-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRisk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
Background:
Diarrhea is a leading cause of childhood morbidity and mortality in sub-Saharan Africa. Data on risk factors for mortality are limited. We conducted hospital-based surveillance to characterize the etiology of diarrhea and identify risk factors for death among children hospitalized with diarrhea in rural western Kenya.Methods and Findings:
We enrolled all children <5 years old, hospitalized with diarrhea (≥3 loose stools in 24 hours) at two district hospitals in Nyanza Province, western Kenya. Clinical and demographic information was collected. Stool specimens were tested for bacterial and viral pathogens. Bivariate and multivariable logistic regression analyses were carried out to identify risk factors for death. From May 23, 2005 to May 22, 2007, 1,146 children <5 years old were enrolled; 107 (9%) children died during hospitalization. Nontyphoidal Salmonella were identified in 10% (118), Campylobacter in 5% (57), and Shigella in 4% (42) of 1,137 stool samples; rotavirus was detected in 19% (196) of 1,021 stool samples. Among stools from children who died, nontyphoidal Salmonella were detected in 22%, Shigella in 11%, rotavirus in 9%, Campylobacter in 5%, and S. Typhi in <1%. In multivariable analysis, infants who died were more likely to have nontyphoidal Salmonella (adjusted odds ratio [aOR] = 6·8; 95% CI 3·1–14·9), and children <5 years to have Shigella (aOR = 5·5; 95% CI 2·2–14·0) identified than children who survived. Children who died were less likely to be infected with rotavirus (OR = 0·4; 95% CI 0·2–0·8). Further risk factors for death included being malnourished (aOR = 4·2; 95% CI 2·1–8·7); having oral thrush on physical exam (aOR = 2·3; 95% CI 1·4–3·8); having previously sought care at a hospital for the illness (aOR = 2·2; 95% CI 1·2–3·8); and being dehydrated as diagnosed at discharge/death (aOR = 2·5; 95% CI 1·5–4·1). A clinical diagnosis of malaria, and malaria parasites seen on blood smear, were not associated with increased risk of death. This study only captured in-hospital childhood deaths, and likely missed a substantial number of additional deaths that occurred at home.Conclusion:
Nontyphoidal Salmonella and Shigella are associated with mortality among rural Kenyan children with diarrhea who access a hospital. Improved prevention and treatment of diarrheal disease is necessary. Enhanced surveillance and simplified laboratory diagnostics in Africa may assist clinicians in appropriately treating potentially fatal diarrheal illness.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001256
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001256Summary
Background:
Diarrhea is a leading cause of childhood morbidity and mortality in sub-Saharan Africa. Data on risk factors for mortality are limited. We conducted hospital-based surveillance to characterize the etiology of diarrhea and identify risk factors for death among children hospitalized with diarrhea in rural western Kenya.Methods and Findings:
We enrolled all children <5 years old, hospitalized with diarrhea (≥3 loose stools in 24 hours) at two district hospitals in Nyanza Province, western Kenya. Clinical and demographic information was collected. Stool specimens were tested for bacterial and viral pathogens. Bivariate and multivariable logistic regression analyses were carried out to identify risk factors for death. From May 23, 2005 to May 22, 2007, 1,146 children <5 years old were enrolled; 107 (9%) children died during hospitalization. Nontyphoidal Salmonella were identified in 10% (118), Campylobacter in 5% (57), and Shigella in 4% (42) of 1,137 stool samples; rotavirus was detected in 19% (196) of 1,021 stool samples. Among stools from children who died, nontyphoidal Salmonella were detected in 22%, Shigella in 11%, rotavirus in 9%, Campylobacter in 5%, and S. Typhi in <1%. In multivariable analysis, infants who died were more likely to have nontyphoidal Salmonella (adjusted odds ratio [aOR] = 6·8; 95% CI 3·1–14·9), and children <5 years to have Shigella (aOR = 5·5; 95% CI 2·2–14·0) identified than children who survived. Children who died were less likely to be infected with rotavirus (OR = 0·4; 95% CI 0·2–0·8). Further risk factors for death included being malnourished (aOR = 4·2; 95% CI 2·1–8·7); having oral thrush on physical exam (aOR = 2·3; 95% CI 1·4–3·8); having previously sought care at a hospital for the illness (aOR = 2·2; 95% CI 1·2–3·8); and being dehydrated as diagnosed at discharge/death (aOR = 2·5; 95% CI 1·5–4·1). A clinical diagnosis of malaria, and malaria parasites seen on blood smear, were not associated with increased risk of death. This study only captured in-hospital childhood deaths, and likely missed a substantial number of additional deaths that occurred at home.Conclusion:
Nontyphoidal Salmonella and Shigella are associated with mortality among rural Kenyan children with diarrhea who access a hospital. Improved prevention and treatment of diarrheal disease is necessary. Enhanced surveillance and simplified laboratory diagnostics in Africa may assist clinicians in appropriately treating potentially fatal diarrheal illness.
: Please see later in the article for the Editors' SummaryIntroduction
Diarrhea is a major cause of morbidity and mortality among children <5 y old in sub-Saharan Africa [1],[2]. Of the estimated 4·2 million deaths in children <5 y old in Africa in 2008, diarrhea caused the largest proportion (19%); followed by pneumonia (18%), and malaria (16%) [3]. The number of childhood deaths only decreased by 4% in Africa from 2000–2003 to 2008, suggesting that interventions for these diseases are lacking in Africa [3],[4].
New tools to combat severe illness and death from rotavirus gastroenteritis have been developed [5]–[7]; recently, the World Health Organization (WHO) recommended the use of rotavirus vaccines worldwide [8]. However, besides the WHO recommendation in 2004 to use zinc for the treatment of diarrhea in children [9], limited progress has been made in the development of new effective prevention and treatment measures for other diarrheal diseases, even as availability and use has declined for the most practical interventions to prevent mortality, oral rehydration solution (ORS) and appropriate infant feeding practices during diarrheal illness [10]–[12]. Understanding the frequency, and relative severity, of diarrheal pathogens in children in sub-Saharan Africa, as well as the antimicrobial susceptibilities of bacterial diarrheal pathogens is critical to better tailor treatment regimens and stimulate new approaches for the prevention of childhood diarrhea.
Detailed information on the etiology and risk factors for fatal childhood diarrhea in sub-Saharan Africa is sparse [13]. Previous mortality studies among young children in various settings in Africa have identified young age, co-morbidity, poor nutritional status, dehydration, lack of breastfeeding, thrush, immunosuppression, prolonged diarrheal duration before hospital admission, extended hospitalization, previous hospital discharges, inter alia, as risk factors for death [14]–[20]. None of these studies identified diarrheal etiologies. Due to the scarcity of data on pathogen-specific childhood diarrheal deaths in Africa, estimates of the relative contribution of infectious diarrheal etiologies to mortality are unavailable [13].
To address the lack of data examining a range of specific infectious agents and risk factors for childhood diarrheal mortality, we carried out a cohort study among children <5 y old in western Kenya to characterize the etiologies and risk factors associated with death during hospitalization for diarrhea.
Materials and Methods
Setting
Bondo and Siaya District Hospitals are two large government-operated district hospitals in Nyanza Province, western Kenya; together they serve mainly a rural population of ∼750,000 people [21]. In 2003, 88% of households in this region lacked access to safe potable water, and 26% lacked latrines [22]. Nyanza Province has one of the nation's lowest immunization rates, the highest infant (125 per 1,000 live births) and child (227 per 1,000 live births) mortality rates, and the highest reported HIV prevalence (15% among persons aged 15–49 y); malaria infection and malnutrition are common [22],[23].
Hospital-Based Surveillance
Data were prospectively collected on admission and at death or discharge for all children <5 y old hospitalized with diarrhea at Bondo and Siaya District Hospitals from May 23, 2005 to May 22, 2007. Diarrhea was defined as ≥3 loose stools within 24 h occurring in the previous 5 d. Bloody diarrhea was defined as the presence of visible blood in stool. All enrolled children were assessed clinically and treated according to the Kenya Ministry of Health (MoH) pediatric standard of care by MoH clinicians not affiliated with the study.
After explaining the study and obtaining written informed consent, trained study staff interviewed caretakers in Dholuo using a standardized supplemental diarrheal questionnaire, collecting information about patient demographics, and the child's clinical diarrheal history; caretakers' written informed consent was also sought to collect, store, and test a stool specimen from their child. The enrolled child's diarrheal treatment and outcome were derived from standardized interviews with the attending clinician, and from medical record abstraction. All reference to children who died or survived specifically relates to in-hospital death or survival. For children with multiple diarrhea admissions, only the last diarrheal episode for which the child sought care was included. In addition to the supplemental diarrhea specific questionnaire, information was collected via trained study staff of the Kenya Medical Research Institute (KEMRI)/Centers for Disease Control and Prevention (CDC) Health and Demographic Surveillance System (HDSS) inpatient surveillance program. The HDSS staff captured routine data on the enrolled child's overall clinical course, exam by the attending physician, anthropometric measurements, health-seeking behavior, treatments before the hospital visit, and laboratory findings, including malaria status. From January 1 to May 22, 2007, HDSS data were not collected at Bondo District Hospital. All enrolled children presenting with diarrhea had the supplemental diarrhea questionnaire administered; additional HDSS data were available for enrolled children who resided in the HDSS study area.
The admission diagnosis was captured by a physician on physical examination of the child at the point of initial admission to the hospital. At the time of discharge or death the physician reviewed the patient records and indicated the diagnosis on discharge/death considering all additional information available during the hospitalization, for example results from diagnostic testing, etc. We defined dehydration on physical exam as sunken eyes, loss of skin turgor, or sunken fontenelle. Fast breathing was defined as ≥60 breaths per minute for children <2 mo old, ≥50 breaths per minute for children 2–11 mo and ≥40 breaths per minute for children 12–59 mo old [24]. The presence and density of malaria parasites was determined by blood smear. After assessment of the median parasite density, high parasite density was defined as ≥20,000 parasites per µl of blood. Severe anemia was defined as a hemoglobin concentration <5 g/dl. HIV counseling and testing were not routinely offered at the time of the study; therefore HIV testing results are not available for participants. For study purposes, classification of HIV/AIDS was based on a clinical diagnosis of “immune suppression syndrome,” or documented antiretroviral therapy on medical records.
Malnutrition was defined as a z-score of <−2 for weight-for-length/height, length/height-for-age, or weight-for-age. Z-scores were calculated using a WHO SAS macro and the WHO Child Growth Standards as the reference population [25]. A restricted analysis was carried out with missing and biologically implausible values excluded [25],[26].
Laboratory Methods
Whole stool specimens and/or rectal swabs placed in Cary-Blair transport medium were cultured for Salmonella, Shigella, Campylobacter, and Vibrio species by standard techniques, and tested for rotavirus by enzyme-linked immunoassay (EIA) (catalogue number 696004, Meridian Biosciences, Inc.) at the KEMRI/CDC laboratory in Kisumu, Kenya. Campylobacter isolates were tested for hippurate hydrolysis to identify C. jejuni (hippurate positive) from C. coli (hippurate negative and grew on Campylobacter selective media at 42°C) or Campylobacter species. Vibrio cholerae isolates were assessed for the O1 serogroup using commercial antisera (catalogue number LL-13916, Lee Labs, Becton-Dickinson) [27]. Antimicrobial susceptibilities of Salmonella, Shigella, and Vibrio isolates to a panel of antimicrobial agents (VWR International) (amoxicillin-clavulanic acid; ampicillin; ceftriaxone; chloramphenicol; ciprofloxacin; gentamicin; kanamycin; nalidixic acid; streptomycin; sulfisoxazole; tetracycline; trimethoprim-sulfamethoxazole) was determined by the disk diffusion method at the KEMRI/CDC laboratory in Kisumu, Kenya [28]. Isolates with moderate or intermediate susceptibility were classified as susceptible. Laboratory results were communicated to each hospital.
The CDC laboratories in Atlanta serotyped nontyphoidal Salmonella and Shigella isolates (catalogue number 294821, Denka Seiken Co. LTD), and tested V. cholerae isolates by PCR for cholera toxin (ctxA) [29], biotype (tcpA) [30], and species-specific gene sequences [31],[32]. Specimens EIA-positive for rotavirus from children who died were subtyped using semi-nested reverse-transcription PCR (RT-PCR) targeting two outer capsid protein genes, VP7 (G-genotype) and VP4 (P-genotype) at the KEMRI laboratories in Nairobi, Kenya [33].
Stool specimens from all 107 children who died, and because of cost and logistical constraints, specimens from a subset of 107 children who survived were tested at the CDC laboratories in Atlanta, GA by multiplex PCR for the genes of enteroaggregative Escherichia coli (EAEC) [34], enteropathogenic E. coli (EPEC) [35],[36], enterotoxigenic E. coli (ETEC) [37], enteroinvasive E. coli (EIEC) [38], Shiga toxin-producing E. coli (STEC) [36], and for norovirus, sapovirus, and astrovirus by real-time TaqMan RT-PCR [39],[40].
Data Management
Data were recorded on optical character recognition enabled forms, scanned into a database using Teleform version 9 software (Verity, Inc., 2003), and subjected to validity checks.
Statistical Analysis
Statistical analyses were performed in SAS version 9·2 (SAS Institute, Inc.). Categorical variables were compared with χ2 or Fisher exact tests, and continuous variables with the Kruskal-Wallis rank-sum test. Odds ratios (OR) with 95% CI were calculated. Exact 95% CIs were computed where applicable. Logistic regression was used for multivariable analysis. The best subset selection method was used to select a final multivariable model from an initial set of variables that had a p-value of ≤0·2 on bivariate analysis. Final selection was based on a significance level of 0.05. The best selection method in SAS version 9·2 uses the algorithm of Furnival and Wilson [41] to find the subsets with the highest likelihood score statistic for models with 1, 2, 3, and so on, explanatory variables. We forced relevant variables into the model, and then selected the most parsimonious model with the highest likelihood score statistic. The influence of each two-way interaction on the main effects and other interaction terms, including age, were assessed.
Testing for diarrheagenic E. coli, norovirus, sapovirus, or astrovirus was completed for all 107 children who died, and because of resource limitations, a matching set of 107 survivors. Separate from the main cohort study analysis, to examine if these additional pathogens were independently associated with death relative to other etiologies, each child who died was matched to one child who survived on the basis of age in months, identification of nontyphoidal Salmonella or Shigella, and weight-for-age z-score to control for these factors. A conditional logistic regression model was fit using the approach described above. Matched odds ratios (mORs) with 95% CI were calculated.
Table 1 lists the data collected for each of the subsets and the associated denominators used in the analysis.
Tab. 1. List of the data collected for each of the subsets, and the associated denominators. 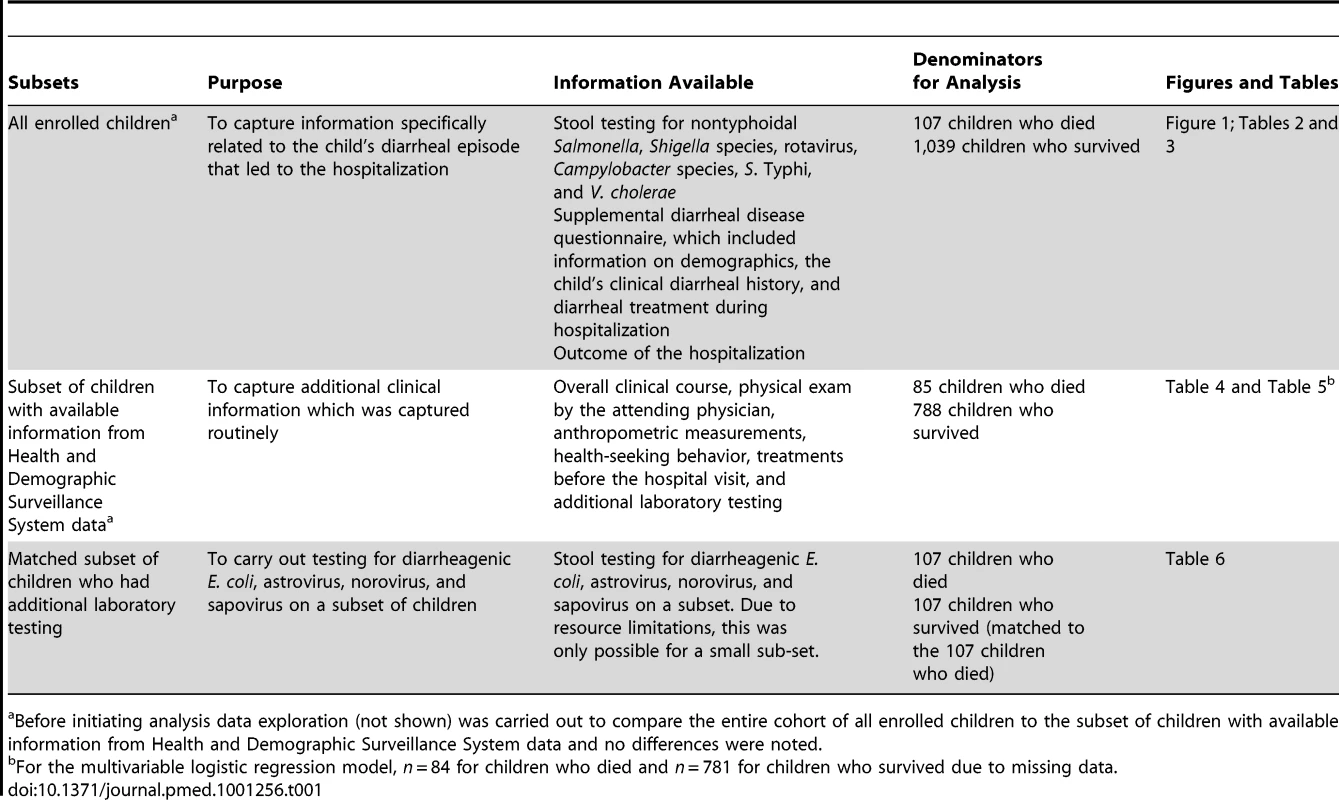
Before initiating analysis data exploration (not shown) was carried out to compare the entire cohort of all enrolled children to the subset of children with available information from Health and Demographic Surveillance System data and no differences were noted. Ethical Review
The study protocol was approved by the Institutional Review Board of the US CDC, the KEMRI Scientific Steering Committee (SSC), and the KEMRI National Ethical Review Committee (ERC).
Results
From May 23, 2005 to May 22, 2007, 1,146 children <5 y old hospitalized with diarrhea were enrolled; 508 (44%) were female. The participation rate was 90%; among those who declined, the reasons were the caretaker was in a hurry (87%), refused (8%), or was unavailable (5%). No caretakers with severely ill children refused participation. Of 1,146 enrolled children (757 and 389 at Siaya District Hospital and Bondo District Hospital, respectively), 107 died during hospitalization, for an in-hospital case fatality ratio (CFR) of 9·3% (9·4% and 9·3% at Siaya District Hospital and Bondo District Hospital, respectively). The sex-specific CFR among children with diarrhea who died during hospitalization was 10·2% for females (52 of 508) and 8·6% for males (55 of 638), p = 0·4. The median age at presentation was 9 mo old for children who died and those who survived (Table 2). Eight enrolled children were neonates, of whom none died.
Tab. 2. Demographic and clinical characteristics, enteric pathogens identified, and pathogen-specific CFRs for 1,146 children <5 y old hospitalized with diarrhea, by children who died (n = 107) and survived (n = 1,039), western Kenya 2005–2007. 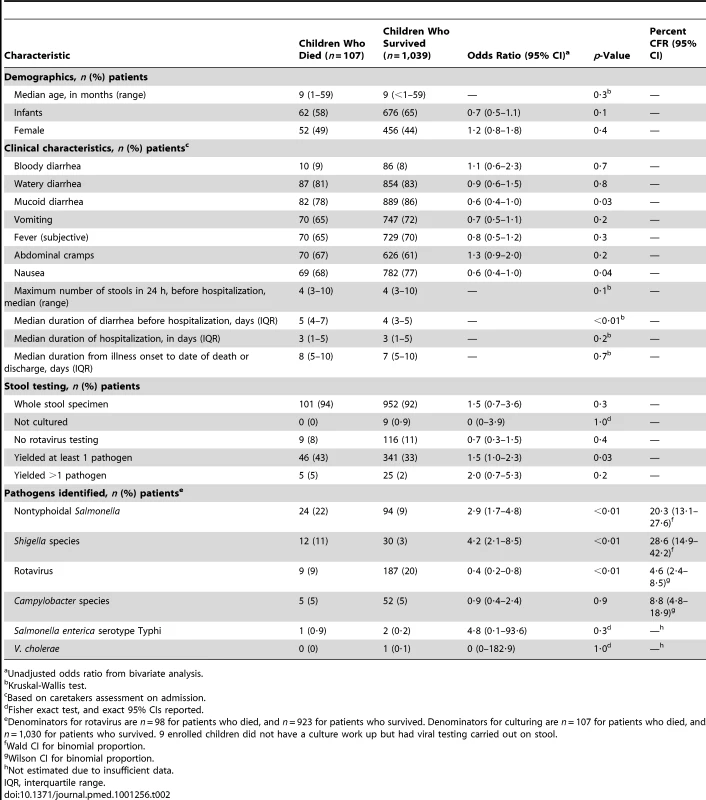
Unadjusted odds ratio from bivariate analysis. Reported symptoms for children who died and those who survived were similar. Overall, bloody diarrhea was recorded in 9% of children who died, and 8% who survived (OR = 1·1; 95% CI 0·6–2·3). Children who died had a significantly longer median duration of diarrhea before hospitalization than children who survived (5 versus 4 d, p<0·01), and both groups had similar median durations of hospitalization (3 versus 3 d, p = 0·2), and of diarrheal illness (8 versus 7 d, p = 0·7) (Table 2).
In total, 92% of specimens tested were whole stool specimens and the remainder were rectal swabs. A higher proportion of children who died than survived had an enteric pathogen identified in their stool (43% versus 33%, OR = 1·5; 95% CI 1·0–2·3) (Table 2).
Children who died were less likely to have rotavirus detected in stool than those who survived (9% versus 20%, OR = 0·4; 95% CI 0·2–0·8). The pathogen-specific CFR for rotavirus was 4·6% (CI 2·4–8·5). The pathogen-specific CFRs were highest for Shigella (28·6%, CI 14·9–42·2), and nontyphoidal Salmonella (20·3%, CI 13·1–27·6) (Table 2).
The highest age-specific all-cause CFRs were among children 2 to 4 y old (Figure 1). When stratified by age groups that were based on developmental stages, breastfeeding and the weaning ages, nontyphoidal Salmonella and Shigella infections were associated with increased mortality among infants 0–11 mo, and Shigella infection was associated with death among children 24–59 mo. No association between any one pathogen examined and increased mortality was seen in children 12–23 mo of age. Although overall rotavirus was most prevalent in infants, those who died were less likely to test positive for rotavirus than survivors. The highest proportion of rotavirus among children who died was in the 24–59-mo-old age category, where 19% had rotavirus identified in their stool. On unadjusted bivariate analysis there was a significant interaction between nontyphoidal Salmonella (p = 0.007) and age, and rotavirus and age (0.03) (Table 3).
Fig. 1. Age distribution (bars) and age-specific CFR (line) of 1,146 children hospitalized with diarrhea, western Kenya 2005–2007. 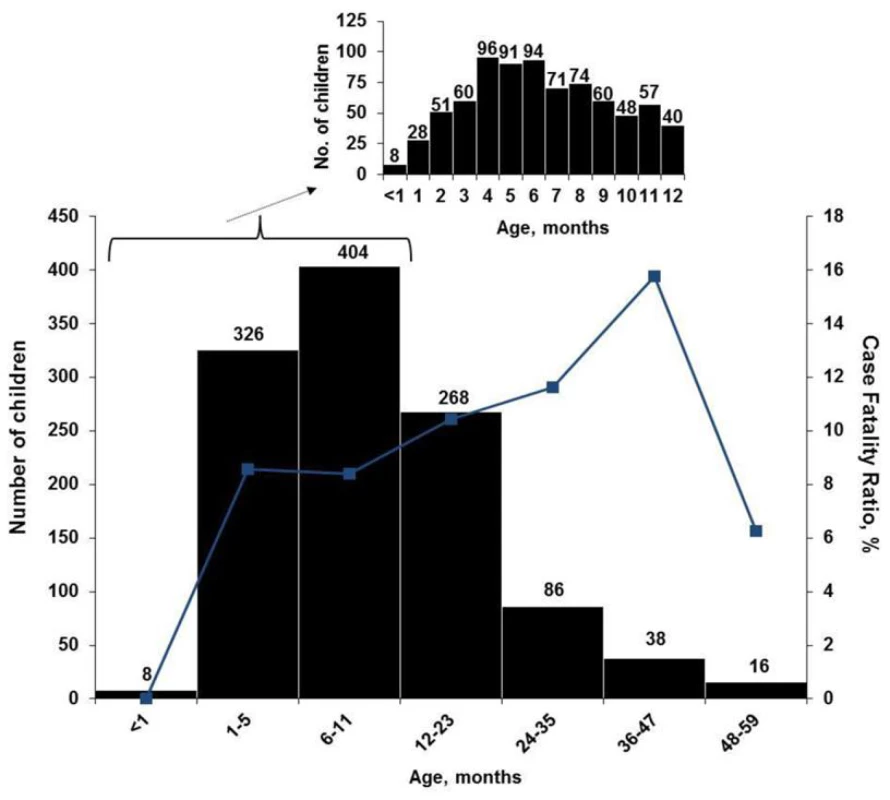
Inset, age distribution by 1-mo periods, for children aged <1 to 12 mo old (n = 778). Tab. 3. Select enteric pathogens identified by age group among enrolled children <5 y old hospitalized with diarrhea and had a stool specimen cultured and/or tested for viral pathogens, by children who died (n = 107) and survived (n = 1,039), western Kenya 2005–2007. 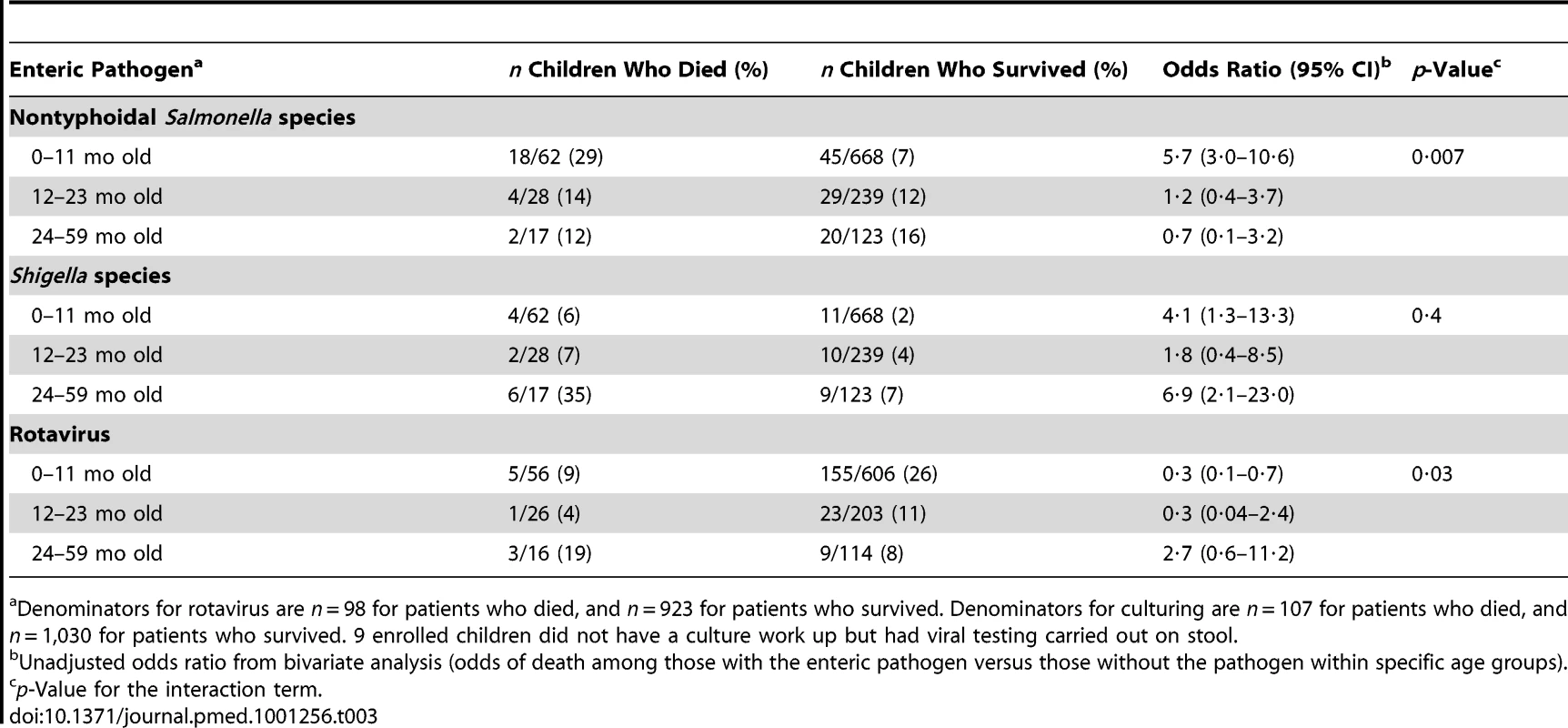
Denominators for rotavirus are n = 98 for patients who died, and n = 923 for patients who survived. Denominators for culturing are n = 107 for patients who died, and n = 1,030 for patients who survived. 9 enrolled children did not have a culture work up but had viral testing carried out on stool. Sub-typing results are shown in Table S1. Serotyping of the nontyphoidal Salmonella isolates revealed that 75% of the isolates among children who died and 70% among those who survived where S. Typhimurium. Shigella species distribution among children who died versus those who survived was somewhat different. For Campylobacter isolations, 80% of isolates from children who died and 83% from survivors were C. jejuni. Rotavirus genotyping results were available for four (44%) of the nine children who died with rotavirus; three were genotype G2P[4] and one was P[6] (Table S1).
We observed no significant differences between survivors and decedents in the proportion of nontyphoidal Salmonella or Shigella isolates that were resistant to any of the antimicrobial agents tested.
Subset of Children with Available Information from HDSS
Risk factors for death identified from the bivariate analysis of a subset of 85 (79%) children who died and 788 (76%) children who survived and had HDSS information available included the following: having a clinical diagnosis of malnutrition on admission or at discharge/death; being malnourished as assessed via anthropometry; oral thrush on physical exam; a clinical diagnosis of dehydration on admission, physical exam, or discharge/death; returning to the hospital for further treatment or being referred for further treatment from another health facility; and being taken to the hospital by a relative who was not the child's biological parent. Being awake and interactive or irritable on physical exam was protective against death (Table 4).
Tab. 4. Clinical information for children <5 y old hospitalized with diarrhea in rural western Kenya (HDSS subset: 85 children who died, 788 children who survived). 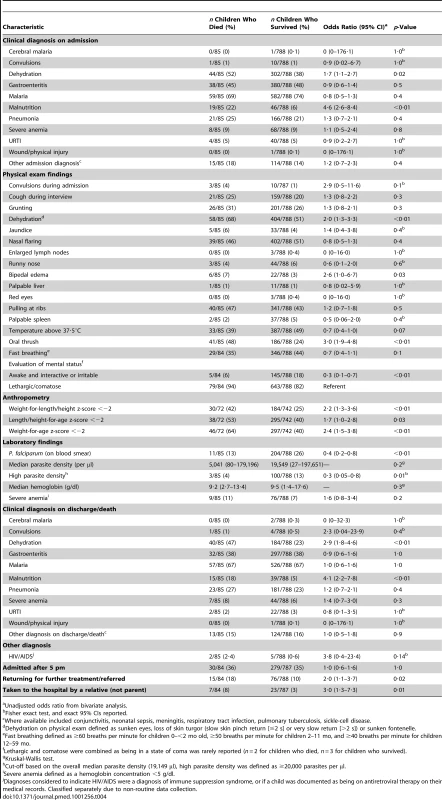
Unadjusted odds ratio from bivariate analysis. Having a clinical diagnosis of malaria, or having Plasmodium falciparum parasites on blood smear, and having a high malaria parasite density were not associated with increased risk of death (Table 4).
While a higher proportion of enrolled children who died than survived were diagnosed with HIV/AIDS (2·4% versus 0·6%, p = 0·14), the difference was not statistically significant (Table 4).
Previously seeking care for the current diarrheal illness was associated with death. No specific pre-hospitalization treatment or in-hospital treatment for the current hospitalization was identified as a risk or protective factor for death during the child's hospitalization (Table S2).
Twelve main-effect variables were selected for the final multivariable logistic regression model. Given the significant interaction of nontyphoidal Salmonella and age observed in the unadjusted bivariate analysis, the interaction term of nontyphoidal Salmonella and age was included in the model. Of the main effects, six were independently associated with an increased odds of death among enrolled children during hospitalization: nontyphoidal Salmonella species isolated from the stool of infants (adjusted OR [aOR] = 6·8; 95% CI 3·1–14·9), Shigella species isolated from stool (aOR = 5·5; 95% CI 2·2–14·0), having a clinical diagnosis of malnutrition on admission (aOR = 4·2; 95% CI 2·1–8·7), having a diagnosis of dehydration on discharge or death (aOR = 2·5; 95% CI 1·5–4·1), having oral thrush on physical exam (aOR = 2·3; 95% CI 1·4–3·8), and having previously sought care at a hospital for the current diarrheal illness (aOR = 2·2; 95% CI 1·2–3·8) (Table 5). Being awake and interactive or irritable as opposed to being lethargic or in a coma was associated with a reduced odds of death in the model (aOR = 0·3; 95% CI 0·1–0·9). We assessed all pairwise interactions, and other main effects, and none reached the 0.05 level of significance in the multivariable analysis.
Tab. 5. Factors independently associated with an increased or decreased risk of death among children <5 y old hospitalized with diarrhea in a multivariable logistic regression analysis, western Kenya 2005–2007. 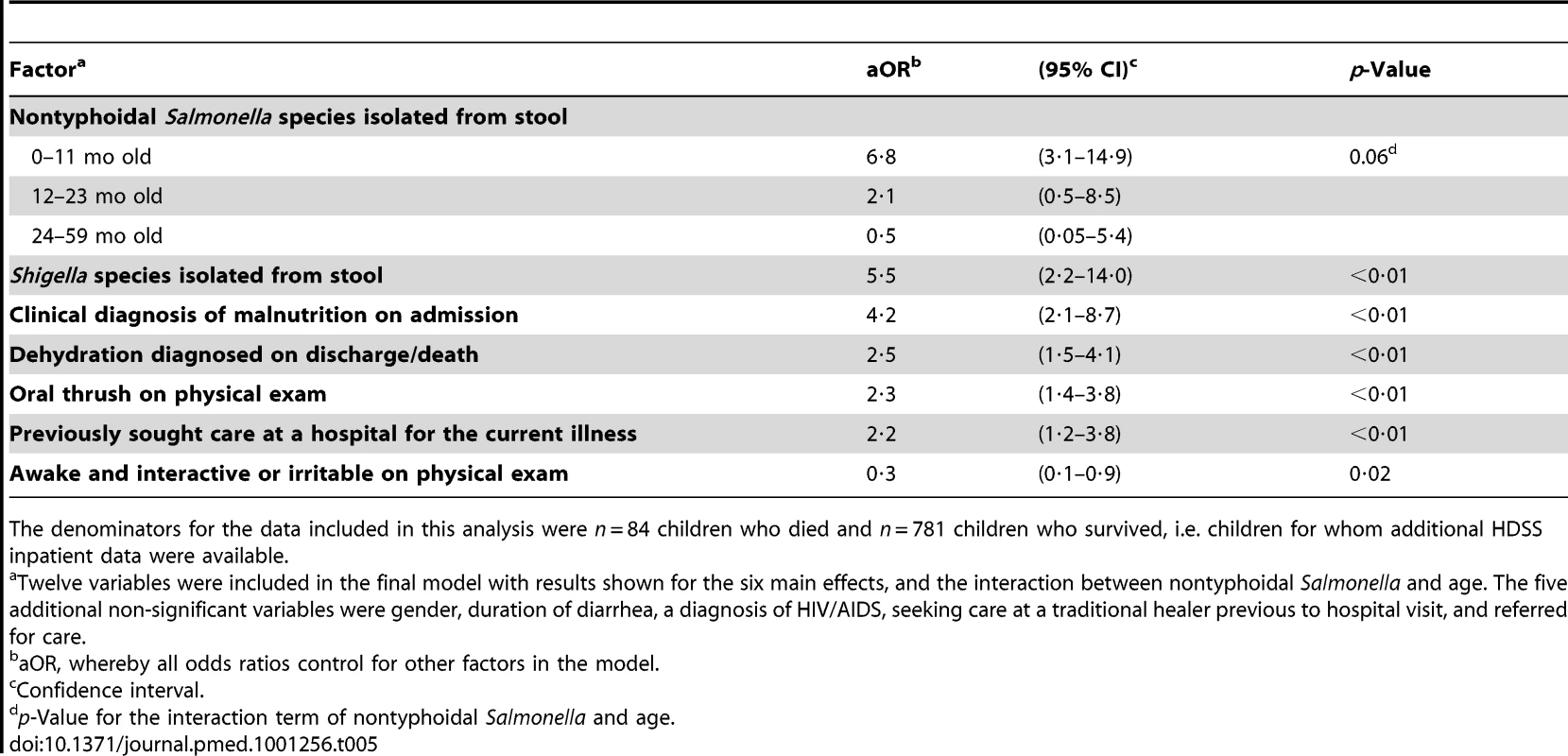
The denominators for the data included in this analysis were n = 84 children who died and n = 781 children who survived, i.e. children for whom additional HDSS inpatient data were available. Matched Subset of Children Who Had Additional Laboratory Testing
In the matched conditional logistic regression analysis of 107 children who died and 107 matched survivors, a higher proportion of children who survived than died had enteroaggregative E. coli (EAEC) (36% versus 30%), atypical EPEC (19% versus 9%), typical EPEC (19% versus 8%), enterotoxigenic E. coli (ETEC) (10% versus 6%), norovirus (9% versus 4%,), and sapovirus (8% versus 2%) identified. The same proportion of children who died and survived tested positive for astrovirus (7% versus 7%). No shiga toxin-producing E. coli (STEC) or enteroinvasive E. coli (EIEC) was identified. In the model, diarrheagenic E. coli, norovirus, astrovirus, and sapovirus were not found significantly more in decedents compared with survivors (Table 6). An unmatched analysis is also shown in Table 6 and provided similar results. No significant interactions with age and the enteric pathogens assessed in the subset were identified.
Tab. 6. Matched conditional logistic regression analysis of pathogen-specific risk factors for death among children <5 y old who died during hospitalization with diarrhea (n = 107), compared with children <5 y old with diarrhea who survived hospitalization (n = 107), western Kenya 2005–2007. 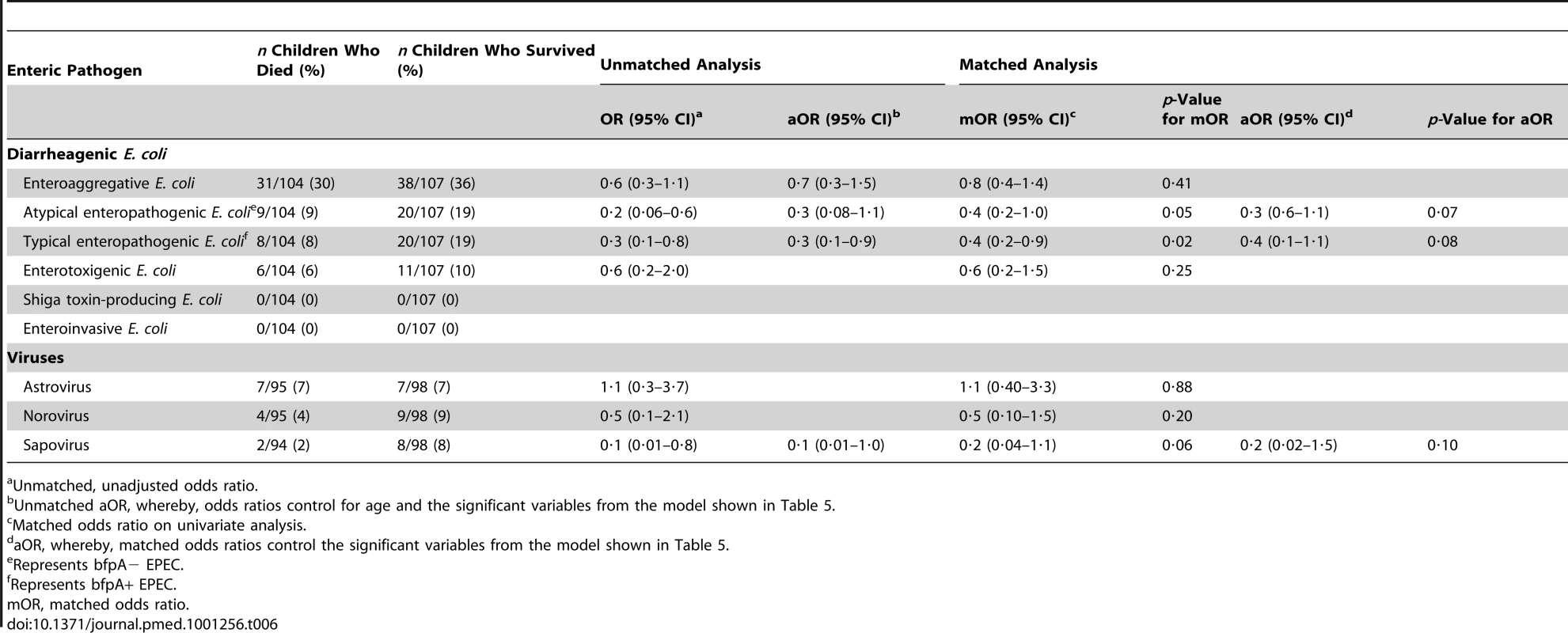
Unmatched, unadjusted odds ratio. Discussion
Although diarrhea is a major cause of mortality in young children in developing countries, few studies comprehensively examine infectious diarrheal etiologies associated with death [13]. Our study is unique because it examines risk factors for childhood diarrheal mortality including a range of diarrheal etiologies in a setting of a high infant and child mortality, and high malaria and HIV prevalence. Rotavirus was the most common etiology of diarrhea in hospitalized children in this rural area but was not the most frequently identified pathogen among in-hospital fatalities. The pathogen-specific CFR for rotavirus was lower than for other enteric pathogens, particularly nontyphoidal Salmonella and Shigella species. Children who died were more likely to have had nontyphoidal Salmonella, or Shigella infections than children who survived.
The children who died while hospitalized for diarrhea were vulnerable for several reasons. They had a significantly longer duration of diarrhea before reaching the hospital, and were more likely to be returning to the hospital, a marker for severe illness, or inadequate treatment/premature discharge on previous admission, as observed in previous studies [16],[17]. The median age of death was 9 mo old. Those with nontyphoidal Salmonella infections, which were associated with 22% of all deaths, were particularly young (median age 7 mo). This young age coincides with the critical weaning period when foods are introduced and Salmonella-specific maternal antibody is lost with consequent elevated risk of diarrhea [42]–[44]. Children who died had other co-morbidities, which were identified as independent risk factors for death, such as malnutrition, oral thrush (which can be associated with HIV/AIDS infection), and dehydration, as has been previously documented [14],[15],[18]. In general, all children in this study had substantially more stunting and wasting and a higher proportion were underweight than children in communities in Nyanza Province [45]. Of note, a lower or similar prevalence of a diarrheal pathogen among children who died compared to survivors does not necessarily indicate that infection did not contribute to mortality, but that the CFRs were lower for such pathogens than for others.
Most cases of non-bloody, non-septic bacterial diarrhea do not require antimicrobial therapy and resolve with symptomatic support (e.g., oral rehydration); however, >75% of enrolled children were treated in-hospital with an antimicrobial drug. It has been demonstrated in a previous study [46] and in the current study (unpublished data) that the utility of the commonly available antimicrobials for treating bacterial diarrhea in this area is substantially limited by reduced antimicrobial susceptibility, particularly for Shigella and nontyphoidal Salmonella. Training and oversight on judicious use of antimicrobial drugs, and enhanced access to laboratory diagnostics for diarrheal diseases, including capacity for blood culture, are warranted to appropriately treat potentially fatal diarrhea.
The study hospitals intermittently ran out of stocks of many critical and life-saving supplies for the treatment of diarrhea, such as ORS, and pediatric intravenous (IV) fluids, needles, or tubing during the study. In addition, in a study assessing community availability of ORS carried out in the area during the same time period, there was a documented lack of widespread availability of ORS packets with only 4% of shops and 48% of pharmacies in the area having ORS available for sale, resulting in very limited community access to life-saving treatment for dehydration outside of the health facility [47],[48].
This study was subject to several important limitations. It only captured in-hospital childhood deaths, and likely missed a substantial number of additional deaths that occurred at home. In resource-limited settings there are inherent biases in studying the etiology of diarrheal deaths in hospitals because rotavirus can be successfully treated with hydration relative to bacterial agents, which may require effective antimicrobial therapy, necessitating knowledge of the causative agent and its antimicrobial susceptibility, which is often not feasible in such settings. Since most diarrheal deaths occur at home where rehydration is less accessible, the etiologic picture of overall childhood diarrheal deaths could be different if community deaths were assessed. HIV counseling and testing were not routinely offered at the time of the study; therefore HIV testing results are not available for participants. Where available, we relied on HIV diagnosis based on clinical features, which may be subject to biases in assessing the factors contributing to diarrheal disease among participants since HIV infection at early stages may have been missed and not all data were routinely captured. Also, given the number of infectious enteric pathogens and clinical factors assessed our model may not have been able to differentiate between clinical factors that had similar effect sizes. The study did not capture other potentially relevant information, such as whether illness was associated with bacteremia (blood cultures were not done), breastfeeding status, and did not specifically ask about pre-hospitalization ORS use. Assessing the prognostic performance of the factors associated with mortality in the detection of patients at high risk of death, as has been carried out in previous mortality studies [49], would be important in future analysis, as would expanding the testing panel to include Cryptosporidium, Giardia, and other enteric agents. With regard to the sensitivity of the tests used, culture is the gold standard for the detection of bacterial agents, and the limit of detection of the viral RT-PCR assays ranges from 10–100 viral particles/reaction.
Since vaccines for most bacterial diarrheal diseases are in the distant future, and roll-out of rotavirus vaccines worldwide is as yet limited, expedited implementation of the new Kenyan Ministry of Public Health and Sanitation (MoPHS) policy on the control and management of diarrheal diseases in children <5 y old is critical [50]. The strategy focuses on home-based case management, including promotion of ORS and zinc use, prompt and effective health facility-based case management, diarrhea prevention through improved nutrition, water, sanitation, and hygiene, and the introduction of rotavirus vaccine, behavior change communication, and logistics management.
The national supply chain management of critical diarrhea treatment supplies such as ORS, pediatric IV fluids, and zinc, should be strengthened, and enforced systematic inventory monitoring of these supplies should take place at health facilities. The implementation of an improved supply chain, which is contained in the new MoPHS policy [50], will help improve the quality of inpatient pediatric care and prevent unnecessary diarrheal deaths.
The findings of particular clinical relevance are that immediate priority should be given to the management of children presenting to the hospital with diarrhea who are at high risk of death, including those who have previously sought care at a health facility for their illness, are dehydrated, have oral thrush, and are malnourished. In addition to receiving appropriate diarrhea case management, malnourished children with diarrhea should be provided nutritional rehabilitation. Further to identifying children at high risk for death from diarrhea in the hospital, this study can help inform policy makers on priority areas for interventions to reduce childhood diarrhea requiring hospitalization or resulting in death, such as the use of zinc for diarrhea management, reemphasis on community level promotion of ORS, water, sanitation and hygiene interventions, and the development and roll-out of new enteric vaccines.
Supporting Information
Zdroje
1. Boschi-PintoCYoungMBlackRE 2010 The Child Health Epidemiology Reference Group reviews of the effectiveness of interventions to reduce maternal, neonatal and child mortality. Int J Epidemiol 39 Suppl 1 i3 i6
2. UNICEF/WHO 2009 Diarrhea: Why children are still dying and what can be done Geneva The United Nations Children's Fund/World Health Organization 1 58
3. BlackRECousensSJohnsonHLLawnJERudanI 2010 Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375 1969 1987
4. BryceJBoschi-PintoCShibuyaKBlackRE 2005 WHO estimates of the causes of death in children. Lancet 365 1147 1152
5. MadhiSACunliffeNASteeleDWitteDKirstenM 2010 Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 362 289 298
6. SantoshamM 2010 Rotavirus vaccine–a powerful tool to combat deaths from diarrhea. N Engl J Med 362 358 360
7. ArmahGESowSOBreimanRFDallasMJTapiaMD 2010 Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 376 606 614
8. World Health Organization 2009 Meeting of the Immunization Strategic Advisory Group of Experts, April 2009 — conclusions and recommendations. Wkly Epidemiol Rec 84 220 236
9. WHO/UNICEF 2004 Joint Statement on clinical management of acute diarrhea Geneva WHO/UNICEF
10. Boschi-PintoCBahlRMartinesJ 2009 Limited progress in increasing coverage of neonatal and child-health interventions in Africa and Asia. J Health Popul Nutr 27 755 762
11. Fischer WalkerCLFontaineOYoungMWBlackRE 2009 Zinc and low osmolarity oral rehydration salts for diarrhoea: a renewed call to action. Bull World Health Organ 87 780 786
12. RamPKChoiMBlumLSWamaeAWMintzED 2008 Declines in case management of diarrhoea among children less than five years old. Bull World Health Organ 86 E F
13. Boschi-PintoCLanataCFMendozaWHabteD 2006 Diarrheal disease. JamisonDTFeachemRGMakgobaMWBosERBainganaFK Disease and mortality in sub-Saharan Africa Washington (D.C.) World Bank 107 234
14. MogesTHaidarJ 2009 Management and outcome of severely malnourished children admitted to Zewditu Memorial Hospital, Ethiopia. East Afr J Public Health 6 162 167
15. SunguyaBFKoolaJIAtkinsonS 2006 Infections associated with severe malnutrition among hospitalised children in East Africa. Tanzan Health Res Bull 8 189 192
16. MoisiJCGatakaaHBerkeleyJAMaitlandKMturiN 2011 Excess child mortality after discharge from hospital in Kilifi, Kenya: a retrospective cohort analysis. Bull World Health Organ 89 725 732A
17. GriffinPMRyanCANyaphisiMHargrett-BeanNWaldmanRJ 1988 Risk factors for fatal diarrhea: a case-control study of African children. Am J Epidemiol 128 1322 1329
18. IrenaAHWambaziMLengaV 2011 Diarrhea is a major killer of children with severe acute malnutrition admitted to inpatient set-up in Lusaka, Zambia. Nutr J 10 110
19. GirmaBBerhaneY 2011 Children who were vaccinated, breast fed and from low parity mothers live longer: a community based case-control study in Jimma, Ethiopia. BMC Public Health 11 197 203
20. ZanoniBCPhungulaTZanoniHMFranceHFeeneyME 2011 Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS One 6 e22706 doi:10.1371/journal.pone.0022706
21. Kenya National Bureau of Statistics 2010 Population and Housing Statistics, Population Projections by Province, 1999–2010 Nairobi, Kenya Kenya National Bureau of Statistics
22. Kenya National Bureau of Statistics 2004 Kenya Demographic and Health Survey 2003 Calverton (Maryland) Kenya National Bureau of Statistics, Kenya Ministry of Health and ORC Macro
23. AdazuKLindbladeKARosenDHOdhiamboFOfwareP 2005 Health and demographic surveillance in rural western Kenya: a platform for evaluating interventions to reduce morbidity and mortality from infectious diseases. Am J Trop Med Hyg 73 1151 1158
24. World Health Organization 1995 The management of acute respiratory infections in children: practical guidelines for outpatient care Geneva World Health Organization
25. World Health Organization 2010 WHO child growth standards, SAS macros Geneva World Health Organization
26. World Health Organization 2006 WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development Geneva World Health Organization
27. EwingWH 1986 Identification of enterobacteriaciae. 4th ed New York Elsevier Science
28. Clinical Laboratory Standards Institute (CLSI) 2005 Performance standards for antimicrobial susceptibility testing: 15th informational supplement. CLSI document M100-S15 Wayne (Pennsylvania) CLSI
29. FieldsPIPopovicTWachsmuthKOlsvikO 1992 Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J Clin Microbiol 30 2118 2121
30. KeaslerSPHallRH 1993 Detecting and biotyping Vibrio cholerae O1 with multiplex polymerase chain reaction. Lancet 341 1661
31. GhoshCNandyRKDasguptaSKNairGBHallRH 1997 A search for cholera toxin (CT), toxin coregulated pilus (TCP), the regulatory element ToxR and other virulence factors in non-01/non-0139 Vibrio cholerae. Microb Pathog 22 199 208
32. NandiBNandyRKMukhopadhyaySNairGBShimadaT 2000 Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J Clin Microbiol 38 4145 4151
33. HullJJTeelENKerinTKFreemanMMEsonaMD 2011 United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr Infect Dis J 30 S42 S47
34. SchmidtHKnopCFrankeSAleksicSHeesemannJ 1995 Development of PCR for screening of enteroaggregative Escherichia coli. J Clin Microbiol 33 701 705
35. GunzburgSTTornieporthNGRileyLW 1995 Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J Clin Microbiol 33 1375 1377
36. PatonAWPatonJC 1998 Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol 36 598 602
37. SchultszCPoolGJvan KetelRde WeverBSpeelmanP 1994 Detection of enterotoxigenic Escherichia coli in stool samples by using nonradioactively labeled oligonucleotide DNA probes and PCR. J Clin Microbiol 32 2393 2397
38. SethabutrOVenkatesanMMurphyGSEampokalapBHogeCW 1993 Detection of Shigellae and enteroinvasive Escherichia coli by amplification of the invasion plasmid antigen H DNA sequence in patients with dysentery. J Infect Dis 167 458 461
39. VegaEBarclayLGregoricusNWilliamsKLeeD 2011 CaliciNet: A novel surveillance network for norovirus gastroenteritis outbreaks in the United States. Emerg Infect Diseases 17 1389 1395
40. OkaTKatayamaKHansmanGSKageyamaTOgawaS 2006 Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J Med Virol 78 1347 1353
41. FurnivalGMWilsonRW 1974 Regressions by leaps and bounds. Technometrics 16 499 511
42. HarrisJRGreeneSKThomasTKNdivoROkandaJ 2009 Effect of a point-of-use water treatment and safe water storage intervention on diarrhea in infants of HIV-infected mothers. J Infect Dis 200 1186 1193
43. LanataCFBlackRE 2008 Diarrheal diseases. Semba RD, Bloem MW, editors. Nutrition and Health in Developing Countries. 2nd ed New York Humana Press 139 178
44. MacLennanCAGondweENMsefulaCLKingsleyRAThomsonNR 2008 The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest 118 1553 1562
45. Kenya National Bureau of Statistics 2009 Kenya Demographic and Health Survey 2008–2009 Preliminary Report Calverton (Maryland) Kenya National Bureau of Statistics, ORC Macro
46. BrooksJTOchiengJBKumarLOkothGShapiroRL 2006 Surveillance for bacterial diarrhea and antimicrobial resistance in rural western Kenya, 1997–2003. Clin Infect Dis 43 393 401
47. BolasSOlsonCKinneryNMathingauAOdidiB 2008 Availability of oral rehydration solution packets at small shops in two areas of Kenya, 2007. Am J Trop Med Hyg 79 30
48. RamPOlsonCKinneryNBlumLBooreA 2008 Recommendation of oral rehydration solution for diarrhea case management by pharmacy workers in Kenya, 2007. Am J Trop Med Hyg 79 30
49. BerkeleyJARossAMwangiIOsierFHAMohammedM 2003 Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. Br Med J 326 1 6
50. Kenya Ministry of Public Health and Sanitation 2010 Policy guidelines on control and management of diarrhoeal diseases in children below five years in Kenya. 1–11 Nairobi Kenya Ministry of Public Health and Sanitation
Štítky
Interní lékařství
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing WorldČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
-
Všechny články tohoto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



