-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAn Entomopathogenic Nematode by Any Other Name
article has not abstract
Published in the journal: . PLoS Pathog 8(3): e32767. doi:10.1371/journal.ppat.1002527
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1002527Summary
article has not abstract
Introduction
Among the diversity of insect-parasitic nematodes, entomopathogenic nematodes (EPNs) are distinct, cooperating with insect-pathogenic bacteria to kill insect hosts. EPNs have adapted specific mechanisms to associate with and transmit bacteria to insect hosts. New discoveries have expanded this guild of nematodes and refine our understanding of the nature and evolution of insect–nematode associations. Here, we clarify the meaning of “entomopathogenic” in nematology and argue that EPNs must rapidly kill their hosts with the aid of bacterial partners and must pass on the associated bacteria to future generations.
Strangers, Acquaintances, and Enemies
Nematode–arthropod associations are plentiful and range from beneficial to antagonistic [1], [2]. These associations have been divided into at least four categories: 1) phoretic (nematodes are transported by an insect), 2) necromenic (nematodes obtain nutrition from insect cadavers), 3) facultative parasitism, and 4) obligate parasitism (see Sudhaus 2008 for a more detailed breakdown [3]). It is thought that insect parasitism evolves in this sequence, with parasites evolving from non-parasitic insect associates (Figure 1A) [1], [3]. Nematodes also interact with bacteria in at least three ways: 1) trophism (nematodes eat bacteria), 2) parasitism (pathogens cause nematode diseases if not resisted), and 3) mutualism (nematodes and bacteria cooperate). Here, we consider entomopathogenic nematodes, which employ bacteria to kill insects.
Fig. 1. Evolution of nematode–insect associations and the entomopathogenic nematode life cycle. 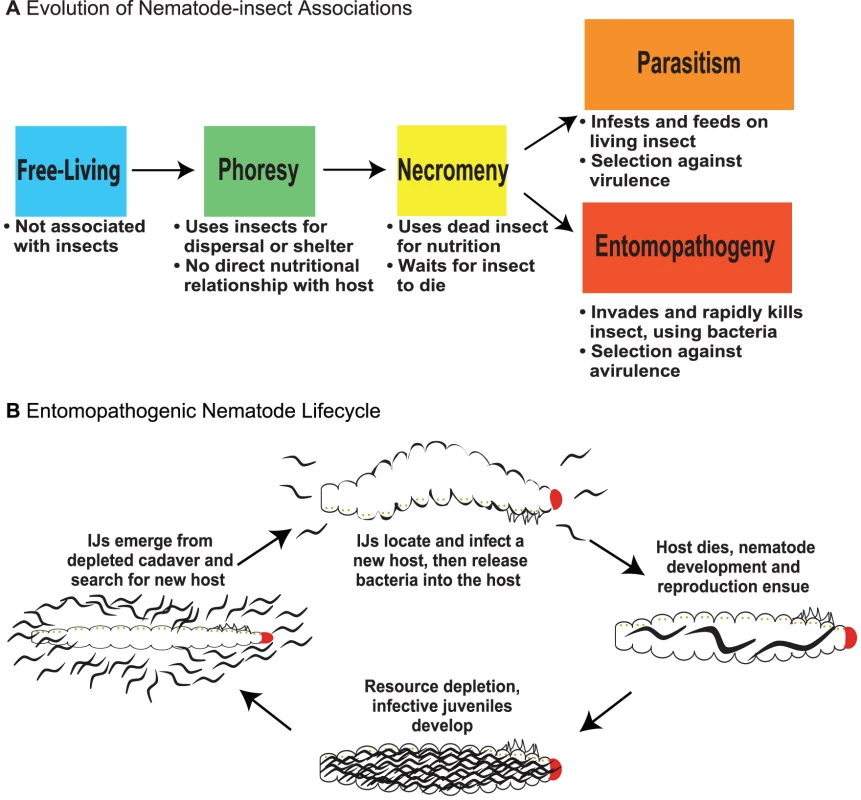
(A) The evolution of nematode–insect associations. Free-living: microbotrophic nematodes not known to associate with arthropods, vertebrates, plants, or fungi; only perhaps transiently associated with insects. Phoresy: a relationship where nematodes are adapted to use insects for dispersal or shelter but have no direct nutritional relationship to them. Necromeny: a relationship where nematodes are adapted to use saprophytic insect cadavers as a food resource but do not participate in insect death. Parasitism: a relationship where nematodes are adapted to use living insects directly for nutrition, likely inflicting some level of harm or even causing eventual death of the host. Entomopathogeny: a relationship where nematodes cooperate with insect-pathogenic bacteria to cause rapid insect disease and death and then feed and develop on the insect and bacterial resources. The distinction between parasitism and entomopathogeny is based on salient features including use of pathogenic bacteria and direction of selection (against virulence or avirulence), either making the nematodes more or less immediately harmful to their host. (B) The life cycle of entomopathogenic nematodes. The IJ stage is a developmentally arrested third larval stage and is the only free-living stage; all other stages exist exclusively within the host. EPN IJs carry symbiotic bacteria and search for potential insect hosts. They enter a host, gain access to the hemolymph, and release their bacterial symbiont. The symbiont plays a critical role in overcoming host immunity. The nematodes develop and reproduce in the resulting nutrient-rich environment until population density is high and resources begin to deplete, at which point new IJs develop and disperse, carrying the symbiotic bacteria to new hosts [5]. Entomopathogenic Nematodes
The term entomopathogenic is widely used in parasitology and pathology, usually referring “to microorganisms and viruses capable of causing disease in an insect host” [4]. Nematodes in Steinernematidae and Heterorhabditidae associate with pathogenic bacteria to kill insect hosts, usually within 48 hours of infection. The hallmarks of this specific type of parasitism by nematodes, known as entomopathogeny, are 1) carriage of pathogenic bacteria by infective juvenile (IJ) nematodes (also known as dauer juveniles); 2) active host-seeking and -penetration by IJs; 3) release of the bacteria into the insect hemolymph; 4) death of the insect, and nematode reproduction and bacterial proliferation driven by cadaver-nutrient utilization; 5) reassociation of the pathogenic bacteria with new generations of IJs; and 6) emergence of IJs from the nutrient-depleted cadaver as they search for new insect hosts (Figure 1B) [5], [6]. Nematode parasites of this kind are known as EPNs.
Recently, other nematode species have been shown to use pathogenic bacteria to parasitize insect hosts. Two Oscheius species, Oscheius chongmingensis and Oscheius carolinensis, and Caenorhabditis briggsae have been identified as potential insect pathogens by baiting soil for nematodes using insect larvae as prey, a common approach used for finding EPNs [7]–[11]. All of these have been found to associate with insect pathogenic bacteria of the genus Serratia, while O. carolinensis may have additional associates [9]–[12]. O. chongmingensis and C. briggsae require their bacterial partners to cause host death, and to grow and reproduce within killed insects, and emerging dauer juveniles are associated with the vectored pathogen [10], [11]. Ongoing studies suggest that these species are EPNs, though their classification as entomopathogens has been contested both semantically and conceptually in the literature and scientific meetings (e.g., the November 2010 NemaSym NSF RCN meeting and the July 2011 Society of Nematologists meeting) [13]–[15].
History, Context, and Formal Criteria
The term entomopathogenic first appeared in the nematology literature in reference to the bacterial symbionts of Steinernema and Heterorhabditis [16]. Bacteria are considered entomopathogenic when their LD50 is <10,000 cells injected into the hemocoel [17]. Some pathogens associated with Steinernema and Heterorhabditis have LD50s<10 cells when injected, but this varies with different hosts and these bacteria are not known to infect insects without the aid of their nematode partners [18]. “Entomopathogenic” was applied to nematodes in 1981 and again in 1986 [19], [20], a use that gained momentum in 1988 [21]. This gradual, social use of the term entomopathogenic without formal definition complicates its application to emerging nematode–bacteria partnerships. Indeed, the convenience of this descriptor is currently that it applies to both partners as a complex, rather than only the nematodes or bacteria. The only clearly identifiable EPN definition that we are aware of was proposed informally [4], [22]. This definition focuses on mutualism with bacteria and on the exclusivity of the IJ as the free-living stage. We find the use of these criteria incomplete since they do not consider rapid death, which is necessary to differentiate EPNs from phoretic, necromenic, or other less virulent forms of parasitism, and the inclusion of a stage-specific requirement in defining EPNs is unnecessary. Since convention provides no standard to assess classification of EPNs, and because “entomopathogenic” was meant to differentiate insect-parasitic nematodes that serve as vectors of bacteria and to reinforce the link between nematology and insect pathology [2], we formally suggest two criteria: 1) the nematodes use a symbiotic relationship with bacteria to facilitate pathogenesis, which implies that the association is non-transient, though not necessarily obligate, and 2) insect death is sufficiently rapid that it can be unequivocally distinguished from phoretic, necromenic, and other parasitic associations (i.e., <120 h), a time frame that also implies efficient release of the pathogen by the nematode vector [17]. These criteria are based on early investigations of EPNs and what we consider the fundamental principles of the EPN lifestyle [1], [2]. We intend this discussion to provide a more thorough evaluation of the defining characteristics of EPNs, though our criteria overlap with, but are not as restrictive as, the previous definition [4], [22].
Koch's postulates can be used to establish pathogenicity of the nematode–bacterium complex or either partner alone, and we suggest that partner association across generations is particularly important in this evaluation [23]. To establish genetic heritability, genes must be passed through the F1 generation to the F2 generation; for example, a mule inherits, but does not pass on, traits inherited from its paternal donkey and maternal horse parents. Similarly, we argue that for an EPN association to be stable, nematodes must not only infect and kill an insect and produce progeny, but must also produce progeny that depart the carcass carrying the pathogenic bacteria. This does not require that the association be obligate—subsequent generations that thrive in non-insect environments may lose the symbiotic bacteria—but we believe it is crucial that symbiont transmission from the infecting parental generation to emerging nematodes from at least two subsequent insect infections be clearly established to distinguish nematode carriage of the bacteria or bona fide association from transient cuticle hitchhiking. Also, in associating, each partner must benefit from the association. At a minimum, the bacteria should increase overall nematode fitness by assisting in insect killing, nutrient liberation, or scavenger deterrence, and the nematodes should provide the bacteria with access to the insect host either by delivery to otherwise inaccessible host cavities or tissues, or by increasing dispersal range through direct carriage. Though EPNs must be capable of infecting and killing insect hosts, this does not preclude them from also, opportunistically, acting as scavengers or from competing with other EPNs for already killed insects [24], [25]. An additional cautionary point here is that the symbiont transmission rate and the stability of nematode–bacterium associations themselves have been well characterized in representative taxa [26], [27], but these details are unclear in most of the 75 EPN species reported to date [7].
Insect host killing within five days of infection is an appropriate requirement and implies selection for virulence or at least selection against avirulence, differentiating entomopathogeny from other forms of parasitism such as those used by mermithids and allantonematids. “Potentially pathogenic” bacteria, microbes that cause septicemia at low inocula when in the hemocoel but that lack mechanisms for actively invading the hemocoel [17], usually cause death within two to four days in common laboratory larvae such as Galleria mellonella, though larger or adult insect hosts, such as mole crickets or Manduca sexta, take longer to succumb, depending on the size of the nematode founding population and which pathogenic bacterium is used [18]. Rapid death caused by EPNs reflects pathogenicity of the bacterial partner with possible contributions from the nematode and relies on efficient release of the bacteria into the hemolymph.
Specialization of EPNs
When considering appropriate criteria that define EPNs, it is tempting to use the particular details that are known for only a few representative taxa. Instead, we avoided specifics in favor of fundamental principles that underlie the associations, and observed that many interesting and often dogmatic EPN characteristics are less widespread than we expected. For example, specialization with particular bacteria is a hallmark EPN characteristic, and monospecificity between one nematode and one genus of bacteria or even one symbiont species is commonly observed among these taxa [7]. However, growing evidence of promiscuous relationships between EPNs and their bacterial symbionts suggests that this may not be as common as originally thought (e.g., [28]–[30]). Although most Heterorhabditis and Steinernema symbionts localize to the nematode intestine, there are excellent examples of nematode–bacteria symbioses in other body sites (e.g., [31]). Of note, Paenibacillus nematophilus associates on the cuticle of Heterorhabditis spp., and, relevant to this discussion, O. carolinensis is associated with insect pathogenic Serratia marcescens on its exterior cuticle [12], [30]. Also, dogma dictates that these associations are obligate, since Steinernema and Heterorhabditis symbionts are generally not free-living, and S. carpocapsae's symbiont is auxotrophic for nicotinic acid, which is not available in the environment [32]. However, Photorhabdus asymbiotica may be free-living (e.g., [33]). Also, most nematodes require their symbionts for growth and reproduction, but exceptions have been observed (e.g., [34], [35]). There are also differences between biological characteristics of the two nematode taxa. For example, Heterorhabditis maternally transmit symbionts by a sophisticated multistep process, while Steinernema have specialized host structures within which they carry their symbionts [28], [29]. Also, some Steinernema infect and kill insect hosts even in the absence of pathogenic bacteria, at least in laboratory conditions, but Heterorhabditis nematodes have not been reported to have this behavior. Finally, as we mentioned above, symbiont transmission to new generations varies widely in the few taxa where it has been studied from >95% to ∼10% [35], [36]. Together, these findings reveal that Steinernema and Heterorhabditis are highly adapted to entomopathogeny and showcase adaptations likely to emerge as a result of long-term commitment to the entomopathogenic lifestyle, even though the biological basis for their symbiotic association with bacteria differs significantly [5], [37]. The exceptions and differences that have been observed for all of these hallmark characteristics highlight why specializations should not be used to exclude newly described associations, and emphasize that applying observations from a few representative members to whole clades can be problematic. Indeed, few species in either genus have been thoroughly explored, and we caution against assuming a priori these specializations to be true of all or even most steinernematids or heterorhabditids (e.g., [38]).
Classification of Newly Described Associations
According to the standards we propose above, C. briggsae may not be an EPN. IJs recovered from dead insects seem able to reinfect new hosts but are less virulent in G. mellonella as a complex than injection of the bacteria alone, suggesting either inefficient release of the pathogen or some antagonism by the nematode vector. This may reflect that C. briggsae is somewhere between necromenic and entomopathogenic, that it is a nascent entomopathogen and not yet efficient, or that G. mellonella is a poor host. However, symbiont heritability has not been demonstrated, and the nature of C. briggsae's bacterial association remains unresolved [10], [11], [39]. Because C. briggsae has not met the suggested criteria, it should not be considered an EPN, facultative or otherwise, until heritability of the pathogenic bacteria is demonstrated and more is known about bacterial release and speed of host death. Our suggested criteria have been tested and met for both O. chongmingensis and O. carolinensis [9], [10], [12]. Therefore, these taxa should be considered EPNs even though further research is required to determine the nature and heritability of their bacterial associations, and whether they are obligate or facultative EPNs.
Symbiosis and Entomopathogeny
Nematode–bacterium partnerships that do not explicitly fulfill the requirements to be classified as EPNs are still of extraordinary interest since they may represent developing, nascent partnerships, but they should not be considered entomopathogens. Our understanding of parasitism and its evolution is continually refined as biodiversity is explored and ecology and evolution become increasingly emphasized among established and satellite model systems. We have suggested specific and restricted use of the term entomopathogenic in nematology, which will facilitate unambiguous communication. Among the 20 or more parasitic lineages of nematodes, entomopathogeny is a unique type of insect parasitism not found among vertebrate - or plant-parasitic nematodes. Recent work indicates that entomopathogeny has arisen at least three times within Nematoda, and that recently described species (O. chongmingensis and O. carolinensis) may represent nascent stages of EPN evolution. These developments emphasize the tremendous specialization exhibited by Heterorhabditis and Steinernema and increase their usefulness as models for the evolution of symbiosis and parasitism.
Zdroje
1. PoinarGOJr 1983 The natural history of nematodes Engelwood Cliffs Prentice Hall
2. GauglerRKayaHK 1990 Entomopathogenic nematodes in biological control. GauglerRKayaHK Boca Raton CRC Press
3. SudhausW 2008 Evolution of insect parasitism in rhabditid and diplogastrid nematodes. MakarovSEDimitrijevicRN Advances in arachnology and developmental biology Vienna-Belgrade-Sofia SASA 143 161
4. OnstadDWFuxaJRHumberRAOestergaardJShapiro-IlanDI 2006 An abriged glossary of terms used in intertebrate pathology. Society for Invertebrate Pathology
5. ChastonJGoodrich-BlairH 2010 Common trends in mutualism revealed by model associations between invertebrates and bacteria. FEMS Microbiol Rev 34 41 58
6. KayaHKGauglerR 1993 Entomopathogenic nematodes. Annu Rev Entom 38 181 206
7. NguyenKBHuntDJ 2007 Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts. HuntDJPerryRN Leiden-Boston Brill
8. BeddingRAAkhurstRJ 1975 A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21 109 110
9. ZhangCLiuJSunJYangSAnX 2008 Heterorhabditidoides chongmingensis gen. nov., sp. nov. (Rhabditida: Rhabditidae), a novel member of the entomopathogenic nematodes. J Invertebr Pathol 98 153 168
10. YeWMTorres-BarraganACardozaYJ 2010 Oscheius carolinensis n. sp (Nematoda: Rhabditidae), a potential entomopathogenic nematode from vermicompost. Nematology 12 121 135
11. AbebeEJumbaMBonnerKGrayVMorrisK 2010 An entomopathogenic Caenorhabditis briggsae. J Exp Biol 213 3223 3229
12. Torres-BarraganASuazoABuhlerWGCardozaYJ 2011 Studies on the entomopathogenicity and bacterial associates of the nematode Oscheius carolinensis. Biol Control 59 123 129
13. RaeRSommerRJ 2011 Bugs don't make worms kill. J Exp Biol 214 1053
14. AbebeEBonnerKGrayVThomasWK 2011 Response to ‘Bugs don't make worms kill’. J Exp Biol 214 1053 1054
15. StockSPBirdDMGhedinEGoodrich-BlairH 2011 Abstracts of the second nematode-bacteria symbioses meeting. J Nematol 43 In press
16. ThomasMTPoinarGOJr 1979 Xenorhabdus gen. nov., a genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. Int J of Syst Bacteriol 29 352 360
17. BucherGE 1960 Potential bacterial pathogens of insects and their characteristics. J Invertebr Pathol 2 172 193
18. ForstSNealsonK 1996 Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol Rev 60 21 43
19. SpiridonovSE 1981 On auxiliary excretory system of an entomopathogenic nematode Heterorhabditis bacteriophora (Rhabditida). Zool Zhurnal 60 1887 1888
20. AkhurstRJ 1986 Xenorhabdus nematophilus subsp. beddingii (Enterobacteriaceae): a new subspecies of bacteria mutualistically associated with entomopathogenic nematodes. Int J Syst Bacteriol 36 454 457
21. GauglerR 1988 Ecological considerations in the biological control of soil-inhabiting insects with entomopathogenic nematodes. Agric Ecosyst Environ 24 351 360
22. GrewalPSEhlersR-UShapiro-IlanDI 2005 Glossary of terms. GrewalPSEhlersR-UShapiro-IlanDI Nematodes as biocontrol agents. CABI Publishing xvii xviii
23. LaceyLA 1997 Manual of techniques in insect pathology San Diego Academic Press
24. San-BlasEGowenSR 2008 Facultative scavenging as a survival strategy of entomopathogenic nematodes. Int J Parasitol 38 85 91
25. San-BlasEGowenSRPembrokeB 2008 Scavenging or infection? Possible host choosing by entomopathogenic nematodes. Nematology 10 251 259
26. PoinarGOJr 1979 Nematodes for biological control of insects Boca Raton CRC Press
27. HanREhlersRU 2000 Pathogenicity, development, and reproduction of Heterorhabditis bacteriophora and Steinernema carpocapsae under axenic in vivo conditions. J Invertebr Pathol 75 55 58
28. KimYKimKSeoJShresthaSKimHH 2009 Identification of an entomopathogenic bacterium, Serratia sp. ANU101, and its hemolytic activity. J Microbiol Biotechnol 19 314 322
29. EnrightMRGriffinCT 2004 Specificity of association between Paenibacillus spp. and the entomopathogenic nematodes, Heterorhabditis spp. Microb Ecol 48 414 423
30. BabicIFischer-Le SauxMGiruadEBoemareN 2000 Occurence of natural dixenic associations between symbiont Photorhabdus luminescens and bacteria related to Ochrobactrum spp. in tropical entomopathogenic Heterorhabditis spp. (Nematoda, Rhabditida). Microbiol 146 709 718
31. PolzMFFelbeckHNovakRNebelsickMOttJA 1992 Chemoautotrophic, sulfur-oxidizing symbiotic bacteria on marine nematodes: morphological and biochemical characterization. Microb Ecol 24 313 329
32. OrchardSSGoodrich-BlairH 2004 Identification and functional characterization of a Xenorhabdus nematophila oligopeptide permease. Appl Environ Microbiol 70 5621 5627
33. GerrardJGJoyceSAClarkeDJffrench-ConstantRHNimmoGR 2006 Nematode symbiont for Photorhabdus asymbiotica. Emerg Infect Dis 12 1562 1564
34. SicardMRamoneHLe BrunNPagesSMouliaC 2005 Specialization of the entomopathogenic nematode Steinernema scapterisci with its mutualistic Xenorhabdus symbiont. Naturwissenschaften 92 472 476
35. AkhurstRJ 1983 Neoaplectana species: Specificity of association with bacteria of the genus Xenorhabdus. Exp Parasitol 55 258 263
36. CowlesCEGoodrich-BlairH 2008 The Xenorhabdus nematophila nilABC genes confer the ability of Xenorhabdus spp. to colonize Steinernema nematodes. J Bacteriol 190 4121 4128
37. Goodrich-BlairHClarkeDJ 2007 Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Mol Microbiol 64 260 268
38. BlaxterM 1998 Caenorhabditis elegans is a nematode. Science 282 2041 2046
39. AbebeEAkeleFAMorrisonJCooperVThomasKT 2011 An insect pathogenic symbiosis between Caenorhabditis and Serratia. Virulence 2 158 161
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- An Entomopathogenic Nematode by Any Other Name
- Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen
- A Foot in the Door for Dermatophyte Research
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
- Indifferent, Affectionate, or Deceitful: Lifestyles and Secretomes of Fungi
- Mutation and Selection of Prions
- Sleeping with the Enemy: How Intracellular Pathogens Cope with a Macrophage Lifestyle
- Taste for Blood: Hemoglobin as a Nutrient Source for Pathogens
- Direct Recognition of by the NK Cell Natural Cytotoxicity Receptor NKp46 Aggravates Periodontal Disease
- A20 () Deficiency in Myeloid Cells Protects against Influenza A Virus Infection
- Anthrax Lethal Factor Cleavage of Nlrp1 Is Required for Activation of the Inflammasome
- Differential Function of Lip Residues in the Mechanism and Biology of an Anthrax Hemophore
- PK-sensitive PrP Is Infectious and Shares Basic Structural Features with PK-resistant PrP
- A Peptidoglycan Fragment Triggers β-lactam Resistance in
- Capsule Type of Determines Growth Phenotype
- Additive Function of MARTX and VvhA Cytolysins Promotes Rapid Growth and Epithelial Tissue Necrosis During Intestinal Infection
- A Novel Mouse Model of Egg-Induced Immunopathology
- Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with
- The Novel Transporter Dur31 Is a Multi-Stage Pathogenicity Factor
- Short ORF-Dependent Ribosome Shunting Operates in an RNA Picorna-Like Virus and a DNA Pararetrovirus that Cause Rice Tungro Disease
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Foot in the Door for Dermatophyte Research
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- An Entomopathogenic Nematode by Any Other Name
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



