-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
There is great interindividual variability in HIV-1 viral setpoint after seroconversion, some of which is known to be due to genetic differences among infected individuals. Here, our focus is on determining, genome-wide, the contribution of variable gene expression to viral control, and to relate it to genomic DNA polymorphism. RNA was extracted from purified CD4+ T-cells from 137 HIV-1 seroconverters, 16 elite controllers, and 3 healthy blood donors. Expression levels of more than 48,000 mRNA transcripts were assessed by the Human-6 v3 Expression BeadChips (Illumina). Genome-wide SNP data was generated from genomic DNA using the HumanHap550 Genotyping BeadChip (Illumina). We observed two distinct profiles with 260 genes differentially expressed depending on HIV-1 viral load. There was significant upregulation of expression of interferon stimulated genes with increasing viral load, including genes of the intrinsic antiretroviral defense. Upon successful antiretroviral treatment, the transcriptome profile of previously viremic individuals reverted to a pattern comparable to that of elite controllers and of uninfected individuals. Genome-wide evaluation of cis-acting SNPs identified genetic variants modulating expression of 190 genes. Those were compared to the genes whose expression was found associated with viral load: expression of one interferon stimulated gene, OAS1, was found to be regulated by a SNP (rs3177979, p = 4.9E-12); however, we could not detect an independent association of the SNP with viral setpoint. Thus, this study represents an attempt to integrate genome-wide SNP signals with genome-wide expression profiles in the search for biological correlates of HIV-1 control. It underscores the paradox of the association between increasing levels of viral load and greater expression of antiviral defense pathways. It also shows that elite controllers do not have a fully distinctive mRNA expression pattern in CD4+ T cells. Overall, changes in global RNA expression reflect responses to viral replication rather than a mechanism that might explain viral control.
Published in the journal: . PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000781
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000781Summary
There is great interindividual variability in HIV-1 viral setpoint after seroconversion, some of which is known to be due to genetic differences among infected individuals. Here, our focus is on determining, genome-wide, the contribution of variable gene expression to viral control, and to relate it to genomic DNA polymorphism. RNA was extracted from purified CD4+ T-cells from 137 HIV-1 seroconverters, 16 elite controllers, and 3 healthy blood donors. Expression levels of more than 48,000 mRNA transcripts were assessed by the Human-6 v3 Expression BeadChips (Illumina). Genome-wide SNP data was generated from genomic DNA using the HumanHap550 Genotyping BeadChip (Illumina). We observed two distinct profiles with 260 genes differentially expressed depending on HIV-1 viral load. There was significant upregulation of expression of interferon stimulated genes with increasing viral load, including genes of the intrinsic antiretroviral defense. Upon successful antiretroviral treatment, the transcriptome profile of previously viremic individuals reverted to a pattern comparable to that of elite controllers and of uninfected individuals. Genome-wide evaluation of cis-acting SNPs identified genetic variants modulating expression of 190 genes. Those were compared to the genes whose expression was found associated with viral load: expression of one interferon stimulated gene, OAS1, was found to be regulated by a SNP (rs3177979, p = 4.9E-12); however, we could not detect an independent association of the SNP with viral setpoint. Thus, this study represents an attempt to integrate genome-wide SNP signals with genome-wide expression profiles in the search for biological correlates of HIV-1 control. It underscores the paradox of the association between increasing levels of viral load and greater expression of antiviral defense pathways. It also shows that elite controllers do not have a fully distinctive mRNA expression pattern in CD4+ T cells. Overall, changes in global RNA expression reflect responses to viral replication rather than a mechanism that might explain viral control.
Introduction
There has been a recent effort to identify the genomic determinants of susceptibility to HIV-1 infection, control of viral replication, and disease progression [1]. Genetic analyses have identified over the years a number of validated variants in candidate genes, while a recent genome-wide association study [2] highlighted the dominant role of variants in the MHC region in the control of viral setpoint (the steady state of viral replication after infection) and disease progression. Other genome-wide studies [3]–[5] confirmed the variants identified in the first genome-wide analysis. These variants collectively explain up to about 13% of the variation in viral setpoint, indicating that other biological determinants of control have yet to be identified. Here our focus is on determining the contribution of variable gene expression to viral control, and to relate it to genomic DNA polymorphism.
There have been a number of transcriptome studies in HIV-1 target cells (CD4+ T cells, monocytes/macrophages), non-targets such as NK cells and B cells, and of dendritic cells and total peripheral blood mononuclear cells (PBMCs) (reviewed in [6], and recent publications [7]–[11]). These studies provide insight into gene expression changes associated with virus replication and persistence. Studies are limited by the number of genes interrogated, or by the number of individuals investigated. These limits notwithstanding, microarray data have yielded novel mechanisms of HIV-mediated pathogenesis. Transcriptome analyses of cell lines transfected with individual viral proteins or mutant viruses have also been reported (reviewed in [6]).
This study aims at coupling a large scale assessment of gene expression in purified CD4+ T cells from HIV-1 infected individuals, with genome-wide genotype data tested for association with viral setpoint. Integrating gene expression data with results from genome-wide association studies may help prioritize fine-mapping efforts and provide shortcuts to disease biology [12]. Therefore, the goals of the study are the description of the expression program associated with HIV-1 in vivo, the identification of mRNAs that are differentially expressed in individuals that present effective control of viral replication, and the search for cis-acting variation in differentially expressed genes. Expression polymorphism due to single nucleotide polymorphisms (SNPs) that influence mRNA levels has received increasing attention for the understanding of phenotypes in health and disease (reviewed in [12]). Genome-wide screens, most generally done in cell lines, have established the relevance of cis-acting SNPs in expression polymorphism [13]. However, little is known regarding expression polymorphism and HIV-1 disease.
In order to have power to detect correlations, we have considered a large sample set of purified CD4+ T cells from individuals with known date of infection and carefully determined viral load results. Transcription analysis was done at the time of viral setpoint, so that samples are representative of the steady-state replication for a given individual, and across the full range of viral setpoint in an infected population. For a large subset of participants, we also established the transcription profile after initiation of antiretroviral therapy (ART) to assess the modulation of expression upon effective control of viremia. Thus, this study represents a first attempt at assessing, genome-wide, the genotype-to-transcriptome-to-clinical phenotype associations in HIV-1 disease.
Results
Transcriptome profile and viral setpoint analysis
We identified 298 hybridization probes that were significantly correlated with viral load (FDR-adjusted p-value<0.01). This resulted in a list of 260 genes, since multiple probes are used for some of the genes. The majority of these (n = 209) were positively associated with viral setpoint, while a smaller group (n = 51) was negatively associated (Supplementary Table S1). We used (unsupervised) clustering to group the expression profiles of the samples for these 260 genes, and found that they showed distinct behavior in individuals with effective virus control (reflected in low viral setpoint) as compared with individuals showing poor control of viral replication (Figure 1). In an analysis that considered viral load at the precise date of transcriptome analysis instead of setpoint, the results were comparable, implying that the expression profile is representative for the period of analysis (three months to three years after seroconversion), Figure 1. The analysis included various parameters as covariates (clinical center, gender, age, CD4 T cell viability and laboratory date, and microarray chip batch - sentrix ID). The CD4 T cell value at the time of sampling was found to be closely correlated with viral setpoint (Pearson's correlation of −0.5 and a p-value = 1.195e-10), which made difficult to separate their effects on the data. The 149 genes that are shared between analysis using CD4 T cell count, and the analysis using viral setpoint are indicated in Supplementary Table S1.
Fig. 1. Transcriptome analysis in CD4+ T cells from HIV-infected untreated individuals. 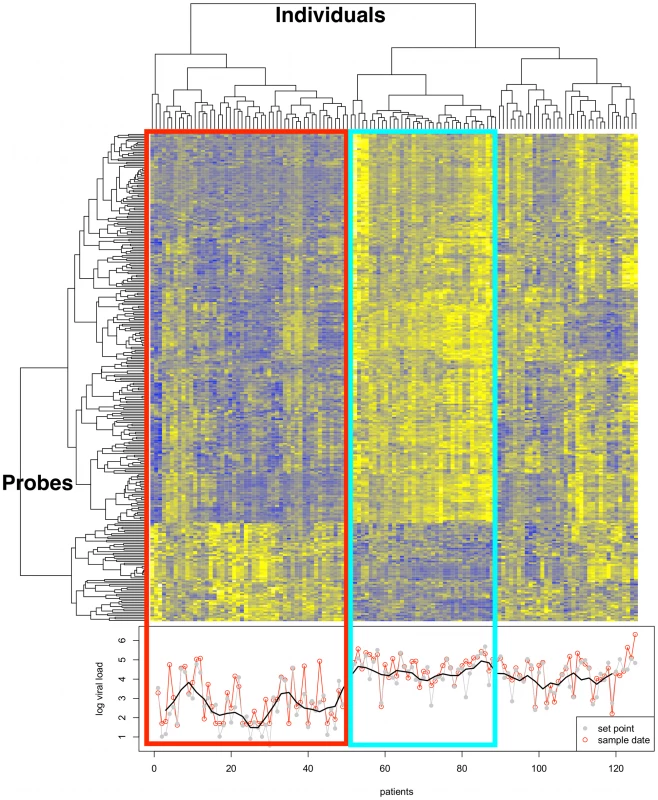
Gene clusters are presented on the left. In total, 260 genes are differentially expressed (at adjusted p<0.01) in association with viral load in CD4+ T cells during in vivo HIV-1 infection. Patient clusters are presented at the top for untreated individuals. Clustering was performed on the Spearman correlation coefficient. The phenotype is presented at the bottom, as log10 viral setpoint in gray, and log10 viral load at time of sample collection in red. A smooth of the setpoint viral load values is depicted by the black line. The red rectangle surrounds a cluster of individuals characterized by low viral load (mean Log10 viral setpoint = 2.6), and including several “elite controllers” – individuals that spontaneous control viral replication in the absence of treatment. The blue rectangle identifies a cluster of individuals with high viral setpoint (mean Log10 viral setpoint = 4.4). The remaining clusters illustrate the heterogeneity of transcription profile across the range of viral load values. The main gene clusters exhibiting a positive correlation with viral setpoint (i.e., increasing gene expression with increasing viral load), as defined by STRING, DAVID and IPA, were the interferon pathway, the proteasome, and cell cycle genes (Figure 2 and Supplementary Table S2, S3). Conversely, among genes that exhibited a negative correlation with viral setpoint (Supplementary Table S1B), no pathway enrichment was identified. A separate analysis that used a gene-by-gene modeling approach resulted in a list of significant genes that was shorter (44 genes) but highly concordant with the output of the empirical Bayes analysis described above (Supplementary Table S4): we therefore used the empirical Bayes results for subsequent analyses. Because the CD4+T cell composition may vary depending on the degree of viral replication [7], we re-analyzed the data controlling for CD25 expression (encoding IL2RA as marker of activation), or CD62L, CD40L, CD11a, and CD27 (markers that distinguish naive from memory CD4+ T cells). Although several additional significant genes were found using each of the above markers as covariates, the overall expression profile did not vary significantly (see for example data from analysis adjusted by CD25 in Supplementary Table S5). These analyses indicate the existence of a clear expression program associated with high viral load, but fail to identify definite gene networks associated with viral control.
Fig. 2. Predicted interaction networks of genes differentially expressed during HIV-1 infection. 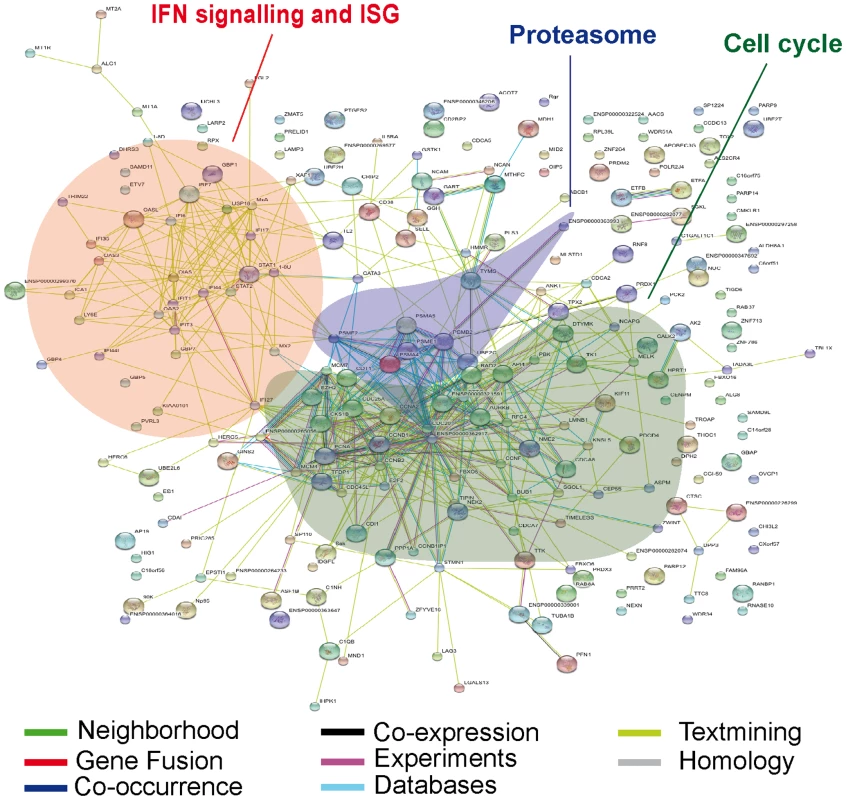
Differentially expressed genes are depicted: links have been predicted using STRING (http://string.embl.de/). Predicted interactions are depicted according to the type of available evidence. The interactions (see color labels) include direct (physical) and indirect (functional) associations; they are derived from four sources: genomic context, high-throughput experiments, conserved coexpression, and previous knowledge from literature. Analysis of genes of the interferon response pathways
We observed a linear association between increasing expression of interferon signaling and interferon-stimulated genes (ISGs) and increasing viral setpoint. We compiled a list of 40 genes implicated in the interferon response [14] (Supplementary Table S6). Seventeen genes were significantly associated with viral setpoint after FDR adjustment at the 0.01 level, and 12 were associated at a p-value of 0.05. These 29 genes comprise most of the signaling and ISGs, but notably exclude the interferon genes themselves and the interferon receptors (Figure 3). This analysis points to a de-regulated interferon response that associates with an ineffective antiviral response.
Fig. 3. Differential expression of genes of the interferon response. 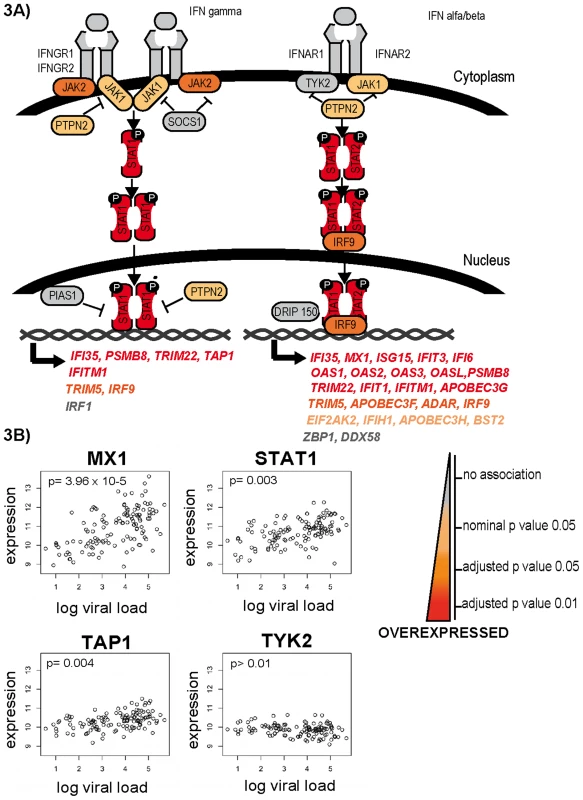
Representative genes of the interferon response pathway are shown in panel A. From grey to red, increasing differential expression with increasing viral setpoint. Selected genes are shown in panel B. While genes associated with interferon receptors, such as TYK2, are not differentially expressed, signaling molecules such STAT1 and interferon-stimulated genes such as MX1 and TAP1 are significantly upregulated with increasing viral load. Analysis of genes associated with HIV-1 life cycle and pathogenesis
We similarly examined in detail a list of selected genes reported to be involved in HIV-1 life cycle or pathogenesis (see Methods for explanation of candidate selection) [15]. Of this list, 138 genes were matched to probes, with four having a FDR-adjusted significant association with viral setpoint, p-value <0.01: TRIM22, IRF7, RANBP1, and APOBEC3G. An additional 12 genes had FDR-adjusted p-values <0.05, and a further 26 had nominal p-values <0.05 (Supplementary Table S7). Genes of the intrinsic cellular defense against retroviruses (TRIM5α, TRIM22, TRIM19/PML, APOBEC3G, APOBEC3F, APOBEC3H, PPIA/Cyclophilin A, BST2/Tetherin) were all upregulated with increasing viral load, which is consistent with their general dependence on the interferon pathways. A number of chemokines and chemokine receptors were also positively modulated with increasing viremia. We also identified differentially expressed genes that are present in both the current analysis and studies that used siRNA or shRNA to identify HIV-1 dependency factors [16]–[19] (Supplementary Table S8).
Changes in transcriptome profile after treatment
The significant association of a number of genes and pathways with viral setpoint was further assessed by observing the changes in transcriptional profile in CD4+ T cells after viral suppression. We found statistical support for differential expression of 247 probes (FDR-adjusted p-value <0.01) between treated and untreated-noncontroller individuals. The list of genes involved had an extensive overlap with the list of genes associated with viral setpoint in the transcriptome analysis above (Supplementary Table S9). The list also shares 97 genes with the recent study by Li et al. [20] on changes in the lymph node transcriptome profile upon initiation of ART. This analysis indicates that successful treatment appears able to recapitulate the cellular state of a well-controlled individual, since we did not find support for any probes being differently expressed between successfully treated and untreated-controller individuals.
Comparison with uninfected individuals
To compare the treated and untreated individuals with uninfected individuals, we clustered the expression profiles from samples from a selected group of individuals, including elite controllers (viral load <50 copies/ml), samples from successfully treated individuals and their paired untreated samples, and from the three uninfected individuals (measured in triplicate, one triplicate failed analysis). For this, we restricted analysis to the 260 genes found to be differently expressed by viral setpoint. As shown in Figure 4, both the successfully treated and uninfected individuals tended to cluster with the controllers individuals. Two of the individuals from healthy donors were most tightly grouped with several of the untreated individuals that have the lowest level of virus at setpoint (i.e. the elite controllers), while one uninfected individual showed a profile that is less extreme, but still most similar to the viral control profile. Treated individuals also preferentially grouped with the viral control pattern, although the majority showed a mid-range expression level and a smaller fraction grouped with elite controllers and uninfected individuals. A bootstrapping analysis showed support (p-value 0.06) for the consistency of the top-level groupings with one group containing all the uninfected individuals and the majority of the treated and elite controller individuals, while the other group contained mostly non-controller individuals. This indicates that the expression levels of individuals with the best viral control closely resemble those of uninfected individuals.
Fig. 4. Transcriptome analysis in CD4+ T cells from HIV-infected individuals before and after viral suppression. 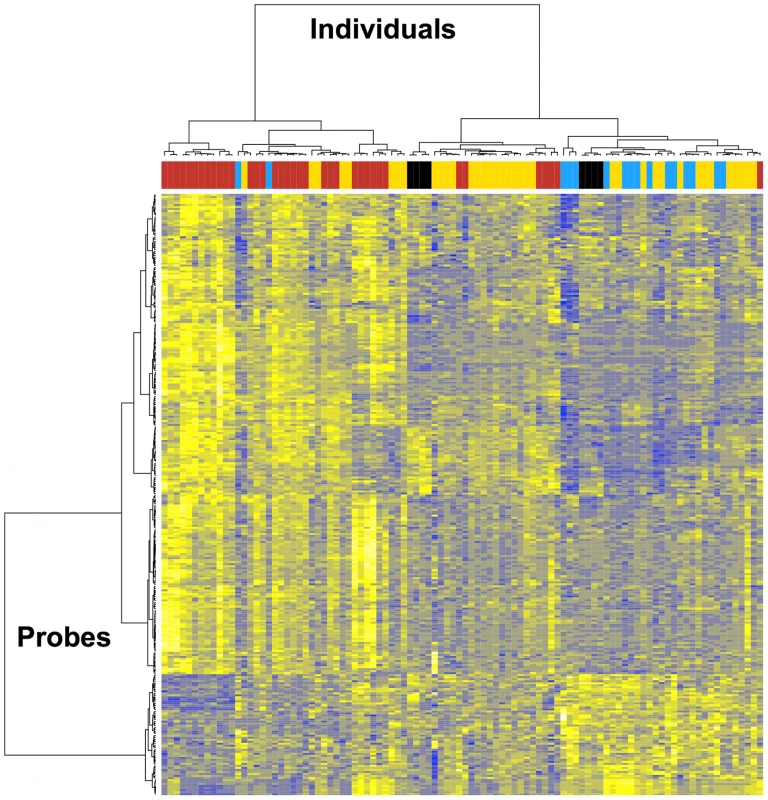
Analysis was restricted to the 260 genes found to be differently expressed by viral setpoint. Gene clusters are presented on the left. Patient clusters are presented at the top. In red, transcriptome profile before viral suppression, and in yellow, transcriptome profile after viral suppression with effective treatment in 37 individuals with pre- and post-treatment initiation samples. In blue, transcriptome profile of 16 elite controllers. In black, transcriptome profile from 3 HIV-negative healthy controls (8 samples). Screen for cis-acting SNPs regulating transcript expression levels
Among a total of 1.3 million association tests comparing 399,626 gene-centric SNPs (some SNPs were within 100 kb of multiple transcripts) with 28,828 individual probes measuring a total of 18,059 unique transcripts, we detected 782 study-wide significant associations (SNP-probe associations) below the threshold p-value of 3.8×10−8. Stepwise linear regression was used to prune out redundant associations of SNPs with a particular probe. This step resulted in evidence for cis-regulation of 208 unique probes, 157 of which were regulated by multiple SNPs in high linkage disequilibrium between SNPs included in the analysis (51 signals of unique SNPs with a transcript, and 731 signals arising from the regulation of 157 transcripts by multiple non-unique SNPs). These 208 associations included 193 SNPs that modulate 190 genes in CD4+ T cells, with the overlap occurring because of probe cross-hybridization, and also several probes detecting the same gene (Supplementary Table S10).
This list of study-wide significant associations was compared to the list of genes whose expression was found associated with viral load at setpoint. Among genes under differential expression during HIV-1 infection, several showed evidence for cis-regulation (Supplementary Table S11) but only one, involving the interferon stimulated OAS1, reached study-wide significance. OAS1 was found to be regulated by an intronic SNP (rs3177979) located near exon 6 (Supplementary Figure S1). Lower expression was associated with the rs3177979 GG genotype. The association was detectable in treated and untreated individuals; however the expression level was lower in samples from treated individuals. The association of this SNP with OAS1 transcript expression is also detectable in PBMCs collected from uninfected controls [21].
We did not observe an association of OAS1 rs3177979 with viral setpoint in the study (untreated) population. However, given the potential interest of genetic polymorphism in OAS1, we also assessed the association between rs3177979 and HIV-1 outcomes in a large population of 2362 individuals [5]. The association p-values were 0.05 for an association of the OAS1 SNP and viral setpoint and 0.09 for HIV-1 disease progression, but differences were subtle: mean HIV-1 load was 4.11 log10 viral copies/ml for the AA genotype, 4.07 for AG, and 4.01 for GG. Because rs3177979 is in linkage disequilibrium with rs10774671, a SNP associated with a splicing variant ([22] and Text S1) reported to have greater activity against West Nile virus [23], we re-genotyped the population for this putative functional SNP, without finding any stronger association: we have therefore no definitive evidence of an association of cis-acting genetic variation in OAS1 with HIV-1 viral control or disease progression.
One additional gene, RANBP1, encoding a Ran GTPase-binding protein that interferes with Rev-mediated expression of HIV-1 [24], presented both increased expression at higher setpoint, and a cis-acting SNP (rs2008591) that modulates its expression (Supplementary Table S11). We assessed the association between rs2008591 and viral setpoint and disease progression in the large population of 2362 individuals [5]. Here, rs2008591 did not associate with viral setpoint (p = 0.45) or disease progression (p = 0.35). Overall, these analyses identified a significant number of cis-acting genetic variants influencing gene expression in CD4+ T cells; however, expression polymorphism, genome-wide or among genes that are modulated during HIV-1 infection, did not contribute in a significant fashion to viral control.
Discussion
This study represents the largest effort to date to characterize the mRNA expression profile in CD4+ T cells in vivo in HIV-1 infected individuals. The study population, only including individuals with known date of seroconversion or elite controllers, represents the complete range of viral load control: from undetectable viral load to sustained high levels of viral replication. The study also analyzed changes in transcriptome upon successful antiretroviral therapy. In addition, we searched for cis-acting variants – SNPs that would possibly associate with the observed differences in gene expression in the course of HIV-1 infection. Overall, changes in RNA expression reflect responses to viral replication rather than a mechanism that might explain control of viral replication. As such, the reactive transcriptome profile we observed shares common responses with other viral infections, eg. to dengue virus [25]–[28] (Supplementary Table S12).
In vivo HIV-1 infection results in a distinctive mRNA transcriptome profile in CD4+ T cells that involves 260 genes in an analysis that differentiates individuals with high and those with low viral setpoint. Under conditions of high viral load, there is a distinct upregulation of the interferon pathways, cell cycle and the ubiquitin-proteasome degradation machinery. The study confirms and extends previous analyses of in vitro infection of T cell lines, or of CD4+ T cells in vivo that were performed on a limited number of individuals [7]–[10],[29],[30].
This study underscores that the observed increase in transcription of ISGs is not associated with a better control of viremia [7]. This contrasts with the reported efficacy and possible therapeutic role of interferon (IFN-α, IFN-α2β) suggested by results from in vitro studies, while exogenous administration of interferon in clinical trials led to doubts about its efficacy in the clinical setting (reviewed in [31]). Our observations lend support to the hypothesis that interferon activation plays a deleterious role in retroviral pathogenesis, as proposed by many recent reports (reviewed in [31]). Elevated ISG expression is associated with disease progression in pathogenic SIV infection of non-human primates [32]–[35], while the type I interferon response subsided after peak viral load during non-pathogenic infection [36],[37]. Sedaghat et al. [7] compared the transcriptional programs of in vivo-activated CD4+ T cells from untreated HIV-positive individuals with those of activated CD4+ T cells from HIV-negative individuals. From this study, they concluded that CD4+ T cells from infected individuals are in a hyperproliferative state that is modulated by type I interferons, and that this would lead, during chronic infection, to CD4+ T-cell preferential differentiation and depletion. Imbeault et al. [10] suggested that interferon could lead to a sustained increase in p53 mRNA levels and therefore to a higher susceptibility of CD4+ T cells to pro-apoptotic signals. Herbeuval and Shearer [31] proposed that interferon, through binding to its receptor on primary CD4+ T cells would result in membrane expression of the TNF-Related Apoptosis-Inducing Ligand, TRAIL, death molecule leading to the selective death of HIV-exposed CD4+ T cells. More recently, Sato et al. [38] showed that type I interferon induce proliferation and exhaustion in hematopoietic stem cells; chronic and excessive type I interferon signaling may cause hematopoietic stem cells reduction. Overall, interferon response appears a poorly effective antiretroviral mechanism, and may actually contribute to HIV-1 disease [7],[39].
Among genes previously associated with HIV-1 pathogenesis, the analysis identified a number of significant associations, in particular for genes of the intrinsic cellular defense against retroviruses. Many of these respond to interferon, and thus have the same profile of increased expression with increasing viral load as ISG. Thus, these genes appear ineffective both by their poor specificity against HIV-1 and by the apparent limited response of HIV-1 to increasing titration of the transcripts. We also analysed genes issued from four genome-wide siRNA/shRNA screens [16]–[19]. Fifteen genes that were associated with decreased cellular permissiveness to infection after silencing, were upregulated with increasing viremia in vivo in the current study. They deserve further inspection for a role in HIV-1 pathogenesis. Although the scope of the present work was not to complete a meta-analytical study of all available genome-wide transcriptome studies and siRNA screens [40], we are aware of the interest to progressively integrate large scale datasets [41].
We aimed at identifying patterns of gene expression associated with effective viral control. However, the nature of the analysis could not establish whether high levels of viral replication would lead to the observed transcriptional profile, or whether genetic modifiers of transcriptional profile were determinants for the control of viral replication. This was addressed first by comparing the transcriptional profile of CD4+ T cell from elite controllers with that of successfully treated individuals and healthy donors. Here, we observed that the expression profile of genes associated with active viral replication was, after effective treatment, similar to that of individuals with spontaneous control of viral replication, and close to that of healthy donors. This suggests that infection drives gene expression rather than the contrary. Second, we tested the hypothesis that genetic variants influence expression levels of genes, thus leading to differences in viral control. The analysis identified a number of variants that would possibly act in cis to modulate gene expression – most notably a variant in OAS1 that has been associated with improved control of West Nile virus infection [23]. It may be argued that if a variant influences expression of a gene, and if expression of that gene correlates with viral load, then the two analyses will be partially redundant. However, we emphasize that this approach allows for independent information because the variation in expression of few if any genes is determined exclusively by cis-acting variants. In addition, the identification of strong cis-acting variants would contribute to disentangle causation and correlation. Thus if a gene expression correlates with viral load, it could be that the change in expression is a response to the amount of virus, or it that the gene directly controls the viral level. In the former case, a cis-acting variant will show no association with viral load, whereas in the latter it will. In the present study, none of the candidate cis-acting SNPs, or SNPs in the implicated genes was associated with differences in viral setpoint in a genome-wide association analysis. These results do not contradict current evidence of mechanisms of viral control through differences in expression levels of particular genes, most notably CCR5 [42]. Rather, the analysis indicates that polymorphisms in genes implicated in the differential expression programs do not represent a strong source of variation at the population level.
There are a number of technical and conceptual limits to the study. The study failed to identify a transcriptome profile characteristic of elite controllers. This may be attributed to the large scale approach, as the current technology covers a total of 25,440 annotated human genes. While this allows for pathway or network analyses, it may fail in the identification of subtle expression changes, in particular at the level of the individual gene. On one hand, the analysis would require greater study power (ie, additional elite controllers) to compensate the penalty of correction for multiple testing. On the other hand, the precision of several of analyses described earlier in this section could be improved through the added resolution of new technology such as RNA-Seq [31], or the targeted multiplexed measurement of gene expression in selected pathways [43]. High-throughput deep sequencing results in a superior dynamic range, and allows quantitative analysis of coding and non-coding region transcripts, such as small RNAs. It should also be pointed out that the use of cryopreserved cells may result in changes in the transcriptome and in transcript stability. However, this allowed the investigation of a large number of samples from seroconverting individuals in batch analyses. We argue that the internal consistency of the results and the general agreement across studies supports the robust nature of the transcription profiles that were generated.
In conclusion, while this study suggests that the generalized upregulation of ISG, an important component of viral defense, does not lead to consistently improved viral control throughout the course of infection, it does not implicate any specific gene expression network in viral control. There are several possible explanations for these observations. First, the most important cellular populations for determining control may be effector cells such as CD8+ T cells or NK cells whose expression patterns have not been evaluated here. Second, the key expression patterns that determine eventual control may be only detectable early in infection and thus largely missed in studies focusing on cells taken during the setpoint period. These possibilities argue strongly that the next phase of expression work in the study of HIV-1 control must focus on large scale analysis of isolated populations of effector cells taken from individuals as early in the course of infection as possible and in a standardized fashion. We believe the approach taken here provides a general template for such studies.
Materials and Methods
Ethics statement
Study participants were followed in the Swiss HIV Cohort Study (www.shcs.ch). The Genetics Project of the Swiss HIV Cohort Study was approved by the ethics committees of all participating centers, and the permission for genomic work was approved by the Institutional Review Board/Ethics Committee of the University Hospital of Lausanne. Participants gave written, informed consent for genetic testing.
Participants
198 HIV-1 infected individuals from the Swiss HIV Cohort study with a known date of seroconversion (n = 182), or elite controllers (n = 16) were included in the study. Seroconversion was defined on the basis of a documented positive test and date and a documented negative test less than two years before the first positive test. The viral setpoint was calculated for each participant by using a median of 4 (range 2 to 8) plasma HIV-1 RNA determinations obtained in the absence of antiretroviral treatment between 3 months and 3 years after seroconversion, as previously described [2]. See Text S1 for the detail definition of viral setpoint and of elite controllers. When available, HIV-1 infected participants contributed samples during stable viral setpoint before and under effective ART (median [IQR] from treatment initiation to sample collection was 1297 (434–2730) days). In addition three healthy blood donors provided three control samples used as biological replicas. Quality control steps at the level of cellular viability, RNA integrity, microarray and hybridization quality, and data analysis led to a final number of 190 samples from 153 participants and 8 samples from 3 healthy controls (68% of valid samples, 78% of successful recruitment). The demographic characteristics of the patients and the flow chart of enrollment and sample validation is presented in Text S1. Representative examples of QC checks are presented in Supplementary Figure S2.
Cell isolation and RNA extraction
CD4+ T cells were positively selected from frozen PBMCs (median time [IQR] of cryopreservation was 616 [333–1448] days) using magnetically labeled CD4 microbeads and subsequent column purification according to the manufacturer's protocol (Miltenyi Biotec). CD4+ T cell purity, verified by flow cytometry, was 95.6% (86.4–98.1%) [median (range)]. CD4+ T cell viability was assessed by the trypan blue dye exclusion method using the Vi-CELL (Beckman Coulter). Total RNA was extracted from purified CD4+ T cells using mirVana miRNA isolation kit (Ambion) according to the manufacturer's protocol for total RNA extraction. RNA amount was estimated by spectrophotometry using the Nanodrop 1000 (Thermo Fisher). RNA quality was determined by Agilent RNA 6000 pico kit on an Agilent 2100 Bioanalyzer. We used cryopreserved samples because of the interest to analyse a large population of seroconverting individuals during the precise window of stable viral setpoint. Samples were collected between 1995 and 2007, and investigated in 2008. The median (range) of CD4+ T cell viability for samples that were successfully analysed was 78.5% (IQR 70.5–85.3). Viability was minimally dependent on time of cryopreservation, and more dependent on collection center. These covariates were included in the analyses (see below).
Transcriptome analysis and genome-wide genotyping
200 ng of total RNA was amplified and labeled using the Illumina TotalPrep RNA Amplification kit (Ambion). cRNA quality was assessed by capillary electrophoresis on Agilent 2100 Bioanalyzer. Expression levels of over 48,000 mRNA transcripts were assessed by the Human-6 v3 Expression BeadChips (Illumina). Hybridization was carried out according to the manufacturer's instructions. Genome-wide SNP data had been generated from genomic DNA using the HumanHap550 Genotyping BeadChip (Illumina) with 555,352 SNPs [2].
Selection of candidate genes for subanalysis
We screened the literature for genes associated with biology of HIV-1 (reviewed in [44]–[47] and recent studies [15],[48],[49]), as well as HIV-1 dependency factors emerging from genome-wide siRNA screens [16]–[19], and genes considered polymorphic and involved in HIV-1 pathogenesis (compiled in www.hiv-pharmacogenomics.org). For the three large siRNA screens, that resulted in over 600 candidates, we restricted analysis to (i) genes identified in at least two of three screens, or to (ii) genes with SNPs that reached a nominal significant p value in a recent genome-wide association study of determinants of susceptibility to HIV-1 [2].
Data pre-processing
Bead summary data was output from Illumina's BeadStudio software without background correction, as this has previously been shown to have detrimental effects [50]. Data pre-processing, including a variance-stabilizing transformation [51] and robust-spline normalization were applied as implemented in the lumi package [52] of R. Four outlier samples identified based on aberrant expression of control probes and aberrant median-interquartile range values compared to other samples were removed.
Differential expression analysis
We applied an empirical Bayes analysis approach within a linear mixed-model framework to identify associations between variation in gene expression and in viral setpoint. The Empirical Bayes approach has been developed to model the variation profiles of all genes and use that information as prior knowledge to better estimate the variance of each gene expression [53]–[55]. In addition, we used a more conservative gene-by-gene modeling approach for result comparison with the empirical Bayes approach. We controlled for variation caused by gender, age, CD4+ T cell viability, location of sample collection, and laboratory batch effects. Effect of chip batch was modeled as a random effect; all others were fixed or continuous. All samples from untreated individuals were tested for association of expression with viral setpoint. We used a false discovery rate (FDR) method [56] to control for multiple testing. Probes selected for further analysis had an FDR-adjusted p-value <0.01. A separate analysis compared expression in samples from treated and untreated individuals, using a similar mixed-model approach as above, but also incorporating viral load as a factor in the analysis.
We tested for effect of treatment by separately comparing samples from treated individuals to each of the untreated groups, using the limma (linear models for microarray data) package in R with FDR adjustment as above. This analysis explicitly excluded samples from the same individuals because the statistical approach did not allow control for both the correlation between paired samples and the strong correlation (batch) effect of chip. To compare samples from treated and untreated individuals with samples from uninfected controls, we clustered the expression profiles for a selected group of individuals. We performed 1000 replicate clusterings on the Pearson correlation coefficient, using the “ward” clustering method as implemented in the pvclust package in R.
Pathway and network analyses
The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) (http://string.embl.de/) was used to identify known and predicted interactions (derived from four sources: genomic context, high-throughput experiments, co-expression, and previous knowledge). DAVID Bioinformatic resources (http://david.abcc.ncifcrf.gov/) using the annotation sources GOTERM-BP (biological process), and GOTERM-MF (molecular function) identified functional categories [57]. Ingenuity Pathway Analysis 7.0 (IPA) (http://www.ingenuity.com/) was used for the analysis of pathway enrichment. Analysis was limited to genes significantly associated with viral load (FDR p-value <0.01).
Screen for cis-acting SNPs regulating transcript expression levels in HIV-infected CD4+ T cells
Normalized expression data was exported for all untreated, HIV-1 infected individuals (n = 125). Only probes that targeted fully annotated genes were included in the analysis. A principal components analysis was run to assess batch effects. The cis-screen consisted of a scan for common SNPs, within 100 kb of the defined gene start and stop positions, for effects on transcript expression levels. The analysis was limited to SNPs with a minor allele frequency greater than 0.04, requiring at least ten alleles to be present to detect associations with a low false positive rate. This analysis was performed using a standard linear regression, incorporating age, gender, and 11 eigenstrat axes to correct for population stratification. In total, there were 1,330,529 tests run, therefore using a Bonferroni correction, a p<3.8×10−8 was used to declare a statistically significant association.
Microarray data accession number
All microarray results have been deposited in the Gene Expression Omnibus database (GSE18233).
Supporting Information
Zdroje
1. TelentiA
GoldsteinDB
2006 Genomics meets HIV. Nat Rev Microbiol 4 9 18
2. FellayJ
ShiannaKV
GeD
ColomboS
LedergerberB
2007 A Whole-Genome Association Study of Major Determinants for Host Control of HIV-1. Science 317 944 947
3. DalmassoC
CarpentierW
MeyerL
RouziouxC
GoujardC
2008 Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: the ANRS Genome Wide Association 01 study. PLoS ONE 3 e3907 doi:10.1371/journal.pone.0003907
4. LimouS
LeCS
CoulongesC
CarpentierW
DinaC
2009 Genomewide Association Study of an AIDS-Nonprogression Cohort Emphasizes the Role Played by HLA Genes (ANRS Genomewide Association Study 02). J Infect Dis 199 419 426
5. FellayJ
GeD
ShiannaKV
ColomboS
LedergerberB
2009 Common Genetic Variation and the Control of HIV-1 in Humans. PLoS Genet 5 e1000791 doi:10.1371/journal.pgen.1000791
6. GiriMS
NebozhynM
ShoweL
MontanerLJ
2006 Microarray data on gene modulation by HIV-1 in immune cells: 2000–2006. J Leukoc Biol 80 1031 1043
7. SedaghatAR
GermanJ
TeslovichTM
CofrancescoJJr
JieCC
2008 Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol 82 1870 1883
8. HyrczaMD
KovacsC
LoutfyM
HalpennyR
HeislerL
2007 Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J Virol 81 3477 3486
9. ChunTW
JustementJS
LempickiRA
YangJ
DennisGJr
2003 Gene expression and viral prodution in latently infected, resting CD4+ T cells in viremic versus aviremic HIV-infected individuals. Proc Natl Acad Sci U S A 100 1908 1913
10. ImbeaultM
OuelletM
TremblayMJ
2009 Microarray study reveals that HIV-1 induces rapid type-I interferon-dependent p53 mRNA up-regulation in human primary CD4+ T cells. Retrovirology 6 5
11. GiriMS
NebozyhnM
RaymondA
GekongeB
HancockA
2009 Circulating monocytes in HIV-1-infected viremic subjects exhibit an antiapoptosis gene signature and virus - and host-mediated apoptosis resistance. J Immunol 182 4459 4470
12. NicaAC
DermitzakisET
2008 Using gene expression to investigate the genetic basis of complex disorders. Hum Mol Genet 17 R129 R134
13. VeyrierasJB
KudaravalliS
KimSY
DermitzakisET
GiladY
2008 High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet 4 e1000214 doi:10.1371/journal.pgen.1000214
14. BowieAG
UnterholznerL
2008 Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol 8 911 922
15. OrtizM
GuexN
PatinE
MartinO
XenariosI
2009 Evolutionary Trajectories of Primate Genes Involved in HIV Pathogenesis. Mol Biol Evol 26 2865 2875
16. BrassAL
DykxhoornDM
BenitaY
YanN
EngelmanA
2008 Identification of host proteins required for HIV infection through a functional genomic screen. Science 319 921 926
17. KonigR
ZhouY
EllederD
DiamondTL
BonamyGM
2008 Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell 135 49 60
18. ZhouH
XuM
HuangQ
GatesAT
ZhangXD
2008 Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 4 495 504
19. YeungML
HouzetL
YedavalliVS
JeangKT
2009 A genome-wide short hairpin RNA screening of jurkat T-cells for human proteins contributing to productive HIV-1 replication. J Biol Chem 284 19463 19473
20. LiQ
SmithAJ
SchackerTW
CarlisJV
DuanL
2009 Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J Immunol 183 1975 1982
21. HeinzenEL
GeD
CroninKD
MaiaJM
ShiannaKV
2008 Tissue-specific genetic control of splicing: implications for the study of complex traits. PLoS Biol 6 e1000001 doi:10.1371/journal.pbio.1000001
22. Bonnevie-NielsenV
FieldLL
LuS
ZhengDJ
LiM
2005 Variation in antiviral 2′,5′-oligoadenylate synthetase (2′5′AS) enzyme activity is controlled by a single-nucleotide polymorphism at a splice-acceptor site in the OAS1 gene. Am J Hum Genet 76 623 633
23. LimJK
LiscoA
McDermottDH
HuynhL
WardJM
2009 Genetic variation in OAS1 is a risk factor for initial infection with West Nile virus in man. PLoS Pathog 5 e1000321 doi:10.1371/journal.ppat.1000321
24. ZolotukhinAS
FelberBK
1997 Mutations in the nuclear export signal of human ran-binding protein RanBP1 block the Rev-mediated posttranscriptional regulation of human immunodeficiency virus type 1. J Biol Chem 272 11356 11360
25. FinkJ
GuF
LingL
TolfvenstamT
OlfatF
2007 Host gene expression profiling of dengue virus infection in cell lines and patients. PLoS Negl Trop Dis 1 e86 doi:10.1371/journal.pntd.0000086
26. LongHT
HibberdML
HienTT
DungNM
VanNT
2009 Patterns of gene transcript abundance in the blood of children with severe or uncomplicated dengue highlight differences in disease evolution and host response to dengue virus infection. J Infect Dis 199 537 546
27. SimmonsCP
PopperS
DolocekC
ChauTN
GriffithsM
2007 Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J Infect Dis 195 1097 1107
28. UbolS
MasrinoulP
ChaijaruwanichJ
KalayanaroojS
CharoensirisuthikulT
2008 Differences in global gene expression in peripheral blood mononuclear cells indicate a significant role of the innate responses in progression of dengue fever but not dengue hemorrhagic fever. J Infect Dis 197 1459 1467
29. WuJQ
DwyerDE
DyerWB
YangYH
WangB
2008 Transcriptional profiles in CD8+ T cells from HIV+ progressors on HAART are characterized by coordinated up-regulation of oxidative phosphorylation enzymes and interferon responses. Virology 380 124 135
30. WenW
ChenS
CaoY
ZhuY
YamamotoY
2005 HIV-1 infection initiates changes in the expression of a wide array of genes in U937 promonocytes and HUT78 T cells. Virus Res 113 26 35
31. HerbeuvalJP
ShearerGM
2007 HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol 123 121 128
32. ReinhartTA
FallertBA
PfeiferME
SanghaviS
CapuanoSIII
2002 Increased expression of the inflammatory chemokine CXC chemokine ligand 9/monokine induced by interferon-gamma in lymphoid tissues of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. Blood 99 3119 3128
33. AbelK
Alegria-HartmanMJ
RothaeuslerK
MarthasM
MillerCJ
2002 The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol 76 8433 8445
34. KhatissianE
ToveyMG
CumontMC
MonceauxV
LebonP
1996 The relationship between the interferon alpha response and viral burden in primary SIV infection. AIDS Res Hum Retroviruses 12 1273 1278
35. BosingerSE
HosiawaKA
CameronMJ
PersadD
RanL
2004 Gene expression profiling of host response in models of acute HIV infection. J Immunol 173 6858 6863
36. LedererS
FavreD
WaltersKA
ProllS
KanwarB
2009 Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog 5 e1000296 doi:10.1371/journal.ppat.1000296
37. MandlJN
BarryAP
VanderfordTH
KozyrN
ChavanR
2008 Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med 14 1077 1087
38. SatoT
OnaiN
YoshiharaH
AraiF
SudaT
2009 Interferon regulatory factor-1 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med doi:10.1038/nm.1973
39. BoassoA
ShearerGM
2008 Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol 126 235 242
40. BushmanFD
MalaniN
FernandesJ
D'OrsoI
CagneyG
2009 Host cell factors in HIV replication: meta-analysis of genome-wide studies. PLoS Pathog 5 e1000437 doi:10.1371/journal.ppat.1000437
41. TelentiA
2009 HIV-1 host interactions - integration of large scale datasets. F1000 Biology Reports 1 71
42. MartinMP
DeanM
SmithMW
WinklerC
GerrardB
1998 Genetic acceleration of AIDS progression by a promoter variant of CCR5. Science 282 1907 1911
43. GeissGK
BumgarnerRE
BirdittB
DahlT
DowidarN
2008 Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26 317 325
44. SwansonCM
MalimMH
2008 SnapShot: HIV-1 proteins. Cell 133 742, 742
45. GoffSP
2007 Host factors exploited by retroviruses. Nat Rev Microbiol 5 253 263
46. NisoleS
StoyeJP
SaibA
2005 TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol 3 799 808
47. HarrisRS
LiddamentMT
2004 Retroviral restriction by APOBEC proteins. Nat Rev Immunol 4 868 877
48. LoeuilletC
DeutschS
CiuffiA
RobyrD
TaffeP
2008 In vitro whole-genome analysis identifies a susceptibility locus for HIV-1. PLoS Biol 6 e32 doi:10.1371/journal.pbio.0060032
49. NeilSJ
ZangT
BieniaszPD
2008 Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451 425 430
50. DunningMJ
Barbosa-MoraisNL
LynchAG
TavareS
RitchieME
2008 Statistical issues in the analysis of Illumina data. BMC Bioinformatics 9 85
51. LinSM
DuP
HuberW
KibbeWA
2008 Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res 36 e11
52. DuP
KibbeWA
LinSM
2008 lumi: a pipeline for processing Illumina microarray. Bioinformatics 24 1547 1548
53. SmythGK
2004 Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3 Article3
54. FengS
WolfingerR
ChuZ
GibsonG
McGrawL
2006 Empirical Bayesian analysis of variance component models for microarray data. J Agric Biol Environ Stats 11 197 209
55. LönnstedtI
SpeedT
2002 Replicated microarray data. Stat Sinica 12 31 46
56. BenjaminiY
HochbergY
1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57 289
57. DennisGJr
ShermanBT
HosackDA
YangJ
GaoW
2003 DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4 3
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR AvidityČlánek Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



