-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaPediatric Oncology as the Next Global Child Health Priority: The Need for National Childhood Cancer Strategies in Low- and Middle-Income Countries
article has not abstract
Published in the journal: . PLoS Med 11(6): e32767. doi:10.1371/journal.pmed.1001656
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1001656Summary
article has not abstract
Summary Points
-
As is already the case in high-income countries, cancer represents the leading cause of non-accidental death among children in a growing number of middle-income countries
-
Meaningful declines in global childhood cancer mortality will require moving beyond the current situation through the establishment of national childhood cancer strategies
-
Key components of such strategies include financial coverage, accreditation of childhood cancer centers, mandatory childhood cancer reporting and registration, development of national standards of care, and the creation of national childhood cancer governing bodies
-
Challenges to implementing such strategies include a paucity of implementation research, formal policy evaluation, and costing data
-
The ideal structure of such strategies in low-income countries is currently unknown, given severe resource constraints, deficits in infrastructure, and competing health needs
Introduction
While the last several decades have witnessed tremendous advances in cure rates for childhood cancer, these improvements have not translated to low-and-middle-income countries (LMICs), where the majority of children reside [1]. In this article, we outline why pediatric cancer should now be considered a global child health priority, describe the need for national childhood cancer strategies (NCCS), and highlight necessary policy components to reduce LMIC pediatric cancer mortality rates.
Pediatric Cancer as a Global Child Health Priority
Major shifts in the magnitude and causes of childhood mortality have occurred in many LMICs, including 106 countries with accelerated declines in childhood mortality from 1990 to 2011; 80% of this decline was due to reductions in death from infectious causes [2]. A large, and growing, proportion of global childhood mortality is therefore due to non-communicable disease [3],[4]. Indeed, 6.0% and 18.6% of deaths among children ages 5 to 14 years in lower - and upper-middle-income countries (MICs), respectively, are due to cancer [5]. As is already the case in high-income countries (HICs), cancer represents the leading cause of non-accidental death among children in a growing number of MICs [6],[7]. In absolute terms, of the 175,000 children diagnosed with cancer annually, an estimated 150,000 live in LMICs [5]. Even this figure represents a substantial underestimate given the endemic under-diagnosis and under-registration of LMIC children with cancer [1].
Unlike many adult malignancies, most pediatric cancers are not associated with modifiable risk factors and are not amenable to population-based screening and prevention programs [8]. Decreasing childhood cancer mortality thus requires accurate diagnosis followed by effective treatment. Fortunately, such treatment exists; in HICs over 80% of children with malignancies are cured [6],[9],[10]. Even simple, low-intensity treatment regimens can cure a significant portion of patients. About half of children with Burkitt lymphoma, the most common childhood malignancy in parts of sub-Saharan Africa, are curable with three to six doses of single-agent cyclophosphamide, demonstrating the achievements possible in even the most resource-limited settings [11]. Preliminary evidence suggests that such treatment is very cost effective [12].
In HICs, the dominant paradigm is to deliver pediatric cancer treatment through a limited number of treatment centers (and associated satellites) in which resources and expertise are concentrated. By contrast, in the majority of LMICs, care is currently delivered without any overarching structure or policy. Though centers of excellence exist in many LMICs, and some benefit from “twinning” partnerships with HIC centers [13]–[15], the absence of explicit national pediatric strategies results in a lack of access to care for the vast majority of LMIC children with cancer [5],[16].
Building National Childhood Cancer Strategies
Meaningful declines in global childhood cancer mortality will require moving beyond the current situation through the establishment of NCCS. For maximal impact, NCCS should include several key policy components, as outlined in Box 1 and detailed below. While examples of such strategies are rare in LMICs, notable exceptions include the recent expansion of Seguro Popular in Mexico, which is used as an illustration throughout this article [17]–[19].
Box 1. Key Components of National Childhood Cancer Strategies in Low - and Middle-Income Countries
-
Financial coverage of childhood cancer treatment – Limiting financial burdens on caregivers is essential to increasing access to lifesaving therapies.
-
Accreditation of childhood cancer centers – Treating institutions should be accredited based on infrastructure, patient volumes, and reporting ability. Financial incentives may be tied to accreditation, and assistance given to centers wishing to achieve accreditation standards.
-
Mandatory childhood cancer reporting and registration – Childhood cancer registries should be created in order to allow for informed resource allocation and the evaluation of childhood cancer policy implementation.
-
Development of national standards of care – National treatment protocols should be developed which take into account local capabilities and realities. Financial incentives may be tied to the use of such protocols.
-
Creation of a national childhood cancer governing body – A multidisciplinary body should be created and tasked with monitoring the above policy components as well as with ongoing policy evaluation.
Financial Coverage of Childhood Cancer Treatment
Effective pediatric cancer control requires financial support for families without adequate resources or private health insurance [16]. In jurisdictions with nascent universal health care systems, financial support may be accomplished through the expansion of such systems to include childhood malignancies. Policymakers may begin by covering specific malignancies depending on prevalence and available financial resources. When prioritizing financial coverage in a specific setting, other considerations may include the level of supportive care necessary during treatment, achievable cure rates, and the need for other treatment modalities, including surgery and radiation.
In Mexico, a Fund for Protection against Catastrophic Expenditures began to cover childhood acute lymphoblastic leukemia (ALL) in 2006 and all other pediatric malignancies in 2008 [18],[20]. Current efforts in China to build comprehensive insurance programs that also cover childhood cancer hold great promise, but are still in their infancy. Financial coverage, though crucial, is by itself insufficient for maximal impact, and can even create perverse incentives when regulatory and monitoring infrastructure is insufficient.
Financial incentives must be carefully structured to encourage the most desirable outcomes. Lump sum payments per patient diagnosed, as was initially done in Mexico, provided no incentive to reduce the incidence of relapse, abandonment of therapy, or toxic death. This misalignment of incentives may partially explain why rates of toxic death remained high even after health insurance was expanded [20]. Dividing remuneration into smaller sums payable as a patient reaches each treatment phase may represent a solution. However, the effectiveness and cost-effectiveness of such strategies have not been evaluated.
Accreditation of Childhood Cancer Centers
In the absence of organized health care systems, childhood cancer treatment may be provided in a single jurisdiction by an extraordinarily high number of centers of varying quality. For example, in Colombia, pediatric oncology care for the estimated 1,800 children diagnosed with cancer each year is currently provided by 165 centers. Many centers lack sufficient expertise, resources, or patients. Expanding financial coverage may exacerbate this phenomenon, as individuals or small institutions begin to treat pediatric oncology patients for monetary gain. Financial remuneration should therefore be provided only to centers accredited on the basis of available infrastructure, patient volume, the ability to report outcomes, and the presence of trained oncology staff members, including at least one certified pediatric oncologist, pediatric oncology nurses, and other members of the multidisciplinary team. By 2009, 47 Mexican hospitals had been certified by the Ministry of Health in this way, and were therefore eligible to receive financial compensation [21]. Ideally, regional referral systems allowing more complicated cases to be treated in highly specialized institutions would also be developed.
Mandatory Childhood Cancer Reporting and Registration
In 2006, only 4% of Asian populations and 1% of sub-Saharan African populations were covered by high-quality population-based cancer registries [22]. Though the effort required to build such registries is considerable, the establishment of childhood cancer population-based registries may be more feasible than adult registries, since the number of pediatric patients is a small fraction of the total cancer cases. Efforts to build such pediatric registries are currently underway in Guatemala and El Salvador. These registries can evolve from hospital-based registries that have already undergone the necessary processes to ensure accurate data collection, as demonstrated in Argentina [23].
Such registries, when combined with mandatory reporting of childhood cancer cases, are integral to national strategies for several reasons. Population-based registries document the burden of disease, allowing for informed resource planning and allocation. Collecting outcome data also allows for the impact of childhood cancer policies to be evaluated. Finally, the documentation of where childhood cancer patients are treated will identify centers with poor outcomes, which can then be eligible for increased resources, corrective action, or in extreme cases, closure.
Development of National Standards of Care
One of the achievements of pediatric oncology in recent decades is the refinement of risk stratification systems, allowing for an assessment of the aggressiveness of a particular child's cancer and for treatment intensity to be matched to disease risk, thus reducing both under-treatment and over-treatment [24],[25]. Avoiding overtreatment is crucial in LMICs, since it carries with it an increased risk of toxic death from causes such as infection and hemorrhage [26],[27]. At some point, any benefit in disease control by intensifying treatment will be outweighed by an increase in toxicity. Finding the balance point for each malignancy at each pediatric cancer center is key to optimizing therapy and curing the maximum number of children possible. This ideal point depends not only upon the malignancy in question, but also upon a particular center's ability to provide supportive care to prevent and manage treatment complications [27],[28].
In many LMIC centers, supportive care capabilities lag behind those in HICs. Implementing unmodified treatment protocols designed for HICs in LMIC centers where supportive care is less developed therefore inevitably leads to high levels of toxicity [27]. For example, in the Dominican Republic, a relatively intensive HIC treatment regimen was used in 91 children with acute lymphoblastic leukemia (ALL) between 2005–2007, resulting in a 2-year survival of 40% and a toxic death rate of 32%. Decreasing treatment intensity improved overall 2-year survival to 70% and decreased the toxic death rate to 8% [28].
National treatment protocols that have been designed and adapted to local circumstances by appropriate experts are therefore integral to maximizing cure rates. In Mexico, the use of such protocols was mandatory, and tied to the accreditation process [20].
Creation of a National Childhood Cancer Governing Body
Finally, a body with the ultimate responsibility of creating and implementing the national strategy is required. This committee should include clinicians, epidemiologists, and policymakers, and be either part of, or associated with, the Ministry of Health. Responsibilities of the body would include accrediting treatment centers, developing and updating national standards of care, planning new infrastructural capacity in jurisdictions with need, workforce training and support, ensuring stable drug supplies, and ongoing policy evaluation.
Challenges to Implementation
Policy Evaluation
A major challenge to the implementation of NCCS is the absence of formal impact assessments. While the principles outlined above are based in sound theory and extensive practical experience, rigorous policy evaluation is lacking. Indeed, preliminary results of the impact of Seguro Popular in Mexico are mixed. While the proportion of eligible cases funded by the Fund for Protection against Catastrophic Expenditures increased from 3.3% to 55.3% between 2006 and 2009, the overall number of children treated did not seem to change [20],[21]. The shifting of cost burdens from families to government may simply precede increases in the number of patients treated, though this has not yet been proven. Though rates of treatment abandonment decreased and overall cure rates increased over the same time period, whether this was directly due to improved financial coverage is unknown. Opportunity and ancillary costs (e.g., travel) remain significant caregiver burdens that may explain persistent regional inequalities [20],[21]. Indeed, such costs are a significant burden even in HICs with universal health care systems, and are likely to play an even larger role in LMICs [29]. Finally, the outcomes of most children treated outside Seguro Popular institutions remain unknown. As new strategies are realized in additional jurisdictions, formal policy evaluation must be incorporated.
Cost and Cost-Effectiveness Data
Second, the cost of implementing such policies has not been described. While childhood cancer treatment is likely to be highly cost-effective given the large number of potential years of life saved [12], rigorous costing studies are required to better inform health policymakers. It is worth noting that pediatric oncology may represent a unique funding model in its ability to attract private resources that would otherwise have remained outside the health sector. For example, in Guatemala an initial outlay of funds from an HIC twinning partner was subsequently leveraged into additional resources from both government and private donors (Figure 1). Similar success may be possible in other settings.
Fig. 1. Funding sources of the Unidad Nacional de Oncologia Pediátrica of Guatemala. 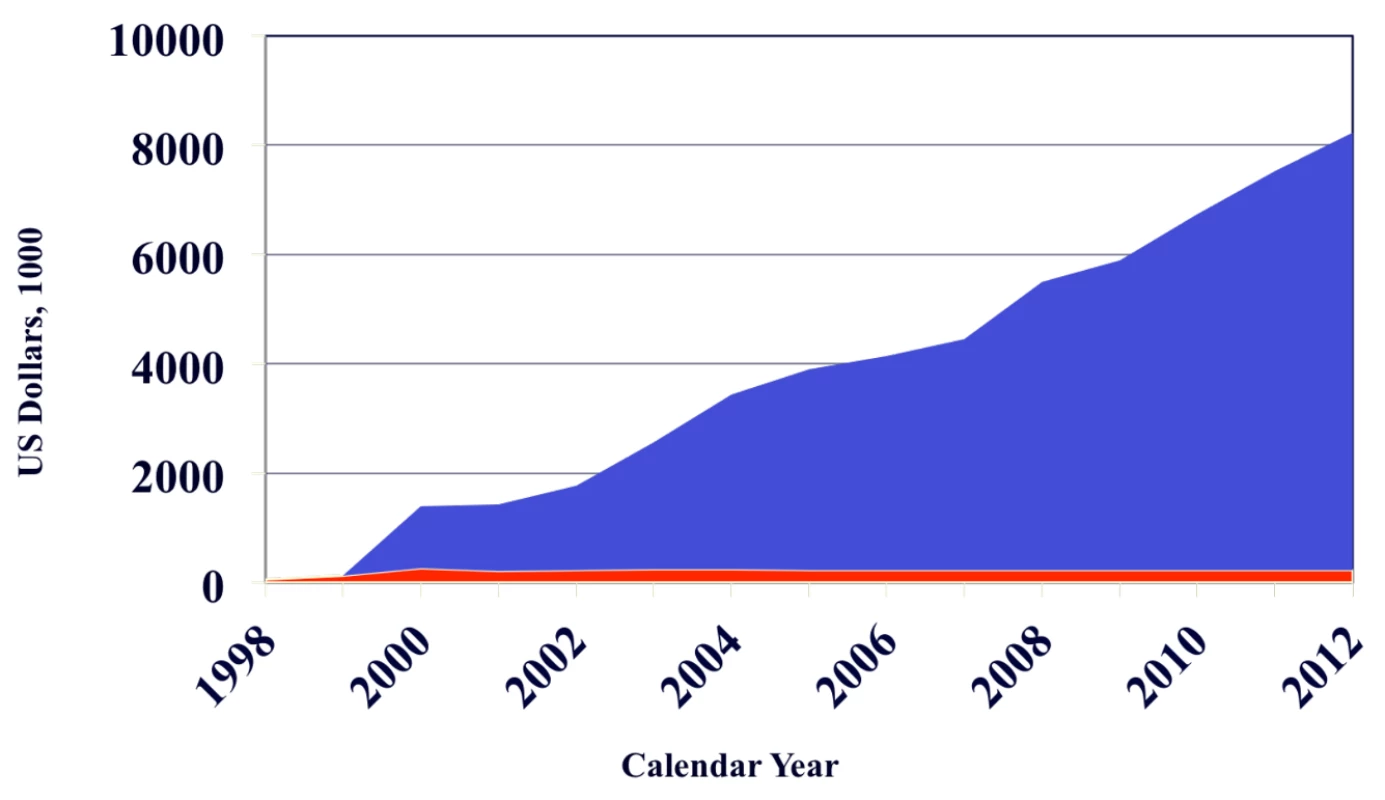
The red area indicates funding from St. Jude Children's Research Hospital and the blue area funding from all other sources. An initial outlay of funds from St. Jude was subsequently leveraged into additional resources from both government and private donors. The creation of an independent fundraising organization (Fundación Ayúdame a Vivir, http://ayuvi.org.gt) was essential to this outcome. Similar successes may be possible in other settings; the role and best use of seed funding from HIC centers requires further investigation. Implementation Research
Third, implementation research is required. Descriptions of how NCCS came to be seen as priorities by policymakers and governmental officials in Chile, Mexico, and China would be of tremendous use to other LMICs. To date, advocacy by grassroots nongovernmental organizations, caregivers, and local medical leaders have been responsible for the creation of childhood cancer policies, as opposed to directives from national and supra-national agencies such as the World Health Organization. Ideally, top-down and bottom-up efforts would be coordinated and integrated to achieve maximal impact (Figure 2).
Fig. 2. Top-down and bottom-up approaches to improve pediatric cancer care. 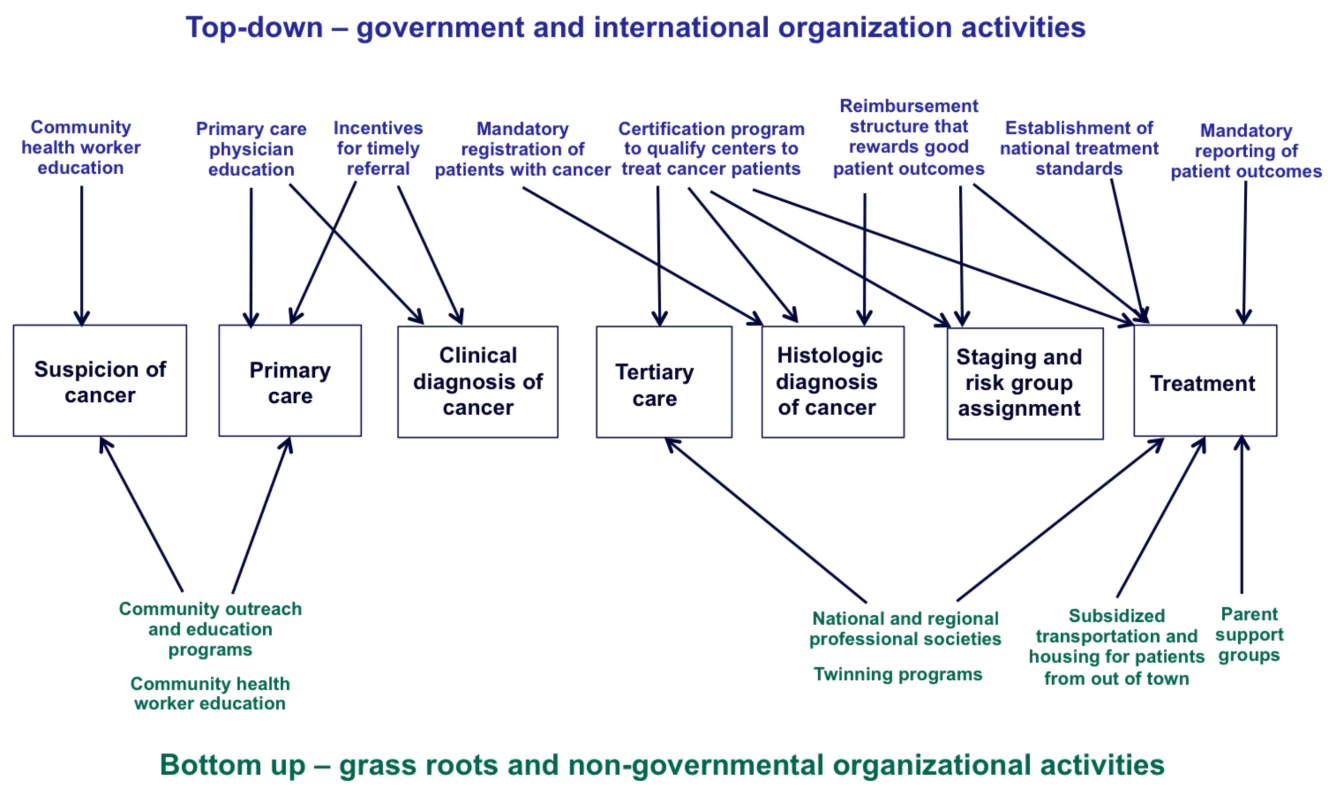
Low-Income Countries
Many of the above policy recommendations are best suited to and most easily implemented in MICs. Low-income countries (LICs) face additional challenges, given severely constrained resources, limited infrastructure, and significant competing health concerns. Nonetheless, several examples of successful childhood cancer treatment in LICs exist [11],[15]. How health policy can support such LIC efforts while still taking into account LIC-specific realities should be the focus of health policy and health economics research efforts.
Correcting Misconceptions
Finally, and perhaps most importantly, many laypeople, policymakers, and decision makers may see childhood cancer treatment as far too complex and costly for most LMICs. Currently, most private and public international funds are directed towards adult cancer. It is worth remembering however that the successful control of HIV was also seen as tremendously complex and costly, and therefore beyond the capabilities of most LMIC health systems. Through international coordination of advocacy, research, and policy, the latest report on HIV/AIDS concluded that “remarkable increases in access to life-saving antiretroviral therapy” continued to be seen, though substantial effort was of course still required [30]. We believe that similar achievements are feasible for children with cancer in LMICs.
Conclusions
Pediatric malignancies account for a growing proportion of overall global childhood mortality, justifying renewed efforts to improve cure rates for this population in resource-limited settings. NCCS offer the best hope of reaching this goal, as summarized in Box 2. While the financial coverage of pediatric oncology care should be an integral part of any such strategy, maximal impact will require additional policies addressing the structural aspects of care delivery and the creation of childhood cancer registries.
Box 2. Key Recommendations
-
National childhood cancer strategies should be designed and implemented in LMICs, with key components as outlined in the text and in Box 1.
-
The implementation and impact of existent national strategies in LMICs should be evaluated; new national strategies should include an evaluation component.
-
Additional data on the cost and cost-effectiveness of such strategies are needed.
-
Health policy and health economics research is required to determine how to best adapt these recommendations to account for the additional challenges inherent to LICs.
Zdroje
1. HowardSC, MetzgerML, WilimasJA, QuintanaY, PuiC-H, et al. (2008) Childhood cancer epidemiology in low-income countries. Cancer 112 : 461–472.
2. LozanoR, WangH, ForemanKJ, RajaratnamJK, NaghaviM, et al. (2011) Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet 378 : 1139–1165.
3. LiuL, JohnsonHL, CousensS, PerinJ, ScottS, et al. (2012) Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379 : 2151–2161.
4. PattonGC, CoffeyC, CappaC, CurrieD, RileyL, et al. (2012) Health of the world's adolescents: a synthesis of internationally comparable data. Lancet 379 : 1665–1675.
5. MagrathI, Steliarova-FoucherE, SidneiE, RibeiroRC, HarifM, et al. (2013) Pediatric cancer in low-income and middle-income countries. Lancet Oncol 14 : 3104–3116.
6. EllisonLF, PoganyL, MeryLS (2007) Childhood and adolescent cancer survival: a period analysis of data from the Canadian Cancer Registry. Eur J Cancer 43 : 1967–1975.
7. SiegelR, NaishadhamD, JemalA (2013) Cancer statistics, 2013. CA: Cancer J Clin 63 : 11–30.
8. SchillingFH, SpixC, BertholdF, ErttmannR, FehseN, et al. (2002) Neuroblastoma screening at one year of age. N Engl J Med 346 : 1047–1053.
9. SmithMA, SeibelNL, AltekruseSF, RiesLAG, MelbertDL, et al. (2010) Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28 : 2625–2634.
10. PuiC-H, PeiD, PappoAS, HowardSC, ChengC, et al. (2012) Treatment outcomes in black and white children with cancer: results from the SEER database and St. Jude Children's Research Hospital, 1992 through 2007. J Clin Oncol 30 : 2005–2012.
11. HarifM, BarsaouiS, BenchekrounS, BouhasR, DoumbeP, et al. (2008) Treatment of B-cell lymphoma with LMB modified protocols in Africa - Report of the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer 50 : 1138–1142.
12. BhaktaN, MartiniukALC, GuptaS, HowardSC (2012) The cost-effectiveness of treating paediatric cancer in low-income and middle-income countries: a case-study approach using acute lymphocytic leukaemia in Brazil and Burkitt lymphoma in Malawi. Arch Dis Child 98 : 155–160.
13. AntillonF, BaezF, Barrantes ZamorraJC, FuLC, MorenoB, et al. (2005) AMOR: a proposed cooperative effort to improve outcomes of childhood cancer in Central America. Pediatr Blood Cancer 45 : 107–110.
14. MostertS, SitaresmiMN, GundyCM, JanesV, Sutaryo, et al. (2010) Comparing childhood leukaemia treatment before and after the introduction of a parental education programme in Indonesia. Arch Dis Child 95 : 20–25.
15. IsraelsT, BorgsteinE, PidiniD, ChagalukaG, de KrakerJ, et al. (2012) Managment of children with a Wilms tumor in Malawi, sub-saharan Africa. J Pediatr Hematol Oncol 34 : 606–610.
16. RibeiroRC, Steliarova-FoucherE, MagrathI, LemerleJ, EdenT, et al. (2008) Baseline status of paediatric oncology care in ten low-income or mid-income countries receiving My Child Matters support: a descriptive study. Lancet Oncol 9 : 721–729.
17. Jasso-GutierrezL, Duran-ArenasL, Flores-HuertaS, Cortes-GalloG (2012) Recommendations to improve healthcare of neonates with respiratory insufficiency beneficiares of Seguro Popular. Salud Publica Mex 54: S57–S64.
18. KnaulFM, Gonzalez-PierE, Gomez-DantesO, Garcia-JuncoD, Arreola-OrnelaH, et al. (2012) The quest for universal health coverage: acheiving social protection for all in Mexico. Lancet 380 : 1259–1279.
19. Rivera-LunaR, Correa-GonzalezC, Altamirano-AlvarezE, Sanchez-ZubietaF, Cardenas-CardosR, et al. (2012) Incidence of childhood cancer among Mexican children registered under a public medical insurance program. Int J Cancer 132 : 1646–1650.
20. Perez-CuevasR, DoubovaSV, Zapata-TarresM, Flores-HernandezS, FrazierL, et al. (2013) Scaling up cancer care for children without medical insurance in developing countries: the case of Mexico. Pediatr Blood Cancer 60 : 196–203.
21. RibeiroRC (2013) Impact of the Mexican government's system of social protection for health, or Seguro Popular, on pediatric oncology outcomes. Pediatr Blood Cancer 60 : 171–172.
22. FerlayJ, ShinH-R, BrayF, FormanD, MathersC, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127 : 2893–2917.
23. PujolCJA, BertoneCL, AcostaLD (2014) Morbidity and mortality rates for childhood cancer in Argentina 2006–2008. Arch Argent Pediatr 112 : 50–54.
24. PuiC-H, RobisonLL, LookAT (2008) Acute lymphoblastic leukemia. Lancet 371 : 1030–1043.
25. MarisJM (2010) Recent advances in neuroblastoma. N Engl J Med 362 : 2202–2211.
26. CreutzigU, ZimmermannM, ReinhardtD, DworzakM, StaryJ, et al. (2004) Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol 22 : 4384–4393.
27. GuptaS, AntillonFA, BonillaM, FuL, HowardSC, et al. (2011) Treatment-related mortality in children with acute lymphoblastic leukemia in Central America. Cancer 117 : 4788–4795.
28. HungerSP, ReyesD, NegrinO, MonteroM, De la RosaL, et al. (2011 (abstr.)) Decreased early mortality and increased survival with less intensive therapy for acute lymphoblastic leukemia (ALL) in the Dominican Republic. Pediatr Blood Cancer 57 : 761.
29. TsimicalisA, StevensB, UngarWJ, McKeeverP, GreenbergML, et al. (2012) A prospective study to determine the costs incurred by families of children newly diagnosed with cancer in Ontario. Psychooncology 21 : 1113–1123.
30. World Health Organization (2013) Global update on HIV treatment 2013: results, impact and opportunities: WHO report in partnership with UNICEF and UNAIDS. Geneva: WHO.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 6- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Nutraceutikum Armolipid Plus podle klinických důkazů zlepšuje lipidový profil − metaanalýza
- Snižuje terapie betablokátory kardiovaskulární benefit aerobního cvičení u pacientů s arteriální hypertenzí?
-
Všechny články tohoto čísla
- Evidence for the Selective Reporting of Analyses and Discrepancies in Clinical Trials: A Systematic Review of Cohort Studies of Clinical Trials
- Health Care in Danger: Deliberate Attacks on Health Care during Armed Conflict
- Melanocytic Nevi as Biomarkers of Breast Cancer Risk
- Blood Transfusions following Trauma: Finding an Evidence-Based Vein
- Antiretroviral Therapy for Refugees and Internally Displaced Persons: A Call for Equity
- Association between Cutaneous Nevi and Breast Cancer in the Nurses' Health Study: A Prospective Cohort Study
- Efficacy of Pneumococcal Nontypable Protein D Conjugate Vaccine (PHiD-CV) in Young Latin American Children: A Double-Blind Randomized Controlled Trial
- Pediatric Oncology as the Next Global Child Health Priority: The Need for National Childhood Cancer Strategies in Low- and Middle-Income Countries
- Place and Cause of Death in Centenarians: A Population-Based Observational Study in England, 2001 to 2010
- HIV among People Who Inject Drugs in the Middle East and North Africa: Systematic Review and Data Synthesis
- Association between Melanocytic Nevi and Risk of Breast Diseases: The French E3N Prospective Cohort
- Patient-Safety-Related Hospital Deaths in England: Thematic Analysis of Incidents Reported to a National Database, 2010–2012
- Pushback: The Current Wave of Anti-Homosexuality Laws and Impacts on Health
- HIV Treatment-as-Prevention Research at a Crossroads
- Red Blood Cell Transfusion and Mortality in Trauma Patients: Risk-Stratified Analysis of an Observational Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Melanocytic Nevi as Biomarkers of Breast Cancer Risk
- Antiretroviral Therapy for Refugees and Internally Displaced Persons: A Call for Equity
- Efficacy of Pneumococcal Nontypable Protein D Conjugate Vaccine (PHiD-CV) in Young Latin American Children: A Double-Blind Randomized Controlled Trial
- Blood Transfusions following Trauma: Finding an Evidence-Based Vein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



