-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaA Key Role for the Urokinase Plasminogen Activator (uPA) in Invasive Group A Streptococcal Infection
Recruitment of the serine protease plasmin is central to the pathogenesis of many bacterial species, including Group A streptococcus (GAS), a leading cause of morbidity and mortality globally. A key process in invasive GAS disease is the ability to accumulate plasmin at the cell surface, however the role of host activators of plasminogen in this process is poorly understood. Here, we demonstrate for the first time that the urokinase-type plasminogen activator (uPA) contributes to plasmin recruitment and subsequent invasive disease initiation in vivo. In the absence of a source of host plasminogen activators, streptokinase (Ska) was required to facilitate cell surface plasmin acquisition by GAS. However, in the absence of Ska, host activators were sufficient to promote cell surface plasmin acquisition by GAS strain 5448 during incubation with plasminogen or human plasma. Furthermore, GAS were able mediate a significant increase in the activation of zymogen pro-uPA in human plasma. In order to assess the contribution of uPA to invasive GAS disease, a previously undescribed transgenic mouse model of infection was employed. Both C57/black 6J, and AlbPLG1 mice expressing the human plasminogen transgene, were significantly more susceptible to invasive GAS disease than uPA−/− mice. The observed decrease in virulence in uPA−/−mice was found to correlate directly with a decrease in bacterial dissemination and reduced cell surface plasmin accumulation by GAS. These findings have significant implications for our understanding of GAS pathogenesis, and research aimed at therapeutic targeting of plasminogen activation in invasive bacterial infections.
Published in the journal: . PLoS Pathog 9(7): e32767. doi:10.1371/journal.ppat.1003469
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003469Summary
Recruitment of the serine protease plasmin is central to the pathogenesis of many bacterial species, including Group A streptococcus (GAS), a leading cause of morbidity and mortality globally. A key process in invasive GAS disease is the ability to accumulate plasmin at the cell surface, however the role of host activators of plasminogen in this process is poorly understood. Here, we demonstrate for the first time that the urokinase-type plasminogen activator (uPA) contributes to plasmin recruitment and subsequent invasive disease initiation in vivo. In the absence of a source of host plasminogen activators, streptokinase (Ska) was required to facilitate cell surface plasmin acquisition by GAS. However, in the absence of Ska, host activators were sufficient to promote cell surface plasmin acquisition by GAS strain 5448 during incubation with plasminogen or human plasma. Furthermore, GAS were able mediate a significant increase in the activation of zymogen pro-uPA in human plasma. In order to assess the contribution of uPA to invasive GAS disease, a previously undescribed transgenic mouse model of infection was employed. Both C57/black 6J, and AlbPLG1 mice expressing the human plasminogen transgene, were significantly more susceptible to invasive GAS disease than uPA−/− mice. The observed decrease in virulence in uPA−/−mice was found to correlate directly with a decrease in bacterial dissemination and reduced cell surface plasmin accumulation by GAS. These findings have significant implications for our understanding of GAS pathogenesis, and research aimed at therapeutic targeting of plasminogen activation in invasive bacterial infections.
Introduction
An emerging theme in bacterial pathogenesis is sequestration of host plasminogen during disease initiation [1]. This has inspired research to develop therapeutic inhibitors of bacterial plasminogen activation and recruitment [2], [3], [4], [5]. To be successful, such strategies require a comprehensive understanding of how bacteria interact with the host fibrinolytic system. Group A streptococcus (GAS) is a globally significant human pathogen, responsible for 600,000 cases of invasive infection each year, approximately 25% of which are fatal [6]. The ability of GAS to accumulate cell surface plasmin activity is an essential step in the initiation of invasive disease [7], [8], but the mechanistic basis of this virulence property has yet to be fully elucidated. While in vitro analyses suggest a role for host plasminogen activators in GAS disease [9], [10], [11], this hypothesis has yet to be conclusively demonstrated in vivo.
The glycoprotein plasminogen is found in plasma and extracellular fluids at concentrations of approximately 2 µM. Activation of plasminogen leads to the generation of the serine protease plasmin. Plasmin is able to degrade fibrin clots, connective tissue, extracellular matrix (ECM) and adhesion proteins. Additionally, activation of pro-metalloproteases by plasmin results in degradation of the collagen structural components of the ECM, leading to widespread tissue destruction [12]. Conversion of plasminogen to plasmin can be facilitated by both host and bacterial activators. The major circulating inhibitor of plasmin is α2-antiplasmin. However, surface bound plasmin is less susceptible to inactivation by α2-antiplasmin [13]. GAS secrete the plasminogen activator streptokinase (Ska), which is highly specific for human plasminogen [14]. The contribution of Ska to GAS virulence is well established, however previous studies have shown that deletion of ska from the GAS chromosome significantly, but not completely, attenuates GAS virulence [9], [10], [11]. This implies a role for host activators. There are two distinct eukaryotic activators of plasminogen, urokinase-type plasminogen activator (uPA) and tissue plasminogen activator (tPA). uPA is primarily involved in cell-associated plasminogen activation. The zymogen pro-uPA can be activated by a variety of proteases, including plasmin [15], [16], [17]. Cleavage of the inactive form of the urokinase plasminogen activator pro-uPA by cell bound plasmin generates the active two-chain uPA. This feedback activation results in a significant increase in plasmin activation within biological systems [18]. uPA is localized on the surface of human cells that contribute to epithelial and innate immune defense against bacterial infection, including keratinocytes, neutrophils and macrophages [19]. Furthermore, uPA is upregulated in response to bacterial sepsis, and elevated uPA levels can be correlated to poor patient outcome [20], [21]. Here we utilise a series of isogenic GAS mutants, in conjunction with a newly developed mouse model of infection, to assess the role of uPA in invasive GAS disease.
Results
Host plasminogen activators are sufficient for the acquisition of cell surface plasmin by GAS
Streptokinase, encoded by the ska gene, is a GAS activator of human plasminogen [14]. To study streptokinase-dependent and -independent interactions of GAS with the host plasminogen activation system, we used wild-type (WT) GAS strain (5448) isolated from a patient with necrotizing fasciitis and toxic shock syndrome. This strain belongs to the globally-disseminated serotype M1T1 clone that is the leading cause of invasive GAS infections in recent decades [22]. An isogenic streptokinase-deficient mutant of this strain (5448Δska), which was generated by allelic replacement of the ska gene with a chloramphenicol acetyltransferase (cat) gene, has been described previously [5]. This mutant was subsequently complemented by reinsertion of the wild-type ska gene in place of cat (5448*). PCR confirmed the genetic manipulations, and western blot analysis showed that 5448Δska lacked streptokinase expression whereas WT strain 5448 and complemented strain 5448* expressed equivalent levels of the protein. The three GAS strains grew equivalently in bacteriologic media, expressed equivalent levels of surface hyaluronic acid capsule, and bound equivalent amounts of plasminogen following incubation in human plasma (Fig S1). As predicted, when incubated in human plasma, the WT 5448 and complemented 5448* GAS strains were able to accumulate plasmin activity at their cell surface, however, deletion of ska from the GAS chromosome resulted in a significant, but partial attenuation in plasmin accumulation (Fig. 1A). This suggests that endogenous host derived plasminogen activators and plasminogen, present in the human plasma, contribute to cell surface plasmin acquisition by GAS. Upon incubation of GAS with human Glu-plasminogen and active human uPA, the 5448Δska mutant accumulated surface plasmin activity equivalent to the WT parent strain (Fig. 1B), suggesting that host-derived uPA can contribute to cell surface plasmin acquisition by GAS. Similarly, GAS were able to acquire cell surface plasmin activity in the presence of human Glu-plasminogen and human tPA (Fig S2), supporting the hypothesis that host plasminogen activators are mediators of plasmin acquisition by GAS [7].
Fig. 1. Cell surface plasmin acquisition in the absence of streptokinase. 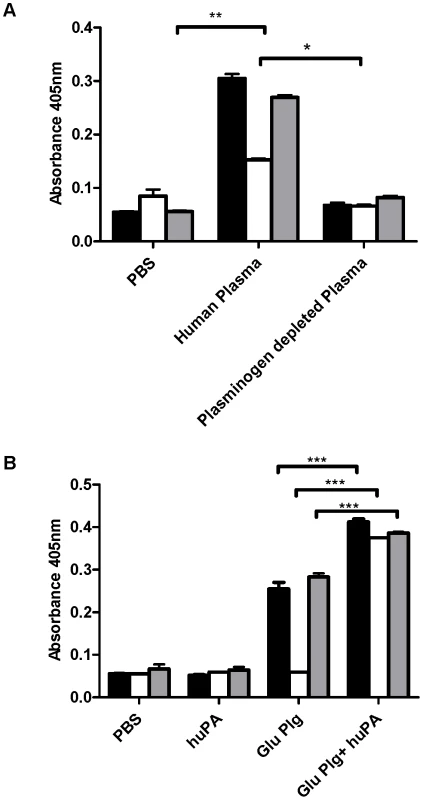
A GAS strains 5448 (black fill), 5448Δska (no fill) and 5448* (grey fill) readily acquire cell surface plasmin activity during a 3 h incubation in human plasma, but not plasminogen-depleted plasma or PBS. B In the absence of streptokinase, uPA can mediate cell surface plasmin acquisition by GAS in vitro. Data is representative of 2 independent experiments. Error bars indicate SEM (n = 3), asterisks indicate statistical significance as determined by unpaired two-tailed students t-test P<0.005 (**), P<0.001 (***). GAS facilitates enhanced uPA generation in plasma
uPA is expressed as the zymogen pro-uPA, which has limited plasminogen activating potential [23], [24]. Activation of pro-uPA occurs via plasmin mediated proteolytic cleavage of the zymogen. Activation of pro-uPA to uPA is enhanced when the plasmin source is localised to the cell surface, and the reciprocal activation of pro-uPA by plasmin and hence co-localised plasminogen by uPA is an important mechanism in the regulation of plasmin activity [25]. Plasmin localised at the GAS cell surface, where it is protected from α2-antiplasmin inhibition [26], may therefore facilitate enhanced uPA activation in plasma. uPA activity levels in plasma were monitored using the uPA specific fluorescence substrate Z-Gly-Gly-Arg-AMC over 2 hours. The initial rate of uPA activity was greater in plasma containing either 5448, 5448* or 5448Δska compared with plasma alone (Fig. 2). In the presence of 1×107 colony forming units (CFU), the initial rate of pro-uPA activation was found to be 10.6(+/−0.928) fluorescence units (fu)/min, 9.812 (+/−0. 957) fu/min , and 8.635 (+/−1.078) fu/min for 5448, 5448* and 5448Δska, respectively, compared with a rate of 4.921(+/−0.0.215) fu/min in plasma alone. The concentration of active uPA in plasma at t30 was determined to be 0.183 (+/−0.035) nM in the presence of 5448, 0.166 (+/−0.031) nM in the presence of 5448*, 0.144 (+/−0.023) nM in the presence of 5448Δska and 0.054 (+/−0.018) nM in plasma alone. These data suggest the presence of endogenous pro-uPA in human plasma that is readily activatable by GAS-bound plasmin.
Fig. 2. GAS facilitates enhanced uPA activity in plasma. 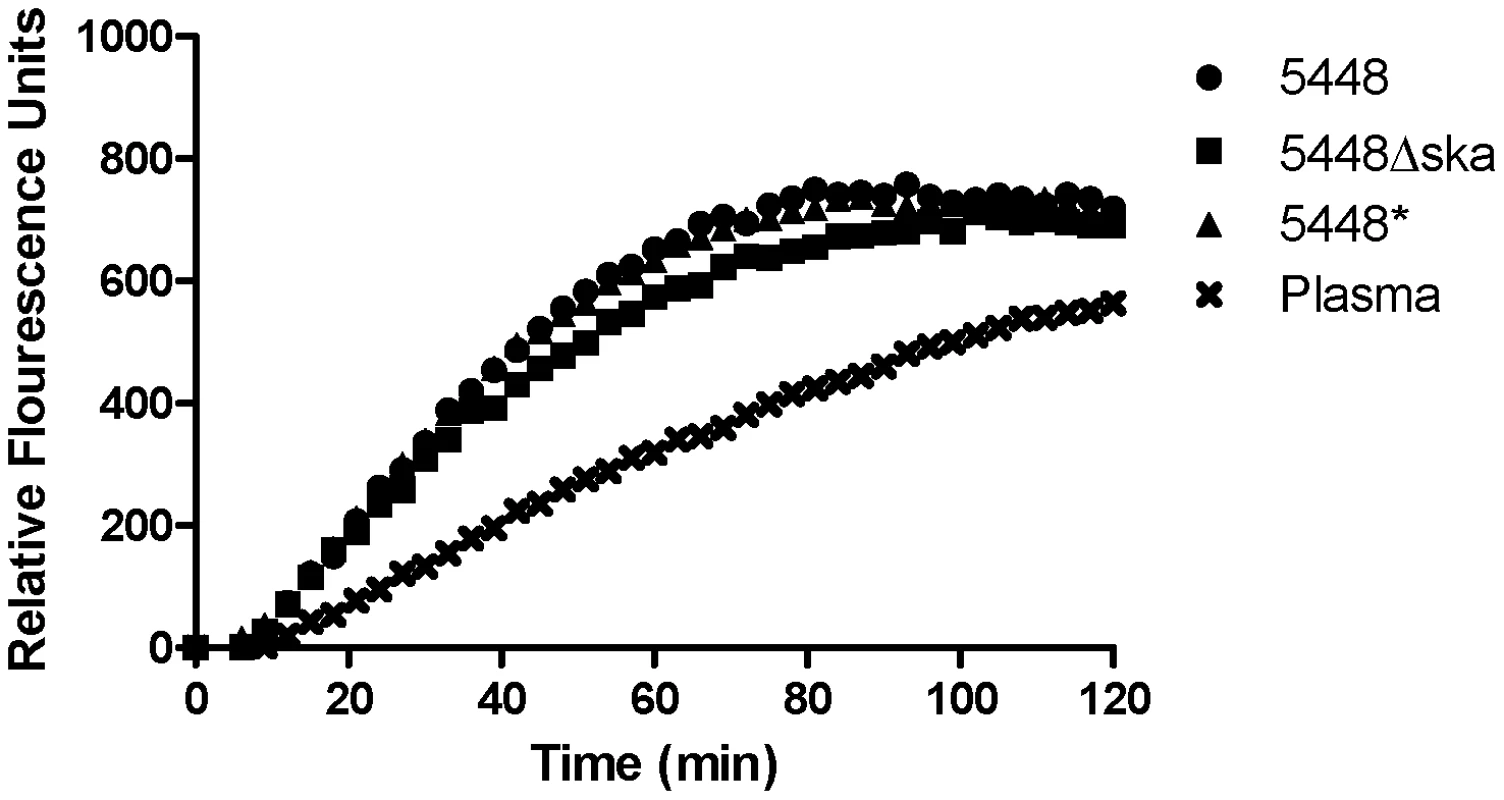
Endogenous uPA activity in human plasma was measured using the uPA specific fluorogenic substrate Z-Gly-Gly-Arg-AMC, in the presence or absence of GAS strains 5448, 5448* and 5448Δska. Data is representative of four independent experiments, performed in duplicate. Background fluorescence has been subtracted from all values. uPA plays a central role in invasive GAS infection
Using a knockout mouse lacking uPA, we assessed the role of host uPA in the development of invasive GAS disease. Following intradermal infection with WT GAS strain 5448 at a high dose (1×109 colony forming units/dose), C57 black/6J mice displayed significantly higher mortality (P = 0.04) than C57 black/6JuPA−/− mice (Fig. 3A). Recognizing that the interaction between streptokinase and plasminogen is species specific, and that the presence of human plasminogen increases the severity of invasive GAS infection in the murine model [7], [8], [9], [11], we crossed the humanized plasminogen mouse line AlbPLG1 with C57 black/6JuPA−/− mice to establish AlbPLG1/uPA−/− infection model. The ability of GAS to acquire cell surface plasmin activity in the presence of human plasminogen and mouse uPA confirmed the ability of mouse uPA to activate human plasminogen at the GAS cell surface (Fig. 3B), and survival of mice infected intradermally with GAS (1×109 colony forming units/dose) increased significantly in mice deficient in uPA (Fig. 3C). Deletion of uPA did not completely eliminate virulence of GAS in this model, which may be explained by the presence of the host plasminogen activator tPA. As with uPA, mouse tPA will activate human plasminogen at the GAS cell surface (Fig S2B). However, mortality was reduced from 80% to 20% in the absence of both uPA and streptokinase (Fig. 3C).
Fig. 3. uPA contributes to bacterial disease dissemination in vivo. 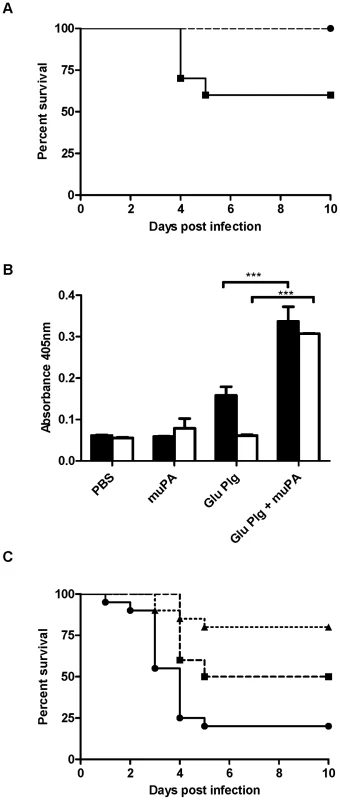
A Cohorts of 10 age and sex matched mice were subcutaneously infected with 1×109 CFU of GAS strain 5448 or 5448Δska. C57 black/6J uPA −/− mice (dashed line) showed a significant increase in survival (P<0.05) compared to C57 black/6J mice (solid line). B Mouse uPA can mediate activation of human plasminogen, and human plasmin acquisition by 5448 (black fill) and 5448Δska (no fill). C AlbPLG1/uPA −/− mice infected with 5448 (dashed line) or 5448Δska (dotted line) showed a significant increase in survival (P<0.01) compared to AlbPLG1 mice infected with 5448 (solid line). Survival data is combined from two independent experiments (n = 20), and significance was determined by log-rank test. Plasmin acquisition data is representative of two independent experiments, error bars indicate SEM (n = 3). Asterisks indicate statistical significance as determined by unpaired two-tailed students t-test, P<0.05 (*), P<0.001 (***). Loss of virulence in the AlbPLG1/uPA−/− mouse model correlates with decreased bacterial dissemination and a decrease in cell surface plasmin acquisition by GAS
The ability of pathogens to sequester plasmin has been implicated in bacterial dissemination during systemic infection [9]. To determine if uPA mediated plasmin acquisition contributes to GAS dissemination, we compared the ability of WT GAS strain 5448 to disseminate during infection of AlbPLG1 and AlbPLG1/uPA−/− mice. In comparison to AlbPLG1 mice, significantly lower numbers of bacteria were detected in the blood (P<0.05) and spleen (P<0.05) of AlbPLG1/uPA−/− mice 72 h post-infection (Fig. 4A). The number of bacteria isolated from the subcutaneous site of infection was similar in the two mouse lines, indicating that differences did not reflect differential ability of the bacteria to survive at the site of infection. This was further supported by the finding that there was no significant difference in lesion size between AlbPLG1 and AlbPLG1/uPA−/− mice (Fig. 4B). GAS acquired significantly lower levels of cell surface plasmin ex vivo in plasma from AlbPLG1/uPA−/− compared with AlbPLG1 mice (Fig. 4C). These data clearly indicate that uPA contributes to cell surface plasmin acquisition and bacterial dissemination in invasive GAS disease.
Fig. 4. Bacterial dissemination in vivo correlates with uPA mediated plasmin acquisition. 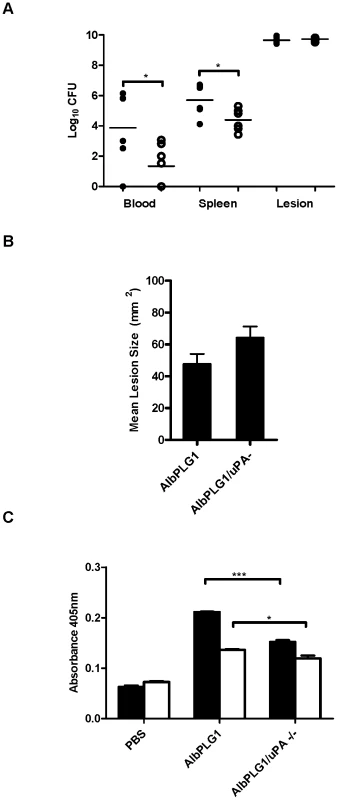
A Bacterial counts in the bloodstream and spleen of mice were significantly higher in AlbPLG1 mice (black circles) than in AlbPLG1/uPA −/− mice (white circles). B AlbPLG1 and AlbPLG1/uPA −/− mice develop equivalent lesions 3 days post-inoculation with GAS strain 5448. C GAS strains 5448 (black fill) and 5448Δska (no fill) accumulate significantly lower levels of cell surface plasmin ex vivo in AlbPLG1/uPA−/− compared with AlbPLG1 plasma. Dissemination and lesion size data is combined from 3 independent experiments (n = 6). Plasmin acquisition data is representative of two independent experiments, error bars indicate SEM (n = 3). Asterisks indicate statistical significance as determined by unpaired two-tailed students t-test, P<0.05 (*), P<0.001. Discussion
Despite increased research efforts over the past 20 years, GAS remains a significant human pathogen, with continued outbreaks of serious GAS infection globally [6]. Interaction with the host protease plasmin is central to the onset of invasive GAS disease [1]. A major consequence of plasmin acquisition in GAS infection is to facilitate bacterial escape from fibrin networks. uPA can facilitate efficient fibrin clearance in the absence of both uPAR and tPA [27], and the results of the current study provide for the first time, evidence that uPA mediated plasmin acquisition facilitates widespread systemic infection by GAS.
GAS were able to establish a subcutaneous infection at the site of inoculation, but showed a decreased propensity for dissemination from the site of local infection and systemic disease initiation in the absence of uPA. This reduced virulence could be directly correlated to reduced bacterial plasmin acquisition in plasma from uPA−/ − mice. Previously, uPA deficiency has been linked to impaired wound healing [27], [28], [29]. No significant difference was seen 3 days post infection in lesion sizes for AlbPLG1 and AlbPLG1/uPA−/− mice. This may reflect the contribution of numerous secreted GAS virulence factors to localised inflammation and lesion development. Comparison of lesion size between AlbPLG1 and AlbPLG1/uPA−/− mice was not performed at later time points due to poor survival of AlbPLG1 mice following GAS infection, however, it appears that in this model, lesion size is not indicative of the propensity of GAS to initiate systemic disease. The finding that virulence was not fully attenuated in the humanised plasminogen uPA−/ − background is not unexpected, given the well established role for streptokinase in GAS virulence, and the presence of the other major host plasminogen activator tPA in this model. However, GAS virulence was further attenuated following deletion of ska, providing further evidence of a role for co-operative plasminogen activation in GAS invasive disease.
The contribution of streptokinase to plasminogen-dependant GAS virulence has been well documented [9], and data presented here clearly show that in the absence of host activators, streptokinase is an absolute requirement for cell surface plasmin acquisition by GAS strain 5448. However, previous studies suggest that even in the absence of streptokinase, GAS are able to acquire cell surface plasmin activity [9], [10]. Following deletion of ska from the chromosome, GAS strain 5448 acquired cell surface plasmin activity in both human plasma and in the presence of plasminogen and uPA. This study therefore demonstrates that streptokinase is not essential for sequestration of plasmin by GAS. The ability of GAS to access a source of plasmin in the absence of streptokinase may have relevance at stages of infection where streptokinase expression is downregulated, or when streptokinase is degraded by other GAS virulence factors such as SpeB. The GAS protease SpeB has been shown to degrade key mediators of plasminogen acquisition, including streptokinase, but not receptors for plasmin and plasminogen such as the streptococcal enolase [8]. uPA mediated plasmin acquisition may therefore be critical during early infection when SpeB is abundant [11], providing a source of plasmin for recruitment to the cell surface. uPA can be detected in normal human plasma and is expressed at the leading edge of keratinocytes and macrophages during wound healing [19], [30]. Additionally, uPA stored in intracellular vesicles in neutrophils is released into the extracellular space following activation [31]. The abundance of migrating and inflammatory cells during GAS infection therefore represents a significant source of uPA that can contribute to plasmin acquisition by GAS. Furthermore, receptor bound plasmin is resistant to α2-antiplasmin inhibition, enhancing the activation of receptor bound pro-uPA [32]. Plasmin bound to GAS cell surface receptors may therefore further amplify uPA mediated proteolysis. The finding in this study that GAS are able to enhance uPA generation in plasma supports a scenario in which plasmin localised to the GAS surface enhances activation of pro-uPA to uPA, effectively creating an activation cascade leading to enhanced proteolysis [18].
Upregulation of uPA in bacterial meningitis is associated with poor patient outcome and breaching of the blood cerebrospinal fluid barrier [21], and uPA is upregulated in response to numerous bacterial infections, including bacterial sepsis [20], [33]. Whilst the ability of bacteria to degrade clots and ECM using uPA activated plasmin has been demonstrated repeatedly in vitro (reviewed in [1]), the contribution of uPA to GAS virulence has not been established in vivo. We now show for the first time, that uPA mediated plasminogen activation contributes to systemic GAS disease in vivo using a novel model of GAS infection AlbPLG1/uPA−/− mice.
It has been suggested that targeting the nexus between bacteria and the fibrinolytic system to inhibit bacterial plasmin activation may provide therapeutic benefit [2], [3], [4], [5], and indeed, data presented here support this proposal. However, the development of potential therapeutics is currently hampered by our limited understanding of this process. Clearly, inhibitors targeting streptokinase mediated plasmin acquisition would not be sufficient to prevent bacterial plasmin accumulation by GAS. Understanding the mechanisms behind bacterial interaction with key components of the fibrinolytic system may therefore aid in the development of therapeutics to control GAS infection.
Materials and Methods
Ethics approvals
Permission to collect human blood was obtained from the University of Wollongong Human Ethics Committee (HE08/250). Blood was taken from healthy adult volunteers, who provided informed, written consent. Animal experiments were performed according to the Australian code of practice for the care and use of Animals for scientific purposes, and the NIH Guide for the care and use of laboratory animals. Permission was obtained from the University of Wollongong (AE11/04; AE12/05) and the University of Notre Dame ethics committees. Volunteers provided informed consent before blood samples were obtained.
Bacterial strains and culture conditions
GAS strains were routinely cultured in Todd-Hewitt broth containing 1% yeast (THBY) or grown on horse blood agar (HBA) plates at 37°C. Invasive GAS isolate 5448 has been described previously [8], [11]. A precise, in-frame allelic replacement of the ska gene with cat encoding chloramphenicol transferase was created in GAS wild type strain 5448 using established methods [7]. The mutation was subsequently reversed by replacement of the cat gene with the wildtype ska gene. The resulting strains were designated 5448Δska and 5448* respectively. Briefly, an 854 bp fragment upstream of ska was PCR amplified with primers ska-upF (5′-TGTACCCGCAGTTACCTGATACC-3′) and ska-upRcat (5′-AGAAACCTCCTACTAAAAGTTAAG-3′), the latter containing a 30 bp 5′ overhang homologous to the 5′ end of cat. A 853 bp fragment downstream of ska was amplified by primers ska-downFcat (5′-CCACGATCTTCTAAAATGATG-3′), containing a 30-bp 5′ overhang homologous to the 3′ end of cat, and ska-downR (5′-TGGCTACCAAGAACGCTTGATTG-3′). The upstream and downstream fragments were combined with the 650 bp cat gene in a fusion PCR reaction using primers ska-upF and ska-downR, creating an amplicon in which ska had been precisely replaced with cat. For reversal of the mutation process, primers ska-upF and ska-downR were used to amplify ska from the 5448 chromosome. The resulting fragments were TA cloned into pCR-2.1-TOPO (Invitrogen) and subsequently digested with restriction enzymes BamHI and XbaI, then ligated into the temperature sensitive vector pHY304, to generate the knockout plasmid pskaKO, and the restoral plasmid pHYska. Plasmids were transformed into GAS strain 5448 or 5448Δska by electroporation. Transformants were identified at the permissive temperature of 30°C under erythromycin (4 µg/ml) selection. Transformants were then grown at the non-permissive temperature of 37°C with erythromycin to select for homologous recombination and integration of the plasmid into the genome. Single crossovers were confirmed by PCR analysis. Release of the plasmid was carried out at 30°C with no antibiotic selection. Screening for erythromycin-sensitive colonies was used to identify double crossover events and allelic replacement mutants were confirmed by PCR and DNA sequence analysis.
Hyaluronic acid capsule assay
Overnight GAS cultures were used to inoculate fresh THBY. Cultures were grown to an OD600 nm of 0.5–0.6. Capsule was extracted and assayed using the Stains-All method, as described previously [34].
Western blot analysis of streptokinase expression
GAS strains were screened for streptokinase expression via western blot analysis as described previously [8]. Briefly, GAS strains were cultured overnight in the presence of the SpeB inhibitor E64 (Sigma-Aldrich). Trichloroacetic-precipitated proteins from culture supernatants were assayed for the presence of streptokinase using rabbit polyclonal streptokinase antiserum. The antisera used and the conditions for Western blot analysis have been described in detail elsewhere [8].
Cell surface plasminogen acquisition
GAS (1×107 CFU) were incubated with 500 nM Human Glu-Plg (Haemotologic Technologies) for 2 h at 37 degrees. Following 2× washes with PBS, plasminogen was eluted from the bacterial cell surface using 100 mM Glycine-HCl (pH 2.0) as described previously [7]. The eluent was screened for the presence of plasminogen by western blot analysis using rabbit anti human plasminogen (Calbiochem), goat anti -rabbit IgG HRP conjugate (Invitrogen), and enhanced chemiluminescence detection.
Host activator mediated cell surface plasmin acquisition
The ability of human uPA (Calbiochem) and mouse uPA (Molecular Innovations), or human tPA (Calbiochem) and mouse tPA (Molecular Innovations) to mediate cell surface plasmin acquisition by GAS in the presence of human Glu-plasminogen (Enzyme Research) was determined using an in vitro plasminogen acquisition assay. GAS cultures were grown to mid-log phase (OD600 nm = 0.5), and 1×108 cells harvested by centrifugation at 6,000× g. Cells were washed twice by resuspension in PBS, followed by resuspension in PBS containing Glu-plasminogen (1 mg/ml), uPA (3 units), tPA (3 units), Glu-Plasminogen and uPA/tPA, or PBS alone. Following incubation for 1 h at 37°C, cells were washed twice in PBS to remove any unbound plasmin, and resuspended in PBS. The plasmin activity of this resuspension was determined using the chromogenic substrate S-2251 (2.5 mM; Sigma-Aldrich).
Cell surface plasmin acquisition in human plasma and mouse plasma
Cell surface plasmin activity assays were conducted following incubation of GAS in human or mouse plasma for 3 has described previously [7]. Plasmin activity was determined as above.
uPA activation in human plasma
uPA activity in plasma and plasma containing GAS, was measured using the fluorogenic substrate Z-Gly-Gly-Arg-AMC (Calbiochem). Fluorescence observed in this assay is directly proportional to uPA activity due to the high specificity of the substrate for uPA [35]. The excitation wavelength range of the substrate is 365–380 nm and the emission wavelength range is 430–460 nm. GAS were prepared as described above, and 1×107 CFU of bacteria added to human plasma, and transferred to a fluor plate. Samples were overlayed with an equivalent volume of PBS containing 1 mM Z-Gly-Gly-Arg-AMC to give a final concentration of 0.5 mM of the fluorogenic substrate. A sample to indicate background fluorescence containing GAS, fluorogenic substrate and buffer was included in assay plates. Fluorescence emission was measured immediately using a Fluostar Optima instrument at 37°C (BMG Labtech, Offenburg, Germany). Data was recorded at 3 min intervals over a period of 120 min. The background fluorescence was subtracted from each reading before statistical analysis. Calculation of the initial rate of change in fluorescence min_1 allowed quantitative interpretation of fluorescence data and was generated using the linear region of the graph where fluorescence was plotted against time, over the first 30 min of the assay. The final concentration of uPA generated under each experimental condition was determined from standard curve measuring relative fluorescence over time in the presence of increasing concentrations of uPA.
Animals
AlbPLG1 mice heterozygous for the human plasminogen transgene [9] were bred as described previously [8]. To construct the uPA knockout lines, C57BL/J6 mice or AlbPLG1 mice were backcrossed with or C57BL/J6upa−/− mice, in which upa is replaced with neo [28] >6 times. Mouse genotype was confirmed by PCR following tail snip as described previously [9], [28]
Streptococcal infection model
GAS cultures were grown to mid-log phase (OD600 nm = 0.5). Following centrifugation, bacteria were washed twice, and resuspended in 0.7% (w/v) saline. For survival studies, cohorts of 10 mice were infected subcutaneously with 1×109 colony forming units of 5448, and survival was monitored over a 10-day period. For studies investigating bacterial dissemination, mice (n = 6) were inoculated with 4×108 colony forming units of 5448. 72 h post infection, the lesion (site of infection), blood, and spleen were collected and the number of viable bacteria determined. Lesion size was determined as described previously [10]. In all studies mice were aged between 6 and 12 weeks, and cohorts were matched for age and sex. The number of CFU used for infection was determined by serial dilution of the inoculum post-infection, plating on horse blood agar, and colony counting following overnight incubation at 37°C.
Statistical analysis
Survival data was analysed by log-rank test. All other data was analysed using a two-tailed unpaired students t-test. Statistical analysis was performed using GraphPad Prism 5.00 (GraphPad, San Diego, CA, USA).
Supporting Information
Zdroje
1. Sanderson-SmithM, De OliveiraDP, RansonM, McArthurJD (2012) Bacterial plasminogen receptors: Mediators of a multifaceted relationship. Journal of Biomedicine and Biotechnology 2012 doi: 10.1155/2012/272148
2. McArthurJD, CookSM, VenturiniC, WalkerMJ (2012) The role of streptokinase as a virulence determinant of Streptococcus pyogenes–potential for therapeutic targeting. Curr Drug Targets 13 : 297–307.
3. SunH, XuY, SitkiewiczI, MaY, WangX, et al. (2012) Inhibitor of streptokinase gene expression improves survival after group A streptococcus infection in mice. Proc Natl Acad Sci U S A 109 : 3469–3474.
4. SunH (2011) Exploration of the host haemostatic system by group A streptococcus: implications in searching for novel antimicrobial therapies. J Thromb Haemost 9 Suppl 1 : 189–194.
5. HollandsA, GonzalezD, LeireE, DonaldC, GalloRL, et al. (2012) A bacterial pathogen co-opts host plasmin to resist killing by cathelicidin antimicrobial peptides. J Biol Chem 287 : 40891–7.
6. CarapetisJR, SteerAC, MulhollandEK, WeberM (2005) The global burden of group A streptococcal diseases. Lancet Infect Dis 5 : 685–694.
7. Sanderson-SmithML, DinklaK, ColeJN, CorkAJ, MaamaryPG, et al. (2008) M protein-mediated plasminogen binding is essential for the virulence of an invasive Streptococcus pyogenes isolate. Faseb J 22 : 2715–2722.
8. ColeJN, McArthurJD, McKayFC, Sanderson-SmithML, CorkAJ, et al. (2006) Trigger for group A streptococcal M1T1 invasive disease. Faseb J 20 : 1745–1747.
9. SunH, RingdahlU, HomeisterJW, FayWP, EnglebergNC, et al. (2004) Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science 305 : 1283–1286.
10. KhilJ, ImM, HeathA, RingdahlU, MundadaL, et al. (2003) Plasminogen enhances virulence of group A streptococci by streptokinase-dependent and streptokinase-independent mechanisms. J Infect Dis 188 : 497–505.
11. WalkerMJ, HollandsA, Sanderson-SmithML, ColeJN, KirkJK, et al. (2007) DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med 13 : 981–985.
12. PlowEF, HerrenT, RedlitzA, MilesLA, Hoover-PlowJL (1995) The cell biology of the plasminogen system. Faseb J 9 : 939–945.
13. WimanB, CollenD (1978) On the kinetics of the reaction between human antiplasmin and plasmin. Eur J Biochem 84 : 573–578.
14. MarcumJA, KlineDL (1983) Species specificity of streptokinase. Comp Biochem Physiol B 75 : 389–394.
15. KobayashiH, SchmittM, GoretzkiL, ChucholowskiN, CalveteJ, et al. (1991) Cathepsin B efficiently activates the soluble and the tumor cell receptor-bound form of the proenzyme urokinase-type plasminogen activator (Pro-uPA). J Biol Chem 266 : 5147–5152.
16. OrgelD, SchroderW, Hecker-KiaA, WeithmannKU, KolkenbrockH, et al. (1998) The cleavage of pro-urokinase type plasminogen activator by stromelysin-1. Clin Chem Lab Med 36 : 697–702.
17. CroucherDR, SaundersDN, LobovS, RansonM (2008) Revisiting the biological roles of PAI2 (SERPINB2) in cancer. Nat Rev Cancer 8 : 535–545.
18. EllisV, ScullyMF, KakkarVV (1989) Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. Potentiation by U937 monocytes. J Biol Chem 264 : 2185–2188.
19. RomerJ, LundLR, EriksenJ, RalfkiaerE, ZehebR, et al. (1991) Differential expression of urokinase-type plasminogen activator and its type-1 inhibitor during healing of mouse skin wounds. J Invest Dermatol 97 : 803–811.
20. BeyrichC, LofflerJ, KobsarA, SpeerCP, KneitzS, et al. (2011) Infection of human coronary artery endothelial cells by group B streptococcus contributes to dysregulation of apoptosis, hemostasis, and innate immune responses. Mediators Inflamm 2011 : 971502.
21. WinklerF, KastenbauerS, KoedelU, PfisterHW (2002) Role of the urokinase plasminogen activator system in patients with bacterial meningitis. Neurology 59 : 1350–1355.
22. AzizRK, KotbM (2008) Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg Infect Dis 14 : 1511–1517.
23. EllisV, ScullyMF, KakkarVV (1987) Plasminogen activation by single-chain urokinase in functional isolation. A kinetic study. J Biol Chem 262 : 14998–15003.
24. HusainSS (1991) Single-chain urokinase-type plasminogen activator does not possess measurable intrinsic amidolytic or plasminogen activator activities. Biochemistry 30 : 5797–5805.
25. StillfriedGE, SaundersDN, RansonM (2007) Plasminogen binding and activation at the breast cancer cell surface: the integral role of urokinase activity. Breast Cancer Res 9: R14.
26. LottenbergR, BroderCC, BoyleMD (1987) Identification of a specific receptor for plasmin on a group A streptococcus. Infect Immun 55 : 1914–1918.
27. BuggeTH, FlickMJ, DantonMJ, DaughertyCC, RomerJ, et al. (1996) Urokinase-type plasminogen activator is effective in fibrin clearance in the absence of its receptor or tissue-type plasminogen activator. Proc Natl Acad Sci U S A 93 : 5899–5904.
28. CarmelietP, SchoonjansL, KieckensL, ReamB, DegenJ, et al. (1994) Physiological consequences of loss of plasminogen activator gene function in mice. Nature 368 : 419–424.
29. JogiA, RonoB, LundIK, NielsenBS, PlougM, et al. (2010) Neutralisation of uPA with a monoclonal antibody reduces plasmin formation and delays skin wound healing in tPA-deficient mice. PLoS One 5: e12746.
30. Grondahl-HansenJ, AgerlinN, Munkholm-LarsenP, BachF, NielsenLS, et al. (1988) Sensitive and specific enzyme-linked immunosorbent assay for urokinase-type plasminogen activator and its application to plasma from patients with breast cancer. J Lab Clin Med 111 : 42–51.
31. PlesnerT, PlougM, EllisV, RonneE, Hoyer-HansenG, et al. (1994) The receptor for urokinase-type plasminogen activator and urokinase is translocated from two distinct intracellular compartments to the plasma membrane on stimulation of human neutrophils. Blood 83 : 808–815.
32. RijkenDC, LijnenHR (2009) New insights into the molecular mechanisms of the fibrinolytic system. J Thromb Haemost 7 : 4–13.
33. BaldiA, PecoriniC, RebucciR, SacconeF, CheliF, et al. (2012) Effect of Escherichia coli lipopolysaccharide on u-PA activity and u-PA and u-PAR RNA expression in a bovine mammary epithelial cell line. Res Vet Sci 93 : 758–762.
34. AshbaughCD, WesselsMR (2001) Absence of a cysteine protease effect on bacterial virulence in two murine models of human invasive group A streptococcal infection. Infect Immun 69 : 6683–6688.
35. ZimmermanM, QuigleyJP, AsheB, DornC, GoldfarbR, et al. (1978) Direct fluorescent assay of urokinase and plasminogen activators of normal and malignant cells: kinetics and inhibitor profiles. Proc Natl Acad Sci U S A 75 : 750–753.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- It's No Fluke: The Planarian as a Model for Understanding Schistosomes
- Stress Relief during Host Infection: The Phage Shock Protein Response Supports Bacterial Virulence in Various Ways
- A Key Role for the Urokinase Plasminogen Activator (uPA) in Invasive Group A Streptococcal Infection
- Blocking TLR7- and TLR9-mediated IFN-α Production by Plasmacytoid Dendritic Cells Does Not Diminish Immune Activation in Early SIV Infection
- Enterovirus 71 Binding to PSGL-1 on Leukocytes: VP1-145 Acts as a Molecular Switch to Control Receptor Interaction
- A Multi-targeted Drug Candidate with Dual Anti-HIV and Anti-HSV Activity
- Pertussis: Challenges Today and for the Future
- Emerging Infectious Diseases: Threats to Human Health and Global Stability
- Signalling C-Type Lectins in Antimicrobial Immunity
- : An Emerging Infectious Threat in Transfusion Medicine
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Pertussis: Challenges Today and for the Future
- Emerging Infectious Diseases: Threats to Human Health and Global Stability
- It's No Fluke: The Planarian as a Model for Understanding Schistosomes
- A Multi-targeted Drug Candidate with Dual Anti-HIV and Anti-HSV Activity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



