-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaChemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures
article has not abstract
Published in the journal: . PLoS Pathog 9(10): e32767. doi:10.1371/journal.ppat.1003602
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1003602Summary
article has not abstract
Introduction
Malaria vector control currently relies almost exclusively on killing adult mosquitoes with chemical insecticides. Insecticide-treated nets (ITNs), long-lasting insecticide-treated nets (LLINs), and indoor residual sprays (IRS) aim to repel, disable, and/or kill mosquitoes on contact. While these tools have proven to be extremely successful in reducing disease incidence and mortality [1], insecticide resistance is on the rise and a resurgence of malaria is feared [2]. To mitigate the effects of resistance, the development of new insecticides and formulations for use in LLINs and for IRS remains a research priority [3]. In this paper we argue that, to increase the effectiveness of the chemical arsenal available, we need to consider the relevant microclimatic conditions in which these tools are deployed. We will discuss how temperature in particular can interact with the conventional use of chemicals within houses, and broaden our discussion to consider its potential influence on the use of semiochemicals to lure mosquitoes to traps.
Test Temperatures Are Higher Than Mosquitoes Typically Experience in the Field
The World Health Organization Pesticide Evaluation Scheme (WHOPES), which promotes and coordinates the testing and evaluation of pesticides for public health, specifies laboratory conditions in their guidelines for testing mosquitocidal compounds and products. The recommended temperatures for phase I trials are 25±2°C for testing of LLINs [4] and 27±2°C for IRS and treated bednets [5].
Though temperatures are standardized to improve the reliability and reproducibility of the tests, the ranges chosen are only observed in small geographical areas of sub-Saharan Africa, mainly directly south of the desert (Figure 1, top row). In most malaria transmission settings, the observed mean temperatures range from approximately 18 (cooler highland areas) to 26°C.
Fig. 1. Monthly mean and minimum outdoor and indoor temperatures throughout Africa for January, April, July, and October. 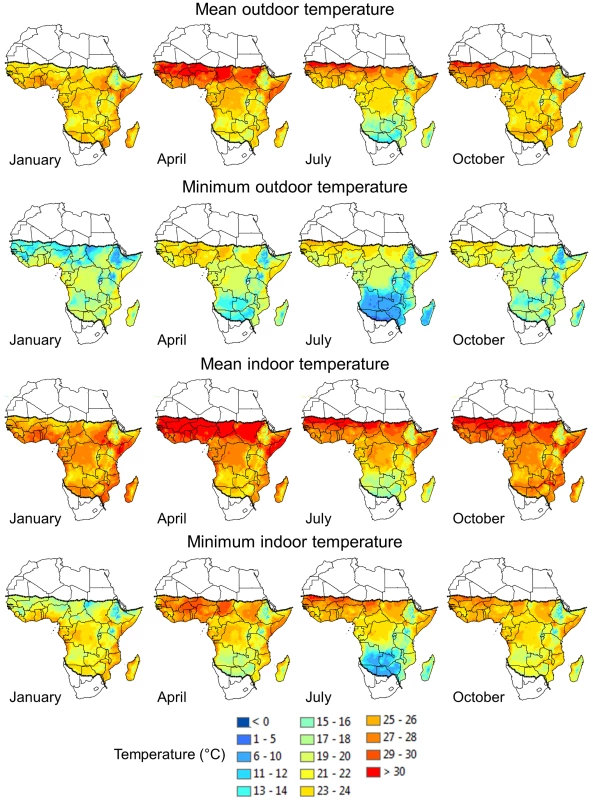
Outdoor monthly mean (top row) and minimum (second row) temperatures. Temperature surfaces were generated by interpolation using weather station data collected between 1960 and 1990. For areas where data records were limited, such as in the Democratic Republic of the Congo, the time period was extended to 2000 (see [45] for details). The current geographical limits of malaria transmission are demarcated by the dotted lines. Indoor monthly mean (third row) and minimum (bottom row) temperatures. Indoor temperature estimates were determined using regression equations that capture the relationship between indoor and outdoor temperatures at different elevations. These regressions were used to convert the outdoor temperature surfaces to matching estimates of indoor temperatures (see [46] for more detailed information). Even more importantly, many vectors of malaria are actively host seeking and blood feeding from dusk until dawn [6], when temperatures are considerably lower than the daily mean. Nighttime minimum temperatures of around 25°C are mostly limited to small areas directly south of the Sahara; in general, minima range from about 13 to 22°C in most malaria transmission zones, depending on season and location (Figure 1, second row).
Temperatures inside houses are generally a few degrees Celsius warmer than those recorded outdoors, and mean indoor temperatures around 25°C can be observed in larger geographic areas (Figure 1, third row). However, indoor minimum temperatures remain well below 25–27°C (Figure 1, bottom row) with large areas experiencing <22°C. It is under these environmental conditions that a mosquito is searching for and biting new hosts.
Susceptible Mosquitoes Could Be More Resistant during Cooler Nighttime Periods
The insecticides used in public health for vector control kill mosquitoes by interfering with nervous system function. But metabolic activity [7], which is involved in degradation of insecticides, and nervous system sensitivity [8] are highly temperature-dependent. As mosquito body temperature changes with its surroundings, environmental temperature has the potential to influence the toxicity of insecticides. This effect is quantified by measuring the temperature coefficient (TC) of an insecticide (Figure 2). A positive TC indicates that an insecticide becomes more toxic as temperature increases; insecticides with a negative TC kill more insects at lower temperatures. Pyrethroids, the dominant insecticide class currently used for malaria control, and DDT, the only organochlorine permitted for IRS, commonly exhibit a negative temperature coefficient. Therefore, in theory, they should perform better under cooler nighttime conditions. On the other hand, carbamates and organophosphates (two and three out of the 12 recommended compounds for IRS, respectively) generally have a positive TC, and may be less efficient under these conditions.
Fig. 2. Temperature coefficients of deltamethrin against different insect species. 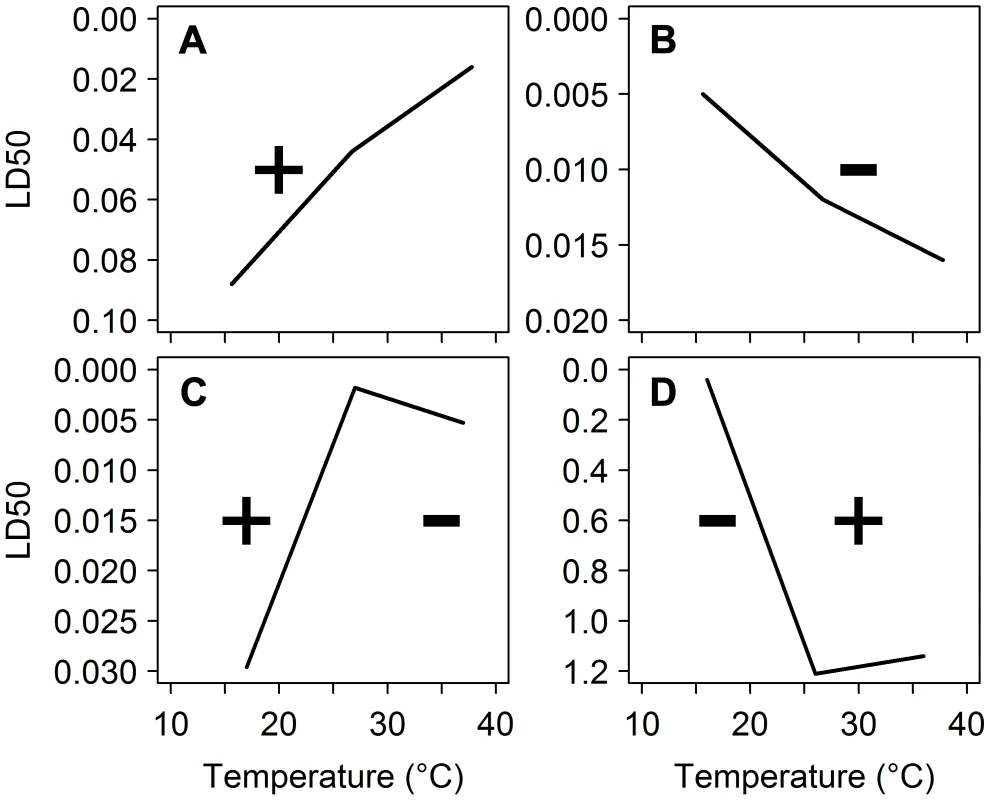
Toxicity (median lethal dose) of deltamethrin to (A) Heliothis virescens (µg/g) [17], (B) Trichoplusia ni (µg/g) [17], (C) Chilo suppressalis (µg/insect) [16], and (D) Triatoma infestans (ng/insect) [47]. Note that the Y-axis is inverted to visualize the temperature coefficient (TC). If the dose required to kill 50% of insects decreases as temperature increases, the insecticide has a positive TC, indicated by +. Negative TC indicated by −. Exceptions to these general TC rules, however, are common. Whether the TC is positive or negative can depend on the insect (e.g., species, developmental stage, age, sex), the chemical tested (e.g., formulation, substrate, dose, duration of exposure) and testing conditions (e.g., temperature range, humidity, time of day). For pyrethroids, the only insecticides currently used on ITNs and LLINs [9], [10] and the dominant insecticide class in IRS [11], a strictly negative relationship with temperature is not always observed. Type II pyrethroids, which have an α-cyano group on the phenoxybenzyl moiety [12], tend to violate this rule. For example, deltamethrin and cypermethrin, which are used in 11 out of the 13 (or 85%) LLINs, have been observed to have positive TCs for mortality in several insect species (Figure 2A, C, D, [13]–[17]). If the same phenomenon applies to malaria mosquitoes, only DDT and two LLINs (those treated with permethrin, a type I pyrethroid) will be most effective during the cooler nighttime periods when a mosquito is active: all other recommended interventions could be less effective at killing vectors.
To the best of our knowledge, there are only two studies that examined the effect of temperature on the toxicity of insecticides on susceptible, adult malaria vectors. Hodjati and Curtis [18] observed a bimodal relationship between temperature and toxicity for permethrin, a type I pyrethroid, against Anopheles stephensi, a primary malaria vector in India (negative between 16 and 22°C, but positive between 22 and 37°C). Over the same range of temperatures, An. gambiae, a major malaria vector in sub-Saharan Africa, displayed a consistently positive TC. This indicates that malaria mosquitoes may not follow the general temperature-toxicity rules. The second study [19] saw a negative TC for DDT and a positive TC for the organophosphate diazinon when An. stephensi was exposed to insecticide residues between 20 and 30°C.
While vector control chemicals are typically applied at concentrations meant to overwhelm variation in susceptibility, evidence from the field shows that the ability of LLINs or IRS to kill mosquitoes can decrease rapidly over time after initial deployment. Although LLINs should retain their insecticidal activity for at least three years under field conditions [20], the mosquitocidal activity of several LLINs is reduced on much shorter time scales [21], [22]. The activity of IRS compounds can decline significantly within the first few months after spraying due to, for example, variation in building materials [22], [23] or in the spraying technique of individual applicators [24]. Thus, there could be periods prior to IRS retreatment or redistribution of new LLINs during which the loss of efficacy from chemical, operational, or environmental factors could be exacerbated by using chemicals that are even less effective under variable temperature conditions.
With approximately 34 dominant anopheline vector species in the world [25], and a variety of recommended chemical products on the market, this lack of data represents a critical gap in our understanding. Although current tools do kill mosquitoes and reduce malaria risk, a better understanding of chemical temperature coefficients could affect the chemical toolbox in two ways: first, it could increase the number of chemicals available for control. By testing insecticidal performance under standard laboratory conditions (25–27°C), there is the possibility that we currently eliminate compounds in the testing phase—especially those with a strongly negative TC—that may perform very well in the field. Second, without information about their action at different temperatures, we may deploy chemicals that will be less efficient than we expect under actual field conditions. Investigating the performance of our vector control tools under different temperature conditions will augment our ability to select the most efficacious tool for a given environment. For insecticidal control of pests in crop systems, it has been acknowledged that knowing a product's temperature coefficient enables pest managers to select a product that is efficacious under the prevailing environmental conditions [26]–[28].
Resistant Mosquitoes May Be More Resistant in the Laboratory
Insecticide resistance is one of the greatest threats to the success of malaria control and elimination campaigns. The WHO currently recommends that the level of resistance in mosquito populations be evaluated at 25±2°C [29]. As with susceptible insects, the mortality of resistant insects can increase or decrease with temperature (e.g., [30], [31]). Hodjati and Curtis [18] showed that resistant An. stephensi mosquitoes were more susceptible to permethrin at 16 and 37°C, compared to 22 and 28°C, where nearly all mosquitoes survived the exposure. In resistant An. gambiae, as in the susceptible strain, susceptibility increased with temperature. This suggests that quantifying resistance under relatively high temperature conditions in the laboratory will not necessarily inform us to what extent a chemical intervention is still effective in the field.
Efficiency of Other (Semio)chemical Interventions Will Also Depend on Environmental Temperature
There is growing evidence that the widespread use of LLINs and IRS is reducing mosquito activity indoors and can drive vector-species composition changes or host-species switching behavior to increase outdoor biting [32]. Alternative interventions that specifically target outdoor biting are needed. One approach is to use chemical compounds to trap or repel mosquitoes, thereby reducing the number of mosquito bites to human hosts. There are reasons to expect that the effectiveness of such odor-baited traps could be affected by environmental temperature.
For odor-baited traps to work, a mosquito needs to detect the odor plume and follow it back to the source. The number of odor molecules of a compound in the gas phase will be reduced when temperatures decrease (see example in [33]). Simply put, there will be less for a mosquito to smell when it is cooler outside. Additionally, odor plume dynamics depend on the stability of the atmosphere, which depends in part on temperature [34]. Although adding a heat source could regulate the release of molecules from a trapping device, the resulting odor plume can be expected to behave differently under cool nighttime conditions than it would under warmer laboratory conditions. In addition, temperature affects several physiological processes involved in insects' odor reception [35], [36]. Lower temperatures can reduce response distance and specificity [37], but also directly impact insect flight behavior by reducing flight speed [38].
So, although these traps seem to work in the field [39], cooler field temperatures may reduce trap efficiency, which has been shown for adult plum curculios, Conotrachelus nenuphar [40]. At present, the behavioral responses of mosquitoes to chemical cues in olfactometers are evaluated at standard insectary conditions, around 26–27°C [41], [42], and there are no WHO guidelines for testing such devices. Again, as malaria mosquitoes host seek and bite only during the cooler evening and night, there might be room for improvement when the actual microclimate observed in the field is considered during laboratory trials.
Conclusions
Chemicals are powerful tools in the control of malaria and other vector-borne diseases such as dengue, leishmaniasis, and Chagas disease [43]. Given that temperature has the potential to affect the toxicity of chemicals used for ITNs, LLINs, and IRS, as well as to alter chemical release from and mosquito response to odor-baited traps, candidate chemicals need to be evaluated under relevant climatic conditions. For the initial development of chemicals to be used in the fight against malaria, we suggest that testing recommendations, currently at 25 to 27±2°C, should include a range of temperatures: 15, 20, 25, and 30°C. Such a change would provide valuable information about how mosquitoes and chemicals will interact under natural field conditions, therefore allowing us to develop more effective tools in the laboratory and to select the tools most likely to be effective in a given local environment. As insecticide resistance monitoring in the field is frequently carried out in areas where malaria is endemic (or epidemic), and these areas are often low-income countries, we suggest adding one additional temperature for these tests: 20°C. This change will give us a better understanding of how well the chemicals currently being used are working to control night-biting vectors. In areas where insecticide resistance has been detected in the mosquito population, such knowledge could be especially valuable. By applying a mixture of chemicals, which may also counter or postpone the development of insecticide resistance in mosquito populations to chemicals used on ITNs, LLINs, and in IRS [3], a given regimen could be efficient across different thermal environments, or in environments with a wide thermal envelope [44]. We believe that considering the temperature coefficient of chemicals from the outset of testing will increase the effectiveness of the chemical toolbox for malaria vector control.
Zdroje
1. WHO (2012) World malaria report 2012. Geneva: World Health Organization.
2. MaxmenA (2012) Malaria surge feared. Nature 485 : 293.
3. AlonsoPL, TannerM (2013) Public health challenges and prospects for malaria control and elimination. Nat Med 19 : 150–155.
4. WHO (2005) Guidelines for laboratory and field testing of long-lasting insecticidal mosquito nets. Geneva: World Health Organization.
5. WHO (2006) Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets. Geneva: World Health Organization.
6. RussellT, GovellaN, AziziS, DrakeleyC, KachurSP, et al. (2011) Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J 10 : 80.
7. MontgomeryJC, MacdonaldJA (1990) Effects of temperature on nervous system: implications for behavioral performance. Am J Physiol Regul Integr Comp Physiol 259: R191–R196.
8. GilloolyJF, BrownJH, WestGB, SavageVM, CharnovEL (2001) Effects of size and temperature on metabolic rate. Science 293 : 2248–2251.
9. WHO (2012) WHO recommended long-lasting insecticidal mosquito nets. Geneva: World Health Organization.
10. WHO (2007) WHO recommended insecticide products treatment of mosquito nets for malaria vector control. Geneva: World Health Organization.
11. WHO (2009) WHO recommended insecticides for indoor residual spraying against malaria vectors. Geneva: World Health Organization.
12. Schleier III JJ, Peterson RKD (2011) Pyrethrins and pyrethroid insecticides. In: López O, Fernándes-Bolaños JG, editors. Green trends in insect control. London: Royal Society of Chemistry. pp. 94–131.
13. JohnsonDL (1990) Influence of temperature on toxicity of two pyrethroids to grasshoppers (Orthoptera: Acrididae). J Econ Entomol 83 : 366–373.
14. ScottJG, MatsumuraF (1983) Evidence for two types of toxic actions of pyrethroids on susceptible and DDT-resistant german cockroaches. Pestic Biochem Physiol 19 : 141–150.
15. SparksTC, PavloffAM, RoseRL, ClowerDF (1983) Temperature-toxicity relationships of pyrethroids on Heliothis virescens (F.) (Lepidoptera: Noctuidae) and Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae). J Econ Entomol 76 : 243–246.
16. LiH, FengT, LiangP, ShiX, GaoX, et al. (2006) Effect of temperature on toxicity of pyrethroids and endosulfan, activity of mitochondrial Na+–K+-ATPase and Ca2+–Mg2+-ATPase in Chilo suppressalis (Walker) (Lepidoptera: Pyralidae). Pestic Biochem Physiol 86 : 151–156.
17. SparksTC, ShourMH, WellemeyerEG (1982) Temperature-toxicity relationships of pyrethroids on three Lepidopterans. J Econ Entomol 75 : 643–646.
18. HodjatiMH, CurtisCF (1999) Effects of permethrin at different temperatures on pyrethroid-resistant and susceptible strains of Anopheles. Med Vet Entomol 13 : 415–422.
19. HadawayAB, BarlowF (1963) The influence of environmental conditions on the contact toxicity of some insecticide deposits to adult mosquitos, Anopheles stephensi Liston. Bull Entomol Res 54 : 329–344.
20. WHO (2007) Insecticide-treated mosquito nets: a position statement. Available: http://www.who.int/malaria/publications/atoz/itnspospaperfinal/en/index.html. Accessed 7 September 2013.
21. LindbladeKA, DotsonE, HawleyWA, BayohN, WilliamsonJ, et al. (2005) Evaluation of long-lasting insecticidal nets after 2 years of household use. Trop Med Int Health 10 : 1141–1150.
22. OkumuF, ChipwazaB, MadumlaE, MbeyelaE, LingambaG, et al. (2012) Implications of bio-efficacy and persistence of insecticides when indoor residual spraying and long-lasting insecticide nets are combined for malaria prevention. Malar J 11 : 378.
23. EtangJ, NwaneP, MbidaJ, PiameuM, MangaB, et al. (2011) Variations of insecticide residual bio-efficacy on different types of walls: results from a community-based trial in south Cameroon. Malar J 10 : 333.
24. MasenduHT, NziramasangaN, MuchechemeraC (2002) Low insecticide deposit rates detected during routine indoor residual spraying for malaria vector control in two districts of Gokwe, Zimbabwe. J Am Mosq Control Assoc 18 : 202–206.
25. KiszewskiA, MellingerA, SpielmanA, MalaneyP, SachsSE, et al. (2004) A global index representing the stability of malaria transmission. Am J Trop Med Hyg 70 : 486–498.
26. MusserFR, SheltonAM (2005) The influence of post-exposure temperature on the toxicity of insecticides to Ostrinia nubilalis (Lepidoptera: Crambidae). Pest Manag Sci 61 : 508–510.
27. MaY-h, GaoZ-l, DangZ-h, LiY-f, PanW-l (2012) Effect of temperature on the toxicity of several insecticides to Apolygus lucorum (Heteroptera: Miridae). J Pestic Sci 37 : 135–139.
28. BoinaDR, OnagbolaEO, SalyaniM, StelinskiLL (2009) Influence of posttreatment temperature on the toxicity of insecticides against Diaphorina citri (Hemiptera: Psyllidae). J Econ Entomol 102 : 685–691.
29. WHO (1998) Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticide on treated surfaces. Geneva: World Health Organization.
30. ScottJG (1987) Effect of temperature on the toxicity of S-bioallethrin andcypermethrin to susceptible and kdr-resistant strains of Blattella germanica (L.) (Dictyoptera: Blattellidae). Bull Entomol Res 77 : 431–435.
31. BrownMA (1987) Temperature-dependent pyrethroid resistance in a pyrethroid-selected colony of Heliothis virescens (F) (Lepidoptera, noctuidae). J Econ Entomol 80 : 330–332.
32. GovellaNJ, FergusonH (2012) Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol 3 : 199–199.
33. RiveronJ, BotoT, AlcortaE (2009) The effect of environmental temperature on olfactory perception in Drosophila melanogaster. J Insect Physiol 55 : 943–951.
34. Stull RB (2000) Meteorology for scientists and engineers. Pacific Grove: Brooks/Cole. 528 p.
35. KodadováB (1996) Resolution of pheromone pulses in receptor cells of Antheraea polyphemus at different temperatures. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 179 : 301–310.
36. BakerTC, HanssonBS, LöfstedtC, LöfqvistJ (1988) Adaptation of antennal neurons in moths is associated with cessation of pheromone-mediated upwind flight. Proc Natl Acad Sci U S A 85 : 9826–9830.
37. LinnC, CampbellM, RoelofsW (1991) The effects of different blend ratios and temperature on the active space of the Oriental fruit moth sex pheromone. Physiol Entomol 16 : 211–222.
38. CharltonRE, KannoH, CollinsRD, CardeRT (1993) Influence of pheromone concentration and ambient temperature on flight of the gypsy moth, Lymantria dispar, in a sustained-flight wind tunnel. Physiol Entomol 18 : 349–362.
39. NjiruB, MukabanaW, TakkenW, KnolsB (2006) Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in western Kenya. Malar J 5 : 39.
40. LeskeyTC, ZhangA (2007) Impact of temperature on plum curculio (Coleoptera: Curculionidae) responses to odor-baited traps. J Econ Entomol 100 : 343–349.
41. SmallegangeRC, Bukovinszkine-KissG, OtienoB, MbadiPA, TakkenW, et al. (2012) Identification of candidate volatiles that affect the behavioural response of the malaria mosquito Anopheles gambiae sensu stricto to an active kairomone blend: laboratory and semi-field assays. Physiol Entomol 37 : 60–71.
42. SeenivasaganT, SharmaKR, PrakashS (2012) Electroantennogram, flight orientation and oviposition responses of Anopheles stephensi and Aedes aegypti to a fatty acid ester-propyl octadecanoate. Acta Trop 124 : 54–61.
43. van den BergH, ZaimM, YadavR, SoaresA, AmeneshewaB, et al. (2012) Global trends in the use of insecticides to control vector-borne diseases. Environ Health Perspect 120 : 577–582.
44. HinksCF, SpurrDT (1991) The efficacy and cost benefits of binary mixtures of deltamethrin combined with other insecticides or synergists against grasshoppers at two temperatures. J Agric Entomol 8 : 29–39.
45. HijmansRJ, CameronSE, ParraJL, JonesPG, JarvisA (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Clim 25 : 1965–1978.
46. BlanfordJI, BlanfordS, CraneRG, MannME, PaaijmansKP, et al. (2013) Implications of temperature variation for malaria parasite development across Africa. Sci Rep 3 : 1300.
47. AlzogarayRA, ZerbaEN (1996) Comparative toxicity of deltamethrin and cis-permethrin on first instars of Triatoma infestans (Hemiptera: Reduviidae). J Med Entomol 33 : 58–62.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of and Invasive Aspergillosis
- Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events
- Emergence of Azole-Resistant Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health
- Fungal Adenylyl Cyclase Acts As a Signal Sensor and Integrator and Plays a Central Role in Interaction with Bacteria
- Sensing of the Microbial Neighborhood by
- Antivirulence Therapy for Animal Production: Filling an Arsenal with Novel Weapons for Sustainable Disease Control
- The Cell Biology of : How to Teach Using Animations
- A Structure-Guided Mutation in the Major Capsid Protein Retargets BK Polyomavirus
- RNA Biology in Fungal Phytopathogens
- , , and the Human Mouth: A Sticky Situation
- The Gene Is Essential for Resistance to Human Serum in
- Unisexual Reproduction Drives Evolution of Eukaryotic Microbial Pathogens
- Bacterial Pathogens Activate a Common Inflammatory Pathway through IFNλ Regulation of PDCD4
- Bats and Viruses: Friend or Foe?
- Protein Trafficking through the Endosomal System Prepares Intracellular Parasites for a Home Invasion
- IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection
- B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Genital Tract Infection
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Chemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures
- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
- Driven Enforced Viral Replication in Dendritic Cells Contributes to Break of Immunological Tolerance in Autoimmune Diabetes
- IL-4Rα-Associated Antigen Processing by B Cells Promotes Immunity in Infection
- A Gammaherpesvirus Uses Alternative Splicing to Regulate Its Tropism and Its Sensitivity to Neutralization
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Epigenetic Dominance of Prion Conformers
- MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells
- The Role of TcdB and TccC Subunits in Secretion of the Tcd Toxin Complex
- A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



