabolic, and epidemiological aspects of childhood obesity with a concise discussion of the relationship between COVID-19 and childhood obesity. The endocrine chapter is focused on childhood obesity pathophysiology and the role of adipocytes and insulin in the mechanism of obesity. The metabolic chapter covered metabolic diseases related to childhood obesity. In contrast, the epidemiological chapter covered the risk factors of childhood obesity and current approaches to the prevention of childhood obesity.
Keywords:
prevention – Childhood obesity – Epidemiology – COVID-19 – risk factors – endocrine role – metabolic role
Introduction
Modern lifestyles demand rapidly prepared and bettertasting food that, as a consequence, has a high caloric density. Technology has dramatically altered lifestyles with less physical activity for children as well as their parents. It has contributed to the worldwide epidemic of obesity. In 2016, the World Health Organization (WHO) reported that about 170 million children under 18 years were overweight or obese1). Since determining body fat is difficult, the diagnosis of obesity is based on body mass index (BMI), which is measured by relating the body weight in kilograms to height in meters squared (kg/m2). During childhood, BMI varies significantly with age2). To define childhood obesity accurately, cutoff values related to age are needed, for example, using reference centiles3). Nevertheless, BMI has been widely utilized to assess childhood obesity at two years of age and older3).
Previous studies have identified non-modifiable and modifiable risk factors for childhood obesity. Nonmodifiable risk factors include genetics4–6), ethnicity7–11) , and parent’s level of education12, 13). Modifiable risk factors include behavior and psychosocial and environmental factors such as high caloric intake14–16), birth weight17–19), physical inactivity13, 20–22), sedentary time22), infant formula19, 23, 24), maternal health25–27), TV viewing and computer usage20, 28, 29), and eating habits30, 31). In rare cases, medical conditions (i.e., Cushing syndrome) may be related to obesity32). The sharp increase in the prevalence of childhood obesity in high-income and most low-to-middle-income countries is due to a sedentary lifestyle combined with the fact that most children are being weaned on food devoid of nutrients33, 34). Newer technologies have introduced cheaper packaged foods which save time but, in return, encourage overeating and inactivity. The long-term side effects are an increased risk of chronic illnesses such as obesity and related comorbidities34). Compared with children five decades ago, modern children are experiencing a behavioral transition due to the development of digital technologies, especially mobile phones and tablets. In addition, the widespread use of social media (i.e., Facebook, Instagram, Twitter, YouTube, and WhatsApp) has altered many aspects of children’s daily lives. This revolution in technology has encouraged children, adolescents, and teenagers to spend more time indoors, eating junk food and drinking sugary beverages while viewing television, playing computer games, and engaging with social media22, 28, 29, 33, 35). Overweight and obesity in children are inversely related to physical activity22, 28, 35, 36), breastfeeding23, 34, 37), developing good eating habits16, 38), eating breakfast39), and sustained consumption of a healthier diet40, 41).
Health risks associated with childhood obesity include dyslipidaemia, hypercholesterolemia, elevated fasting blood sugar, insulin resistance, hypertension42), metabolic syndrome, impaired glucose tolerance43), type 2 diabetes44), cardiovascular disease (CVD)45), orthopedic problems46), non-alcoholic fatty liver disease (NFLD)47) and the development of PCOS48). In addition, social and psychological consequences such as body image disorders, low self-esteem, depression, social isolation, discrimination, and underachievement in school49–53) have been reported among overweight and obese children. COVID-19 increases the risk of stress, anxiety, and depression. These are associated with significant modification in lifestyle towards unhealthy eating patterns, inactivity, and sedentary behaviour. In turn, it may increase the risk of childhood obesity and/or aggravate it. The interaction between the COVID-19 pandemic and childhood obesity has placed an extra burden on healthcare systems and public health professionals worldwide. In addition, children and adolescents have suffered excessively from the COVID-19 pandemic. The strict instructions to decrease disease transmission have placed families and their children under severe health consequences, including psychological and metabolic disturbances54–57). Although the official lockdown that most countries went through had limited disease transmission, this approach has forced families and their children to move to a more sedentary lifestyle and change their way of eating towards more caloric-dense food54–60). Thus, COVID-19 has been considered a risk factor for weight gain and childhood obesity. Obese individuals with COVID-19, on the other hand, faced severe health consequences compared with their lean counterparts61–63).
Understanding the pathophysiology and the risk factors of childhood obesity provides an opportunity to channel resources, research, and appropriate interventions in directions that would be most beneficial in addressing and preventing the problem. This review aims to explore and expand upon the medical characteristics related to childhood obesity, including endocrine, metabolic, and epidemiological aspects, risk factors, and approaches to prevention. The relationship between COVID-19 and childhood obesity has also been discussed.
The pathophysiology of childhood obesity
The mechanisms underlying the development of childhood and adult obesity are similar despite differences in risk factors. Weight gain generally results from a positive energy balance, usually achieved via four scenarios (Table 1).
Tab. 1. Scenarios explaining positive energy balance that lead to weight gain 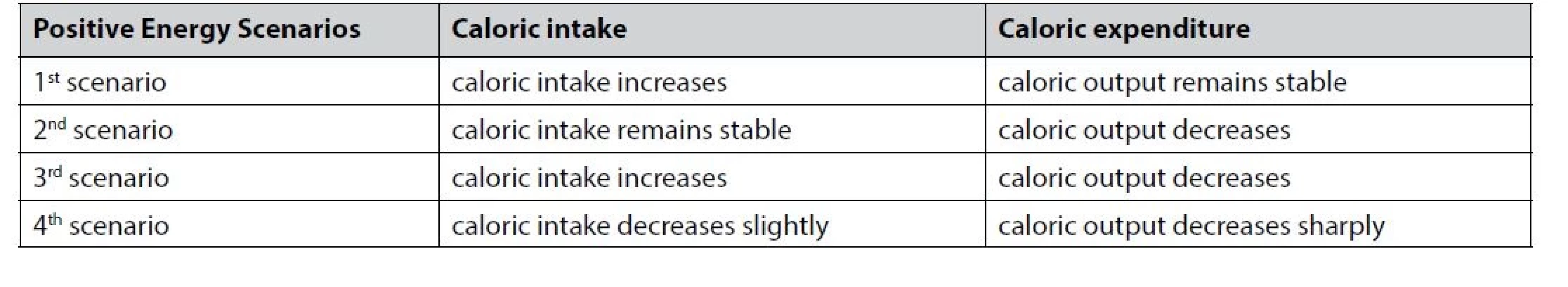
Adipocyte and energy balance
Adipocytes or fat cells play pivotal role in regulating energy balance by storing triglycerides as fat in periods of energy surplus and mobilizing it during periods of energy deprivation. The differentiation of stem cell precursors into adipocytes occurs before birth, and this capacity of the precursor cells continues during adulthood. After birth, adipose tissue expands because of adipocyte hypertrophy and accelerated hyperplasia. The differentiation of new adipocytes occurs in response to the body’s fat storage demands64). The capacity of each fat cell has been pre-determined during the process of cell determination. Adipocyte determination involves the interaction of specific intracellular signaling pathways and the activation of numerous transcription factors, including peroxisome proliferator-activated receptor γ (PPARγ), enhancer binding protein α, β, and δ (C/EBP α, C/EBP β, and C/EBP δ), single transducers and activators of transcription (STATs), the transcriptional factor sterol-regulatoryelement - binding-protein-1 (SREBP1), insulin-like growth factor I (IGF-l), macrophage colony-stimulating factor, fatty acids, prostaglandins and glucocorticoids65).
Adipocyte differentiation, which follows determination, occurs when the specialized cell becomes a lipid-filled adipocyte and undergoes chronological changes in the expression of specific genes that determine the exact adipocyte phenotype. Differentiated adipocytes are predestined to function in energy storage and balance under tight hormonal control. There are two factors affecting differentiation: first, the communication between individual cells, and second, the extracellular environment. One of the consequences of cellular differentiation is the production of cells capable of performing a specialized function65). Adipocytes are fully differentiated cells that are incapable of mitotic division. It suggests that adipocyte hyperplasia arises due to the differentiation of more primitive cells that can be primed to respond to metabolic needs. Therefore, when energy intake exceeds energy expenditure, the body stores most excess energy as triglycerides in the adipocyte, increasing cellular mass or hypertrophy. Increased adipose tissue mass results from increased cell number, size, or both. Hyperplasia and hypertrophy are involved in the pathophysiology of weight gain during childhood66), and adipose tissue dysfunction is associated with metabolic disturbances. Compared with lean children, a significant increase in adipocyte size and number has been reported in obese children at the beginning of early childhood66). In vitro, these changes in adipose tissue constituents are associated with a significant increase in the proliferation of stromal vascular cells and a reduction in basal lipolytic activity66).
During childhood and adolescence, the expansion of fat mass/adipose tissue directly results from adipocyte hyperplasia, while adipocyte hypertrophy is the main contributor during adulthood67). Adipocyte size is important because larger adipocytes secrete more cytokines at maturity66), although fat distribution is also crucial68, 69). The significant increase in inflammatory cytokines is associated with insulin resistance, impaired glucose tolerance, T2DM, and other metabolic disturbances70).
Adipocyte and insulin resistance
Insulin stimulates glucose uptake and inhibits lipolysis, which occurs rapidly through glucose transporter protein (GLUT4) translocation or covalent modification of hormone-sensitive lipase. Insulin resistance is the impaired ability of endogenous and exogenous insulin to control hepatic glucose production and enhance glucose clearance68, 69). Insulin resistance is common in obesity and is associated strongly with visceral fat68, 69). In adipocytes, cellular fat accumulation is governed by two important factors, the capacity of fat cells or adipocyte size and insulin status66). Insulin resistance directly correlates with fat cell capacity or adipocyte size. Thus, the bigger the adipocytes, the higher the insulin resistance, with the opposite also being true. Compared to large adipocytes, small adipocytes display higher rates of insulin-stimulated glucose uptake, higher levels of glucose oxidation, and lower sensitivity to the antilipolytic action of insulin66).
Elevated serum glucose levels following energy intake require an instant response from pancreatic β cells in the form of insulin secretion to meet increased demand71). However, pressure on β cells increases when energy intake exceeds energy expenditure for an extended period. As a consequence, β cells will start to become more sensitive to pituitary gland signals. As a result, β cells will secrete more insulin than required, and this causes hyperinsulinaemia. Higher insulin concentrations in the peri-cellular environment will encourage cellular fat accumulation and inhibit any opportunity for adipocytes to lose fat72).
Adipocyte and metabolic diseases
Abdominal obesity or increased visceral fat depots plays a pivotal role in the pathogenesis of metabolic abnormalities and associated complications, such as type 2 diabetes and artherosclerosis (Fig. 1). Visceral and subcutaneous fat depots respond differently metabolically to a high-fat diet, as the increase in visceral fat is mostly due to adipocyte hypertrophy, while the increase in the subcutaneous fat mass is primarily due to adipocyte hyperplasia73). Abdominal obesity or increased visceral fat is more common in males. Although metabolic health consequences are associated with increased visceral fat in males and females, this is more strongly associated with increased cardiometabolic risk in females74). The international cutoff for a higher risk of metabolic and cardiovascular complications in children (abdominal obesity) is a waist-to-height ratio (WtHR) of 0.5 for both genders and all age groups combined75). However, variations between ethnicities have been reported76). It was previously thought that adipose tissue hyperplasia is limited to early development and that no new cells are recruited after that77, 78). However, new cells are continuously recruited during weight gain79). Thus, the body has to recruit new cells to store excess triglycerides in the abdominal cavity during ectopic fat distribution (Fig. 1). This increase in adipocyte number (hyperplasia) will soon be followed by an increase in cell size (hypertrophy) if there is a significant increase in cytokines, inflammation, and insulin resistance. It, if not treated, will be followed by the development of the metabolic syndrome and associated health risks (Fig. 1). Obesity, especially abdominal obesity, promotes increased risk of elevated fasting blood glucose, insulin resistance, impaired glucose tolerance, type 2 diabetes, hypertension, PCOS, atherosclerosis, and CVD42–48, 80, 81). A recent Swedish cross-sectional study reported a linear independent relationship between fat mass and blood pressure in Swedish 9-year-old children80). Childhood obesity is associated with adulthood obesity and is related to chronic kidney disease (CKD) in obese adults82). Moreover, severe obesity in children and adolescents is related to the development of early stages of chronic kidney disease (CKD)82), although CKD and end-stage renal disease (ESRD) are much more observed with obesity in adults83).
Fig. 1. Mechanisms of metabolic diseases in obesity 
A sedentary lifestyle is associated with increased daily caloric intake and decreased physical activity. With or without genetic interaction, it is well understood that when energy intake exceeds energy expenditure, the body starts to store energy as fat in adipose tissue. Without lifestyle modification, more fat will be deposited in subcutaneous tissues leading to obesity. Obesity is associated with insulin resistance and hyperinsulinaemia. When classical places to store fat are full, the body starts to direct fat to be deposited in unusual areas (abdominal cavity, muscles, and liver). Obesity alters lipid profile (increased triglycerides and total cholesterol) and accelerates the process towards impaired glucose tolerance and type 2 diabetes. Obesity, insulin resistance, and type 2 diabetes are associated with hypertension, atherosclerosis, and cardiovascular disease (CVD). If not well controlled, increased hypertension may cause kidney damage over time, placing the body under a vicious cycle as more kidney damage will raise blood pressure even further, leading to further kidney damage and so on. Obesity, hypertension, diabetes, and CVD are risk factors for chronic kidney disease (CKD) development. Variations in weight gain among children and the increased prevalence of overweight and obesity could also result from a genetic predisposition4). Previously, genome-wide association studies have identified > 60 frequent single nucleotide polymorphisms (SNPs) that are associated with increased BMI84) and increased risk of early onset of obesity85). However, < 3% of the phenotypic variance in BMI has been explained by these loci84). It suggests that other genetic factors may explain this large missing heritability. Maternal health also plays a crucial role in the genesis of childhood obesity. Alteration in the maternal environment caused by disease (i.e., gestational diabetes) or behavior (i.e., smoking) is associated with an increased risk of high birth weight, early childhood overweight and obesity, and obesity during adolescence86, 87).
Childhood obesity and the COVID-19 pandemic
Following the sudden breakout of COVID-19, the WHO declared it a global pandemic55–57, 60). It is well known that physical inactivity and/or sedentary behavior are associated with an increased risk of weight gain and childhood obesity13, 20–22). Worldwide, health systems have implemented strategies that limit physical activity to restrain the spread of COVID-19 (Fig. 2). Because there was no vaccination at that point, and to decrease viral spread, the world relied on non-pharmacological interventions such as closing schools, which later on was followed by the total lockdown in most countries worldwide as an emergency safety procedure to minimize disease transmission as much as possible. Indeed, these approaches have reduced COVID-19 transmission significantly. However, it is contributed to worsening childhood obesity globally54). The official lockdown that most countries applied has placed children at a higher risk of weight gain and obesity74). Instructions such as “Stay at home,” “Stay away,” and “Stay safe” have limited outdoor movement and/or daily exercise and forced children and their families to change their routine life toward a sedentary lifestyle (Fig. 2). Over time, and due to the fear of COVID-19 infection, children and their families were totally controlled by unhealthy behaviors. However, not everyone responded to COVID-19 public health restrictions in the same way, which caused a wide inconsistency in behaviours88).
Fig. 2. The relationship between COVID-19 and childhood obesity 
International efforts to decrease COVID-19 transmission have contributed significantly to worsening childhood obesity. The global lockdown has altered a family’s surrounding environment and moved it towards a sedentary environment. As a result, many families have changed their daily life toward a sedentary lifestyle. Children have switched their way of eating, and their food choices have become more caloric-dense than usual, and with physical inactivity, the result was a significant increase in weight gain and the prevalence of childhood obesity. Moreover, instructions to food sellers and restaurants to open doors and deliver their food have augmented the dependence on unhealthy snacks, more heavily processed and calorie-dense foods55). The former has added an additional burden on public health systems globally. Consequently, this has exacerbated the rising prevalence of childhood obesity worldwide56), which increased the risk of severe COVID-1957).
Assessment of childhood obesity
Different countries have in the past used their standard growth charts and different cutoff points, resulting in a commonly accepted standard not being employed2). However, in 2000 international age - and genderspecific cutoff points for young people aged 2–18 years were published3). The WHO has recommended using age - and gender-specific BMI for age percentiles for children in the United States. Accordingly, children are considered normal or underweight when the BMI is < 85th percentile, overweight when the BMI is between the 85th and the 94th percentile, and obese when the BMI is ≥ 95th percentile. For severe obesity, both a BMI ≥ 1.2 × 95th percentile and the 99th BMI-forage percentile threshold have been used89).
Other available methods to measure adiposity in children include densitometry and dual-energy X-ray absorptiometry, which are more accurate but much more expensive and not practical for population-level surveillance or clinical management. Anthropometric methods, such as measurement of waist circumference, skin fold thickness, and BMI, measure adiposity indirectly90).
Childhood obesity and adult obesity
Much attention has focused on the relationship between childhood obesity and subsequent adolescent and adult obesity. A previous study showed that about 55% of obese children turn out to be obese adolescents, about 80% of obese adolescents will be obese in adulthood, and about 70% will be obese over the age of 3091). Compared to overweight children (< 85th percentile for BMI) who were older than 2 years and less than 5 years, overweight children (> 85th percentile for BMI) have 5 times increased risk of becoming overweight by the age of 12 years92). Furthermore, 80% of children who were overweight at any time from 5 to 11 years and 60% of children who were overweight at the ages of less than 5 years were overweight at age 12 years, while none of the children with a BMI < 50th percentile at the age between 5–11 years became obese at 12 years92). Obesity during adolescence is strongly related to obesity in adulthood93, 94). Obese adolescents were 16 times more likely to become severely obese in young adulthood than normalweight or overweight adolescents [hazard ratio (HR) = 16.0 (95% CI: 12.4–20.5)]93). However, most obese adults were not overweight or obese during childhood and adolescence91). The inability to predict adulthood obesity based on BMI during childhood was previously reported in a study where only 17% and 18% of obese 33-year-old men and women, respectively, had been obese at age 7 years95). It presents a challenge for any prevention program targeting only obese children and adolescents.
Factors influencing the development of childhood obesity
Highlighting the risk factors for childhood obesity is crucial. First, as some of these factors are potentially modifiable, they are essential to consider in preventing and treating childhood obesity. Second, controlling childhood obesity may decrease the prevalence of obesity and associated health risks in childhood and later life.
Daily caloric intake
Weight gain results from consuming food high in sugar and fat, frequent snacking, excessive sugary beverages, and overeating. Factors affecting daily food intake include price, portion size, smell, taste, variety, and accessibility of foods, and the food preparation method14–16, 29). There are also strong cultural influences on the type of food consumed, with some societies abstaining from particular food types or only eating food if it has been prepared in a specific manner14). The sharp increase in the prevalence of childhood obesity could be due to the increase in daily caloric intake and overeating and also the sharp decrease in caloric expenditure because of a sedentary lifestyle96). We suggest four scenarios leading to an energy surplus (Table 1). During the COVID-19 pandemic and due to related restrictions, there was a move towards a more sedentary lifestyle. It was associated with significantly changing children’s behavior and eating habits56). Closing schools and the official lockdown have been related to changes in eating patterns toward dense caloric food54–57). As a result, childhood obesity increased significantly (Fig. 2).
Genetics
Common genetic polymorphisms have been suggested as important determinants of obesity, as an obesity-predisposing genotype is present in 10% of individuals4). An association between salivary amylase (AMY1) gene copy number and reduced risk of obesity has been observed5). This gene conferred 16% protection against obesity in Mexican children (OR per estimated copy = 0.84). A positive correlation between the BMI of Australian parents and their children has been observed in previous studies. Compared to normal-weight parents, overweight and obese parents are more likely to have overweight and/or obese children6). The likelihood of children being obese is 40% when one of their parents is obese. If both parents are obese, the chance that their children will be obese is 70%97). When the genetics of obesity are associated with environmental factors (i.e., sedentary lifestyle), the risk of a child becoming obese increases sharply. It is difficult under these circumstances to determine whether the cause of weight gain is due to genetic or environmental factors or an interaction between the two.
Ethnicity
Ethnic factors have also been reported to be influential7–11). Findings from the United Kingdom Millennium Cohort study showed that Black ethnicity is associated with a 41% increase in the risk of developing obesity during early childhood compared to White ethnicity. In comparison, Indian ethnicity was associated with 37% protection against obesity7). Similarly, a cohort study in the USA found that the highest prevalence of risk factors for obesity during early childhood was among African American children. In contrast, the lowest was among Asian children7). The rate of infant weight gain during the first 9 months of life was the most important contributor to the BMI z-score gap, which was a reliable predictor of BMI z-score at kindergarten entry7). The gap between the highest and the lowest rates of infant weight gain, which was between 14.9% and 70.5%, may explain differences between White children and their racial and/or ethnic minority peers7). African-American and Hispanic children displayed higher predicted mean BMI scores and differing mean BMI trajectories compared to White children, adjusting for time-dependent and independent predictors9). Around the age of 11 years, Hispanic boys (HB) and African-American girls (AAG) continuously had the highest prevalence of overweight and obesity (HB: 52.5%, AAG: 49.1% around age of11) and mean BMI after adiposity rebound than their counterparts8). The diverse socioeconomic and cultural profiles of all ethnic groups may explain the disparities in the prevalence of overweight and childhood obesity10, 13).
Physical inactivity
Inactivity or sedentary behavior is associated with an increased risk of childhood obesity13, 20–22, 98), and this association continues to exist even after controlling for all potential confounders98). Inactivity is associated with a significant decrease in energy expenditure. Children with a sedentary lifestyle often have a high birth weight and belong to families at high risk (i.e., overweight mothers and maternal smoking). In addition, they are more likely to be from families with low income or low educational achievement, both of which are risk factors or childhood obesity independent of ethnicity13). On the other hand, regular physical activity protects against childhood obesity35). Among primary school children, daily physical activity for ≥ 60 minutes is associated with a 50% and 80% decreased risk of becoming overweight and developing abdominal obesity, respectively35). Restrictions during the COVID-19 pandemic have been associated with a significant decrease in physical activity54–57). It resulted in weight gain and an increase in the prevalence of childhood obesity54, 55, 57, 59), as shown in Figure 2.
Birth weight
High birth weight is a risk factor for becoming overweight and obese in early childhood17–19, 99), adolescence86), and adulthood100). High birth weight is associated with maternal obesity18). A previous US study found that each 1kg increase in birth weight is associated with a 30% increase in the likelihood of being overweight during the ages of 11–14 years, even when maternal BMI and other relevant covariates are controlled for86). Results from a large US cohort study (NHANES) showed that children weighing ≥ 4000 g at birth become overweight at the age of 4.5 years101). High birth weight babies have a 2.5-fold increased risk of becoming overweight or obese at 9 years compared with babies of normal weight99).
Infant formula
Children who have never breastfed or had mixed formula and breastfeeding have a > 90%, and a > 50% increased risk of being overweight and/or obese, respectively, independent of parental BMI19). A recent Canadian study24) reported a two-fold increase in the risk of becoming overweight in the first year of life in infants who were exclusively formula fed in comparison to breastfed infants [OR = 2.04 (95% CI: 1.25–3.32)]. There is international consensus that exclusive breastfeeding is associated with significant and sustained protection against childhood obesity11, 19, 23, 24, 34, 37).
Parental educational level
Parental education level is negatively correlated with childhood obesity12, 13, 102). The more educated the parent, the less likely they or their children are to be obese and vice versa12, 13). High parental education level was associated with intakes of low-sugar and low-fat foods among children. In contrast, low parental education level was associated with increased consumption of high-sugary food and dietary fat102). The UK Millennium Cohort study reported that higher academic qualifications were associated with a 37% protection against early childhood obesity [OR = 0.63 (95% CI: 0.52–0.77)] if the primary caregiver left school after age 16 compared to leaving at age 1613). Paradoxically, two recent studies from Vietnam have reported that overweight and obesity are more likely to be seen in children of mothers with higher education and income levels35, 103), while low maternal education was associated with higher odds of being underweight88). These results suggest that the effect of parental educational level varies across the world, being protective against childhood obesity in some countries12, 13) while being a risk factor in others35, 103).
Maternal health
An overweight or obese mother is associated with an increased risk of early childhood obesity18). An alteration of the maternal environment negatively impacts the mother’s health and increases the risk of the child becoming overweight26, 104). Gestational weight gain is associated with an increased risk of high birth weight and adolescent obesity25, 87). The importance of the fetal environment on childhood obesity, later on, can be used to motivate optimal maternal glucose control during pregnancy as a means of reducing the risk of obesity among offspring26). In addition, being born to a mother with gestational diabetes mellitus was associated with a 40% increased risk of becoming overweight during adolescence. The risk was sustained even after controlling for all related confounders86). The association between gestational diabetes mellitus and obesity during childhood was slightly decreased (30% vs. 40%) when birth weight was considered, suggesting that the association of gestational diabetes mellitus with later obesity could partially be explained by its influence on birth weight86). These results support the pivotal role of altered maternal-fetal glucose metabolism in developing obesity in offspring. Maternal smoking during pregnancy is associated with a more than twofold increase in childhood obesity21). A healthy diet during pregnancy is associated with healthier fetal growth resulting in a significant decrease in neonatal cardiometabolic risk, morbidity, and mortality rates105). Contrarily, poor nutrition during pregnancy, especially during the last semester, is associated with poor fetal growth, resulting in a significant increase in the risk of neonatal adiposity106). It was found to be associated with higher blood pressure and cardiometabolic risk107).
Environmental factors
Factors related to lifestyle, culture, and socioeconomic status significantly impact the prevalence of childhood obesity by influencing eating behavior and physical activity. Exposure to an environment with a highcalorie diet is associated with an increased risk of obesity. The risk may become higher when high caloric intake is coupled with inactivity. Childhood BMI can be predicted by parental BMI. The risk of a 10-year-old child becoming obese is double when both parents are obese108). Similar findings were observed in preschool children who had overweight parents109). Therefore, the family environment is an important factor that can either promote or prevent childhood obesity. Irregular familial eating habits and parental conflicts may influence weight gain110, 111). In addition, parental characteristics and psychological problems also have a crucial impact on childhood obesity112). Overweight and obesity is 54% more prevalent among school children of divorced parents than children of married parents [RR = 1.54 (95% CI: 1.21–1.95)], while abdominal obesity increased 89% in the former [RR = 1.89 (95% CI: 1.35–2.65)]. Computer usage increased the odds of overweight and high abdominal adiposity by 4.5 and 3.9 times, respectively35). Patterns associated with childhood obesity include TV viewing28, 29). TV viewing among children not only encourages eating but also encourages eating when not hungry. An early UK cohort study showed that children who watch TV for four to eight hours per week at age three, have a significantly higher risk of developing obesity by age seven100). Previous studies have reported that the increased duration of TV viewing is strongly associated with childhood weight gain20, 29, 113), while having a TV in the child’s bedroom aggravates the problem. TV viewing for long periods at a time not only uses little energy but also encourages snacking113). Finally, lockdown restrictions associated with the COVID-19 pandemic have stimulated sedentary lifestyle factors, which caused significant environmental changes as it limited physical activity and encouraged inactivity and daily caloric intake (Fig. 2). It caused significant weight gain and increased childhood obesity worldwide54 – 57).
Other factors
Endocrine causes of obesity are identified in less than one percent of children and adolescents with obesity. These disorders include hypothyroidism, cortisol excess (Cushing syndrome or glucocorticoid therapy), growth hormone deficiency, and acquired hypothalamic lesions32). In unordinary circumstances, such as the breakout of an infectious disease (i.e., COVID-19) and to decrease disease transmission, an expected response may include prohibiting gatherings and limiting outdoor movement. The former may cause a dramatic change in the manner of living by promoting a more sedentary lifestyle, and our experience with the COVID-19 pandemic has provided excellent evidence56, 114, 88). As a result, the prevalence of obesity increases for children and their parents, and the COVID-19 experience is concrete evidence. Thus, a similar breakout in the future may cause similar outcomes54, 55, 57, 59, 60).
Health consequences of childhood obesity
The sharp increase in the prevalence of childhood obesity has affected males and females equally. Childhood obesity is associated with serious metabolic42–48) and psychosocial consequences49–53). Although health risks associated with obesity appear more frequently in adults, cardiovascular comorbidities such as hypertension and dyslipidaemia are seen in obese children. They are linked to increased cardiovascular risk in young adults115). In a large Chinese cohort study of 7203 schoolchildren aged 6 to 8 years, the incidence of hypertension was 50.1 % and 70 % among overweight and obese children, respectively116). As in adults, cardio-metabolic risks are strongly associated with abdominal obesity42, 45). Endocrine comorbidities of obesity in children include hyperlipidaemia, hypercholesterolaemia, insulin resistance, impaired glucose tolerance, diabetes mellitus43, 44), hyperandrogenism with early onset PCOS in girls accelerated linear growth and bone age, as well as early sexual maturation in girls and delayed onset sexual maturation in boys. Abdominal obesity is associated with an increased risk of insulin resistance, hyperinsulinaemia, and hyperandrogenism117). Menstrual disorders have been observed frequently in obese adolescents. Insulin resistance, hyperinsulinaemia, and hyperandrogenism are the major elements in the development of PCOS (Fig. 3). Obese children are also predisposed to non-alcoholic fatty liver disease (NAFLD)47). When subcutaneous adipose tissue is overloaded, the body directs fat to be stored in the liver. In severe cases, it can lead to fibrosis, cirrhosis, and liver failure. Laboratory abnormalities seen with NAFLD include an elevation in the liver enzymes alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and gammaglutamyl transferase118). These abnormalities resolve with weight loss118). Obese children have a higher risk of developing valgus knee alignment46), asthma119), and sleep apnea120).
Fig. 3. The development of PCOS in the obese adolescent 
Obesity is a key feature of metabolic diseases. Obesity is associated with insulin resistance and hyperinsulinaemia. Without treatment, obese individuals will create a vicious cycle as insulin resistance will end with hyperinsulinaemia. Insulin resistance and hyperinsulinaemia are both associated with metabolic disturbances. The former is related to visceral fat (abdominal obesity), hyperandrogenism (high LH/FSH ratio), high level of free testosterone, and low level of sex hormone binding globulin (SHBG) which are all considered risk factors for follicle development arrest, leading to polycystic ovary syndrome (PCOS). Childhood obesity is also associated with psychosocial consequences49–53). Teasing and bullying are commonly reported by obese children49–51). Childhood obesity contributes to low self-esteem and body dissatisfaction among schoolchildren and can affect academic performance53, 120). Compared to children of normal weight, obese and overweight children were four times more likely to report having problems at school121). Finally, obese children are more likely to develop psychosocial problems such as anxiety, withdrawal from peer interaction, depression, and suicide52). A large prospective US study examined 9374 adolescents in grades 7 to 12 and found that increased BMI was associated with depression in the first year of follow-up. Depression scores were highest in children with the highest BMI122). These adverse consequences can be life-threatening and warrant early prevention strategies.
Previous and most recent studies have provided reliable evidence that COVID-19 pandemic has complicated child health in general, promoting child obesity and related consequences54, 56, 58–63, 88, 123, 124). In general, obese individuals have responded poorly to COVID-19 antiviral treatments123), and the severity of COVID-19 was found to be related to obesity125, 126). It could be due to poor antibody responses because of the adiposity124). Previous studies found escalating worries due to COVID-19 among individuals with obesity. It includes anxiousness about contracting the virus due to potential unfavorable outcomes and fear of death58, 61, 125, 126). It has led some to remain at home and abstain from outdoor movement, gathering, and social contact. Consequently, the risks of mental health deterioration, weight gain, and obesity have increased58). Moreover, at hospital admission, obese people generally required special assistance, including mechanical ventilation, hospitalization, and intensive care unit admission62, 63) . These observations highlight major challenges healthcare systems face as a consequence of the increasing prevalence of childhood obesity.
Prevention of childhood obesity
Childhood obesity is a preventable disease since most of the factors influencing it can be addressed (Table 2). To improve health outcomes, a well-designed prevention program for childhood obesity must consider the following aspects. First, once genetic risk factors have been excluded, environmental risk factors, which are mostly modifiable, including behavior and parental practices, can be modified21, 28, 29, 110–113). Thus, a successful program will require parental and societal participation to change the environment surrounding the child. Second, maternal health has a direct impact on the risk of infants and children becoming overweight or obese25–27). Thus, improving women’s health before and during pregnancy, parturition, and breastfeeding is crucial. Third, a significant decrease in the prevalence of overweight and obesity among breastfed children has been reported23, 34, 37). While milk formula feeding is associated with an increased risk of overweight and obesity among infants and children19, 23, 24), breastfeeding is associated with a protective effect (Table 2). Fourth, overweight and obesity are decreased in children with healthy eating habits16, 39). Fifth, overweight and obesity are less prevalent among families and children consuming a Mediterranean or other healthy diet40, 102). Last, overweight and obesity in children are inversely associated with physical activity35, 36).
Tab. 2. Preventive approaches against childhood overweight and obesity 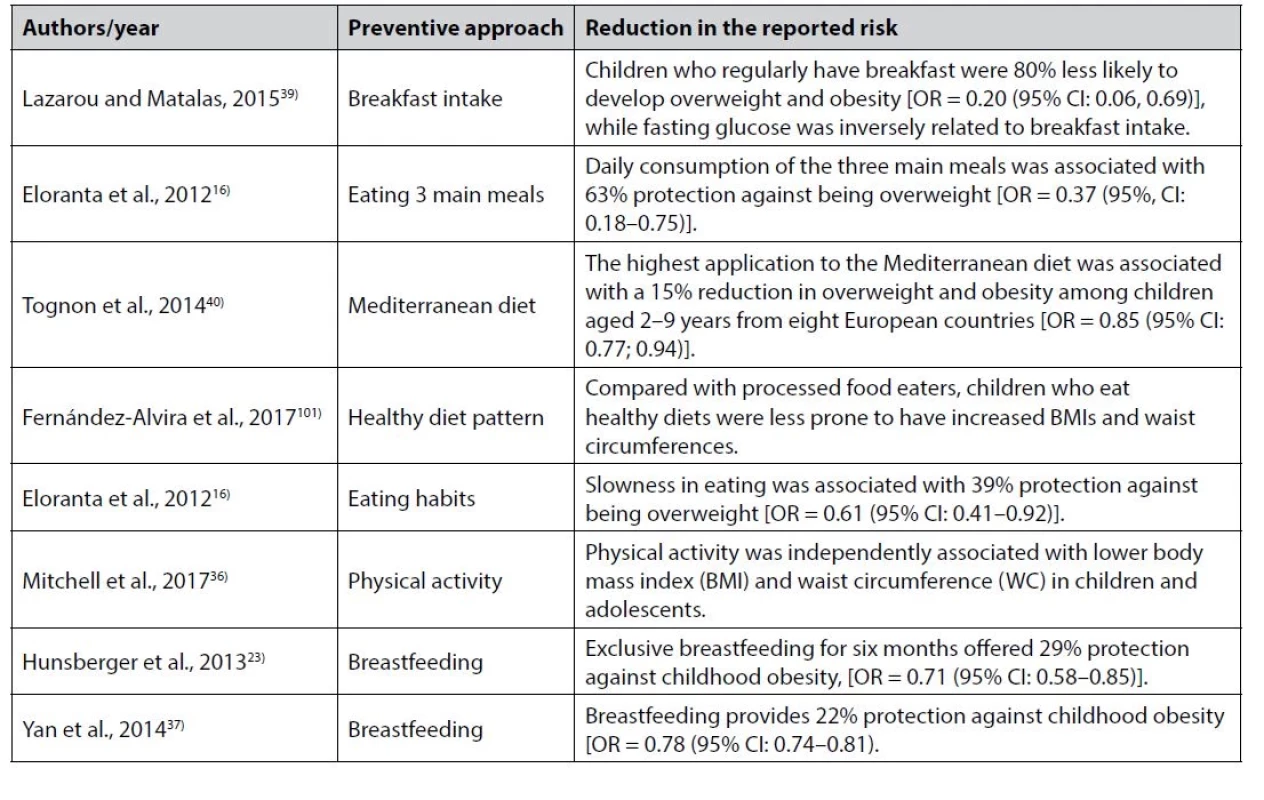
Consequently, the following recommendations should be considered. First, maintaining healthy weight gain during pregnancy, according to the American Institute of Medicine. Recommendations are for women with a normal body mass index (BMI) to gain 11.5–16.0 kg, overweight women to gain 7.0–11.5 kg, and obese women at least 6 kg25). Gestational diabetes should be managed, and smoking should be avoided. Second, breastfeeding should be encouraged for the first six months. Third, children should be encouraged to follow a sustainable healthy diet. Fourth, encourage eating three regular meals. Regular breakfast consumption during childhood protects against obesity during adolescence and young adulthood30), while skipping breakfast is associated with an increased risk of developing obesity31). Moreover, a significant association was previously detected between breakfast skipping and increased insulin resistance127) and type 2 diabetes128). In adults, breakfast skipping promotes fat accumulation and obesity as it disrupts energy metabolism by increasing energy retention and decreasing energy expenditure. Compared to those who have a breakfast (control group), a randomized crossover study of adult men has observed an increase in 24-h glucose levels without any change in energy expenditure following breakfast skipping129). Compared to White Americans, African Americans are less likely to eat breakfast during adolescence, and young adulthood and are shown to be at higher risk of developing obesity30). Fifth, encourage children to create the right eating habits (Table 2); for example, slowly eating was associated with a 39% protection against childhood obesity16). Last, encourage children to increase physical activity. Primary schools should include physical activity in their daily timetable. In addition, parents should motivate for outside physical activities for their children and/or encourage children to walk or cycle to school. To find efficient ways of creating a positive image for the various child health stakeholders such as parents, neighbors, and schools, the media, food advertisers, and food companies should take responsibility and work together towards promoting healthier lifestyles to prevent childhood obesity.
A simple prevention program may suggest avoiding risk factors (i.e., high caloric, high-fat diet and inactivity) and increasing exposure to factors associated with a protective effect (i.e., promoting the consumption of a healthier diet and exercise). However, the willingness to accept high-calorie food restrictions and exposure to a healthier lifestyle is influenced mainly by parents, schools, and neighborhoods (Fig. 4). In addition, the awareness of health risks associated with obesity may improve child behavior toward a healthier lifestyle. As most parents and children are not necessarily aware of all the factors linked to childhood obesity and associated health risks, a starting point in any prevention program should be to educate parents, children, and directors of educational institutes. The aim should be to highlight the epidemiology, risk factors, and associated health risks of obesity to educate society and increase awareness (Fig. 4). It may help to minimize the time that children are exposed to certain risk factors and increase the exposure time to protective factors.
Fig. 4. Child behavior and childhood obesity 
In an ordinary situation, a child’s behavior is controlled by environmental factors, including, firstly, home, where a child spends most of his/her time and can modify and refine his/her attitude towards healthier behavior. However, if a child is surrounded by passive lifestyle factors (obesity-related factors), he/she may develop a sedentary behavior. Second, school affects a child’s behavior as he/ she spends a significant amount of time and may build his/her character towards developing healthy behavior and increases his/her awareness regarding risks related to obesity. Third, the neighborhood is also important as a child meets his/her friend and copies and/ or ape their attitudes. Thus, promoting healthy instructions in neighboring communities is also important to decrease child obesity. Genetic risk factors are non-modifiable, and therefore a feasible prevention program targeting obese children with genetic risk factors should focus on controlling environmental factors such as eating habits. Obese mothers should be encouraged to follow preventive approaches (Table 2) and avoid or decrease the child’s exposure time to risk factors (i.e., milk formula and inactivity)16, 38). Preventive approaches and strategies for older children and adolescents may include promoting healthier eating habits (Table 2) and a more active lifestyle (limiting television viewing). Daily exercise can be promoted by encouraging cycling or walking to school. Other approaches may include restricting the intake of sugary beverages and processed snacks. To apply these behavioral strategies, other measures, such as modifying the surrounding environment, can be integrated. To increase physical activity, sporting equipment should be available at school, community sports facilities, and at home. The internet and television can be used to highlight the causes of childhood obesity and associated health risks. They may also provide the correct instruction for preventing childhood obesity and increase awareness among children, parents, and society leaders. Finally, COVID-19-related lockdown and restrictions have disrupted the daily routine of children and adolescents, moved it towards a sedentary lifestyle, and encouraged the consumption of high-caloric dense food114). The former has promoted weight gain, increased the prevalence rate of childhood obesity (as shown in Figure 2), and placed obese children at higher risk of severe COVID-19 infection54, 55, 62, 63). Therefore, to decrease weight gain and the severity of the infection, there is an urgent need for public health interventions to complement efforts to improve a healthy lifestyle. These may include (but are not limited to): encouraging physical activity and healthy eating in the general population during the upcoming COVID-19 waves and subsequent lock-down events, especially for children and families at risk.
Conclusion
Childhood obesity is a serious medical condition that affects children and adolescent males and females. It begins when energy balance is disturbed, and as a consequence, cellular fat accumulation is increased. A continuous fat deposition will lead to insulin resistance, forcing the body to deposit fat in unusual places (abdominal cavity and liver). Childhood obesity is a multi-factorial disorder with major contributions from genetic and environmental factors. Increased caloric intake and decreased physical activity may explain the growing prevalence of childhood obesity worldwide. Factors contributing to childhood obesity include modifiable factors such as daily caloric intake, inactivity, and birth weight and non-modifiable factors such as genetics, ethnicity, and parental education level. Due to the COVID-19 pandemic, children and their families have been forced to spend more time at home. It caused an increase in the prevalence of weight gain and obesity. Furthermore, obese children are at higher risk for severe COVID-19 infection compared with non-obese. It has placed children under serious health consequences. There is a strong relationship between childhood obesity and adolescent obesity; however, the relationship between childhood obesity and adulthood obesity is still debatable, as most obese adults are not obese children. The former highlights the pivotal impact of environmental factors contributing to the sharp increase in the prevalence of obesity in general. Obesity during childhood may predispose the child to numerous health risks, which can be serious if the obesity is not addressed. There is a wide range of potential childhood obesity prevention interventions, from educating families to educational institutions applying prevention strategy measures. To achieve optimal growth, development, and health and to combat the growing prevalence of childhood obesity, infants should be exclusively breastfed for the first six months of life. Public health campaigns and social media should be integrated to include programs addressing childhood obesity and its associated risks. Preventing childhood obesity can be achieved by educating children, parents, families, and educational institutions.
Acknowledgment
We would like to thank Dr. Nasheen Naidoo from the Department of Pathology, Tygerberg Hospital and Stellenbosch Faculty of Medicine and Health Sciences, Cape Town, South Africa for the critical reading of this article.
Conflict of interest: none.
Received June 30, 2022 / Accepted December 15, 2022
A. T. Ali • F. Al-ani • Dr. Osamah Al-ani
Faculty of Medicine, Odessa National Medical University
Valikhovs’kyi Ln, 2
650 00 Odessa, Ukraine
e-mail: oalani@europe.com
Zdroje
1. WHO. Report of the Commission on Ending Childhood Obesity. Implantation plan: executive summary. Geneva: World Health Organization; 2017 (WHO/NMH/PND/ ECHO/17.1). Licence: CC BY-NC-SA 3.0 IGO.
2. Cole T. J., Freeman J. V., Preece M. A. Body mass index reference curves for the UK, 1990. Arch. Dis. Child. 1995; 73(1), 25–29.
3. Cole T. J., Bellizzi M. C., Flegal K. M., Dietz W. H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320(7244), 1240.
4. Herbert A., Gerry N. P., McQueen M. B., et al. A common genetic variant is associated with adult and childhood obesity. Science 2006; 312(5771), 279–283.
5. Mejıa-Benıtez M. A., Bonnefond A., Yengo L., et al. Beneficial effect of a high number of copies of salivary amylase AMY1 gene on obesity risk in Mexican children. Diabetologia 2015; 58(2), 290–294.
6. Burke V., Beilin L. J., Dunbar D. Family lifestyle and parental body mass index as predictors of body mass index in Australian children: a longitudinal study. Int. J. Obes. Relat. Metab. Disord. 2001; 25(2), 147–157.
7. Isong I. A., Rao S. R. Bind M. A., et al. Racial and ethnic disparities in early childhood obesity. Pediatrics 2018; 141(1), pii: e20170865.
8. Min J., Wen X., Xue H., Wang Y. Ethnic disparities in childhood BMI trajectories and obesity and potential causes among 29,250 US children: Findings from the Early Childhood Longitudinal Study-Birth and Kindergarten Cohorts. Int. J. Obes. (London) 2018; 42(9), 1661–1670.
9. Guerrero A. D., Mao C., Fuller B., et al. Racial and Ethnic Disparities in Early Childhood Obesity: Growth Trajectories in Body Mass Index. J. Racial Ethn. Health Disparities 2016; 3(1), 129–137.
10. Zilanawala A., Davis-Kean P., Nazroo J., et al. Race/ ethnic disparities in early childhood BMI, obesity and overweight in the United Kingdom and United States. Int. J. Obes. 2015; 39(3), 520–911.
11. Hawkins S. S., Cole T. J., Law C. Millennium Cohort Study Child Health Group. An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK Millennium Cohort Study. J. Epidemiol. Community Health 2009; 63(2), 147–155.
12. Lombardo F. L., Spinelli A., Lazzeri G., et al. Severe obesity prevalence in 8 - to 9-year-old Italian children: a large population-based study. Eur. J. Clin. Nutr. 2015; 69(5), 603–608.
13. Brophy S., Cooksey R., Gravenor M. B., et al. Risk factors for childhood obesity at age 5: analysis of the Millennium Cohort Study. BMC Public Health 2009; 9, 467.
14. Ali A. T., Crowther N. J. Factors predisposing to obesity: A review of the literature. JEMDSA 2009; 14(2), 81–84.
15. Maffeis C., Grezzani A., Perrone L., Del Giudice E. M., Saggese G., Tatò L. Could the savory taste of snacks be a further risk factor for overweight in children? J. Pediatr. Gastroenterol. Nutr. 2008; 46(4), 429–437.
16. Eloranta A. M., Lindi V., Schwab U., et al. Dietary factors associated with overweight and body adiposity in Finnish children aged 6–8 years: the PANIC Study. Int. J. Obes. (London), 2012; 36(7), 950–955.
17. Rios-Castillo I., Cerezo, S., Corvalán C., et al. Risk factors during the prenatal period and the first year of life associated with overweight in 7-year-old low-income Chilean children. Matern. Child. Nutr. 2015; 11(4), 595 – 605.
18. Tchoubi S., Sobngwi-Tambekou J., Noubiap J. J., et al. Prevalence and risk factors of overweight and obesity among children aged 6-59 months in Cameroon: A multistage, stratified cluster sampling nationwide survey. PLoS One 2015; 10(12), e0143215.
19. Zhang J., Himes J. H., Guo Y., et al. Birth weight, growth and feeding pattern in early infancy predict overweight/ obesity status at two years of age: a birth cohort study of Chinese infants. PLoS One 2013; 8(6), e64542.
20. Tremblay M. S., Willms J. D. Is the Canadian childhood obesity epidemic related to physical inactivity? Int. J. Obes. Relat. Metab. Disord. 2003; 27(9), 1100–1105.
21. Shi Y., De Groh M., Morrison H. Perinatal and early childhood factors for overweight and obesity in young Canadian children. Can. J. Public Health 2013; 104(1), e69–74.
22. Carson V., Tremblay M. S., Chastin S. F. M. Cross-sectional associations between sleep duration, sedentary time, physical activity, and adiposity indicators among Canadian preschool-aged children using compositional analyses. BMC Public Health 2017; 17(Suppl 5), 848.
23. Hunsberger M., Lanfer A., Reeske A., et al. Infant feeding practices and prevalence of obesity in eight European countries – the IDEFICS study. Public Health Nutr. 2013; 16(2), 219–227.
24. Forbes J. D., Azad M. B., Vehling L., et al. association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2018; 172(7), e181161.
25. Oken E., Kleinman K. P., Belfort M. B., et al. Associations of gestational weight gain with short - and longer - term maternal and child health outcomes. Am. J. Epidemiol. 2009; 170(2), 173–180.
26. Battista M. C., Hivert M. F., Duval K., et al. Intergenerational cycle of obesity and diabetes: how can we reduce the burdens of these conditions on the health of future generations? Exp. Diabetes Res. 2011; 2011, 596060.
27. Mitanchez D., Yzydorczyk C., Siddeek B., et al. The offspring of the diabetic mother-short - and long-term implications. Best Pract. Res. Clin. Obstet. Gynaecol. 2015; 29(2), 256–269.
28. Cox R., Skouteris H., Rutherford L., et al. Television viewing, television content, food intake, physical activity and body mass index: a cross-sectional study of preschool children aged 2–6 years. Health Promot. J. Austr. 2012; 23(1), 58–62.
29. Alghadir A. H., Gabr S. A., Iqbal Z. A. Television watching, diet and body mass index of school children in Saudi Arabia. Pediatr. Int. 2016; 58(4), 290–294.
30. Merten M. J., Williams A. L., Shriver L. H. Breakfast consumption in adolescence and young adulthood: parental presence, community context, and obesity. J. Am. Diet. Assoc. 2009; 109(8), 1384–1391.
31. Affenito S. G., Thompson D. R., Barton B. A., et al. Breakfast consumption by African-American and white adolescent girls correlates positively with calcium and fiber intake and negatively with body mass index. J. Am. Diet. Assoc. 2005; 105(6), 938–945.
32. Reinehr T., Hinney A., de Sousa G., et al. Definable somatic disorders in overweight children and adolescents. J. Pediatr. 2007; 150, 613.
33. Reilly J. J., Armstrong J., Dorosty A. R., et al. Early life risk factors for obesity in childhood: cohort study. BMJ 2005; 330(7504), 1357–1363.
34. Bammann K., Peplies J., de Henauw S., et al. Early life course risk factors for childhood obesity: the IDEFICS case-control study. PLoS One 2014; 9(2), e86914.
35. Ngan H. T. D., Tuyen L. D., Phu P. V., et al. childhood overweight and obesity amongst primary school children in Hai Phong City, Vietnam. Asia Pac. J. Clin. Nutr. 2018; 27(2), 399–405.
36. Mitchell J. A., Dowda M., Pate R. R., et al. Physical activity and pediatric obesity: a quantile regression analysis. Med. Sci. Sports Exerc. 2017; 49(3), 466–473.
37. Yan J., Liu L., Zhu Y., et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 2014; 14, 1267.
38. Hesketh K., Waters E., Green J., et al. Healthy eating, activity and obesity prevention: a qualitative study of parent and child perceptions in Australia. Health Promot. Int. 2005; 20(1), 19–26.
39. Lazarou C., Matalas A. L. Breakfast intake is associated with nutritional status, Mediterranean diet adherence, serum iron and fasting glucose: the CY Families study. Public Health Nutr. 2015; 18(7), 1308–1316.
40. Tognon G., Hebestreit A., Lanfer A., et al. Mediterranean diet, overweight and body composition in children from eight European countries: cross-sectional and prospective results from the IDEFICS study. Nutr. Metab. Cardiovasc. Dis. 2014; 24(2), 205–213.
41. Bertoli S., Leone A., Vignati L., et al. Adherence to the Mediterranean diet is inversely associated with visceral abdominal tissue in Caucasian subjects. Clin. Nutr. 2015; 34(6), 1266–1272.
42. Kelishadi R., Mirmoghtadaee P., Najafi H., et al. Systematic review on the association of abdominal obesity in children and adolescents with cardio-metabolic risk factors. J. Res. Med. Sci. 2015; 20(3), 294–307.
43. Morandi A., Maschio M., Marigliano M., et al. Screening for impaired glucose tolerance in obese children and adolescents: a validation and implementation study. Pediatr. Obes. 2014; 9(1): 17–25.
44. Temneanu O. R., Trandafir, L. M., Purcarea M. R. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J. Med. Life 2016; 9(3), 235–239.
45. Manco M., Nobili V., Alisi A., et al. Arterial stiffness, thickness and association to suitable novel markers of risk at the origin of cardiovascular disease in obese children. Int. J. Med. Sci. 2017; 14(8), 711–720.
46. Bout-Tabaku S., Shults J., Zemel B. S., et al. obesity is associated with greater valgus knee alignment in pubertal children, and higher body mass index is associated with greater variability in knee alignment in girls. J. Rheumatol. 2015; 42(1), 126–133.
47. Félix D. R., Costenaro F., Gottschall C. B., et al. Non-alcoholic fatty liver disease (Nafld) in obese children - effect of refined carbohydrates in diet. BMC Pediatr. 2016; 16(1), 187.
48. Pinola P., Lashen H., Bloigu A., et al. Menstrual disorders in adolescence: a marker for hyperandrogenaemia and increased metabolic risks in later life? Finnish general population - based birth cohort study. Hum. Reprod. 2012; 27(11), 3279–3286.
49. McCormack L. A., Laska M. N., Gray C., et al. Weight-related teasing in a racially diverse sample of sixth-grade children. J. Am. Diet. Assoc. 2011; 111(3), 431–436.
50. Bang K. S., Chae S. M., Hyun M. S., et al. The mediating effects of perceived parental teasing on relations of body mass index to depression and self-perception of physical appearance and global self-worth in children. J. Adv. Nurs. 2012; 68(12), 2646–2653.
51. Morales D. X., Prieto N., Grineski S. E., et al. Race/ ethnicity, obesity, and the risk of being verbally bullied: a National Multilevel Study. J. Racial Ethn. Health Disparities 2019; 6(2), 245–253.
52. Sutaria S., Devakumar D., Yasuda S. S., et al. Is obesity associated with depression in children? Systematic review and meta-analysis. Arch. Dis. Child. 2019; 104(1), 64–74.
53. Sobol-Goldberg S., Rabinowitz J. Association of childhood and teen school performance and obesity in young adulthood in the US National Longitudinal Survey of Youth. Prev. Med. 2016; 89, 57–63.
54. la Fauci G., Montalti M., di Valerio Z., et al. Obesity and COVID-19 in children and adolescents: reciprocal detrimental influence – systematic literature review and meta-analysis. Int. J. Environ. Res. Public Health 2022; 19, 7603.
55. Rundle A. G., Park Y., Herbstman J. B., Kinsey E. W., Wang Y. C. COVID-19-related school closings and risk of weight gain among children. Obesity (Silver Spring) 2020; 28(6), 1008–1009.
56. Carroll N., Sadowski A., Laila A., et al. The impact of COVID-19 on health behavior, stress, financial and food security among middle to high income Canadian families with young children. Nutrients 2020; 12(8).
57. de Luis Roman D. A., Izaola O., Primo Martin D., et al. effect of lockdown for COVID-19 on self-reported body weight gain in a sample of obese patients. Nutr. Hosp. 2020; 37(6), 1232–1237.
58. Grannell A., le Roux C. W., McGillicuddy D. I am terrified of something happening to me the lived experience of people with obesity during the COVID-19 pandemic. Clin Obes. 2020; 10(6), e12406.
59. Stavridou A., Kapsali E, Panagouli E, et al. obesity in children and adolescents during Covid-19 pandemic. Children (Basal) 2021; 8(2), 135.
60. Khan MA, Moverley Smith JE. “Covibesity” a new pandemic. Obes Med. 2020; 19, 100282.
61. Vallis M., Glazer S. Protecting individuals living with overweight and obesity: attitudes and concerns towards COVID-19 vaccination in Canada. Obesity (Silver Spring) 2021; 29(7), 1128–1137.
62. Wei Y. Y., Wang R. R., Zhang D. W., et al. Risk factors for severe COVID-19: Evidence from 167 hospitalised patients in Anhui, China. J. Infect. 2020; 81(1), e89–e92.
63. Simonnet A., Chetboun M., Poissy J., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus - 2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020; 28(7), 1195–1199.
64. Gregoire F. M., Smas C. M., Sul H. S. Understanding adipocyte differentiation. Physiol. Rev. 1998; 78(3), 783–809.
65. Ali A. T., Hochfeld W. E., Myburgh R., Pepper M. S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013; 92(6–7), 229–236.
66. Muir L. A., Neeley C. K., Meyer K. A., et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring) 2016; 24(3), 597–605.
67. Jo J., Gavrilova O., Pack S., et al. Hypertrophy and /or hyperplasia: dynamics of adipose tissue growth. PLoS Comput. Biol. 2009; 5(3), e1000324.
68. Ali A. T., Crowther N. J. Body fat distribution and insulin resistance. SAMJ 2005; 95(11), 878–880.
69. Ali A. T., Ferris W. F., Naran N. H., Crowther N. J. Insulin resistance in the control of body fat distribution: a new hypothesis. Horm. Metab. Res. 2011; 43(2), 77–80.
70. Landgraf K., Rockstroh D., Wanger I. V., et al. evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes 2015; 64(4), 1249–1261.
71. Satoh T. Molecular mechanism for the regulation of insulin stimulated glucose uptake by small guanosine triphosphatases in skeletal muscle and adipocytes. Int. J. Mol. Sci. 2014; 15(10), 18677–18692.
72. Guilherme A., Virbasius J. V, Puri V., Czech M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008; 9(5), 367–77.
73. Joe A. W. B., Yi L., Even Y., Vogl A. W., Rossi F. M. V. Depots - specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 2009; 27, 2563–2570.
74. Schorr M., Dichtel L. E., Gerweck A. V., Valera R. D., Torriani M., Miller K. K., Bredella M. A. Sex differences in body composition and association with cardiometabolic risk. Biol. Sex Differ. 2018; 9(1), 28.
75. Bacopoulou F., Efthymiou V., Landis G., Rentoumis A., Chrousos G. P. Waist circumference, waist-to-hip ratio and waist-to-height ratio reference percentiles for abdominal obesity among Greek adolescents. BMC Pediatr. 2015; 15, 50.
76. Kruger H. S., Faber M., Schutte A. E., Ellis S. M. A proposed cutoff point of waist-to-height ratio for metabolic risk in African township adolescents. Nutrition 2013; 29(3), 502–507.
77. Drolet R., Richard C., Sniderman A. D., et al. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int. J. Obes. 2008; 32, 283–291.
78. Spalding K. L., Arner E., Westermark P. O., et al. Dynamics of fat cell turnover in humans. Nature 2008; 453, 783–787.
79. Jo J., Guo J., Liu T., Mullen S., Hall K. D., Cushman S. W., Periwal V. Hypertrophy-driven adipocyte death overwhelms recruitment under prolonged weight gain. Biophysical J. 2010; 99(11), 3535–3544.
80. Henriksson P., Sandborg J., Henstrom M., et al. Body composition, physical fitness and cardiovascular risk factors in 9-year-old children. Sci. Rep. 2022; 12, 2665.
81. Aris I. M., Rifas-Shiman S. L., Li L. J., et al. Patterns of body mass index milestones in early life and cardiometabolic risk in early adolescence. Int. J. Epidemiol. 2019; 48(1), 157–167.
82. Aarestrup J., Blond K., Vistisen D., Jørgensen M. E., Frimodt-Møller M., Jensen B. W., et al. Childhood body mass index trajectories and associations with adult-onset chronic kidney disease in Denmark: A population-based cohort study. PLoS Med. 2022; 19(9), e1004098.
83. Rhee C. M., Ahmadi S. F., Kalantar-Zadeh K. The dual roles of obesity in chronic kidney disease: A review of the current literature. Curr. Opin. Nephrol. Hypertens. 2016; 25(3), 208–216.
84. Berndt S. I., Gustafsson S., Mägi R., et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 2013; 45(5), 501–512.
85. Bradfield J. P., Taal H. R., Timpson N. J., et al. A genome - wide association meta-analysis identifies new childhood obesity loci. Nat. Genet. 2012; 44(5), 526–531.
86. Gillman M. W., Rifas-Shiman S., Berkey C. S., Field A. E., Colditz G. A. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003; 111(3), e221–226.
87. Oken E., Rifas-Shiman S. L., Field A. E., et al. Maternal gestational weight gain and offspring weight in adolescence. Obstet. Gynecol. 2008; 112(5), 999–1006.
88. Robinson E, Boyland E., Chisholm A., et al. Obesity, eating behavior and physical activity during COVID-19 lockdown: a study of UK adults. Appetite 2021; 156, 104853.
89. Lo J. C., Maring B., Chandra M., et al. prevalence of obesity and extreme obesity in children aged 3–5 years. Pediatr. Obes. 2014; 9(3), 167–175.
90. Boeke C. E., Oken E., Kleinman K. P., Rifas-Shiman S. L., Taveras E. M., Gillman M. W. Correlations among adiposity measures in school-aged children. BMC Pediatr. 2013; 13, 99.
91. Simmonds M., Llewellyn A., Owen C. G., Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes. Rev. 2016; 17(2), 95–107.
92. Nader P. R., O’Brien M., Houts R., et al. Identifying risk for obesity in early childhood. Pediatrics 2006; 118(3), e594–601.
93. The N. S., Suchindran C., North K. E., Popkin B. M., Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA 2010; 304(18), 2042–2047.
94. Rooney B. L., Mathiason M. A., Schauberger C. W. Predictors of obesity in childhood, adolescence, and adulthood in a birth cohort. Matern. Child. Health J. 2011; 15(8), 1166–1175.
95. Power C., Lake J. K., Cole T. J. Body mass index and height from childhood to adulthood in the 1958 British born cohort. Am. J. Clin Nutr. 1997; 66, 1094–1101.
96. Gibbs B. B., Brach J. S., Byard T., et al. Reducing sedentary behavior versus increasing moderate-to-vigorous intensity physical activity in older adults. J. Aging Health 2017; 29(2), 247–267.
97. Adams J. P., Murphy P. G. Obesity in anaesthesia and intensive care. BJA 2000; 85, 91–108.
98. Mitchell J. A., Mattocks C., Ness A. R., et al. Sedentary behavior and obesity in a large cohort of children. Obesity (Silver Spring) 2009; 17(8), 1596–1602.
99. Hirschler V., Bugna J., Roque M., Gilligan T., Gonzalez C. Does low birth weight predict obesity/overweight and metabolic syndrome in elementary school children? Arch. Med. Res. 2008; 39(8), 796–802.
100. Reilly J. J., Armstrong J., Dorosty A. R., et al. Early life risk factors for obesity in childhood: cohort study. BMJ 2005; 330(7504), 1357.
101. Guo S. S., Chumlea W. C. Tracking of body mass index in children in relation to overweight in adulthood. Am. J. Clin. Nute. 1999; 70(1), 145S–148S.
102. Fernández-Alvira J. M., Mouratidou T., Bammann K., et al. Parental education and frequency of food consumption in European children: the IDEFICS study. Public Health Nutr. 2013; 16(3), 487–498.
103. Hoang N. T. D., Orellana L., Le T. D., et al. Anthropometric status among 6¯9-year-old school children in rural areas in Hai Phong City, Vietnam. Nutrients 2018; 10(10), pii: E1431.
104. Ensenauer R., Brandlhuber L., Burgmann M., et al. Obese nondiabetic pregnancies and high maternal glycated hemoglobin at delivery as an indicator of offspring and maternal postpartum risks: The Prospective PEACHES Mother-Child Cohort. Clin. Chem. 2015; 61(11), 1381–1390.
105. Maffoni S., de Giuseppe R., Stanford F. C., Cena H. Folate status in women of childbearing age with obesity: a review. Nutr. Res. Rev. 2017; 30(2), 265–271.
106. Shapiro A. L., Kaar J. L., Crume T. L., et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int. J. Obes. (London) 2016; 40(7), 1056–1062.
107. Aris I. M., Bernard J. Y., Chen L. W., et al. Infant body mass index peak and early childhood cardiometabolic risk markers in multi-ethnic Asian birth cohort. Int. J. Epidemiol. 2017; 46, 513–525.
108. Whitaker R. C., Wright J. A. Pepe M. S., Seidel K. D., Dietz W. H. Predicting obesity in young adulthood from childhood and parental obesity. New Engl. J. Med. 1997; 337(13), 869–873.
109. Jiang J., Rosenqvist U., Wang H., Greiner T., Ma Y., Toschke A. M. Risk factors for overweight in 2 - to 6-year-old children in Beijing, China. Int. J. Pediatr. Obes. 2006; 1(2), 103–108.
110. Johannsen D. L., Johannsen N. M., Specker B. L. Influence of parents’ eating behaviours and child feeding practices on children’s weight status. Obesity (Silver Spring) 2006; 14(3), 431–439.
111. Tao Z. L., Zhong W. F. The correlation of Chinese mothers’ eating attitudes and psychological characteristics with their children’s eating attitudes, as well as the gender effect on eating attitudes of children. Eat. Weight Disord. 2008; 13, 149–156.
112. Biehl A., Hovengen R., Grøholt E. K., Hjelmesæth J., Strand B. H., Meyer H. E. Parental marital status and childhood overweight and obesity in Norway: a nationally representative cross-sectional study. BMJ Open 2014; 4(6), e004502.
113. Dennison B. A., Erb T. A., Jenkins P. L. Television viewing and television in bedroom associated with overweight risk among low-income preschool children. Pediatrics 2002; 109(6), 1028–1035.
114. Coulthard H., Sharps M., Cunliffe L., van den Tol A. Eating in the lockdown during the Covid 19 pandemic; self-reported changes in eating behaviour, and associations with BMI, eating style, coping and health anxiety. Appetite 2022; 161, 105082.
115. Berenson G. S., Srinivasan S. R., Bao W., Newman W. P., Tracy R. E., Wattigney W. A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa heart study. New Engl. J. Med. 1998; 338, 1650–1656.
116. Wang J., Zhu Y., Jing J., et al. Relationship of BMI to the incidence of hypertension: a 4 years’ cohort study among children in Guangzhou, 2007–2011. BMC Public Health 2015; 15, 782.
117. Ali A. T., Al-ani O., Al-ani F., Guidozzi F. Polycystic ovary syndrome and metabolic disorders: A review of the literature. Afr. J. Reprod. Health 2022; 26(8), 89–99.
118. Feldstein A. E., Charatcharoenwitthaya P., Treeprasertsuk S., Benson J. T., Enders F. B., Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study up to 20 years. Gut 2009; 58, 1538–1544.
119. Jones M. H., Roncada C., Fernandes M. T. C., et al. Asthma and obesity in children are independently associated with airway dysanapsis. Front. in Pediatr. 2017; 5, 270.
120. Alonso-Álvarez M. L., Cordero-Guevara J. A., Terán-Santos J., et al. Obstructive sleep apnea in obese community - dwelling children: the NANOS study. Sleep 2014; 37(5), 943–949.
121. Schwimmer J. B., Burwinkle T. M., Varni J. W. Health related quality of life of severely obese children and adolescents. JAMA 2003; 289(14), 1813–1819.
122. Goodman E., Whitaker R. C. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics 2002; 110, 497–504.
123. Kosaraju R., Guesdon W., Crouch M. J., et al. B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon murine influenza infection. J. Immunol. 2017; 198(12), 4738–4752.
124. Milner J. J., Beck M. A. The impact of obesity on the immune response to infection. Proc. Nutr. Soc. 2012; 71(2), 298–306.
125. Zachariah P., Johnson C. L., Halabi K. C., et al. Epidemiology, clinical features, and disease severity in patients with Coronavirus Disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatrics 2020; 174(10), e202430.
126. Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., Stachel A. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin. Infect. Dis. 2020; 71(15), 896–897.
127. Smith K. J., Gall S. L., McNaughton S. A., Blizzard L., Dwyer T., Venn A. J. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am. J. Clin. Nutr. 2010; 92, 1316–1325.
128. Mekary R. A., Giovannucci E., Willett W. C., van Dam R. M., Hu F. B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012; 95, 1182–1189.
129. Kobayashi F., Ogata H., Omi N., Nagasaka S., Yamaguchi S., Hibi M., Tokuyama K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes. Res. Clin. Pr. 2014; 8, e201–e298.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2023 Číslo 1- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Entry and competition of retail pharmacies: A case study of OTC drugs sales and ownership deregulation1
- Childhood obesity: causes, consequences, and prevention
- Contribution to the concept of polypharmacy II. Prescription and use of medicines
- New trends in advanced parkinson disease stage therapy
- Are some COVID-19 vaccines better than others regarding the short-term side effects?
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- New trends in advanced parkinson disease stage therapy
- Childhood obesity: causes, consequences, and prevention
- Contribution to the concept of polypharmacy II. Prescription and use of medicines
- Entry and competition of retail pharmacies: A case study of OTC drugs sales and ownership deregulation1
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



