-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCauses of Acute Hospitalization in Adolescence: Burden and Spectrum of HIV-Related Morbidity in a Country with an Early-Onset and Severe HIV Epidemic: A Prospective Survey
Background:
Survival to older childhood with untreated, vertically acquired HIV infection, which was previously considered extremely unusual, is increasingly well described. However, the overall impact on adolescent health in settings with high HIV seroprevalence has not previously been investigated.Methods and Findings:
Adolescents (aged 10–18 y) systematically recruited from acute admissions to the two public hospitals in Harare, Zimbabwe, answered a questionnaire and underwent standard investigations including HIV testing, with consent. Pre-set case-definitions defined cause of admission and underlying chronic conditions. Participation was 94%. 139 (46%) of 301 participants were HIV-positive (median age of diagnosis 12 y: interquartile range [IQR] 11–14 y), median CD4 count = 151; IQR 57–328 cells/µl), but only four (1.3%) were herpes simplex virus-2 (HSV-2) positive. Age (median 13 y: IQR 11–16 y) and sex (57% male) did not differ by HIV status, but HIV-infected participants were significantly more likely to be stunted (z-score<−2 : 52% versus 23%, p<0.001), have pubertal delay (15% versus 2%, p<0.001), and be maternal orphans or have an HIV-infected mother (73% versus 17%, p<0.001). 69% of HIV-positive and 19% of HIV-negative admissions were for infections, most commonly tuberculosis and pneumonia. 84 (28%) participants had underlying heart, lung, or other chronic diseases. Case fatality rates were significantly higher for HIV-related admissions (22% versus 7%, p<0.001), and significantly associated with advanced HIV, pubertal immaturity, and chronic conditions.Conclusion:
HIV is the commonest cause of adolescent hospitalisation in Harare, mainly due to adult-spectrum opportunistic infections plus a high burden of chronic complications of paediatric HIV/AIDS. Low HSV-2 prevalence and high maternal orphanhood rates provide further evidence of long-term survival following mother-to-child transmission. Better recognition of this growing phenomenon is needed to promote earlier HIV diagnosis and care.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(2): e32767. doi:10.1371/journal.pmed.1000178
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000178Summary
Background:
Survival to older childhood with untreated, vertically acquired HIV infection, which was previously considered extremely unusual, is increasingly well described. However, the overall impact on adolescent health in settings with high HIV seroprevalence has not previously been investigated.Methods and Findings:
Adolescents (aged 10–18 y) systematically recruited from acute admissions to the two public hospitals in Harare, Zimbabwe, answered a questionnaire and underwent standard investigations including HIV testing, with consent. Pre-set case-definitions defined cause of admission and underlying chronic conditions. Participation was 94%. 139 (46%) of 301 participants were HIV-positive (median age of diagnosis 12 y: interquartile range [IQR] 11–14 y), median CD4 count = 151; IQR 57–328 cells/µl), but only four (1.3%) were herpes simplex virus-2 (HSV-2) positive. Age (median 13 y: IQR 11–16 y) and sex (57% male) did not differ by HIV status, but HIV-infected participants were significantly more likely to be stunted (z-score<−2 : 52% versus 23%, p<0.001), have pubertal delay (15% versus 2%, p<0.001), and be maternal orphans or have an HIV-infected mother (73% versus 17%, p<0.001). 69% of HIV-positive and 19% of HIV-negative admissions were for infections, most commonly tuberculosis and pneumonia. 84 (28%) participants had underlying heart, lung, or other chronic diseases. Case fatality rates were significantly higher for HIV-related admissions (22% versus 7%, p<0.001), and significantly associated with advanced HIV, pubertal immaturity, and chronic conditions.Conclusion:
HIV is the commonest cause of adolescent hospitalisation in Harare, mainly due to adult-spectrum opportunistic infections plus a high burden of chronic complications of paediatric HIV/AIDS. Low HSV-2 prevalence and high maternal orphanhood rates provide further evidence of long-term survival following mother-to-child transmission. Better recognition of this growing phenomenon is needed to promote earlier HIV diagnosis and care.
: Please see later in the article for the Editors' SummaryIntroduction
In industrialised countries trauma and behavioural disorders, such as substance abuse, obesity, and sexually transmitted infections account for most adolescent morbidity [1]. Chronic diseases, trauma, oncology, and mental disorders account for the majority of hospital admissions [2],[3]. In Africa, research on adolescent morbidity has been limited and has predominantly focused on reproductive health, reflecting the high incidence of sexually transmitted infections and obstetric problems among young people [4],[5].
As the HIV epidemic matures, long-term survival of HIV-infected infants to adolescence following vertical transmission is increasingly being recognised in African countries [6],[7]. Symptomatic HIV in older children and adolescents is a growing problem in clinical practice but there are few data describing the burden of HIV and its contribution to ill-health and mortality in this age group [8],[9]. In Southern Africa—the global region that has experienced the most severe adult HIV epidemic [10]—a substantial epidemic of HIV-infected long-term survivors of vertical infection is anticipated during the coming decade, with implications for adolescent health [11].
The spectrum of HIV-related disease varies considerably between adults and children, but has not been well-defined for adolescents. Although opportunistic infections predominate in all age groups, chronic noninfectious clinical conditions may be more prominent in HIV-infected adolescents than in adults and infants [9]–[12]. In clinical studies of HIV infection, children are often classified as aged 0–14 y and adults as 15–49 y, and thus distinctive features of HIV-associated morbidity among adolescents may be missed [13]–[15]. The aim of this study was to investigate the prevalence of HIV and the spectrum of morbidity among hospitalised adolescents aged 10–18 y in Harare, Zimbabwe.
Methods
Study Participants
Harare Central Hospital and Parirenyatwa Hospital are the main public sector hospitals in Harare and cater to two-thirds of Harare's population; the remaining one-third using private health care facilities. The cost of hospitalisation in public sector facilities is partially subsidised by the government. Malaria transmission does not occur in Harare. Between September 2007 and April 2008, patients admitted to either hospital were enrolled consecutively the following day. Recruitment was limited to weekdays and to a maximum of five patients per site per day for logistical ease. Patients aged between 10 and 18 y admitted with any acute medical or surgical condition, including trauma, were eligible. Patients were excluded if moribund (i.e., likely to die within the next few hours), requiring intensive care admission, or admitted for obstetric or elective reasons, or previously enrolled into the study.
Data Collection
Standardised investigations, summarised in Figure 1, included social and clinical history, height, weight, and Tanner puberty staging, and laboratory investigations (full blood count, herpes simplex virus-2 [HSV-2] serology, provider-initiated HIV testing and counselling [PITC], and CD4+ lymphocyte [CD4+] count for HIV-infected participants). Where diagnostic HIV testing was declined, participants and their guardians were asked to consent to unreported HIV-testing for study purposes. In addition, all participants with febrile, wasting, or respiratory illness had a standardised infectious screen (blood culture, blood films for malaria, cryptococcal antigen testing [serum 1∶8 dilution], two sputum specimens, and chest radiography). If Pneumocystis jirovecii infection was suspected, patients underwent sputum induction with nebulised hypertonic saline.
Fig. 1. Study recruitment procedure for eligible participants. 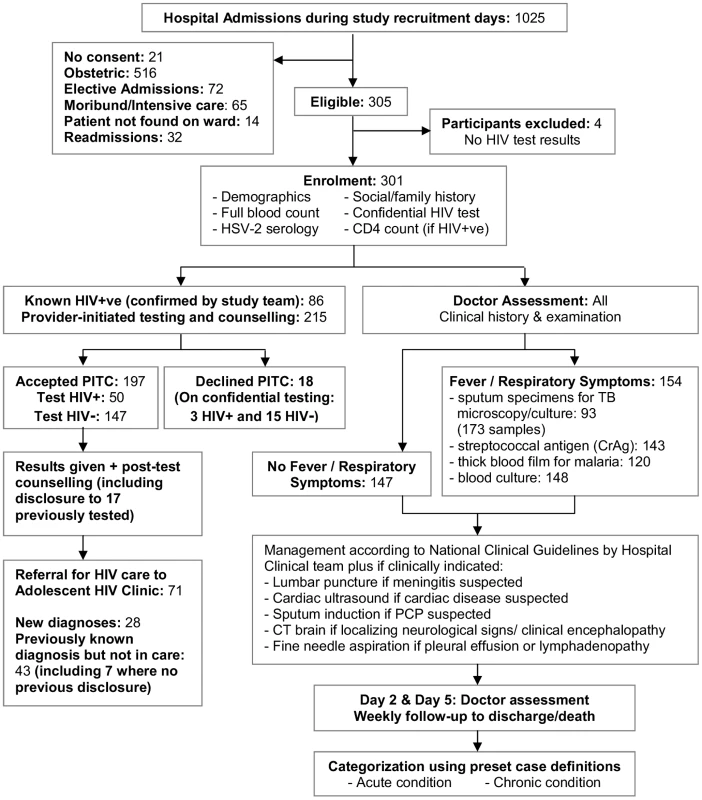
Otherwise investigations followed standard hospital guidelines including lumbar puncture for suspected meningitis and cerebral computed tomography (CT) for focal neurological signs or suspected encephalopathy. Participants were followed for the duration of their hospital stay. Pre-set diagnostic algorithms defined the definitive or presumptive cause of admission, and any underlying chronic conditions (Text S1). The Adult World Health Organization (WHO) Classification was used to stage HIV infection [16].
Laboratory Methods
HIV serology used parallel testing with Abbott Determine and SD Bioline. Discordant samples were resolved using Vironostika. HSV-2 antibodies were detected using recombinant IgG antigen (HerpeSelect, Focus Technologies; cut-off for positivity: OD>1.1). CD4 counts were determined by flow cytometry (CyFlow counter, Partec).
Blood culture used Myco/F Lytic (MFL, Becton Dickinson) [17], inspected daily using a handheld UV Woods lamp. Bacterial pathogens were identified by Gram staining and culture on conventional media, with biochemical tests for confirmation. Concentrated decontaminated sputum specimens and positive blood cultures were examined under fluorescent microscopy (Auramine-O) and cultured for mycobacteria (Lowenstein-Jensen media). CSF and positive blood cultures were investigated for Cryptococcus using India ink contrast staining, culture on Sabouraud media, and cryptococcal antigen testing (IMMY, Alpha Laboratories). Induced sputum was stained with Grocott's silver stain. Thick blood films for malaria were examined using Giemsa staining.
Statistical Analysis
Data were analysed with STATA software, version 10.0 (STATA Corporation). Continuous variables were compared using Student's t-test for normally distributed variables and Mann Whitney U test for variables not normally distributed. Categorical variables were compared using the Chi-squared (χ2) or Fisher's exact test as appropriate. Z-scores for height - and weight-for-age were calculated using British 1990 Growth Reference Curves, which provide data over the age of 10 y [18]. z-Scores of <−2 were considered to represent stunting and wasting, respectively [18].
Multivariate logistic regression was used for analysis of risk factors for mortality [19]. Risk factors were considered in two groups: socio-demographic (sex, age, orphanhood, difficulty raising clinic fees, tuberculosis [TB] or illness in a household member, food shortage, type of care-giver) and clinical (stage of HIV, previous TB, antiretroviral therapy [ART] status, stunting, wasting, pubertal delay, chronic disease, anaemia, poor performance scores, self-perceived poor health, broad diagnosis category). An initial model included socio-demographic factors and all factors that reached statistical significance at p<0.05 were included in a multivariate model. Factors that remained independently associated with death were retained. The association between each factor in the clinical group and death was assessed by adding each factor into the multivariate model that included the subset of independently significant socio-demographic factors. The multivariate model was built that included the subset of socio-demographic factors in the first multivariate model plus any clinical factors that were significant after adjusting for the socio-demographic factors. The final multivariate logistic regression model was reached by excluding single factors sequentially until all remaining factors were statistically significant.
Ethical Considerations
Written consent was obtained from all participants and from guardians of participants aged below 16 y. Onward referral for HIV care services was made for all testing HIV-positive. Guardians were encouraged to disclose HIV status to HIV-infected participants who did not know their status. Ethical approval for the study protocol was obtained from the Ethics Committees of the London School of Hygiene and Tropical Medicine and the Joint Research Ethics Committee at the University of Zimbabwe, Medical Research Council of Zimbabwe (MRCZ), Harare and Parirenyatwa Hospital Ethics Committees and the Institutional Review Board of the Biomedical Research and Training Institute.
Results
Baseline Participant Characteristics and HIV Diagnosis
Of 1,025 total adolescent hospital admissions, 340 were eligible. 301 participants were recruited, with a refusal rate of 6% (Figure 1). HIV prevalence was 46%. The median age was 13 y (interquartile range [IQR]: 11–16), and 43% of participants were female, with no significant association of age or sex by HIV status (Table 1). Four (1.3%) participants tested HSV-2 positive, of whom two were HIV-positive. HIV-infected participants were less likely to be married (1% versus 9%, p<0.032), although the comparison was based on small numbers.
Tab. 1. Baseline demographic, clinical, and growth characteristics of adolescents admitted to hospital (n = 301 unless specified otherwise). 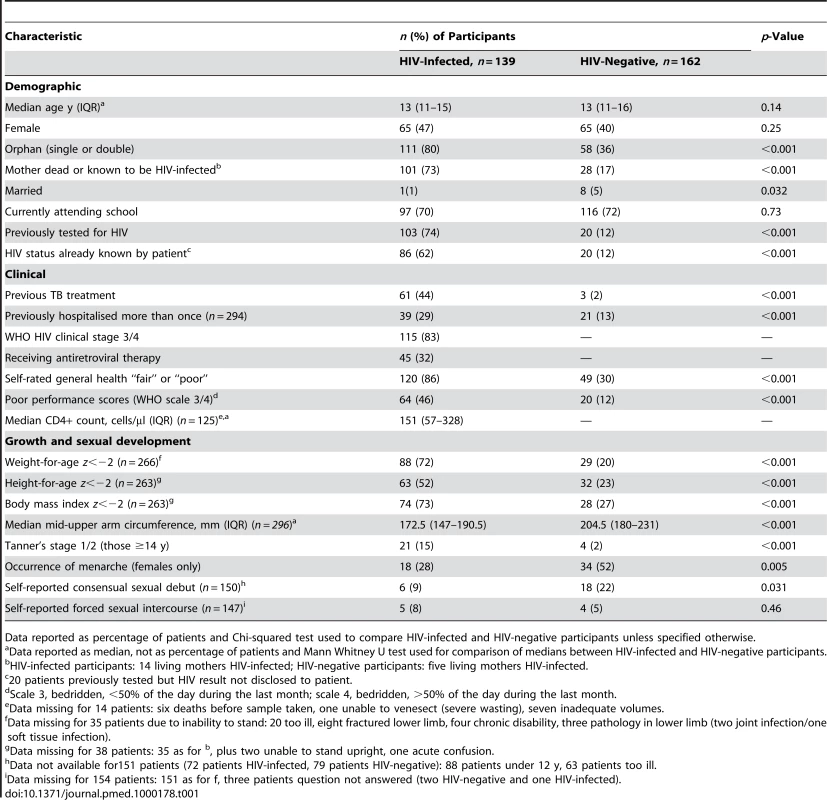
Data reported as percentage of patients and Chi-squared test used to compare HIV-infected and HIV-negative participants unless specified otherwise. The median age at diagnosis of HIV infection was 12 y (IQR: 11–14). Of the 139 participants who were HIV-positive, 86 (62%) had tested prior to admission and knew their HIV status. Fifty participants tested positive following PITC; of these, 17 had tested HIV-positive previously but had not been told of their HIV infection. All guardians, however, agreed to disclosure to the participant with assistance of study counsellors. Only 18 (6%) participants declined PITC, of whom three were HIV-positive on unreported HIV testing and are likely to remain unaware of their HIV infection.
HIV-infected participants were significantly more likely to be orphans, previously treated for tuberculosis, previously hospitalised more than once, stunted, and pubertally immature (Tanner's stage 1/2 for participants aged 14 y or older, and girls less likely to have undergone menarche) (Table 1).
HIV-infected patients were profoundly immunosuppressed at presentation: 83% had WHO stage 3 or 4 disease and 58% had CD4+ T lymphocyte counts <200 cells/µl. Only 44 (43%) of the HIV-infected participants who had previously had an HIV test were on ART (for a median duration of 121 d).
Causes of Hospitalisation and CD4+ Counts by Diagnostic Group
The most frequent diagnosis among HIV-infected participants was infection with tuberculosis, pneumonia, cryptococcosis, and blood stream infections being the most frequent diagnoses. Among HIV-negative participants, the commonest cause of admission was trauma, followed by acute exacerbations of chronic medical conditions, predominantly cardiac (Table 2). The median duration of stay in hospital for HIV-infected participants was 9 d (IQR 6–16 d) and for HIV-negative participants 7 d (IQR 4–18 d).
Tab. 2. Causes of admission among adolescents admitted to hospital. 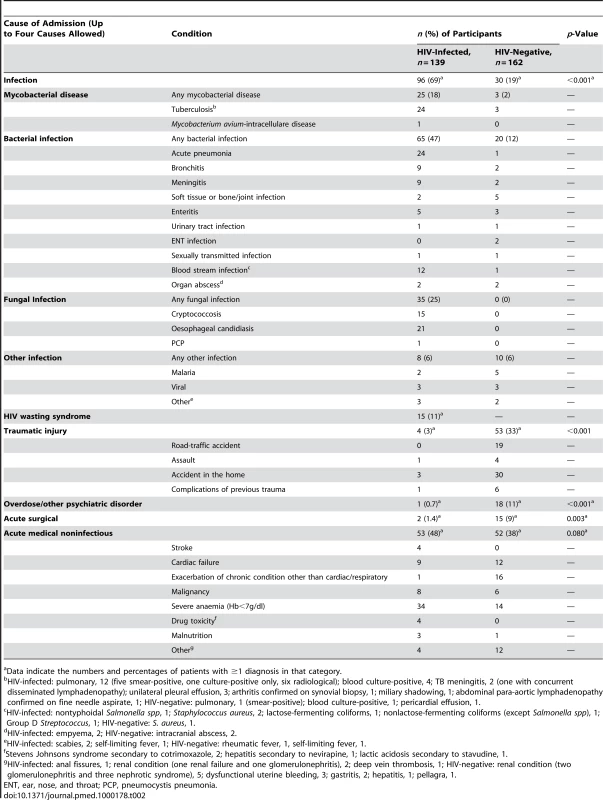
Data indicate the numbers and percentages of patients with ≥1 diagnosis in that category. The highest median CD4 counts were found in patients presenting with trauma or acute surgical causes conditions (455 and 628 cells/µl). Patients with blood stream infections, oesophageal candidiasis, and HIV-wasting syndrome had the lowest median CD4 counts (85, 77, and 34 cells/µl, respectively).
Disease-Specific Microbiological Findings
113 (81%) and 41 (25%) of HIV-infected and HIV-negative admissions (p<0.001) met criteria for the standardised infectious screen (see Methods), of whom 20 (18%) and two (5%) had positive blood cultures, respectively. The most frequently identified pathogens in HIV-infected participants were nontyphoidal Salmonella species and M. tuberculosis. Cryptococcus spp. were identified in blood culture in three patients, in cerebrospinal fluid (CSF) culture in five patients, and seven patients had a positive cryptococcal antigen (CrAg) only.
There were 27 TB diagnoses of which 13 were pulmonary TB: six were sputum smear - positive, one was culture-positive only, and six were radiological diagnoses (smear - and culture-negative pulmonary disease with failure to respond to broad-spectrum antibiotics, but response to TB treatment at 1 mo). M. tuberculosis was identified in blood culture in five patients.
Chronic Clinical Conditions
84 (28%) participants had underlying chronic medical conditions other than HIV (26% in HIV-infected versus 29% in HIV-negative, p<0.56). In addition, 70% of HIV-infected participants had chronic skin complaints, although rarely responsible for admission (Table 3). Admission as a result of acute exacerbation of a chronic condition accounted for 26 (19%) and 44 (27%) admissions in HIV-infected and HIV-negative participants, respectively (p<0.082). Chronic lung disease and cardiac disease were the most common serious HIV-related complications (Table 3). In HIV-negative participants, rheumatic heart disease was the most common chronic condition.
Tab. 3. Chronic conditions among adolescents admitted to hospital. 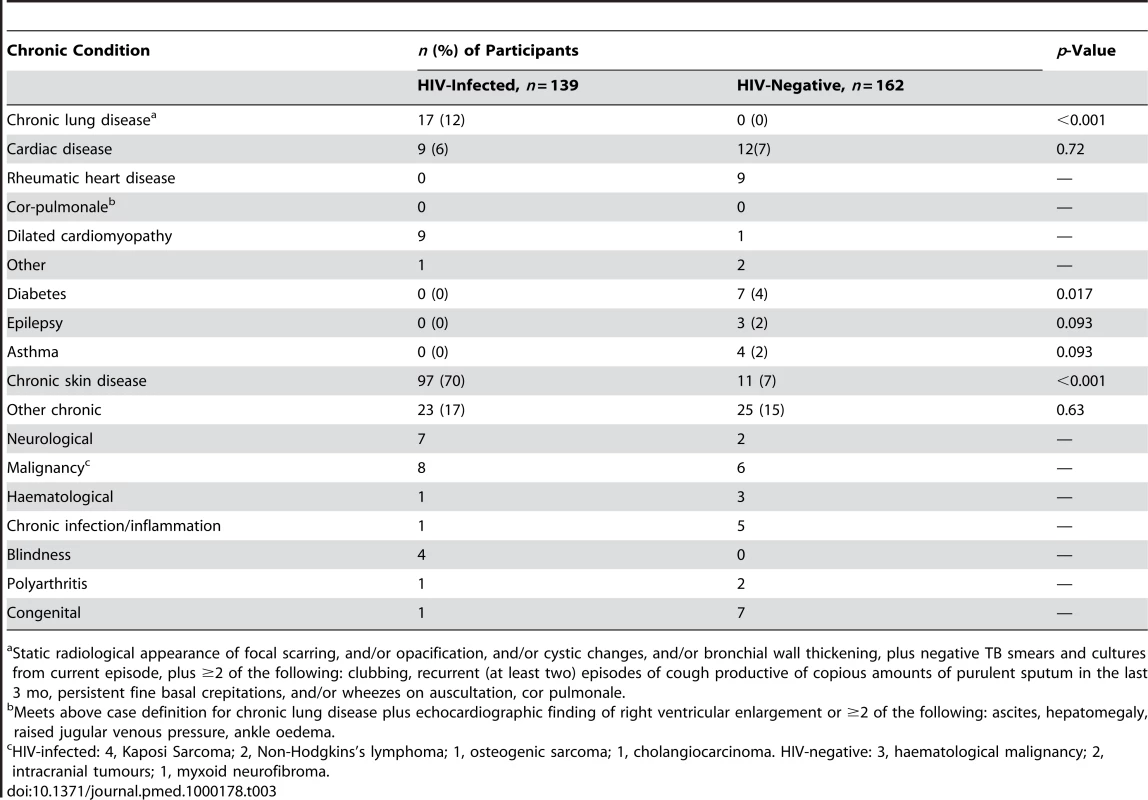
Static radiological appearance of focal scarring, and/or opacification, and/or cystic changes, and/or bronchial wall thickening, plus negative TB smears and cultures from current episode, plus ≥2 of the following: clubbing, recurrent (at least two) episodes of cough productive of copious amounts of purulent sputum in the last 3 mo, persistent fine basal crepitations, and/or wheezes on auscultation, cor pulmonale. Causes of and Risk Factors for Death in Hospital
32/139 (23%) HIV-infected participants died in hospital compared with 11/162 (7%) HIV-negative participants (sex - and age-adjusted odds ratio [OR] for death for HIV-infected patients 3.7, 95% [confidence interval] CI 1.7–7.8, p<0.001). Death was significantly associated with low CD4+ T lymphocyte count (74 versus 183 cells/µl, p<0.0029). The highest case-fatality rates among HIV-infected participants were from HIV wasting syndrome (53%), any malignancy (50%), and drug toxicity (50%), and among HIV-negative participants the highest case-fatality rates were for malignancy (83%) (Table 4).
Tab. 4. Causes of death among adolescents admitted to hospital. 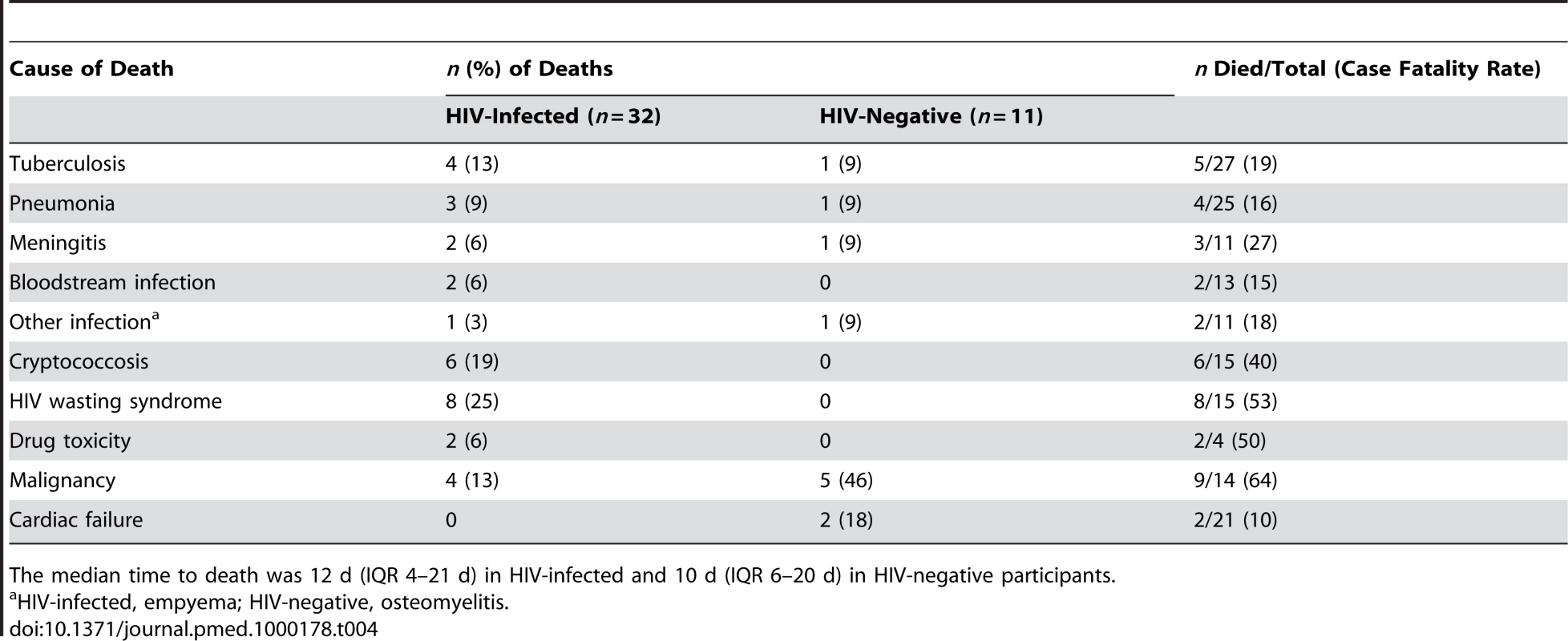
The median time to death was 12 d (IQR 4–21 d) in HIV-infected and 10 d (IQR 6–20 d) in HIV-negative participants. Of the considered socio-demographic risk factors, younger age and having a primary care-giver who was not the parent were associated with an increased risk of death (Table 5). After adjusting for these factors, advanced HIV disease, severe anaemia, TB treatment in the past, poor self-rated health, a chronic disease (except chronic skin disease), pubertal delay (Tanner puberty stage 1/2 in those aged 14 y or above), poor performance scores, wasting, and stunting were all associated with increased risk of death. In the final multivariate model, WHO stage 4 HIV infection (OR 2.8, 95% CI 1.1–7.1; p<0.032), chronic disease (OR 2.8, 95% CI 1.3–6.0; p<0.009), pubertal delay (OR 4.0, 95% C.I 1.4–11.6; p<0.011), and poor performance scores (OR 6.9, 95% C.I 3.0–15.8; p<0.001) remained independently associated with increased risk of death.
Tab. 5. Risk factors for death among adolescents admitted to hospital (n = 301 unless specified). 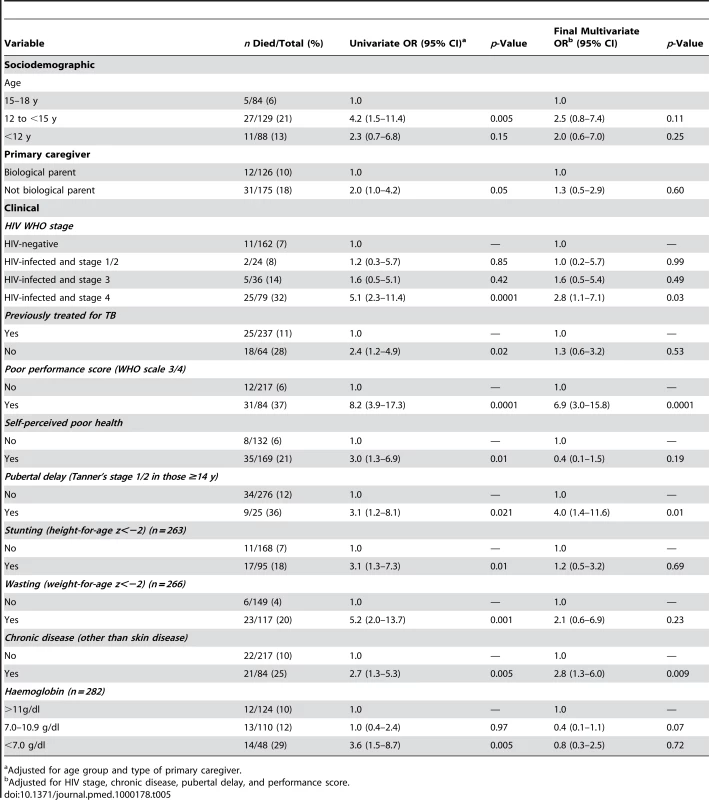
Adjusted for age group and type of primary caregiver. Discussion
The main finding of this study is that HIV is now the single most common cause of acute admission and in-hospital death among adolescents in Harare. HIV-infected adolescents were profoundly immunosuppressed, and the median CD4 count (51 cells/µl) was similar to that reported in other studies of hospitalised African adults in the pre-ART era [20],[21]. The spectrum of HIV-associated infections was also similar to that reported in African adults in the pre-ART era [15]. However, adolescents had an additional and heavy burden of chronic complications—such as growth failure and lung and cardiac disease—that have typically been reported in vertically HIV-infected children [22]–[24]. In this study, underlying chronic complications were associated with an increased risk of in-hospital death.
We found an equal sex distribution of HIV infection, low rates of self-reported sexual debut, and a much lower prevalence of HSV-2 infection than would be anticipated for sexually acquired HIV in southern Africans [25]. These findings, along with strong associations with orphanhood and the chronic complications discussed above, and negative association with marriage (given that marriage at young age increases risk of HIV for young African girls [26]), all favour long-term survival following mother-to-child transmission as the source of HIV infection in most of our participants. Also notable is the fact that Zimbabwe has had an unusually good safety record for preventing percutaneous HIV from early on in the HIV epidemic [27], and population-based HIV prevalence surveys among older children in southern Africa have documented substantial HIV prevalence rates with equal gender distribution [28],[29]. The widely held perception is that HIV progresses rapidly in HIV-infected infants with a median survival of 2 y [30], and thus survival to late childhood and adolescence with untreated HIV has been considered extremely unusual. Our study, however, demonstrates that long-term survival occurs and is now a major cause of morbidity among adolescents.
Diagnosis of HIV infection was frequently delayed in this age group until presentation with advanced HIV disease. A substantial minority had not been diagnosed with HIV before hospitalisation, and most others reported relatively recent diagnosis following a prolonged history of recurrent infections. The beneficial effects of early diagnosis and ART on reducing the risk of opportunistic infections and chronic complications, reducing mortality, and improving growth are well-recognised in children [31]. In addition to the high risk of irreversible complications, older age at diagnosis and delay in starting ART may potentially result in suboptimal immune response [32],[33] and blunted catch-up growth and pubertal development [34],[35].
Although the phenomenon of long-term survival in HIV-infected infants is well recognised in Zimbabwe, several factors unique to this age group may be creating both demand and supply-side barriers to diagnostic testing: in contrast to adults, there are no free-standing counselling and testing services for under 16-y-olds in Zimbabwe, and there are also legal barriers to testing without the permission of the legal guardian, who may be incapacitated or absent. Reluctance of guardians to disclose the true nature of the underlying illness was also apparent in that a substantial minority of our HIV-infected participants had not been told their previous test results. Clear advice to health professionals to offer PITC and to assist guardians with disclosure may improve timely diagnosis and adherence to subsequent ART [36].
Our study was hospital-based and therefore likely to be biased towards sicker patients and more severe HIV-associated complications. One of the study sites was based at a referral hospital, which may have resulted in a higher proportion of specialist diagnoses such as cancer. Both hospitals had ART clinics, but only nine (6%) of the HIV-infected participants were admitted from these. The vast majority of our participants (87%) were referred directly from primary health care clinics or through hospital casualty departments, and the only local alternatives to these two hospitals are private facilities. Our results are, therefore, likely to be representative of the pattern of acute severe morbidity and mortality in Harare. Judging by the high numbers of adolescents attending HIV care clinics throughout Zimbabwe [37], our results may also be more generally representative. Zimbabwe is unusual in having had high HIV prevalence in antenatal clinic attendees from the early 1990s [38], and so may be a few years ahead of other countries in the region with respect to the subsequent epidemic of adolescent survivors.
HIV-related morbidity and mortality, most likely reflecting long-term survival from the paediatric HIV epidemic in the 1990s, is now a major cause of adolescent morbidity and mortality in Harare. Recognition of the high burden of HIV in acutely unwell older children and adolescents is needed to stimulate earlier diagnosis and improve access to HIV care for this previously neglected age group. Our results strongly support implementation of PITC for all patients in this age group attending health facilities, ideally at the primary care as well as hospital level, so that diagnosis is made and treatment can be started before patients are critically ill.
Supporting Information
Zdroje
1. SellsCW
BlumRW
1996 Morbidity and mortality among US adolescents: an overview of data and trends. Am J Public Health 86 513 519
2. CaflischM
AlvinP
2000 Management of adolescents in pediatric hospitals. A national survey. Arch Pediatr 7 732 737
3. RosenEU
1988 Adolescent health problems–can paediatricians in the RSA cope? S Afr Med J 73 337 339
4. ObasiAI
BaliraR
ToddJ
RossDA
ChangaluchaJ
2001 Prevalence of HIV and Chlamydia trachomatis infection in 15–19-year olds in rural Tanzania. Trop Med Int Health 6 517 525
5. AgyeiWK
EpemaEJ
LubegaM
1992 Contraception and prevalence of sexually transmitted diseases among adolescents and young adults in Uganda. Int J Epidemiol 21 981 988
6. StoverJ
WalkerN
GrasslyNC
MarstonM
2006 Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Infect 82 iii45 iii50
7. MarstonM
ZabaB
SalomonJA
BrahmbhattH
BagendaD
2005 Estimating the net effect of HIV on child mortality in African populations affected by generalized HIV epidemics. J Acquir Immune Defic Syndr 38 219 227
8. WalkerAS
MulengaV
SinyinzaF
LishimpiK
NunnA
2006 Determinants of survival without antiretroviral therapy after infancy in HIV-1-infected Zambian children in the CHAP Trial. J Acquir Immune Defic Syndr 42 637 645
9. FerrandRA
LuethyR
BwakuraF
MujuruH
MillerRF
2007 HIV infection presenting in older children and adolescents: a case series from Harare, Zimbabwe. Clin Infect Dis 44 874 878
10. UNAIDS 2008 Report on the global HIV/AIDS epidemic Geneva UNAIDS
11. FerrandRA
CorbettEL
WoodR
HargroveJ
NdhlovuCE
2009 AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. Aids 23 2039 2046
12. ShahI
2005 Age related clinical manifestations of HIV infection in Indian children. J Trop Pediatr 51 300 303
13. BakakiP
KayitaJ
Moura MachadoJE
CoulterJB
TindyebwaD
2001 Epidemiologic and clinical features of HIV-infected and HIV-uninfected Ugandan children younger than 18 months. J Acquir Immune Defic Syndr 28 35 42
14. BobatR
CoovadiaH
MoodleyD
CoutsoudisA
1999 Mortality in a cohort of children born to HIV-1 infected women from Durban, South Africa. S Afr Med J 89 646 648
15. GrantAD
DjomandG
SmetsP
KadioA
CoulibalyM
1997 Profound immunosuppression across the spectrum of opportunistic disease among hospitalized HIV-infected adults in Abidjan, Cote d'Ivoire. AIDS 11 1357 1364
16. World Health Organization 2005 Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance - African Region Geneva World Health Organization
17. ArchibaldLK
McDonaldLC
AddisonRM
McKnightC
ByrneT
2000 Comparison of BACTEC MYCO/F LYTIC and WAMPOLE ISOLATOR 10 (lysis-centrifugation) systems for detection of bacteremia, mycobacteremia, and fungemia in a developing country. J Clin Microbiol 38 2994 2997
18. ColeTJ
1997 Growth monitoring with the British 1990 growth reference. Arch Dis Child 76 47 49
19. VictoraCG
HuttlySR
FuchsSC
OlintoMT
1997 The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol 26 224 227
20. CorbettEL
ChurchyardGJ
CharalambosS
SambB
MoloiV
2002 Morbidity and mortality in South African gold miners: impact of untreated disease due to human immunodeficiency virus. Clin Infect Dis 34 1251 1258
21. GrantAD
SidibeK
DomouaK
BonardD
Sylla-KokoF
1998 Spectrum of disease among HIV-infected adults hospitalised in a respiratory medicine unit in Abidjan, Cote d'Ivoire. Int J Tuberc Lung Dis 2 926 934
22. JeenaPM
CoovadiaHM
ThulaSA
BlytheD
BuckelsNJ
1998 Persistent and chronic lung disease in HIV-1 infected and uninfected African children. Aids 12 1185 1193
23. ArpadiSM
2000 Growth failure in children with HIV infection. J Acquir Immune Defic Syndr 25 S37 S42
24. BuchaczK
RogolAD
LindseyJC
WilsonCM
HughesMD
2003 Delayed onset of pubertal development in children and adolescents with perinatally acquired HIV infection. J Acquir Immune Defic Syndr 33 56 65
25. WeissHA
BuveA
RobinsonNJ
Van DyckE
KahindoM
2001 The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. AIDS 15 S97 S108
26. GregsonS
NyamukapaCA
GarnettGP
MasonPR
ZhuwauT
2002 Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet 359 1896 1903
27. LopmanBA
GarnettGP
MasonPR
GregsonS
2005 Individual level injection history: a lack of association with HIV incidence in rural Zimbabwe. PLoS Med 2 e37 doi:10.1371/journal.pmed.0020037
28. GomoE
RusakanikoS
MashangeW
MutswangaJ
ChandiwanaB
2005 Household survey of HIV-prevalence and behaviour in Chimanimani District, Zimbabwe Cape Town, South Africa Human Social Research Council
29. BrookesH
ShisanaO
RichterL
2004 The national household HIV prevalence and risk survey of South African children Cape Town Human Social Research Council
30. NewellML
CoovadiaH
Cortina-BorjaM
RollinsN
GaillardP
2004 Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 364 1236 1243
31. SohCH
OleskeJM
BradyMT
SpectorSA
BorkowskyW
2003 Long-term effects of protease-inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet 362 2045 2051
32. WalkerAS
DoerholtK
SharlandM
GibbDM
2004 Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV. Paediatric Study Aids 18 1915 1924
33. NewellML
PatelD
GoetghebuerT
ThorneC
2006 CD4 cell response to antiretroviral therapy in children with vertically acquired HIV infection: is it associated with age at initiation? J Infect Dis 193 954 962
34. Bakeera-KitakaS
McKellarM
SniderC
KekitiinwaA
PiloyaT
2008 Antiretroviral therapy for HIV-1 infected adolescents in Uganda: assessing the impact on growth and sexual maturation. Pediatr Infect Dis J 3 97 104
35. KekitiinwaA
LeeKJ
WalkerAS
MagandaA
DoerholtK
2008 Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr 49 384 392
36. Bikaako-KajuraW
LuyirikaE
PurcellDW
DowningJ
KaharuzaF
2006 Disclosure of HIV status and adherence to daily drug regimens among HIV-infected children in Uganda. AIDS Behav 10 S85 S93
37. FerrandRA
LoweS
WhandeB
MunaiwaL
LanghaugL
2009 Survey of children accessing HIV services in a high HIV prevalence setting: time for HIV-infected adolescents to count? Bull World Health Organ In press
38. Ministry of Health and Child Welfare 2007 Zimbabwe national HIV and AIDS estimates 2007 Harare, Zimbabwe Ministry of Health and Child Welfare
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 2- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Effectiveness of Non-nucleoside Reverse-Transcriptase Inhibitor-Based Antiretroviral Therapy in Women Previously Exposed to a Single Intrapartum Dose of Nevirapine: A Multi-country, Prospective Cohort Study
- The Global Research Neglect of Unassisted Smoking Cessation: Causes and Consequences
- Adolescent HIV—Cause for Concern in Southern Africa
- Impact of Antiretroviral Therapy on Incidence of Pregnancy among HIV-Infected Women in Sub-Saharan Africa: A Cohort Study
- Automated Detection of Infectious Disease Outbreaks in Hospitals: A Retrospective Cohort Study
- Event Rates, Hospital Utilization, and Costs Associated with Major Complications of Diabetes: A Multicountry Comparative Analysis
- Pretreatment CD4 Cell Slope and Progression to AIDS or Death in HIV-Infected Patients Initiating Antiretroviral Therapy—The CASCADE Collaboration: A Collaboration of 23 Cohort Studies
- Can Broader Diffusion of Value-Based Insurance Design Increase Benefits from US Health Care without Increasing Costs? Evidence from a Computer Simulation Model
- Causes of Acute Hospitalization in Adolescence: Burden and Spectrum of HIV-Related Morbidity in a Country with an Early-Onset and Severe HIV Epidemic: A Prospective Survey
- Developing Global Maps of the Dominant Vectors of Human Malaria
- Guidance for Developers of Health Research Reporting Guidelines
- Packages of Care for Attention-Deficit Hyperactivity Disorder in Low- and Middle-Income Countries
- A New Policy on Tobacco Papers
- Ghostwriting at Elite Academic Medical Centers in the United States
- Measuring hsCRP—An Important Part of a Comprehensive Risk Profile or a Clinically Redundant Practice?
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Packages of Care for Attention-Deficit Hyperactivity Disorder in Low- and Middle-Income Countries
- Measuring hsCRP—An Important Part of a Comprehensive Risk Profile or a Clinically Redundant Practice?
- Developing Global Maps of the Dominant Vectors of Human Malaria
- Guidance for Developers of Health Research Reporting Guidelines
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



