-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaPrevalence, Distribution, and Impact of Mild Cognitive Impairment in Latin America, China, and India: A 10/66 Population-Based Study
Background:
Rapid demographic ageing is a growing public health issue in many low - and middle-income countries (LAMICs). Mild cognitive impairment (MCI) is a construct frequently used to define groups of people who may be at risk of developing dementia, crucial for targeting preventative interventions. However, little is known about the prevalence or impact of MCI in LAMIC settings.Methods and Findings:
Data were analysed from cross-sectional surveys established by the 10/66 Dementia Research Group and carried out in Cuba, Dominican Republic, Peru, Mexico, Venezuela, Puerto Rico, China, and India on 15,376 individuals aged 65+ without dementia. Standardised assessments of mental and physical health, and cognitive function were carried out including informant interviews. An algorithm was developed to define Mayo Clinic amnestic MCI (aMCI). Disability (12-item World Health Organization disability assessment schedule [WHODAS]) and informant-reported neuropsychiatric symptoms (neuropsychiatric inventory [NPI-Q]) were measured. After adjustment, aMCI was associated with disability, anxiety, apathy, and irritability (but not depression); between-country heterogeneity in these associations was only significant for disability. The crude prevalence of aMCI ranged from 0.8% in China to 4.3% in India. Country differences changed little (range 0.6%–4.6%) after standardization for age, gender, and education level. In pooled estimates, aMCI was modestly associated with male gender and fewer assets but was not associated with age or education. There was no significant between-country variation in these demographic associations.Conclusions:
An algorithm-derived diagnosis of aMCI showed few sociodemographic associations but was consistently associated with higher disability and neuropsychiatric symptoms in addition to showing substantial variation in prevalence across LAMIC populations. Longitudinal data are needed to confirm findings—in particular, to investigate the predictive validity of aMCI in these settings and risk/protective factors for progression to dementia; however, the large number affected has important implications in these rapidly ageing settings.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 9(2): e32767. doi:10.1371/journal.pmed.1001170
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001170Summary
Background:
Rapid demographic ageing is a growing public health issue in many low - and middle-income countries (LAMICs). Mild cognitive impairment (MCI) is a construct frequently used to define groups of people who may be at risk of developing dementia, crucial for targeting preventative interventions. However, little is known about the prevalence or impact of MCI in LAMIC settings.Methods and Findings:
Data were analysed from cross-sectional surveys established by the 10/66 Dementia Research Group and carried out in Cuba, Dominican Republic, Peru, Mexico, Venezuela, Puerto Rico, China, and India on 15,376 individuals aged 65+ without dementia. Standardised assessments of mental and physical health, and cognitive function were carried out including informant interviews. An algorithm was developed to define Mayo Clinic amnestic MCI (aMCI). Disability (12-item World Health Organization disability assessment schedule [WHODAS]) and informant-reported neuropsychiatric symptoms (neuropsychiatric inventory [NPI-Q]) were measured. After adjustment, aMCI was associated with disability, anxiety, apathy, and irritability (but not depression); between-country heterogeneity in these associations was only significant for disability. The crude prevalence of aMCI ranged from 0.8% in China to 4.3% in India. Country differences changed little (range 0.6%–4.6%) after standardization for age, gender, and education level. In pooled estimates, aMCI was modestly associated with male gender and fewer assets but was not associated with age or education. There was no significant between-country variation in these demographic associations.Conclusions:
An algorithm-derived diagnosis of aMCI showed few sociodemographic associations but was consistently associated with higher disability and neuropsychiatric symptoms in addition to showing substantial variation in prevalence across LAMIC populations. Longitudinal data are needed to confirm findings—in particular, to investigate the predictive validity of aMCI in these settings and risk/protective factors for progression to dementia; however, the large number affected has important implications in these rapidly ageing settings.
: Please see later in the article for the Editors' SummaryIntroduction
Ageing [1] and the health transition in low - and middle-income countries (LAMICs) are responsible for an unprecedented increase in the prevalence and societal impact of noncommunicable diseases, including dementia [2]. Large numbers of people with dementia currently live in LAMICs [3],[4] with prevalence estimates comparable to those of the Western world [5]. At present, disease-modifying drugs are not available [6] and symptomatic medications have been found to have only modest benefit [7]. Primary prevention of dementia is therefore of great importance [8].
Mild cognitive impairment (MCI) is an intermediate state between normal cognitive ageing and dementia [9]. Identification of MCI is thought to be crucial to early intervention. Indeed, in some studies MCI is associated with an increased risk of dementia [10], as well as with future disability [11] and mortality [12]. Such associations, however, do vary according to the nature of the sample (clinical versus population-based), the case definition of MCI applied, the assessment procedures used for operationalizing component criteria [13]–[15], and, potentially, the cultural background of participants [16],[17]. A recent review also suggested that MCI is associated with neuropsychiatric symptoms, cited as being of potential importance for defining subgroups at higher risk of developing dementia in the future [18].
In community-dwelling older adults the prevalence of amnestic MCI (aMCI), defined according to Petersen's revised criteria [10], ranges between 2.1% [19] and 11.5% [20] and is most commonly found to be around 3%–5% [21]–[33] with few exceptions in older samples [20],[34]–[36]. Reports of the community prevalence of aMCI have been predominantly derived from European and North American populations. To our knowledge, very few population-based studies have been published from LAMICs and those from Asia are controversial. Specifically, estimates of aMCI prevalence were similar to those found in Western countries in Kolkata, India (6%) [37] and in Chongqing, China (4.5%) [29], but higher prevalences were reported by Lee and colleagues in Malaysia (15.4%) [38] and by Kim et al. in South Korea (9.7%) [39].
Estimating the population prevalence of MCI in LAMICs is a public health priority as rapid demographic ageing is predicted to result in a large majority of people residing in these regions being at risk of dementia and cognitive decline. If so, this will have significant implications with regard to social support and future health care costs, especially as systems are not in place to cope with increased neurodegenerative disease and health resources at present are already extremely limited.
In this study, using data from the cross-sectional phase of the 10/66 Dementia Research Group (DRG) programme on dementia, noncommunicable diseases and ageing in LAMICs [40], we operationalized the Mayo Clinic–defined aMCI [10] construct and then estimated the prevalence of this condition in eight LAMICs, in addition to its sociodemographic correlates and associations with disability and neuropsychiatric symptoms.
Methods
Ethics Statement
Written informed consent, or witnessed oral consent in case of illiteracy, or next of kin written agreement in case of incapacity, was obtained from all participants. The appropriate Research Ethics Committees at King's College London and at all local countries approved the study protocol and the consent procedures.
Sample
The 10/66 study has been described previously [40]. In brief, the study consisted of a series of cross-sectional one-phase geographic catchment area surveys, carried out in eight urban and rural sites in Peru, Mexico, China, and India, and in three urban sites in Cuba, the Dominican Republic, and Venezuela, between January 2003 and November 2007. The target sample size was 2,000 participants per country, in order to allow estimation of a typical dementia prevalence of 4.5% (SE 0.9%) with 80% power. All community-resident individuals aged 65+ y were eligible for inclusion. Using a process of full household enumeration, all residents aged 65+ y within catchment areas were approached by means of door-knocking and a reliable informant was required for inclusion. Being younger than 65 y was the only exclusion criteria, and weighted sampling procedures were not applied.
Measurements
All participants completed the 10/66 standardized assessment at their place of residence. This consisted of participant and informant interviews and a physical examination, described in full elsewhere in an open-access publication [40]. Participant interviews included questionnaire measures of sociodemographic status, education and childhood environment, social networks and support, self-report measures of common physical disorders, health service use, and lifestyles (smoking, alcohol intake, diet, exercise), in addition to a fully structured diagnostic interview for mental disorder (Geriatric Mental State [GMS], described below). Physical examinations included measures of resting blood pressure, anthropometric measures, and a structured neurological examination. A battery of cognitive assessments was administered (described below) and an informant interview included structured questionnaires on cognitive decline and neuropsychiatric symptoms (both described below), as well as questions on care arrangements, caregiver strain and distress, financial implications of caregiving, and support received. The 10/66 study protocol was translated into Spanish, Tamil, and Mandarin, and minor adaptations were made by local clinicians fluent in English. Validation statistics for the assessments and procedures have been published [41]. The protocol included the GMS Examination [42],[43], an informant interview on all participants, a neurological examination, and a neuropsychological battery that comprised the following:
(1) The participant interview section of the Community Screening Instrument for Dementia (CSI “D”) [44]. This was developed as a screening instrument for dementia for use in cross-cultural settings in combination with the informant interview. The cognitive assessment covers multiple domains, including orientation to time and place, language, memory, praxis, and abstract thinking. It deliberately excludes literacy-dependent items. A memory subscale was derived from the CSI “D” using the items addressing immediate and delayed recall of a three word list, recall of the name of the interviewer, and recall of five elements of a short story (logical memory). (2) The Modified Consortium to Establish a Registry for Alzheimer's Disease (CERAD) ten-word-list learning task [45]. Six words: butter, arm, letter, queen, ticket, and grass were taken from the original CERAD battery English language list. Pole, shore, cabin, and engine were replaced with corner, stone, book, and stick, which were deemed more culturally appropriate for all sites in the 10/66 pilot phase (a wider sample that included the survey sites). In the learning phase, the list is read to the participant. Next, the participant is asked to immediately recall the words that they remember. This process is repeated three times, giving an immediate word list memory score, with a maximum total of 30. After a 5-min delay, the participant is again asked to recall the ten words with encouragement but no cues, giving a word list delayed recall score with a maximum total score of 10.
Demographic correlates analyzed against aMCI were age, gender, education, and number of assets. Participants' gender and stated age were recorded. Age was confirmed by the interviewer from official documentation and informant report, and any discrepancies resolved through further questions and clarification and, ultimately, by consensus within the research team. Illiteracy (inability to read and/or write), level of education (none/did not complete primary/completed primary/secondary/tertiary), and number of household assets (car, television, refrigerator, telephone, plumbed toilet, water, and electricity mains) were also recorded.
The impact of aMCI was quantified through investigating associations with disability and neuropsychiatric symptoms. Participant interviews included the 12-item WHO disability assessment schedule (WHODAS-12) [46], which assesses five activity-limitation domains (communication, physical mobility, self-care, interpersonal interaction, life activities and social participation). Two questions with scores ranging from 0 (no difficulty) to 4 (extreme difficulty) cover each domain, and the global standardized score ranges from 0 (not disabled) to 100 (maximum disability). Details on the WHODAS 2.0 validity and psychometric properties can be found elsewhere [47],[48]. The informant interview, as well as administering structured CSI “D” questions (regarding decline in memory or intelligence, activities of daily living, social and occupational functioning used for dementia diagnoses—summarized below and applied as an exclusion criteria), also included the neuropsychiatric inventory (NPI-Q) [49], and the following binary symptom categories were selected for analyses of associations with aMCI: depression, anxiety, apathy, irritability.
For analyses of associations of aMCI with disability and neuropsychiatric symptoms, the following covariates available in the dataset were used for adjusted models in addition to the four sociodemographic variables described above: depression (GMS), self-reported limiting physical impairments (arthritis, visual difficulties, hearing difficulties, respiratory disorders, heart problems, gastrointestinal problems, fainting episodes, limb paralysis, skin disorders), self-reported hypertension, self-reported stroke, psychotic disorder (GMS), self-reported regular pain.
Case Definition of aMCI
Mayo Clinic–defined aMCI was diagnosed on the basis of the following criteria: (1) objective memory impairment beyond that expected for age; (2) subjective memory complaint; (3) no, or only mild impairment in core activities of daily living, and (4) no dementia. Each criterion was operationalized as follows.
Objective memory impairment
A composite memory score was created using results from the memory subscale of the CSI “D” [44], immediate and delayed word recall scores from the modified CERAD ten-word list [50]. For all tasks impaired performance was defined as a score 1.5 standard deviation (SD) or more below the mean adjusted for age and education. The 1.5-SD definition stems from that applied to define “abnormal memory performance” by Peterson et al. in 1999 [9], and has been recently recommended also by a National Institute on Aging-Alzheimer's Association workgroup [51]. Operationalization of MCI in other population-based studies has consistently followed this definition [25],[33],[52],[53], which has also been used to define other constructs such as “Cognitive Impairment No Dementia” [54]. The CERAD word list has been used in previous research [25]. Although, there have been controversies surrounding the MCI entity itself [55]–[58], they have not to our knowledge focused on the 1.5-SD threshold. Norms were derived from controls without dementia from the 24-centre 10/66 pilot study, which had found minimal geographic variation [41]. Participants were excluded if hearing impairment had prevented cognitive assessment.
Subjective memory impairment
An ordinal scale ranging from 0 to 6 was created by summing item scores from relevant questions in the GMS including: (1) Have you had any difficulty with your memory (0, no; 1, yes)? (2) Have you tended to forget names of your family or close friends/where you have put things (for each question: 0, no/transient; 1, noticed most days per week; 2, noticed daily)? (3) Do you have to make more efforts to remember things than you used to (0, no; 1, yes)? Using this scale, subjective memory impairment was defined as present when an individual scored three or more: the definition that has been used in all previous research to use this scale [59],[60].
Normal activities of daily living/instrumental activities of daily living
On the basis of responses from the CSI “D” informant interview, normal activities of daily living (ADL)/instrumental activities of daily living (IADLs) were defined as very mild or no impairment in either carrying out household chores, pursuing hobbies, using money, feeding, dressing, or toileting. The definition of impairment did not include problems arising only from physical impairments.
No dementia
Diagnoses of dementia were applied using the 10/66 dementia algorithm and Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria [61]. Participants meeting either criterion were excluded from the analyzed sample (both aMCI cases and controls).
Statistical Analysis
Analyses were carried out on the 10/66 data archive release 2.1. All analyses used STATA version 10.1 [62]. As mentioned above, participants with dementia were excluded from all analyses as has been standard practice in MCI epidemiological research. Sample characteristics across countries were described including age, gender, education, number of household assets, global disability scores (WHODAS-12) [46], and NPI-Q symptoms [49].
In order to determine the potential impact of aMCI we assumed that, while both activities of daily living (ADLs) and instrumental activities of daily living (IADLs) would be expected to be intact in people with aMCI, subtle functional impairment may already be present as well as possibly nonspecific and mild behavioral and psychological symptoms of dementia (BPSD) [18]. Zero-inflated negative binomial regression (ZINB) count models were used to assess the association between aMCI and WHODAS-12 disability and NPI-Q scores using identical models to those previously reported for these samples [63]. We used zero-inflated models to deal with skewness in the distribution of the scores characterized by excessive zeros (inflation). The model distinguishes a group whose members have always zero counts (referred to as “certain zero”), from one in which members have either zero or positive counts. ZINB includes a logistic part to model the probability that a zero comes from the first group versus the second group and a negative binomial part to model the counts within the second group. Log-scale coefficients were exponentiated and 95% confidence intervals back-transformed. We determined the appropriateness of the ZINB model against a standard negative binomial model using the Vuong test postestimation and adjusted for the relevant covariates listed above, followed by Poisson regression models to generate prevalence ratios for NPI-Q symptoms as binary-dependent variables. ZINB models were further compared to zero-inflated Poisson models and in every country the test of the dispersion parameter (labelled alpha in Stata and theta by some other sources) was significant at the 0.001 level, indicating ZINB as more appropriate in all cases. Behavioural/psychological outcomes, depression, anxiety, apathy, irritability were modelled separately against aMCI as an independent variable for illustrative purposes, with no attempt to adjust given symptoms for the other three, accepting that these are related constructs.
Prevalence of aMCI was reported for each country by age and gender and adjusted for household clustering. Direct standardization, using the whole sample as the reference population, was used to compare prevalence estimates across countries after adjustment for age, gender, and education. For each country associations with age (continuous variable), gender, education (ordinal variable), and number of household assets (ordinal variable) on aMCI prevalence were calculated using mutually adjusted (as appropriate) prevalence ratios (PRs), with robust 95% confidence intervals (using the “robust” syntax in Stata to take into account household clustering: model robust standard errors [64],[65]), using Poisson working models.
To determine the pooled effects for all analyses, the statistical outputs for each country were combined into fixed-effect meta-analyses. Random effect models were not used as we wished to summarise the countries within this study rather than generalise to a hypothetical population of centres. We then calculated Cochrane Q heterogeneity and Higgins' I2 (95% CIs). The latter statistics set the degree of heterogeneity between studies that is not explained by chance and is expressed as a percentage with values up to 25%, 50%, and over 75% representing mild, moderate, and high heterogeneity, respectively [66].
Results
The results were derived from a total of 15,376 participants aged 65+ and without dementia across the different countries. Response rates (i.e., participation rates for all potentially eligible residents within the defined geographic catchments) were higher than 80% in all countries. Missing data on the variables of interest were present in less than 1% of the sample. Descriptive data by country are displayed in Table 1. Age was not evenly distributed across groups (65–69, 70–74, 75–79, and 80+ y) across countries, the samples from Venezuela, China, and India being slightly younger. In all countries more women participated than men. Educational level was highest in Cuba, and the number of household assets was lowest in Mexico and India.
Tab. 1. Sociodemographic characteristics of participants by country. 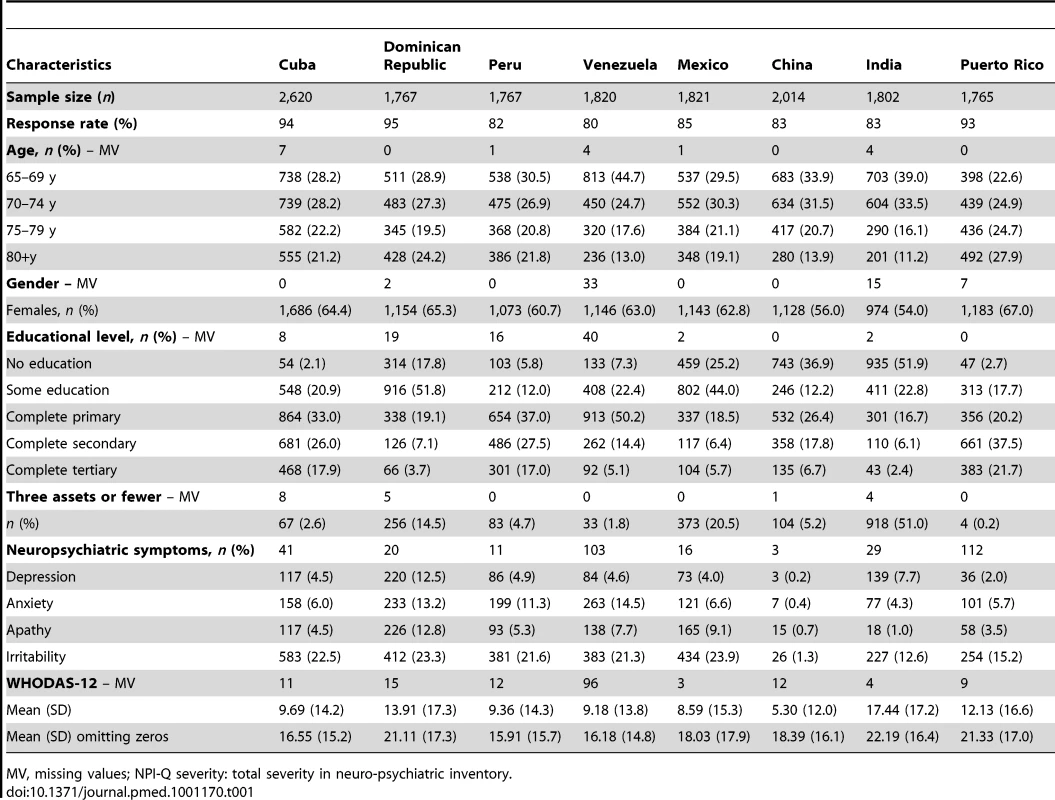
MV, missing values; NPI-Q severity: total severity in neuro-psychiatric inventory. In each country there was a statistically significant zero-inflation in the distributions of WHODAS-12 scores (Vuong test for the whole sample, z = 45.29, p<0.001) that confirmed the better fit of ZINB over negative binomial alone. Associations between aMCI, disability, and neuropsychiatric symptoms are summarized in Table 2 along with meta-analytical fixed-effect method-pooled estimates, and between-country heterogeneity. After adjustment, disability was significantly higher in aMCI cases compared to the remainder in Peru, India, and Dominican Republic, although was lower in China. The pooled fixed-effect model meta-analytical estimate indicated a positive association with disability although there was moderate to high heterogeneity in these associations between countries. After adjustment aMCI cases were more likely to have informant-rated anxiety, irritability, and apathy symptoms, with no significant between-country heterogeneity. However, there was no overall association with informant-rated depression in pooled estimates although the individual prevalence ratio was significant in Peru.
Tab. 2. Association between aMCI and disability (WHODAS-12), and the association between aMCI and neuropsychiatric symptoms (NPI–Q; depression, anxiety, apathy, and irritability). 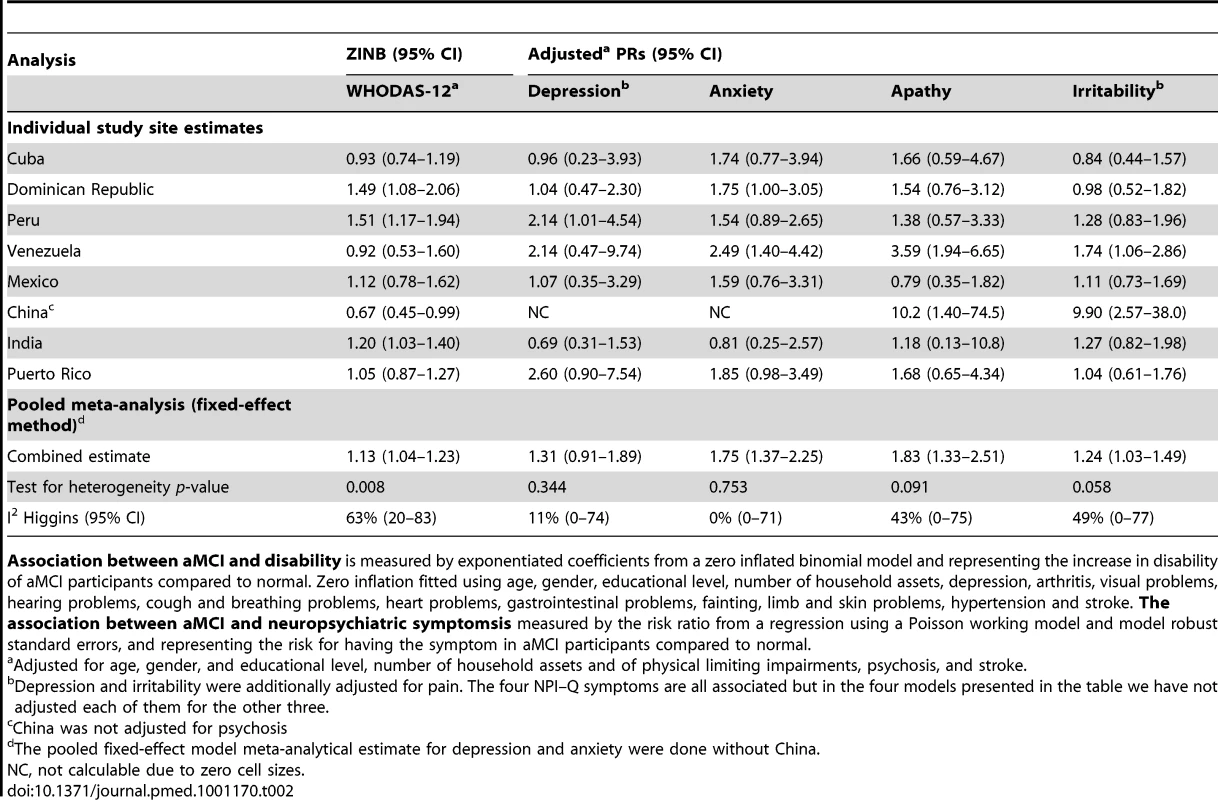
Association between aMCI and disability is measured by exponentiated coefficients from a zero inflated binomial model and representing the increase in disability of aMCI participants compared to normal. Zero inflation fitted using age, gender, educational level, number of household assets, depression, arthritis, visual problems, hearing problems, cough and breathing problems, heart problems, gastrointestinal problems, fainting, limb and skin problems, hypertension and stroke. The association between aMCI and neuropsychiatric symptomsis measured by the risk ratio from a regression using a Poisson working model and model robust standard errors, and representing the risk for having the symptom in aMCI participants compared to normal. The prevalence of aMCI ranged from 0.8% in China to 4.3% in India, and changed very little after direct standardization for age, gender, and education level, as displayed in Table 3. Adjusted PRs (95% CI) from Poisson regression models for independent associations with age, gender, education, and assets are shown in Table 4. No pooled associations were found with age or education but there was a modest association with male gender and fewer assets. Overall little heterogeneity was found between nations in these associations.
Tab. 3. Prevalence of aMCI by country, gender, and age group. 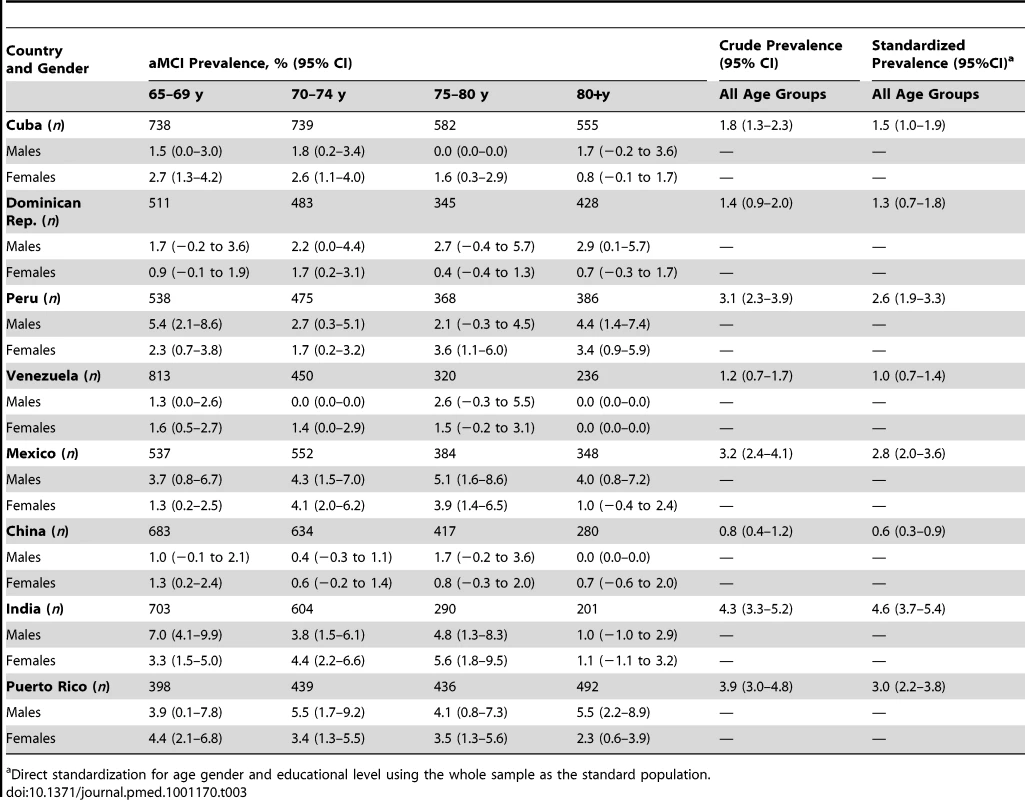
Direct standardization for age gender and educational level using the whole sample as the standard population. Tab. 4. Mutually adjusted (95% CI) for the independent effects of age, gender, education, and assets on aMCI prevalence. 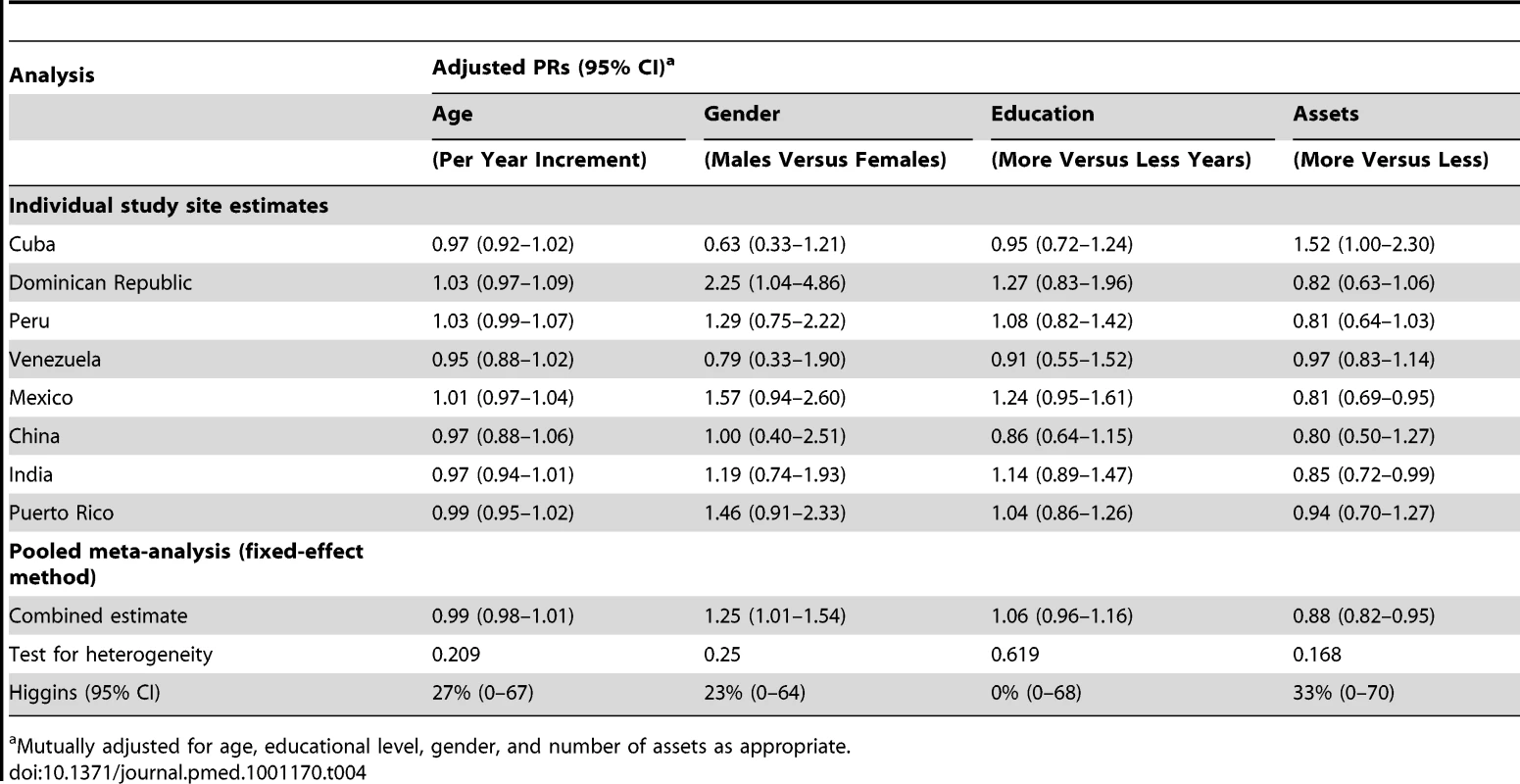
Mutually adjusted for age, educational level, gender, and number of assets as appropriate. Discussion
Using data from a large series of cross-sectional surveys applying standard sampling and measurements, we estimated the community prevalence of Mayo Clinic–defined aMCI in six countries in Latin America, China, and India. To our knowledge this is the first study to attempt to make direct comparisons of prevalence estimates of aMCI across diverse cultures and world regions. Differences in prevalence between countries were marked and ranged from 0.8% (China) to 4.3% (India), i.e., greater than five-fold variation. After direct standardization for age, gender, and education, using the whole population as the reference, these differences were not markedly attenuated.
Inconsistencies in aMCI prevalence observed between the 10/66 study centres are likely to be due to components of the aMCI diagnosis itself. In a cross-cultural context, these support questions previously raised concerning its conceptual basis [67] and/or operationalization outside clinical settings [68]. However, aMCI has been reported to be associated with increased mortality in a prospective study [12], and differences in aMCI-associated survival between country sites cannot be excluded as a factor influencing variation in prevalence. It should be noted that the 10/66 dementia diagnosis showed much higher sensitivity than the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria in both pilot and clinical validation 10/66 studies [41],[61]. Compared to numerous aMCI prevalence reports from community-based sites in Finland (5.3%) [26], Italy (4.9%) [69], Japan (4.9%) [32], the US (6%) [30], South Korea (9.7%) [39], Malaysia (15.4%) [38], and India (6%) [37], both the crude and adjusted aMCI prevalence reported here are relatively low. However, the estimates are similar to those reported by the British MRC CFAS study (2.5%) [15] and to estimates for aMCI prevalence in community samples from Southern France (3.2%) [33], the US (3.8%) [25], and Germany (3.1%) [70]. Low aMCI prevalence in our Latin American sites contrast with the aMCI prevalence (ranging between 3.8% and 6.3% depending on age) reported amongst American Caribbean Hispanics [31]. Differential mortality may explain these differences, but a potential role of the environment and lifestyle in the increased risk of MCI amongst Hispanic immigrants in North America cannot be excluded. Crude aMCI prevalence in India (4.3%) is similar to the figure described by Das and colleagues in Kolkata [37]. Prevalence in China was the lowest (0.6%), similar only to that described in the VITA study in Vienna [27] and markedly lower than that reported in a recent study from Chongqing (4.5%) [29]. Overall, the results suggest that there is very little consistency in prevalence of aMCI across world regions. When considered between studies, this may well reflect diagnostic issues arising from a lack of specific criteria for the operationalization of MCI (i.e., cognitive batteries and specific cut-off scores for impairment) as well as unmeasured differences and cultural variations potentially relevant for some components of the aMCI construct (such as subjective memory impairment, as described below). The objective for the analyses here was to standardize the assessments as much as possible in order to gain a clearer idea of international variation. The fact that substantial heterogeneity remains suggests important variation in constructs underlying the definition. These will be considered further below.
Female gender, increased age, lower education, and lower socioeconomic status are associated with dementia [71] and have been described in association with MCI [31]. In our study, however, the effects of age and education on aMCI prevalence were negligible across study sites, with no between-country heterogeneity in this respect. It is important to bear in mind that age - and education-standardised normative data were used to define aMCI and the lack of association supports the robustness of the norms, although for education, it might also reflect lower variance in the exposure or weaker underlying associations between education and other risk factor profiles in these samples. Lower socioeconomic status remained associated with aMCI and this may be an additional marker, beyond education, of relevant social disadvantage. The observed association with male gender contrasts with the higher reported age-adjusted prevalence of dementia in women compared to men [71], but could reflect the effect of dementia case exclusion consistent with Mayo Clinic Study of Aging reports that women experience a transition from normal cognition directly to dementia at a later age but more abruptly [20].
As described earlier, a key consideration with aMCI applied as a construct in international research is its cross-cultural validity. An advantage of the 10/66 study was that identical measures were taken and identical algorithms applied for diagnosis across the study sites and the protocols for cognitive assessments in the 10/66 study were the result of a long and painstaking process of development and validation [41]. However, a construct such as subjective memory impairment is potentially subject to cultural influences and may underlie between-site variation. For example, between sites, people with objectively lower performance on cognitive assessments may be more or less likely to admit to memory difficulties. Since this is a component of the most commonly used definitions of aMCI/MCI, these cultural variations may be reflected in differing prevalences. However, despite the differences in prevalences of aMCI between sites, associations with disability were relatively consistent, providing support for the cross-cultural applicability of the aMCI construct. They did not suggest, for example, that only more severe forms of aMCI were being identified in China where prevalence was lowest, compared to India where it was highest (particularly since disability was lower rather than higher in China in those with aMCI compared to the remainder of the sample). Associations between aMCI and disability should be viewed with caution since activities of daily living impairment is an exclusion criterion for the former. Lower likelihood of reporting difficulties in China would be unlikely to account for the negative association observed between aMCI and disability in that site because under-reporting would have to be differential between those with/without aMCI. There is very limited evidence from population-based studies on the occurrence and characteristics of neuropsychiatric symptoms that may accompany MCI [18]. While we did not find any association between aMCI and depressive symptoms, our findings of a significant association between aMCI and anxiety, apathy, and irritability are largely consistent with those from the Cardiovascular Health Study and the Mayo Clinic longitudinal study on aging in the US [72],[73], the Kungsholmen study in Sweden [74], and a small study from Thailand [75]. However, it should be borne in mind that individual behavioural/psychological symptoms were not mutually adjusted as outcomes and the independence of observed associations in Table 2 cannot be assumed.
Strengths of the study include the very large sample size and the wide range of populations sampled in terms of culture, economy, and population characteristics. Moreover, internal validity was maintained through rigorously prevalidated and standardised measurements applied consistently between countries in addition to common algorithms used to define aMCI. There are some limitations. The samples were drawn from specific geographic catchment areas and cannot be assumed to be representative of the source nation/site. No attempt was made to differentiate urban and rural status in this analysis because not all sites recruited from both settings. The study was cross-sectional in design and the impact of survival cannot be evaluated. Furthermore, within the aMCI category, participants who had developed this late in life could not be distinguished from those for whom it was a stable lifetime trait. Finally, aMCI diagnosis was determined without clinical judgement, which is difficult to obtain in large population-based studies and unfeasible in most of our study sites. Although aMCI was originally derived as a diagnosis for secondary or tertiary care clinical settings, it is being increasingly applied in epidemiological research and data from community samples is an important supplement, particularly if future community-level interventions are planned to prevent progression to dementia. Our analysis here is intended to extend this particular evidence base. Follow-up is currently underway in most 10/66 sites, which will provide further data on predictive validity.
This is one of the first studies, to our knowledge, to investigate the prevalence of aMCI in LAMICs, where the large majority of older people and people with dementia currently live [3],[4]. Longitudinal data are needed to clarify further the predictive validity of the aMCI case-definition applied here and to evaluate the extent to which it can be applied as a risk marker for further cognitive decline or dementia. In addition, further evaluation is needed of the associations with disability and neuropsychiatric symptoms since our findings do suggest higher than expected comorbidity and there are large absolute numbers of older people with aMCI in these rapidly ageing and populous world regions.
Zdroje
1. UN, Affairs DoEaS 2006 World population prospects: 2006 revision. New York Population Division, UN Secretariat
2. MathersCDLoncarD 2006 Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3 e442 doi:10.1371/journal.pmed.0030442
3. FerriCPPrinceMBrayneCBrodatyHFratiglioniL 2005 Global prevalence of dementia: a Delphi consensus study. Lancet 366 2112 2117
4. PrinceMJacksonJCAlbaneseESousaRMFerriCP 2009 World Alzheimer Report. London King's College London
5. Llibre RodriguezJJFerriCPAcostaDGuerraMHuangY 2008 Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet 372 464 474
6. CummingsJL 2004 Treatment of Alzheimer's disease: current and future therapeutic approaches. Rev Neurol Dis 1 60 69
7. KaduszkiewiczHZimmermannTBeck-BornholdtHPvan den BusscheH 2005 Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. BMJ 331 321 327
8. WilcockGK 2004 Primary prevention of dementia. Psychiatry 3 35 36
9. PetersenRCSmithGEWaringSCIvnikRJTangalosEG 1999 Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56 303 308
10. PetersenRCStevensJCGanguliMTangalosEGCummingsJL 2001 Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56 1133 1142
11. PurserJLFillenbaumGGPieperCFWallaceRB 2005 Mild cognitive impairment and 10-year trajectories of disability in the Iowa Established Populations for Epidemiologic Studies of the Elderly cohort. J Am Geriatr Soc 53 1966 1972
12. HunderfundALRobertsROSlusserTCLeibsonCLGedaYE 2006 Mortality in amnestic mild cognitive impairment: a prospective community study. Neurology 67 1764 1768
13. PalmerKBackmanLWinbladBFratiglioniL 2003 Detection of Alzheimer's disease and dementia in the preclinical phase: population based cohort study. BMJ 326 245
14. PanzaFCapursoCD'IntronoAColaciccoAMCapursoA 2007 Heterogeneity of mild cognitive impairment and other predementia syndromes in progression to dementia. Neurobiol Aging 28 : 1631-1632; discussion 1633-1634
15. StephanBCMatthewsFEMcKeithIGBondJBrayneC 2007 Early cognitive change in the general population: how do different definitions work? J Am Geriatr Soc 55 1534 1540
16. ArnaizEAlmkvistOIvnikRJTangalosEGWahlundLO 2004 Mild cognitive impairment: a cross-national comparison. J Neurol Neurosurg Psychiatry 75 1275 1280
17. XuGMeyerJSHuangYChenGChowdhuryM 2004 Cross-cultural comparison of mild cognitive impairment between China and USA. Curr Alzheimer Res 1 55 61
18. ApostolovaLGCummingsJL 2008 Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord 25 115 126
19. PalmerKBackmanLWinbladBFratiglioniL 2008 Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry 16 603 611
20. PetersenRCRobertsROKnopmanDSGedaYEChaRH 2010 Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 75 889 897
21. BusseABischkopfJRiedel-HellerSGAngermeyerMC 2003 Subclassifications for mild cognitive impairment: prevalence and predictive validity. Psychol Med 33 1029 1038
22. DlugajMWeimarCWegeNVerdePEGerwigM 2010 Prevalence of mild cognitive impairment and its subtypes in the Heinz Nixdorf Recall study cohort. Dement Geriatr Cogn Disord 30 362 373
23. GamaldoAAAllaireJCSimsRCWhitfieldKE 2010 Assessing mild cognitive impairment among older African Americans. Int J Geriatr Psychiatry 25 748 755
24. GanguliMChangCCSnitzBESaxtonJAVanderbiltJ 2010 Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry 18 674 683
25. GanguliMDodgeHHShenCDeKoskyST 2004 Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology 63 115 121
26. HanninenTHallikainenMTuomainenSVanhanenMSoininenH 2002 Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta Neurol Scand 106 148 154
27. JungwirthSWeissgramSZehetmayerSTraglKHFischerP 2005 VITA: subtypes of mild cognitive impairment in a community-based cohort at the age of 75 years. Int J Geriatr Psychiatry 20 452 458
28. KochanNASlavinMJBrodatyHCrawfordJDTrollorJN 2010 Effect of different impairment criteria on prevalence of “objective” mild cognitive impairment in a community sample. Am J Geriatr Psychiatry 18 711 722
29. LiJWangYJZhangMXuZQGaoCY 2011 Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 76 1485 1491
30. LopezOLJagustWJDeKoskySTBeckerJTFitzpatrickA 2003 Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 60 1385 1389
31. ManlyJJBell-McGintySTangMXSchupfNSternY 2005 Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol 62 1739 1746
32. MeguroKIshiiHYamaguchiSIshizakiJSatoM 2004 Prevalence and cognitive performances of clinical dementia rating 0.5 and mild cognitive impairment in Japan. The Tajiri project. Alzheimer Dis Assoc Disord 18 3 10
33. RitchieKArteroSTouchonJ 2001 Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 56 37 42
34. DickersonBCSperlingRAHymanBTAlbertMSBlackerD 2007 clinical prediction of alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry 64 1443 1450
35. RappSRLegaultCHendersonVWBrunnerRLMasakiK 2010 Subtypes of mild cognitive impairment in older postmenopausal women: the Women's Health Initiative Memory Study. Alzheimer Dis Assoc Disord 24 248 255
36. YaffeKMiddletonLELuiLYSpiraAPStoneK 2011 Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol 68 631 636
37. DasSKBosePBiswasADuttABanerjeeTK 2007 An epidemiologic study of mild cognitive impairment in Kolkata, India. Neurology 68 2019 2026
38. LeeLKShaharSChinAVMohd YusoffNARajabN 2011 Prevalence of gender disparities and predictors affecting the occurrence of mild cognitive impairment (MCI). Arch Gerontol Geriatr 54 185 191
39. KimKWParkJHKimMHKimMDKimBJ 2011 A nationwide survey on the prevalence of dementia and mild cognitive impairment in South Korea. J Alzheimers Dis 23 281 291
40. PrinceMFerriCPAcostaDAlbaneseEArizagaR 2007 The protocols for the 10/66 dementia research group population-based research programme. BMC Public Health 7 165
41. PrinceMAcostaDChiuHScazufcaMVargheseM 2003 Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet 361 909 917
42. CopelandJRDeweyMEGriffiths-JonesHM 1986 A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med 16 89 99
43. CopelandJRPrinceMWilsonKCDeweyMEPayneJ 2002 The Geriatric Mental State Examination in the 21st century. Int J Geriatr Psychiatry 17 729 732
44. HallKSHendrieHCBrittainHMNortonJAJr.RodgersDD 1993 The Development of a dementia screening interview in two distinct languages. International Journal of Methods in Psychiatric Research 3 1 28
45. WelshKAButtersNMohsRCBeeklyDEdlandS 1994 The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 44 609 614
46. UstunTBKostanjsekNChatterjiS Rehm J Measuring health and disability: manual for WHO Disability Assessment Schedule (WHODAS 2.0). Geneva World Health Organization In press
47. RehmJÜstünTBSaxenaSNelsonCBChatterjiS 1999 On the development and psychometric testing of the WHO screening instrument to assess disablement in the general population. Int J Meth Psych Res 8 110 122
48. SousaRMDeweyMEAcostaDJotheeswaranATCastro-CostaE 2010 Measuring disability across cultures–the psychometric properties of the WHODAS II in older people from seven low - and middle-income countries. The 10/66 Dementia Research Group population-based survey. Int J Methods Psychiatr Res 19 1 17
49. KauferDICummingsJLKetchelPSmithVMacMillanA 2000 Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 12 233 239
50. GurujeOUnverzargtFWOsuntokunBOHendrieHCBaiyewuO 1995 The CERAD Neuropsychological Test Battery: norms from a Yoruba-speaking Nigerian sample. West Afr J Med 14 29 33
51. AlbertMSDekoskySTDicksonDDuboisBFeldmanHH 2011 The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7 270 279
52. LarrieuSMLetenneurLPOrgogozoJMMFabrigouleCPAmievaHP 2002 Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 59 1594 1599
53. PalmerKWang H-X, BackmanLWinbladBFratiglioniL 2002 Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. Am J Psychiat 159 436 442
54. GrahamJERockwoodKBeattieBLEastwoodRGauthierS 1997 Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 349 1793
55. GauthierSTouchonJ 2005 Mild cognitive impairment is not a clinical entity and should not be treated. Arch Neurol 62 1164 1166 discussion 1167
56. PetersenRCMorrisJC 2005 Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 62 1160 1163 discussion 1167
57. RaschettiRAlbaneseEVanacoreNMagginiM 2007 Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med 4 e338 doi:10.1371/journal.pmed.0040338
58. WhitehousePJMoodyHR 2006 Mild cognitive impairment: A ‘hardening of the categories’? Dementia 5 11 25
59. GanguliMChandraVGilbyJERatcliffGSharmaSD 1996 Cognitive test performance in a community-based nondemented elderly sample in rural India: the Indo-U.S. Cross-National Dementia Epidemiology Study. Int Psychogeriatr 8 507 524
60. KimJMStewartRPrinceMShinISYoonJS 2003 Diagnosing dementia in a developing nation: an evaluation of the GMS-AGECAT algorithm in an older Korean population. Int J Geriatr Psychiatry 18 331 336
61. PrinceMJde RodriguezJLNoriegaLLopezAAcostaD 2008 The 10/66 Dementia Research Group's fully operationalised DSM-IV dementia computerized diagnostic algorithm, compared with the 10/66 dementia algorithm and a clinician diagnosis: a population validation study. BMC Public Health 8 219
62. STATA 2007 Stata Statistical Software: Release 10. LPS College Station (Texas) StataCorp. LP 2007
63. SousaRMFerriCPAcostaDAlbaneseEGuerraM 2009 Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet 374 1821 1830
64. LumleyTKronmalRMaS 2006 Relative risk regression in medical research: models, contrasts, estimators, and algorithms. Technical report 293, UW Biostatistics Working Paper Series. Available: http://www.bepress.com/uwbiostat/paper293. Accessed 8 January 2012.
65. G Zou 2004 A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159 702 706
66. HigginsJPThompsonSG 2002 Quantifying heterogeneity in a meta-analysis. Stat Med 21 1539 1558
67. RitchieKTouchonJ 2000 Mild cognitive impairment: conceptual basis and current nosological status. Lancet 355 225 228
68. MatthewsFEStephanBCBondJMcKeithIBrayneC 2007 Operationalization of mild cognitive impairment: a graphical approach. PLoS Med 4 1615 doi:10.1371/journal.pmed.0040304
69. TognoniGCeravoloRNucciaroneBBianchiFDell'AgnelloG 2005 From mild cognitive impairment to dementia: a prevalence study in a district of Tuscany, Italy. Acta Neurol Scand 112 65 71
70. BusseABischkopfJRiedel-HellerSGAngermeyerMC 2003 Mild cognitive impairment: prevalence and predictive validity according to current approaches. Acta Neurol Scand 108 71 81
71. FratiglioniLWinbladBvon StraussE 2007 Prevention of Alzheimer's disease and dementia. Major findings from the Kungsholmen Project. Physiol Behav 92 98 104
72. LyketsosCGLopezOJonesBFitzpatrickALBreitnerJ 2002 Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 288 1475 1483
73. GedaYERobertsROKnopmanDSPetersenRCChristiansonTJ 2008 Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 65 1193 1198
74. PalmerKBergerAKMonasteroRWinbladBBackmanL 2007 Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology 68 1596 1602
75. MuangpaisanWIntalapapornSAssantachaiP 2008 Neuropsychiatric symptoms in the community-based patients with mild cognitive impairment and the influence of demographic factors. Int J Geriatr Psychiatry 23 699 703
Štítky
Interní lékařství
Článek : Transmission ?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 2- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Nutraceutikum Armolipid Plus podle klinických důkazů zlepšuje lipidový profil − metaanalýza
- Snižuje terapie betablokátory kardiovaskulární benefit aerobního cvičení u pacientů s arteriální hypertenzí?
-
Všechny články tohoto čísla
- Beyond the Numbers: Describing Care at the End of Life
- Engaging Men in Prevention and Care for HIV/AIDS in Africa
- Mobile Phone Text Messaging: Tool for Malaria Control in Africa
- Homocysteine and Coronary Heart Disease: Meta-analysis of Case-Control Studies, Avoiding Publication Bias
- Socioeconomic Factors and All Cause and Cause-Specific Mortality among Older People in Latin America, India, and China: A Population-Based Cohort Study
- The Evolving Landscape of the Economics of HIV Treatment and Prevention
- Complexity in Non-Pharmacological Caregiving Activities at the End of Life: An International Qualitative Study
- : Transmission ?
- Why Does Mental Health Not Get the Attention It Deserves? An Application of the Shiffman and Smith Framework
- Prevalence, Distribution, and Impact of Mild Cognitive Impairment in Latin America, China, and India: A 10/66 Population-Based Study
- Association between Clean Delivery Kit Use, Clean Delivery Practices, and Neonatal Survival: Pooled Analysis of Data from Three Sites in South Asia
- Characterisation of Hospital Ward–Based Transmission Using Extensive Epidemiological Data and Molecular Typing
- The Activities of Current Antimalarial Drugs on the Life Cycle Stages of : A Comparative Study with Human and Rodent Parasites
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Activities of Current Antimalarial Drugs on the Life Cycle Stages of : A Comparative Study with Human and Rodent Parasites
- Association between Clean Delivery Kit Use, Clean Delivery Practices, and Neonatal Survival: Pooled Analysis of Data from Three Sites in South Asia
- Prevalence, Distribution, and Impact of Mild Cognitive Impairment in Latin America, China, and India: A 10/66 Population-Based Study
- Characterisation of Hospital Ward–Based Transmission Using Extensive Epidemiological Data and Molecular Typing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



