-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaHeart Failure Care in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
Background:
Heart failure places a significant burden on patients and health systems in high-income countries. However, information about its burden in low - and middle-income countries (LMICs) is scant. We thus set out to review both published and unpublished information on the presentation, causes, management, and outcomes of heart failure in LMICs.Methods and Findings:
Medline, Embase, Global Health Database, and World Health Organization regional databases were searched for studies from LMICs published between 1 January 1995 and 30 March 2014. Additional unpublished data were requested from investigators and international heart failure experts. We identified 42 studies that provided relevant information on acute hospital care (25 LMICs; 232,550 patients) and 11 studies on the management of chronic heart failure in primary care or outpatient settings (14 LMICs; 5,358 patients). The mean age of patients studied ranged from 42 y in Cameroon and Ghana to 75 y in Argentina, and mean age in studies largely correlated with the human development index of the country in which they were conducted (r = 0.71, p<0.001). Overall, ischaemic heart disease was the main reported cause of heart failure in all regions except Africa and the Americas, where hypertension was predominant. Taking both those managed acutely in hospital and those in non-acute outpatient or community settings together, 57% (95% confidence interval [CI]: 49%–64%) of patients were treated with angiotensin-converting enzyme inhibitors, 34% (95% CI: 28%–41%) with beta-blockers, and 32% (95% CI: 25%–39%) with mineralocorticoid receptor antagonists. Mean inpatient stay was 10 d, ranging from 3 d in India to 23 d in China. Acute heart failure accounted for 2.2% (range: 0.3%–7.7%) of total hospital admissions, and mean in-hospital mortality was 8% (95% CI: 6%–10%). There was substantial variation between studies (p<0.001 across all variables), and most data were from urban tertiary referral centres. Only one population-based study assessing incidence and/or prevalence of heart failure was identified.Conclusions:
The presentation, underlying causes, management, and outcomes of heart failure vary substantially across LMICs. On average, the use of evidence-based medications tends to be suboptimal. Better strategies for heart failure surveillance and management in LMICs are needed.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(8): e32767. doi:10.1371/journal.pmed.1001699
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001699Summary
Background:
Heart failure places a significant burden on patients and health systems in high-income countries. However, information about its burden in low - and middle-income countries (LMICs) is scant. We thus set out to review both published and unpublished information on the presentation, causes, management, and outcomes of heart failure in LMICs.Methods and Findings:
Medline, Embase, Global Health Database, and World Health Organization regional databases were searched for studies from LMICs published between 1 January 1995 and 30 March 2014. Additional unpublished data were requested from investigators and international heart failure experts. We identified 42 studies that provided relevant information on acute hospital care (25 LMICs; 232,550 patients) and 11 studies on the management of chronic heart failure in primary care or outpatient settings (14 LMICs; 5,358 patients). The mean age of patients studied ranged from 42 y in Cameroon and Ghana to 75 y in Argentina, and mean age in studies largely correlated with the human development index of the country in which they were conducted (r = 0.71, p<0.001). Overall, ischaemic heart disease was the main reported cause of heart failure in all regions except Africa and the Americas, where hypertension was predominant. Taking both those managed acutely in hospital and those in non-acute outpatient or community settings together, 57% (95% confidence interval [CI]: 49%–64%) of patients were treated with angiotensin-converting enzyme inhibitors, 34% (95% CI: 28%–41%) with beta-blockers, and 32% (95% CI: 25%–39%) with mineralocorticoid receptor antagonists. Mean inpatient stay was 10 d, ranging from 3 d in India to 23 d in China. Acute heart failure accounted for 2.2% (range: 0.3%–7.7%) of total hospital admissions, and mean in-hospital mortality was 8% (95% CI: 6%–10%). There was substantial variation between studies (p<0.001 across all variables), and most data were from urban tertiary referral centres. Only one population-based study assessing incidence and/or prevalence of heart failure was identified.Conclusions:
The presentation, underlying causes, management, and outcomes of heart failure vary substantially across LMICs. On average, the use of evidence-based medications tends to be suboptimal. Better strategies for heart failure surveillance and management in LMICs are needed.
Please see later in the article for the Editors' SummaryIntroduction
In high-income countries (HICs), heart failure is a well-recognized public health problem representing a significant burden for patients and healthcare systems [1],[2]. For example, in the UK and US, heart failure is one of the leading causes of hospitalisation, and despite recent advances, outcomes remain poor [3]–[6]. Of those hospitalised for heart failure in the UK, about 10% will die during admission [6]. In the US, between 20% and 27% of those who survive to discharge will be re-admitted within 30 d [7], whilst 5-y mortality rates range between 40% and 65% amongst the US, UK, Netherlands, and Sweden [2]–[4],[8],[9]. The costs associated with heart failure care are also substantial. In many HICs, heart failure typically consumes 1%–2% of healthcare resources [2], mainly because of repeated admissions to hospitals and prolonged inpatient stays.
With demographic changes and the epidemiological transition to non-communicable diseases [10],[11], heart failure is expected to become a major public health issue in low - and middle-income countries (LMICs). Yet systematic evidence for its current burden to patients and health services is limited [1],[2],[12]. In fact, the last review of the burden of heart failure in LMICs, conducted over ten years ago, found no population studies and concluded that published data on heart failure epidemiology were almost entirely absent from most populations across the world [12]. As a result, many of our assumptions regarding the current burden of this condition worldwide are based on extrapolations from studies conducted in HICs, which may not be appropriate [1],[2].
Therefore, we sought to conduct a systematic review of both published and unpublished data regarding the patterns of heart failure presentation, management, and outcomes in LMICs.
Methods
This systematic review was designed and undertaken according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13]. A study protocol describing the methodology has been published previously [14]. In brief, we searched Medline, Embase, Global Health Database, and WHO regional databases for articles published between 1 January 1995 and 30 March 2014 with the subject terms “heart failure” or “cardiomyopathies” or any related terms AND “incidence”, “prevalence”, “cause*”, “etiology”, “aetiology”, “epidemiolog*”, “burden”, “management”, “treatment”, “prevent*”, “population based”, “community”, “trends”, “survey”, “surveillance”, “mortality”, “morbidity”, “fatalit*”, or “attack rate”. Relevant studies from LMICs on the epidemiology, diagnosis, management, and outcomes of heart failure were included. There were no language restrictions. We also scrutinised the reference lists of study reports and review articles, and inquired among our collaborators and international heart failure experts about any additional databases or studies of which they may be aware. We further searched the Institute for Health Metrics and Evaluation's Global Health Data Exchange as well as the websites of regional and country-specific societies of cardiology to identify further datasets.
Figure 1 summarises the retrieval and selection process for studies and relevant databases. After removing duplicate reports, two reviewers independently screened all titles and abstracts for their potential eligibility and extracted data using a pre-designed form. Studies were eligible for inclusion if they reported on heart failure patients from LMICs as defined by the World Bank [15]. Studies must have reported on at least 100 cases and contained relevant information on demographic characteristics, prevalence, case fatality, underlying aetiology, or management of patients with heart failure. Studies confined to subgroups of patients with heart failure (for example, those that included only dilated cardiomyopathy or heart failure as a complication of acute myocardial infarction) were excluded, as were studies that clearly did not include a representative sample of patients from the setting chosen (for example, studies that selected people referred to an echocardiography department, or studies that excluded adult populations) [14]. Investigators of multinational studies that had not reported findings by country were contacted for country-specific data.
Fig. 1. Data acquisition flowchart. 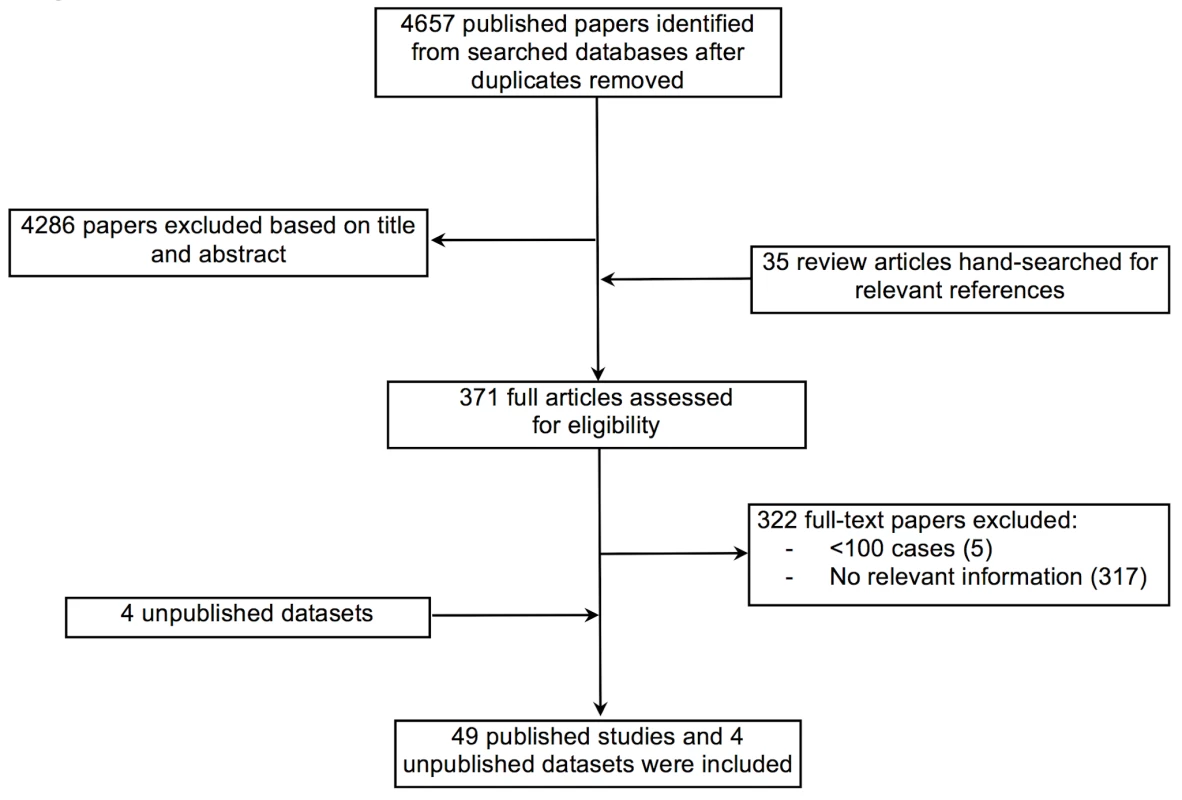
Quality Assessment
In order to capture a comprehensive overview of heart failure in LMICs, a wide range of studies, each with differing objectives and designs, were included. Studies meeting the minimum quality requirement, as specified below, for inclusion were analysed for both methodological limitations and reporting quality, using items from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [16] (Tables 1–6). Specifically, the sample size of each study, the location and type of healthcare facility, diagnostic methods used, and patient selection criteria were documented. In addition, we assessed each study's specific methodological strengths and weaknesses as well as likely external validity.
Tab. 1. Characteristics of Africa region studies and databases included. 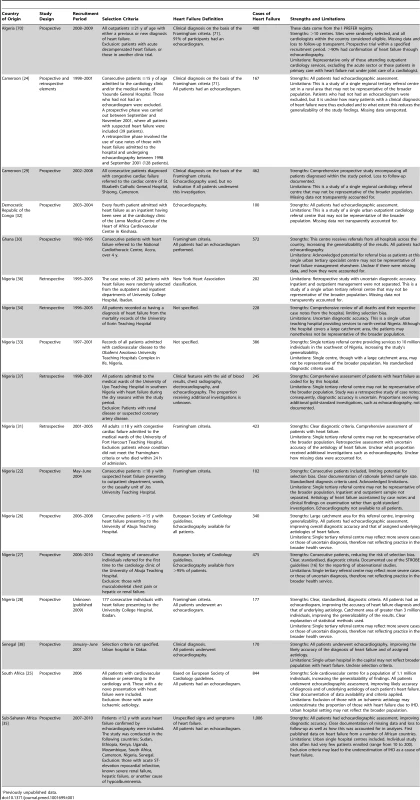
Previously unpublished data. Tab. 2. Characteristics of Americas region studies and databases included. 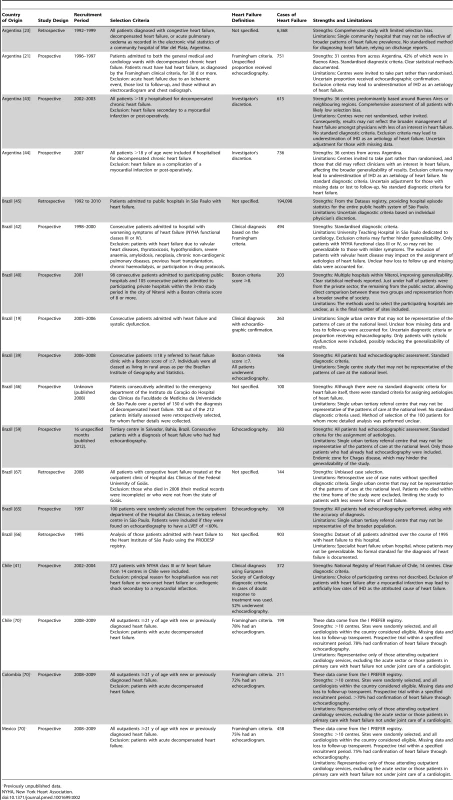
Previously unpublished data. Tab. 3. Characteristics of Eastern Mediterranean region studies and databases included. 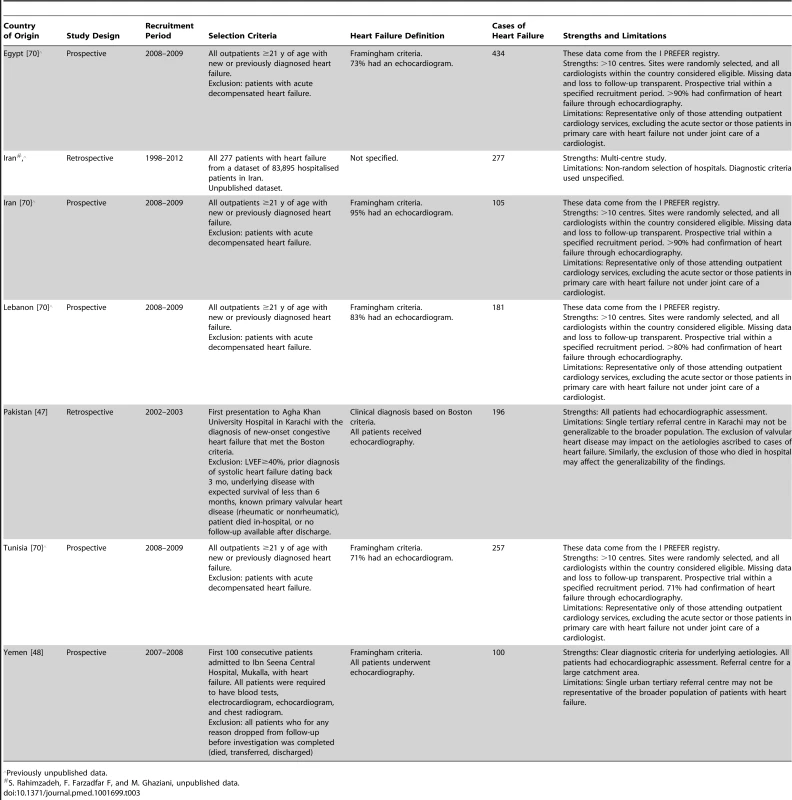
Previously unpublished data. Tab. 4. Characteristics of Europe region studies and databases included. 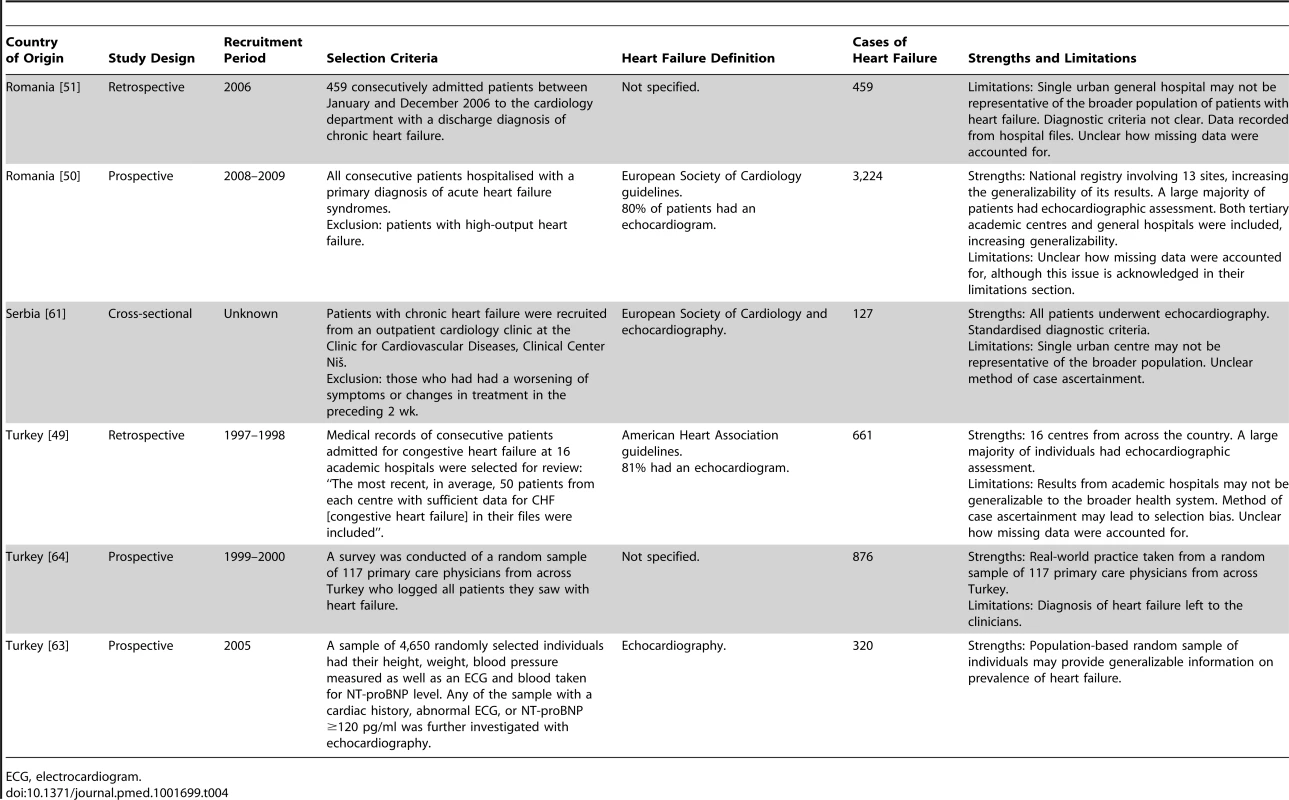
ECG, electrocardiogram. Tab. 5. Characteristics of South East Asia region studies and databases included. 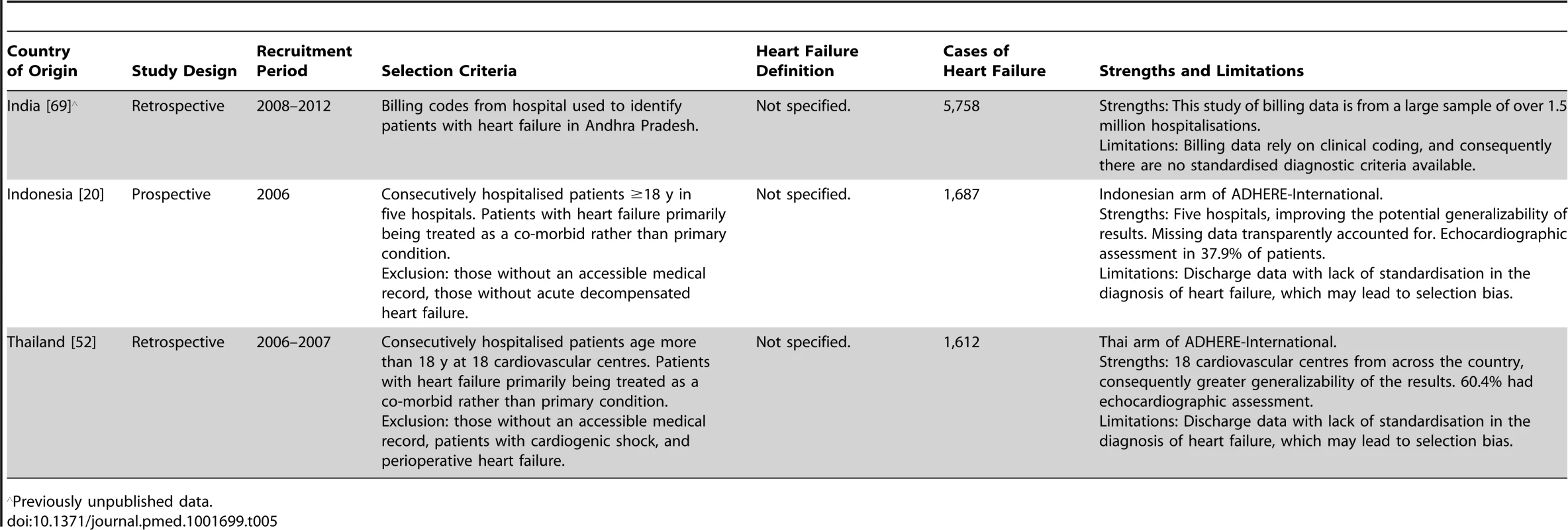
Previously unpublished data. Tab. 6. Characteristics of Western Pacific region studies and databases included. 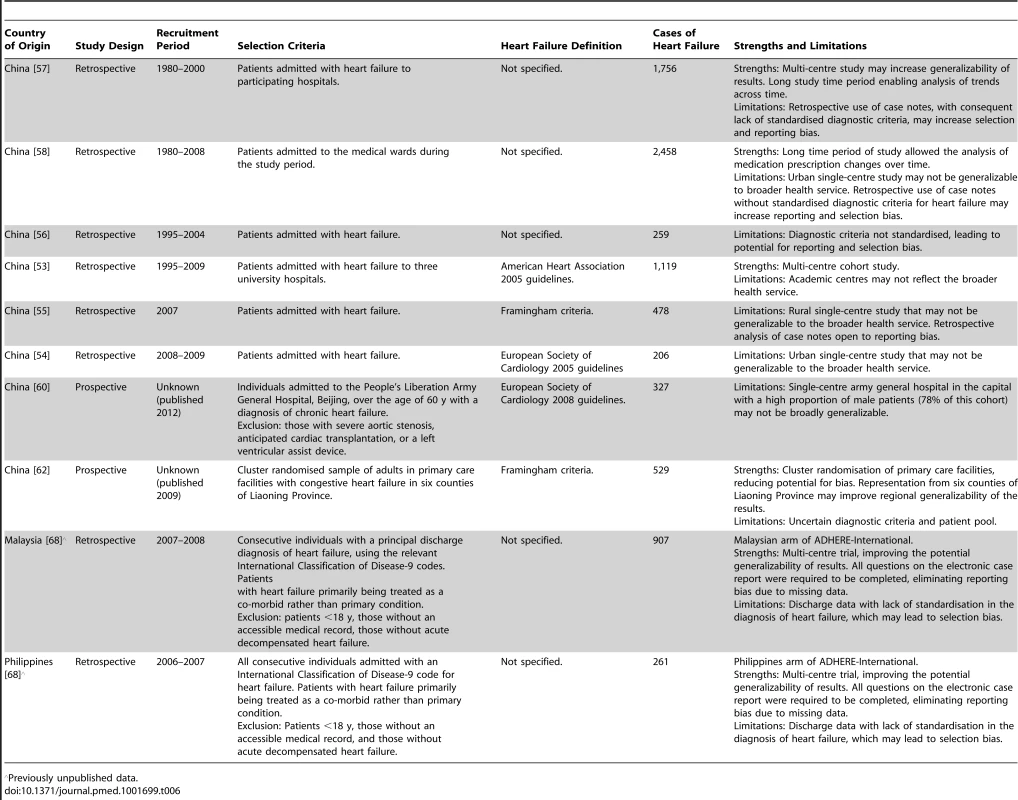
Previously unpublished data. Statistical Analysis
Study-specific data on percentages are presented as forest plots with exact binomial 95% confidence intervals (CIs). These percentages were pooled, by World Health Organization region (Table 7) and across regions, using the random effects method of DerSimonian and Laird [17]. Heterogeneity between studies was quantified by the I2 statistic and tested using Cochran's Q test. Means were rarely reported with an estimate of variability, and, consequently, we weighted individual means by study size in pooled analyses, and present the pooled mean and the range of means.
Tab. 7. Included countries grouped by World Health Organization region. 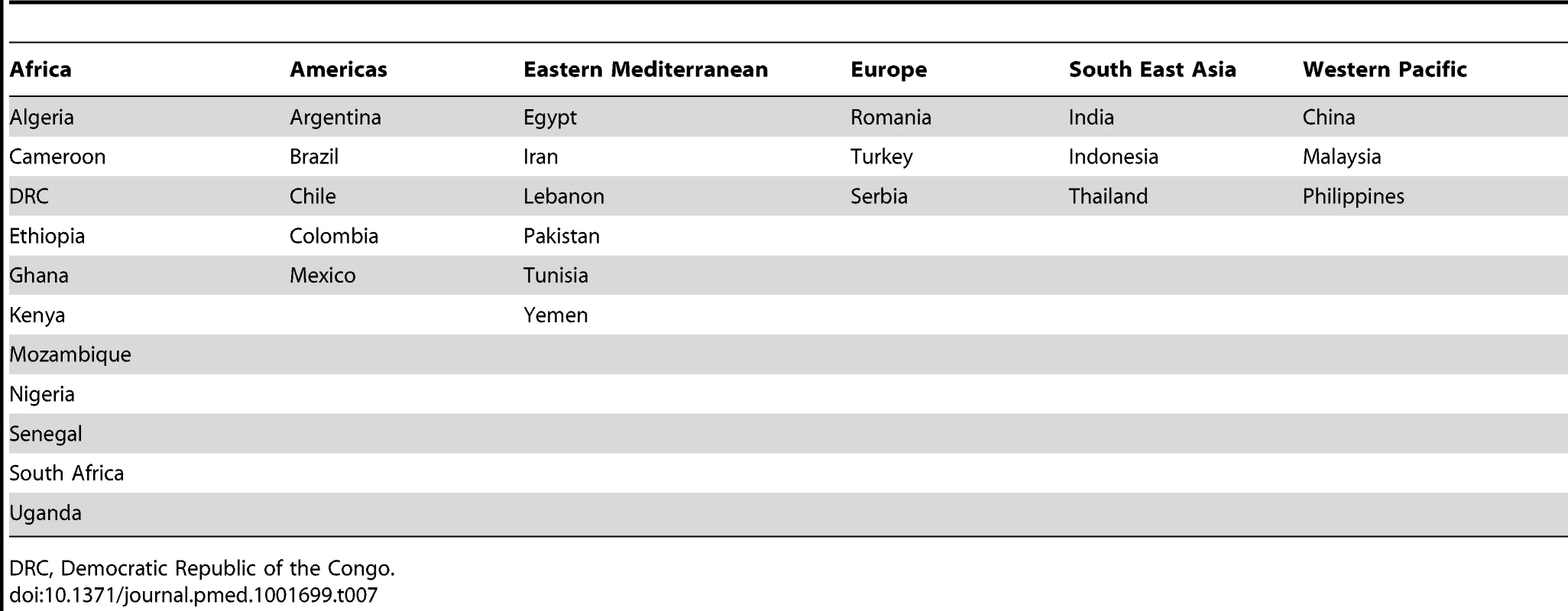
DRC, Democratic Republic of the Congo. Patients presenting acutely to hospitals may differ in many respects from those that are seen in clinics for chronic management. When pooling the data we therefore indicate the setting of each study in all forest plots. Studies from community primary care or outpatient clinics were designated as non-acute, and studies from inpatient populations, acute. Studies reporting both inpatient and outpatient data were included in the non-acute category. Additional subgroup analyses were performed by level of country income and by study time period. For income level analyses, studies were divided into low-income, low-middle-income, and upper-middle-income groups according to World Bank [15] country classification at the final year of the recruitment period of the study. The relationship between a study's mean age at admission for heart failure and the human development index (HDI) [18] for the country involved was estimated with linear regression analysis; the HDI was taken for the closest year to the final year of patient recruitment for the studies representing each country. The HDI is a composite measure of development produced by the United Nations Development Programme that incorporates life expectancy, education, and gross national income per capita [18]. Random effects meta-regression was performed to investigate study year as an explanation for the between-study heterogeneity in causes of heart failure, management, and in-hospital mortality. Corresponding bubble plots were drawn, with the size of each bubble inversely proportional to the estimated variance in the respective study.
Statistical analyses were done using R version 3.0.2 and Stata version 11.2.
Results
Geographic Distribution and Study Description
Overall, 49 published studies [19]–[67] and four unpublished datasets ([68]–[70]; S. Rahimzadeh, F. Farzadfar F, M. Ghaziani, unpublished data) were included; their geographical distribution is presented in Figure 2, and key study characteristics, divided by WHO region, are summarised in Tables 1–6. We obtained unpublished country datasets from the Acute Decompensated Heart Failure Registry (ADHERE)–International [68] regarding Malaysia and the Philippines, as well as the Identification of Patients with Heart Failure and Preserved Systolic Function (I PREFER) registry [70] including Iran, Lebanon, Egypt, Tunisia, Algeria, Chile, Colombia, and Mexico. Additional unpublished data were contributed from Iran (S. Rahimzadeh, F. Farzadfar F, and M. Ghaziani, unpublished data) and India [69].
Fig. 2. Geographic distribution of studies on heart failure in lowand middle-income countries. 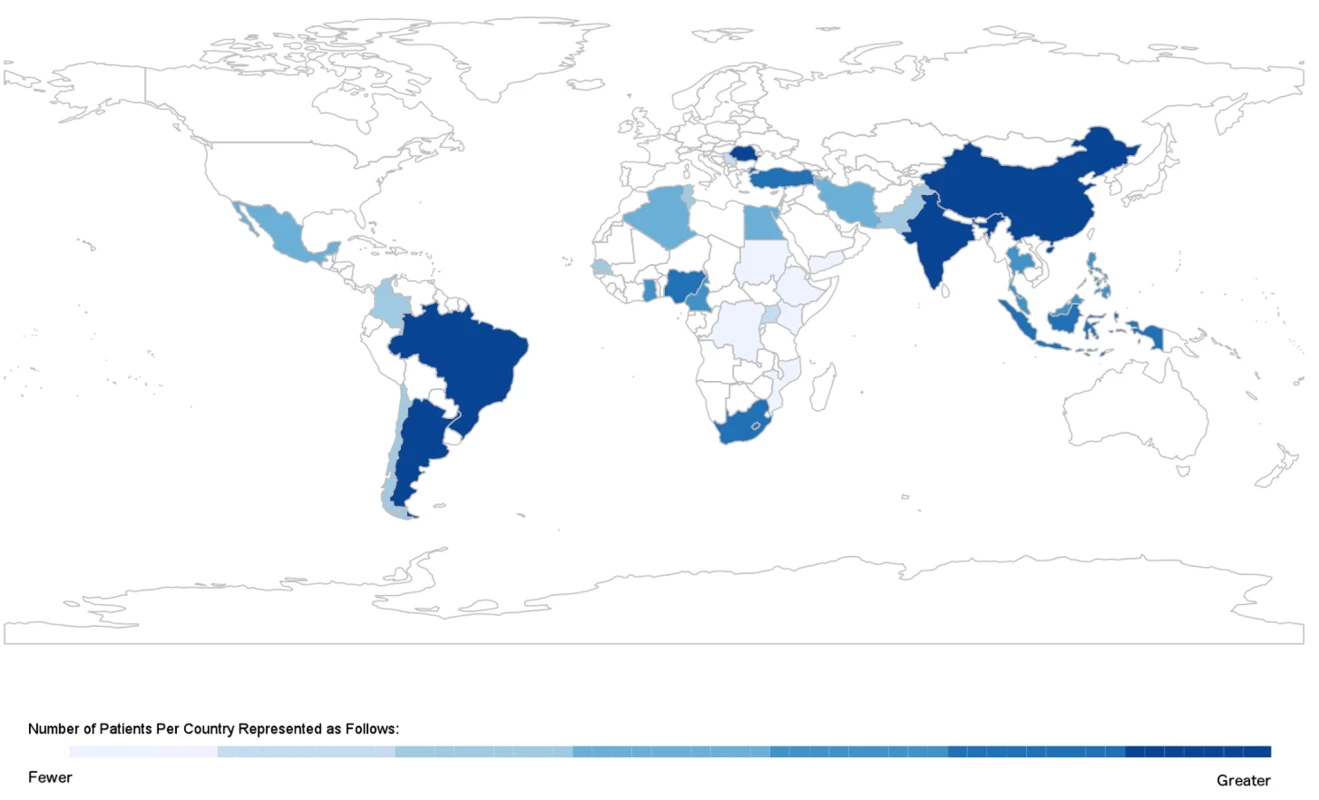
Most studies were based in a single hospital, although 21 datasets documented multi-centre studies in Algeria [70], Argentina [21],[43],[44], Brazil [40],[45], Chile [41],[70], Colombia [70], China [55],[57],[62], Egypt [70], Indonesia [20], India [69], Iran (S. Rahimzadeh, F. Farzadfar F, and M. Ghaziani, unpublished data; [70]), Lebanon [70], Thailand [52], Malaysia [68], Mexico [70], Philippines [68], Romania [50], Tunisia [70], Turkey [49],[63],[64], and a further nine countries in sub-Saharan Africa [35]. Four studies involved both inpatient and outpatient data [22],[24],[26],[36], six studies referred solely to patients seen at outpatient clinics [27],[39],[61],[66],[67],[70], three studies described heart failure in primary care settings [62]–[64], and the remainder reported solely on inpatient populations. One study was a population-based assessment of the prevalence of heart failure in Turkey [63].
Case Identification and Ascertainment
The studies together included 237,908 episodes of heart failure hospitalisation. The median number of cases across all studies was 386 (range: 100–194,098). Diagnosis of heart failure was established according to the Framingham criteria [71] in 12 studies [21],[22],[24],[28]–[31],[42],[48],[55],[62],[70]. European Society of Cardiology guidelines were used in eight studies [25]–[27],[41],[50],[54],[60],[61], the Boston criteria [72] in three studies [39],[40],[47], and the American Heart Association guidelines in two studies [49],[53], and the diagnosis was left to the investigator's or examining physician's discretion in 26 studies ([19],[20],[23],[32]–[38],[43]–[46],[51],[52],[56]–[58],[64],[65],[67]–[69]; S. Rahimzadeh, F. Farzadfar F, and M. Ghaziani, unpublished data). One study diagnosed all cases of heart failure solely using echocardiography [59]. Information on the use of additional investigative tools, including echocardiography, chest radiography, and electrocardiography, was provided in 28 studies [19]–[21],[24]–[26],[28],[31],[35],[39],[41]–[43],[46],[48]–[50],[51],[54],[56],[57],[59],[61],[63],[64],[66],[70],[73]. Of these, 14 studies performed echocardiography on all patients [19],[24]–[26],[28],[35],[46],[48],[53],[54],[59],[61],[63],[66]. The mean left ventricular ejection fraction (LVEF) was documented in 18 studies, reporting data from Algeria [70], Egypt [70], Tunisia [70], Cameroon [24], Ethiopia [35], Sudan [35], Mozambique [35], Kenya [35], Uganda [35], Senegal [35], South Africa [25], Nigeria [26],[28], Brazil [19],[39],[42],[46],[66], Chile [41],[70], Colombia [70], Mexico [70], Romania [50], Serbia [61], Turkey [49], Iran [70], Lebanon [70], Indonesia [20], Thailand [52], and China [53]. Across all studies, mean LVEF was 40% (range: 27%–57%) (Table 8). Hospitalised patients had a mean LVEF of 38% (27%–57%), with a corresponding figure of 48% (29%–55%) in non-acute settings.
Tab. 8. Characteristics of patients, by region. 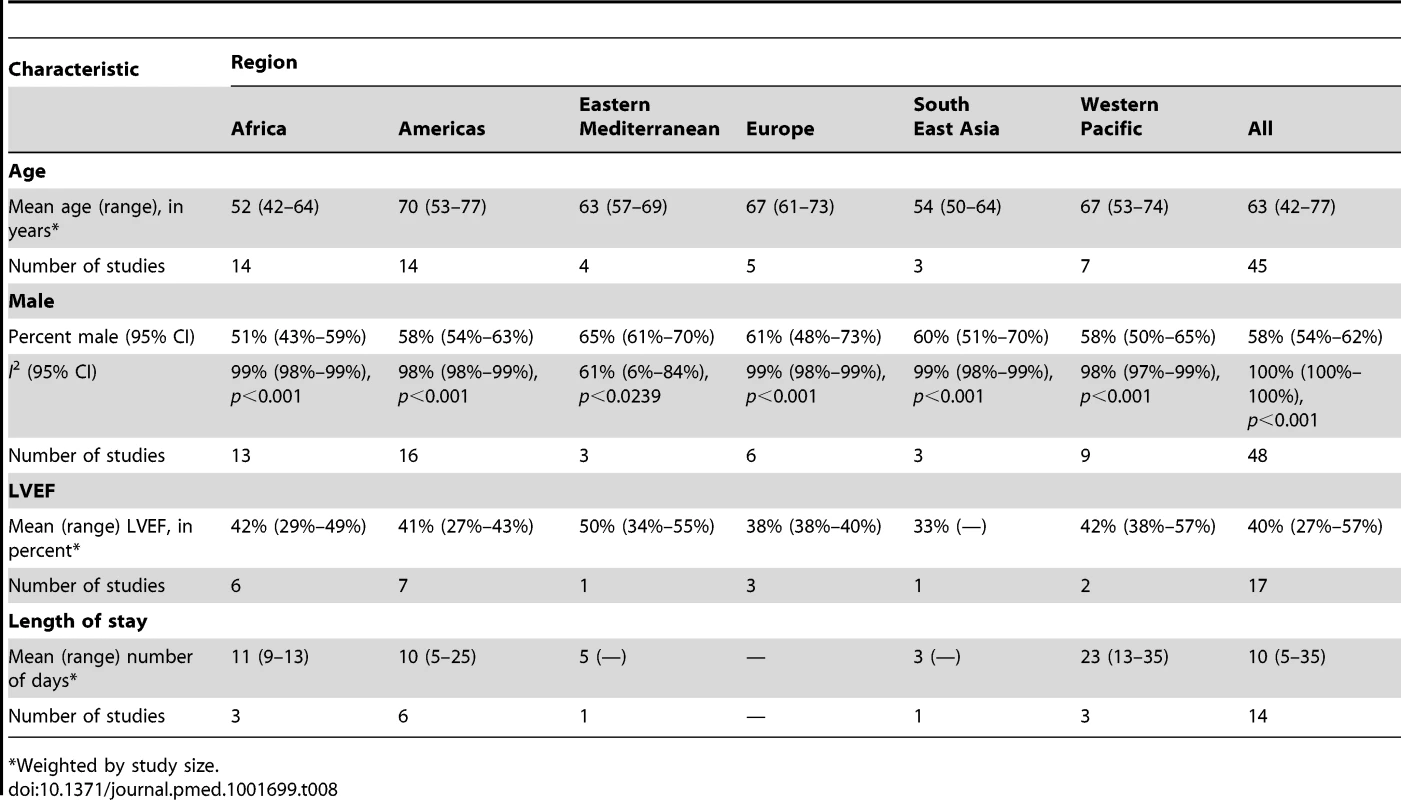
*Weighted by study size. Demographic Characteristics
The demographic characteristics of patients and outcomes by region are shown in Table 8. The corresponding data by country are shown in Table 9. Men made up 58% (95% CI: 54%–62%) of study participants (Figure 3). The mean age of patients for each region ranged from just over 52 y (range: 42–64) in Africa to 70 y (range: 53–77) in the Americas, and when combined across all regions was 63 y (range: 42–77). The mean age of patients on admission rose with the country income level. In low-income countries, the corresponding figure was 50 y (range: 42–58), rising to 60 y (range: 50–74) in low-middle-income countries, and reaching 70 y (range: 54–77) in upper-middle-income countries. Mean age also correlated with the HDI across countries (r = 0.71, p<0.001) (Figure 4).
Fig. 3. Male patients by region. 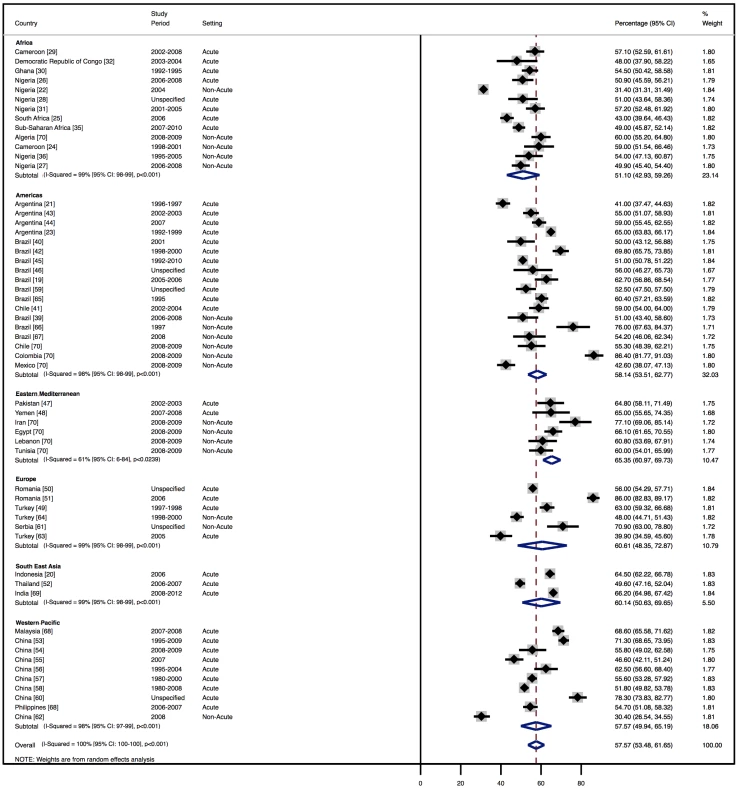
Fig. 4. Correlation of age and human development index, by country. 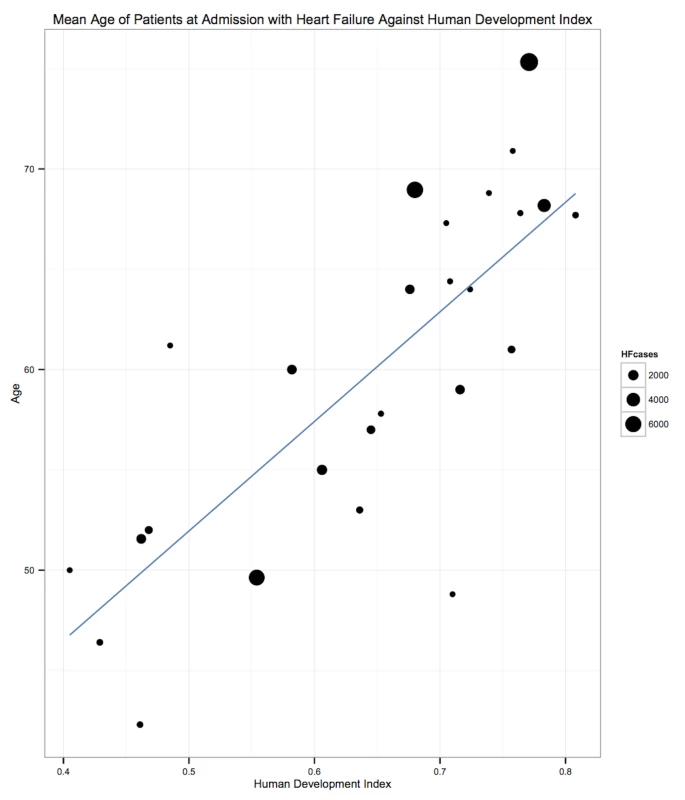
r = 0.71, p<0.001. The HDI is a measure produced by the United Nations Development Programme that incorporates gross national income per capita, life expectancy, and time spent in education. It serves as a single statistic that provides a comparable measure of development across nations. HF, heart failure. Tab. 9. Characteristics of patients, by country. 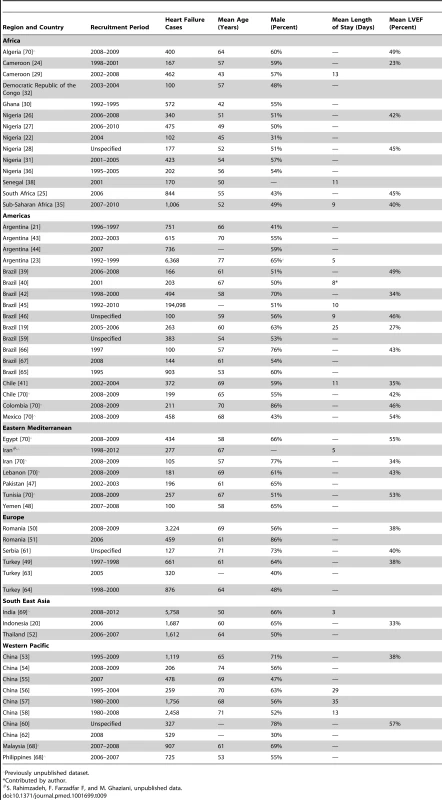
Previously unpublished dataset. Causes of Heart Failure
Although most studies made a clear distinction between aetiologies and co-morbidities, the categories reported were highly variable, and multiple causes were often attributed to individual cases of heart failure. Across all LMICs, non-communicable diseases, and in particular ischaemic heart disease (IHD) and hypertension, are the leading causes of heart failure (Table 10). However, there is heterogeneity between the regions. IHD is the most commonly reported cause of heart failure in all regions except Africa and the Americas (Figures 5 and 6). In the Americas hypertension and IHD are responsible for a similar percentage of documented cases, at 31% (95% CI: 19%–43%, I2 99%, p for heterogeneity <0.001) and 33% (95% CI: 27%–38%, I2 96%, p<0.001), respectively. In Africa, 8% (95% CI: 5%–11%, I2 98%, p<0.001) of heart failure is due to IHD, with hypertension the dominant cause, responsible for 46% (95% CI: 36%–55%, I2 98%, p<0.001) of cases. Cardiomyopathies cause 24% (95% CI: 20%–29%, I2 99%, p<0.001) of heart failure cases across LMICs taken together (Figure 7). Idiopathic, hypertrophic, and restrictive cardiomyopathies are reported across all countries; however, other specific types of cardiomyopathies showed substantial regional variation. Peri-partum and HIV-associated cardiomyopathies were reported only in Africa. By contrast, Chagas cardiomyopathy remains a Latin American phenomenon [21],[43]. Valvular heart disease is responsible for 18% (95% CI: 15%–22%, I2 98%, p<0.001) of cases of heart failure across LMICs (Figure 8).
Fig. 5. Aetiology of heart failure: ischaemic heart disease by region. 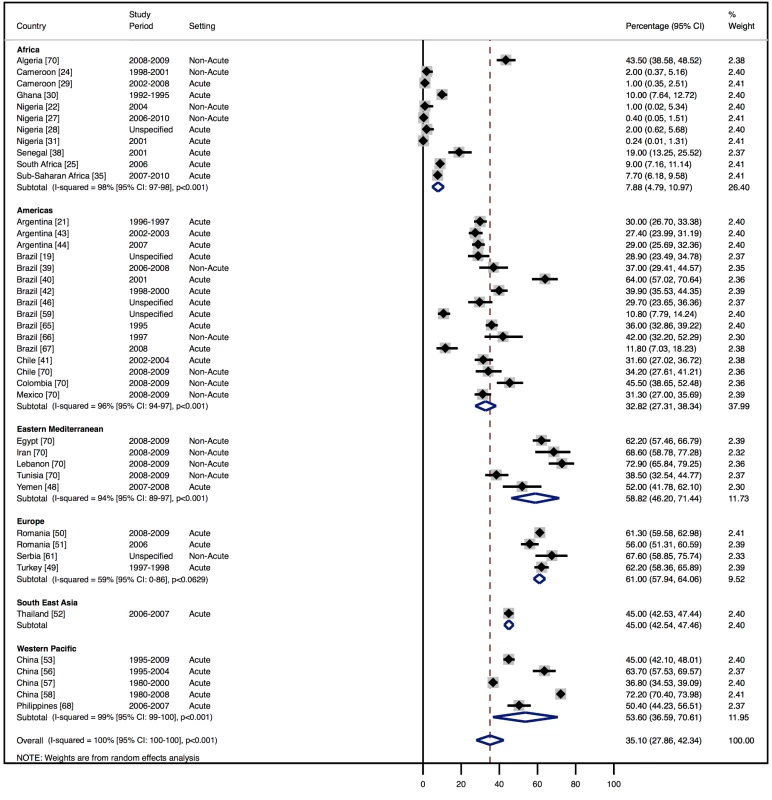
Percentage of heart failure cases with a documented cause of IHD. Fig. 6. Aetiology of heart failure: hypertension by region. 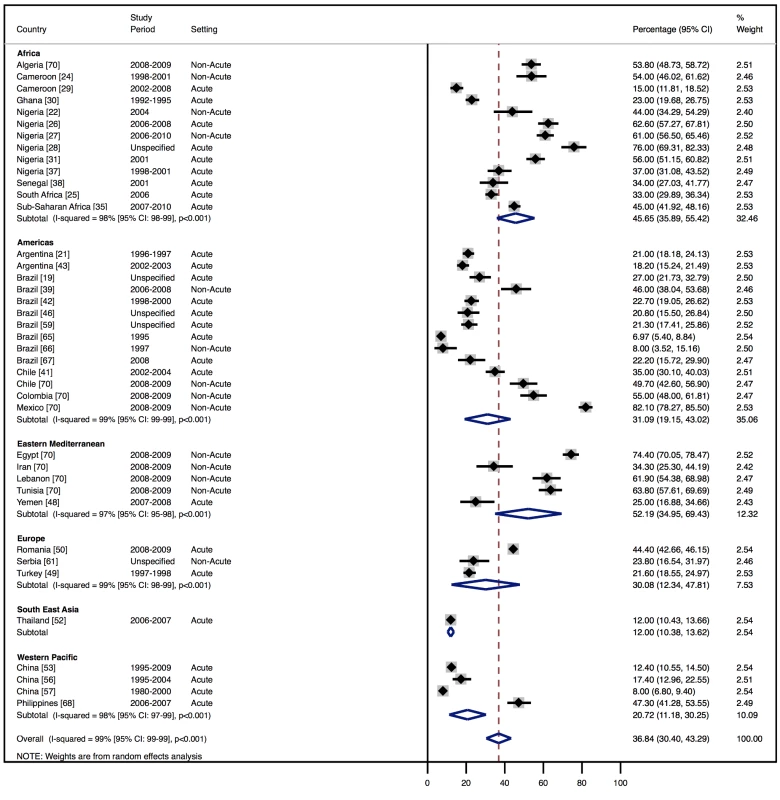
Percentage of heart failure cases with a documented cause of hypertension. Fig. 7. Aetiology of heart failure: cardiomyopathies by region. 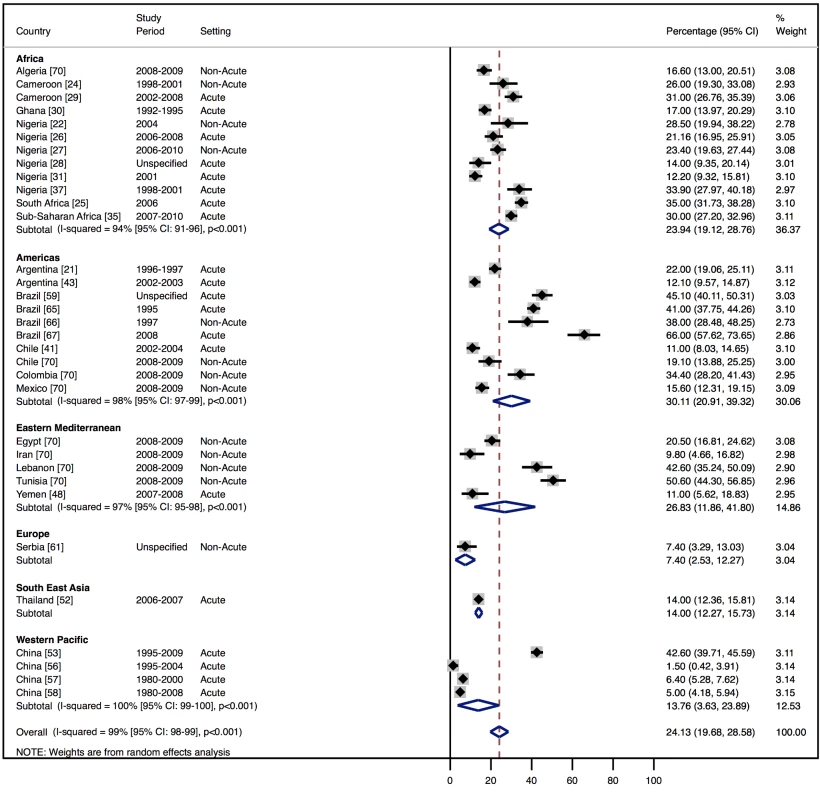
Percentage of heart failure cases with a documented cause of cardiomyopathy. Fig. 8. Aetiology of heart failure: valvular heart disease by region. 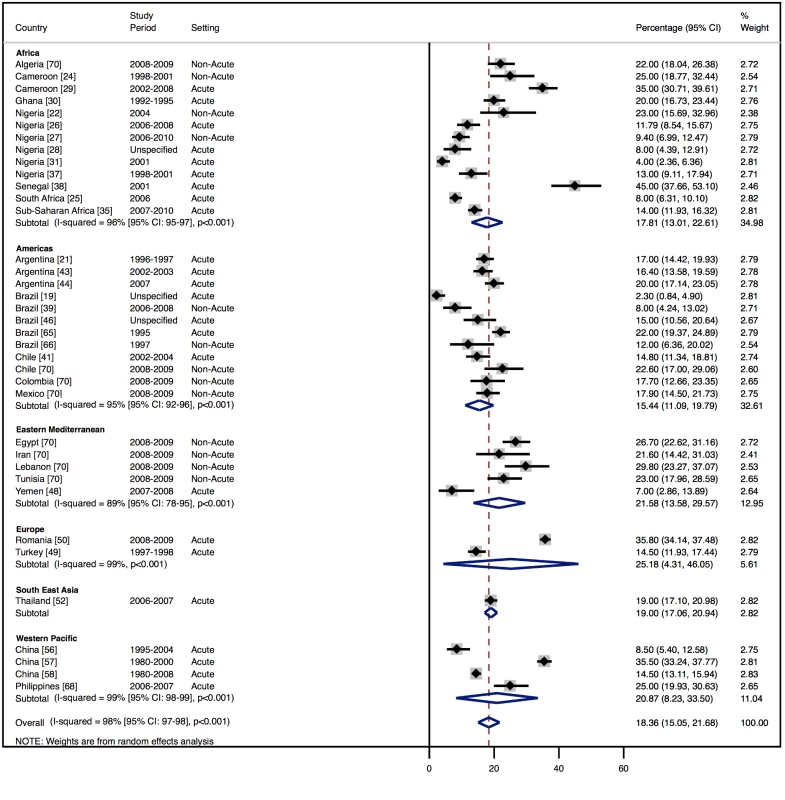
Percentage of heart failure cases with a documented cause of valvular heart disease. Tab. 10. Reported causes of heart failure, by region. 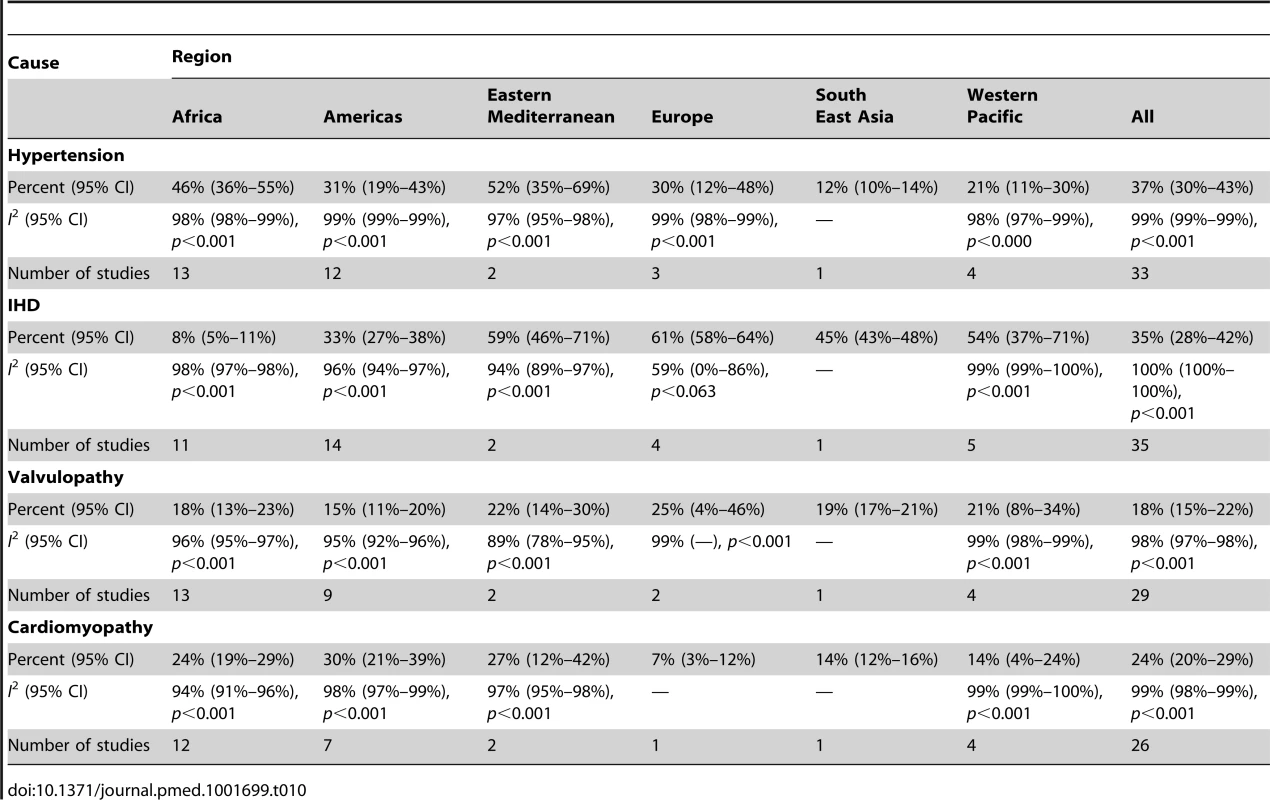
Management of Heart Failure
Amongst all studies, the management of heart failure varies considerably between regions and within regions, as well as between studies from the same country (Table 11). The most commonly prescribed treatments are loop and/or thiazide diuretics, prescribed for 69% (95% CI: 60%–78%, I2 100%, p<0.001) of individuals in LMICs worldwide (Figure 9). Angiotensin-converting enzyme inhibitors (ACEIs) are used in 57% (95% CI: 49%–64%, I2 100%, p<0.001) of cases, beta-blockers in 34% (95% CI: 28%–41%, I2 100%, p<0.001), and mineralocorticoid receptor antagonists in 32% (95% CI: 25%–39%, I2 100%, p<0.001) (Figures 10–12).
Fig. 9. Diuretic use by region. 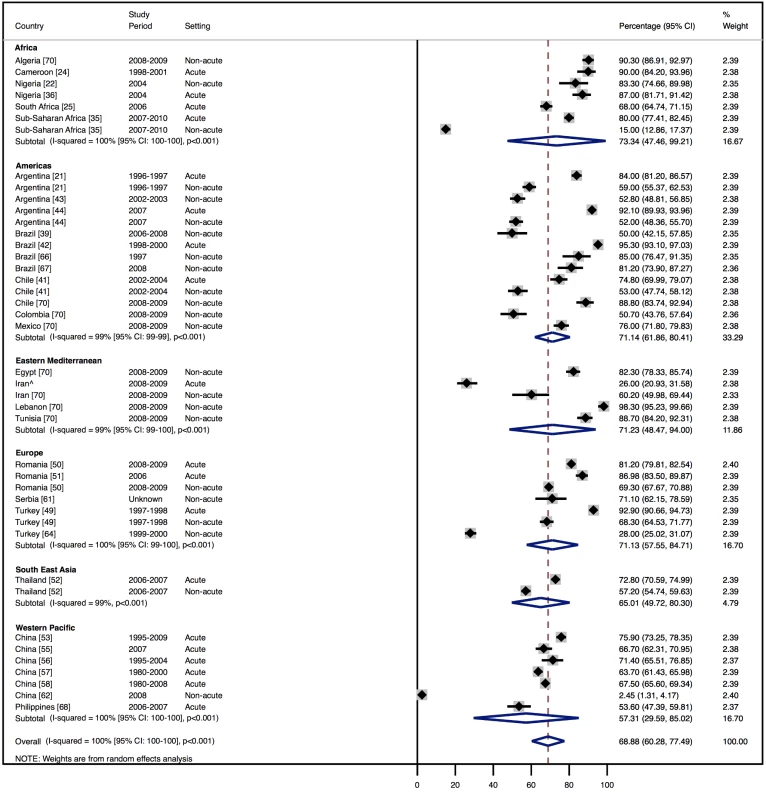
Loop and/or thiazide diuretics. ∧Rahimzadeh S, Farzadfar F, Ghaziani M (2013) Iranian hospital data project (unpublished data). Fig. 10. Angiotensin-converting enzyme inhibitor use by region. 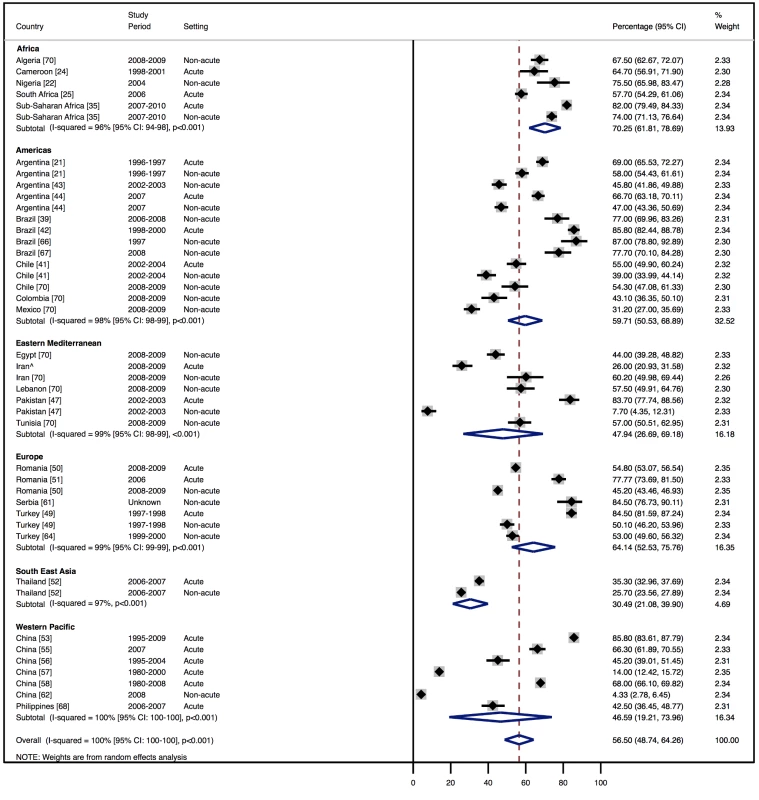
∧Rahimzadeh S, Farzadfar F, Ghaziani M (2013) Iranian hospital data project (unpublished data). Fig. 11. Beta-blocker use by region. 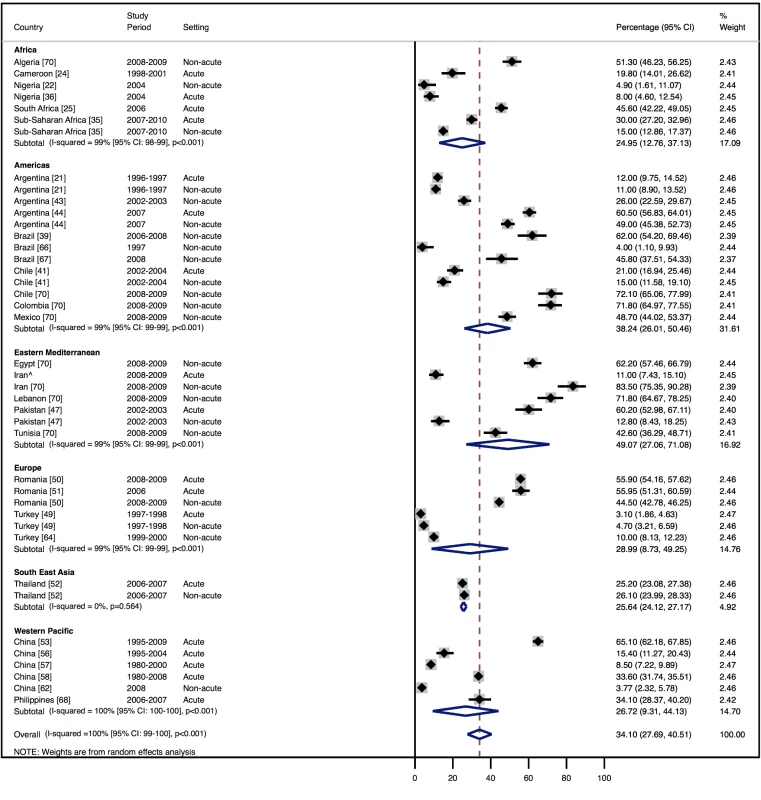
∧Rahimzadeh S, Farzadfar F, Ghaziani M (2013) Iranian hospital data project (unpublished data). Fig. 12. Mineralocorticoid receptor antagonist use by region. 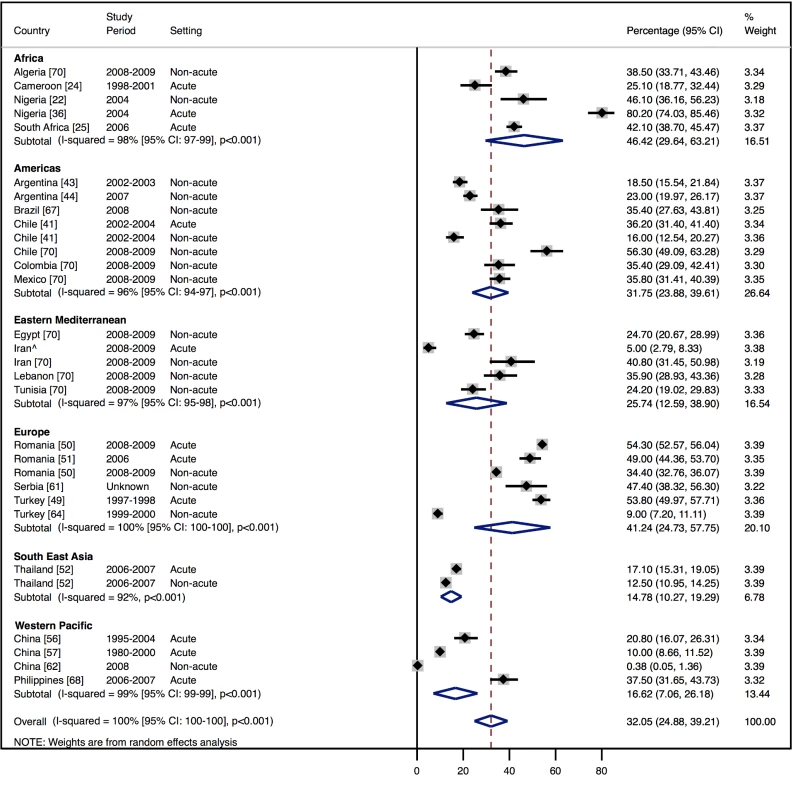
∧Rahimzadeh S, Farzadfar F, Ghaziani M (2013) Iranian hospital data project (unpublished data). Tab. 11. Reported management of heart failure, by region. 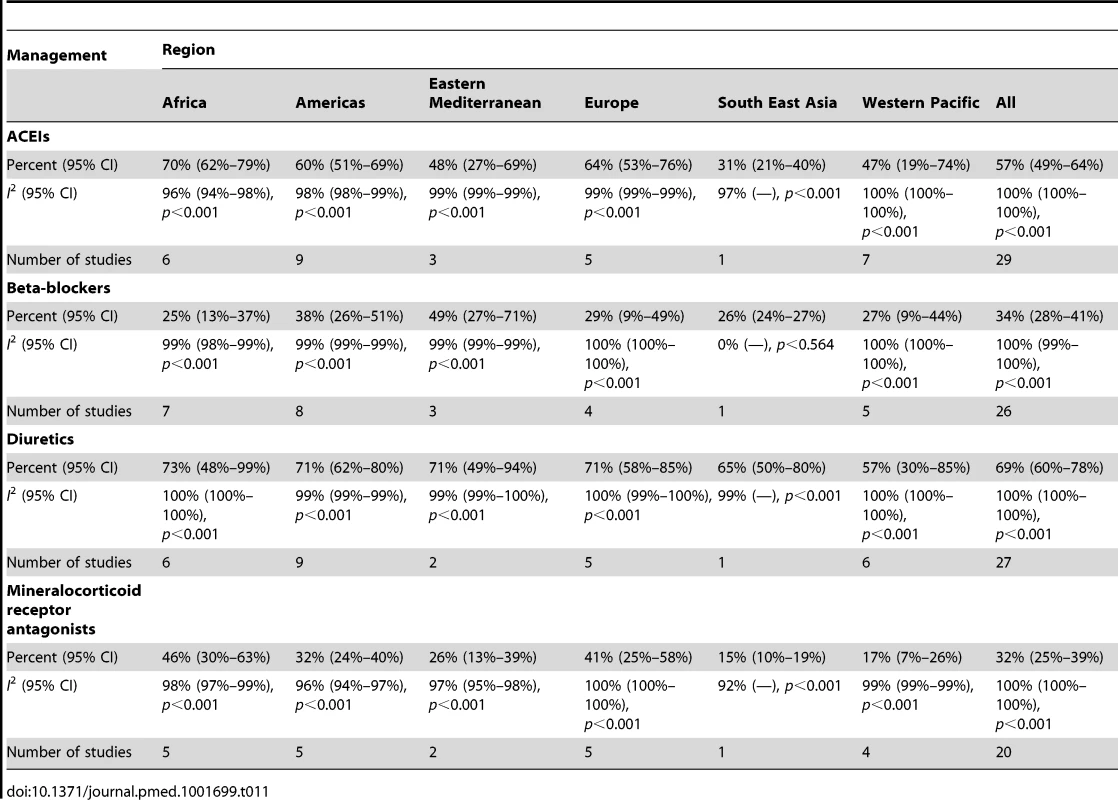
Outcomes
Across LMICs, patients admitted with heart failure remained in hospital for a mean of 10 d (Table 8). The mean hospital stay ranged from 3 d in India to 23 d amongst studies from China. Wide differences were observed amongst Argentinian and Brazilian studies. In Argentina, length of stay varied between studies from 5 d to 25 d, with an overall mean of 7 d. In Brazil, the range was between 9 and 25 d, with an overall mean of 10 d (see Table 9 for individual study data).
In-hospital mortality was 8% (95% CI: 6%–10%, I2 99%, p<0.001) (Figure 13) across the 23 studies that reported this measure ([20],[21],[23],[29],[31],[33]–[35],[40],[41],[43]–[46],[48],[50],[52],[56]–[58],[68]–[69]; S. Rahimzadeh, F. Farzadfar F, and M. Ghaziani, unpublished data), with no significant association with the country-level length of stay. Four studies reported longer-term outcome data, showing comparable mortality rates post-discharge: THESUS-HF, a multi-centre study of heart failure across nine countries of sub-Saharan Africa, found that mortality from heart failure was 4.2% in hospital and 17.8% at 6 mo after hospital discharge [35]. In Brazil, Barretto and colleagues reported a mortality rate of 8.8% in hospital and 25.8% at 1 y [19], whilst in Pakistan, after almost a year of follow-up, a similar mortality rate of 27.5% was recorded [47]. Of the Brazilian cohort studied by de Campos Lopes and colleagues, 44% were alive at 21 mo of follow-up [42].
Fig. 13. In-hospital mortality rates by region. 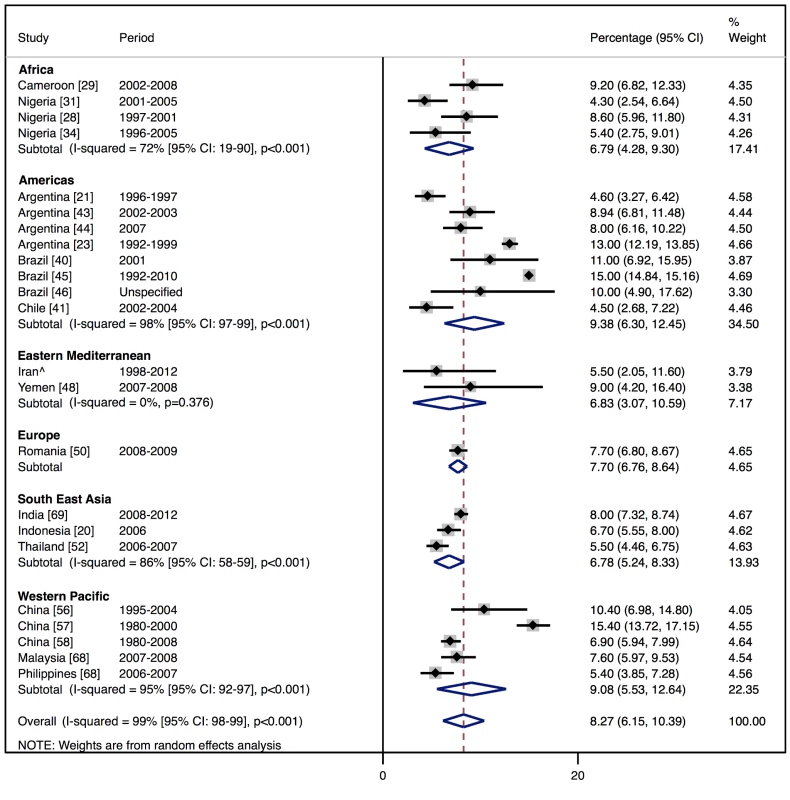
∧Rahimzadeh S, Farzadfar F, Ghaziani M (2013) Iranian hospital data project (unpublished data). Data relating to heart failure as a proportion of total hospital admissions were available for five countries. Across these countries, heart failure accounted for 2.2% (range: 0.3%–7.7%) of total admissions. Brazil was the only LMIC with nationwide registry data compiled for all patients with heart failure treated by its public health system [74]. Here, heart failure was responsible for 2.2% of total hospitalisations across the country [74]. In India, heart failure accounted for only 0.37% of cases from a sample of 1,551,410 hospitalisations, as derived from billing data in Andhra Pradesh [69]. Out of a representative sample of 38,926 hospital admissions in Iran, 0.3% were identified as having heart failure as the primary cause of admission (S. Rahimzadeh, F. Farzadfar F, and M. Ghaziani, unpublished data). By contrast, 5.8% of total hospital admissions in Cameroon were due to heart failure [24],[29], and 7.7% in Argentina [23].
In sub-Saharan Africa, the total number of cardiovascular admissions was reported, rather than total hospital admissions. In Nigeria, heart failure accounted for 31% of cardiovascular cases presenting to hospital [27], with corresponding figures of 38% in Senegal [38] and 47% in Soweto, South Africa [75].
Population-level data regarding the prevalence of heart failure were available in only one study, from Turkey [63]. Here an absolute prevalence of 2.9% for heart failure was found across the sample [63].
Effect of Time on Heterogeneity of Outcomes
Meta-regression was performed to investigate the potential effect of the time period in which each study was undertaken on between-study heterogeneity in the causes, management, and outcomes of heart failure.
A statistically significant effect was observed between the study time period and hypertension as a cause of heart failure, which rose by 2.5% per year (95% CI: 1.4%–3.6%, p<0.001) between 1990 and the late 2000s (Figure 14). There was no evidence to suggest that study time period had a significant effect on the other main causes of heart failure (IHD: 0.05%, 95% CI: −1.4% to 1.5%, p<0.95; cardiomyopathies: 0.65%, 95% CI: −0.3% to 1.6%, p<0.19; valvular heart disease: −0.04%, 95% CI: −0.7% to 0.6%, p<0.89) (Figures 15–17).
Fig. 14. Meta-regression of hypertension against study period. 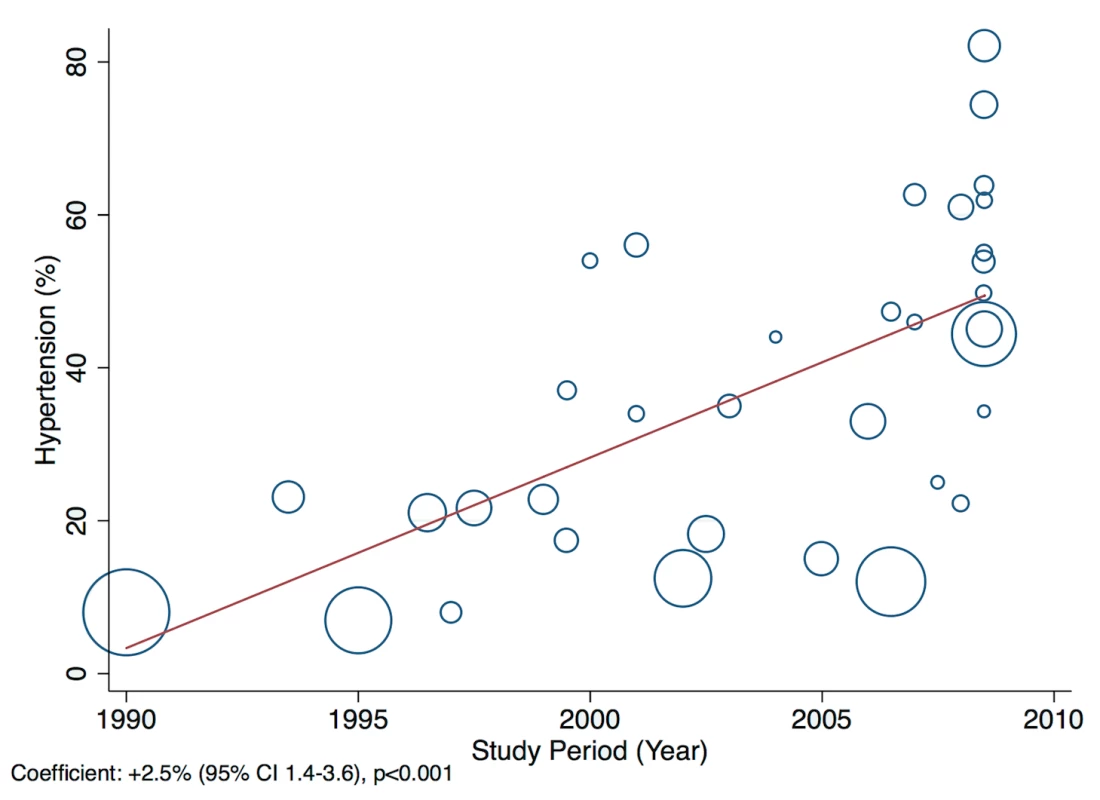
Fig. 15. Meta-regression of ischaemic heart disease against study period. 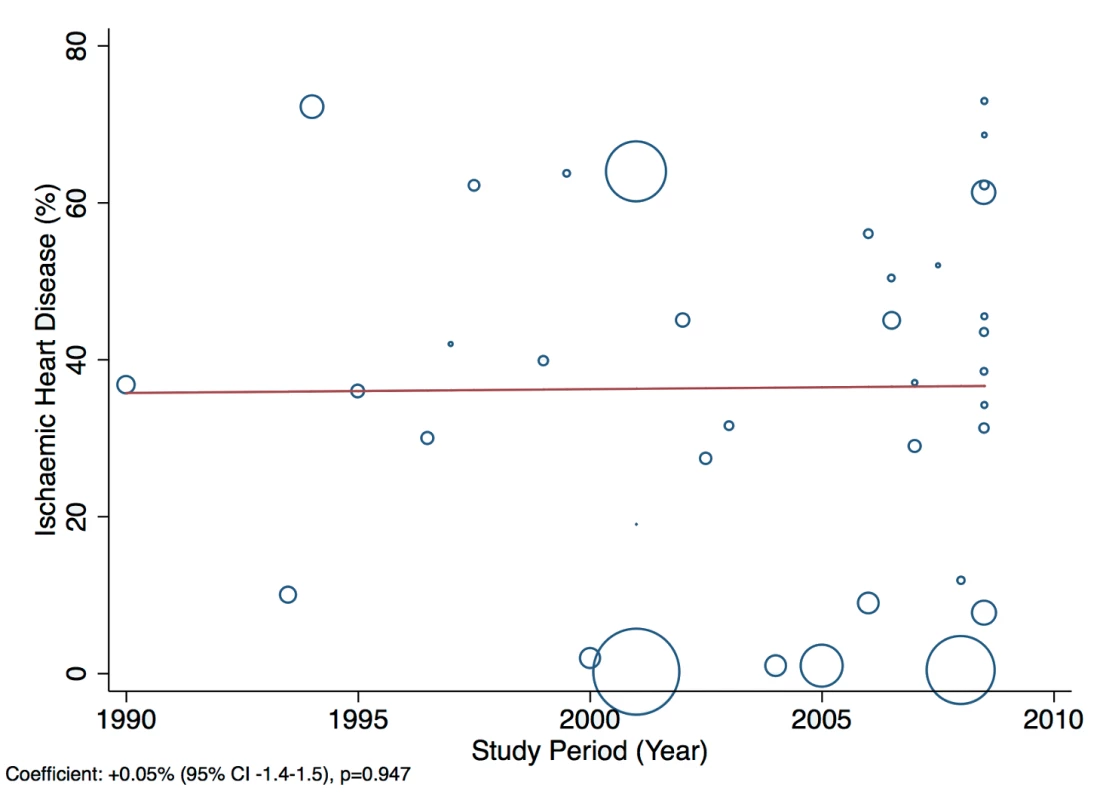
Fig. 16. Meta-regression of cardiomyopathies against study period. 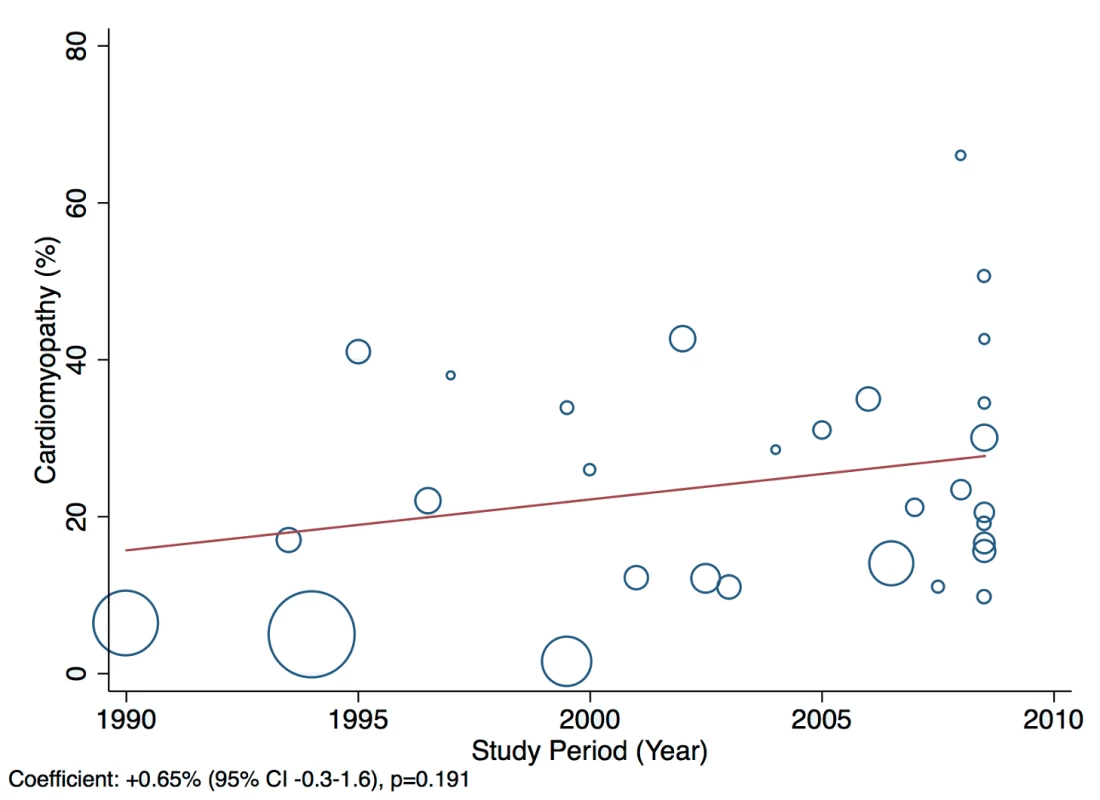
Fig. 17. Meta-regression of valvular heart disease against study period. 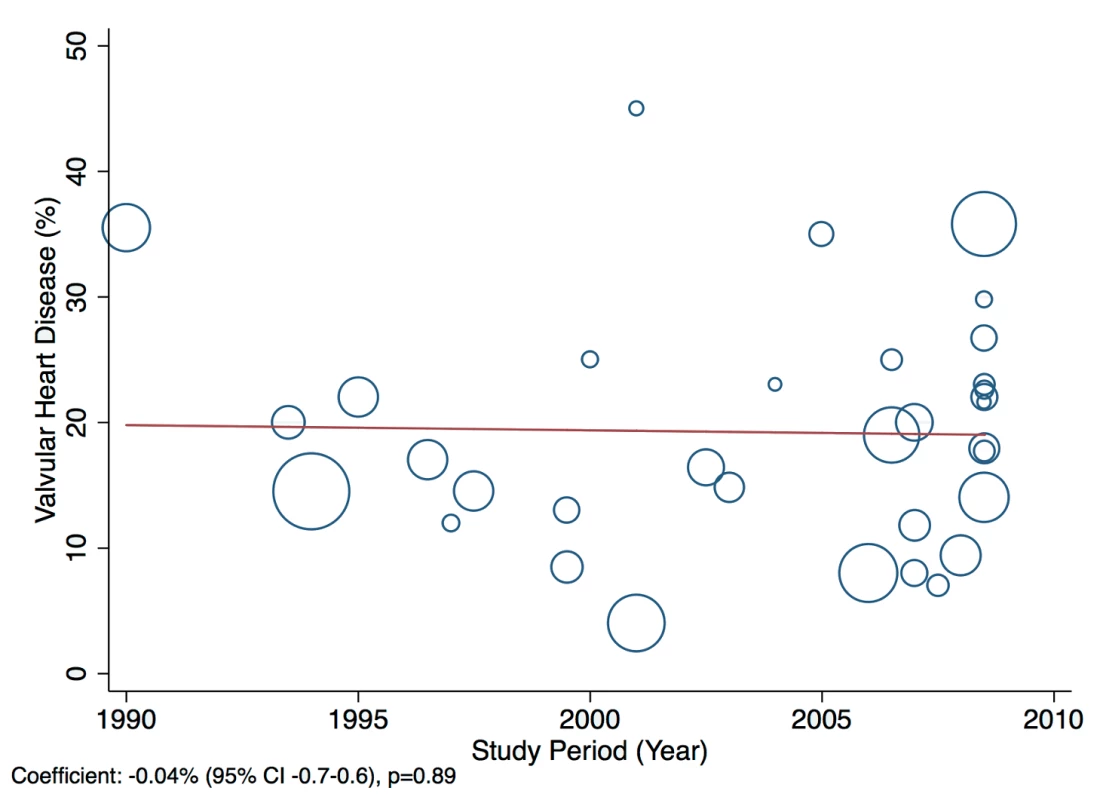
The reported utilization rates for medical treatments of heart failure did not differ significantly over time, with the exception of beta-blockers, which showed an increase of 2.8% per year (95% CI: 1.5%–4.1%, p<0.001) (Figure 18). Corresponding figures were −0.4% per year (95% CI: −1.8% to 0.98%, p = 0.56) for ACEI use and 0.67% (95% CI: −0.9% to 2.2%, p = 0.38) for mineralocorticoid receptor antagonist use, with loop and/or thiazide diuretic use changing by −0.49% per year (95% CI: −1.9% to 0.9%, p = 0.49) (Figures 19–21).
Fig. 18. Meta-regression of beta-blocker use against study period. 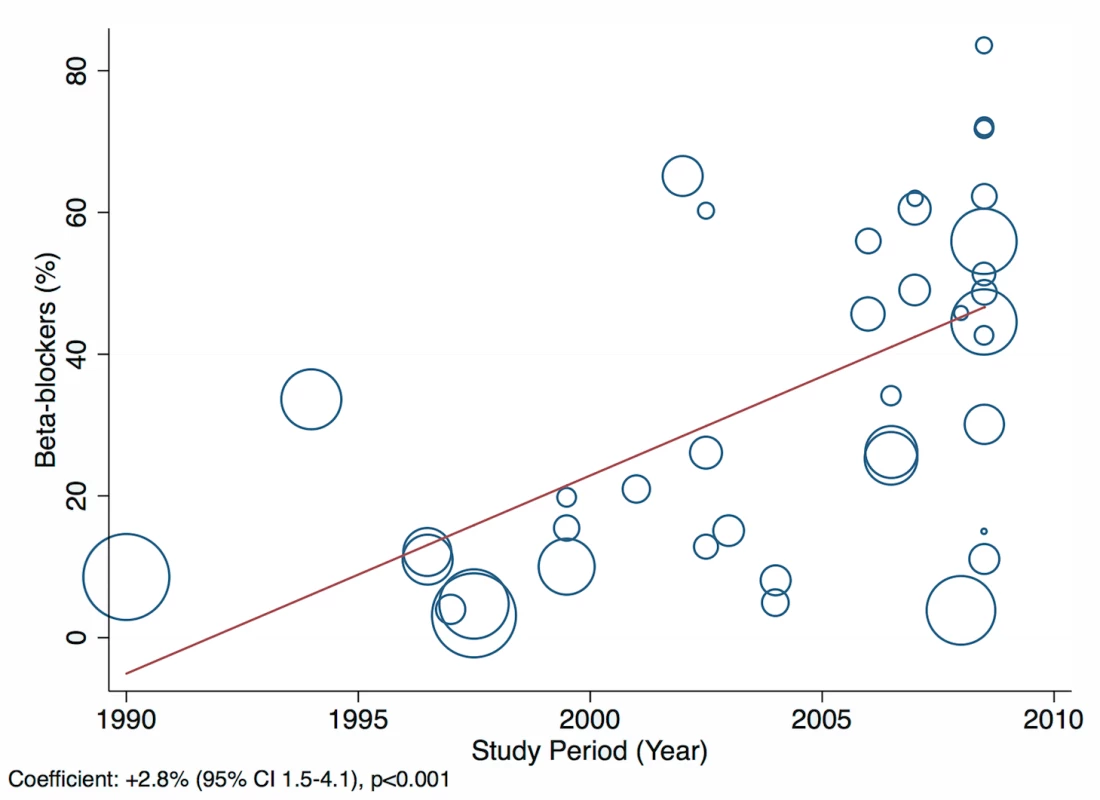
Fig. 19. Meta-regression of angiotensin-converting enzyme inhibitor use against study period. 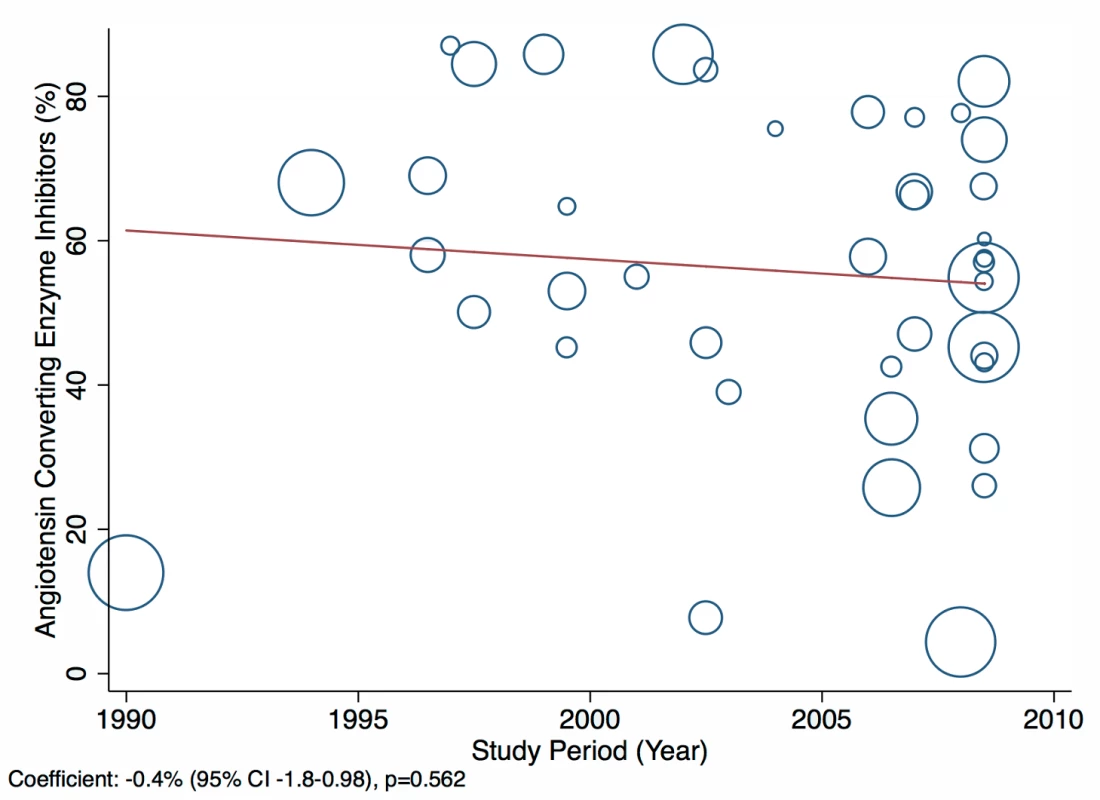
Fig. 20. Meta-regression of mineralocorticoid receptor antagonist use against study period. 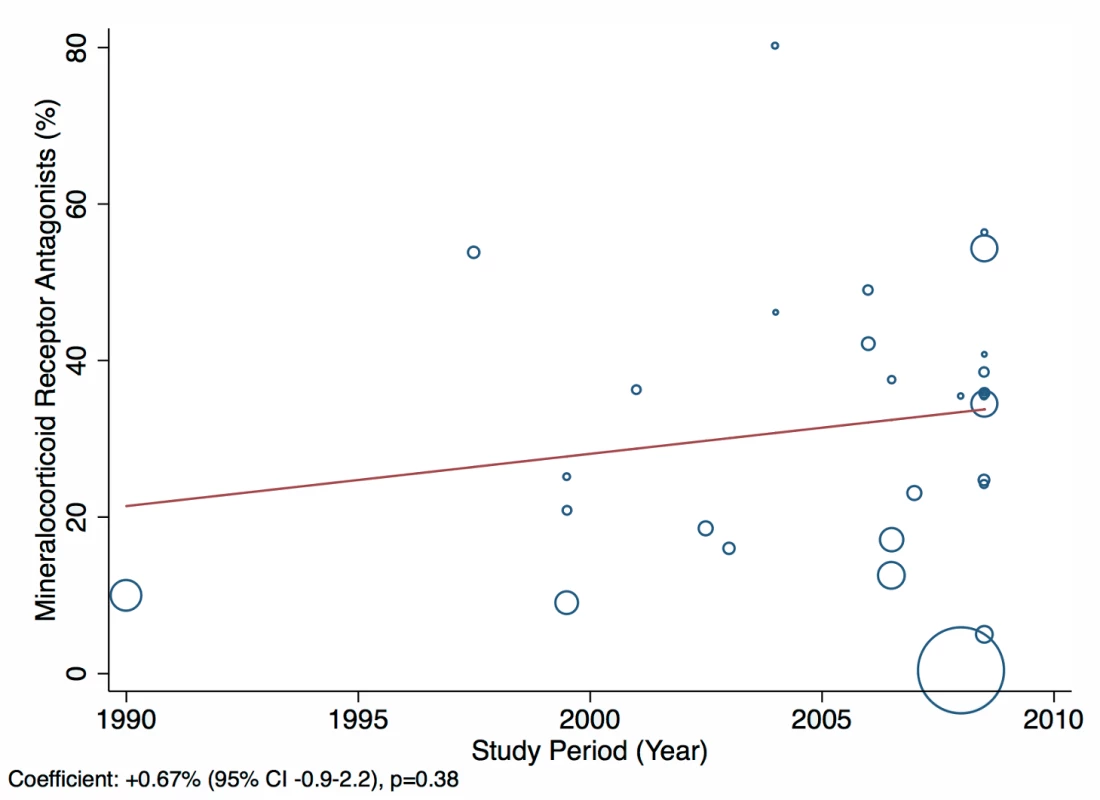
Fig. 21. Meta-regression of diuretic use against study period. 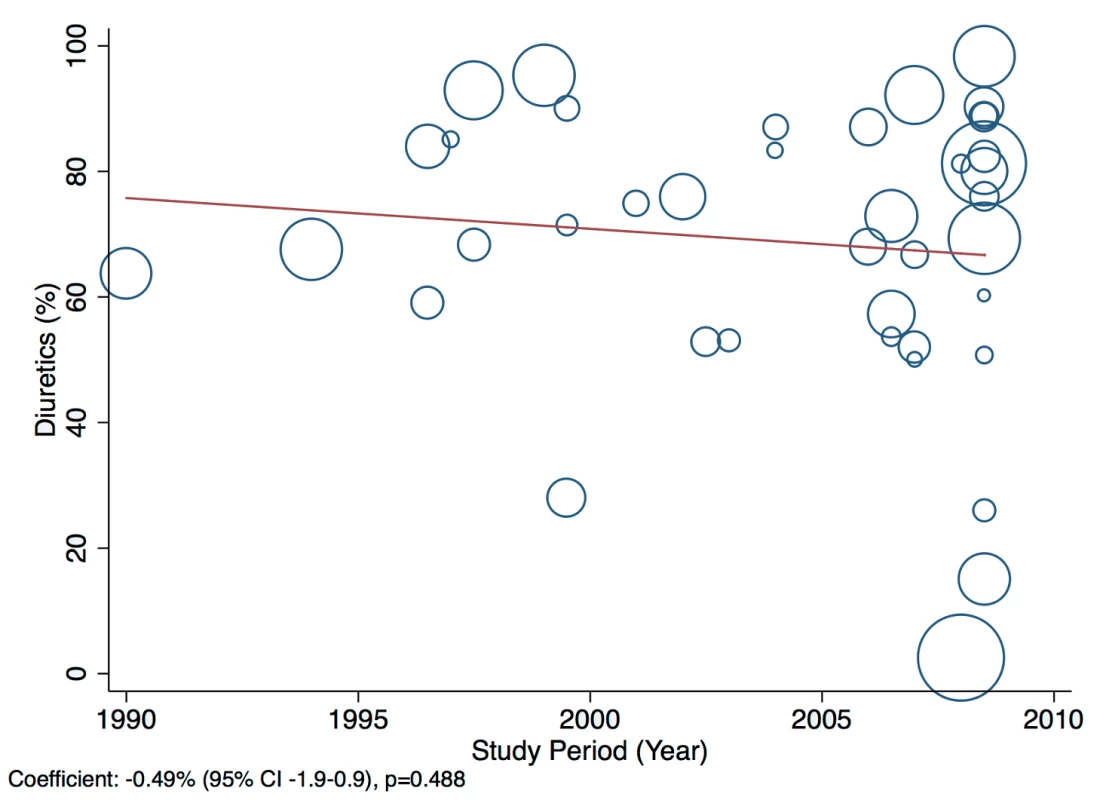
There was also some evidence to suggest in-hospital mortality rate declined by 0.28% per year between 1990 and 2010 (95% CI: −0.54% to −0.012%, p = 0.042) (Figure 22).
Fig. 22. Meta-regression of in-hospital mortality rates against study period. 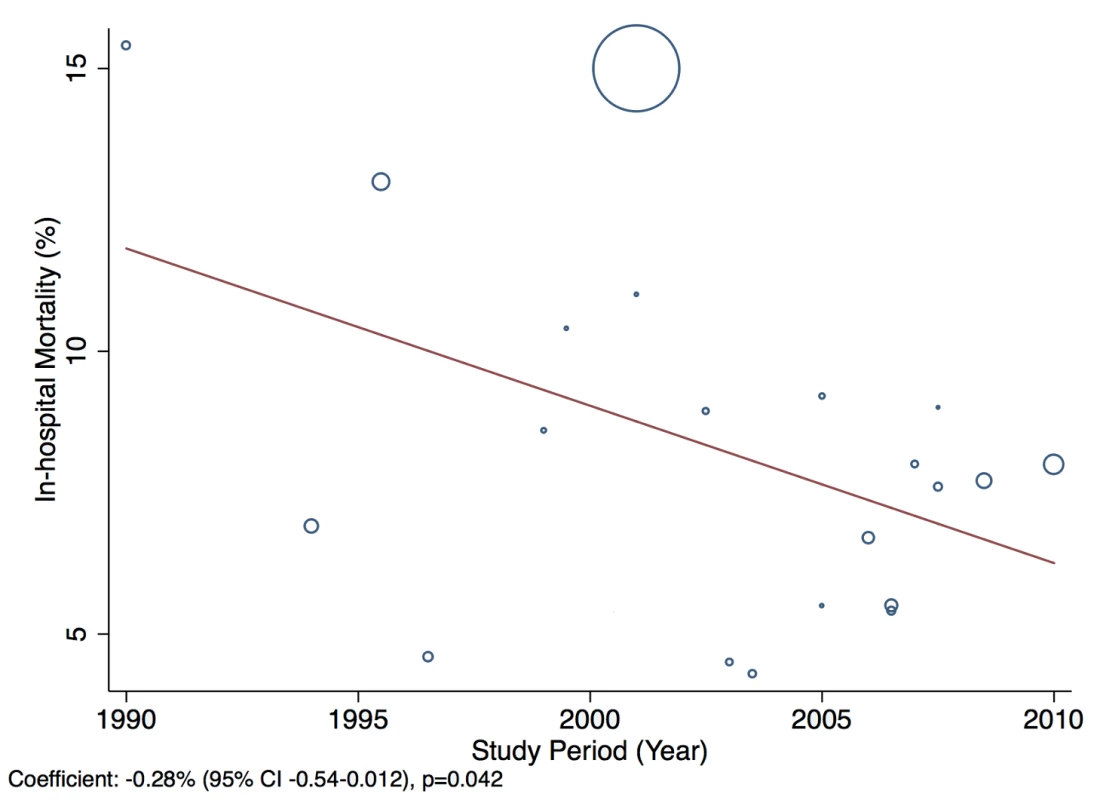
Discussion
Our study presents, to our knowledge, the most comprehensive review to date and the first pooled analysis of the burden of heart failure in LMICs worldwide, collating data on over 230,000 episodes from 31 countries, with representation from all world regions. We found that heart failure is already a major burden to populations and health services in LMICs, where it makes up an average of 2.2% of hospital admissions, affecting more men than women. Reflecting the broad range of countries included and their differing levels of socio-economic development, there are wide variations in patient characteristics and the causes of heart failure and its management. Nonetheless, noticeable similarities can be discerned both between the included LMICs themselves and between these LMICs and HICs.
Across all LMICs from which data were available, the mean age of patients was 63 y, which is over a decade younger than in studies from HICs [76],[77]. The observed differences in age between the countries correlated strongly with the differences in HDI across them. Alongside this is the graded rise in the mean age of patients from represented low-income, low-middle-income, and upper-middle-income countries, from 50 y in the former to 60 y and 70 y, respectively. Thus, the age of presentation in upper-middle-income countries comes close to that in HICs (70 y in the EuroHeart Failure Survey II across 30 countries in Europe [76] and 72 y in ADHERE in the US [77]).
Substantial inter-regional variation is present in the causes ascribed to individual cases of heart failure. Heart failure is a syndrome made up of a constellation of signs and symptoms, with additional features present on further investigation. Given that a number of its aetiological underpinnings are often potential co-morbidities, disentangling one from the other is fraught with challenges, particularly in low-resource environments without recourse to a broad range of investigative tools [31]. Although 80% of studies from the Americas, Western Pacific, and Europe reporting aetiologies for heart failure documented the use of additional investigative tools, only 50% of studies from Africa did so. Nevertheless, our results are broadly consistent with the patterns of risk factors reported by Khatibzadeh and colleagues in their recent review of the worldwide risk factors for heart failure [78], as well as those of the Global Burden of Disease Study [10]. It is of note that preventable non-communicable diseases, in particular IHD and hypertension, are responsible for the large majority of cases worldwide.
Current guidelines worldwide stress the importance of ACEIs, beta-blockers, and mineralocorticoid receptor antagonists in the management of heart failure with reduced LVEF, with loop/thiazide diuretics given for symptom relief. Across the 29 studies from which management data were available, few studies reported the LVEF of patients, and fewer still separated data by LVEF. Overall mean LVEF was 40%: 38% amongst inpatients and 48% amongst those in non-acute settings. Consequently, it is not possible to make strong conclusions about the adherence of practice to evidence-based practices worldwide, but it is evident that management diverges considerably between regions and remains suboptimal on average. Data from the EuroHeart Failure Survey II of 30 high-income European countries also demonstrated poor medical management [76]. In this study, the mean LVEF of patients was 38%, and just over one-third of patients had a LVEF>45% [76]. Here, 71% of individuals were prescribed ACEIs, 48% a mineralocorticoid receptor antagonist, and 61% a beta-blocker at discharge [76]. The corresponding figures across our dataset are 57%, 32%, and 34%, respectively.
Across represented LMICs, patients admitted with heart failure had a poorer immediate prognosis than those in many HICs. However, as is the case for HICs, the estimates from LMICs varied substantially, although we found the difference between the two outlying regions in terms of prognosis, the Americas and South East Asia, was not statistically significant (p = 0.27). On average, the in-hospital mortality rate was 8.3% in LMICs, compared to 6.7% in the EuroHeart Failure II Survey [76] and 4% in ADHERE in the US [77]. Such differences, and the wide heterogeneity both within LMICs and between LMICs and HICs, may be due to different thresholds for hospitalisation or differences in patient characteristics, treatment strategies, or hospital characteristics. Reports of outcomes after hospital discharge were available from some studies, and these were more comparable to estimates from HICs [4],[6],[19],[42],[47].
Remarkable regional variation exists in the incidence of heart failure admissions to hospital. Of particular note is the low rate of reported admissions for heart failure in India and Iran. Unpublished data from India, based on the hospital billing codes assigned to patients from a sample of just under 1,551,410 admissions, showed an incidence of 0.37% [69]. Similarly, 0.3% of all hospital admissions were attributed to heart failure in a registry of over 80,000 hospitalisations across a number of hospitals in Iran (S. Rahimzadeh, F. Farzadfar F, and M. Ghaziani, unpublished data). These figures are an order of magnitude smaller than what is reported in HICs. There are several possible reasons for this observation. For example, it may be that in these countries, hospitals are still largely used for procedure-related activities, as opposed to pure medical management. In such a setting, treatment of medical conditions, such as heart failure, is much more likely to take place in the outpatient setting, for which data from India and Iran are lacking. Overall, the population-level incidence and prevalence of heart failure, despite its significance and dominance amongst cardiovascular diseases presenting to hospitals worldwide, remains largely unknown. Similarly, few data regarding the direct and indirect costs of heart failure are available in LMICs, information that is vital in understanding and measuring the value of different health service configurations and novel interventions.
This review collates data over a time period of almost 20 y, which may be one explanation for the degree of heterogeneity in results between studies. However, when study period was analysed using meta-regression against the causes, management, and outcomes of heart failure, only three statistically significant effects were found. These included a rising percentage of patients in whom hypertension was reported as a contributing cause of heart failure, an increasing trend in the reported prescription of beta-blockers over time, and a substantial decline in in-hospital death rates (see Figures 14, 18, and 22). Although these associations are plausible and—in case of beta-blocker use and mortality rates—encouraging, they should be interpreted cautiously because of the potential for confounding.
Limitations
The data included are derived from a heterogeneous group of studies that set out with differing research goals. Variation in the methodologies used, particularly in methods of standardising the diagnosis and assessment of heart failure, may impact on some of the findings. These factors likely explain the high estimates of between-study variation that we found. Such variation may lead to underestimation of the true prevalence of heart failure, as well as inaccuracies in the causes ascribed to cases of heart failure. Our study includes individuals from three groups: those with their first presentation with acute heart failure, those with acute decompensation of chronic heart failure, and those with stable chronic heart failure seen in the outpatient clinic setting. Differences between healthcare systems may mean that the characteristics of patients seen in various settings may differ between countries, whilst adherence to gold-standard management may be more common amongst those with stable chronic heart failure seen in outpatient settings staffed by cardiologists than amongst those with acute heart failure treated in hospitals staffed by general internal physicians. In analysing these patients we have focussed on the evidence-based medical management methods common to all three groups. Combining data from 1995 to 2014, this study summarises management techniques over an almost 20-y period, an approach that may underestimate adherence to current management standards. However, when evaluated with meta-regression, the heterogeneity in a management variable was rarely found to be explained by changes over time. Another limitation of our study is that our data are derived from studies conducted for the most part in urban tertiary referral centres, which may not reflect the broader picture of heart failure in other hospitals and the community. Finally, despite the large number of studies included, information from some regions and for some outcomes was limited. In countries where few data are available, these results may not be truly reflective of the population and should therefore be interpreted as only a guide to the true prevalence, causes, and management of heart failure.
Conclusion
This review shows that heart failure places a considerable burden on health systems in LMICs, and affects a wide demographic profile of patients in these countries. Non-communicable diseases dominate the causes of heart failure across LMICs, although infectious valvular diseases and cardiomyopathies continue to impose a significant burden. Together, this suggests a double burden of communicable and non-communicable diseases for countries in the midst of epidemiological transition. In addition, we have identified high in-hospital mortality and wide variation and significant suboptimal use of pharmacological therapies. Further population-level studies, with clear case and outcome definitions, are needed for a more accurate assessment of heart failure in LMICs.
Supporting Information
Zdroje
1. MosterdA, HoesAW (2007) Clinical epidemiology of heart failure. Heart 93 : 1137–1146 doi:10.1136/hrt.2003.025270
2. BuiAL, HorwichTB, FonarowGC (2011) Epidemiology and risk profile of heart failure. Nat Rev Cardiol 8 : 30–41 doi:10.1038/nrcardio.2010.165
3. HobbsFDR, RoalfeAK, DavisRC, DaviesMK, HareR (2007) Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J 28 : 1128–1134 doi:10.1093/eurheartj/ehm102
4. LevyD, KenchaiahS, LarsonMG, BenjaminEJ, KupkaMJ, et al. (2002) Long-term trends in the incidence of and survival with heart failure. N Engl J Med 347 : 1397–1402 doi:10.1056/NEJMoa020265
5. RogerVL, GoAS, Lloyd-JonesDM, AdamsRJ, BerryJD, et al. (2011) Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123: e18–e209 doi:10.1161/CIR.0b013e3182009701
6. National Institute for Cardiovascular Outcomes Research (2011) National heart failure audit: April 2010–March 2011. London: University College London. Available: http://www.hqip.org.uk/assets/NCAPOP-Library/Heart-Failure-Audit-Report-NICOR-2010-2011.pdf. Accessed 21 July 2014.
7. JencksSF, WilliamsMV, ColemanEA (2009) Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 360 : 1418–1428 doi:10.1056/NEJMsa0803563
8. ZarrinkoubR, WettermarkB, WändellP, MejhertM, SzulkinR, et al. (2013) The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 15 : 995–1002 doi:10.1093/eurjhf/hft064
9. BleuminkGS, KnetschAM, SturkenboomMCJM, StrausSMJM, HofmanA, et al. (2004) Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J 25 : 1614–1619 doi:10.1016/j.ehj.2004.06.038
10. LozanoR, NaghaviM, ForemanK, LimS, ShibuyaK, et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 : 2095–2128 doi:10.1016/S0140-6736(12)61728-0
11. MurrayCJL, VosT, LozanoR, NaghaviM, FlaxmanAD, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 : 2197–2223 doi:10.1016/S0140-6736(12)61689-4
12. MendezGF, CowieMR (2001) The epidemiological features of heart failure in developing countries: a review of the literature. Int J Cardiol 80 : 213–219.
13. MoherD, LiberatiA, TetzlaffJ, AltmanDG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097 doi:10.1371/journal.pmed.1000097
14. BennettDA, EliaszTK, ForbesA, KiszelyA, KhoslaR, et al. (2012) Study protocol: systematic review of the burden of heart failure in low - and middle-income countries. Syst Rev 1 : 59 doi:10.1186/2046-4053-1-59
15. World Bank (2014) World DataBank [database]. Available: http://databank.worldbank.org/data/databases.aspx. Accessed 7 March 2013.
16. Von ElmE, AltmanDG, EggerM, PocockSJ, GotzschePC, et al. (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335 : 806–808 doi:10.1136/bmj.39335.541782.AD
17. DerSimonianR, LairdN (1986) Meta-analysis in clinical trials. Control Clin Trials 7 : 177–188.
18. United Nations Development Programme Human Development Reports (2014) Human Development Index (HDI). Available: http://hdr.undp.org/en/statistics/hdi/. Accessed 17 November 2013.
19. BarrettoACP, Del CarloCH, CardosoJN, MorgadoPC, MunhozRT, et al. (2008) Hospital readmissions and death from heart failure—rates still alarming. Arq Bras Cardiol 91 : 309–314.
20. SiswantoB, RadiB, KalimH (2010) Heart failure in NCVC Jakarta and 5 hospitals in Indonesia. CVD Prev Control 5 : 35–38 doi:10.1016/j.cvdpc.2010.03.005
21. AmarillaGA, CarballidoR, TacchiCD, FariasEF, PernaER, et al. (1999) Insuficiencia cardiaca en la Republica Argentina. Variables relacionadas con mortalidad intrahospitalaria. Resultados preliminares del protocolo CONAREC VI. Rev Argent Cardiol 67 : 53–62.
22. LaabesEP, ThacherTD, OkeahialamBN (2008) Risk factors for heart failure in adult Nigerians. Acta Cardiol 63 : 437–443 doi:10.2143/AC.63.4.2033041
23. DíazA, FerranteD, BadraR, MoralesI, BecerraA, et al. (2007) Seasonal variation and trends in heart failure morbidity and mortality in a South American community hospital. Congest Heart Fail 13 : 263–266.
24. KingueS, DzudieA, MenangaA, AkonoM, OuankouM, et al. (2005) [A new look at adult chronic heart failure in Africa in the age of the Doppler echocardiography: experience of the medicine department at Yaounde General Hospital]. Ann Cardiol Angéiologie 54 : 276–283 doi:10.1016/j.ancard.2005.04.014
25. StewartS, WilkinsonD, HansenC (2008) Predominance of heart failure in the Heart of Soweto Study cohort emerging challenges for urban African communities. Circulation 118 : 2360–2367 doi:10.1161/CIRCULATIONAHA.108.786244
26. OjjiDB, AlfaJ, AjayiSO, MamvenMH, FalaseAO (2009) Cardiovascular topics pattern of heart failure in Abuja, Nigeria: an echocardiographic study. Cardiovasc J Afr 20 : 349–352.
27. OjjiD, StewartS, AjayiS, ManmakM, SliwaK (2013) A predominance of hypertensive heart failure in the Abuja Heart Study cohort of urban Nigerians: a prospective clinical registry of 1515 de novo cases. Eur J Heart Fail 15 : 835–842 doi:10.1093/eurjhf/hft061
28. AdebayoAK, AdebiyiAA, OladapoOO, OgahOS, AjeA, et al. (2009) Characterisation of heart failure with normal ejection fraction in a tertiary hospital in Nigeria. BMC Cardiovasc Disord 9 : 52 doi:10.1186/1471-2261-9-52
29. Tantchou TchoumiJC, AmbassaJC, KingueS, GiambertiA, CirriS, et al. (2011) Occurrence, aetiology and challenges in the management of congestive heart failure in sub-Saharan Africa: experience of the Cardiac Centre in Shisong, Cameroon. Pan Afr Med J 8 : 11.
30. AmoahAG, KallenC (2000) Aetiology of heart failure as seen from a National Cardiac Referral Centre in Africa. Cardiology 93 : 11–18 doi:10.1159/000006996
31. OnwuchekwaAC, AsekomehGE (2009) Pattern of heart failure in a Nigerian teaching hospital. Vasc Heal Risk Manag 5 : 745–750.
32. Longo-MbenzaB, MambuneHFA, KasiamJB, VitaEK, FueleSM, et al. (2007) Relationship between waist circumference and cholesterol in central Africans with congestive heart failure. West Afr J Med 26 : 183–190.
33. AdedoyinRA, AdesoyeA (2005) Incidence and pattern of cardiovascular disease in a Nigerian teaching hospital. Trop Doct 35 : 104–106 doi:10.1258/0049475054037075
34. ChijiokeA, KoloP (2009) Mortality pattern at the adult medical wards of a teaching hospital in sub-Saharan Africa. Int J Trop Med 4 : 27–31.
35. DamascenoA, MayosiBM, SaniM, OgahOS, MondoC, et al. (2012) The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med 172 : 1386–1394 doi:10.1001/archinternmed.2012.3310
36. OmoleO, OgunbayoOO, FasanmadeAA (2011) A ten year study of management of chronic heart failure in a tertiary hospital in the South West Nigeria. Int J Pharm Technol 3 : 1520–1536.
37. AnsaVO, EkottJU, EssienIO, BasseyEO (2008) Seasonal variation in admission for heart failure, hypertension and stroke in Uyo, South-Eastern Nigeria. Ann Afr Med 7 : 62–66.
38. ThiamM (2003) Insuffisance cardiaque en milieu cardiologique africain. Bull Soc Pathol Exot 96 : 217–218.
39. BalieiroHM, OsugueRK, RangelSP, BrandãoR, BalieiroTL, et al. (2009) Clinical and demographic profile and quality indicators for heart failure in a rural area. Arq Bras Cardiol 93 : 637–642.
40. TavaresLR, VicterH, LinharesJM, BarrosCM De, OliveiraMV, et al. (2004) Epidemiology of decompensated heart failure in the city of Niterói: EPICA–Niterói Project. Arq Bras Cardiol 82 : 125–128.
41. CastroPG, VukasovicJLR, GarcésES, SepúlvedaLM, FerradaMK, et al. (2004) Insuficiencia cardíaca en hospitales chilenos: resultados del Registro Nacional de Insuficiencia Cardíaca, Grupo ICARO. Rev Med Chile 132 : 655–662.
42. De Campos LopesCB, YamadaAT, AraújoF, Pereira BarretoAC, MansurAJ (2006) Socioeconomic factors in the prognosis of heart failure in a Brazilian cohort. Int J Cardiol 113 : 181–187 doi:10.1016/j.ijcard.2005.11.009
43. RizzoM, ThiererJ, FrancesiaA, BettatiMI, TernsPP, et al. (2004) Registro Nacional de Internación por Insuficiencia Cardíaca 2002–2003. Rev Argent Cardiol 72 : 333–340.
44. FairmanE, ThiererJ, RodríguezL, BlancoP, GuettaJ, et al. (2009) 2007 national registry of admissions due to heart failure. Rev Argent Cardiol 77 : 33–39.
45. GodoyHL, SilveiraJA, SegallaE, AlmeidaDR (2011) Hospitalization and mortality rates for heart failure in public hospitals in São Paulo. Arq Bras Cardiol 97 : 402–407.
46. ManginiS, SilveiraFS, SilvaCP, GrativvolPS, SeguroLF, et al. (2007) Decompensated heart failure in the emergency department of a cardiology hospital. Arq Bras Cardiol 90 : 400–406.
47. JafaryFH, KumarM, ChandnaIE (2007) Prognosis of hospitalized new-onset systolic heart failure in Indo-Asians—a lethal problem. J Card Fail 13 : 855–860 doi:10.1016/j.cardfail.2007.07.005
48. BahajAA (2010) Clinical characteristic and in-patients mortality among 100 patients with heart failure admitted to Ibn Seena Central Hospital. Iraqi J Med Sci 8 : 60–68.
49. ErginA, KemalEN, ÜnalŞ, AbdullahD, SeyfeliE (2004) Epidemiological and pharmacological profile of congestive heart failure at Turkish academic hospitals. Anadolu Kardiyol Derg 4 : 32–38.
50. ChioncelO, VinereanuD, DatcuM, IonescuDD, CapalneanuR, et al. (2011) The Romanian Acute Heart Failure Syndromes (RO-AHFS) registry. Am Heart J 162 : 142–53.e1 doi:10.1016/j.ahj.2011.03.033
51. ZdrengheaD, PopD, PenciuO, ZdrengheaM (2009) Drug treatment of heart failure patients in a general Romanian hospital. Rom J Intern Med 47 : 227–233.
52. LaothavornP, HengrussameeK (2010) Thai Acute Decompensated Heart Failure Registry (Thai ADHERE). CVD Prev 5 : 89–95 doi:10.1016/j.cvdpc.2010.06.001
53. ShiC, WangL, HuD, LiJ, ZhuT, et al. (2010) Prevalence, clinical characteristics and outcome in patients with chronic heart failure and diabetes. Chin Med J (Engl) 123 : 646–650.
54. WeiL, SuY, FuL, ZhuZ-Y, LuG-L, et al. (2010) Analysis of multiple etiological factors and type of chronic heart failure in elderly patients. Med J Natl Defend Forces Southwest China 20 : 259–261.
55. SunX-Q, LiuS-L, LangY-J, SuiY-Y, FuY, et al. (2009) Retrospective analysis of inpatients with heart failure in rural areas of Liaoning province. J China Med Univ 38 : 852–854.
56. LiS-Y, SongF-F, ChenH-J (2007) Clinical analysis of 259 senile heart failure inpatients in tropical zone. China Trop Med 7 : 1169–1171.
57. Kang'AnC (2002) Retrospective investigation of hospitalised patients with heart failure in some parts of China in 1980, 1990 and 2000. Chin J Cardiol 30 : 450–454.
58. AnF, WangL, YangY (2010) Retrospective analysis of medication treatment in 2458 patients with chronic heart failure. Chin J Prev Control Chronic Non-Commun Dis 18 : 606–608.
59. Dos ReisFB, FernandesAMS, De AndradeG, BitencourtA, NevesF, et al. (2012) Influencia de la etiologia sobre la mortalidad en la insuficiencia cardiaca con funcion sistolica preservada en una poblacion con alta prealencia de cardiopatia chagascia. Rev Argent Cardiol 81 : 246–250 doi:10.7775/rac.es.v81.i3.1417
60. FuS-H, ZhuB, ZhangY-X, YiS-Y, LiuY, et al. (2012) Chronic kidney disease: an independent risk factor of all-cause mortality for elderly Chinese patients with chronic heart failure. J Geriatr Cardiol 9 : 355–360 doi:10.3724/SP.J.1263.2012.04121
61. StanojevićD, ApostolovićS, Janković-TomaševićR, Šalinger-MartinovićS, PavlovićM, et al. (2012) Prevalence of renal dysfunction and its influence on functional capacity in elderly patients with stable chronic heart failure. Vojn Pregl 69 : 840–845.
62. SunX, LiJ, LangY, SuiY, FuY, et al. (2009) Investigation of epidemiologic features and treatment of chronic heart failure in some rural parts of Liaoning province. Zhongguo Shiyong Neike Zazhi 29 : 1000–1002.
63. DegertekinM, ErolC, ErgeneO, TokgozogluL, AksoyM, et al. (2012) [Heart failure prevalence and predictors in Turkey: HAPPY study.]. Turk Kardiyol Dern Ars 40 : 298–308 doi: 10.5543/tkda.2012.65031
64. ClelandJGF, Cohen-SolalA, AguilarJC, DietzR, EastaughJ, et al. (2002) Management of heart failure in primary care (the IMPROVEMENT of Heart Failure Programme): an international survey. Lancet 360 : 1631–1639.
65. BarrettoAC, NobreMR, WajngartenM, CanesinMF, BallasD, et al. (1998) Insuficiência cardíaca em grande hospital terciário de São Paulo. Arq Bras Cardiol 71 : 15–20.
66. BarrettoAC, WajngartenM, Serro-AzulJB, PierriH, NussbacherA, et al. (1997) Tratamento medicamentoso de insuficiência cardíaca em hospital terciário de São Paulo. Arq Bras Cardiol 69 : 375–379.
67. NogueiraPR, RassiS, CorrêaKDS (2010) Epidemiological, clinical e therapeutic profile of heart failure in a tertiary hospital. Arq Bras Cardiol 95 : 392–398.
68. AthertonJJ, HaywardCS, Wan AhmadWA, KwokB, JorgeJ, et al. (2012) Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International-Asia Pacific). J Card Fail 18 : 82–88 doi:10.1016/j.cardfail.2011.09.003
69. Roa M, Kadam S, Sathyanarayana TN, Shidhaye R, Shidhaye R, et al. (2009) A rapid evaluation of the Rajiv Aarogyasri community health insurance scheme, Andhra Pradesh. Hyderabad: Indian Institute of Public Health.
70. Magaña-SerranoJA, AlmahmeedW, GomezE, Al-ShamiriM, AdgarD, et al. (2011) Prevalence of heart failure with preserved ejection fraction in Latin American, Middle Eastern, and North African Regions in the I PREFER study (Identification of Patients With Heart Failure and PREserved Systolic Function: an epidemiological regional study). Am J Cardiol 108 : 1289–1296 doi:10.1016/j.amjcard.2011.06.044
71. McKeePA, CastelliWP, McNamaraPM, KannelWB (1971) The natural history of congestive heart failure: the Framingham study. N Engl J Med 285 : 1441–1446 doi:10.1056/NEJM197112232852601
72. CarlsonKJ, LeeDC-S, GorollAH, LeahyM, JohnsonRA (1985) An analysis of physicians' reasons for prescribing long-term digitalis therapy in outpatients. J Chronic Dis 38 : 733–739 doi:10.1016/0021-9681(85)90115-8
73. MoscavitchS-D, GarciaJL, RosaLF, PestanaPR, MoraesLV, et al. (2009) Insuficiência cardíaca: estarão as diretrizes incorporadas na rede de cuidados primários? Rev Port Cardiol 28 : 683–696.
74. Brazil Minister of Health (2014) Datasus [database]. Available: http://www2.datasus.gov.br/DATASUS/index.php. Accessed 7 December 2013.
75. SliwaK, WilkinsonD, HansenC, NtyintyaneL, TibazarwaK, et al. (2008) Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet 371 : 915–922.
76. NieminenMS, BrutsaertD, DicksteinK, DrexlerH, FollathF, et al. (2006) EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 27 : 2725–2736 doi:10.1093/eurheartj/ehl193
77. AdamsKF, FonarowGC, EmermanCL, LeJemtelTH, CostanzoMR, et al. (2005) Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 149 : 209–216 doi:10.1016/j.ahj.2004.08.005
78. KhatibzadehS, FarzadfarF, OliverJ, EzzatiM, MoranA (2012) Worldwide risk factors for heart failure: A systematic review and pooled analysis. Int J Cardiol 168 : 1186–1194 doi:10.1016/j.ijcard.2012.11.065
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 8- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- VIDEO: Jak zacházet s osobními ochrannými pracovními prostředky (OOPP)
- Snižuje terapie betablokátory kardiovaskulární benefit aerobního cvičení u pacientů s arteriální hypertenzí?
-
Všechny články tohoto čísla
- Observational Studies: Getting Clear about Transparency
- Scaling up Rural Sanitation in India
- Intervention Synthesis: A Missing Link between a Systematic Review and Practical Treatment(s)
- From Intense Rejection to Advocacy: How Muslim Clerics Were Engaged in a Polio Eradication Initiative in Northern Nigeria
- Ethics, Economics, and the Use of Primaquine to Reduce Falciparum Malaria Transmission in Asymptomatic Populations
- Hand Sanitiser Provision for Reducing Illness Absences in Primary School Children: A Cluster Randomised Trial
- Protective Efficacy and Safety of Three Antimalarial Regimens for the Prevention of Malaria in Young Ugandan Children: A Randomized Controlled Trial
- Heart Failure: Gaps in Knowledge and Failures in Treatment
- Staffing of Healthcare Workers and Patient Mortality: Randomized Trials Needed
- Associations between Stroke Mortality and Weekend Working by Stroke Specialist Physicians and Registered Nurses: Prospective Multicentre Cohort Study
- Women's Access and Provider Practices for the Case Management of Malaria during Pregnancy: A Systematic Review and Meta-Analysis
- Stress Hyperglycaemia in Hospitalised Patients and Their 3-Year Risk of Diabetes: A Scottish Retrospective Cohort Study
- The Effect of India's Total Sanitation Campaign on Defecation Behaviors and Child Health in Rural Madhya Pradesh: A Cluster Randomized Controlled Trial
- Heart Failure Care in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Women's Access and Provider Practices for the Case Management of Malaria during Pregnancy: A Systematic Review and Meta-Analysis
- Observational Studies: Getting Clear about Transparency
- Heart Failure Care in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
- Scaling up Rural Sanitation in India
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



