-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFungal Biofilms
article has not abstract
Published in the journal: . PLoS Pathog 8(4): e32767. doi:10.1371/journal.ppat.1002585
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002585Summary
article has not abstract
Introduction
Biofilms are a principal form of microbial growth and are critical to development of clinical infection. They are responsible for a broad spectrum of microbial infections in the human host. Many medically important fungi produce biofilms, including Candida [1], Aspergillus [2], Cryptococcus [3], Trichosporon [4], Coccidioides [5], and Pneumocystis [6]. In this review we emphasize common features among fungal biofilms, and point toward genes and pathways that may have conserved roles.
Biofilm cell communities are more resistant to antifungal drugs than planktonic cells. Contributing factors include biofilm structural complexity, presence of extracellular matrix (ECM), metabolic heterogeneity intrinsic to biofilms, and biofilm-associated up-regulation of efflux pump genes. The actual fold increase in resistance varies with both the drug and species. Candida albicans and Candida parapsilosis biofilms are relatively resistant to fluconazole, amphotericin B, nystatin, voriconazole, and others. Aspergillus fumigatus biofilms are relatively resistant to itraconazole and, to some extent, to caspofungin. Cryptococcal biofilms are unaffected by fluconazole and voriconazole, and biofilms of Trichosporon asahii display elevated resistance to amphotericin B, caspofungin, voriconazole, and fluconazole. Azole and amphotericin B therapies are ineffective against Pneumocystis carinii biofilms. Biofilm-associated resistance mechanisms have been characterized in C. albicans and A. fumigatus and include drug binding by ECM and production of persister cells [2], [7] (see supplementary references for this section in Text S1). Persister cells represent only a fraction of the population, and probably reflect its metabolic heterogeneity. These mechanisms may pertain to other fungi as well.
Fungal Pathogen Biofilm Architecture
Biofilms are complex surface-associated cell populations embedded in an ECM that possess distinct phenotypes compared to their planktonic cell counterparts. Nutrients, quorum-sensing molecules, and surface contact are contributory factors. C. albicans biofilms are comprised primarily of yeast-form and hyphal cells, both of which are required for biofilm formation [1]. Formation is a sequential process involving adherence to a substrate (either abiotic or mucosal surface), proliferation of yeast cells over the surface, and induction of hyphal formation [1]. ECM accumulates as the biofilm matures, and seems to contribute to cohesion [8]. C. albicans biofilms form on numerous abiotic [9] and biotic surfaces [10]–[12]. In denture stomatitis, a combination of biotic mucosal (the host) and abiotic surface (the denture) biofilm formation exists [13]. Other Candida spp. including C. tropicalis, C. parapsilosis, and C. glabrata form ECM-containing biofilms but do not produce true hyphae [14].
Aspergillus biofilms can form both on abiotic and biotic surfaces [15]. The initial colonizing cells that adhere to the substrate are conidia. Mycelia (the hyphal form) develop as the biofilm matures [15]. ECM that binds the biofilm together [15] has been observed in vitro [15] and in vivo [16]. Hyphal organisation is different in the two forms of A. fumigatus biofilm infection: aspergilloma infections present an intertwined ball of hyphae; aspergillosis infections present individual separated hyphae [16]. Hyphae of C. albicans and of A. fumigatus can form pores or channels through biotic surfaces [17], [18].
The emerging fungal pathogen T. asahii forms biofilms comprised of yeast and hyphal cells embedded in matrix [4], as do those of Coccidioides immitis [5]. C. neoformans forms biofilms consisting of yeast cells on many abiotic substrates [3], and shed capsular polysaccharide forms the ECM. Although C. neoformans forms hyphae in the course of mating, no hyphae have been observed in C. neoformans biofilms to date. Similarly, Pneumocystis species do not produce hyphal structures as part of their biofilms [6]. Thus, hyphal formation is not a uniform feature of fungal biofilms.
Genetic Determinants of Fungal Biofilm Formation
Transcription factors play fundamental roles in both positive and negative regulation of biofilm formation through regulation of hyphal formation and cell surface proteins responsible for adherence [1]. Bcr1, a C2H2 zinc finger transcription factor, is a critical determinant of C. albicans biofilm formation in all environments studied to date [10], [12], [13], [19]. Bcr1 seems to be a conserved regulator of biofilm formation, because the Bcr1 ortholog of C. parapsilosis is required for biofilm formation as well [20]. Ace2, another C2H2 zinc finger transcription factor, also contributes to C. albicans biofilm formation, probably through its role in adherence as well as hypha formation [21]. The C. albicans transcription factor Efg1, a global regulator of cell surface protein genes and hyphal formation [1], is required for biofilm formation as well. The orthologs or best hits of Bcr1, Ace2, and Efg1, including C. glabrata CAGL0E06116g, CAGL0M04323g, and CAGL0L01771g, and C. parapsilosis CPAG00564, CPAG00148, and CPAG00178, are good candidates for biofilm regulators in those species. Transcription factors with analogous roles to Bcr1, Ace2, and Efg1 of C. albicans in A. fumigatus may be identified amongst the 124 uniquely expressed or upregulated transcription factors identified in biofilm culture by Gibbons et al., 2011 [22]. The A. fumigatus transcription factor LAEA, a regulator of cell type and secondary metabolism gene clusters in A. fumigatus, is highly upregulated in biofilms [22], and it remains to be seen whether it may influence biofilm phenotypes.
Cell wall proteins are of particular interest in biofilm formation. Besides its expected role in adherence, the cell wall may have a sensory role that promotes adherence-induced responses [23]. Numerous cell wall protein genes that may function in these capacities are upregulated early in C. albicans and A. fumigatus biofilm formation [22], [24], [25]. C. albicans cell surface proteins have been reviewed authoritatively (see Text S1). Among the upregulated A. fumigatus surface proteins are the hydrophobins RodA, RodB, RodD, and RodE. RODB is thought to play the most crucial role with its expression increased over 4,000-fold in biofilm versus planktonic growth conditions [22]. Ten other putative adhesins have been identified by Gibbons et al., 2011 [22]. It is possible these Aspergillus proteins have functions analogous to known adhesins in C. albicans.
Gene Expression Portrait of Fungal Biofilms
Biofilm cells have phenotypes distinct from planktonic cells, and this difference is reflected in greatest detail at the gene expression level. Detailed gene expression profiling comparisons, conducted in both C. albicans and A. fumigatus, have revealed substantial changes in gene expression between biofilm and planktonic cells [22], [26]. Changes in transcription factor expression is characteristic of C. albicans biofilm formation in vitro and in vivo [24]–[26], suggesting biofilm formation to be a highly regulated process. Similarly, almost 50% of the predicted transcription factors of A. fumigatus, including many with roles in asexual and sexual development, are upregulated in biofilms compared to planktonic cells.
Although biofilms are thought to include dormant cells, biofilms of C. albicans and A. fumigatus have increased expression of genes involved in protein synthesis. These genes encode ribosomal proteins, protein turnover, and translation factors as well as ribosomal proteins, indicating increased protein translation and ribosome production in biofilms to be a feature of biofilms [22], [25], [26]. If indeed biofilm cells are nutrient limited, these particular gene expression features may optimize recycling of cellular constituents.
Upregulation of multi-drug resistance transporter genes is common to A. fumigatus (MDR1, MDR2, MDR4) and C. albicans (MDR1, CDR1, CDR2) biofilms in vitro [22]. C. albicans MDR1 and CDR2 are upregulated in in vivo biofilms, as is PDR16, which is increased in fluconazole-resistant cells that overexpress CDR1 and CDR2 [25]. Phase dependency of these transporters exists in vivo for C. albicans CDR1 and in A. fumigatus for MDR4 [25], [27]. Additionally, ergosterol gene expression may account for increased drug resistance of biofilms. Genes involved in sterol biosynthesis are upregulated in A. fumigatus and C. albicans biofilms [22], [25], [26]. Increases in ERG gene expression as well as multi-drug resistance transporters has been correlated with increased azole resistance in C. albicans patient isolate samples, though their contribution to biofilm-specific azole resistance has not been detected in mature biofilms (see Text S1).
Increased expression of adherence genes is also a property of biofilm cells. ALS1 is the most upregulated of the known adherence genes of C. albicans under biofilm conditions. Garcia-Sanchez et al. (2004) [26] highlight that the ALS genes are differentially expressed in biofilms and have autonomous contributions in the biofilm transcriptome. Nett et al. (2009) [25] observed differential expression of ALS genes at different stages of biofilm formation and potential for overlap of function in vivo. A similar pattern of differential adhesin expression is seen in vitro in the A. fumigatus biofilm environment [22]. The inducing signal for biofilm adherence genes is clearly an area of interest as a basic biological question as well as a direction for prospective therapeutic development.
A significant number of primary metabolism genes, including those for amino acid synthesis, in particular sulfur amino acid biosynthesis, and nucleotide synthesis, are upregulated in C. albicans biofilms in vitro [24], [26] and in vivo [25], relative to in planktonic cells in vitro. Many are regulated by GCN4, a transcriptional activator required for biofilm formation [26]. Genes involved in amino acid metabolism are also upregulated in A. fumigatus biofilms including amino acid permeases, transporters, and amino peptidases. Secondary metabolism gene upregulation is significant in A. fumigatus biofilms, possibly due to upregulation of LAEA, a secondary metabolism regulator [22]. Altered metabolic gene expression may reflect nutrient limitation, but the rapid kinetics of induction (in C. albicans at least [24]) may reflect a different regulatory signal.
Many cell wall biogenesis genes are induced in the biofilm environment. Altered expression of genes for β-1,3 glucan synthesis and modification are features of in vivo C. albicans biofilms including FKS1, BGL2, and XOG1 [25]. Given the connection between the β-glucan pathway and biofilm matrix production, these may also contribute to ECM production. Nett et al. (2009) [25] highlight downregulation of β-1,3 glucan degrading enzymes in 24-hour biofilms and suggest this functions in glucan conservation for matrix production. In contrast, altered expression of α - and β-1,3 glucan synthesis genes is not observed in A. fumigatus biofilms. Although it is not directly reflected by the expression of polysaccharide synthase genes, the presence of α-1,3 glucan, galactosaminogalactan, and galactomannan in the mycelial extacellular matrix is correlated to the aerial growth of the mycelium of A. fumigatus [16]. Expression of more than 50% of cell wall genes investigated in A. fumigatus is, however, altered in the biofilm habitat, including upregulation of the ROD genes. Thus, these two organisms both restructure their cell surfaces in biofilms, though they may use different mechanisms to achieve that outcome.
Mating Type and Fungal Biofilms
Genetic exchange is a feature of bacterial biofilms, mediated in part by extracellular DNA. Although extracellular DNA has been detected in C. albicans biofilms [28], the main mechanism of biofilm-associated genetic exchange involves mating and cell fusion. Most biofilm studies have been conducted with nonmating a/α cells, but biofilm formation of the mating-capable cell types, a/a and α/α, has revealed a unique regulatory pathway intimately tied to pheromone signalling. In order to mate, C. albicans must go through a switch from the white to opaque cell type. Upon switching, α/α opaque cells release a mating pheromone that induces a mating response in a/a opaque cells and vice versa. Pheromone release also induces an adhesive phenotype among the mating-incompetent a/a white cells [29], leading to mixed biofilm formation and ultimately mating [30].
Notably, genes upregulated specifically in white cells in response to pheromone exposure specify primarily cell wall and surface proteins [29]. Several of these genes contribute to a/a-α/α biofilm formation [29]. The configuration of the mating type locus also seems to affect global biofilm properties [30], which may result from distinct signalling pathways [30]. If C. albicans has distinct ways to make a biofilm, it seems likely that other fungi will as well.
Perspective
Fungal biofilms reflect a range of architectures. Regulators of biofilm formation may be conserved even among disparate biofilm architectures. From detailed analysis in C. albicans and A. fumigatus, there are numerous candidate genes that could be investigated in other biofilm-forming fungi. In addition to hyphal gene expression, characteristic biofilm gene expression patterns include increased expression of transcription factors and protein synthesis genes. Differential adhesin expression, upregulation of cell wall genes, and increased primary metabolism are features of the biofilm environment. Studies of mating pheromone effects on adherence highlight how a small portion of biofilm constituents can have a significant impact on biofilm formation. The presence of highly drug tolerant persister cells in biofilms (discussed above) is another illustration of the contribution of cell heterogeneity to overall biofilm properties. How other heterogeneous properties among biofilm cells may contribute to the overall development and integrity of pathogenic fungal biofilms will be an interesting question for future research.
Fig. 1. Common features of fungal biofilms. 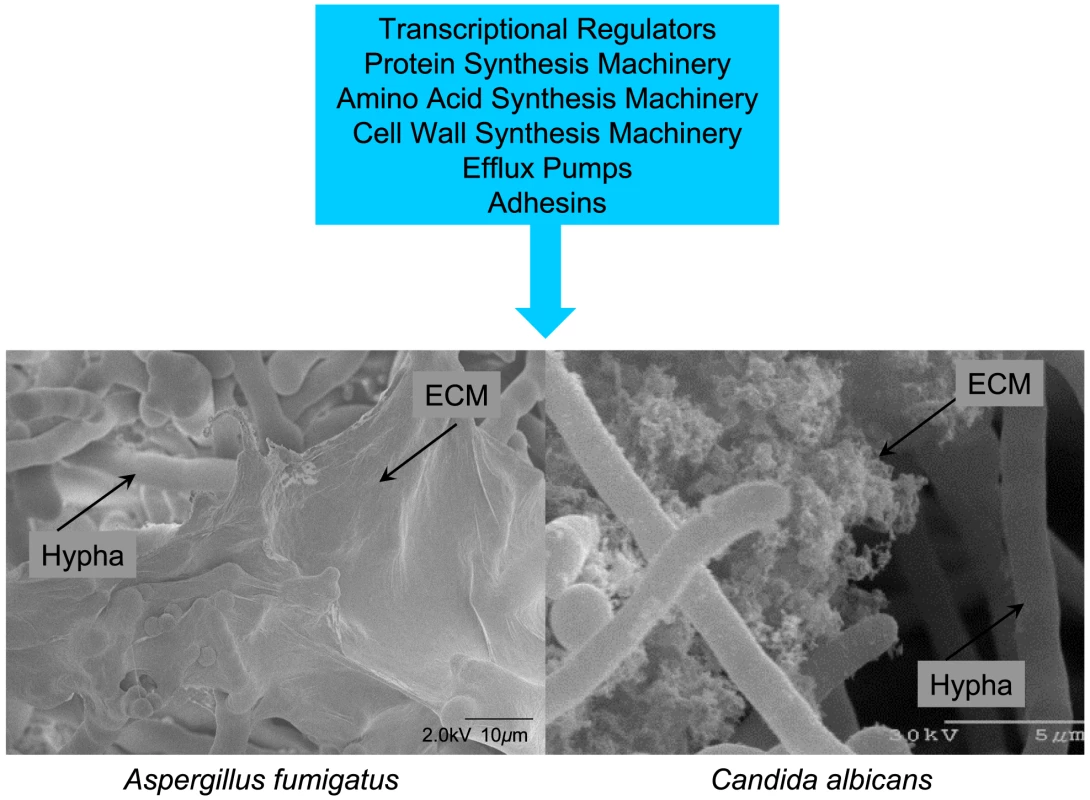
Gene expression has been compared between planktonic cells and biofilm cells of both A. fumigatus and C. albicans. The major functional categories of genes upregulated in biofilms are summarized in the blue box. The micrographs below show a cryo SEM view of an A. fumigatus biofilm (left; Stephanie Guadagnini, Anne Beauvais, and J.P. Latge, Institut Pasteur, Paris, France) and a scanning EM view of C. albicans in vitro biofilm cells (right; Fanning, Suhan, and Mitchell, unpublished). In both cases, extracellular matrix (ECM) is evident. Supporting Information
Zdroje
1. FinkelJSMitchellAP 2011 Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9 109 118
2. BeauvaisAMullerFM 2009 Biofilm formation in Aspergillus fumigatus. LatgeJPSteinbachWJ Aspergillus fumigatus and aspergillosis Washington (D.C.) ASM Press 149 157
3. MartinezLRCasadevallA 2007 Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol 73 4592 4601
4. Di BonaventuraGPompilioAPiccianiCIezziMD'AntonioD 2006 Biofilm formation by the emerging fungal pathogen Trichosporon asahii: development, architecture, and antifungal resistance. Antimicrob Agents Chemother 50 3269 3276
5. DavisLECookGCostertonJW 2002 Biofilm on ventriculo-peritoneal shunt tubing as a cause of treatment failure in coccidioidal meningitis. Emerg Infect Dis 8 376 379
6. CushionMTCollinsMSLinkeMJ 2009 Biofilm formation by Pneumocystis spp. Eukaryot Cell 8 197 206
7. LaFleurMDKumamotoCALewisK 2006 Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother 50 3839 3846
8. Al-FattaniMADouglasLJ 2006 Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55 999 1008
9. AndesDNettJOschelPAlbrechtRMarchilloK 2004 Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun 72 6023 6031
10. HarriottMMLillyEARodriguezTEFidelPLJrNoverrMC 2010 Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156 3635 3644
11. KamaiYKubotaMHosokawaTFukuokaTFillerSG 2001 New model of oropharyngeal candidiasis in mice. Antimicrob Agents Chemother 45 3195 3197
12. Dongari-BagtzoglouAKashlevaHDwivediPDiazPVasilakosJ 2009 Characterization of mucosal Candida albicans biofilms. PLoS ONE 4 e7967 doi:10.1371/journal.pone.0007967
13. NettJEMarchilloKSpiegelCAAndesDR 2010 Development and validation of an in vivo Candida albicans biofilm denture model. Infect Immun 78 3650 3659
14. SilvaSNegriMHenriquesMOliveiraRWilliamsDW 2011 Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol 19 241 247
15. MowatEWilliamsCJonesBMcChlerySRamageG 2009 The characteristics of Aspergillus fumigatus mycetoma development: is this a biofilm? Med Mycol 47 Suppl 1 S120 126
16. LoussertCSchmittCPrevostMCBalloyVFadelE 2010 In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol 12 405 410
17. SinghalDBakerLWormaldPJTanL 2011 Aspergillus fumigatus biofilm on primary human sinonasal epithelial culture. Am J Rhinol Allergy 25 219 225
18. LermannUMorschhauserJ 2008 Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans. Microbiology 154 3281 3295
19. DwivediPThompsonAXieZKashlevaHGangulyS 2011 Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLoS ONE 6 e16218 doi:10.1371/journal.pone.0016218
20. DingCButlerG 2007 Development of a gene knockout system in Candida parapsilosis reveals a conserved role for BCR1 in biofilm formation. Eukaryot Cell 6 1310 1319
21. MulhernSMLogueMEButlerG 2006 Candida albicans transcription factor Ace2 regulates metabolism and is required for filamentation in hypoxic conditions. Eukaryot Cell 5 2001 2013
22. GibbonsJGBeauvaisABeauRMcGaryKLLatgeJP 2011 Global transcriptome changes underlying colony growth in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot Cell 11 68 78
23. KumamotoCA 2008 Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat Rev Microbiol 6 667 673
24. MurilloLANewportGLanCYHabelitzSDunganJ 2005 Genome-wide transcription profiling of the early phase of biofilm formation by Candida albicans. Eukaryot Cell 4 1562 1573
25. NettJELepakAJMarchilloKAndesDR 2009 Time course global gene expression analysis of an in vivo Candida biofilm. J Infect Dis 200 307 313
26. Garcia-SanchezSAubertSIraquiIJanbonGGhigoJM 2004 Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot Cell 3 536 545
27. RajendranRMowatEMcCullochELappinDFJonesB 2011 Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob Agents Chemother 55 2092 2097
28. MartinsMUppuluriPThomasDPClearyIAHenriquesM 2010 Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia 169 323 331
29. SahniNYiSDanielsKJSrikanthaTPujolC 2009 Genes selectively up-regulated by pheromone in white cells are involved in biofilm formation in Candida albicans. PLoS Pathog 5 e1000601 doi:10.1371/journal.ppat.1000601
30. YiSSahniNDanielsKJLuKLSrikanthaT 2011 Alternative mating type configurations (a/alpha versus a/a or alpha/alpha) of Candida albicans result in alternative biofilms regulated by different pathways. PLoS Biol 9 e1001117 doi:10.1371/journal.pbio.1001117
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Systematic Review of Mucosal Immunity Induced by Oral and Inactivated Poliovirus Vaccines against Virus Shedding following Oral Poliovirus Challenge
- The Arbuscular Mycorrhizal Symbiosis: Origin and Evolution of a Beneficial Plant Infection
- Modelling the Evolutionary Dynamics of Viruses within Their Hosts: A Case Study Using High-Throughput Sequencing
- Structural Basis of Cytotoxicity Mediated by the Type III Secretion Toxin ExoU from
- Fungal Biofilms
- The Problem of Auto-Correlation in Parasitology
- T Regulatory Cells Control Susceptibility to Invasive Pneumococcal Pneumonia in Mice
- Small-Molecule Inhibitors of Dengue-Virus Entry
- The Baculovirus Uses a Captured Host Phosphatase to Induce Enhanced Locomotory Activity in Host Caterpillars
- Matrix Metalloprotease 9 Mediates Neutrophil Migration into the Airways in Response to Influenza Virus-Induced Toll-Like Receptor Signaling
- Necrotrophism Is a Quorum-Sensing-Regulated Lifestyle in
- Modeling of the N-Glycosylated Transferrin Receptor Suggests How Transferrin Binding Can Occur within the Surface Coat of
- The Role of Cofactors in Prion Propagation and Infectivity
- The Role of Mast Cells in the Defence against Pathogens
- The Accessory Genome as a Cradle for Adaptive Evolution in Pathogens
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Accessory Genome as a Cradle for Adaptive Evolution in Pathogens
- Systematic Review of Mucosal Immunity Induced by Oral and Inactivated Poliovirus Vaccines against Virus Shedding following Oral Poliovirus Challenge
- The Arbuscular Mycorrhizal Symbiosis: Origin and Evolution of a Beneficial Plant Infection
- Modelling the Evolutionary Dynamics of Viruses within Their Hosts: A Case Study Using High-Throughput Sequencing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



