-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaOriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
During embryogenesis an entire organism develops from a single cell. This process is vital for the formation of life, thus cell division occurs with a very distinct orientation and pattern that is tightly controlled by several signaling pathways. The mechanisms underlying these pathways are complex and not yet fully understood. In the roundworm Caenorhabditis elegans, a common genetic model, the patterns and orientations in which cells divide in the embryo have been well characterized offering an ideal model to study the molecular mechanisms involved. Here, we show that the signal mediated by the adhesion G protein-coupled receptor LAT-1 is based on cAMP. This second messenger is essential for the orientation of distinct cell division planes in the early embryo. Studies based on a lat-1 knockout mutant reveal that LAT-1 signaling affects the levels of the second messenger cAMP in the cells via a specific G protein. Thereby the receptor is activated by an intrinsic sequence. This pathway is the first one clearly shown to involve a G protein-coupled receptor-dependent G-protein signal in orientation of embryonic cell division, offering a novel level of regulation of this process among other described pathways.
Published in the journal: . PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005624
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005624Summary
During embryogenesis an entire organism develops from a single cell. This process is vital for the formation of life, thus cell division occurs with a very distinct orientation and pattern that is tightly controlled by several signaling pathways. The mechanisms underlying these pathways are complex and not yet fully understood. In the roundworm Caenorhabditis elegans, a common genetic model, the patterns and orientations in which cells divide in the embryo have been well characterized offering an ideal model to study the molecular mechanisms involved. Here, we show that the signal mediated by the adhesion G protein-coupled receptor LAT-1 is based on cAMP. This second messenger is essential for the orientation of distinct cell division planes in the early embryo. Studies based on a lat-1 knockout mutant reveal that LAT-1 signaling affects the levels of the second messenger cAMP in the cells via a specific G protein. Thereby the receptor is activated by an intrinsic sequence. This pathway is the first one clearly shown to involve a G protein-coupled receptor-dependent G-protein signal in orientation of embryonic cell division, offering a novel level of regulation of this process among other described pathways.
Introduction
Spindle and cleavage plane orientation play a central role in many aspects of development as well as homeostasis of organs and organisms. Alignment of spindles during cell division is achieved by interactions of the spindle apparatus with the cell cortex. The machinery linking tubulin to the cortex and supplying force to move the spindle is well characterized [1–3]. However, the molecular mechanisms balancing forces in a specific direction are complex and less well understood. In the early Caenorhabditis elegans embryo a large network of only partially characterized signaling pathways including Wnt proteins [4] and PAR proteins [5–7] is engaged in the control of spindle orientations during asymmetric and symmetric cell divisions. These pathways form the basis for the invariant embryonic cell lineage of the nematode with highly reproducible cleavage planes and axes in the early embryo. A sequence of directed cell divisions establishes the particular geometry of early blastomeres, eventually creating an 8-cell stage embryo which contains AB4 descendants after the third round of cell cleavage [8]. At this stage, the Wnt/Frizzled (Fz) is involved in spindle rotation of one of the dividing AB descendants to ensure the proper contact of its daughter cells to neighboring blastomeres [9–11]. In the subsequent round of cell division, the Notch pathway induces the left-right asymmetry of the embryo via specific contacts of two of the then eight AB descendants [12,13]. Recently, we have shown that in addition to MOM-2/Wnt and MOM-5/Fz, the latrophilin homolog LAT-1 is a novel receptor required for the coordination of spindles and thus, division plane orientation at the 8-cell stage in the C. elegans embryo [14]. Some evidence even indicates a function of LAT-1 in parallel to components of the Wnt pathway [14]. In lat-1(ok1465) homozygous nematodes the spindle of ABal, one of the AB4 cells, is turned almost perpendicular to the anterior-posterior axis in which the cell normally divides. This tilted orientation causes not only one but both daughter cells to touch the neighboring blastomere MS [14] resulting in embryonic lethality as a consequence of incorrect cell-cell contacts. Thus, LAT-1 is involved in orienting cleavage planes in an anterior-posterior direction in descendants of the ABal blastomere. However, it remained elusive how the latrophilin homolog exerts this function.
Latrophilins were initially identified as targets for latrotoxin, a component of the black widow spider´s (Latrodectus mactans) toxin [15] and are described as modulators of neurotransmitter release [16,17]. They belong to the class of adhesion G protein-coupled receptors (aGPCRs), a family of seven transmembrane (7TM) receptors with emerging roles in cell and tissue polarity. One of the best understood members of this class, flamingo/CELSR, has been shown to be involved in the planar cell polarity pathway in mice as well as Drosophila melanogaster [18,19]. aGPCRs form the second largest class of GPCRs. Despite their essential physiological functions, especially in development and neurobiology [18,20–22], the signaling mechanisms of the majority of these receptors remain poorly understood, and for most family members agonists and downstream effectors are unknown [23,24]. Our previous work showed that LAT-1 mediates two distinct signals, one transduced via the 7TM and the C terminus, while the other requires only the extracellular N terminus containing the GPCR-autoproteolysis inducing (GAIN) domain and its integral motif, the GPCR proteolytic site (GPS) [25], which are characteristic for many aGPCRs [26,27]. During embryonic development, LAT-1 conveys its signal cell autonomously via the 7TM and the C terminus raising essential questions regarding the molecular details and effectors of the signaling cascades triggered by LAT-1.
Here, we have identified the mechanisms of LAT-1 signaling required to mediate the coordination of anterior-posterior tissue polarity in the early C. elegans embryo. We show that a Gs protein-signaling cascade is the key pathway. Following activation by a tethered agonist, LAT-1 elevates levels of the second messenger cyclic AMP (cAMP) via interaction with a Gs protein. By increasing intracellular cAMP levels LAT-1 controls spindle orientation in the dividing cell. We discuss how polarity is realized by the respective cascade and the non-polar cAMP signal.
Results
LAT-1 signals via the Gs protein/adenylyl cyclase/cAMP pathway
Structure-function analyses have previously shown that the correct alignment of cell division planes of some AB descendants in the early C. elegans embryo is mediated by the latrophilin homolog LAT-1 via the 7TM-dependent signaling mode of the receptor [14,25]. This could be indicative of a classical GPCR signal via heterotrimeric G proteins. To investigate this hypothesis, we heterologously expressed wild-type lat-1 in COS-7 cells (Fig 1A and S1 Fig) and tested for functional G-protein coupling of the receptor to the three major G proteins Gs, Gq and Gi, which are highly conserved between metazoan species [28]. As no interaction partner of LAT-1 has been described which is capable of triggering G protein-mediated signaling of the receptor, its coupling abilities were determined by measuring its basal activity. In the absence of agonists an equilibrium between inactive and active GPCR conformation exists, with only a few receptors residing in the active state [29]. GPCR overexpression increases the amount of receptors in general and thus, in each state. At a certain threshold basal activity of the GPCRs in the active state can be detected and signaling pathways measured, which are normally only activated upon agonist stimulation. The signaling abilities of orphan GPCRs have been frequently identified by taking advantage of this capacity [30,31]. Using this well-established system we first tested the involvement of the Gs protein/adenylyl cyclase pathway in LAT-1 signal transduction by measuring the formation of cAMP. Cells overexpressing lat-1 showed a receptor amount-dependent increase of cAMP levels with a maximum of 1.4-fold (Fig 1B), suggesting that LAT-1 transduces its signal via Gs proteins. For the characterization of the G protein-coupling abilities of LAT-1 to Gi and Gq proteins we performed inositol trisphosphate (IP) accumulation assays. No increase of cellular IP levels was detected (Fig 1C), indicating that LAT-1 does not couple to Gq proteins under basal conditions in this assay. It was reported earlier that a chimera, in which the C-terminal 4 amino acids of Gαi (Gαqi4) are substituted with the corresponding ones from a Gαq subunit, reroutes the intracellular signals towards a Gq pathway modulating IP concentration [32]. Co-expression of lat-1 with this chimera did not cause an increase of IP concentration, indicating that Gi-protein coupling appears also improbable (Fig 1C). Applying the same concept by co-expressing a Gαqs4 chimera with lat-1, which redirects the Gs protein signal towards a Gq-signaling cascade, we detected an increase in basal IP levels when compared to COS-7 cells transfected with empty vector (Fig 1C). By being able to reroute the Gs protein signal we verified that the LAT-1-mediated elevation of cAMP levels was due to Gs-protein coupling and not a result of a secondary effect or Gβγ signaling. Therefore, we conclude that LAT-1 very likely activates the Gs protein/adenylyl cyclase signaling pathway.
Fig. 1. LAT-1 couples to Gαs, but not to Gαi or Gαq. 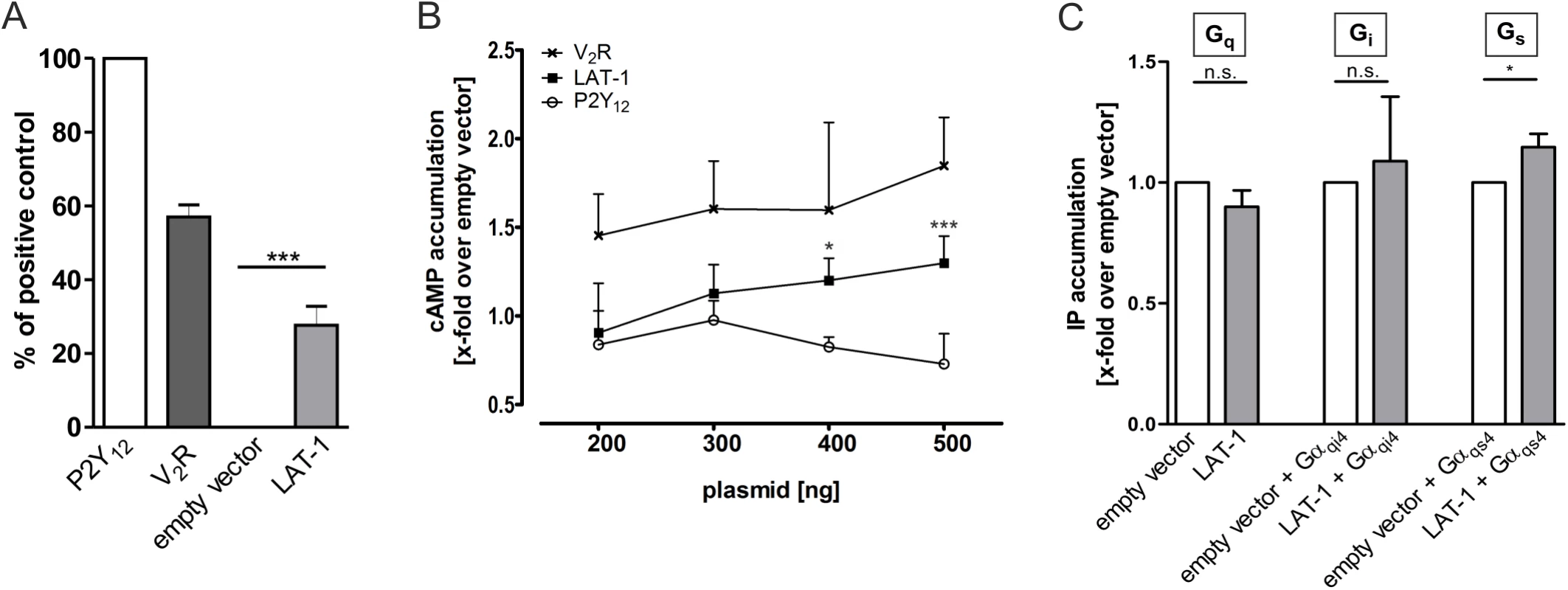
(A) COS-7 cells were transfected with 500 ng of empty vector (pcDps) or plasmid encoding either human ADP receptor P2Y12, the human vasopressin type 2 receptor V2R or LAT-1. 48 hours post transfection, surface expression levels were determined with a cell surface ELISA. Expression of the GPCR human vasopressin type 2 receptor V2R is shown as a comparison. Data are displayed as percentage of P2Y12 (positive control) and given as means ± SD of five independent experiments, each performed in triplicate. The non-specific OD value (empty vector) is 0.02 ± 0.01 (set 0%) and the OD value of P2Y12 is 0.95 ± 0.05 (set 100%). *** p < 0.001. (B) To test for functional coupling of LAT-1 to Gs proteins, COS-7 cells were transfected with increasing amounts of empty control vector (pcDps) or plasmid encoding LAT-1, human vasopressin type 2 receptor V2R, or human P2Y12, respectively, and cAMP levels were measured 48 hours later by cAMP accumulation assay. cAMP concentrations are shown as fold change over empty control vector, cAMP levels: 6.2 ± 2.3 nM (200 ng); 6.1 ± 2.5 nM (300 ng); 5.1 ± 2.6 nM (400 ng); 6.1 ± 4.5 nM (500 ng). LAT-1 but not P2Y12 causes an increase in cAMP levels. The Gs-protein coupling V2R served as a positive control and the predominantly Gi-protein coupling P2Y12 as negative control. Data are given as means ± SD of five independent experiments, each performed in triplicate. * p < 0.05; *** p < 0.001. (C) For analyses of Gq-, Gi- and Gs-protein coupling, IP accumulation assays were performed detecting Gαq-mediated increase in IP levels. To measure functional coupling of LAT-1 to Gαi, a chimeric Gαqi4 protein was applied to reroute a Gi-mediated signal to a Gαq-mediated pathway. Similarly, to validate Gs-protein coupling IP accumulation assays were performed using a Gαqs4 chimera. For each assay, 1.5 μg of plasmid containing lat-1 cDNA were transfected (for Gi- and Gs-coupling, co-transfection with 100 ng of the respective chimeric protein was applied). No signal was detected for Gq or Gi protein-signaling pathways, but for Gs-protein coupling. Basal IP levels are: 220 ± 34 CPM/well (empty vector); 306 ± 20 CPM/well (empty vector + Gαqi4); 394 ± 53 CPM/well (empty vector + Gαqs4). Data are given as means ± SD of five independent experiments, each performed in triplicate. n.s. not significant; * p < 0.05. Anterior-posterior cell division in the early embryo depends on cAMP-mediated LAT-1 signaling
The question which of the 21 Gα subunits in C. elegans is activated by LAT-1 is difficult to address as for many of them no effectors or signals are described. One likely candidate is GSA-1, the closest homolog of Gαs in C. elegans displaying 66% identity to mammalian Gαs proteins [33], which is also expressed in embryos [34]. gsa-1(pk75) homozygous animals survive embryogenesis but arrest in larval development [34], which may be explained by a maternal contribution of the embryonic functions. To elucidate a possible interaction of lat-1 with gsa-1 in early embryonic development we knocked down maternally and zygotically contributed gsa-1 activity using RNA interference (RNAi).
In embryos homozygous for lat-1(ok1465), subsequently referred to as lat-1, the ABal division plane was tilted towards a position almost perpendicular to the anterior-posterior axis (90.3°±17.9°, means ± SD, n = 18, p < 0.001 Fig 2B and 2E) whereas the wild-type orientation was more oblique (128.2°± 8°, means ± SD, n = 14; angles measured towards the posterior; Fig 2A and 2E).
Fig. 2. Division plane defects in lat-1 mutant embryos are rescued by elevation of intracellular cAMP via a Gs protein/adenylyl cyclase pathway. 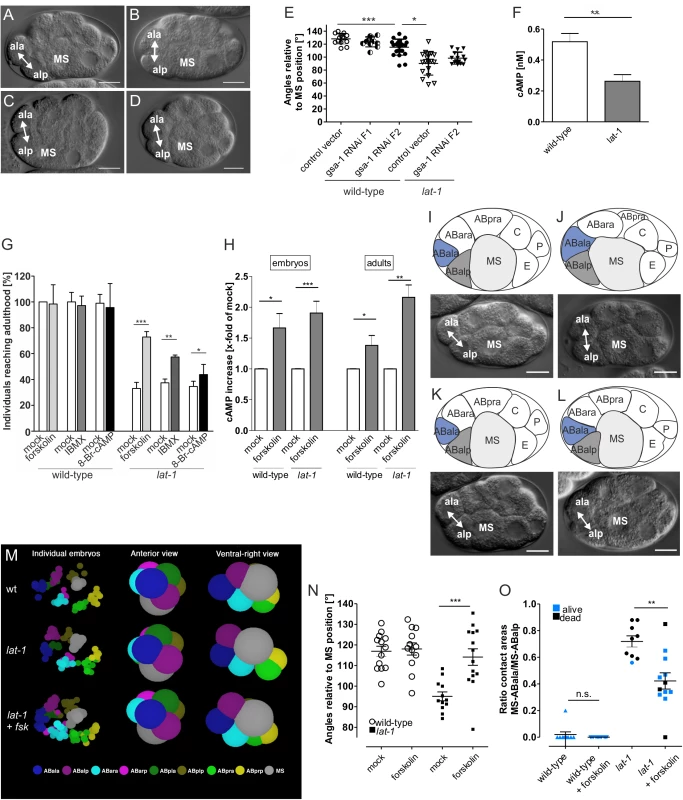
(A-D) gsa-1 RNAi in AB4 embryos reveals a similar phenotype to that of lat-1, scale bars = 10 μm. In wild-type embryos treated with the RNAi control vector L4440 ABal divides in an anterior-posterior direction (A) whereas in treated lat-1 mutants the division plane is almost perpendicular to MS (B). gsa-1 RNAi by feeding over two generations leads to a turning of the F2 ABal division plane in a direction similar to that in lat-1 mutants (C). F2 lat-1; gsa-1(RNAi) embryos show a similar ABal division plane to lat-1 mutants (D). (E) Cell division plane angles of ABal relative to MS after knockdown of gsa-1. Feeding of gsa-1 RNAi on wild-type nematodes resulted in an ABal division plane angle significantly different to the one of control vector-fed wild-type embryos in the F2 generation. Embryos of the F1 generation did not show a significant different angle. Knockdown of gsa-1 in lat-1 mutants in the F2 generation did not change division plane angles. The vector L4440 served as negative control. Data are shown as means ± SD. * p < 0.05; *** p < 0.001; n ≥ 11. (F) cAMP levels are reduced in lat-1 embryos compared to a control wild-type population. cAMP levels were measured after lysis (15 μg protein) by cAMP accumulation assay. Assays were performed in three independent experiments, data are given as means ± SD. ** p < 0.01. (G) Rescue of developmental lethality in lat-1 nematodes treated with compounds promoting cAMP accumulation: forskolin (80 μM), IBMX (10 mM) and 8-Br-cAMP (0.25 mM). Mothers and subsequently offspring of wild-type and lat-1 individuals were incubated and individuals reaching adulthood were scored (n = 410). As controls, nematodes were incubated in solvent without drug (mock). Data are shown as means ± SD. * p < 0.05; ** p < 0.01; *** p < 0.001. (H) Forskolin increases cAMP levels in C. elegans embryos and adults. lat-1 mutants and subsequently embryos were incubated with 80 μM forskolin. Embryos displayed an increase in cAMP levels compared to control animals. Similarly, mutant individuals at the L4 stage also showed an elevation in cAMP concentration. cAMP levels were measured after lysis by cAMP accumulation assay. As controls, nematodes were treated with 0.8% DMSO lacking forskolin (mock). Basal cAMP levels of mock control are: 0.70 ± 0.14 nM (wild-type embryos); 0.37 ± 0.19 nM (lat-1 embryos); 1.9 ± 0.7 nM (wild-type adults); 2.3 ± 0.8 nM (lat-1 adults). Assays were performed in three independent experiments, data are given as means ± SD. * p < 0.05; ** p < 0.01; *** p < 0.001. (I-L) Relative positions of ABala, ABalp, and MS in an AB4 embryo. Schematic representation (top) and DIC microscopy (bottom), scale bars = 10 μm. In wild-type embryos (I) and embryos incubated in 80 μM forskolin for 120 minutes (K) ABal divides in an anterior-posterior direction with only ABalp forming an interface with MS. In lat-1 mutants the division plane is skewed resulting in ABala and ABalp contacting MS (J). lat-1 embryos treated with 80 μM forskolin for 120 minutes display wild-type division and cell interfaces (L). (M) Arrangement of blastomeres in the 12-cell stage embryo. 3D representation of the data shown in Fig 2I-L using a wild-type, a lat-1 embryo and a lat-1 embryo treated with 80 μM forskolin (fsk) show that defects in anterior-posterior division plane alignment in lat-1 mutant embryos are changed towards wild-type cleavage orientations upon forskolin treatment. Left-hand side shows the positions of the 8-AB descendants and the MS blastomere are shown for the individual embryos. The right-hand side is two views of the mean positions of the blastomeres. The spheres are enlarged to convey an impression of the contacts. Forskolin turns the ABal spindle in the mutant in a more anterior direction. (N) Cell division plane angles of ABal relative to MS of untreated wild-type/lat-1 controls and wild-type/lat-1 embryos after incubation of mothers and subsequently embryos for 48 hours in 80 μM forskolin. Upon forskolin treatment, mutant embryos display a wild-type division angle. As controls, nematodes were treated with 0.8% DMSO lacking forskolin (mock). Data are shown as average angles ± SD, *** p < 0.001; n ≥ 12. (O) Forskolin reduces the contact length of ABala to MS. Ratio of relative contact lengths of ABala to MS and ABalp to MS cells lat-1 embryos untreated (control, n = 10) and after incubation of mothers and subsequently embryos for 48 hours in 80 μM forskolin (n = 11). In wild-type (n = 10) and forskolin-treated wild-type (n = 11) embryos ABala does not contact MS. Data were calculated from pixel analyses utilizing DIC images and are shown as means ± SD, ** p < 0.01. When knocking down gsa-1 in wild-type nematodes by feeding dsRNA to young adult hermaphrodites we observed no embryonic lethality in the offspring. However, the embryos still displayed a minor turning of the ABal spindle towards the direction typical for lat-1 (123.5° ± 7.7°, means ± SD, n = 11; Fig 2E). It appeared that RNAi affected the progeny (F1) more drastically by rendering the adult hermaphrodites almost sterile. Only few embryos could be recovered as most hermaphrodites did not contain any embryo, some, however, one to five. In these second generation (F2) embryos, the orientation of the ABal division plane was more similar to that of lat-1 mutants (115.3° ± 12.2°, means ± SD, n = 12; Fig 2C and 2E).
In contrast to F1 embryos, the cleavage direction of F2 embryos was significantly different from wild-type embryos (p < 0.001). Although some F2 embryos showed a cleavage angle of 87°, which is typical for lat-1 (Fig 2E), no entire similarity to lat-1 was reached. The fact that the RNAi effect is only pronounced in the F2 generation suggests that either inactivation of the gsa-1 mRNA is very inefficient and/or slow or that oocytes already contain a sufficient amount of maternally contributed GSA-1 to rescue embryos in the absence of endogenous mRNA. Analyses of mRNA levels by qPCR revealed a 3 - to 6-fold reduction of gsa-1 transcript in all RNAi-treated samples compared to untreated levels (S2A Fig). Protein levels of GSA-1 are significantly reduced 36 hours after onset of RNAi treatment, albeit not fully depleted (S2B and S2C Fig) suggesting that GSA-1 protein is still available in the cells but at lower levels than in wild-type animals.
Although the cleavage direction of gsa-1 RNAi embryos is the same as in lat-1 mutants this is not a stringent indication that both proteins function in the same pathway, they could still function in parallel. As lat-1(ok1465) is a null mutant, one should expect that an additional inactivation of GSA-1 should not have an effect on the cleavage direction of ABal, if they act in the same pathway. However, a synergistic effect should be visible if they work in different pathways. To analyze whether LAT-1 and GSA-1 function sequentially or independently lat-1 mutants were fed with bacteria expressing dsRNA for the gsa-1 sequence, which yielded a cleavage direction of ABal in F2 embryos that was not significantly altered compared to lat-1 embryos (97.7° ± 8.8°, means ± SD, n = 13, p > 0.1; Fig 2D and 2E). This is consistent with the notion that GSA-1 is the G protein downstream of LAT-1.
To investigate the physiological relevance of the LAT-1-dependent Gs protein-mediated cAMP signal in C. elegans, we first measured cAMP levels in embryos. Interestingly, the cAMP level in a population of lat-1 embryos was significantly reduced (0.26 nM) compared with wild-type embryos (0.52 nM) (Fig 2F). Next, we tested if an elevation of cAMP rescues lethality of lat-1 worms, a consequence of the tilted ABal division plane. Consistent with the maternal and zygotic requirement of LAT-1 [14] we incubated L4 larvae and subsequently developing embryos with different compounds promoting or mimicking elevation of cAMP levels: the adenylyl cyclase activator forskolin, the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) and the stable cAMP analogue 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP). Treatment with any of these compounds led to an amelioration of embryonic lethality with forskolin having the strongest effect by increasing the survival rate from 33% to 73% (Fig 2G). As forskolin activates adenylyl cyclases and thus, potentially exhibits toxicity we tested various concentrations of forskolin and found that 80 μM had an optimal effect whereas higher concentrations were detrimental on C. elegans. As shown in Fig 2H, forskolin elevated cAMP in wild-type and in lat-1 hermaphrodites. Interestingly, we did not observe any involvement of cAMP in the LAT-1 function depending exclusively on the N terminus. As loss of this function leads to reduced fertility, we investigated the effect of elevated cAMP levels on brood size of lat-1 mutants. We did not detect any rescue of reduced brood size upon treatment with forskolin (S3A Fig), independently of time and duration of drug application (S3B Fig), suggesting that the 7TM-independent function of LAT-1 involved in fertility does not rely on a Gs/adenylyl cyclase-mediated cAMP signal.
We next investigated if forskolin corrects the defective ABal spindle orientation observed in lat-1 mutant embryos (95°± 7°, means ± SD, n = 12, p < 0.0005; Fig 2J and 2N) into the wild-type direction (117° ± 9°, means ± SD, n = 13; angle measured towards the posterior; Fig 2I and 2N). Upon application of 80 μM forskolin ABal spindles in lat-1 mutants returned to the oblique position (114° ± 10°, means ± SD, n = 14, p > 0.5) (Fig 2K–2N).
As a result of faulty cleavage plane orientation in lat-1 mutant embryos both daughters, ABalp and ABala, retain equal contact to MS after division [14] (Fig 2J) whereas in wild-type embryos only the posterior daughter ABalp remains in contact with the MS blastomere. The anterior daughter ABala is displaced to the most anterior position in the embryo (Fig 2I). The arrangement of cells at the 12-cell stage does not solely depend on the cleavage direction of ABal but also on that of ABar, which under specific circumstances may push ABala away from MS, whose position also varies in embryos. Therefore, we investigated the contact lengths of the ABala and ABalp blastomeres with MS. The ratio of the two lengths was used as a measure for cell position. In wild-type embryos ABala normally does not contact MS (ratio of 0.00 ± 0.00), whereas in lat-1 mutant embryos it is shifted to 0.72 ± 0.03 (Fig 2O). Forskolin strongly reduced the contact of ABala to MS. Embryos in which the contact length ratio of ABala-MS to ABalp-MS was smaller than 0.45, mostly survive. However, due to potential independent roles of LAT-1 in later processes and detrimental effects of forskolin, occasionally embryos with ratios smaller than this cut-off still died. These observations suggest that suppression of lethality in lat-1 mutants generally occurs by correcting the aberrant cleavage direction of the ABal blastomere in the mutant. It appears surprising that forskolin, which acts on all adenylyl cyclases, has such a specific effect. Initially we suspected that the drug may universally randomize spindle orientation and thus, ABala occasionally would not touch MS. However, the standard deviation for the angle of ABal cleavage is ± 9° in untreated wild-type embryos and ± 10.2° after forskolin treatment, indicating that the drug does not randomize cleavage directions. Thus, the suppression of the lat-1 phenotype by forskolin is very specific, suggesting that by activating adenylyl cyclases and subsequently raising cellular cAMP concentrations, it specifically mimics the process normally controlled by LAT-1.
Further, cAMP being the central element in this process is also supported by the fact that decreasing its levels by treating very early wild-type embryos with the adenylyl cyclase inhibitor 2',5'-dideoxyadenosine (ddA) leads to a specific partial phenocopy of lat-1 mutants resulting in embryonic lethality (S4 Fig).
Taken together, these results indicate that LAT-1 implements signaling via cAMP in vivo. As the lat-1 phenotype is rescued by elevation of cAMP which is not restricted to a certain cellular compartment the division plane orientation in an anterior-posterior direction is likely to be molecularly realized by components downstream of this second messenger.
LAT-1 functions in embryonic development upon activation by a tethered peptide agonist
We next asked how LAT-1 is activated to trigger the signaling cascade to ensure correct cell division plane orientation. Previously, we have postulated that an interaction between the GPS and 7TM domain is essential for LAT-1 activation [25]. Very recently, Liebscher et al. have shown for the aGPCR GPR126 in zebrafish that the sequence immediately C-terminal of the cleavage site in the GPS (the Stachel sequence) acts as an internal tethered agonist to activate GPR126 [35]. We hypothesized that in LAT-1 a similar sequence would also have agonistic properties. To test this hypothesis, we analyzed peptides of varying lengths comprising the sequence C-terminal of the GPS cleavage site (Fig 3A) for their ability to activate LAT-1 in vitro by measuring cAMP levels. Peptides of 12 and 13 amino acids (CP12, CP13) increased cAMP levels in lat-1-transfected COS-7 cells above basal levels, with CP12 displaying the highest agonistic activity by increasing the concentration 1.6-fold (Fig 3B). In contrast, a CP12-derived peptide with positions +1 (T530 in the full length receptor) and +3 (F532 in the full length receptor) mutated to alanine did not display any activity (CMP12) demonstrating that agonistic activity is highly sequence-specific (Fig 3B). The agonistic peptides were able to activate a chimeric LAT-1 with the extracellular N terminus exchanged for that of the rat muscarinic M3 acetylcholine receptor (M3R ECD::LAT-1, S5 Fig) suggesting that the sequence directly interacts with the 7TM.
Fig. 3. An agonistic sequence C-terminal of the GPS cleavage site activates LAT-1 in vitro. 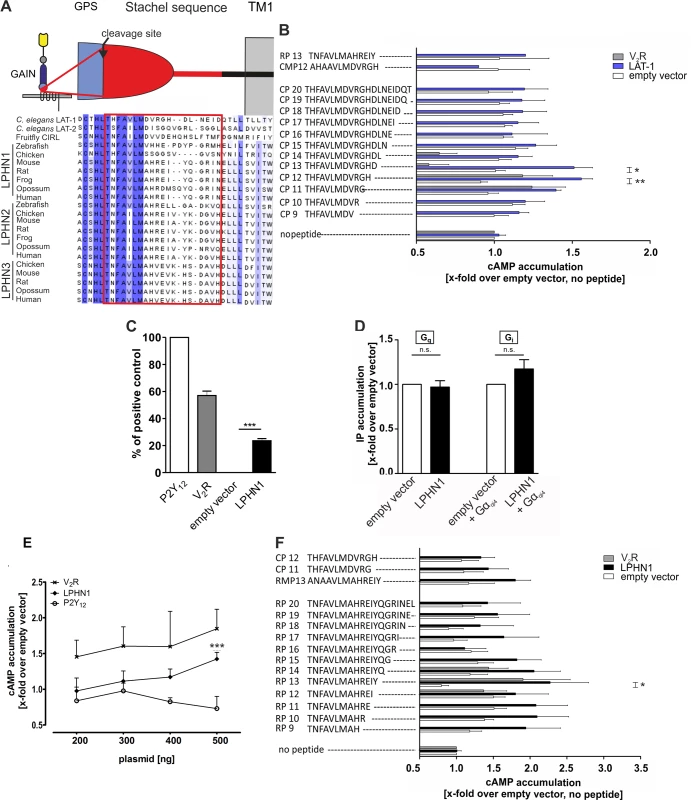
(A) Evolutionary conservation of the putative agonistic region C-terminal of the GPCR proteolytic site (GPS) cleavage site in different latrophilin homologs. The GPS is part of the GPCR-autoproteolysis inducing (GAIN) domain, a characteristic feature of most aGPCRs which is located N-terminal of the first transmembrane domain (TM1). Sequences were retrieved from NCBI/GenBank and aligned utilizing Jalview 2 [36]. (B, F) Peptide-stimulated cAMP response of LAT-1 (B) and rat LPHN1 (F). COS-7 cells transfected with 0.2 μg empty control vector (pcDps) or plasmid encoding the latrophilin homolog, respectively, were stimulated with 1 mM peptide and cAMP levels were measured by cAMP accumulation assay. A mutated peptide served as negative control (CMP12/RMP13). As control for peptide specificity, the human vasopressin type 2 receptor (V2R) was used, which does not respond to any of the peptides tested. Basal cAMP levels (empty vector, no peptide) are 5.3 ± 1.3 nM. Data are given as means ± SD of five independent experiments, each performed in triplicate. * p < 0.05; ** p < 0.01. (C) LPHN1 is expressed in COS-7 cells. Cells were transfected with 500 ng/well of empty vector (pcDps) or plasmid encoding either rat LPHN1, human P2Y12, or human V2R. After 48 hours, surface expression levels were determined using surface ELISA and displayed as percentage of positive control P2Y12. Expression of the human vasopressin type 2 receptor V2R is shown as a comparison. The non-specific OD value (empty vector) was 0.06 ± 0.03 (set 0%) and the OD value of P2Y12 was 1.28 ± 0.12 (set 100%). Data are given as means ± SD of at least three independent experiments. *** p < 0.001. (D) LPHN1 does not yield a basal signal for Gαi- or Gαq-protein coupling. Testing Gi and Gq protein-signal specificity of rat LPHN1, IP accumulation assays were performed to detect Gq protein-mediated increase in IP levels. To analyze functional Gi coupling, the chimeric Gαqi4 protein was applied to reroute a potential Gi-protein pathway to the Gi protein-mediated signaling cascade. For each assay, 1.5 μg/well of plasmid encoding LPHN1 was transfected (for Gi-protein coupling, co-transfection with 100 ng of chimeric Gαqi4 protein was performed). No activation of any of the signaling pathway was observed. Basal IP levels are: 220 ± 34 CPM/well (empty vector); 234 ± 39 CPM/well (empty vector + Gαqi4). Data are given as means ± SD of three independent experiments, each performed in triplicate. n.s. not significant. (E) LPHN1 couples to Gαs. COS-7 cells were transfected with increasing amounts of empty control vector (pcDps) or plasmid encoding either rat LPHN1, human V2R or human P2Y12, and cAMP levels were measured after 48 hours. cAMP concentrations are shown as fold change over empty control vector, basal cAMP levels: 6.2 ± 2.3 nM (200 ng); 6.1 ± 2.5 nM (300 ng); 5.1 ± 2.6 nM (400 ng); 6.1 ± 4.5 nM (500 ng). The Gs-protein coupling V2R served as positive and the predominantly Gi-protein coupling P2Y12 as negative control. Data are given as means ± SD of three independent experiments, each performed in triplicate. *** p < 0.001. As our initial experiments tested G protein-coupling abilities of LAT-1 only under non-stimulated conditions (Fig 1C) we performed these assays in the presence of the agonistic peptide CP12 to elucidate coupling to Gi and Gq proteins. However, we were unable to obtain any results with these chimeric G proteins. Due to rather low LAT-1 expression levels compared to mammalian Gi or Gq-coupled receptors we cannot fully exclude the possibility of coupling to other G protein families additionally to Gs proteins.
We next tested whether activation by a tethered agonist is conserved between species. In mammals, three latrophilin homologs exist. LPHN1 (ADGRL1) shows the highest conservation to LAT-1 overall and within the potential agonistic sequence (Fig 3A). Like LAT-1, this receptor is expressed in COS-7 cells (Fig 3C) and slightly activates the Gs-signaling pathway, but not a Gq - or Gi-signaling cascade in the absence of an agonist (Fig 3D and 3E). Further analyses showed that, similar to C. elegans LAT-1, rat LPHN1 was activated by a peptide representing the sequence C-terminal of the cleavage site. A peptide of 13 amino acids length (RP13) displayed the most efficient agonistic properties and increased basal cAMP levels 2.3-fold (Fig 3F). However, we did not observe any cross-activation of LAT-1 with the rat Stachel sequence and vice versa (Fig 3B and 3F). We also tested if the agonistic peptides are able to trigger a Gq or Gi pathway via rat LPHN1. Upon stimulation with the agonistic peptide RP13 coupling of the receptor to a Gq protein was observed in an IP accumulation assay (S6 Fig). These data suggest that the activation mechanism involving the Stachel sequence is conserved between species, but the implementation of the mechanism is sequence-specific, at least between distantly related latrophilin orthologs.
To assess the agonistic properties of the Stachel sequence in vivo and its impact on LAT-1 signaling in embryonic development we employed rescue experiments utilizing lat-1 mutant animals expressing a chimeric receptor. In this receptor the LAT-1 GPS and thus, the entire Stachel sequence is exchanged for the GPS of C. elegans LAT-2 (Fig 4A). This receptor has been shown to retain activity to complement the fertilization defect in lat-1 mutants, but does not rescue the tissue polarity phenotype [25], ensuring that it is devoid of any potential activation by the Stachel sequence. In this assay, soaking of hermaphrodites with the agonistic peptides CP11, CP12 and CP13 efficiently rescued the tissue polarity phenotype in the early embryo, demonstrating that all three peptides are also able to activate LAT-1 in vivo (Fig 4B). CP13 had the strongest effect (46 ± 12%) compared to untreated controls (26 ± 7%). Peptides with no agonistic activity in vitro (CP9, CP10, CP14, RP13, CMP12) did also not display any activity in vivo (Fig 4B). To test whether this effect is specific to LAT-1 signaling, we also treated lat-1 mutants with the respective peptides but did not detect any rescue (Fig 4B). We did not observe any effect of these peptides on wild-type nematodes, which might be due to the fact that receptor signaling is tightly controlled in vivo or that an increase in activity levels is not reflected in a specific phenotype. To further control for specificity we introduced mutations in the Stachel sequence of the full length LAT-1 protein (T530A and F532A, similarly to the mutations described in CMP12). Consistent with the in vitro experiments no rescue of developmental lethality was detected when expressing this construct in lat-1 mutant nematodes (Fig 4C). To ensure that the mutant protein is functional we assessed the expression of the lat-1T530A/F532A::gfp construct, which is indistinguishable from a wild-type receptor (Fig 4D). Biochemical activity was confirmed by the rescue of the reduced fertility in lat-1 mutants, therefore confirming the receptor´s 7TM-independent functionality (Fig 4D) [25]. These data show that the sequence immediately C-terminal of the GPS cleavage site in LAT-1 is an agonistic region essential for receptor activation and thus, crucial for LAT-1 signaling in embryonic development.
Fig. 4. The agonistic sequence of LAT-1 triggers receptor function in vivo. 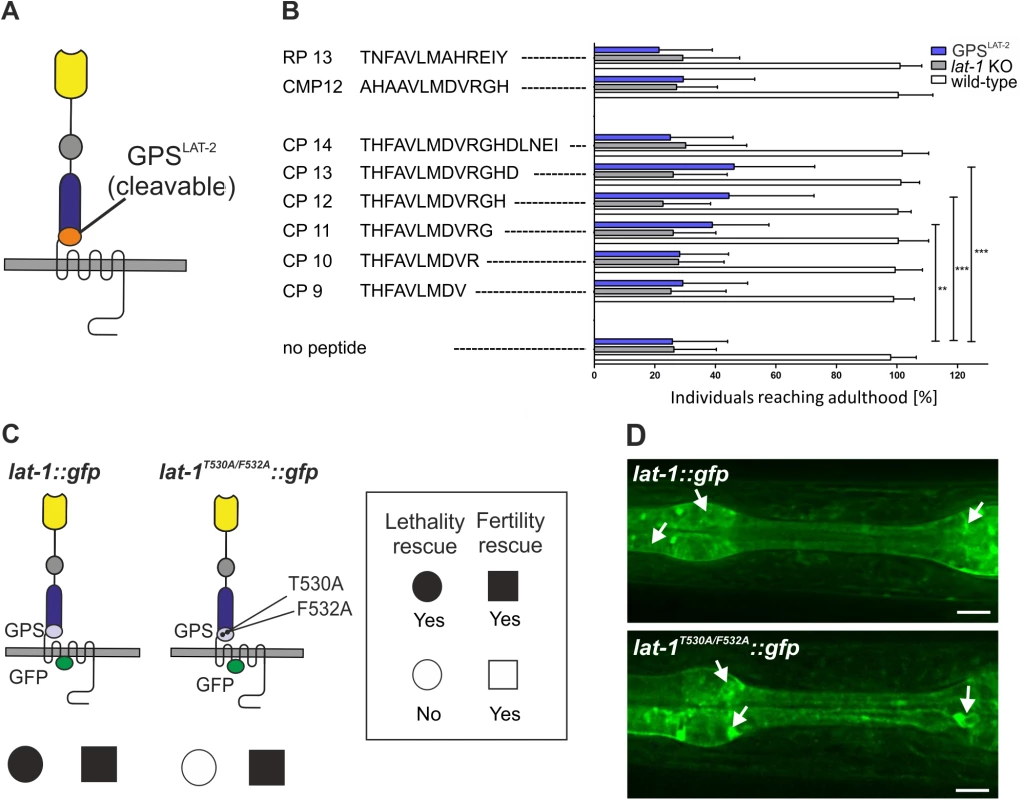
(A) Domain architecture of LAT-1 with an exchange of the GPS for the LAT-2 GPS. (B) Peptide-mediated rescue of lethality in lat-1 mutant nematodes expressing lat-1 with a lat-2 GPS. Mothers and subsequently offspring were soaked in 0.1 mM of peptide and individuals reaching adulthood were scored (n ≥ 350). No response to any peptide was observed in lat-1 null mutant animals. Negative controls: mutated peptide CMP12 and a peptide derived from rat LPHN1 (RP13). Data are shown as means ± SD, **p<0.01; ***p<0.001. (C) Transgenically expressed lat-1T530A/F532A::gfp exclusively rescues fertility defects of lat-1 mutants. Nematodes expressing lat-1T530A/F532A::gfp in a lat-1 mutant background display lethality but no fertility defects compared to a lat-1::gfp transgenic control. (D) Expression and protein localization of lat-1::gfp (top) is indistinguishable from lat-1T530A/F532A::gfp (bottom). Arrows indicate expression. Discussion
Oriented cell division and spindle orientation in early C. elegans embryogenesis are controlled by complex signaling pathways involving GPCRs such as the four frizzled family Wnt receptors [9,37–39] and in which the role for heterotrimeric G proteins has been firmly demonstrated in several elegant studies [40,41]. Gotta and Ahringer showed that proper spindle directionality of the cleavages in the 4-cell C. elegans embryo depends on Gß/γ and that Gα signaling is required for spindle placement in the 1-cell embryo [41]. However, there is evidence that the activity of the G proteins is modulated mostly in a GPCR-independent manner via G-protein regulators [42] and GEF proteins [43]. A GPCR-dependent and G protein-mediated signaling pathway has not been unambiguously defined despite some clear indications [44,45]. In the present study, we demonstrate that a GPCR-dependent G protein-mediated signal based on the adhesion GPCR LAT-1, which is involved in orienting spindles in an anterior-posterior direction in ABal descendants [14], is an essential mechanism for controlling oriented cell division. We provide functional evidence that LAT-1 couples to a Gαs protein which activates an adenylyl cyclase, thereby elevating cellular concentrations of the second messenger cAMP in vitro (Figs 1B and 3B). Similar coupling abilities were observed for the latrophilin ortholog rat LPHN1 (Fig 3E and 3F). consistent with analyses showing that this receptor triggers elevated cAMP levels upon treatment with α-latrotoxin in vitro [46]. In accordance with this study we also observe that LAT-1 triggers a change in basal IP levels, albeit only upon stimulation with an agonistic peptide (S6B Fig).
In vivo analyses have revealed that the increase of cAMP levels is a key signal for the anterior-posterior orientation of cleavage planes in the ABal cell in the C. elegans embryo. Treatment of lat-1 mutant nematodes with the adenylyl cyclase activator forskolin raises cAMP independent of the receptor to a point where cell division plane orientation is sufficiently restored and subsequent lethality is rescued (Fig 2H–2O). The small contact lengths between ABala and MS cells observed in some cases does not seem to have a detrimental effect on developing embryos. The incomplete reduction of contacts lengths to wild-type levels might be due to limitations in accessibility of forskolin to the embryo. Limited drug uptake may explain the partial rescue of lethality by the different compounds affecting cAMP levels (Fig 2G). However, it is also possible that lethality cannot be rescued to wild-type levels as it might be a result of different LAT-1 functions. As neither drug accessibility nor uptake rate or half-life of each compound in the organism could be resolved, we were unable to determine the exact time point and duration at which the signal is required. However, consistent with previous work demonstrating maternal and zygotic requirement of the receptor [14], treatment of L4 hermaphrodites and subsequently embryos with the respective drug was sufficient for rescue (Fig 2G).
Consistently with the putative role of LAT-1 as a regulator of cAMP, lat-1 mutant embryos display decreased levels of the second messenger. We cannot exclude that this decrease is exclusively a consequence of absence of LAT-1 signals in the cells of the early embryo. As there is evidence for the receptor to mediate effects in other cells as well [14], it is conceivable that these are also mediated via cAMP.
Combined with the functional in vitro data our analyses on rescue of lat-1 mutant defects by stimulating a Gs-mediated signal suggest that a GPCR cascade via a cAMP regulation is one essential pathway for the coordination of anterior-posterior cell division plane orientation in embryogenesis.
Our data indicate that the G protein involved in this cascade is GSA-1 (Fig 2A–2E). The major pathways involved in polarity decisions in the early embryo such as Notch/Delta and Wnt/Fz have been shown to mediate signals via routes different from G proteins [4,13]. However, the Wnt/Fz pathway component APC in C. elegans feeds into Rac [45] which supports a scenario in which spindle directions are also regulated by differential G-protein signaling. The signals mediated by these G proteins remain widely elusive, but involvement of different intracellular signals warrants a precise regulation and avoids intersection of the multitude of signaling pathways required to ensure tightly controlled oriented cell division. It could be speculated that LAT-1 contributes an additional signal via a Gs protein/adenylyl cyclase introducing a new level of regulation.
Interestingly, our data suggest that the cAMP-based signal mediating this polarity is not polar and thus, LAT-1 is not required to be asymmetrically localized prior to ABal cell division. Consistently, no asymmetrical distribution of LAT-1 has been found previously [14]. This is in contrast to asymmetric protein localization that has been described for many components of pathways involved in planar cell polarity (PCP) or Wnt/Fz signaling [3] but in accordance with some anterior-posterior tissue polarity models [47]. However, it is well possible that the signal transduced by LAT-1 to mediate an effect on polarity is polarized further downstream through effectors of cAMP such as protein kinases A (PKA) or A kinase anchoring proteins (AKAP). This effect has been shown for a cAMP-dependent PKA in the establishment of neuronal polarity [48,49]. It is also conceivable that the polarized effect of LAT-1 signaling is promoted by a temporal cue which could not be investigated in this study.
In order to transduce signals LAT-1 is activated by a tethered agonist downstream of the GPS. In vitro and in vivo analyses identified a sequence of 12 amino acids exhibiting agonistic properties (Figs 3B and 4B). These findings are in accordance with recent data on GPR126 and GPR133 which revealed similar agonistic regions, termed Stachel sequences [24]. The biological implications of both studies are intriguing as they raise the hypothesis that several aGPCR share the same mechanism of activation.
Our data suggest that the activation mechanism is evolutionary conserved as latrophilin orthologs in rat and C. elegans both display an intrinsic agonistic sequence of a similar lengths. However, the tethered agonist of one latrophilin receptor is highly sequence-specific (Fig 3F), it is not able to activate its ortholog despite 59% identity/76% similarity of both agonistic motifs. As mutations at the conserved positions +1 and +3 within the Stachel sequence are sufficient to abolish LAT-1 function this specificity is likely to be conferred by structural properties. Consistently, an exchange of the GPS for the one of the paralog LAT-2 (69% identity/87% similarity within the Stachel sequence) results in a loss of LAT-1 function in development [25]. These data are also in concordance with the study identifying the Stachel sequence of GPR126 in which no cross-activation of Stachel sequence-derived agonistic peptides from GPR126 and another aGPCR, GPR133, is observed despite a high amino acid identity among the agonistic regions [24]. However, cross-activation between certain aGPCR orthologs or closely related aGPCRs via the Stachel sequence cannot be excluded and might have interesting implications.
Interestingly, the Stachel sequence identified in both latrophilin orthologs corresponds exactly to the respective C-terminal section of the GPS. These findings support our previous structure-function analyses which provided evidence for an interaction between LAT-1 GPS and 7TM domain [25]. Future studies need to focus on the details of the activation mechanism and clarify how the interaction is induced as well as which region of the 7TM is a potential interaction site. As we have previously shown that cleavage at the GPS is not essential for LAT-1 function, a model in which the tethered agonist functions after receptor cleavage is unlikely. Conformational changes within the GPS-containing GAIN domain upon binding to extracellular proteins could be the stimulus for exposure of the sequence within the GPS. The 7TM-independent function of LAT-1 is not based on a cAMP signal. However, the separation of this function from the 7TM-dependent function is likely to be conferred by the tethered agonist.
In summary, our results show that a GPCR-dependent G protein-signaling cascade based on LAT-1 is involved in oriented cell division in the early C. elegans embryo. LAT-1 activates a Gs protein/adenylyl cyclase signaling pathway, probably via GSA-1. By regulating cAMP levels, the receptor controls coordination of anterior-posterior cleavage plane orientation in the ABal cell. The data support a model in which LAT-1 resides in an inactive state while the Stachel sequence is not interacting with the 7TM domain (Fig 5). We hypothesize that an unknown extracellular cue causes the tethered agonist to contact the 7TM domain resulting in an increase of cAMP levels. This signal then promotes coordination of anterior-posterior cleavage plane orientation after the fourth round of cell divisions. Future studies need to focus on the effectors of the cAMP signal and how polar division plane orientation is coordinated on a molecular level by the identified non-polar signal.
Fig. 5. Model for LAT-1 signaling in the early embryo. 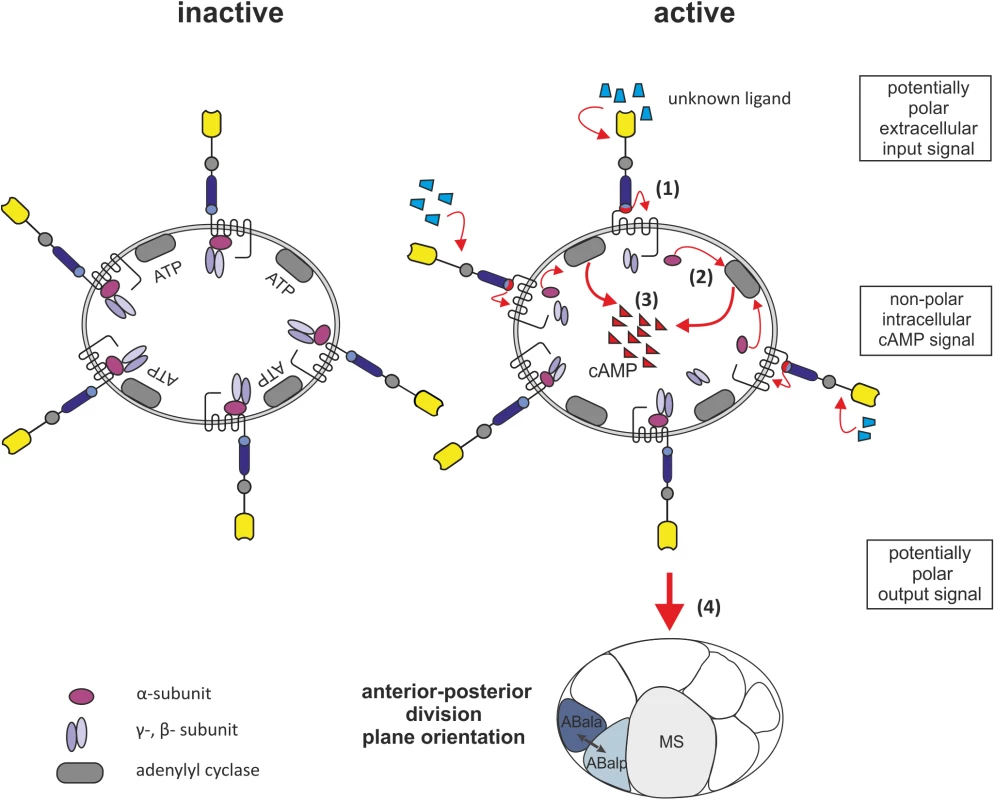
LAT-1 resides in an inactive state while the Stachel sequence is not interacting with the 7TM domain. Upon structural changes of the N terminus e.g. binding of an as yet unknown extracellular ligand, the tethered agonist contacts the 7TM domain (1). The Gs protein, likely to be GSA-1, then activates the adenylyl cyclase (2) resulting in an increase of cAMP levels (3). This signal then promotes coordination of anterior-posterior cleavage plane orientation after the fourth round of cell division (4). The cAMP signal within the cell is non-polar. Materials and Methods
C. elegans strains
C. elegans strains were cultured and manipulated according to standard protocols [50]. Wild-type worms were C. elegans variety Bristol, N2. The allele lat-1(ok1465) was generated by the C. elegans gene knockout consortium and provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The following transgenes have been previously described: aprEx157[lat-1(lat-2 GPS) (pSP75) rol-6(su1006) pBSK] [25], aprEx77[lat-1::gfp (pSP5) rol-6(su1006) pBSK] [14]. The transgene aprEx185[lat-1T530A/F532A(pSP94) rol-6(su1006) pBSK] was generated for this study (for details see Supporting Experimental Procedures).
In vitro functional assays
For functional assays, COS-7 cells were transiently transfected. To determine total as well as cell surface expression of receptors carrying N-terminal HA and C-terminal FLAG tags, indirect cellular enzyme-linked immunosorbent assays (ELISA) were employed [51]. cAMP concentrations were measured using the ALPHAScreen cAMP assay kit (PerkinElmer Life Sciences) according to the manufacturer's protocol. IP formation was determined as previously described [31]. Assay data was analyzed with GraphPad Prism version 5.0 (GraphPad Software). Statistics were performed using a two-way ANOVA in combination with Bonferroni as post-hoc test. For details see Supporting Experimental Procedures.
Drug and peptide experiments
All compounds were obtained from Sigma Aldrich. Forskolin was dissolved in DMSO to 10 mM, 3-isobutyl-1-methylxanthine (IBMX) in Dent´s buffer to 10 mM, 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP) in 1 N ammonium hydroxide to 250 mM and 2',5'-dideoxyadenosine (ddA) was diluted in DMSO to 100 mM prior to final dilution in Dent´s buffer. Peptides were synthesized (see Supporting Experimental Procedures) and dissolved in DMSO to 100 mM prior to final dilution in M9. Worms were incubated in 80 μM forskolin, 10 mM IBMX, 0.25 mM 8-Br-cAMP, 1 mM ddA or 0.1 mM peptide solution, respectively, containing E. coli OP50.
RNA interference
RNAi of gsa-1 was carried out using a feeding clone. The open reading frame of gsa-1 was amplified from total cDNA using primers RNAi_1/RNAi_2 (for primer sequences see S1 Table) and cloned into pCR4 (Life Technologies). It was then cloned into the NotI/SpeI sites of L4440 [52]. Feeding by RNAi was performed as previously described using the E. coli strain HT115 [53]. Embryos of the F1 and the F2 generation fed with the RNAi clone described were analyzed for spindle orientation in dividing blastomeres using 4D microscopy.
Quantitative PCR (qPCR)
Nematodes were collected and approximately 2,000 hermaphrodites were placed in TRIzol (Thermo Fisher Scientific). Total RNA was extracted following the manufacturer´s protocol. cDNA was obtained from 1 μg RNA using Omniscript RT kit (Qiagen) and random hexamer primers. qPCR analysis of gsa-1 was performed with primers lat1_1034F/lat1_1035R using a LightCycler PCR machine and GoTaq® qPCR Master Mix (Promega) according to manufacturer´s protocol. As internal reference genes the following were used: act-1 (primers SP1/SP2), cdc-42 (primers SP3/SP4), eif-3 (primers SP7/SP8), tba-1 (primers SP9/SP10). For primer sequences see S1 Table. Data analysis was performed utilizing MxPro QPCR Software (Agilent Technologies).
Western blot analysis
Approximately 2,000 hermaphrodites were placed in 100 μl M9 containing protease inhibitor (Roche) and sonicated with 15 30 s pulses in a Bioruptor Standard (Diagenode). Approximately 20 μl of sample were boiled in Laemmli buffer for 5 minutes. For the mammalian cell control, 3 × 105 HEK293 cells were lysed in Laemmli buffer for 5 minutes. Protein was subject to electrophoresis as described previously [54] using a 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a nitrocellulose membrane (Amersham). Blots were probed with rabbit anti-Gsα antibody (Merck Millipore) at 1 : 1,000 dilution overnight at 4°C and subsequently incubated for 2 hours at room temperature with a horseradish-peroxidase-conjugated goat anti-rabbit antibody (Sigma Aldrich) at 1 : 10,000 dilution. Western blots were developed by an enhanced chemiluminescence (ECL) detection system (Thermo Fisher Scientific). For detection of actin as loading control, membranes were stripped in Stripping buffer (1% SDS, 0.1 M Tris pH 6.8, 0.175% β-mercaptoethanol) for 30 minutes at 50°C, blocked and probed with mouse anti-actin (Merck Millipore) at 1 : 1,000 dilution, and then incubated with horseradish-peroxidase-conjugated rabbit anti-mouse (Sigma Aldrich) 1 : 10,000 and processed as described above. Antibody signals were quantified by densitometric analysis using ImageJ software [55].
Microscopy
4D DIC imaging and quantitative evaluation of division plane angles were performed as previously described using SIMI Biocell software (SIMI Reality Motion Systems) [56]. Embryos were dissected from young adult hermaphrodites incubated for 120 minutes in Dent`s buffer, 80 μM forskolin or respective solvents as control. Live images were taken with a Zeiss Axioplan 2e and a Zeiss Examiner. Z-stacks with spatial spacing of 1 μm were taken every 35 ms for 300 min. Confocal and fluorescent images were collected with Zeiss LSM5 and LSM510 Meta setups.
Lethality rescue assay
The lethality rescue assay was conducted as previously described [14]. Fifty L4 hermaphrodites were transferred into wells of a 72-well flat-bottom Terasaki plates (Greiner Bio-One) containing OP50 and allowed to lay eggs for 24 hours at 22°C. Five to ten eggs were transferred into fresh wells with corresponding solutions and incubated at 22°C. The number of dead/surviving embryos was scored 24 hours later, the number of adult animals 48 hours later on an inverted microscope. Data were examined with an unpaired two-tailed t test for each genotype and condition.
Supporting Information
Zdroje
1. Gillies TE, Cabernard C (2011) Cell division orientation in animals. Curr Biol 21: R599–609. doi: 10.1016/j.cub.2011.06.055 21820628
2. Lu MS, Johnston CA (2013) Molecular pathways regulating mitotic spindle orientation in animal cells. Development 140 : 1843–1856. doi: 10.1242/dev.087627 23571210
3. Segalen M, Bellaiche Y (2009) Cell division orientation and planar cell polarity pathways. Semin Cell Dev Biol 20 : 972–977. doi: 10.1016/j.semcdb.2009.03.018 19447051
4. Gonczy P, Rose LS (2005) Asymmetric cell division and axis formation in the embryo. WormBook. 2007/12/01 ed. pp. 1–20.
5. Kemphues K (2000) PARsing embryonic polarity. Cell 101 : 345–348. 10830161
6. Pellettieri J, Seydoux G (2002) Anterior-posterior polarity in C. elegans and Drosophila—PARallels and differences. Science 298 : 1946–1950. 12471246
7. Tsou MF, Ku W, Hayashi A, Rose LS (2003) PAR-dependent and geometry-dependent mechanisms of spindle positioning. J Cell Biol 160 : 845–855. 12642612
8. Sulston JE, Schierenberg E, White JG, Thomson JN (1983) The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100 : 64–119. 6684600
9. Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, et al. (1997) Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 90 : 707–716. 9288750
10. Thorpe CJ, Schlesinger A, Carter JC, Bowerman B (1997) Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell 90 : 695–705. 9288749
11. Walston T, Tuskey C, Edgar L, Hawkins N, Ellis G, et al. (2004) Multiple Wnt signaling pathways converge to orient the mitotic spindle in early C. elegans embryos. Dev Cell 7 : 831–841. 15572126
12. Hutter H, Schnabel R (1994) glp-1 and inductions establishing embryonic axes in C. elegans. Development 120 : 2051–2064. 7925009
13. Priess JR (2005) Notch signaling in the C. elegans embryo. WormBook. 2007/12/01 ed. pp. 1–16.
14. Langenhan T, Prömel S, Mestek L, Esmaeili B, Waller-Evans H, et al. (2009) Latrophilin signaling links anterior-posterior tissue polarity and oriented cell divisions in the C. elegans embryo. Dev Cell 17 : 494–504. doi: 10.1016/j.devcel.2009.08.008 19853563
15. Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, et al. (1997) alpha-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron 18 : 925–937. 9208860
16. Sudhof TC (2001) alpha-Latrotoxin and its receptors: neurexins and CIRL/latrophilins. Annu Rev Neurosci 24 : 933–962. 11520923
17. Willson J, Amliwala K, Davis A, Cook A, Cuttle MF, et al. (2004) Latrotoxin receptor signaling engages the UNC-13-dependent vesicle-priming pathway in C. elegans. Curr Biol 14 : 1374–1379. 15296755
18. Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, et al. (2003) Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol 13 : 1129–1133. 12842012
19. Strutt H, Strutt D (2008) Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol 18 : 1555–1564. doi: 10.1016/j.cub.2008.08.063 18804371
20. Monk KR, Oshima K, Jors S, Heller S, Talbot WS (2011) Gpr126 is essential for peripheral nerve development and myelination in mammals. Development 138 : 2673–2680. doi: 10.1242/dev.062224 21613327
21. Shima Y, Kengaku M, Hirano T, Takeichi M, Uemura T (2004) Regulation of dendritic maintenance and growth by a mammalian 7-pass transmembrane cadherin. Dev Cell 7 : 205–216. 15296717
22. Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, et al. (2004) G protein-coupled receptor-dependent development of human frontal cortex. Science 303 : 2033–2036. 15044805
23. Langenhan T, Aust G, Hamann J (2013) Sticky signaling—adhesion class G protein-coupled receptors take the stage. Sci Signal 6: re3. doi: 10.1126/scisignal.2003825 23695165
24. Liebscher I, Schöneberg T, Prömel S (2013) Progress in demystification of adhesion G protein-coupled receptors. Biol Chem 394 : 937–950. doi: 10.1515/hsz-2013-0109 23518449
25. Prömel S, Frickenhaus M, Hughes S, Mestek L, Staunton D, et al. (2012) The GPS motif is a molecular switch for bimodal activities of adhesion class G protein-coupled receptors. Cell Rep 2 : 321–331. doi: 10.1016/j.celrep.2012.06.015 22938866
26. Prömel S, Langenhan T, Arac D (2013) Matching structure with function: the GAIN domain of adhesion-GPCR and PKD1-like proteins. Trends Pharmacol Sci 34 : 470–478. doi: 10.1016/j.tips.2013.06.002 23850273
27. Arac D, Boucard AA, Bolliger MF, Nguyen J, Soltis SM, et al. (2012) A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. Embo J 31 : 1364–1378. doi: 10.1038/emboj.2012.26 22333914
28. de Mendoza A, Sebe-Pedros A, Ruiz-Trillo I (2014) The evolution of the GPCR signaling system in eukaryotes: modularity, conservation, and the transition to metazoan multicellularity. Genome Biol Evol 6 : 606–619. doi: 10.1093/gbe/evu038 24567306
29. Lefkowitz RJ, Cotecchia S, Samama P, Costa T (1993) Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci 14 : 303–307. 8249148
30. Eggerickx D, Denef JF, Labbe O, Hayashi Y, Refetoff S, et al. (1995) Molecular cloning of an orphan G-protein-coupled receptor that constitutively activates adenylate cyclase. Biochem J 309 (Pt 3): 837–843.
31. Bohnekamp J, Schöneberg T (2011) Cell adhesion receptor GPR133 couples to Gs protein. J Biol Chem 286 : 41912–41916. doi: 10.1074/jbc.C111.265934 22025619
32. Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR (1993) Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature 363 : 274–276. 8387644
33. Park JH, Ohshima S, Tani T, Ohshima Y (1997) Structure and expression of the gsa-1 gene encoding a G protein alpha(s) subunit in C. elegans. Gene 194 : 183–190. 9272860
34. Korswagen HC, Park JH, Ohshima Y, Plasterk RH (1997) An activating mutation in a Caenorhabditis elegans Gs protein induces neural degeneration. Genes Dev 11 : 1493–1503. 9203577
35. Liebscher I, Schon J, Petersen SC, Fischer L, Auerbach N, et al. (2014) A Tethered Agonist within the Ectodomain Activates the Adhesion G Protein-Coupled Receptors GPR126 and GPR133. Cell Rep 9 : 2018–2026. doi: 10.1016/j.celrep.2014.11.036 25533341
36. Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25 : 1189–1191. doi: 10.1093/bioinformatics/btp033 19151095
37. Herman MA, Wu M (2004) Noncanonical Wnt signaling pathways in C. elegans converge on POP-1/TCF and control cell polarity. Front Biosci 9 : 1530–1539. 14977564
38. Ruvkun G, Hobert O (1998) The taxonomy of developmental control in Caenorhabditis elegans. Science 282 : 2033–2041. 9851920
39. Sawa H, Lobel L, Horvitz HR (1996) The Caenorhabditis elegans gene lin-17, which is required for certain asymmetric cell divisions, encodes a putative seven-transmembrane protein similar to the Drosophila frizzled protein. Genes Dev 10 : 2189–2197. 8804313
40. Bergmann DC, Lee M, Robertson B, Tsou MF, Rose LS, et al. (2003) Embryonic handedness choice in C. elegans involves the Galpha protein GPA-16. Development 130 : 5731–5740. 14534142
41. Gotta M, Ahringer J (2001) Distinct roles for Galpha and Gbetagamma in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nat Cell Biol 3 : 297–300. 11231580
42. Tsou MF, Hayashi A, Rose LS (2003) LET-99 opposes Galpha/GPR signaling to generate asymmetry for spindle positioning in response to PAR and MES-1/SRC-1 signaling. Development 130 : 5717–5730. 14534135
43. Afshar K, Willard FS, Colombo K, Johnston CA, McCudden CR, et al. (2004) RIC-8 is required for GPR-1/2-dependent Galpha function during asymmetric division of C. elegans embryos. Cell 119 : 219–230. 15479639
44. Fievet BT, Rodriguez J, Naganathan S, Lee C, Zeiser E, et al. (2013) Systematic genetic interaction screens uncover cell polarity regulators and functional redundancy. Nat Cell Biol 15 : 103–112. doi: 10.1038/ncb2639 23242217
45. Cabello J, Neukomm LJ, Gunesdogan U, Burkart K, Charette SJ, et al. (2010) The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol 8: e1000297. doi: 10.1371/journal.pbio.1000297 20126385
46. Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, et al. (1997) Alpha-latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem 272 : 21504–21508. 9261169
47. Zallen JA (2007) Planar polarity and tissue morphogenesis. Cell 129 : 1051–1063. 17574020
48. Barnes AP, Solecki D, Polleux F (2008) New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr Opin Neurobiol 18 : 44–52. doi: 10.1016/j.conb.2008.05.003 18514505
49. Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM (2007) LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 129 : 565–577. 17482549
50. Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94. 4366476
51. Schöneberg T, Schulz A, Biebermann H, Gruters A, Grimm T, et al. (1998) V2 vasopressin receptor dysfunction in nephrogenic diabetes insipidus caused by different molecular mechanisms. Hum Mutat 12 : 196–205. 9711877
52. Timmons L, Fire A (1998) Specific interference by ingested dsRNA. Nature 395 : 854. 9804418
53. Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J (2001) Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2: RESEARCH0002.
54. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 : 680–685. 5432063
55. Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9 : 671–675. 22930834
56. Bischoff M, Schnabel R (2006) A posterior centre establishes and maintains polarity of the Caenorhabditis elegans embryo by a Wnt-dependent relay mechanism. PLoS Biol 4: e396. 17121454
Štítky
Genetika Reprodukční medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 10
-
Všechny články tohoto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



