-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
In a retrospective cohort study, Bethan Davies and colleagues estimate the impact of non-NAAT testing on risk of subsequently developing pelvic inflammatory disease.
Published in the journal: . PLoS Med 15(1): e32767. doi:10.1371/journal.pmed.1002483
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002483Summary
In a retrospective cohort study, Bethan Davies and colleagues estimate the impact of non-NAAT testing on risk of subsequently developing pelvic inflammatory disease.
Introduction
Sexually transmitted C. trachomatis (chlamydia) is the most prevalent sexually transmitted infection (STI) with an estimated 68,455,000 incident cases globally in women in 2012 [1,2]. Chlamydia is the subject of intensive control efforts in many high-income settings [3]. The aim of diagnosing and treating chlamydia is 2-fold: to reduce the risk of progression to complications in the individual (including pelvic inflammatory disease [PID]) and to reduce the risk of transmission to another individual (including neonates).
The method of chlamydia diagnosis has advanced over time. Antigen-based diagnostic tests were introduced in the 1980s to replace culture [4]. Some of these antigen-based tests were relatively labour intensive and nucleic acid amplification tests (NAATs) were developed and increasingly robotized to increase the volume of tests that could be undertaken. The additional advantage of NAATs is that they have a real-world sensitivity of 90%–96%, which leads to a lower proportion of untreated infections (false negative tests) compared to antigen-based methods (direct immunofluorescence (IF) or direct fluorescent antibody (DFA) and enzyme-linked immunosorbent assay (ELISA) or enzyme immunoassay (EIA) sensitivity 65%–75% compared to NAAT) [5,6].
To minimise the risk of undertreatment due to false negative tests, NAATs have been the recommended test type for the diagnosis of chlamydia since the early 2000s [7–9]. However, antigen-based methods remain in use in many settings, often due to resource constraints. They are also widely available for purchase online and sold over the counter in pharmacies, including for home testing [3]. The cost and availability of ASSURED (affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and delivered to end-users) diagnostic tests also hampers the implementation of chlamydia case management guidelines in low - and middle-income settings [2]. The WHO recommends syndromic management of vaginal discharge for low-resource settings and this has been widely implemented where expensive diagnostic tests are not available [10–13].
There are important health consequences from less sensitive diagnostic tests and syndromic diagnosis. Infected people with false negative tests are falsely reassured, undiagnosed, untreated, and remain at risk of complications and onward transmission of chlamydia whilst uninfected people with false positive tests are incorrectly diagnosed and given unnecessary treatment.
We hypothesise that the increased risk of undiagnosed chlamydia infection following the use of non-NAATs will lead to an observable higher risk of PID in women who test negative using non-NAATs compared to NAATs. We aim to estimate the risk of PID following an undiagnosed chlamydia infection in women tested with a non-NAAT.
Methods
Ethics statement
The Danish Chlamydia Study was approved by the Danish Data Protection Agency (J.nr. 2010-41-4866, J.nr. 2012-331-0228 and J.nr. 2015-41-4344).
Study design
The Danish Chlamydia study is a purpose-generated dataset of all chlamydia tests performed in public health laboratories in Denmark (including Greenland) between 1st January 1992 and 2nd November 2011. A full description of this dataset has been previously published [14]. For the present study we extracted all chlamydia test records from women aged 15–34 years that were performed between 1st January 1998 and 31st December 2001, the interval when non-NAATs were replaced by NAATs as the most common test type (S1 Fig). In Denmark, the contribution of NAATs to all non-NAAT/NAAT chlamydia tests was 23.5% in 1998 and 66.01% in 2001, and there were 696,987 female residents aged 15–34 years in 2000 [15]. Clinical Microbiology Laboratories (CMLs) switched from non-NAATs to NAATs at individually determined times, with the change-over occurring during the study period for most CMLs. The choice of chlamydia test was made by the CML not by the requesting clinicians and there were no changes to recommendations for chlamydia testing during the study period.
We excluded chlamydia tests that: (a) had an ambiguous result (defined as not positive or negative e.g., “inconclusive” or missing data) or (b) used a test type other than NAAT (defined as PCR, SDA, TMA, LCR, DNA, DNA/RNA) or non-NAAT (defined as ELISA, IF, “antigen”) (examples of excluded test types: unknown, microscopy, culture). We then limited the dataset to the first chlamydia test per woman in this time interval (the index test), preferentially selecting positive tests and NAATs if multiple tests were performed on the same date.
We linked chlamydia test records to hospital healthcare records from the Danish National Patient Register (1993–2012) using the study ID number, which is anonymously linked to the unique Danish patient identification number (key held securely and not accessible to researchers) that is recorded in all administrative healthcare records [16]. Each woman’s first recorded healthcare presentation (outpatient, emergency department, and inpatient) for PID was identified (defined as International Classification of Diseases version 10 (ICD-10) A18·1; A51·4; A52·7; A54·2; A56·1; N70-74·8, ICD-10 coding introduced in Denmark in 1994). Episodes of PID were defined as “previous” if they occurred before the index chlamydia test, “same day” if they occurred on the same date as the index chlamydia test, and “12 months” if they occurred between one and 365 days after the index test. We excluded women who had a history of PID before their index chlamydia test and women who were diagnosed with PID on the same day as their index test.
We defined a priori exposure categories as follows: age at chlamydia test (15–24 years; 25–34 years), year of chlamydia test (1998/1999 and 2000/2001) and chlamydia test positivity (yes/no). Following peer review, we defined two additional explanatory variables: location of the laboratory processing the chlamydia test, defined as (a) STI clinic within their catchment (yes/no), (b) “no” STI clinic as a single group with “yes” STI clinic divided into the five separate laboratories; repeat chlamydia test, defined using the method outlined above and limited to tests performed ≥30 and ≤365 days after the index test and ≤1 day before a PID diagnosis categorised as no/negative/positive. We also categorised age and year into multiple categories and as continuous variables.
Statistical analysis
For the cohort overall and by chlamydia test type we describe age, year, chlamydia test positivity, laboratory area, repeat chlamydia test, and incidence of PID by 12 months. We used Chi-squared tests to compare the proportion of women in each category by chlamydia test type.
We estimated the risk of PID following an undiagnosed chlamydia infection in women tested using a non-NAAT. To do this, we estimated “i” the proportion of women tested with a non-NAAT who had an undiagnosed infection, under the assumption that NAAT positivity is equal to true population chlamydia positivity (where i = [NAAT positivity] − [non-NAAT positivity]) and “j” the excess proportion of women tested with a non-NAAT who were diagnosed with PID (where j = [risk of PID following a non-NAAT]–[risk of PID following a NAAT]). We then make the assumption that all the observed excess cases of PID in women tested with a non-NAAT (j) occurred in the proportion with an undiagnosed chlamydia infection (i) to estimate “p” the risk of PID following an undiagnosed chlamydia infection in women tested using a non-NAAT (where p = [j]/[i]). Finally we estimate “r”, the number of excess cases of PID that would be observed per 100,000 women tested using a non-NAAT compared to a NAAT (where r = [i]*[p]*100,000).
We used logistic regression to determine the association between chlamydia test type and PID by 12 months adjusted for age, year, and laboratory area. We repeated the analysis stratified by chlamydia test result due to the known difference in the performance of NAAT and non-NAAT diagnostic tests. For each sub-cohort, we compared the risk of PID at 12 months by test type using a difference between two proportions test and adjusted logistic regression analysis. We then repeated this logistic regression analysis including repeat chlamydia test as an explanatory variable. PID is a relatively rare event in this cohort, therefore we refer to the resulting odds of PID as “risks” in the results and discussion because probability is a more natural measure.
Results
Generation of the study dataset is illustrated in Fig 1. There were 272,105 women resident in Denmark aged 15–34 years who had an eligible chlamydia test during the study period (1998–2001) and no documented previous history of PID. The mean age of women on the date of their index chlamydia test (i.e., at entry to the study cohort) was 24.80 years (standard deviation 5.06, range 15–34 years). Under half of this cohort were tested using a NAAT (n = 121,857, 44.78%), and overall, chlamydia test positivity was 6.38% (n = 17,353) (Table 1). A repeat chlamydia test was performed in 61,890 women, of which 5.63% (n = 3,484) were positive. The five laboratories with an STI clinic in their catchment area performed 76.20% (n = 207,331) of the index chlamydia tests. Two of these five laboratories did not perform non-NAATs during the study period (S1 Table). Compared to women tested using a non-NAAT, women tested using a NAAT were more likely to be younger, tested after 1998, tested by laboratories in areas without an STI clinic, to have a positive index test, and to have a positive repeat test (Table 1).
Fig. 1. Cohort formation. 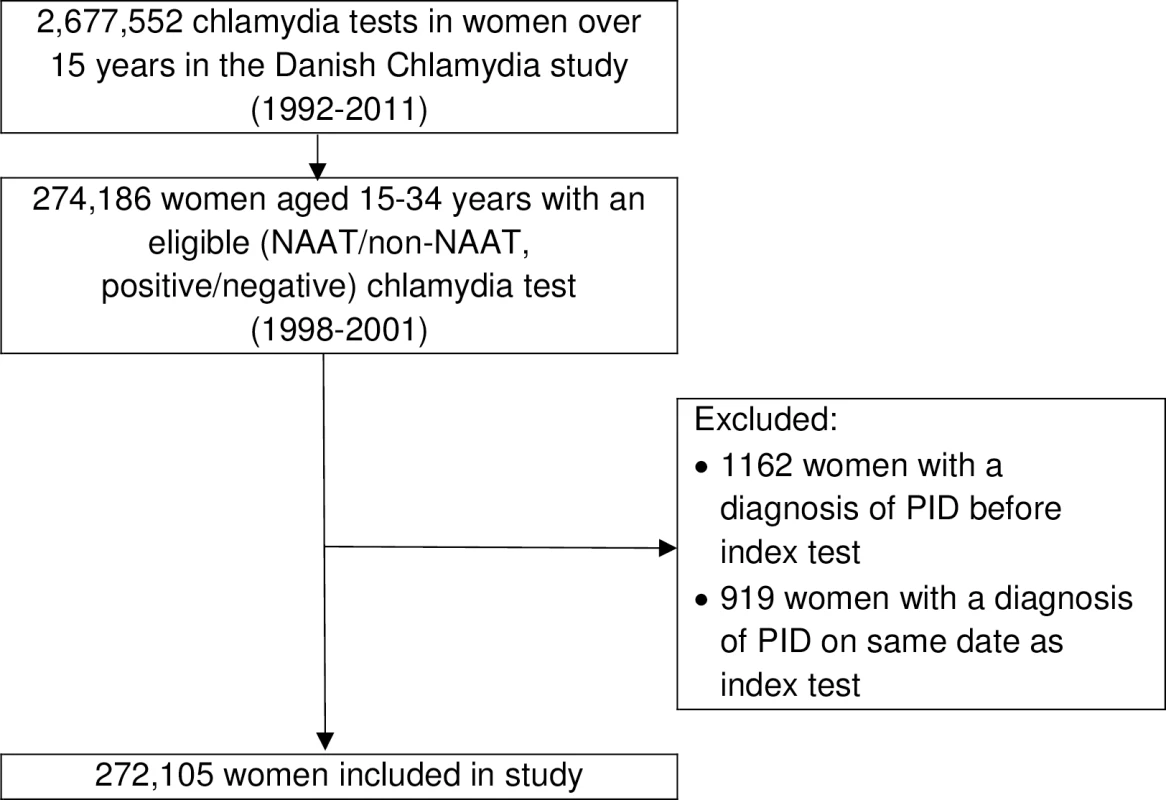
NAAT, Nucleic Acid Amplification Test; PID, pelvic inflammatory disease. Tab. 1. Description of the study cohort (1998–2001) by chlamydia test type. 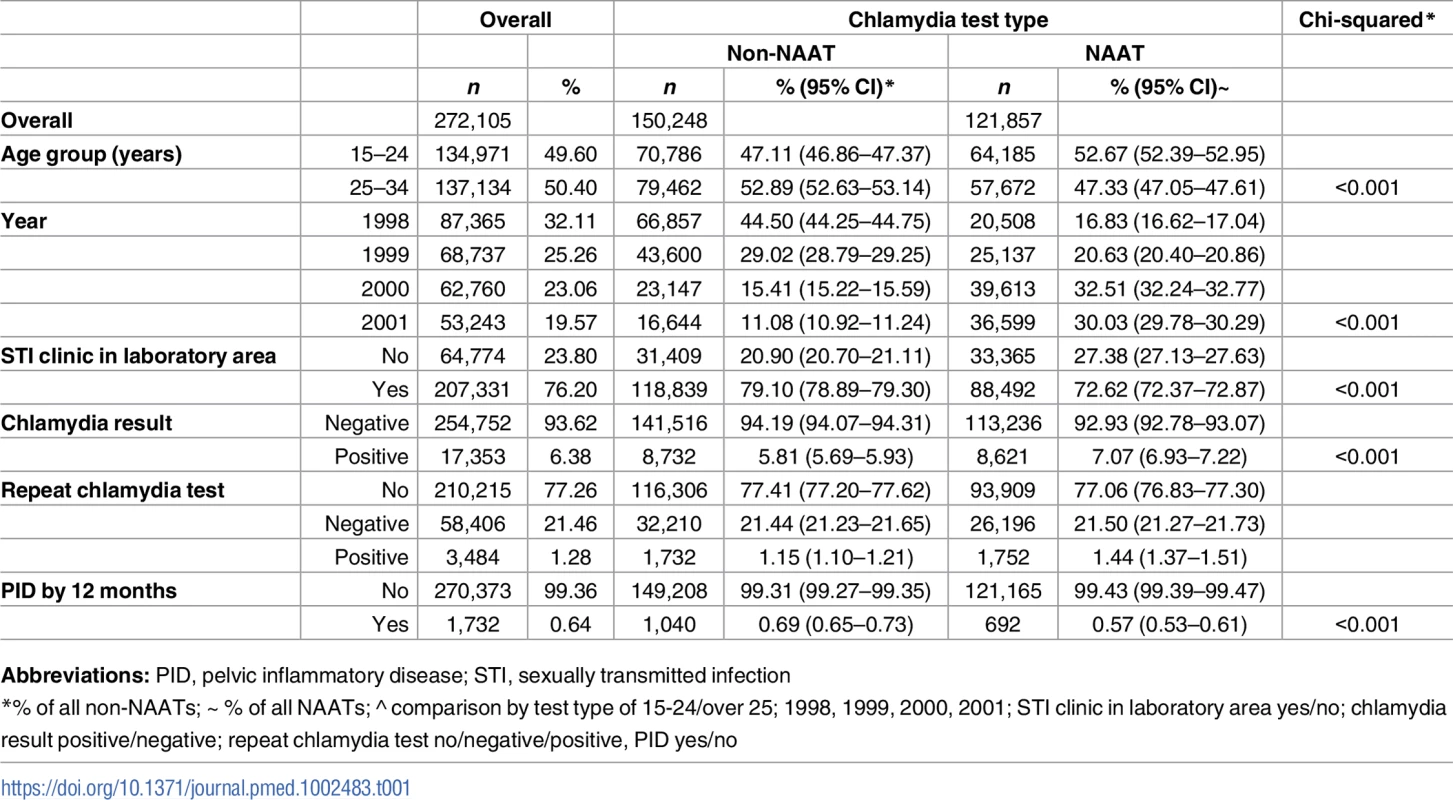
Abbreviations: PID, pelvic inflammatory disease; STI, sexually transmitted infection Overall, 1,732 (0.64%) women had a hospital healthcare presentation for PID within 12 months of their chlamydia test. Over half (52.19% [n = 904]) of women diagnosed with PID were inpatients, 25.40% (n = 440) were outpatients and 20.44% (n = 354) were treated in emergency departments (the location was unknown for 1.96% [n = 34]). There was no difference in the proportion of women with a positive test who progressed to PID within 12 months by test type (0.78% non-NAAT and 0.81% NAAT, p = 0.805, Fig 2), but a higher proportion of women progressed to PID following a negative non-NAAT compared to a negative NAAT (0.69% non-NAAT and 0.55% NAAT, p < 0.001).
Fig. 2. Crude risk of PID by 12 months by test result and test type. 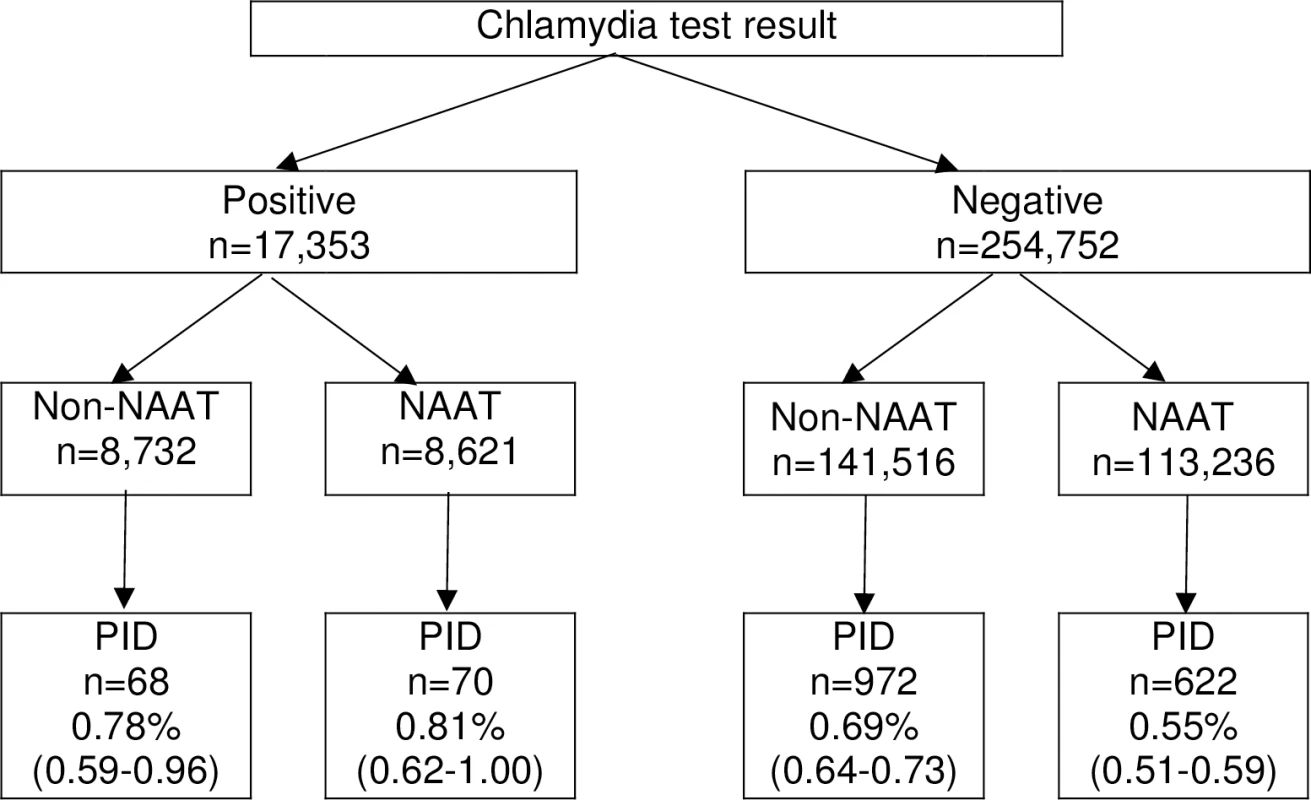
NAAT, Nucleic Acid Amplification Test; PID, pelvic inflammatory disease. Test positivity was 18% higher following a NAAT compared to a non-NAAT (absolute difference 1.26%, equal to 7.07% minus 5.81%). Therefore, compared to women tested with a NAAT, we estimate that 1.26% (equal to the difference in test positivity) of women tested using a non-NAAT had an undiagnosed chlamydia infection and an additional 0.12% (equal to 0.69% minus 0.57%) were diagnosed with PID. Therefore, the estimated risk of progression from undiagnosed chlamydia infection to PID within 12 months is 9.52% (95% CI 9.30–9.68), and there would be an estimated 120 excess cases of PID per 100,000 women tested with a non-NAAT compared to a NAAT.
The unadjusted risk of PID within 12 months of a chlamydia test was significantly higher in women tested with a non-NAAT, tested in 1998 compared to 2001, aged over 25, and with a positive rather than a negative index test (odds ratio [OR] 1.27 [95% CI 1.07–1.52]) (Table 2). The unadjusted risk of PID in the overall cohort became significantly higher at age 29 (S2 Table). The adjusted risk of PID was 32% higher in women over 25 compared with under 25 years (AOR 1.32 [1.20–1.46]), and 14% lower in women tested in 2001 compared with 1998 (AOR 0.86 [0.74–1.00]) and following a NAAT rather than a non-NAAT (AOR 0.86 [0.78–0.96]).
Tab. 2. Unadjusted and adjusted logistic regression analysis of PID by 12 months by chlamydia test type, age, year of test and laboratory area, overall, and stratified by chlamydia status. 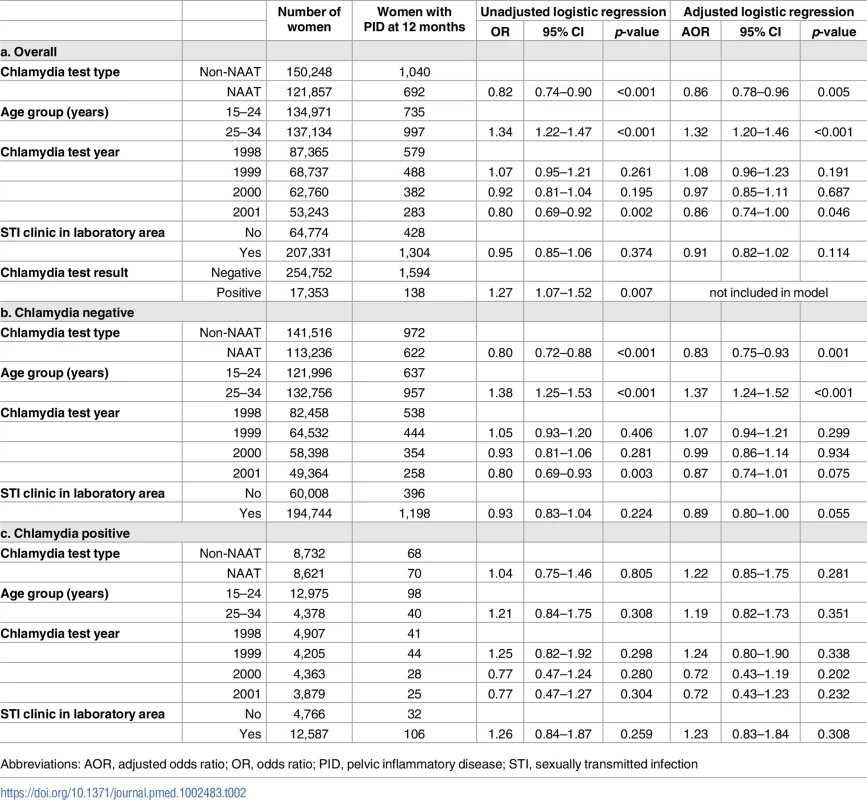
Abbreviations: AOR, adjusted odds ratio; OR, odds ratio; PID, pelvic inflammatory disease; STI, sexually transmitted infection Stratifying the analysis by test result demonstrated that for women with a positive test (presumably treated) there was no difference in the risk of PID by 12 months by test type (AOR 1.22 [0.85–1.75]). However, women with a negative test (presumably untreated) had a 17% lower risk of PID following a NAAT compared to a non-NAAT (AOR 0.83 [0.75–0.93]).
In a further stratified model including the first repeat chlamydia test within 12 months, women with a positive index test had a 62% lower risk of PID following a negative repeat test compared to women without a repeat test (AOR 0.38 [0.26–0.57]) (S3 Table). For women with a negative index test, having a repeat test was not significantly associated with PID.
Discussion
Women with a negative chlamydia test had a 17% higher adjusted risk of PID (absolute difference 0.14% [0.69% compared to 0.55%]) by 12 months if they were tested using a non-NAAT compared to a NAAT, but there was no difference in the risk of PID following a positive chlamydia test, supporting our hypothesis. This is presumably due to the higher proportion of false negative tests from less sensitive non-NAATs and we estimate that 18% of chlamydia infections in women tested with a non-NAAT went undiagnosed. This study suggests that the risk of progression to PID following an untreated infection was 9.52%. We quantify the health impact of using less sensitive chlamydia diagnostic tests as an excess 120 cases of PID per 100,000 women tested, which provides further evidence for restricting the use of non-NAATs and other less sensitive diagnostic tests.
This is the first published comparison of the risk of PID following chlamydia testing that takes into account diagnostic test type. There are several factors that have the potential to contribute to the observed excess risk of PID following a negative non-NAAT compared to a NAAT. Firstly, a higher proportion of women tested with non-NAATs were from the older age group compared to the younger age group. PID risk is known to increase with age but the increased risk of PID remained after adjustment for age [17]. Secondly, non-NAATs were more common in the earlier time interval but it is unlikely that there were PID risk factors (e.g., prevalence of non-chlamydia causes of PID) specific to this period that resolved by 2001 and the association remained in the adjusted analysis. Thirdly, there is the potential for bias in the application of chlamydia diagnostic test type by PID risk or other unmeasured confounders. However, the choice of diagnostic test was undertaken in the laboratory (not by the clinician), and each laboratory usually changed from non-NAATs to NAATs at a discrete time as machinery was replaced, therefore with only a short test overlap until the old test was closed down. The association remained after adjustment for the presence of an STI clinic in the catchment of laboratories.
Therefore, the most likely explanation for a difference in the risk of PID by test type following a negative chlamydia test is the difference in test performance and the proportion of undiagnosed infections. In this cohort, chlamydia test positivity was 18% higher in women tested with a NAAT compared to those tested with a non-NAAT (7.07% compared to 5.81%), which is broadly in keeping with the reported 65%–75% sensitivity of non-NAATs compared to NAATs [5,6].
NAATs are universally recommended for the diagnosis of chlamydia in sexually active adults but their use is infrequent in a significant minority of European countries (n = 9/28) [3]. Data collected from 28 European countries in 2012 by the European Centre for Disease Prevention and Control found that NAATs were unavailable in the public sector in five countries and at least four countries used non-NAATs for the majority of chlamydia diagnostic tests. It is likely that the higher price of NAATs compared to non-NAATs is at least partly contributing to the reported deviation from international guidance. In addition to use by public-sector authorities, EIA chlamydia tests are easily available for purchase online and in-person in many countries. It can be difficult to access information about the test, including its performance, which raises challenging questions about the regulation of online diagnostic tests.
Syndromic management of vaginal discharge is widely applied in low-resource settings where diagnostic tests are unavailable [13]. This approach generally leads to an overdiagnosis of chlamydia in uninfected women and undertreatment of those who are asymptomatic (estimated to be 80% of all infections) [2]. A recent expert commission supports the development of point-of-care tests (POCTs) for use in low-resource settings as a major player in the pathway to improved chlamydia control [2]. But current efforts are hampered by the poor performance of POCTs (sensitivity in women 22.7%–93.8%, specificity 89.0%–100%) [2]. The WHO is planning to develop guidance on STI laboratory diagnosis and screening in 2017/2018. We hope that this will address the challenge of balancing the risks of using less sensitive diagnostic tests against syndromic management approaches in the context of resource availability and chlamydia control impact [9].
Of further relevance to chlamydia control policy, our supplementary analysis suggests that the age at which the risk of PID increased was 29 years, which is older than the cutoff (25 years) commonly used in chlamydia testing guidelines. This interesting finding warrants further exploration.
In this analysis we made crude assumptions about the underlying prevalence of chlamydia infection and the number of women at risk of PID in the non-NAAT group to estimate that the risk of progression to PID following an undiagnosed infection was 9.52% (9.30–9.68). This estimate is remarkably consistent with the most robust observed risk of 9.46% (2.79–16.13) from the Prevention Of Pelvic Infection (POPI) Randomised Controlled Trial (RCT) [18]. This similarity in result would suggest that the estimates are approximating a true underlying biological risk. However, cohort studies of the risk of complications following chlamydia infection are hampered by the unknown impact of unmeasured confounders including undiagnosed repeat chlamydia infections, other incident STIs, and diagnostic biases. A further methodological challenge is that all subsequent diagnoses of PID within the timeframe are attributed to the incident chlamydia infection, which is likely to overestimate the true biological association. It is important to acknowledge that some of these limitations can be considered to apply equally within the POPI RCT as women were not repeatedly tested for STIs during follow-up.
We agree that parameter estimates obtained from cohort studies should be interpreted cautiously. However, this is the second example of the Danish Chlamydia Study producing estimates of the risk of complications following chlamydia that are consistent with other study designs. In a recent analysis, our estimate of the population excess fraction of chlamydia on PID was consistent with estimates from a multi-parameter evidence synthesis [19]. We suggest that the consistency between our estimates and those of others support the view that cohort studies are a valid and informative study design in this context.
For completeness, we should consider that NAATs are not without their critics. Hadgu and Sternberg argue that the true specificity of NAATs is lower than that commonly reported and that the risk of progression to PID is lower following NAATs compared to other diagnostic test types because NAATs can detect small quantities of genetic material that may be associated with infection of lower pathogenic potential [20]. Our study does not support this hypothesis as we found no difference in the risk of progression to PID following a positive diagnostic test (NAAT 0.81% [0.62–1.00] versus non-NAAT 0.78% [0.59–0.96]).
The data used in this study was drawn from an unselected dataset that contains complete ascertainment of chlamydia tests performed in public laboratories in Denmark (1992–2011). There are no private microbiology laboratories in Denmark, therefore the study cohort is representative of the national population of women aged 15–34 years who were tested for chlamydia. However, within this representative cohort, there is the potential for a systematic difference in the clinical risk profile of women tested by CML because only five CMLs had an STI clinic in their catchment at the time of the study. These CMLs are situated in large metropolitan areas. In the study dataset the proportion of tests performed using a NAAT varied from 5.5% to 100% for the CMLs with STI clinics in their catchment, but as a group this proportion was more similar to those observed in CMLs without STI clinics and the overall cohort (42.7%; 51.5%, and 44.8% respectively). We are not able to explore this potential bias further because data on the source of the chlamydia test (e.g., STI clinic or primary care) or the clinical indication for the test (e.g., symptomatic or asymptomatic) is not available. To control for potential confounding between test type and PID, CML was included in the adjusted analysis.
We applied exclusion criteria to improve the accuracy of chlamydia exposure categorisation. The coding in the original laboratory data did not allow identification of specific manufacturers’ tests, therefore we categorised chlamydia test type broadly into NAAT and non-NAAT (excluding culture and microscopy). Our analysis assumes a homogenous performance of diagnostic tests within a category.
A four-year time interval that spanned the introduction of NAATs in Denmark was chosen to maximise the study population size whilst limiting the potential for other relevant secular changes. The study cohort represents approximately 39.0% of Denmark’s female population, 15–34 years (n = 696,987 on 1st January 2000) [15,21]. Generalisation of the findings from this analysis to the contemporary setting may be compromised if intervening updates to chlamydia testing policies have led to a change in the composition (demographic or risk) of the population being tested for chlamydia.
We used a broad ICD-10 definition of PID that is consistent with comparable research. The ICD-10 codes included in the final dataset were all within the range N70–74.4. Healthcare presentations with a diagnostic code for PID were obtained from the hospital setting (emergency department, outpatient and inpatient). Data on cases of PID that were only seen in primary/community care were not available, therefore our estimates of the absolute risk of PID following chlamydia are likely to underestimate the true risk. We consider that the PID events included in this study will represent the more severe disease in the population and therefore potentially the most significant events in terms of future reproductive health. The incomplete ascertainment is unlikely to be biased by chlamydia test type. We excluded women who were diagnosed with PID on the same date as their index chlamydia test as this is independent of chlamydia test type. We also excluded women with a past history of PID to reduce any potential biases in future risk.
Chlamydia exposure status in this cross-sectional study is based on a single diagnostic test result and we assume that all diagnosed infections were treated. We did not apply exclusion criteria based on women’s previous history of chlamydia testing or infection. We assume that the distribution of both diagnosed and undiagnosed previous chlamydia infection (before 1998) would be independent of chlamydia test type.
This analysis also assumes that there is a causal relationship between the index chlamydia infection (diagnosed or undiagnosed) and subsequent PID. To improve this assumption, we limited episodes of PID to those within 12 months of the chlamydia test as the estimated duration of an untreated chlamydia infection is in the order of 15 months and RCTs report the risk of PID at 12 months after the test [22,23]. This assumption is likely to overestimate the measured strength of association. We were able to adjust the analysis for test year, age, laboratory area, and repeat chlamydia test but data on other confounders were not available (e.g., indication for the chlamydia test (symptomatic or asymptomatic); antibiotic use; individual level incidence of other causes of PID (e.g., other STIs); healthcare seeking behaviour). It is unlikely that these confounders are associated with diagnostic test type.
Conclusion
NAATs have been the recommended test type for the diagnosis of chlamydia in sexually active adults for over a decade due to their superior performance over non-NAATs. We found that women with a negative chlamydia test had a 17% higher risk of PID by 12 months if they were tested using a non-NAAT compared to a NAAT and estimate that using non-NAATs will result in an additional 120 cases of PID per 100,000 tested women. This is presumably due to the higher proportion of false negative tests. This study has quantified the positive impact on women’s reproductive health from using accurate chlamydia diagnostic tests and provides further evidence for restricting the use of inferior non-amplified assays including antigen-based diagnostic tests.
Supporting Information
Zdroje
1. Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS ONE. 2015;10(12):e0143304. doi: 10.1371/journal.pone.0143304 26646541; PubMed Central PMCID: PMC4672879.
2. Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17(8):e235–e79. Epub 2017/07/14. doi: 10.1016/S1473-3099(17)30310-9 28701272.
3. European Centre for Disease Prevention and Control. Chlamydia control in Europe: a survey of Member States 2012. Stockholm ECDC, 2014 February 2014. Report No.
4. Schachter J. Rapid diagnosis of sexually transmitted diseases—speed has a price. Diagnostic microbiology and infectious disease. 1986;4(3):185–9. Epub 1986/03/01. 3082583.
5. Chernesky MA. The laboratory diagnosis of Chlamydia trachomatis infections. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale / AMMI Canada. 2005;16(1):39–44. Epub 2007/12/27. 18159527; PubMed Central PMCID: PMCPmc2095010.
6. Newhall WJ, Johnson RE, DeLisle S, Fine D, Hadgu A, Matsuda B, et al. Head-to-head evaluation of five chlamydia tests relative to a quality-assured culture standard. J Clin Microbiol. 1999;37(3):681–5. Epub 1999/02/13. 9986831; PubMed Central PMCID: PMC84517.
7. British Association for Sexual Health and HIV. 2006 UK National Guideline for the Management of Genital Tract Infection with Chlamydia trachomatis. 2006.
8. Lanjouw E, Ouburg S, de Vries HJ, Stary A, Radcliffe K, Unemo M. 2015 European guideline on the management of Chlamydia trachomatis infections. International journal of STD & AIDS. 2016;27(5):333–48. Epub 2015/11/27. doi: 10.1177/0956462415618837 26608577.
9. World Health Organisation. WHO Guidelines for the treatment of Chlamydia trachomatis. Geneva, Switzerland: WHO, 2016 ISBN 978 92 4 154971 4.
10. Adachi K, Nielsen-Saines K, Klausner JD. Chlamydia trachomatis Infection in Pregnancy: The Global Challenge of Preventing Adverse Pregnancy and Infant Outcomes in Sub-Saharan Africa and Asia. BioMed Research International. 2016;2016 : 21. doi: 10.1155/2016/9315757 27144177
11. Cliffe SJ, Tabrizi S, Sullivan EA. Chlamydia in the Pacific region, the silent epidemic. Sexually transmitted diseases. 2008;35(9):801–6. Epub 2008/06/27. doi: 10.1097/OLQ.0b013e318175d885 18580823.
12. Otieno FO, Ndivo R, Oswago S, Ondiek J, Pals S, McLellan-Lemal E, et al. Evaluation of syndromic management of sexually transmitted infections within the Kisumu Incidence Cohort Study. International journal of STD & AIDS. 2014;25(12):851–9. doi: 10.1177/0956462414523260. 24516075
13. World Health Organisation. Guidelines for the management of sexually transmitted infections. Geneva, Switzerland: WHO, 2003 ISBN 92 4 154626 3.
14. Davies B, Turner KM, Frolund M, Ward H, May MT, Rasmussen S, et al. Risk of reproductive complications following chlamydia testing: a population-based retrospective cohort study in Denmark. Lancet Infect Dis. 2016;16(9):1057–64. doi: 10.1016/S1473-3099(16)30092-5 27289389.
15. Statbank Denmark. Population 1. January by sex, age and time: Statistics Denmark 2017 [cited 2017 17.10.17]. Available from: http://www.statistikbanken.dk/statbank5a/default.asp?w=1920.
16. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scandinavian journal of public health. 2011;39(7 Suppl):30–3. doi: 10.1177/1403494811401482 21775347.
17. Public Health England. Health Protection Report: weekly report. PHE, 2015 23 June 2015. Report No.: Contract No.: 22.
18. Oakeshott P, Kerry S, Aghaizu A, Atherton H, Hay S, Taylor-Robinson D, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ. 2010;340(20378636).
19. Davies B, Turner KM, Leung S, Yu BN, Frolund M, Benfield T, et al. Comparison of the population excess fraction of Chlamydia trachomatis infection on pelvic inflammatory disease at 12-months in the presence and absence of chlamydia testing and treatment: Systematic review and retrospective cohort analysis. PLoS ONE. 2017;12(2):e0171551. doi: 10.1371/journal.pone.0171551 28199392.
20. Hadgu A, Sternberg M. Reproducibility and specificity concerns associated with nucleic acid amplification tests for detecting Chlamydia trachomatis. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2009;28(1):9–15. doi: 10.1007/s10096-008-0586-3 18642036.
21. Statistics Denmark FOLK1A: POPULATION AT THE FIRST DAY OF THE QUARTER BY REGION, SEX, AGE AND MARITAL STATUS 2017 [cited 2017 13 July 2017]. Available from: http://www.statbank.dk/statbank5a/default.asp?w=1600.
22. Price MJ, Ades AE, Angelis DD, Welton NJ, Macleod J, Soldan K, et al. Mixture-of-exponentials models to explain heterogeneity in studies of the duration of Chlamydia trachomatis infection. Statistics in medicine. 2013;32(9):1547–60. Epub 2012/09/06. doi: 10.1002/sim.5603 22949217.
23. Gottlieb SL, Xu F, Brunham RC. Screening and treating Chlamydia trachomatis genital infection to prevent pelvic inflammatory disease: interpretation of findings from randomized controlled trials. Sexually transmitted diseases. 2013;40(2):97–102. doi: 10.1097/OLQ.0b013e31827bd637 23324973.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Flexofytol® – přírodní revoluce v boji proti osteoartróze kloubů
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
-
Všechny články tohoto čísla
- What about drinking is associated with shorter life in poorer people?
- Life course socioeconomic position, alcohol drinking patterns in midlife, and cardiovascular mortality: Analysis of Norwegian population-based health surveys
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study
- Sexually transmitted infections in the era of antiretroviral-based HIV prevention: Priorities for discovery research, implementation science, and community involvement
- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Association between intake of less-healthy foods defined by the United Kingdom's nutrient profile model and cardiovascular disease: A population-based cohort study
- Progression of the first stage of spontaneous labour: A prospective cohort study in two sub-Saharan African countries
- The cost-effectiveness of alternative vaccination strategies for polyvalent meningococcal vaccines in Burkina Faso: A transmission dynamic modeling study
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
- Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies
- Long-term trends in mortality and AIDS-defining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle- and high-income countries in Europe and Thailand: A cohort study
- What’s coming for health science and policy in 2018? Global experts look ahead in their field
- Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study
- Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: Findings from a multistage sampling-based survey
- From macro- to microfactors in health: Social science approaches in research on sexually transmitted infections
- The WHO 2016 verbal autopsy instrument: An international standard suitable for automated analysis by InterVA, InSilicoVA, and Tariff 2.0
- Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



