-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaWhen genes move, genomes collide
article has not abstract
Published in the journal: . PLoS Genet 14(4): e32767. doi:10.1371/journal.pgen.1007286
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1007286Summary
article has not abstract
The ultimate test of whether two diverged populations are, in fact, good species is whether they will maintain their distinctness in sympatry [1, 2]. This requires the action of one or more reproductive isolating mechanisms. Identifying traits and alleles that underlie reproductive isolation has therefore been a major focus of speciation genetics research [3, 4]. In addition to informing the basis of reproductive isolation, alleles underlying interspecific incompatibilities can provide information concerning how functional evolutionary divergence unfolds.
The genus Mimulus has long served as a model for the study of reproductive isolation [5, 6]. Much of this work has focused on Mimulus guttatus and M. nasutus. These recently diverged species have broadly overlapping geographic ranges, and—when sympatric—M. nasutus’ ancestry frequently introgresses into M. guttatus [7–9]. Because hybridization and introgression in this species pair are common, the genetic mechanisms preventing their fusion is of great interest. In this issue, Zuellig and Sweigart [10] map the genetic basis of an inviability phenotype that only manifests in F2 M. nasutus × M. guttatus hybrids.
Zuellig and Sweigart [10] identify the precise alleles of hybrid seed inviability in a pair of recently related and naturally hybridizing species of Mimulus (Fig 1A). After two bulked segregant analyses to identify the incompatibilities, and some impressive snooping in the unassembled portion of the M. guttatus reference genome, Zuellig and Sweigart identify 2 genes involved in the defect: hl13 (Migut.M02023) and hl14 (Migut.O00467). These genes are duplicates of plastid transcriptionally active chromosome 14 (pTAC14)―a gene known to be essential for proper chloroplast development in Arabidopsis thaliana [11]. A functional copy of pTAC14 is found on chromosome 14 (hl14) of both the M. guttatus reference genome and the M. guttatus studied samples, but the gene is missing from M. nasutus’ chromosome 14. In contrast, both species have a syntenic copy of pTAC14 (hl13) on chromosome 13, but the M. guttatus allele includes a frameshift mutation and is not expressed. As a result, some F2 hybrids and advanced backcrossed seedlings will be homozygous for the nonfunctional M. guttatus’ copy of pTAC14 at hl13 and M. nasutus’ null allele on chromosome 14; these individuals will lack a functional pTAC14 gene, leaving them unable to photosynthesize and therefore inviable (Fig 1B).
Fig. 1. The history of the M. guttatus–M. nasutus species pair, and the evolution of the hl13/hl14 incompatibility. 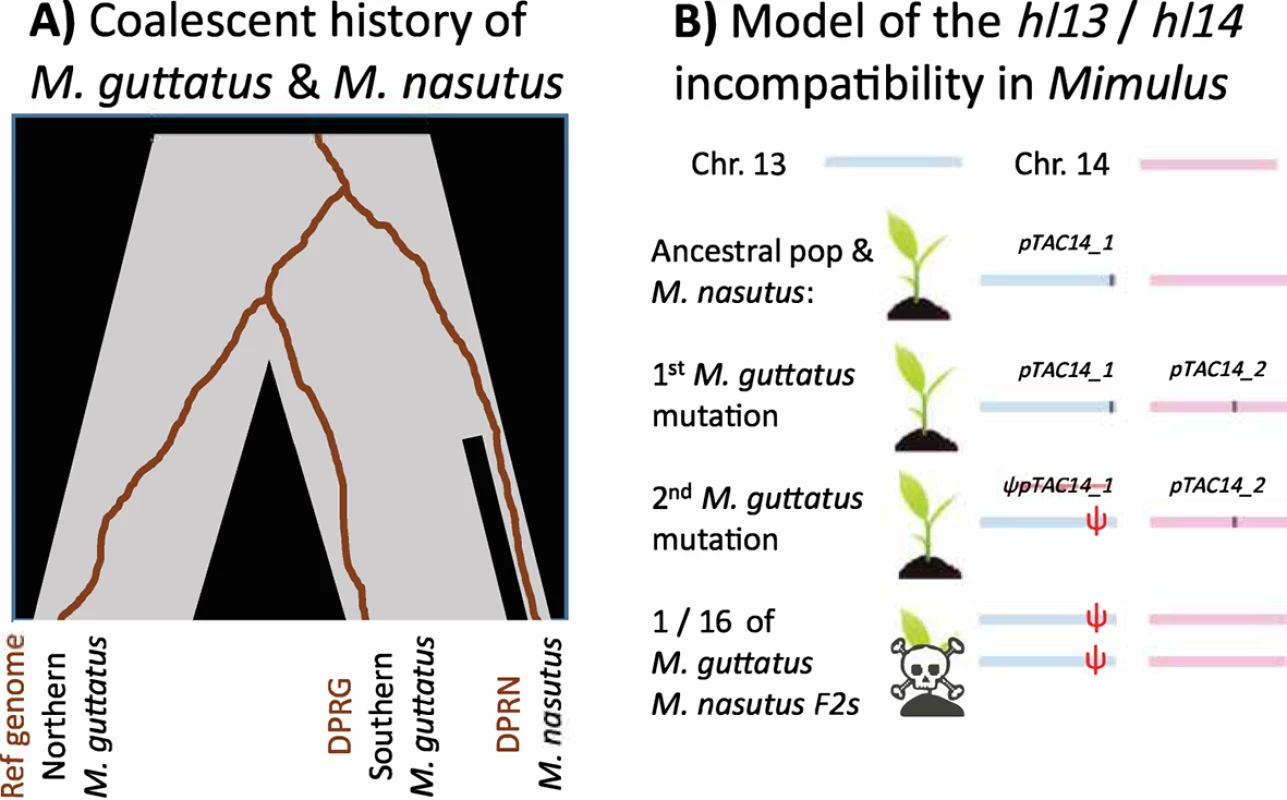
(A) Southern M. guttatus populations (including the sample DPRG studied by Zuellig and Sweigart) are more closely related to M. nasutus (sample DPRN) than to northern M. guttatus (including the M. guttatus reference genome). Nonetheless, DPRG is more similar to the reference strain at hl13 and hl14—the loci underlying the hybrid incompatibility identified by Zuellig and Sweigart—than M. nasutus (shown by the brown coalescent genealogies). (B) Zuellig and Sweigart found that (1) pTAC14 was ancestrally located in chromosome 13 in Mimulus, (2) a copy moved to chromosome 14 in M. guttatus, and (3) the initial copy then lost function in M. guttatus. While all F1s are viable, one-sixteenth of F2s inherit a chromosome 14 without pTAC14 and a chromosome 13 with a nonfunctional pTAC14. Because pTAC14 is a critical photosynthetic gene, these seedlings are inviable. The idea that divergent resolution of gene duplicates could result in the inviability of hybrids inheriting null alleles has been long hypothesized by theory [12–16]. The results are an intuitive consequence of gene movement and meiotic segregation. Moyle et al. [12] first formalized this model of stepwise gene movement leading to hybrid incompatibility―first a gene is duplicated, then it loses function in its original location, and, effectively, the only remaining copy is in a different chromosome. The nature of Mendelian segregation ensures hybrid defects that affect only a proportion of hybrids (in this case no F1 is affected, while one-sixteenth of the F2s are).
Previous studies also have lent support for this model. In the Drosophila simulans–D. melanogaster species pair, the transposition of the gene JYalpha from the fourth to the third chromosome in D. simulans causes sterility in the hybrid males [17]. A similar result has been found between accessions of the selfing plant A. thaliana from Columbia (Col) and Cape Verde Island (Cvi)—the histidinol-phosphate aminotransferasegene exists on chromosome 1 but not chromosome 5 in Cvi, and on chromosome 5 but not chromosome 1 in Col, and F2s lacking histidinol-phosphate aminotransferasegene are inviable [18]. Unlike M. guttatus and M. nasutus, none of these species pairs hybridize in nature.
The results from Zuellig and Sweigart have three important implications, each offering new research directions. First, this study will allow researchers to ask how incompatibility due to gene movement contributes to genome variation and differentiation in natural populations. For example, future studies and/or reexamination of genomic patterns of introgression between these species [9] could examine whether M. nasutus’ ancestry is elevated around hl14 and depleted around hl13 in sympatric M. guttatus, as would be expected if incompatible alleles are selected against upon introgression [19–21]. Additional directions could address how hl13/hl14 acts in concert with the other reproductive isolating mechanisms and mapped incompatibilities (e.g., [22]) to prevent the fusion of these species in sympatry.
Second, both the process of duplication of pTAC14 and nonfunctionalization are plausibly neutral, suggesting that reproductive isolation may have arisen by a neutral process. In fact, Zuellig and Sweigart find no strong evidence that natural selection is responsible for either pTAC14‘s duplication or degeneration, and as such the authors suggest that this incompatibility has a neutral origin. While this hypothesis is plausible, it clearly needs more scrutiny. Further study of the effects of the alternative functional copies, and additional population genomic studies of the history of selection on hl13 and hl14, could inform the evolutionary question of how and why genes relocate.
Finally, the recency of the split between these species and the extensive natural variation in M. guttatus mean that the hl13/hl14 incompatibility provides an excellent opportunity to identify the factors shaping the alternative resolution of alternative paralogs. This question is particularly interesting because of the evolutionary history of the species’ split; the M. guttatus population studied by Zuellig and Sweigart is more closely related to M. nasutus than it is to the M. guttatus reference genome with which it shares hl13 and hl14 alleles [8]. This observation―that the gene trees underlying hybrid incompatibilities do not match the population tree—may seem counterintuitive, but is potentially consistent with recent results from theoretical population genetics [23].
Overall, Zuellig and Sweigart’s results provide empirical evidence that the evolution of gene duplicates might be involved in reproductive isolation in young species that hybridize in nature. Systematic efforts like Zuellig and Sweigart’s will reveal to what extent gene movement is a prevalent force in the formation of species. More generally, their study provides a map route to understand what is the role of hybrid incompatibilities in keeping naturally co-occurring―and potentially hybridizing―species apart.
Zdroje
1. Coyne JA, Orr HA (2004) Speciation. Sunderland, MA: Sinauer Associates, Inc.
2. Harrison RG (2012) The language of speciation. Evolution 66(12):3643–3657. doi: 10.1111/j.1558-5646.2012.01785.x 23206125
3. Maheshwari S, Barbash DA (2011) The genetics of hybrid incompatibilities. Annu Rev Genet 45(1):331–355.
4. Nosil P, Schluter D (2011) The genes underlying the process of speciation. Trends Ecol Evol 26(4):160–167. doi: 10.1016/j.tree.2011.01.001 21310503
5. Vickery RK (1964) Barriers to gene exchange between members of the Mimulus guttatus complex. Source Evol 18(1):52–69.
6. Wu CA, Lowry DB, Cooley AM, Wright KM, Lee YW, Willis JH (2008) Mimulus is an emerging model system for the integration of ecological and genomic studies. Heredity (Edinb) 100(2):220–230.
7. Sweigart AL, Willis JH (2003) Patterns of nucleotide diversity in two species of Mimulus are affected by mating system and asymmetric introgression. Evolution (N Y) 57(11):2490–2506.
8. Brandvain Y, Kenney AM, Flagel L, Coop G, Sweigart AL (2014) Speciation and Introgression between Mimulus nasutus and Mimulus guttatus. PLoS Genet 10(6):e1004410. doi: 10.1371/journal.pgen.1004410 24967630
9. Kenney AM, Sweigart AL (2016) Reproductive isolation and introgression between sympatric Mimulus species. Mol Ecol 25(11):2499–2517. doi: 10.1111/mec.13630 27038381
10. Zuellig M,Sweigart AL (2018) Gene duplicates cause hybrid lethality between sympatric species of Mimulus. PLoS Genet 14(4): e1007130. https://doi.org/10.1371/journal.pgen.1007130
11. Gao Z-P, Yu QB, Zhao TT, Ma Q, Chen GX, Yang ZN (2011) A Functional Component of the Transcriptionally Active Chromosome Complex, Arabidopsis pTAC14, Interacts with pTAC12/HEMERA and Regulates Plastid Gene Expression. Plant Physiol 157(4):1733–1745. doi: 10.1104/pp.111.184762 22010110
12. Moyle LC, Muir CD, Han M V., Hahn MW (2010) The contribution of gene movement to the “two rules of speciation.” Evolution 64(6):1541–1557. doi: 10.1111/j.1558-5646.2010.00990.x 20298429
13. Werth CR, Windham MD (1991) A model for divergent, allopatric speciation of polyploid Pteridophytes resulting from silencing of duplicate-gene expression. Am Nat 137(4):515–526.
14. Lynch M, Force A (2000) The probability of duplicate gene preservation by subfunctionalization. Genetics 154(1):459–473. 10629003
15. Muller HJ (1942) Isolating mechanisms, evolution and temperature. Biol Symp 6 : 71–125
16. Dobzhansky T (1937) Genetics and the Origin of Species. New York: Columbia University Press.
17. Masly JP, Presgraves DC (2007) High-resolution genome-wide dissection of the two rules of speciation in Drosophila. PLoS Biol 5(9):e243. doi: 10.1371/journal.pbio.0050243 17850182
18. Bikard D, Patel D, Le Metté C, Giorgi V, Camilleri C, Bennett MJ et al. (2009) Divergent evolution of duplicate genes leads to genetic incompatibilities within A. thaliana. Science (80-) 323(5914):623–626. doi: 10.1126/science.1165917 19179528
19. Bank C, Bürger R, Hermisson J (2012) The limits to parapatric speciation: Dobzhansky-Muller incompatibilities in a continent-Island model. Genetics 191(3):845–863. doi: 10.1534/genetics.111.137513 22542972
20. Höllinger I, Hermisson J (2017) Bounds to parapatric speciation: A Dobzhansky–Muller incompatibility model involving autosomes, X chromosomes, and mitochondria. Evolution (N Y) 71(5):1366–1380.
21. Turissini DA, Matute DR (2017) Fine scale mapping of genomic introgressions within the Drosophila yakuba clade. PLoS Genet 13(9):e1006971. doi: 10.1371/journal.pgen.1006971 28873409
22. Ferris KG, Barnett LL, Blackman BK, Willis JH (2017) The genetic architecture of local adaptation and reproductive isolation in sympatry within the Mimulus guttatus species complex. Mol. Ecol, 26 : 208–224. doi: 10.1111/mec.13763 27439150
23. Wang RJ, Hahn MW (2018) Speciation genes are more likely to have discordant gene trees. bioRxiv. doi: 10.1101/244822
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2018 Číslo 4
Nejčtenější v tomto čísle- Intronic gene mutations cause a splicing defect by a novel mechanism involving U1snRNP binding downstream of the 5’ splice site
- When genes move, genomes collide
- House dust mites use a plant-like siRNA pathway to silence transposable elements
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



