-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Cost-Effectiveness of Low-Cost Essential Antihypertensive Medicines for Hypertension Control in China: A Modelling Study
In a Markov-style simulation model, Andrew Moran and colleagues estimate the reduction in cardiovascular disease and cost-effectiveness of broad provision of antihypertensive medications in China.
Published in the journal: . PLoS Med 12(8): e32767. doi:10.1371/journal.pmed.1001860
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001860Summary
In a Markov-style simulation model, Andrew Moran and colleagues estimate the reduction in cardiovascular disease and cost-effectiveness of broad provision of antihypertensive medications in China.
Introduction
High blood pressure (BP) is the leading risk factor for cardiovascular disease (CVD) in China, and uncontrolled high BP is responsible for more of total disease burden in China than any other single risk factor [1]. Approximately 325 million, or about 30%, of Chinese adults aged 18 y or older have hypertension [2]. Among Chinese adults with hypertension, less than half are aware of their diagnosis, about 34% are treated with medications to lower BP, and less than 28% of those treated are controlled to a goal of <140 mm Hg systolic BP and <90 mm Hg diastolic BP [2]. Though the potential health gains from hypertension control would be enormous, the cost-effectiveness of implementing Chinese BP treatment guidelines has not been assessed.
China’s 2009 national health reform expanded health insurance coverage dramatically, but most patients still pay for outpatient clinic visits and medications out-of-pocket [3–5]. The 2009 reforms introduced a list of “essential” antihypertensive medications with fixed, affordable prices required in government-sponsored primary health facilities [6]. Negotiation for and enforcement of lower drug costs is done at the provincial or municipal level, and enforcement of the “zero profit” rule has not been uniform across the health system [7]. We used the CVD Policy Model-China, a national scale computer simulation model [8,9], to assess the cost-effectiveness of treating hypertension in China, using low-cost medications on the national essential medicines list.
Methods
CVD Policy Model-China Overview
Population-based mathematical simulation models are appropriate for estimating the average value of implementing national clinical practice guidelines. The CVD Policy Model-China is a computer-simulation, state-transition (Markov cohort) mathematical model of coronary heart disease and stroke incidence, prevalence, mortality, non-CVD deaths, and health care costs at the population level in adults aged 35–84 y in China (Fig 1, S1 Text) [8]. The model predicts annual coronary heart disease and stroke incidence and non-CVD mortality among persons without CVD, stratified into cells by age, sex, systolic BP, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol levels, body mass index (BMI), and status of isolated diastolic hypertension, hypertension treatment, chronic kidney disease, smoking, and diabetes mellitus. Simulations projecting CVD in future years incorporate demographic changes and preserve age-related trends in risk factors, event rates, and case fatality. Appropriate to the diagnostic definitions of CVDs, the model assumes that survivors persist in a chronic disease state (linear model without remission). The model also predicts life years, coronary heart disease and stroke events, CVD mortality, and non-CVD mortality among patients with CVD. Each policy model health state and event has an annual cost and quality-of-life adjustment.
Fig. 1. CVD Policy Model-China structure. 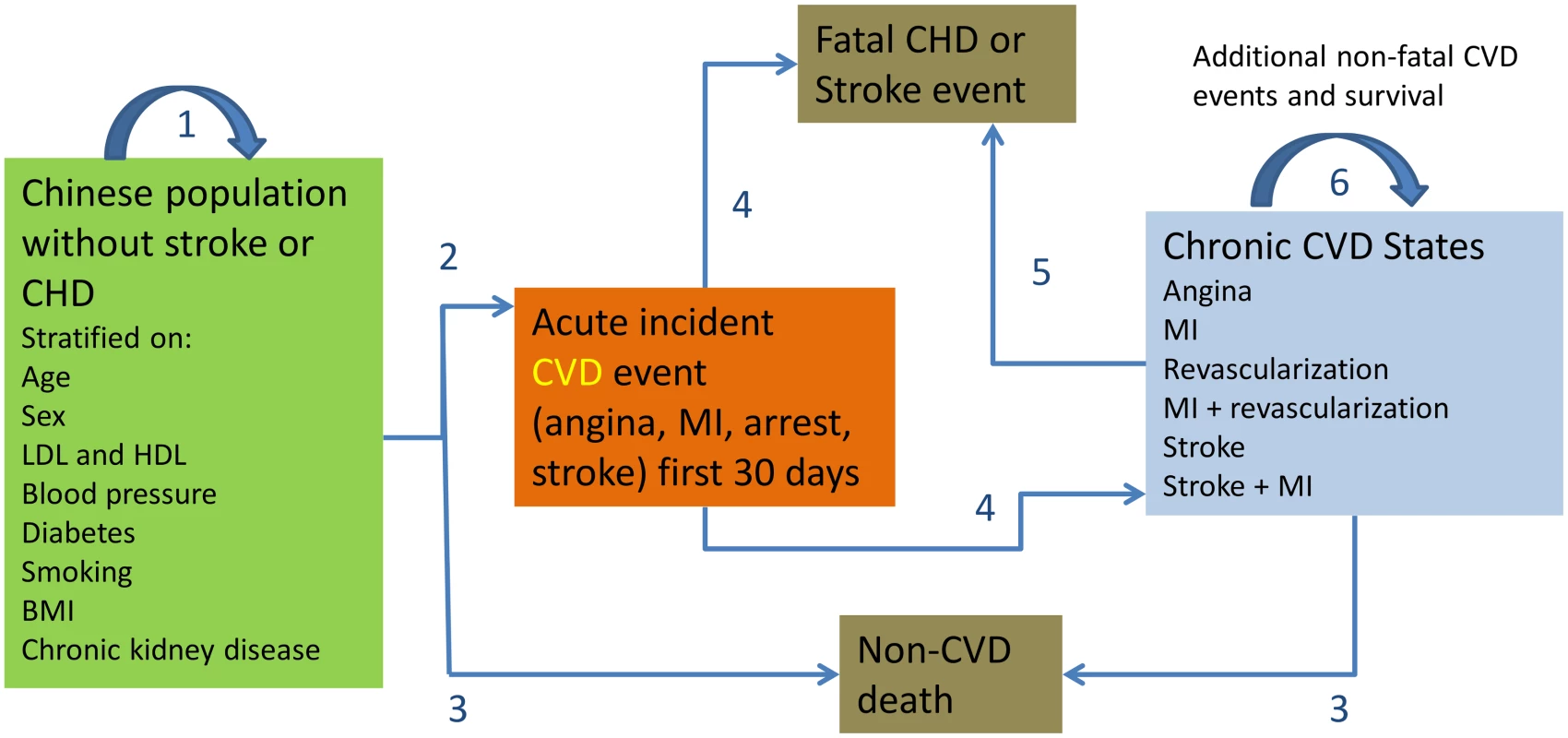
State transitions are numbered in the diagram. Transition 1 = remain in CVD-free state. Transition 2 = incident CVD. Transition 3 = non-CVD death. Transitions 4 and 5 = survival or case fatality. Transition 6 = survival with or without repeat CVD event in chronic CVD patients. National prevalence, joint distributions, and means of risk factors were estimated from the International Collaborative Study of Cardiovascular disease in Asia (InterASIA). This included proportions of Chinese adults with systolic BP of <130, 130–139, 140–159, and ≥160 mm Hg and diastolic BP of <80, 80–89, 90–99, and ≥100 mm Hg, and the proportion with self-reported untreated hypertension. Systolic BP of 140–159 mm Hg and ≥160 mm Hg correspond in both Chinese Society of Hypertension guidelines [10] and European Society of Cardiology guidelines [11] to stage one and ≥stage two hypertension. Isolated diastolic hypertension was categorized into two corresponding categories: diastolic of 90–99 or ≥100 mm Hg, both accompanied by systolic BP of <140 mm Hg. For treatment of isolated systolic hypertension and combined systolic and diastolic hypertension, we simulated a reduction in systolic BP; for treatment of isolated diastolic hypertension, we simulated a reduction in diastolic BP. All of these subcategories of hypertension received the intervention in treatment simulations.
The CVD Policy Model-China defined coronary heart disease as myocardial infarction (ICD-9 410 and 412 or ICD-10 I21 and I22), angina and other coronary heart disease (ICD-9 411, 413, and 414 or ICD-10 I20 and I23–I25), and a fixed proportion of “ill-defined” CVD-coded events and deaths (ICD-9 codes 427.1, 427.4, 427.5, 428, 429.0, 429.1, 429.2, 429.9, and 440.9 or ICD-10 I47.2, I49.0, I46, I50, I51.4, I51.5, I51.9, and I70.9) [12]. Stroke was defined by ICD-9 codes 430–438 (excluding transient ischemic attack) or ICD-10 I60–I69. Starting with coronary heart disease and stroke incidence and prevalence obtained from the China Hypertension Epidemiology Follow-up Study, the CVD Policy Model-China mortality projections were calibrated to fit with age-specific and overall coronary heart disease and stroke mortality numbers for the years 2000–2010 estimated by the World Health Organization (S1 Text).
Risk Factor Risk Coefficients and Model Calibration
Risk factor beta coefficients for LDL, HDL, diabetes, chronic kidney disease, and smoking conditioned on age and sex were estimated from the China Multi-provincial Cohort Study (CMCS) [13] using three distinct competing risk Cox proportional hazard models with coronary heart disease, total stroke, and other-cause death as the outcomes.
We assumed CVD risk reduction is due to BP reduction [14] and that BP is lowered to a similar extent across classes when comparing per-class standard doses [15,16]. We started with observational Prospective Studies Collaboration age-specific relative risks and 95% confidence intervals for coronary heart disease and stroke per 10 mm Hg change in systolic BP or 5 mm Hg diastolic BP (Table 1) [17]. Age-specific relative risk inputs were calibrated to be within ≤0.02 of these estimates and overall relative risks within 95% confidence interval bounds of the summary estimate from a large meta-analysis of randomized clinical trials of hypertension treatment (S1 Table, S2 Table) [14]. The stroke relative risk estimate was found to be close to the pooled estimate from the East Asian trials included in that analysis (0.59 [0.49–0.71], S1 Text). The resulting relative risk assumptions were validated for treatment of systolic BP in ages of 60–74 y by simulating the treatment and placebo groups of the Systolic Hypertension in the Elderly Program (SHEP) trial and comparing simulated relative rate ratios with those observed in the trial (S1 Text, S3 Table).
Tab. 1. Main assumptions for the cost-effectiveness analysis of China hypertension control policy. 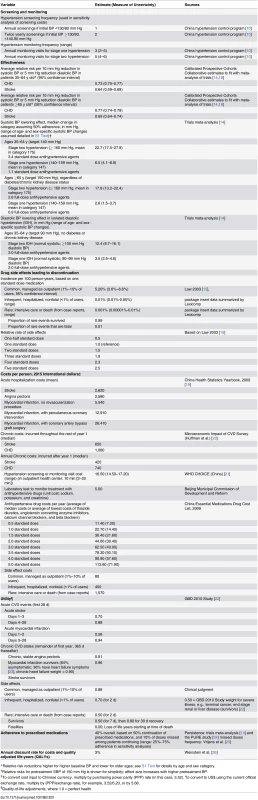
*Relative risk reductions higher for higher baseline BP and lower for older ages; see S1 Text for details by age and sex category. Hypertension Treatment Policy Simulations
BP lowering with treatment and the number of “standard dose” antihypertensive agents needed to meet target BP in untreated hypertensive patients according to pretreatment BP and age were based on a meta-analysis of BP treatment trials (S4 Table) [14]. Among adults with stage two hypertension and the highest pretreatment BP (mean 176 mm Hg systolic or 110 mm Hg diastolic), it was assumed that a small proportion have truly resistant hypertension, and the average treated BP was just above goal (143 mm Hg systolic or 92 mm Hg diastolic). Variance in BP change with antihypertensive treatment was based on standard deviations around the main BP change estimates observed in a meta-analysis [15]. In main simulations, complete discontinuation of prescribed medications in China one year after initiation was assumed to be 50%, based on medication discontinuation rates observed in China and other middle income countries that were sampled in the Prospective Urban Rural Epidemiology (PURE) study [24]. For all simulations, it was assumed that adherent patients miss about 10% of scheduled medication doses, leading to 10% lower effectiveness, but incur the full cost of the scheduled regimen [25]. Based on these assumptions, overall medication adherence was assumed to be 40% in main simulations, similar to a study by another group [27].
A status quo simulation projected total (first ever and repeat) myocardial infarction and stroke events, direct CVD costs, and QALYs for Chinese adults assuming no change in current levels of hypertension treatment over the years 2015–2025. The first intervention step simulated treatment of all untreated patients with existing CVD (secondary prevention). Subsequent simulations progressively added primary prevention treatment of the untreated population without existing CVD and in two steps: first, stage two hypertension alone and second, stage two plus stage one together. Among patients aged 65–84 y with systolic hypertension (with or without diastolic hypertension), those with stage two hypertension required an average of 2.7 standard dose medications to reach a systolic BP goal of 150 mm Hg, and those with stage one hypertension required an average of 0.9 standard dose medications to reach a goal systolic BP of 150 mm Hg (Table 1, S4 Table, and S5 Table). Among patients aged 35–64 y with systolic hypertension (with or without diastolic hypertension), those with stage two hypertension required an average of 3.4 standard dose medications to reach a goal systolic BP of 140 mm Hg, and those with stage one hypertension required an average of 1.2 standard dose medications to reach a goal systolic BP of 140 mm Hg. For patients of all ages with isolated diastolic hypertension, those with stage two isolated diastolic hypertension required an average of 3.0 standard dose medications to reach a goal diastolic BP of 90 mm Hg, and those with stage one isolated diastolic hypertension required an average of 1.0 standard dose medications to reach a goal diastolic BP of 90 mm Hg. Based on equivocal clinical trial evidence to support a lower target for patients with diabetes or chronic kidney disease and consequent changes to recent international guidelines, we assumed the same diagnostic thresholds and targets for patients with or without these conditions [11,28].
Median costs of drugs in four standard antihypertensive drug classes (thiazide diuretic, angiotensin converting enzyme inhibitor, beta blocker, and long-acting calcium channel blocker) in China’s 2009 national essential medicines list were averaged and inflated to 2015 (S1 Text). Combinations of standard dose medications (1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 standard doses) were assigned the cumulative cost of the individual agents because the essential medicines list did not include combination agents priced lower than the cost of two separate drugs. Rates of adverse events from medication side effects were based on a meta-analysis of treatment trial side effect rates for more common events [15] and postmarketing reports for rarer events. Adverse event rates were translated into quality of life impairments, and added costs related to events ranging from transient symptoms accompanied by an office visit (common) to death (rare).
In addition to individual patient treatment costs, we simulated national hypertension control program costs. China’s central government recently financed opportunistic hypertension screening in adults aged ≥35 y in outpatient clinics. Nonetheless, we simulated adding the cost of systematic hypertension screening of adults aged 35–84 y in outpatient clinics: twice yearly for adults without diabetes or chronic kidney disease and systolic BP of 130–139 mm Hg or diastolic BP of 85–89 mm Hg and annually for those below 130 mm Hg systolic and 85 mm Hg diastolic. The expanded screening program was simulated by adding screening visit direct costs (Table 1) [20] for the following groups: (1) people unaware of their hypertension diagnosis aged 35–84 y in 2015, (2) ongoing screening of undiagnosed persons (twice yearly if prior screening result was 130–139/85–89 mm Hg and annually if <130/85 mm Hg), and (3) waves of 35-y-olds in the years 2015–2025. The main cost of implementing China’s zero-profit essential medicines program would be replacing physician’s income derived in the past from adding a personal service fee to every prescription dispensed. Based on policies proposed for Chongching and Tianjin provinces, we assumed that the government would cover the cost of returning 15% of pharmaceutical expenditures as payments to physicians prescribing according to the essential medicines program rules [29]. Lastly, following the findings of a World Health Organization CHOosing Interventions that are Cost Effective (WHO-CHOICE) analysis of program costs, we assumed that a clinic-based prevention program would require an additional 5% of total intervention costs for program administration [30].
Analyses were interpreted from a payer’s perspective. Effectiveness (measured as QALYs gained, reductions in coronary heart disease and stroke events), screening, treatment, monitoring, and total costs (inclusive of acute and chronic CVD treatment costs saved) were simulated over the years 2015–2025 and averaged to annual estimates. All future costs and QALYs were discounted at 3% annually. Incremental cost-effectiveness ratios (ICERs) were calculated by dividing the incremental change in CVD costs by incremental change in QALYs. All results reported as cost saving describe less costly and more effective strategies. Results are reported in 2015 international dollars and 2015 Chinese renminbi (RMB) according to the exchange rate published by the World Bank, based on PPP methods (1.00 yuan = Int$0.28; Int$1.00 = 3.52 yuan). The ICER threshold for cost-effectiveness was based on the WHO-CHOICE-recommended gross domestic product (GDP) per capita-indexed cost-effectiveness threshold of highly cost effective (< 1 x GDP per capita). A cost-effectiveness acceptability curve also assessed the probability of cost-effectiveness over a range of willingness to pay thresholds, including a threshold of 2 x GDP per capita. Basing the conversion rate on PPP, GDP per capita for China was assumed to be Int$11,900 (38,450 RMB)].
Exploratory and Sensitivity Analyses
One-way sensitivity analyses examined higher and lower medication adherence rates, ranges of uncertainty surrounding the relative risk of coronary heart disease and stroke per mm Hg lower BP, the BP lowering effect of antihypertensive medications (mm Hg change), medication costs, and the probability of side effects (Table 1). Three alternate medication cost assumptions were explored: (1) the average of lowest national essential medicines list prices per drug class, (2) median prices from the Shanghai municipality essential medicines list (highest cost assumption), and (3) median prices from the Yunnan province essential medicines list (lowest cost assumption, S1 Text). For stage two hypertension patients without diabetes, mean drug costs ranged from Int$44–Int$46 (Yunnan, 281–295 RMB) to Int$193–Int$236 (Shanghai, 1,227–1,505 RMB); stage one mean drug costs ranged from Int$ 19–Int$22 (Yunnan, 122–137 RMB) to Int$46–Int$92 (Shanghai, 296–589 RMB). An exploratory analysis repeated the main simulations after recalibrating stroke and coronary heart disease incidence to match higher cause-specific mortality targets for stroke and coronary heart disease reported by the China Ministry of Health (S1 Text). Lacking specific data on indirect costs to patients, we tested the sensitivity of the results to possible indirect costs by adding 10% higher cost for treatment and monitoring and adding 50% higher cost to acute CVD event costs. Lastly, we assessed the cost-effectiveness of hypertension treatment, including medication, monitoring, and side effect costs but excluding screening, program administration, and implementation costs.
In a probabilistic (Monte Carlo) analysis, 1,000 random draws were taken of the uncertainty distributions for systolic BP relative risk, BP lowering with treatment, quality of life penalties, total treatment costs (inclusive of medications, monitoring, and side effect costs), case fatality, background CVD treatment costs, population mean BP, hypertension prevalence, and antihypertensive drug use (S1 Text and S6 Table). The uncertainty intervals reported do not include the following sources of uncertainty: variation in program administration costs, Essential Medicines implementation costs, screening costs, or CVD incidence or prevalence, because uncertainty distribution estimates were not available for these inputs. Multivariable probabilistic analyses resulted in 1,000 cost and QALY pairings that were used to calculate 95% uncertainty intervals for incremental costs, QALYs, ICER estimates and the proportion of ICERs that were cost saving or cost-effective, and probability of cost-effectiveness at different willingness-to-pay thresholds.
In order to quantify lifetime benefits of hypertension treatment, we simulated a cohort of 20 million 35–44-y-old adults until death or reaching the age of 84 y. By comparing with a status quo cohort simulation, we tabulated lifetime gains in discounted life years and QALYs gained and costs incurred per person-year of hypertension treatment initiated in untreated eligible adults at whatever age they first met diagnostic criteria. National program costs were not included in the cohort simulation.
Results
If the Chinese government systematically screened adults aged 35–84 years for hypertension, it would require an investment of about Int$962 million (3.4 billion RMB) in 2015 to screen adults unaware of an existing hypertension diagnosis, and about Int$65 billion U.S. annually (231 billion RMB) to screen adults currently without hypertension and all persons becoming 35 years of age after 2015 for incident cases of hypertension during 2015-2025. Assuming use of low-cost medications from the national essential medicines list, treating hypertensive adults with a prior diagnosis of CVD for secondary prevention was projected to prevent about 111,000 cardiovascular events yearly over 2015–2025. Treating all previously untreated adults with stage two hypertension for primary prevention, along with secondary prevention treatment in CVD patients, was projected to avert about 583,000 strokes and 93,000 myocardial infarctions and gain about 934,000 QALYs annually compared with the status quo (Table 2). Treating all hypertension (stage one and stage two; primary and secondary prevention) would prevent about 803,000 CVD events and gain about 1.2 million QALYs annually compared with the status quo.
Tab. 2. Effectiveness and cost-effectiveness of implementing different BP control guidelines in untreated Chinese adults aged 35–84 y with hypertension, averaged from the projections for 2015–2025, the CVD Policy Model-China. 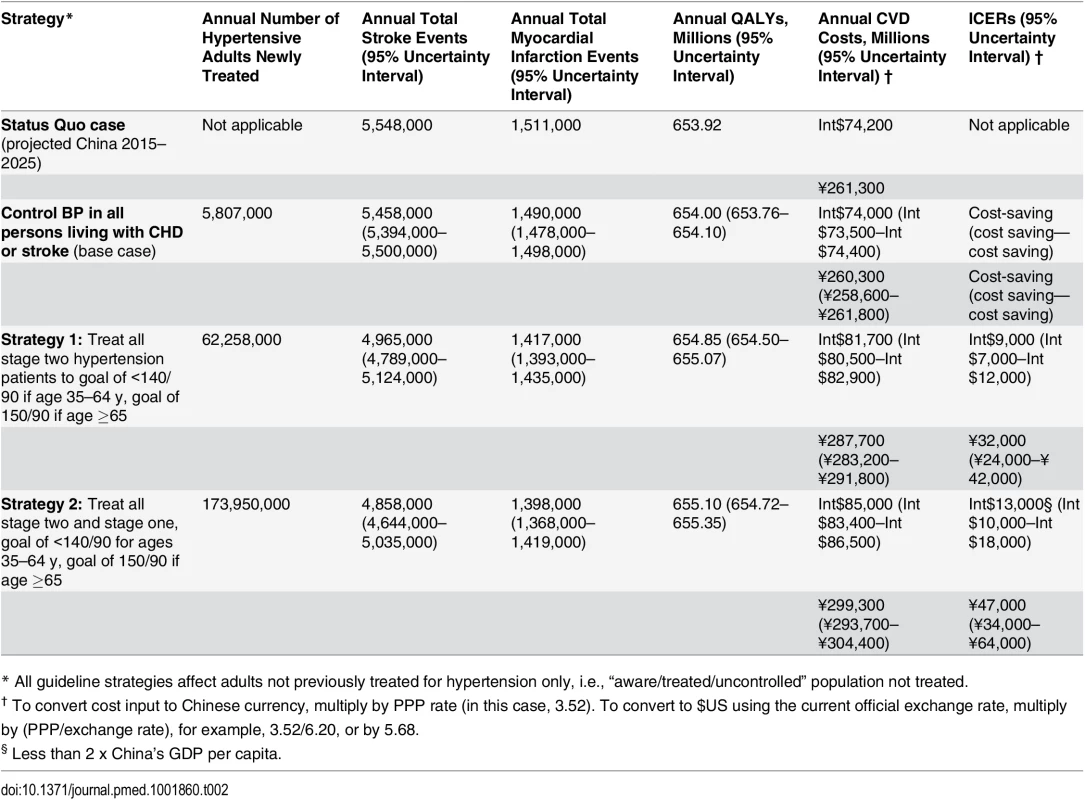
Each successive strategy is compared with the prior strategy. Results are in 2015 international dollars and 2015 Chinese RMB. All results reported as cost-saving describe strategies projected to be less costly and more effective than the prior strategy. Ninety-five percent uncertainty intervals were calculated from the results of 1,000 probabilistic simulations. Treating hypertension in CVD patients was projected to save costs in 100% of probabilistic simulations. Incrementally adding treatment of stage two patients was projected to be cost-effective (Int$9,000 per QALY gained, [95% uncertainty range Int$7,000 to Int$12,000], Table 2 and S7 Table). Treating all untreated hypertensive patients (primary and secondary prevention) was projected to be borderline cost-effective compared with treating stage two and CVD patients alone (about Int$13,000 per QALY gained, [Int$10,000 to Int$18,000]). At a willingness-to-pay threshold of the GDP per capita of China (Int$11,900 in 2015), treating all hypertensives was the most cost-effective strategy in 63% of probabilistic simulations (Fig 2). At thresholds of Int$19,000 and above, treating all hypertensive adults was the most cost-effective strategy in 100% of simulations.
Fig. 2. Cost-effectiveness acceptability curves comparing treating all untreated hypertensive adults (blue) with treating only untreated CVD patients and adults with stage 2 hypertension but without CVD (red). 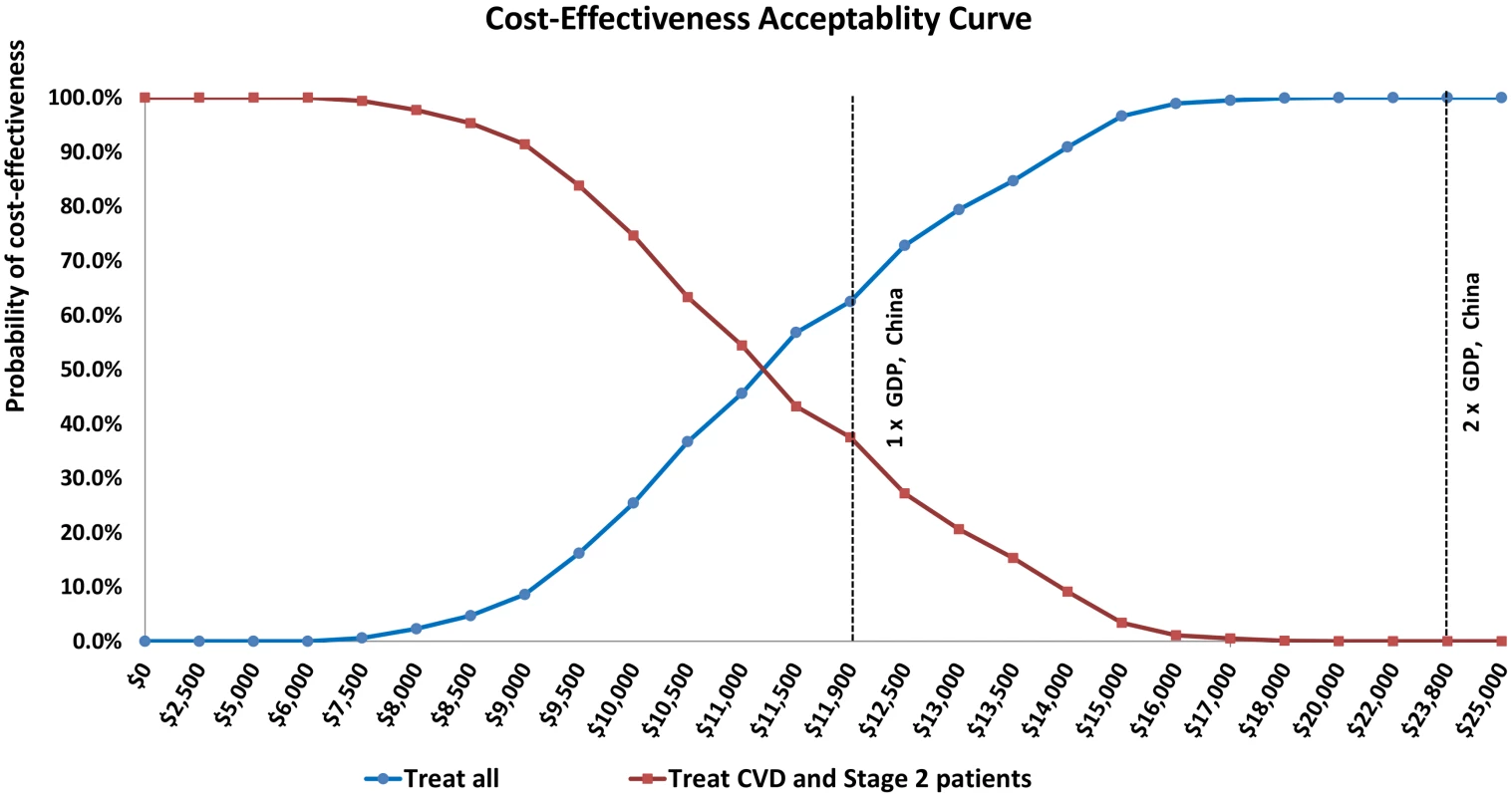
The threshold for cost-effective in China assumed for this analysis is labeled at Int,900 (China’s GDP per capita; conversion to US dollars from Chinese RMB based on PPP). Twice China’s GDP is also labelled at Int,800. Of all one-way sensitivity analyses, assuming 25% medication adherence by patients, high Shanghai drug costs, or low medication efficacy led to the most unfavorable cost-effectiveness results (treating all hypertension about Int$47,000, Int$37,000, and Int$27,000 per QALY gained, respectively, Table 3). Assuming lower medication costs, lower monitoring costs (Int$1 less per visit), or achieving the same health gains using less frequent screening (two fewer visits for stage two, one fewer visit for stage one) led to exceptionally low-cost projections. Adding 10% higher treatment and monitoring costs or 50% acute CVD event costs to reflect possible indirect costs to patients did not change the results substantially. When screening, program administration, and implementation costs were excluded, adding primary prevention treatment of stage two hypertension was cost saving, and treating all hypertensive adults remained cost-effective (Int$12,000 per QALY gained).
Tab. 3. One-way sensitivity analysis of hypertension treatment inputs. 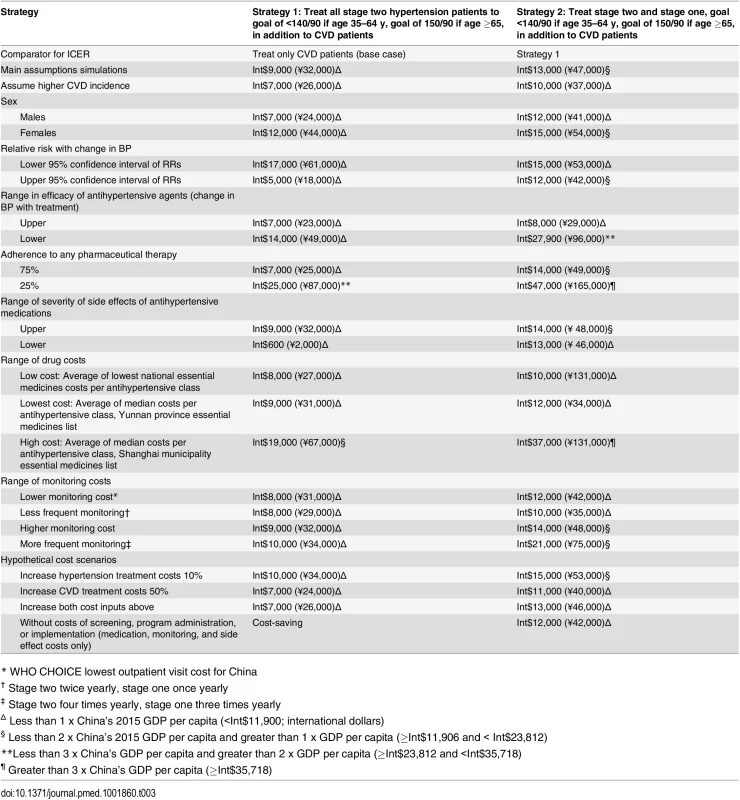
All estimates are ICERs, compared with the prior strategy. Results are in 2015 international dollars (2015 Chinese RMB). All results reported as cost saving describe strategies projected to be less costly and more effective than the prior strategy. For the cohort simulation starting at ages 35–44 y, hypertension treatment starting at the age at meeting diagnostic criteria led to an average lifetime gain of about five healthy days of life (4.8 quality-adjusted d and 4.2 total d) and an average lifetime cost of Int$1.80 (6.34 RMB).
Discussion
In this analysis of hypertension control for CVD prevention in China, we found that controlling hypertension in adults aged 35–84 y could prevent about 800,000 cardiovascular events annually and be borderline cost-effective. Strengthening one pillar of China’s 2009 health reform—affordable essential antihypertensive medications—appeared to be crucial for achieving population-wide hypertension control at low cost.
Very few past studies have estimated the cost-effectiveness of hypertension treatment in China, and to our knowledge, ours is the first to assess cost-effectiveness by balancing program and intervention costs with projected downstream benefits of prevented CVD events. In a mathematical modeling study, Lim et al. estimated that scaling up a multidrug CVD prevention program, including aspirin and a statin along with an antihypertensive medication, would cost about US$55 per patient treated and would be relatively expensive, costing China about one US dollar per capita population in 2006 [27]. That analysis did not report on hypertension treatment specifically and likely overestimated the net cost of the program by not including cost saved by preventing or delaying CVD events. Three cost-effectiveness analyses conducted in China used primary data to demonstrate that implementing community health center-based hypertension management programs is effective and inexpensive, but cost-effectiveness was not measured based on prevented CVD or life-year gains [31–33].
The methods and reporting of this study conform to Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [34] and Quality of Health Economic Studies Instrument standards recommended for cost-effectiveness analyses of CVD risk-factor guidelines [35]. China-specific demographic, epidemiologic, and health care cost data were used whenever possible. Effectiveness assumptions were grounded in a large meta-analysis of randomized antihypertensive medication treatment trials. However, all computer simulation studies are limited by reliance on numerous assumptions derived from diverse study designs and samples. Some model inputs were derived from studies of non-Chinese CVD patients and may not represent the general Chinese population. This analysis was limited in that educational and dietary measures for lowering BP were not compared with pharmacologic treatment, nor was hypertension treatment assessed in combination with treatment of elevated serum cholesterol levels [27,36]. We varied drug costs according to published essential medicines lists using median prices within antihypertensive drug classes, so our medication cost inputs do not reflect the frequency with which specifically priced agents are actually prescribed. We did not model the possibility that the practice of charging additional costs to patients by individual prescribers will persist even as the essential medicines program is implemented. We did not account for all of the costs of scaling up a hypertension treatment program (including training and infrastructure costs), specific out-of-pocket and indirect costs that would be incurred by patients participating in hypertension treatment, or indirect costs avoided as a result of prevented CVD.
While China rapidly expanded health insurance coverage nationally within the past decade, many Chinese adults still have limited access to hypertension screening and follow-up for hypertension treatment and monitoring. For example, in the New Rural Cooperative Medical Scheme, which now covers over 95% of the rural population, most coverage is for inpatient hospitalizations, and the costs of basic medical services, including hypertension education, screening, treatment, and monitoring, are not usually covered [4,37]. The results of our analysis suggest that expanding the scope of hypertension treatment would be borderline cost-effective for a government payer (around China’s per capita GDP) even if the costs of systematic screening of adults ages 35–84-y-old and essential medicines program implementation costs were added to medication and monitoring costs.
Our results were most sensitive to assumptions about medication costs and patient adherence to medications, both of which are influenced by drug pricing. It is estimated that of the 5% of China’s GDP that is spent on health care, 42% is spent on pharmaceuticals, a much higher proportion than is spent in high-income nations (15% on average) [7]. Because medication costs are usually paid out-of-pocket by patients with hypertension, local and national governments do not directly feel the impact of high drug costs [5]. However, high drug costs likely have a big impact at the level of individual households and therefore indirectly on the national economy [19]. Additionally, Chinese patients are reluctant to pay out-of-pocket for antihypertensive medications [5], and studies of Chinese patients have shown that out-of-pocket drug costs reduce medication adherence among patients with hypertension [3] and CVD [38]. Therefore, the Chinese government should work to ensure that the expense of scaling up a national hypertension control program is not borne in large part by patients. A subsidized antihypertensive medications program in Shandong province improved medication adherence dramatically [3] and might be successfully scaled up to a national policy.
Our computer simulation modeling study projected that treating hypertension in untreated Chinese adults could prevent about 800,000 CVD events annually and was cost-effective in over 63% of simulations. Cost-effectiveness was particularly sensitive to medication adherence and antihypertensive drug costs, implying that full implementation of the essential medicines program and subsidized drug costs programs will be important for reaping the benefits of improved hypertension control in China.
Supporting Information
Zdroje
1. Yang G, Wang Y, Zeng Y, Gao GF, Liang X, et al. (2013) Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 381 : 1987–2015. doi: 10.1016/S0140-6736(13)61097-1 23746901
2. Wang J, Zhang L, Wang F, Liu L, Wang H, et al. (2014) Prevalence, awareness, treatment, and control of hypertension in China: results from a national survey. Am J Hypertens 27 : 1355–1361. doi: 10.1093/ajh/hpu053 24698853
3. Yu B, Zhang X, Wang G (2013) Full coverage for hypertension drugs in rural communities in China. Am J Manag Care 19: e22–29. 23379776
4. Zhang M, Meng Y, Yang Y, Liu Y, Dong C, et al. (2011) Major inducing factors of hypertensive complications and the interventions required to reduce their prevalence: an epidemiological study of hypertension in a rural population in China. BMC Public Health 11 : 301. doi: 10.1186/1471-2458-11-301 21569365
5. Tang JL, Wang WZ, An JG, Hu YH, Cheng SH, et al. (2010) How willing are the public to pay for anti-hypertensive drugs for primary prevention of cardiovascular disease: a survey in a Chinese city. Int J Epidemiol 39 : 244–254. doi: 10.1093/ije/dyp213 19491141
6. Yip WC, Hsiao WC, Chen W, Hu S, Ma J, et al. (2012) Early appraisal of China's huge and complex health-care reforms. Lancet 379 : 833–842. doi: 10.1016/S0140-6736(11)61880-1 22386036
7. Tang S, Tao J, Bekedam H (2012) Controlling cost escalation of healthcare: making universal health coverage sustainable in China. BMC Public Health 12 Suppl 1: S8. doi: 10.1186/1471-2458-12-S1-S8 22992484
8. Moran A, Gu D, Zhao D, Coxson P, Wang YC, et al. (2010) Future cardiovascular disease in china: markov model and risk factor scenario projections from the coronary heart disease policy model-china. Circ Cardiovasc Qual Outcomes 3 : 243–252. doi: 10.1161/CIRCOUTCOMES.109.910711 20442213
9. Wang M, Moran AE, Liu J, Coxson PG, Heidenreich PA, et al. (2014) Cost-effectiveness of optimal use of acute myocardial infarction treatments and impact on coronary heart disease mortality in China. Circ Cardiovasc Qual Outcomes 7 : 78–85. doi: 10.1161/CIRCOUTCOMES.113.000674 24425706
10. Liu LS, Writing Group of Chinese Guidelines for the Management of Hypertension (2011) [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi 39 : 579–615. 22088239
11. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, et al. (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34 : 2159–2219. doi: 10.1093/eurheartj/eht151 23771844
12. Lozano R, Murray C.J.L., Lopez A.D., and Satoh T. (2001) Miscoding and misclassification of ischaemic heart disease mortality. Global Programme on Evidence for Health Policy Working Paper No. 12. Geneva, World Health Organization.
13. Liu J, Hong Y, D'Agostino RB Sr., Wu Z, Wang W, et al. (2004) Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA 291 : 2591–2599. 15173150
14. Law MR, Morris JK, Wald NJ (2009) Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338: b1665. doi: 10.1136/bmj.b1665 19454737
15. Law MR, Wald NJ, Morris JK, Jordan RE (2003) Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 326 : 1427. 12829555
16. Turnbull F, Blood Pressure Lowering Treatment Trialists Collaboration (2003) Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 362 : 1527–1535. 14615107
17. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R (2002) Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360 : 1903–1913. 12493255
18. Moran AE, Odden MC, Thanataveerat A, Tzong KY, Rasmussen PW, et al. (2015) Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med 372 : 447–455. doi: 10.1056/NEJMsa1406751 25629742
19. (2009) The Ministry of Health of the People's Republic of China. China's Health Statistics Yearbook 2009. Beijing: the Peking Union Medical College Press
20. Huffman MD, Rao KD, Pichon-Riviere A, Zhao D, Harikrishnan S, et al. (2011) A cross-sectional study of the microeconomic impact of cardiovascular disease hospitalization in four low - and middle-income countries. PLoS One 6: e20821. doi: 10.1371/journal.pone.0020821 21695127
21. (2011) CHOosing Interventions that are Cost Effective (WHO-CHOICE). World Health Organization.
22. Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, et al. (2012) Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 380 : 2129–2143. doi: 10.1016/S0140-6736(12)61680-8 23245605
23. Hellermann JP, Goraya TY, Jacobsen SJ, Weston SA, Reeder GS, et al. (2003) Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol 157 : 1101–1107. 12796046
24. Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, et al. (2011) Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet 378 : 1231–1243. doi: 10.1016/S0140-6736(11)61215-4 21872920
25. Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M (2008) Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 336 : 1114–1117. doi: 10.1136/bmj.39553.670231.25 18480115
26. Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB (1996) Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 276 : 1253–1258. 8849754
27. Lim SS, Gaziano TA, Gakidou E, Reddy KS, Farzadfar F, et al. (2007) Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet 370 : 2054–2062. 18063025
28. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, et al. (2014) 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311 : 507–520. doi: 10.1001/jama.2013.284427 24352797
29. Li Y, Ying C, Sufang G, Brant P, Bin L, et al. (2013) Evaluation, in three provinces, of the introduction and impact of China's National Essential Medicines Scheme. Bull World Health Organ 91 : 184–194. doi: 10.2471/BLT.11.097998 23476091
30. Johns B, Baltussen R, Hutubessy R (2003) Programme costs in the economic evaluation of health interventions. Cost Eff Resour Alloc 1 : 1. 12773220
31. Bai Y, Zhao Y, Wang G, Wang H, Liu K, et al. (2013) Cost-effectiveness of a hypertension control intervention in three community health centers in China. J Prim Care Community Health 4 : 195–201. doi: 10.1177/2150131912470459 23799707
32. Wang X, Li W, Li X, An N, Chen H, et al. (2013) Effects and cost-effectiveness of a guideline-oriented primary healthcare hypertension management program in Beijing, China: results from a 1-year controlled trial. Hypertens Res 36 : 313–321. doi: 10.1038/hr.2012.173 23154592
33. Liang XH, Gu DF, Zhang H, Zhu K, Deng Y, et al. (2011) [The analysis of drug cost and direct medical expense in community health management of hypertensive patients]. Zhonghua Yu Fang Yi Xue Za Zhi 45 : 732–736. 22169696
34. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, et al. (2013) Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 346: f1049. doi: 10.1136/bmj.f1049 23529982
35. Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, et al. (2014) ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation 129 : 2329–2345. doi: 10.1161/CIR.0000000000000042 24677315
36. Rodgers A, Lawes CMM, Gaziano T, Vos T (2006) The Growing Burden of Risk from High Blood Pressure, Cholesterol, and Bodyweight. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P., editor. Disease Control Priorities in Developing Countries. New York: Oxford University Press and the World Bank.
37. Wang H, Yip W, Zhang L, Wang L, Hsiao W (2005) Community-based health insurance in poor rural China: the distribution of net benefits. Health Policy Plan 20 : 366–374. 16143589
38. Niu S, Zhao D, Zhu J, Liu J, Liu Q, et al. (2009) The association between socioeconomic status of high-risk patients with coronary heart disease and the treatment rates of evidence-based medicine for coronary heart disease secondary prevention in China: Results from the Bridging the Gap on CHD Secondary Prevention in China (BRIG) Project. Am Heart J 157 : 709–715 e701. doi: 10.1016/j.ahj.2008.12.009 19332200
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 8- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Antipsychotic Maintenance Treatment: Time to Rethink?
- Point-of-Care Information in Open Access: A Time to Sow?
- Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study
- Assessing the Causal Relationship of Maternal Height on Birth Size and Gestational Age at Birth: A Mendelian Randomization Analysis
- The Cost-Effectiveness of Low-Cost Essential Antihypertensive Medicines for Hypertension Control in China: A Modelling Study
- Open Access to a High-Quality, Impartial, Point-of-Care Medical Summary Would Save Lives: Why Does It Not Exist?
- The Polypill: From Promise to Pragmatism
- The Impact of a One-Dose versus Two-Dose Oral Cholera Vaccine Regimen in Outbreak Settings: A Modeling Study
- Retained in HIV Care But Not on Antiretroviral Treatment: A Qualitative Patient-Provider Dyadic Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Polypill: From Promise to Pragmatism
- Open Access to a High-Quality, Impartial, Point-of-Care Medical Summary Would Save Lives: Why Does It Not Exist?
- The Impact of a One-Dose versus Two-Dose Oral Cholera Vaccine Regimen in Outbreak Settings: A Modeling Study
- Retained in HIV Care But Not on Antiretroviral Treatment: A Qualitative Patient-Provider Dyadic Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



