-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaNINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
The cytoplasmic dynein 1 motor complex is known to be essential for photoreceptor outer segment formation and function. NINL, an important interaction partner of three ciliopathy-associated proteins (lebercilin, USH2A and CC2D2A), was previously shown to associate with this motor complex. In this work, we scrutinize the role of NINL using a combination of affinity proteomics and zebrafish studies, in order to gain insight into the pathogenic mechanisms underlying these three associated hereditary disorders. We identify DZANK1 as an important interaction partner of NINL and show that loss of Ninl, Dzank1, or a combination of both synergistically results in impaired transport of trans Golgi-derived vesicles and, as a consequence, defective photoreceptor outer segment formation. Using affinity proteomics, we demonstrate that NINL and DZANK1 associate with complementary subunits of the cytoplasmic dynein 1 complex. Our results support a model in which the NINL-DZANK1 protein module is essential for the proper assembly and folding of the cytoplasmic dynein 1 motor complex, shedding light on the structure and stoichiometry of this important motor complex.
Published in the journal: . PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005574
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005574Summary
The cytoplasmic dynein 1 motor complex is known to be essential for photoreceptor outer segment formation and function. NINL, an important interaction partner of three ciliopathy-associated proteins (lebercilin, USH2A and CC2D2A), was previously shown to associate with this motor complex. In this work, we scrutinize the role of NINL using a combination of affinity proteomics and zebrafish studies, in order to gain insight into the pathogenic mechanisms underlying these three associated hereditary disorders. We identify DZANK1 as an important interaction partner of NINL and show that loss of Ninl, Dzank1, or a combination of both synergistically results in impaired transport of trans Golgi-derived vesicles and, as a consequence, defective photoreceptor outer segment formation. Using affinity proteomics, we demonstrate that NINL and DZANK1 associate with complementary subunits of the cytoplasmic dynein 1 complex. Our results support a model in which the NINL-DZANK1 protein module is essential for the proper assembly and folding of the cytoplasmic dynein 1 motor complex, shedding light on the structure and stoichiometry of this important motor complex.
Introduction
Dysfunction of cilia is the underlying defect in a growing group of pleiotropic genetic disorders, the ciliopathies. Cilia are ubiquitous microtubule-based organelles involved in fluid propulsion (motile cilia) or signal transduction (primary cilia) and ciliopathy-associated proteins localize to various ciliary sub-compartments. Retinal dystrophy is a common clinical feature of ciliopathies where the primary affected retinal cell type is the photoreceptor, which contains a highly specialized primary cilium, consisting of the connecting cilium and axoneme serving as a backbone to the outer segment. For propagation of visual excitation, outer segments are composed of stacks of membranous discs, which are densely packed with the light-sensitive transmembrane receptor rhodopsin and its associated photo-transduction machinery. The membranous disks are organized around the axoneme that is continuous with the connecting cilium. The entire outer segment can thus be regarded as a highly specialized primary cilia compartment. The connecting cilium literally connects the outer segment to the inner segment of the photoreceptor and is the equivalent of a canonical ciliary transition zone. This proximal region of the cilium ensures a tight control of protein access into the ciliary compartment [1–5] through a gate-keeper function, involving several ciliopathy-associated proteins such as NPHP’s [4] and Meckel and Joubert syndrome proteins [6], importins and Ran GTPases [7, 8].
Given the daily renewal of about 10% of the total length of the outer segments in humans [9], photoreceptor cells require intense intracellular trafficking to build their outer segments and to replenish the shed discs. Transmembrane proteins, such as rhodopsin and Usherin are synthesized in the inner segment and subsequently moved from the trans-Golgi network (TGN) towards the base of the ciliary compartment via microtubule-based vesicular transport [10]. This transport involves motor proteins such as the ATPases kinesin and dynein [11, 12]. Specifically, the cytoplasmic dynein 1 motor complex, which consists of two 530 kDa heavy chains, responsible for force production, a group of 74 kDa intermediate chains, 53 to 57 kDa light intermediate chains, and 8 to 21 kDa light chains [13], has been implicated in minus-end directed transport of post-Golgi-derived rhodopsin-containing vesicles [14]. During its transport, the carboxy-terminal domain of rhodopsin binds to the dynein light chain Tctex-type DYNLT1 [14]. In the absence of rhodopsin, small rudimentary photoreceptor outer segments are formed during the first few postnatal weeks. After this period the outer segments vanish and photoreceptors die rapidly. As a consequence, photo-transduction is impaired leading to defects in visual function [15, 16]. A similar defect in photoreceptor morphology and function is observed in the zebrafish cannonball mutant, in which the cytoplasmic dynein motor complex 1 is dysfunctional due to a mutation in the dynein cytoplasmic 1 heavy chain 1 (dync1h1) gene [17]. These findings emphasize the importance of the cytoplasmic dynein motor complex 1 for intracellular trafficking which is essential for photoreceptor development and function. However, the structure of this complex has not been fully elucidated to date.
Previously, we described a retinal ciliopathy-associated protein module consisting of Usherin, Lebercilin (Leber’s congenital amaurosis type 5) and NINLisoB (ninein-like protein isoform B) present at the base of the connecting cilium [18]. In addition, we now identified a physical interaction between NINLisoB and the ciliopathy protein CC2D2A, involved in Joubert and Meckel syndrome, two important ciliopathies (Bachmann-Gagescu, et al co-submission). Furthermore, NINL was found to associate with several members of the cytoplasmic dynein 1-dynactin motor complex and polo-like kinase 1 and was found to function in microtubule nucleation by recruitment of gamma-tubulin ring complexes [19]. However, the function of NINL in photoreceptors is still elusive.
In the current study, we investigate the role of NINL and its associated protein complex in the retina using a combination of proteomics and in vivo studies in zebrafish. We identify a central role for NINL and its novel interaction partner DZANK1 in vesicle transport towards the photoreceptor outer segments. Knockdown in zebrafish larvae of either ninl or dzank1 or synergistically at sub-phenotypic doses, leads to abnormal outer segment morphology, mislocalization of rhodopsin, accumulation of vesicular structures and consequently, loss of visual function. We further demonstrate that NINL and DZANK1 associate with complementary subunits of the cytoplasmic dynein 1 motor complex, which is essential for proper transport of vesicle-bound proteins towards the base of the photoreceptor connecting cilium and, as a consequence, photoreceptor development in zebrafish. Together, our findings shed light onto the assembly and structure of the cytoplasmic dynein 1 motor complex and link it to several ciliopathy proteins located at the entrance to the ciliary compartment.
Results
DZANK1 interacts with NINLisoB in the retina
Previously, NINLisoB was identified as a key connector of three large retinal ciliopathies [18] (Bachmann-Gagescu et al, co-submission). Its function within the retina, however, has not yet been deciphered. To get insight into the function of NINL, we screened a random-primed bovine retina cDNA library to identify interaction partners for the previously predicted intermediate filament (IF) domain (538-825aa) of NINLisoB [18]. Approximately 70% of the positive clones (> 1,000 clones), could be identified as DZANK1 (double zinc ribbon and ankyrin repeat domains 1), a protein with a yet unknown function. To elaborate on this finding, three overlapping cDNA fragments were cloned: one encoding full length DZANK1 (752aa) and two different deletion constructs, encoding either the zinc finger domains as found in Ran binding proteins (ZNF_RBZ, SMART accession number SM00547, 244-317aa) or the ankyrin repeats known to be involved in protein-protein interactions (ANK, SMART accession number SM00248, 595-681aa) (SMART database; http://smart.embl-heidelberg.de) (Fig 1a). To test whether the interactions of DZANK1 with NINL are isoform-specific, both isoforms of NINL were tested. The transcript encoding NINLisoB lacks exon17 of the originally described NINL gene (encoding NINLisoA), resulting in the in-frame skipping of 349 amino acids after residue 734 [18]. Using dedicated binary yeast two-hybrid assays, we were able to pinpoint the interaction to the ZNF_RBZ domains of DZANK1 and both isoforms of NINL (Fig 1b). Since NINLisoB was originally found in the yeast-two hybrid screen, we continued with this isoform for further confirmations. We performed several in vitro and in vivo binding assays to conclusively validate the interaction between DZANK1 and NINLisoB. In a glutathione S-transferase (GST) pull-down assay, we identified that full-length Strep/FLAG-tagged DZANK1 was pulled down from HEK293T cell lysates by GST-fused NINLisoB_538-825aa and not by GST alone (Fig 1c). To confirm this interaction in cellulo, HEK293T cells were co-transfected with plasmids encoding full length HA-tagged NINLisoB and Strep/FLAG-tagged DZANK1 for immunoprecipitation (IP) assays. With anti-HA antibodies, NINLisoB consistently co-immunoprecipitated with full length DZANK1, but not with the control protein LRRK2 (Fig 1d). Reciprocal IP experiments with anti-FLAG antibodies for IP confirmed these results (Fig 1d’). In addition we performed GST pull-down assays from biologically relevant bovine retinal extracts followed by mass spectrometry (LC-MS/MS) analysis. In total, 445 different proteins were identified of which 59 passed the stringent filtering criteria. GST-fused human NINLisoB_aa538-825 was able to consistently pull-down endogenous bovine DZANK1 (in 4 out of 4 assays) whereas unfused GST (in 0 out of 3 assays) was not, which further confirmed the interaction (S1 Table). Further confirmation of the interaction was obtained through co-localization of eGFP - and mRFP-tagged DZANK1 and NINLisoB in hTERT-RPE1 cells. In singly transfected cells, NINLisoB and DZANK1 were both present at the ciliary base, partially co-localizing with the basal body and the ciliary marker polyglutamylated tubulin (anti-GT335) (Fig 2a and 2b”). DZANK1 also localized along the microtubule network of the cells. Co-expression of NINLisoB and DZANK1 resulted in the co-localization of both proteins, thereby fully retaining the latter to the basal body (Fig 2c”, yellow signal). Importantly, co-localization of endogenous NINL and DZANK1 was also observed by immunohistochemistry in rat retina where NINL was demonstrated at the region of the connecting cilium [18]. Using a specific anti-DZANK1 antibody we show that NINL and DZANK1 co-localize in this region (Fig 2d–2f’).
Fig. 1. Protein-protein interaction studies. 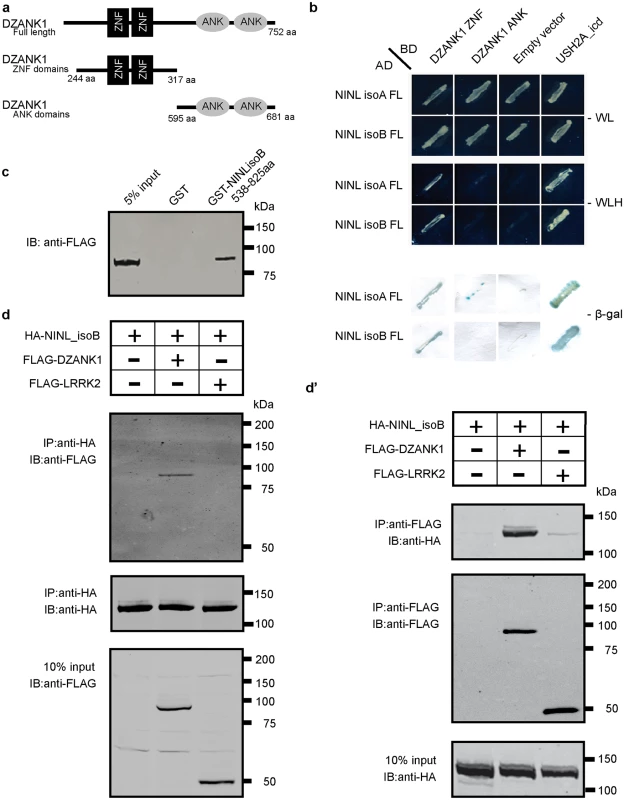
(a) Schematic protein structure of DZANK1. Two different deletion constructs of DZANK1, one consisting of the ZNF_RBZ domains (244-317aa) and the other of the ANK domains (595-681aa), were used for co-transformation in yeast. (b) Results of the co-transformation in yeast. Yeast transformants were selected on low (SD/-Trp/-Leu/-His) and high-stringency (SD/-Trp/-Leu/-His-/-Ade) medium with observed growth, indicating interaction of the tested bait and prey proteins. In addition, the β-galactosidase filter lift assay was performed. The USH2A_intracellular region was used as a positive control. Empty prey vector was used as a negative control. Yeast-two-hybrid analysis revealed a specific interaction between DZANK1 ZNF_RBZ domains and both NINLisoA/B. (c) GST pull-down assays, showing that Strep/FLAG-tagged DZANK1 was efficiently pulled down by GST-fused NINLisoB, but not by GST alone. The first lane shows 5% input of the protein lysate. (d) Co-immunoprecipitation of DZANK1 Full Length (FL) with NINLisoB, but not with LRRK2. The immunoblot (IB) in the top panel shows that HA-tagged NINL co-immunoprecipitated with Strep/FLAG-tagged DZANK1 (lane 2), whereas unrelated FLAG-tagged LRRK2 (lane 3) did not. The anti-HA immunoprecipitates are shown in the middle panel; protein input is shown in the bottom panel. (d’) Reciprocal IP experiments using anti-FLAG antibodies confirmed the co-immunoprecipitation of HA-tagged NINLisoB with Strep/FLAG-tagged DZANK1 (lane 2) and not with LRRK2 (lane 3) shown in the top panel. The anti-FLAG immunoprecipitations are shown in the middle panel; protein input is shown in the bottom panel. ANK: ankyrin repeat domain; ZNF_RBZ: Zinc Finger domain like in Ran-binding proteins; aa: amino acids. Fig. 2. NINL and DZANK1 co-localize at the base of cilia in RPE cells and rat retina. 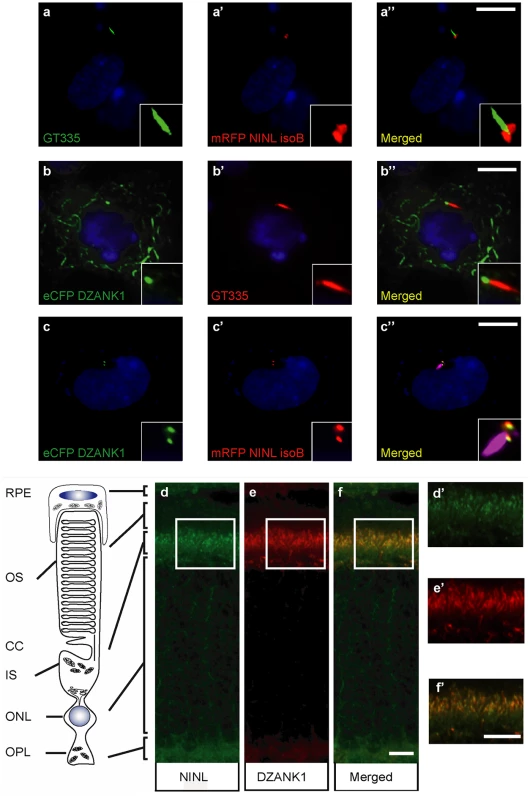
(a-c) Centrosomal co-localization of NINLisoB and DZANK1 in hTERT-RPE1 cells. When expressed alone, mRFP-NINLisoB (red signal in, a-a” and c-c”) was localized to both centrioles at the base of the cilia marked with GT335 (green signal in a’-a” and red signal in b-b”), whereas eYFP–DZANK1 was localized to the basal body and to the microtubule network of the cell (green signal in b-b”). After co-expression of NINLisoB and DZANK1, both proteins were localized at the basal body of the cilia in the centrosome (cilia marked by GT335, cyanid signal), supporting an interaction between the two proteins (c-c”). Nuclei were stained with DAPI (blue signal). (d-f) Co-localization of endogenous NINLisoB and DZANK1 in rat retina. (d-e) Co-immunostaining of NINL and DZANK1 in radial cryo-sections of adult (P20) rat retina with anti-NINL antibodies (green signal; d) and anti-DZANK1 antibodies (red signal; e) showing co-localization (yellow signal: f) in the inner segment (IS) and in the region of the connecting cilium (CC). (d’-f’) show details of the subcellular (co-) localization of NINL and DZANK1 in, respectively, d, e and f. Scale bars represent 10 μm (a-c”), 5 μm (f) and 1 μm (f’). Ninl and dzank1 genetically interact in photoreceptor outer segment morphogenesis and function
To investigate the biological role of NINL and DZANK1 in the retina, we first addressed the suitability of zebrafish as an animal model. tBLASTn searches of the zebrafish genome, using the amino acid sequence of human NINL and DZANK1, revealed a single ortholog for both genes. Although extensive RT-PCR analyses were performed, we were not able to detect the transcript encoding zebrafish Ninl isoform A in RNA obtained from zebrafish larvae (5 days post fertilization (dpf)). Therefore we conclude that under the given conditions the ninl transcript that shows the highest degree of homology with the human NINLisoB encoding transcript is the most prominent. The presence of shorter or alternative transcripts encoding additional zebrafish Ninl isoforms cannot be ruled out, as no 5’ - and 3’-RACE experiments were performed. Subsequently, the effect of ninl and dzank1 knockdown during zebrafish embryonic development was investigated, using gene-specific translation-blocking (atgMOs) and splice-blocking morpholinos (spMOs).
Control MO-injected larvae (10 ng/nl; n = 300 from 2 biological replicates) appeared morphologically normal, and could not be distinguished from uninjected larvae (WT) during the studied developmental period of 4 to 5 dpf (Fig 3a and 3d). In contrast, injection of ninl atgMO revealed a concentration-dependent spectrum of phenotypes (n>200/group from 2 biological replicates; Bachmann-Gagescu et al., companion manuscript). Injection of the optimal concentration of 2 ng/nl ninl atgMO resulted in severe morphological defects including ventrally curved body axis, ventriculomegaly, pronephric cysts, expanded melanophores, small eyes and circling swimming behavior at 4 dpf (n = 300 from 2 biological replicates; Fig 3b and Bachmann-Gagescu et al., companion manuscript). In addition, we observed significantly shorter photoreceptor outer segments (OS) on retinal cryosections from 4dpf ninl morphant larvae stained with boron-dipyrromethene (bodipy) to mark the outer segment membrane disks (mean OS length 1.6+/-0.26 μm in morphants compared to 3.9+/-0.32 μm in wild-type, p<0.0001, unpaired Student’s t-test, n>10 larvae from each group in each of 2 biological replicates) (Bachmann-Gagescu et al, companion manuscript). Co-injection of 150 pg/nl capped MO-resistant mRNA encoding human NINLisoB with 2 ng/nl of ninl atgMO rescued the observed body curvature phenotype (curved body shape in 71% of ninl atgMO injected larvae (n = 207) versus 36% in ninl atgMO + ninl mRNA injected larvae (n = 203), data pooled from 2 biological replicates, p<0.0001, two-tailed Fisher’s exact test) and the OS length (mean OS length in rescued larvae 3.8+/-0.25 μm, p<0.0001, Student’s t-test, n = 10 larvae) (Bachmann-Gagescu et al, co-submission). In addition, the specificity of the observed phenotypes is further confirmed by the fact that a second morpholino against ninl targeting the splice site at the intron14/exon15 junction causing aberrant splicing led to similar phenotypes, including ventriculomegaly, expanded melanophores (4 ng/nl, n = 200) and shortened photoreceptor outer segments as compared to control MO-injected larvae. Finally, using an anti-ninl antibody, we observed substantial decrease of Ninl protein on Western blots and on immuno-histochemistry of retinal cryosections for the ninl atgMO-injected larvae and milder decrease in both assays for the ninl ex15 spMO-injected larvae (Bachmann-Gagescu et al, companion manuscript).
Fig. 3. Morphological, functional and epistatic effects of ninl and dzank1 knockdown in zebrafish retina. 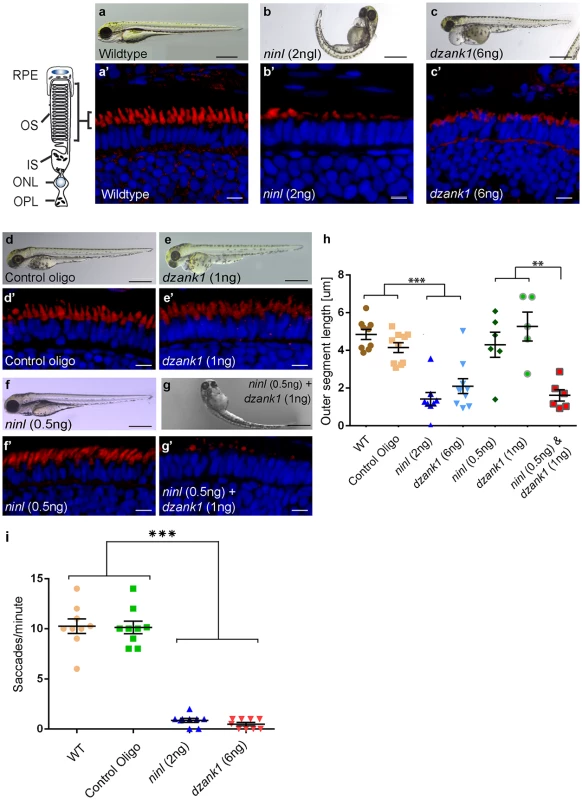
(a-c) Images of 4 dpf living zebrafish. Un-injected controls (WT) appear morphologically normal (a), while embryos injected with 2 ng of ninl atgMO display morphological defects, including ventrally curved body axis and small eyes (b). Embryos injected with 6 ng dzank1 ex8 spMO resulted in expanded melanophores, small eyes and severe pericardial edema (c) (a’-c’) Retinal histology of 4 dpf zebrafish morphants examined by cryo-sections, where bodipy highlights the OS (red) and nuclei are stained with DAPI (blue) in all panels. Outer segments were shortened and dysmorphic in ninl and dzank1 morphants compared to wildtype larvae. (d-g) Ninl interacts genetically with dzank1. Injection of sub-effective dzank1 (1 ng/nl) MO (e’) or ninl (0.5 ng/nl) MO (f’) shows normal OS shape and length in morphologically normal appearing larvae, which could not be distinguished from un-injected embryos (WT) or control MO-injected larvae (d’). Combined injection of sub-effective concentrations of ninl (0.5 ng/nl) and dzank1 (1 ng/nl) MO together results in almost complete absence of OS (g’). (h) Quantification of Outer Segment length, shown as a scatter graph where each datapoint represents the mean OS length in one larva, revealed a significantly decreased length of outer segments in ninl (2ng/nl), dzank1 (6ng/nl) and ninl/dzank1 double morphants as compared to controls. Bars represent the mean value for each treatment group with the Standard error of the mean (SEM) ***P<0.0001, ** P<0.001, unpaired Student’s t-test. (i) Analysis of the Opto Kinetic Response (OKR) showing severely decreased responses in larvae injected with 2 ng ninl atgMO or 6 ng dzank1 ex8 spMO (*** p<0.0001, Student’s t-test). Scale bars represent 500 μm (a-c and e-h) and 15 μm (a’-c’ and e’-h’). RPE, retinal pigment epithelium; OS, Outer Segment; IS, Inner Segment; ONL, Outer Nuclear Layer; OPL, Outer Plexiform Layer. Injection of increasing amounts of a splice-blocking morpholino targeting dzank1 exon8 resulted in small eyes and severe pericardial edema at 4 dpf (S1a Fig, Fig 3c). In addition, dzank1 morphants showed impaired ambulatory activity as predicted before [20]. Based on the incidence of the observed phenotypes and non-quantitative RT-PCR analysis on RNA from morphant larvae (harvested at 2 and 4dpf; S1a and S1g Fig.) we determined that the optimal dose of the dzank1 ex8 spMO was 6 ng/nl. Co-injection of 150 pg/nl capped MO-resistant mRNA encoding human DZANK1 with 6 ng/nl dzank1 ex8 spMO rescued the observed ambulatory activity phenotype (n = 105, 2 biological replicates; S1b and S1e Fig). This observed ambulatory activity phenotype could be fully recapitulated by a second splice blocking morpholino targeting dzank1 exon4 (6 ng/nl, n = 162 out of 200 injected larvae from 2 biological replicates; S2 Fig), further indicating the specificity of the used morpholino. Subsequently, we evaluated the retinal morphology of dzank1 ex8 spMO-treated larvae (4 dpf; n = 40). While retinal lamination was unaffected, significantly shortened photoreceptor outer segments (OS) were observed as compared to controls, as highlighted by boron-dipyrromethene (bodipy) staining of membranes (Fig 3a’–3c’and 3h) (mean OS length 2.1+/-0.39 μm in morphants compared to 4.9+/-0.27 μm in wild-type, p<0.0001, unpaired Student’s t-test, n> 10 larvae from each group in each of 2 biological replicates). The observed photoreceptor outer segment defects fully coincided with the ambulatory activity defects observed in dzank1 ex8 spMO-treated larvae and could be rescued as well (mean OS length in rescued larvae 3.0+/-0.36 μm as compared to 1.9+/-0.25 μm in dzank1 morphants (P = 0.01, unpaired Student’s t-test, n = 13 larvae each group) and 3.5+/-0.25 μm in uninjected larvae (P = 0.3 (NS), unpaired Student’s t-test, n = 12 larvae)(S1f Fig). These phenotypes could also be recapitulated by a second splice blocking MO targeting ninl ex15 or dzank1 ex4 (Bachmann-Gagescu et al., companion manuscript; S2 Fig). These observations are indicative for an essential role of both Ninl and Dzank1 in OS formation and/or maintenance.
To investigate whether there is a functional relationship between Ninl and Dzank1 in this process, sub-effective doses of dzank1 ex8 spMO (1 ng/nl) and ninl atgMO (0.5 ng/nl) were co-injected. While single injections with these sub-phenotypic amounts of MOs caused no discernible phenotypes (no body curvature defects or abnormal swimming behavior for ninl atgMO-injected larvae or no defects in ambulatory activity in dzank1 ex8 spMO-injected larvae) (n = 200/group, 2 biological replicates; Fig 3e and 3f)), co-injection of both MOs resulted in a severely enhanced phenotype including defects in swimming behavior, small eyes and curved tails (Fig 3d–3g). Furthermore, photoreceptor outer segment length measurements on transverse sections of bodipy-stained retina demonstrated significantly impaired OS formation in double morphants (ninl atgMO 0.5 ng/nl + dzank1 ex8 spMO 1ng/nl; mean OS length 1.6+/-0.3 μm, n = 6), as compared to low dose single ninl atgMO-injected (0.5 ng/nl;mean OS length 4.3+/-0.66 μm, n = 6) and dzank1 ex8 spMO-injected larvae (1ng/nl; mean OS length 5.2+/-0.76 μm, n = 5), and as to control MO-injected (mean OS length 4.2+/-0.26 μm, n = 10) and uninjected (WT) larvae (mean OS length 4.8+/-0.28 μm, n = 9) (Fig 3a’, 3d’, 3e’, 3f’, 3g’ and 3h; P<0.001, unpaired Student’s t-test for pairwise comparison between double morphants and each low dose single morphant). These retinal defects in double morphants were similar to those quantified in larvae injected with the optimal doses of dzank1 spMO (6 ng/nl; 2.1+/-0.39 μm, n = 10; Fig 3c’ and 3h) or ninl atgMO (2 ng/nl; 1.6+/-0.26 μm, n = 8; Fig 3b’ and 3h) alone. The functional impact of these morphological changes in the morphant retinas was confirmed by measurements of the optokinetic response (OKR) [21, 22], which tests the ability of zebrafish larvae to visually track rotating stripes [23]. At 4 dpf, ninl and dzank1 morphant larvae displayed a grossly impaired OKR response (0–2 saccades per minute), in contrast to the normal OKR response (5–15 saccades per minute) of control morphants and un-injected larvae from the same clutch (mean number of saccades per minute = 10.3 +/ - 0.7 in uninjected wild-type, 10.1 +/ - 0.6 in control oligo injected, 0.5 +/ - 0.2 in dzank1 morphant and 0.9 +/ - 0.2 in ninl morphant larvae, n = 9/group; P<0.0001 for pairwise comparisons wildtype/control oligo vs each morphant, unpaired Student’s t-test) (Fig 3h). Since spontaneous eye movements in the absence of a stimulus were occasionally registered in all MO-injected larvae, the loss of OKR response is not due to a defect in ocular muscular contraction, but to impaired visual function.
Knockdown of ninl and dzank1 leads to vesicle accumulation in photoreceptor cells
To obtain a more detailed insight into the role of Ninl and Dzank1 in OS morphogenesis, the retinal ultra-structure of ninl, dzank1 morphants and control larvae was studied by transmission electron microscopy (TEM) (for numbers see S5 Table). In control MO-injected embryos (4 dpf), the photoreceptor inner segments (IS) displayed compact ellipsoid regions with clustered mitochondria, a narrow myoid with a Golgi apparatus, while OS presented well-organized, nicely stacked disc structures (Fig 4a), as described for wild-type uninjected larvae [17, 24]. In contrast, in both ninl and dzank1 morphants, IS malformations were found including swollen Golgi complexes with enlarged and distended cisternae, accumulation of vesicle-like structures throughout the IS, large vacuoles and dispersed mitochondria. Occasionally, lysosomal structures were observed (Fig 4e’). OS were either absent, or disrupted. Ultra-structural characteristics of deviant OS were hampered elongation, accumulation of vesicles, polarization defects and deformed discs (Fig 4d and 4e’). Statistical analyses of these defects were based on quantification of the proportion of photoreceptor cells presenting with vesiculated inner segments in the different experimental groups (Fig 4i). No significant difference could be demonstrated between vesiculation in IS of larvae injected with sub-phenotypic doses of dzank1 (1 ng/nl) or ninl (0.5 ng/nl) MO (Fig 4f and 4g) as compared to control MO-injected (10 ng/nl) or wildtype (WT) zebrafish larvae. The percentage of IS in photoreceptors with vesicular structures and/or swollen Golgi complexes in dzank1 (6 ng/nl MO), ninl (2 ng/nl MO) as well as combined (dzank1 1 ng/nl and ninl 0.5 ng/nl) MO-injected zebrafish groups was significantly increased compared to WT and control MO-injected larvae (P<0.01, Student’s t-test). The most significant vesicular increase was observed in the combined MO-injected (dzank1 and ninl) group (Fig 4h and 4h”) (P<0.001, Student’s t-test).
Fig. 4. dzank1 and ninl knockdown leads to accumulation of vesicles and vacuoles in zebrafish photoreceptors. 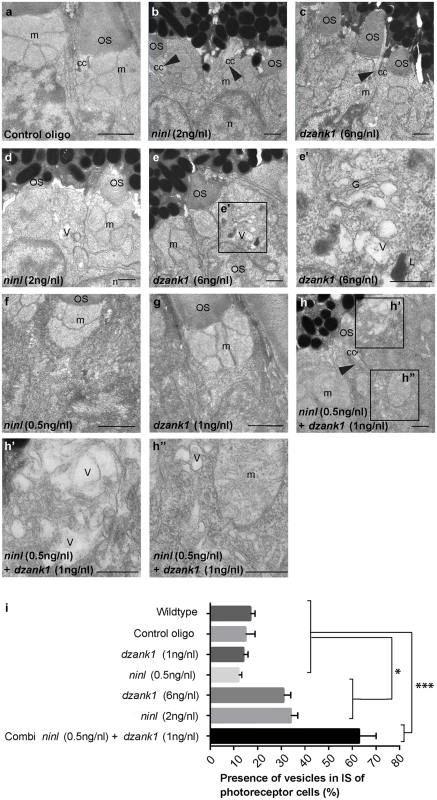
Transmission electron microscopy of control (a), ninl knockdown larvae (b,d) and dzank1 knockdown larvae (c,e). Ninl and dzank1 morphants (b-e) demonstrate absent or shortened and dysmorphic outer segments (OS), whereas connecting cilia (CC) are still intact (arrowheads in b-c,h), and accumulation of vesicular structures (v), highlighted in the boxed area in e (e’). Sub-effective concentration of ninl (f) and dzank1 (g) MO-injection leads to a normal phenotype compared to control MO-injected larvae (a) and uninjected controls. Injection of combined sub-effective concentrations of dzank1 and ninl (h) MO leads to increased vesicle accumulation in OS (h’) and inner segment (h”). (i) Analysis of the presence of vesicles in inner segments of photoreceptor of cells (%). On the Y—axis the different classes are indicated (minimum of 6 eyes per group). (* P<0.01 and *** P<0.001 Student’s t-test). Larvae in all panels are 4 dpf. Scale bars represent 0.5 μm. OS: outer segment, CC: connecting cilium, m: mitochondria, n: nucleus, v: vesicular structures, G: Golgi system, L: lysosome. NINL and DZANK1 associate specifically with complementary subunits of the cytoplasmic dynein 1 motor complex
Mass spectrometry (MS)-based quantitative proteomics was employed to gain further insights into the molecular basis of the defects observed in ninl and dzank1 morphants. N-terminally fused Strep/FLAG-tagged NINLisoA/B and DZANK1 together with their associated, native protein complexes were tandem-affinity-purified from HEK293T cells and the complex components identified by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Besides six actin-binding proteins (ARP1, ARP1B, ARP10, CAPZA1, CAPZA2 and CAPZB) and three subunits of Ca2+/calmodulin-dependent protein kinase II (CaMKII) (CAMK2A, CAMK2D, and CAMK2G), the NINL-associated interactome contained multiple subunits of the cytoplasmic dynein 1-dynactin motor complex (DYNC1H1, DYNC1LI1, DYNC1LI2, DYNCI2, DYNLRB1, DCTN1-4, and DCTN6), which is involved in minus end–directed, microtubule-associated transport (Fig 5a, S2 Table). These results were confirmed by the GST pull-down from bovine retinal extracts in which DZANK1, several subunits of the cytoplasmic dynein 1-dynactin motor complex (DYNC1H1, DYNC1LI1, DCTN1, DCTN2 and DCTN4) and five dynactin-associated actin-binding proteins (ARP1B, ARP10, CAPZA1, CAPZA2 and CAPZB) were found to associate with GST-fused NINLisoB_aa538-825 (S1 Table). We thus confirmed the previously described association of NINL with several subunits of the cytoplasmic dynein 1-dynactin motor complex [19]. Intriguingly, the DZANK1-associated protein complex exclusively contained the two cytoplasmic dynein 1 light chains, DYNLL1 and DYNLL2, which were absent from the NINL interactome. We confirmed the identified interaction between DZANK1 with DYNLL1 and DYNLL2 by reciprocal co-IP experiments in HEK293T cells. In addition, co-expression of mRFP-tagged DYNLL1 or DYNLL2 with eCFP-tagged DZANK1 in ciliated hTERT-RPE1 cells resulted in recruitment of the latter to the basal body and accessory centriole (S3 and S4 Figs). These results suggest that the cytoplasmic dynein 1 motor complex is composed of at least two sub-complexes: one DZANK1-associated sub-complex containing DYNLL1 and DYNLL2, and another NINL-associated sub-complex containing at least DYNC1H1, DYNC1LI1, DYNC1LI2, DYNCI2 and DYNLRB1. In order to better understand the orchestration and dynamics of the NINL-associated motor complex, we performed an elution profile analysis of SDS-induced sub-complexes by quantitative mass spectrometry (EPASIS) [25]. SF-TAP-tagged NINLisoA/isoB was over-expressed in HEK293T cells and affinity-purified using the FLAG moiety of the fusion tag. The native protein complex was sequentially treated with increasing SDS-concentrations to destabilize the interactions and thereby induce the elution of sub-complexes. Besides the dynactin submodule, which showed the most stable association with the NINL complex (S5c Fig; complete elution at ≥ 0.005% SDS) a second sub-module consisting of proteins from the cytoplasmic dynein 1 motor complex [26] was identified (S5c Fig c; elution between 0.001 and 0.01% SDS). This dynein sub-module may dissociate in two additional sub-modules but this finding lacked statistical significance (S5–S8 Figs, S3 and S4 Tables). In addition, CLIP1 (= CLIP-170), PAFAH1B1 (= LIS1), ACAD11 and MRPS27 co-eluted with the dynein module, making them potentially novel candidate components of the dynein 1 motor complex.
Fig. 5. NINL and DZANK1 associate specifically with complementary subunits of the cytoplasmic dynein 1 motor complex. 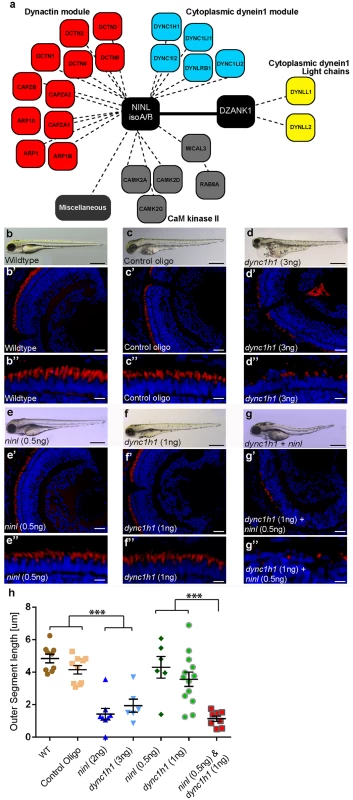
(a) Strep-SILAC and TAP experiments show that DZANK1 interacts specifically with DYNLL1 and DYNLL2 (yellow). NINL associates with components of the dynactin complex (red) and with most components of the dynein 1 motor complex (blue), except for DYNLL1 and DYNLL2. The solid line between NINL and DZANK1 symbolizes a direct interaction, whereas the dashed lines stand for interactions determined by immune and affinity purifications. (b-g”) Genetic interaction between ninl and dync1h1. MO-injection with a high concentration of dync1h1 (3 ng/nl) shows larvae with pericardial edema, small eyes and short OS (d-d”). MO-injection of sub-effective concentrations of ninl (0.5 ng/nl) (e-e”) or dync1h1 (1 ng/nl) (f-f”) generates larvae with a normal phenotype and normal OS length, compared to wildtype larvae (b-b”) and control MO-injected larvae (c-c”). The combination of sub-effective MO concentrations of ninl (0.5 ng/nl) and dync1h1 (1 ng/nl) results in short larvae and virtual absence of outer segments (g-g”). All larvae are 4 dpf. Panels b’-d” and e’-g” are retinal cryo-sections stained with bodipy (red) to highlight the outer segments and DAPI to stain the nuclei. (h) Quantification of photoreceptor outer segment length revealed a significantly decreased length of outer segments in dync1h1 (3ng/nl) and dync1h1/ninl double morphants as compared to controls (*** P<0.0001; two-tailed, unpaired Student’s t-test). Scale bars represent 500 μm (b-g), 50 μm (b’-g’) and 15 μm (b”-g”). Zebrafish Ninl and Dzank1 are required for intracellular dynein-based transport
Dynein 1 heavy chain 1 (dync1h1), encoding a subunit of the cytoplasmic dynein 1 motor complex which mediates minus-end-directed post-Golgi vesicle trafficking towards the basal body, is mutated in the zebrafish cannonball mutant [17]. Similar to our findings in ninl and dzank1 morphants, cannonball mutants form short rudimentary photoreceptor OS, show organelle positioning defects and display a severe accumulation of vesicles in the photoreceptor IS and OS and this phenotype could be recapitulated by a translation blocking morpholino against dync1h1 [17]. Sub-effective concentrations of this previously published dync1h1 MO (1 ng/nl) [17] in combination with the ninl atgMO (0.5 ng/nl) were co-injected and compared to single injected morphants, control MO-injected larvae and uninjected controls (n = 300/group from 2 biological replicates). At 4 dpf, quantification of OS lengths in each group was performed on bodipy-stained cryosections and revealed significantly shortened OS in larvae in the dync1h1 (3 ng/nl; mean OS length 1.95+/-0.4 μm, n = 5; Fig 5d’, 5d” and 5h) and dync1h1/ninl morphant groups (1ng/nl + 0.5 ng/nl; mean OS length 1.413+/-0.2 μm, n = 13; Fig 5g’, 5g” and 5h) as compared to uninjected controls (mean OS length 4.8+/-0.27 μm, n = 9; Fig 5b’, 5b” and 5h), control MO-injected (10 ng/nl; mean OS length 4.2+/-0.26 μm, n = 10; Fig 5c’, 5c” and 5h) and to both low-dose single dync1h1 MO-injected (1 ng/nl; mean OS length 3.6+/-0.44 μm, n = 13; Fig 5f’, 5f” and 5h) and ninl atgMO-injected (0.5 ng/nl; mean OS length 4.3+/-0.66 μm, n = 6; Fig 5e’, 5e” and 5h) groups (P<0.001; two tailed, unpaired Student’s t-test on pairwise comparisons between double morphants and each of the single low-dose morphants), demonstrating a genetic interaction between ninl and dync1h1 and supporting a role for Ninl in cytoplasmic dynein 1-driven intracellular vesicle transport. In addition, localization of rhodopsin, which should be restricted to the OS, was aberrantly observed in the photoreceptor cell body in both ninl and dzank1 morphants (S9 Fig and Bachmann-Gagescu et al. co-submission.).
The observed mislocalization of rhodopsin in these morphants could be explained by defects in intracellular transport. However it could also be the result of defects in OS development and a consequence of the absence of this structure. Although the OS of ninl morphants are severely affected, the connecting cilium of photoreceptor cells in ninl, dzank1 and ninl-dzank1 double morphants was found to be intact (arrow heads in Fig 4b, 4c and 4h), providing the opportunity to test the localization of proteins to this ciliary compartment regardless of the presence of an outer segment. USH2A, a previously published interaction partner of NINL, is a transmembrane protein that is synthesized in the IS and subsequently transported in TGN-derived vesicles towards the base of the connecting cilium. USH2A was previously shown to localize in the peri-ciliary region, which should be independent of the presence of the OS [27]. Therefore, a defect in USH2A localization would likely be due to impaired post-Golgi dynein-based trafficking. Using a zebrafish-specific anti-Ush2a antibody, we observed a strongly reduced immunofluorescent signal in ninl and dzank1 morphants (4 dpf), as compared to control MO-injected larvae (4 dpf) (Fig 6a’–6d’). The localization of centrin, which was used as a marker for the connecting cilium, was unaffected in both controls and morphants (Fig 6a”’–6d”’). Together, these data indicate a defect in the transport of vesicle-bound transmembrane proteins in ninl and dzank1 morphants.
Fig. 6. Impaired Ush2a transport in dzank1 and ninl morphants. 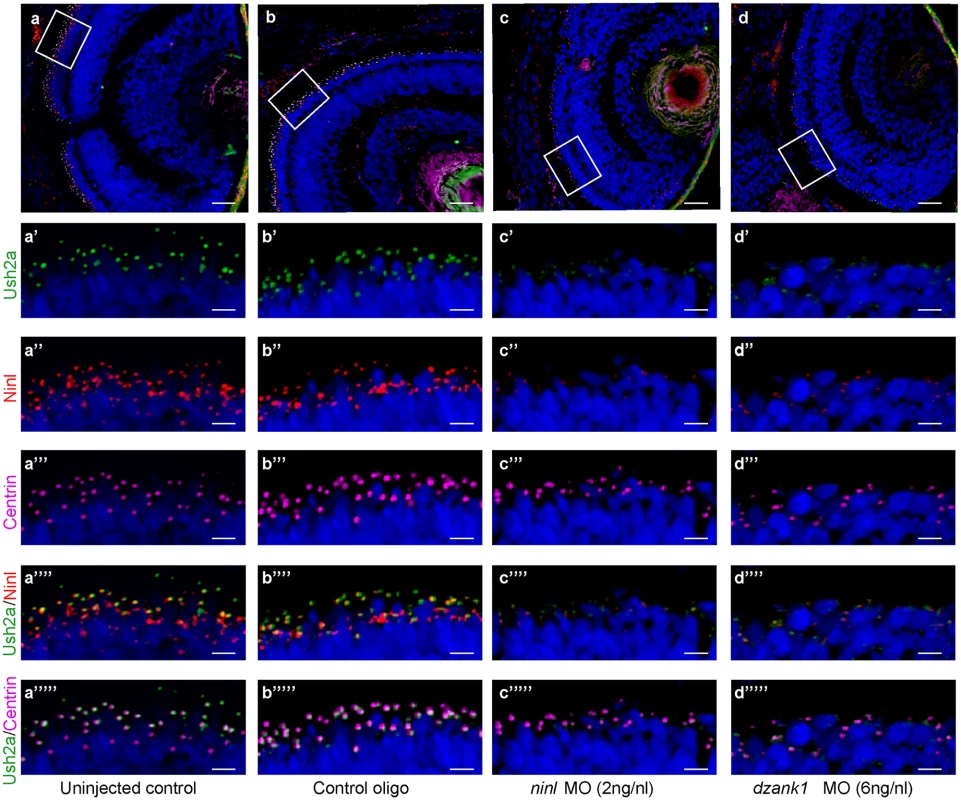
(a-d) Overview of Ninl and Ush2a localization in cryo-sections of 4 dpf zebrafish retina using anti-Ninl (red signal), anti-Ush2a (green signal) and the basal body marker anti-Centrin (cyanid signal). In all images the nuclei are counterstained with DAPI (blue signal). (a-a”“‘) Un-injected wildtype, (b-b”“‘) 10 ng/nl control MO-injected, (c-c”“‘) 2 ng/nl ninl MO-injected and (d-d”“‘) 6 ng/nl dzank1 MO-injected zebrafish retinas. (a’-d’) Anti-Ush2a staining (green signal) is strongly reduced in dzank1 and ninl morphants (c’-d’), while Ush2a is clearly present in wildtype and control MO-injected larvae. (a”-d”). Specific Ninl-immunofluorescence (red signal) was largely abolished in ninl morphants and reduced in dzank1 morphants. (a”‘-d”‘) Centrin (cyanide signal) was observed in wildtype, un-injected control and in both ninl and dzank1 morphants. (a”“-d”“) Co-localization of Ush2a and Ninl (yellow signal) was observed in wildtype and control MO-injected larvae. (a”“‘-d”“‘) Co-localization of Ush2a and Centrin (yellow signal) was seen in all images (WT, Control Oligo, ninl and dzank1 morphants), despite strong reduction of Ush2a immunofluorescence in ninl and dzank1 morphants. Scale bars represent 15 μm, except for (a-d) in which the scale bars represent 50 μm. To determine whether Ninl and/or Dzank1 are required for other dynein-based intracellular transport processes, we monitored dynein 1-mediated melanosome transport in zebrafish skin. Zebrafish alter their skin pigmentation by trafficking melanosomes within melanophores. The melanosome, a lysosome-related organelle, can be shuttled bi-directionally between the cell periphery and the peri-nuclear region by two microtubule-based molecular motors, kinesin ll (anterograde) and dynein 1 (retrograde). Pigment aggregation (retrograde transport) can be stimulated within minutes upon treatment with epinephrine [28]. In the melanosome transport assay, 5 dpf larvae are dark-adapted to display maximum melanophore dispersion (Fig 7a). After addition of epinephrine, the melanosomes rapidly contract and can be visually evaluated for reduction in pigment dispersion (Fig 7a’–7a”). The endpoint is apparent when pigmentation pattern reflects peri-nuclear accumulation of melanosomes (Fig 7a’). A rapid melanosome contraction was seen in control MO-injected and un-injected larvae (Fig 7a–a” and 7d) (ΔT = 1.55 min and ΔT = 1.30 min respectively; n = 20/group), whereas ninl and dzank1 morphants demonstrated significantly delayed dynein 1-mediated melanosome retraction (ΔT = 15.42 min and ΔT = 23.62, n = 20; P<0.001; Student’s t-test (two tailed, unpaired), Fig 7b–7c”’ and 7d), which is indicative for impaired dynein 1-mediated retrograde transport. Taken together, our findings suggest that the Ninl-Dzank1-cytoplasmic Dynein 1 complex is required for the intracellular transport of organelles and vesicles, and is essential for the photoreceptor’s OS formation, maintenance and function.
Fig. 7. ninl and dzank1 knockdown results in epinephrine-induced melanosome retraction delay. 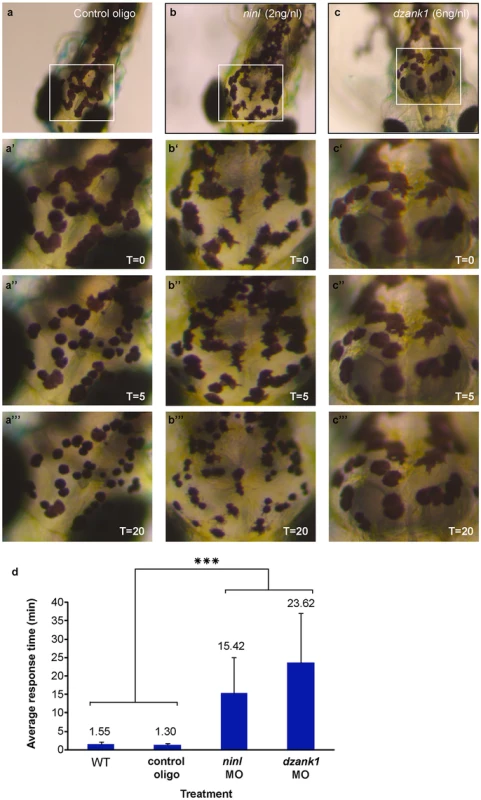
Control MO-injected larvae (10 ng/nl; n = 20) at 5 dpf (a-a”‘), ninl morphant (2 ng/nl; n = 20) (b-b”‘) and dzank1 morphant (6 ng/nl; n = 20) (c-c”‘). White Box denotes the area at higher magnification (40x) (a’-c”). (a-c’) Melanosome pattern of the different larvae before treatment, (a”-c”), 5 min after epinephrine addition and (a”‘-c”‘) 20 min after epinephrine addition, t represents time in minutes. (d) Graphical representation with error bars (standard deviation) demonstrating a significant delay of epinephrine-induced melanosome retrograde trafficking compared with wild-type and control MO-injected (10 ng/nl) larvae. Treatment (control, ninl- or dzank1-morphants) is noted on the x-axis and average response time in minutes is noted on the Y-axis. ***: P<0.001 (two-tailed, unpaired Student’s t-test). Discussion
In this study, a central role for NINL and its novel interaction partner DZANK1 is identified in vesicle transport in photoreceptor cells. We demonstrate that NINL and DZANK1 associate with complementary subunits of the cytoplasmic dynein 1 motor complex and that this complex consists of at least two submodules. In photoreceptor cells, this motor complex has been implicated in post-Golgi vesicle trafficking and organelle positioning [14, 17]. In line with this function, in vivo studies in the zebrafish demonstrate defects in post-Golgi trafficking as revealed by delayed cytoplasmic dynein 1-regulated melanosome transport, defective photoreceptor outer segment formation, abnormal vesicle accumulation within the photoreceptor inner segments and mislocalization of rhodopsin and Ush2a in both ninl and dzank1 morphants.
Cytoplasmic dynein 1 is fundamentally important for embryonic development. Dynein 1 heavy chains are essential for the formation of the motor complex and their absence leads to very early embryonic lethality in mice before E8.5 [29]. In contrast, larvae of the zebrafish cannonball (cnb) mutant lacking Dync1h1 undergo relatively normal early development and remain indistinguishable from wild-type siblings until approximately 3.5 dpf. This remarkable difference between mouse and zebrafish mutants may be explained by the presence of wild-type maternally-derived (yolk-associated) mRNA in zebrafish embryos [30]. Cnb larvae eventually show a reduced eye size, present with small rudimentary photoreceptor outer segments and expanded skin melanophores, show severe organelle positioning defects and die between 6 and 8 dpf [17]. The phenotype of zebrafish depleted for Ninl is remarkably similar to that of the cnb mutant, with small eyes, mispositioned organelles, retinal dystrophy, and expanded melanophores. These overlapping phenotypic characteristics in combination with the observed genetic interaction in the retina between ninl and dync1h1 suggest that the ninl morphant phenotype is caused by dysfunctional cytoplasmic dynein 1-mediated transport.
Mice lacking Dync1li1, a light-intermediate chain subunit of dynein 1, which is structurally less important than heavy chains, survive into adulthood [31]. Their photoreceptors do, however, lack outer segments due to blocked transport of TGN-derived vesicles towards the basal body. In contrast to the ninl morphants, dzank1 morphants show a much milder phenotype and do not present curved bodies or major early developmental defects. DZANK1 associates specifically with two structurally less important dynein 1 light chains, which might explain the less severe phenotype of dzank1 morphant zebrafish larvae. Nonetheless, photoreceptor cells of both ninl and dzank1 morphant zebrafish larvae display severely shortened outer segments, disruption of mitochondria organization and accumulation of vesicles within the inner segments. Given that photoreceptors have extremely high transport requirements due to the significant daily renewal of their outer segments, they are predicted to be more sensitive to defects in intracellular transport, which could explain why loss of dzank1 results predominantly in a photoreceptor phenotype.
The physical position and role of NINL and DZANK1 in the cytoplasmic dynein 1 motor complex remains unknown. Up to now, tandem affinity purification assays from HEK293T cells using different subunits of this motor complex as bait never identified peptides of NINL or DZANK1 (S2 Table). The most likely explanation for this is that NINL and DZANK1 are not expressed in these cells or are expressed only at very low levels. To get the first insights into the dynamics and orchestration of the NINL-associated dynein 1 motor complex, we performed an elution profile analysis of SDS-induced sub-complexes by quantitative mass spectrometry (EPASIS). Despite the fact that the dynein module shows a tendency of being built up of two distinct sub-modules, the statistical significance is lacking. CLIP1 (= CLIP-170), PAFAH1B1 (= LIS1), ACAD11 and MRPS27 co-eluted with the dynein module, making them potentially novel candidate components of the dynein 1 motor complex. LIS1 was previously found to interact with cytoplasmic dynein 1-dynactin [32] and CLIP1 [33] in order to keep dynein in a persistent microtubule-bound state [34]. The role of ACAD11 and MRPS27 in dynein 1 function or dynamics needs to be determined. The dynactin complex was identified as a distinct sub-complex which was most strongly associated with NINL in the EPASIS essay. The lack of clear OS defects in zebrafish mok/dctn1a and dctn1b mutants [17, 35, 36] however, implies that dynein 1 functions independently of dynactin in outer segment morphogenesis. Indeed it was reported that the binding of dynactin1 to dynein 1 is non-essential for the ability of dynein 1 to bind stably to rhodopsin transport vesicles in vitro [14]. Therefore it can be concluded that the ocular phenotypes observed in the ninl morphant are most likely caused by a non-functional dynein 1 complex rather than dysfunction of the dynactin complex.
We previously described the association of NINLisoB with USH2A [18], a protein known to be essential for photoreceptor homeostasis in mice [27, 37]. The retinal defects observed in ninl morphants are more severe than those observed in the Ush2a knockout mouse model, which displays intact outer segments and late-onset mild photoreceptor degeneration [27]. This comparison suggests a transport function for NINLisoB upstream of USH2A towards the apical inner segment. The absence of Ush2a at the photoreceptor periciliary region of ninl morphants is in line with this hypothesis.
DZANK1 protein sequence analysis predicted the presence of two ZNF_RBZ domains and their interaction with RanGDP rather than their involvement in transcription. Ran is an abundant Ras-like GTPase, which plays a role in multiple cellular processes, including modulation of nucleo-cytoplasmic transport of macromolecules larger than ~40 kDa across the nuclear envelope [38]. Further, it has been proposed that a similar complex, consisting of Ran, Ran-binding proteins and importins/exportins plays a role in regulating import of cargo at the base of the cilium [39] and that RanBP2 is involved in processing or transport of opsin [40]. Since the ZNF domains of DZANK1 are highly homologous to the functional domains of RanBP2, DZANK1 might be involved in transport of opsin as well. The observed mislocalization of rhodopsin in photoreceptor cells of dzank1 morphant zebrafish larvae is in line with this. Moreover, a role for DZANK1 in opsin transport and subsequent ciliary entry creates an attractive functional connection between DZANK1 and the Usher protein network, members of which have been suggested to act in opsin vesicle docking at the periciliary region and subsequent transport in the connecting cilium and calyceal processes [41, 42].
In summary, our study provides a deeper insight into the tissue-specific dynamics of the cytoplasmic dynein 1 motor complex, and supports an essential role for this complex in close connection to NINL and DZANK1 in post-Golgi vesicle transport of selective cargo in zebrafish photoreceptor cells.
Methods
Ethics statement
Animal experiments were conducted in accordance with the Dutch guidelines for the care and use of laboratory animals, with the approval of the Animal Experimentation Committee (Dier Experimenten Commissie [DEC]) of the Royal Netherlands Academy of Arts and Sciences (Koninklijke Nederlandse Akademie van Wetenschappen [KNAW] (Protocol # RU-DEC 2012–301).
Yeast two-hybrid interaction assay
To identify the interacting regions between DZANK1 and NINLisoA/B, a GAL4-based Y2H screen (HybriZAP, Stratagene, La Jolla, CA, USA) was used as previously described [43, 44]. Accession IDs: NINLisoA (Q9Y2I6), NINLisoB (XP_005260736), DZANK1 (Q9NVP4), DYNLL1 (NP_001032584), DYNLL2 (Q96FJ2).
Localization studies in cells
The cellular (co)-localization of DZANK1, NINLisoB, DYNLL1 and DYNLL2 was determined by co-transfecting hTERT-RPE1 cells on glass slides, with pcDNA3-mRFP and pcDNA3-eCFP. DZANK1 FL, NINLisoB, DYNLL1 and DYNLL2 were transfected using Effectene Transfection Reagent (Qiagen, Netherlands) according to manufacturer’s instructions. After 48 hours transfection, cells were washed with PBS, fixed with 4% paraformaldehyde (PFA) and mounted with Vectashield containing DAPI (Vector Laboratories, Inc., UK). Images were taken with an Axioplan2 Imaging fluorescence microscope (Zeiss) and processed using Adobe Photoshop version 8.0 (Adobe Systems, USA).
GST pull-down
The GST-fusion proteins were produced by transforming Escherichia coli BL21-DE3 with plasmid pDEST15-NINL (538aa to 825aa), as previously described [43]. Strep/FLAG-tagged DZANK1 or Strep/FLAG-tagged NINLisoB were produced by transfecting HEK293T cells with plasmids encoding N-SF-TAP-hsDZANK1 or NINLisoB, respectively, using the transfection reagent (PEI; Polyethylenimine), according to the manufacturer's instructions. The GST pull-down assay was performed as described previously [43]. For GST pull-down experiments from retinal extracts, retinas were dissected from fresh bovine eyes obtained from the local slaughter house. Retinas were homogenated by sonication on ice for two times 30 s in extraction buffer [10 mM HEPES (pH 7.9), 10 mm NaCl, 3 mm MgCl2, freshly added 1 mm DTT, 1 mm Na3VO4], supplemented with complete protease inhibitor cocktail (Roche Diagnostic). Retinal extracts were incubated overnight at 4°C with the GST fusion proteins immobilized on glutathione sepharose 4B beads. GST fusion proteins were eluted from the glutathion sepharose 4B beads with 100 mM reduced Glutathione (GSH) in 50mM TRIS-HCl (pH 8.0) overnight. Proteins were subsequently precipitated and analyzed by mass spectrometry analysis as described below.
Co-immunoprecipitation in HEK293T cells
HA-tagged NINL and DYNLL1 were expressed by using the mammalian expression vector pcDNA3-HA/DEST, the 3xHA-tagged DYNLL2 by using p3xHA_CMV/DEST, Strep/FLAG-tagged DZANK1 by using pSF-NTAP/DEST and LRRK2 by using p3xFLAG/DEST from the Gateway cloning system (Invitrogen, USA). HEK293T cells were co-transfected, using Effectene Transfection Reagent (Qiagen, USA) according to the manufacturer’s instructions. Twenty-four hours after transfection, the cells were washed with PBS and subsequently lysed in IP lysis buffer (50 mM Tris-HCL pH 7.5, 150 mM NaCl, 1% Triton-X-100 supplemented with complete protease inhibitor cocktail (Roche, Germany)) on ice. HA-tagged molecules were immune-precipitated from cleared lysates at 4°C overnight. Protein-antibody complexes were coupled to ProtA/G beads (Santa Cruz) for 2 hours at 4°C. After incubations, the beads were pelleted and washed three times with lysis buffer. Beads were boiled and proteins were resolved on SDS-PAGE. For western blotting, proteins were electrophoretically transferred onto nitrocellulose membranes, blocked with 5% non-fat dry milk (Biorad) in PBS-T (PBS supplemented with 0.1% Tween) and analyzed with the appropriate primary and secondary antibodies in 0.5% non-fat dry milk in PBS-T. After 4 washes in lysis buffer, the protein complexes were analyzed on immunoblots using the Odyssey Infrared Imaging System (LI-COR, USA). As secondary antibodies, IRDye800 goat-anti-mouse IgG and Alexa Fluor 680 goat-anti-rabbit IgG were used.
Antibodies
The monoclonal antibodies directed against Centrin (1 : 100), Millipore, lot nr: 04–162 and the polyclonal antibodies directed against the cytoplasmic region of USH2AisoB (1 : 100), Novus Biological, lot nr: T00620A02 have been described previously [43, 45]. For the rhodopsin staining anti-rhodopsin, clone 4D2 Millipore lot nr: 2038649 (1 : 1000) was used.
For Western blot and immunohistochemical analyses, antibodies directed against human NINL (aa406-455) were purchased from LSBio, cat. No. LS-C201509 (1 : 100). Antibodies against the C-terminal region of zebrafish Ninl, which were raised in guinea pigs against a GST-fusion protein, encoding a peptide consisting of 403aa to 591aa (Genbank NP_001268727) were used for immunohistochemical analyses. The cDNA, encoding this peptide was amplified by using the forward and reverse primers 5'-GACCAAGCCTGTCAAGAGCG-3' and 5'-GCCCTGAGACTTCAACAAC-3', respectively. The secondary antibodies were goat anti-guinea pig Alexa Fluor 488 and Alexa Fluor 568, goat anti-rabbit Alexa Fluor 488, goat anti-mouse Alexa Fluor 488, Alexa Fluor 568 and Alexa Fluor 647; (all used at a dilution of 1 : 500, all from Molecular Probes-Invitrogen Carlsbad, CA, USA). The latter were diluted together with DAPI in block buffer (2% BSA and 10% Normal Goat serum in PBS).
Zebrafish
Experimental procedures were conducted in accordance with international and institutional guidelines. Wild type adult Tupfel Long fin (TLF) zebrafish were used. The zebrafish eggs were obtained from natural spawning of wild-type breeding fish. Larvae were maintained and raised by standard methods [46]. Translation-blocking ninl (5’-CATCCTCGTCCATCCCACCACATAC-3’), exon15 splice-blocking ninl (5’ - CCCAACACTAAAGAGATACACCAAT-3’), exon4 splice-blocking dzank1 (5’ - CGGCCATCACTGCATCACATTACAA-3’) exon8 splice-blocking dzank1 (5’ - AGGACATCTTTAGAATGATAGACGT-3’) and translation blocking dync1h1 (5’ - CGCCGCTGTCAGACATTTCCTACAC-3’) morpholinos (MOs) were designed by Gene Tools Inc. (USA) and diluted to the appropriate concentration in deionized, sterile water, supplemented with 0.5% phenol red. To determine the most effective dose of the ninl, dzank1 and dync1h1 MOs, 1 nl of diluted MO (containing 2,4,6,8 and 10 ng) was injected into the yolk of one - to two-cell-stage embryos using a Pneumatic Picopump pv280 (World Precision Instruments). A minimum sample size of 50 larvae was used for every condition. After injection, embryos were cultured in E3 embryo medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, supplemented with 0.1% methylene blue) at 28°C and subsequently phenotyped at 4 dpf (days post fertilization). Injected embryos were classified into two classes of phenotypes based on the relative severity as compared to age-matched, standard control (5’-cctcttacctcagttacaatttatac-3’; Gene Tools Inc, USA) MO-injected (10 ng) embryos of the same clutch. Images were taken with an Axioplan2 Imaging fluorescence microscope (Zeiss, Germany) equipped with a DC350FX camera (Zeiss, Germany). To determine the efficiency of splice-blocking, RNA was isolated from 50 control MO injected and 50 dzank1 - (and ninl-) splice MO-injected embryos (2 dpf) using the RNeasy mini kit (Qiagen) according to manufacturer’s instructions. Here, 500 ng of total RNA was used to produce first-strand cDNA. Reverse transcription was performed using the Superscript III cDNA synthesis kit (Life Technologies) according to the manufacturer’s instructions. Subsequently, PCR analysis was performed. Primers used for the analysis of ninl exon15 morphants are 5’-AAGTATGATGGCCTGGATGC-3’ and 5’-GAGATGTCCTTCCGCTCAAC-3’; primers used for the analysis of dzank1 exon4 morphants are 5’-GGCAGCACCTCAAATAATCC-3’ and 5’-CTGAAGGTCGATGGCTAAGG-3’; primers used for the analysis of dzank1 exon8 morphants are 5’-CTCGCTTGACAGCACAAAAC-3’ and 5’-AAAACAGGTCTGGCTTGTCG-3’. Obtained fragments were extracted from a 1% agarose gel using the Nucleospin gel extraction kit (Machery Nagel, USA) and Sanger sequenced. For histological analysis of zebrafish, larvae were fixed in 4% PFA in PBS at 4°C overnight. Embryos were rinsed with PBS and infiltrated in 10% sucrose solution in PBS for two hours. Embryos were positioned (ventral side downwards) in Tissue Tek (Sakura), rapidly frozen in melting isopropyl alcohol and sections (seven μm thickness along the lens/optic nerve axis) were made. Immunohistochemistry was performed using retina sections, derived from four-to-six day old morphants and age-matched control oligo MO-injected zebrafish. The bodipy staining was performed on 5 day old larvae. The sections were washed twice in PBS for 5 minutes, permeabilized with 0.5% triton-x-100 in PBS for two times 10 minutes and followed by three washing steps with PBS for 5 minutes. Sections were then incubated for 10 minutes with bodipy (1 : 100), DAPI and phalloidin/actin (monoclonal Actin, Abcam lotnr: Ab328–500 (1 : 400)) diluted in PBS. Subsequent photoreceptor outer segment length measurements were performed blinded as to their injection status (using ImageJ). Equivalent single confocal sections through each eye were selected and the outer segments from 10 adajcent photoreceptors were measured and averaged for each larvae. P-values were calculated using Student’s t-test (two tailed, unpaired).
Affinity purifications of protein complexes
HEK293T cells were cultured as described previously [47]. For SILAC experiments, HEK293T cells were grown in SILAC DMEM (PAA), supplemented with 3 mM L-Glutamine (PAA), 10% dialyzed fetal bovine serum (PAA), 0.55 mM lysine and 0.4 mM arginine. Light SILAC medium was supplemented with 12C6, 14N2 lysine and 12C6, 14N4 arginine. Heavy SILAC medium was supplemented with either 13C6 lysine and 13C6, 15N4 arginine or 13C6, 15N2 lysine and 13C6, 15N4 arginine. 0.5 mM proline was added to all SILAC media to prevent arginine to proline conversion. All amino acids were purchased from Silantes. For DNA transfections, HEK293T cells were seeded, grown overnight, and then transfected using PEI.
Tandem affinity purification
HEK293T (human embryonic kidney, ATCC) cells were transfected for 48 hours with either SF-TAP-NINL, SF-TAP-DZANK1, using polyethyleneimine (PEI, Polysciences) as a transfection reagent. Following transfection, cells were lysed in lysis buffer containing 30 mM Tris–HCl (pH 7.4), 150 mM NaCl, 0.5% Nonidet-P40 (NP40), freshly supplemented with protease inhibitor cocktail (Roche), phosphatase inhibitor cocktail II and III (Sigma), for 20 minutes at 4°C. The Streptavidin - and FLAG-based tandem affinity purification steps were performed as previously described [47, 48]. 5% of the final eluate was evaluated by SDS-PAGE followed by silver staining, according to standard protocols, while the remaining 95% were subjected to protein precipitation with chloroform and methanol. Protein precipitates were subsequently subjected to mass spectrometry analysis and peptide identification as previously described [25].
One-step Strep affinity purification
For SILAC experiments, one step Strep purifications of SF-TAP-tagged proteins and associated protein complexes was performed as described earlier [49]. HEK293T cells, transiently expressing the SF-TAP-tagged constructs were lysed in lysis buffer, containing 0.5% Nonidet-P40, protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktails II and III (Sigma-Aldrich) in TBS (30 mM Tris-HCl (pH 7.4), 150 mM NaCl), for 20 minutes at 4°C. After sedimentation of nuclei at 10,000 x g for 10 minutes, the protein concentration was determined by a Bradford assay, before equal amounts of each lysate were transferred to Strep-Tactin-Superflow beads (IBA) and were incubated for one hour at 4°C on an end-over-end shaker. Then, the resin was washed three times with wash buffer (TBS containing 0.1% NP-40, phosphatase inhibitor cocktail II and III). The protein complexes were eluted by incubation for 10 minutes in Strep-elution buffer (IBA). The eluted samples were concentrated using 10 kDa cut-off VivaSpin 500 centrifugal devices (Sartorius Stedim Biotech) and pre-fractionated using 1D-SDS-Page. Afterwards, the samples were subjected to in-gel tryptic cleavage as described elsewhere [50].
Protein complex destabilization
For EPASIS, SF-TAP-tagged NINL was over-expressed in HEK293T cells as described above. After 48 hours, cells were lysed as described for SF-TAP analysis and the cleared lysates were incubated with anti-FLAG-M2 agarose resin for 1h. After three washes with wash buffer (TBS containing 0.1% Tergitol-type NP-40 and phosphatase inhibitor cocktails II and III, Sigma-Aldrich), the resin was incubated at 4°C for three minutes with each concentration of SDS (0.001%, 0.005%, 0.01% and 0.02%) in SDS-elution buffer (TBS containing phosphatase inhibitor cocktails II and III). Afterwards, a final elution step with FLAG peptide (200 μg/ml; Sigma-Aldrich) in wash buffer was performed. After every elution step a single wash step was performed. The flow-through was collected and precipitated by methanol-chloroform, before being analyzed by LC-MS/MS.
Mass spectrometry and data analysis
LC-MS/MS analysis was performed on an Ultimate3000 nano RSLC system (Thermo Scientific) coupled to a LTQ Orbitrap Velos or to an LTQ OrbitrapXL mass spectrometer (Thermo Scientific) by a nano spray ion source. Tryptic peptide mixtures were automatically injected and loaded at a flow rate of 6 μl/min in 0.1% trifluoroacetic acid in HPLC-grade water onto a nano trap column (75 μm internal diameter (i.d.) × 2 cm, packed with Acclaim PepMap100 C18, 3 μm, 100 Å; Thermo Scientific). After five minutes, peptides were eluted and separated on the analytical column (75 μm i.d. × 25 cm, Acclaim PepMap RSLC C18, 2μm, 100 Å; Thermo Scientific) by a linear gradient from 2% to 35% of buffer B (80% acteto-nitrile and 0.08% formic acid in HPLC-grade water) in buffer A (2% aceto-nitrile and 0.1% formic acid in HPLC-grade water) at a flow rate of 300 nl/min over 33 minutes for EPASIS samples, respectively over 80 minutes for SF-TAP and SILAC samples. Remaining peptides were eluted by a short gradient from 35% to 95% buffer B in 5 minutes. The eluted peptides were analyzed by a LTQ Orbitrap Velos or a LTQ Orbitrap XL mass spectrometer. From the high resolution MS pre-scan with a mass range of 300 to 1500, the ten most intense peptide ions were selected for fragment analysis in the linear ion trap if they exceeded an intensity of at least 200 counts and if they were at least doubly charged. The normalized collision energy for CID was set to a value of 35% and the resulting fragments were detected with normal resolution in the linear ion trap. The lock mass option was activated; the background signal with a mass of 445.12002 as lock mass. Every ion selected for fragmentation, was excluded for 20 seconds by dynamic exclusion. Non-quantitative MS/MS data were analyzed, using Mascot (version 2.4, Matrix Science, Boston, MA, USA). Mascot was set up to search the human subset of the Swiss Prot database (Release 2013_12, 20274 entries), assuming trypsin as the digestion enzyme. Mascot was searched with a fragment ion mass tolerance of 0.5 Da and a parent ion tolerance of 10.0 PPM. Oxidation of methionine and was specified as variable modification, iodoacetamide derivative of cysteine as fixed. The Mascot results were loaded in Scaffold (version Scaffold_4, Proteome Software Inc., Portland, OR) to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm [51]. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm [52]. Proteins, which contained similar peptides and could not be differentiated based on MS/MS analysis alone, were grouped to satisfy the principles of parsimony.
For SILAC experiments, all acquired spectra were processed and analyzed, using the MaxQuant software [53] (version 1.3.0.5) and the human subset of the human proteome reference set, provided by SwissProt (release 2012_01 534,242 entries) was used for peptide and protein identification. Cysteine carbamidomethylation was selected as fixed modification, methionine oxidation and protein acetylation were allowed as variable modifications. The peptide and protein false discovery rates were set to 1%. Contaminants like keratins were removed. Proteins, identified and quantified by at least two unique peptides were considered for further analysis. The significance values were determined by Perseus tool (part of MaxQuant), using significance A. Label-free quantification and statistical analysis of the EPASIS data were performed as previously described [25] using MaxQuant (version 1.3.0.5). The human subset of the human proteome reference set, provided by SwissProt (Release 2012_01 534,242 entries) was used for peptide and protein identification. Seven biological replicates with five fractions each were performed for the NINL experiment and three biological replicates for the control experiment, resulting in a total number of 50 individual samples being measured. Proteins had to be present in at least 4/7 (57%) repeated experiments to be considered for further analysis. The reproducibility of the experiments was analyzed as already described [25] and is shown in S6 and S7 Figs To assign the proteins to the pre-defined sub-complexes (S4 Table [26, 54]), the EPD threshold was determined by a stepwise parameter search (n = 1000), which resulted in a value of 0.089 (S8 Fig).
Transmission electron microscopy (TEM)
Zebrafish (4 dpf) were fixed at 4°C overnight in a freshly prepared mixture of 2,5% glutaraldehyde and 2% PFA in 0.1 M sodium cacodylate buffer (pH 7.4). After rinsing in buffer, specimens were post-fixated in a freshly prepared mixture, containing 1% osmium tetroxide and 1% potassium ferrocyanide in 0.1 M sodium cacodylate buffer (pH 7.4), at room temperature during 2 hours. After rinsing, tissues were dehydrated through a graded series of ethanol and embedded in epon. Ultrathin (rostrocaudally) sections (70 nm), comprising zebrafish eyes at the optic nerve level, were collected on Formvar coated grids, subsequently stained with 2% uranyl acetate and Reynold’s lead citrate, and examined with a Jeol1010 electron microscope. Using Adobe Photoshop version 8.0, TEM images were adjusted for brightness and contrast. To compare the degree of vesiculation in the inner segments of the various experimental groups, quantitative TEM analysis was accomplished. To this end, 8000x magnification images of the central retina (50% middle arc length) were acquired. For each zebrafish group, several eyes and fields of view in the retina were evaluated (S5 Table). For each field of view, the total number of photoreceptor cells was counted. Finally, each photoreceptor cell was evaluated for presence of vesicles. The statistical significance of differences between two groups was assessed using the independent samples Student’s t-test (SPSS 20.0). Morphant groups were analyzed and compared versus the wild type group, as well as versus the mock-injected group. The statistical significance was set at p < 0.05. Data are presented as means ± SEM.
Zebrafish OKR assay
The OKR was measured by a previously described method [55]. Zebrafish larvae were mounted in an upright position in 3% methylcellulose in a small Petri dish. The Petri dish was placed on a platform surrounded by a rotating drum 8 cm in diameter. A pattern of alternating black and white vertical stripes was displayed on the drum interior (each stripe subtended an angle of 36°C). Larvae (4 dpf) were visualized through a stereomicroscope positioned over the drum and illuminated with fiberoptic lights. Eye movements were recorded while larvae were optically stimulated by the rotating stripes. Larvae were subjected to a protocol of a 30 seconds counterclockwise rotation, a 10 seconds rest, and a 30 seconds clockwise rotation.
Melanosome transport assay
To induce melanosome retraction P5 larvae were exposed to epinephrine (Sigma E4375) at a final concentration of 500 μg/ml. Melanosome retraction was continuously monitored under the microscope and the endpoint was scored when all melanosomes in the head (and the trunk) were perinuclear [28]. P-values were obtained using Student’s t-test (two tailed, unpaired).
Statistical analyses
For all quantifications of zebrafish experiments, the Graphpad Prism6 software (http://www.graphpad.com/scientific-software/prism/) was employed to generate scatter plots, calculate mean values and SEM values, and perform statistical tests. Continuous data was analyzed using two-tailed, unpaired Student’s t-test and categorical data was analyzed using Fisher’s exact test.
Supporting Information
Zdroje
1. Giessl A., Trojan P., Rausch S., Pulvermuller A. & Wolfrum U. Centrins, gatekeepers for the light-dependent translocation of transducin through the photoreceptor cell connecting cilium. Vision Res 46, 4502–4509 (2006).
2. Hu Q. & Nelson W. J. Ciliary diffusion barrier: the gatekeeper for the primary cilium compartment. Cytoskeleton 68, 313–324 (2011).
3. Nachury M. V., Seeley E. S. & Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annual review of cell and developmental biology 26, 59–87 (2010).
4. Omran H. NPHP proteins: gatekeepers of the ciliary compartment. J Cell Biol 190, 715–717 (2010).
5. Szymanska K. & Johnson C. A. The transition zone: an essential functional compartment of cilia. Cilia 1, 10 (2012).
6. Garcia-Gonzalo F. R. et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 43, 776–784 (2011).
7. Dishinger J. F. et al. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nature cell biology 12, 703–710 (2010).
8. Kee H. L. et al. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nature cell biology 14, 431–437 (2012).
9. Kevany B. M. & Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 25, 8–15 (2010).
10. Papermaster D. S., Schneider B. G., DeFoe D. & Besharse J. C. Biosynthesis and vectorial transport of opsin on vesicles in retinal rod photoreceptors. J Histochem Cytochem 34, 5–16 (1986).
11. Hsiao Y. C., Tuz K. & Ferland R. J. Trafficking in and to the primary cilium. Cilia 1, 4 (2012).
12. Madhivanan K. & Aguilar R. C. Ciliopathies: the trafficking connection. Traffic 15, 1031–1056 (2014).
13. Hook P. & Vallee R. B. The dynein family at a glance. J Cell Sci 119, 4369–4371 (2006).
14. Tai A. W., Chuang J. Z., Bode C., Wolfrum U. & Sung C. H. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell 97, 877–887 (1999).
15. Wang J. & Deretic D. Molecular complexes that direct rhodopsin transport to primary cilia. Progress in retinal and eye research 38, 1–19 (2014).
16. Humphries M. M. et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet 15, 216–219 (1997).
17. Insinna C., Baye L. M., Amsterdam A., Besharse J. C. & Link B. A. Analysis of a zebrafish dync1h1 mutant reveals multiple functions for cytoplasmic dynein 1 during retinal photoreceptor development. Neural Dev 5, 12 (2010).
18. van Wijk E. et al. Usher syndrome and Leber congenital amaurosis are molecularly linked via a novel isoform of the centrosomal ninein-like protein. Hum Mol Genet 18, 51–64 (2009).
19. Casenghi M., Barr F. A. & Nigg E. A. Phosphorylation of Nlp by Plk1 negatively regulates its dynein-dynactin-dependent targeting to the centrosome. J Cell Sci 118, 5101–5108 (2005).
20. Pandey A.K. et al. Functionally enigmatic genes: a case study of the brain ignorome. PLoS One 9(2):e88889 (2014).
21. Neuhauss S. C. et al. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. The Journal of neuroscience: the official journal of the Society for Neuroscience 19, 8603–8615 (1999).
22. Brockerhoff S. E. et al. A behavioral screen for isolating zebrafish mutants with visual system defects. Proceedings of the National Academy of Sciences of the United States of America 92, 10545–10549 (1995).
23. Huang Y. Y. & Neuhauss S. C. The optokinetic response in zebrafish and its applications. Frontiers in bioscience: a journal and virtual library 13 (2008).
24. Insinna C. & Besharse J. C. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn 237, 1982–1992 (2008).
25. Texier Y. et al. Elution profile analysis of SDS-induced subcomplexes by quantitative mass spectrometry. Molecular & cellular proteomics: MCP 13, 1382–1391 (2014).
26. Allan V. J. Cytoplasmic dynein. Biochem Soc Trans 39, 1169–1178 (2011).
27. Liu X. et al. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci U S A 104, 4413–4418 (2007).
28. Yen H. J. et al. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet 15, 667–677 (2006).
29. Harada A. et al. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol 141, 51–59 (1998).
30. Pelegri F. Maternal factors in zebrafish development. Dev Dyn 228, 535–554 (2003).
31. Kong S. et al. Dlic1 deficiency impairs ciliogenesis of photoreceptors by destabilizing dynein. Cell research 23 (2013).
32. Smith D. S. et al. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nature cell biology 2, 767–775, doi: 10.1038/35041000 (2000).
33. Tai C. Y., Dujardin D. L., Faulkner N. E. & Vallee R. B. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J Cell Biol 156, 959–968, doi: 10.1083/jcb.200109046 (2002).
34. Toropova K. et al. Lis1 regulates dynein by sterically blocking its mechanochemical cycle. eLife 3, doi: 10.7554/eLife.03372 (2014).
35. Doerre G. & Malicki J. A mutation of early photoreceptor development, mikre oko, reveals cell-cell interactions involved in the survival and differentiation of zebrafish photoreceptors. J Neurosci 21, 6745–6757 (2001).
36. Tsujikawa M., Omori Y., Biyanwila J. & Malicki J. Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc Natl Acad Sci U S A 104, 14819–14824, doi: 10.1073/pnas.0700178104 (2007).
37. Boldt K. et al. Disruption of intraflagellar protein transport in photoreceptor cilia causes Leber congenital amaurosis in humans and mice. J Clin Invest 121, 2169–2180, doi: 10.1172/JCI45627 (2011).
38. Stewart M. Molecular mechanism of the nuclear protein import cycle. Nature reviews. Molecular cell biology 8, 195–208, doi: 10.1038/nrm2114 (2007).
39. Kee H. L. & Verhey K. J. Molecular connections between nuclear and ciliary import processes. Cilia 2, 11, doi: 10.1186/2046-2530-2-11 (2013).
40. Ferreira P. A., Nakayama T. A., Pak W. L. & Travis G. H. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature 383, 637–640, doi: 10.1038/383637a0 (1996).
41. Liu X., Udovichenko I. P., Brown S. D., Steel K. P. & Williams D. S. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci 19, 6267–6274 (1999).
42. Kremer H., van Wijk E., Marker T., Wolfrum U. & Roepman R. Usher syndrome: molecular links of pathogenesis, proteins and pathways. Hum Mol Genet 15 Spec No 2, R262–270, 15/suppl_2/R262 [pii] doi: 10.1093/hmg/ddl205 (2006).
43. van Wijk E. et al. The DFNB31 gene product whirlin connects to the Usher protein network in the cochlea and retina by direct association with USH2A and VLGR1. Hum Mol Genet 15, 751–765, ddi490 [pii] doi: 10.1093/hmg/ddi490 (2006).
44. Roepman R., Schick D. & Ferreira P. A. Isolation of retinal proteins that interact with retinitis pigmentosa GTPase regulator by interaction trap screen in yeast. Methods in enzymology 316, 688–704 (2000).
45. Reiners J., Nagel-Wolfrum K., Jurgens K., Marker T. & Wolfrum U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp Eye Res 83, 97–119, S0014-4835(06)00068-6 [pii] doi: 10.1016/j.exer.2005.11.010 (2006).
46. Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. & Schilling T. F. Stages of embryonic development of the zebrafish. Dev Dyn 203, 253–310, doi: 10.1002/aja.1002030302 (1995).
47. Gloeckner C. J., Boldt K., Schumacher A., Roepman R. & Ueffing M. A novel tandem affinity purification strategy for the efficient isolation and characterisation of native protein complexes. Proteomics 7, 4228–4234 (2007).
48. Boldt K., van Reeuwijk J., Gloeckner C. J., Ueffing M. & Roepman R. Tandem affinity purification of ciliopathy-associated protein complexes. Methods in cell biology 91 (2009).
49. Gloeckner C. J., Boldt K. & Ueffing M. Strep/FLAG tandem affinity purification (SF-TAP) to study protein interactions. Current protocols in protein science / editorial board, John E. Coligan … [et al.] Chapter 19, Unit19 20 (2009).
50. Gloeckner C. J., Boldt K., Schumacher A. & Ueffing M. Tandem affinity purification of protein complexes from mammalian cells by the Strep/FLAG (SF)-TAP tag. Methods in molecular biology 564, 359–372 (2009).
51. Keller A., Nesvizhskii A. I., Kolker E. & Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Analytical chemistry 74, 5383–5392 (2002).
52. Nesvizhskii A. I., Keller A., Kolker E. & Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Analytical chemistry 75, 4646–4658 (2003).
53. Cox J. & Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nature biotechnology 26, 1367–1372 (2008).
54. Schroer T. A. Dynactin. Annual review of cell and developmental biology 20, 759–779 (2004).
55. Phillips J. B. et al. Harmonin (Ush1c) is required in zebrafish Muller glial cells for photoreceptor synaptic development and function. Disease models & mechanisms 4, 786–800 (2011).
Štítky
Genetika Reprodukční medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 10
-
Všechny články tohoto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



