-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaEpigenetic Regulation of a Murine Retrotransposon by a Dual Histone Modification Mark
Large fractions of eukaryotic genomes contain repetitive sequences of which the vast majority is derived from transposable elements (TEs). In order to inactivate those potentially harmful elements, host organisms silence TEs via methylation of transposon DNA and packaging into chromatin associated with repressive histone marks. The contribution of individual histone modifications in this process is not completely resolved. Therefore, we aimed to define the role of reversible histone acetylation, a modification commonly associated with transcriptional activity, in transcriptional regulation of murine TEs. We surveyed histone acetylation patterns and expression levels of ten different murine TEs in mouse fibroblasts with altered histone acetylation levels, which was achieved via chemical HDAC inhibition with trichostatin A (TSA), or genetic inactivation of the major deacetylase HDAC1. We found that one LTR retrotransposon family encompassing virus-like 30S elements (VL30) showed significant histone H3 hyperacetylation and strong transcriptional activation in response to TSA treatment. Analysis of VL30 transcripts revealed that increased VL30 transcription is due to enhanced expression of a limited number of genomic elements, with one locus being particularly responsive to HDAC inhibition. Importantly, transcriptional induction of VL30 was entirely dependent on the activation of MAP kinase pathways, resulting in serine 10 phosphorylation at histone H3. Stimulation of MAP kinase cascades together with HDAC inhibition led to simultaneous phosphorylation and acetylation (phosphoacetylation) of histone H3 at the VL30 regulatory region. The presence of the phosphoacetylation mark at VL30 LTRs was linked with full transcriptional activation of the mobile element. Our data indicate that the activity of different TEs is controlled by distinct chromatin modifications. We show that activation of a specific mobile element is linked to a dual epigenetic mark and propose a model whereby phosphoacetylation of histone H3 is crucial for full transcriptional activation of VL30 elements.
Published in the journal: . PLoS Genet 6(4): e32767. doi:10.1371/journal.pgen.1000927
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000927Summary
Large fractions of eukaryotic genomes contain repetitive sequences of which the vast majority is derived from transposable elements (TEs). In order to inactivate those potentially harmful elements, host organisms silence TEs via methylation of transposon DNA and packaging into chromatin associated with repressive histone marks. The contribution of individual histone modifications in this process is not completely resolved. Therefore, we aimed to define the role of reversible histone acetylation, a modification commonly associated with transcriptional activity, in transcriptional regulation of murine TEs. We surveyed histone acetylation patterns and expression levels of ten different murine TEs in mouse fibroblasts with altered histone acetylation levels, which was achieved via chemical HDAC inhibition with trichostatin A (TSA), or genetic inactivation of the major deacetylase HDAC1. We found that one LTR retrotransposon family encompassing virus-like 30S elements (VL30) showed significant histone H3 hyperacetylation and strong transcriptional activation in response to TSA treatment. Analysis of VL30 transcripts revealed that increased VL30 transcription is due to enhanced expression of a limited number of genomic elements, with one locus being particularly responsive to HDAC inhibition. Importantly, transcriptional induction of VL30 was entirely dependent on the activation of MAP kinase pathways, resulting in serine 10 phosphorylation at histone H3. Stimulation of MAP kinase cascades together with HDAC inhibition led to simultaneous phosphorylation and acetylation (phosphoacetylation) of histone H3 at the VL30 regulatory region. The presence of the phosphoacetylation mark at VL30 LTRs was linked with full transcriptional activation of the mobile element. Our data indicate that the activity of different TEs is controlled by distinct chromatin modifications. We show that activation of a specific mobile element is linked to a dual epigenetic mark and propose a model whereby phosphoacetylation of histone H3 is crucial for full transcriptional activation of VL30 elements.
Introduction
Our present view on transcriptional regulation has substantially advanced in recent decades. The classical model, that the presence of promoter sequences and the availability of transcription factors determine the expression status of corresponding genes, has been extended to a model, in which the accessibility of the DNA is central to transcriptional control. In eukaryotes, DNA is packed and compacted into chromatin with the nucleosome consisting of DNA and histone proteins as the basic unit. The degree of compaction – either into inaccessible heterochromatin or open euchromatin – has major implications for the transcriptional potential of associated DNA. A way to regulate chromatin accessibility is the posttranslational chemical modification of histone proteins. It can alter chromatin structure and switch genes from a transcriptional repressed to an active state and vice versa [1].
The best-studied histone modification is the acetylation of N-terminal lysine residues, which correlates with open chromatin and active gene transcription. In contrast, histone deacetylation is linked to gene silencing and histone deacetylases (HDACs) are considered as transcriptional co-repressors [2]. Only recently, a more general role of histone deacetylation during transcriptional regulation is emerging [3]. The use of chemical HDAC inhibitors and knock out/down technologies resulted in the identification of an increasing number of target genes controlled by histone acetylation in different cellular contexts [4]–[6]. Based on sequence homology, mammalian HDACs have been divided into four classes, of which class I enzymes seem to fulfil more basic cellular functions whereas class II enzymes are thought to play specialised cell-type specific roles. HDACs are often found to interact with proteins important for stable gene silencing via DNA methylation and constitution of heterochromatin, but also with transcriptional regulators mediating only transient repression. Crosstalk between histone acetylation and other epigenetic marks is an important feature of HDAC function [7]. Hence, HDACs are central components of multiple silencing complexes containing additional enzymatic activities such as DNA and histone methylation.
Recent annotation of multiple complete genomes has revealed that a large fraction of eukaryotic genomes consists of repetitive sequences, mainly derived from transposable elements (TEs) [8], [9]. Most of those sequences are remnants of once active TEs now incapable of transposition because of their host-mediated inactivation followed by subsequent functional erosion and the accumulation of mutations and deletions. However, some elements remain intact and constitute a constant threat to the integrity of the host genome. Potentially functional elements can act as insertion-mutagens via targeting protein coding genes or causing chromosome breakage. Even transpositionally inactive TEs have the potential to trigger illegitimate recombination and genome rearrangement, or to influence neighbouring genes by causing alternative splicing, premature termination, and modulating gene expression patterns [10], [11]. Recent estimations suggest that TEs provoke around 0.1% (in humans) or 10–12% (in mice) of all spontaneous germ line mutations, and an increasing number of reports document the contribution of somatic transposon activity to pathological situations [11]–[13]. Consequently, organisms were challenged to evolve multiple lines of defence to restrict TE activity, targeting crucial steps in their life cycle [11], [14]. One of the most efficient host-directed strategies is to block TE transcription. This is achieved applying epigenetic mechanisms such as DNA methylation, modification of chromatin structure, and the action of small RNAs [13], [15]–[19]. Different subclasses of small RNAs (siRNAs, rasiRNAs and piRNAs) seem to guide and target the silencing machinery, while chromatin modifiers and DNA methyltransferases (DNMTs) accomplish the transformation into a heterochromatic, transcriptionally silent state [20]. Numerous proteins, involved in the establishment, maintenance and read-out of DNA methylation and chromatin modification patterns, are crucial for TE silencing in germline and somatic cells [13]. However, the exact contribution of DNA methylation, chromatin remodelling, modification of histones and their interplay has not been completely resolved. Analysis in several model organisms revealed that the different silencing-mechanisms are of varying importance depending on the host organism. Some (e.g. yeast and Drosophila) lack an efficient DNA methylation machinery and therefore depend on alternative silencing pathways, while others (e.g. mammals and plants) widely use DNA methylation in concert with other mechanisms to silence TEs [18], [21].
Despite the tight collaboration of multiple silencing layers, numerous TEs maintain their capacity to escape host-mediated silencing under certain conditions. Different types of internal and external stimuli are able to trigger TE activity in a wide range of organisms. Many of those stimuli can be subsumed as cellular stress and the correlation between TE activity and stress has been in the focus of interest for several decades [22], [23]. Recent examples of mammalian TEs, showing increased transcriptional activity upon external stimuli, include members of all major TE orders: SINEs [24], [25], LINEs [26], and LTR elements [27].
To better understand the role of histone acetylation in transcriptional regulation of TEs in mammalian somatic cells, we monitored the expression of selected, potentially active TEs in mouse fibroblasts with impaired histone deacetylase capacity. In our present study we found that chemical inhibition of class I and II HDACs, but not genetic inactivation of HDAC1 alone, resulted in local hyperacetylation and expression of a defined subset of VL30 LTR retrotransposable elements. Furthermore, we show that not only histone acetylation, but histone phosphorylation in concert with acetylation (phosphoacetylation) was triggering transcriptional induction of VL30 elements. We suggest a model, in which external stress signals cause the activation of MAP kinase pathways, ultimately leading to the phosphoacetylation of histones and efficient transcription of VL30 elements.
Results
To assess the epigenetic control of TEs with respect to histone acetylation, we treated mouse 3T3 fibroblasts with the deacetylase inhibitor trichostatin A (TSA), a compound efficiently inhibiting class I and II HDACs and the DNA methyltransferase inhibitor 5′-Aza-2′-deoxycytidine (Aza-dC). Pilot studies had shown that loss of the class I deacetylase HDAC1 in fibroblasts resulted in significantly reduced total cellular HDAC activity, suggesting a prominent role of this enzyme in histone acetylation in this cell type (data not shown). Therefore we also included immortalised HDAC1-deficient fibroblasts (HDAC1−/−) in this study.
Effects of HDAC1 depletion, TSA, and Aza-dC treatment on mouse fibroblasts
To confirm that our experimental setup indeed perturbed epigenetic states in mouse fibroblasts, we performed the following experiments: firstly, we monitored the expression levels of proteins targeted by chemical or genetic inhibition. Protein expression levels of the class I deacetylases HDAC1, HDAC2, HDAC3 and the maintenance DNA methyltransferase DNMT1 were surveyed via Western blot. As shown in Figure 1A, loss of HDAC1 in immortalized fibroblasts led to upregulation of HDAC2, its closest homologue. This compensatory mechanism may buffer some effects caused by the loss of HDAC1, but still total cellular HDAC activity was diminished by about 30% in HDAC1−/− compared to HDAC1+/+ cell lines (data not shown), demonstrating an important enzymatic function of HDAC1 in fibroblasts. In our experimental setup TSA treatment for 24h did not influence expression of HDAC1, HDAC2 or HDAC3, whereas DNMT1 levels were slightly decreased. A reduction of DNMT1 levels after histone deacetylase inhibitor treatment has been previously reported [28], [29]. Aza-dC treatment resulted in slightly reduced amounts of DNMT1, consistent with earlier reports showing that Aza-dC can trap DNMT1 by covalently linking the enzyme to DNA [30] or lead to proteasomal degradation of the DNMT1 protein [31]. Protein expression of HDAC1, HDAC2 and HDAC3 remained unaffected.
Fig. 1. Effects of HDAC1 depletion, TSA treatment, and Aza-dC treatment on DNA–methylation and histone acetylation patterns in mouse fibroblasts. 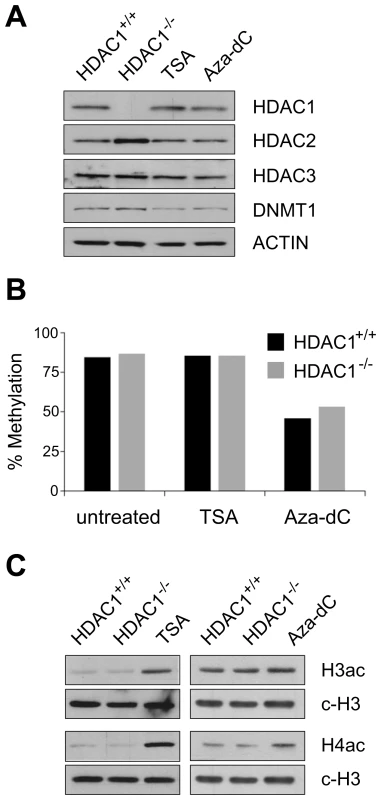
Mouse wildtype fibroblasts were left untreated (HDAC1+/+) or treated with TSA (166nM; 24h for (A) and (B); 3h for (C)) or Aza-dC (1µM for 24h; 24h recovery). In addition untreated HDAC1 deficient fibroblasts (HDAC1−/−) were included in these experiments. (A) Western blot analysis of chromatin modifying enzymes. Proteins were extracted, separated by SDS-PAGE and analysed with antibodies specific for HDAC1, HDAC2 and DNMT1. Equal loading was controlled with an antibody raised against β-ACTIN. (B) DNA methylation of IAP retrotransposons in HDAC1+/+ (black bars) and HDAC1−/− (grey bars) fibroblasts. Both cell lines were treated with TSA or Aza-dC as described above. Average DNA methylation levels of three CpG sites within the LTR were determined employing the Ms-SNuPE technique. (C) Analysis of histone acetylation patterns. Histones were extracted and analysed on Western blots using H3ac and H4ac specific antibodies. Equal loading was monitored with an antibody recognising the C-terminus of histone H3 (c-H3). Next, we evaluated effects on the biological targets of HDACs and DNMT1, i.e. the acetylation states of histones and CpG methylation levels of DNA. To examine CpG methylation levels at defined targets we employed the MS-SNuPE technique [32]. We quantified DNA methylation levels of three CpG sites within the regulatory LTR region of an IAP retrotransposons, highly methylated in somatic cells [33]. Whereas neither loss of HDAC1, nor chemical inhibition of HDACs with TSA for 24h affected levels of DNA methylation, Aza-dC treatment for 24h severely reduced DNA methylation of the IAP LTR region to 50% (Figure 1B).
Furthermore, we analysed histone acetylation levels in wildtype fibroblasts, either untreated (HDAC1+/+) or treated with TSA and Aza-dC and HDAC1-deficient cells (HDAC1−/−). To that end we extracted histones and performed Western blot analyses using antibodies recognising acetylated histones. Previous reports had shown that in murine embryonic stem cells genetic inactivation of Hdac1 was accompanied by hyperacetylation of histones H3 and H4, detectable with antibodies recognising acetylated histone H3 (H3ac) and acetylated histone H4 proteins (H4ac) [34]. In our fibroblast system, however, we failed to detect global hyperacetylation of histones in HDAC1−/− cells as compared to HDAC1+/+ cells using such H3ac or H4ac antibodies (Figure 1C); only when we probed the blots with antibodies specifically recognising the acetylation state of individual lysine residues, we detected weak hyperacetylation occurring at defined residues (data not shown). In contrast to this limited site-specific response, inhibition of all class I and II HDACs with TSA led to a robust and global hyperacetylation detectable with H3ac and H4ac antibodies. Finally, interfering with DNA methylation by Aza-dC treatment moderately enhanced global H3 and H4 acetylation levels (Figure 1C).
Histone acetylation of transposon-associated chromatin
After defining global effects of HDAC and DNMT inhibition in our cell system, we moved on to specifically address the role of histone acetylation in the regulation of TEs. We analysed the acetylation state of chromatin associated with defined TEs in wildtype fibroblasts without treatment (HDAC1+/+), or treated either with TSA or Aza-dC, and HDAC1-deficient fibroblasts (HDAC1−/−). To monitor their acetylation status, we performed chromatin-immunoprecipitation (ChIP) experiments with H3ac and H4ac antibodies. Furthermore, we included antibodies recognising the C-terminal part of histone H3 to survey nucleosome occupancy and unspecific rabbit IgGs to control the specificity of immunoprecipitation reactions. Quantitative Real-Time PCR analyses allowed the quantification of H3 and H4 acetylation levels associated with a representative selection of ten murine TEs, including a DNA transposon (Tigger), non-LTR retrotransposons (LINEs, SINEs), and LTR retrotransposons (VL30, IAP, ETn, MERVL, MT, ORR1) (Table 1). We chose LINEs and SINEs, because they constitute the majority of murine TEs and populate the genome in more than 660,000 and 1.5 million copies, respectively. LTR elements are less abundant, but harbour the most active TEs within the mouse lineage; MERVL, MT and ORR1 elements are expressed during early embryogenesis [35] and VL30, IAP and ETn elements have been responsible for a number of germ line transposition events [12]. The transposon Tigger was included as representative of the class of DNA elements, which is functionally extinct in vertebrates. As shown in Figure 2A (upper panel), loss of HDAC1 did not influence histone H3 acetylation at any transposon type analysed as compared to wildtype cells. In contrast, TSA treatment was sufficient to specifically enhance H3 acetylation at SINE B1 and B2 elements and VL30 LTR elements, the latter ones near the promoter as well as throughout the body of the element. Histone H4 acetylation of TE-associated chromatin was analysed similarly in a subsequent series of experiments. Again, loss of HDAC1 did not alter histone acetylation at tested transposons, but TSA-induced inhibition of all class I and II HDACs led to increased H4 acetylation at all repeat families investigated (Figure 2A; lower panel). Thus, TSA treatment exhibited differential effects on histone H3 and histone H4 acetylation of TEs. Whereas H3 histones were found hyperacetylated at specific elements only, H4 hyperacetylation occurred as a global response. Interestingly, the elements hyperacetylated at H3 upon TSA treatment, SINEs and VL30, also showed highest enrichment of H4 acetylation compared to other TEs.
Fig. 2. Histone acetylation states of transposable elements upon HDAC1 depletion, TSA treatment, and Aza-dC treatment. 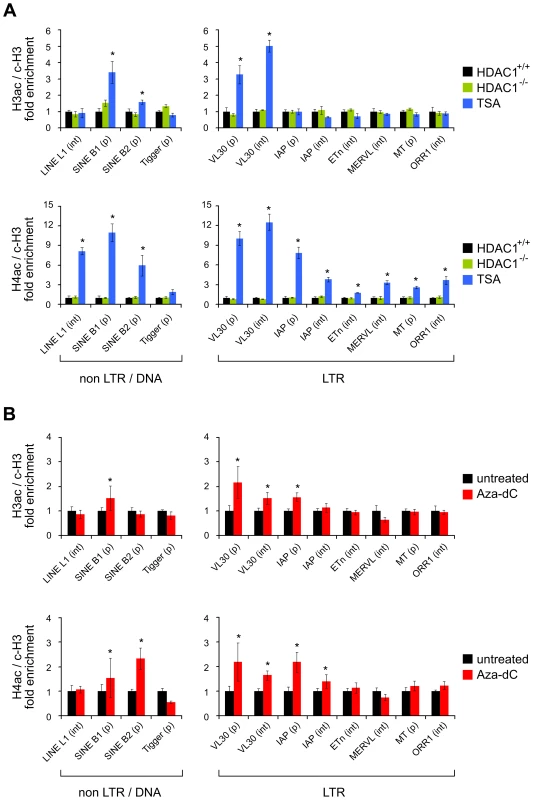
The histone acetylation state of chromatin associated with defined transposable elements was determined via ChIP technique using antibodies specifically recognising acetylated histone H3 (H3ac) and acetylated histone H4 (H4ac). Values were normalised to the nucleosome density as determined via ChIP using antibodies recognising the C-terminal region of histone H3 (c-H3) and are given relative to the acetylation levels in untreated fibroblasts. Control experiments employing unspecific IgG antibodies consistently led to negligible amounts of precipitated material (data not shown). (A) Histone H3 and H4 acetylation levels of a variety of transposable elements in HDAC1−/− (green bars) and TSA (166nM; 24h; blue bars) treated fibroblasts as compared to untreated HDAC1+/+ (black bars) fibroblasts. (p) promoter region ±500bp of transcriptional start site; (int) internal region. N = 3; ± SEM *p<0.05 (paired, one sided student's t-test). (B) Corresponding acetylation levels of transposable elements in Azd-dC treated fibroblasts (1µM; 24h, 24h recovery; red bars). N = 5; ± SEM *p<0.05. Tab. 1. Transposable elements analysed in this study. 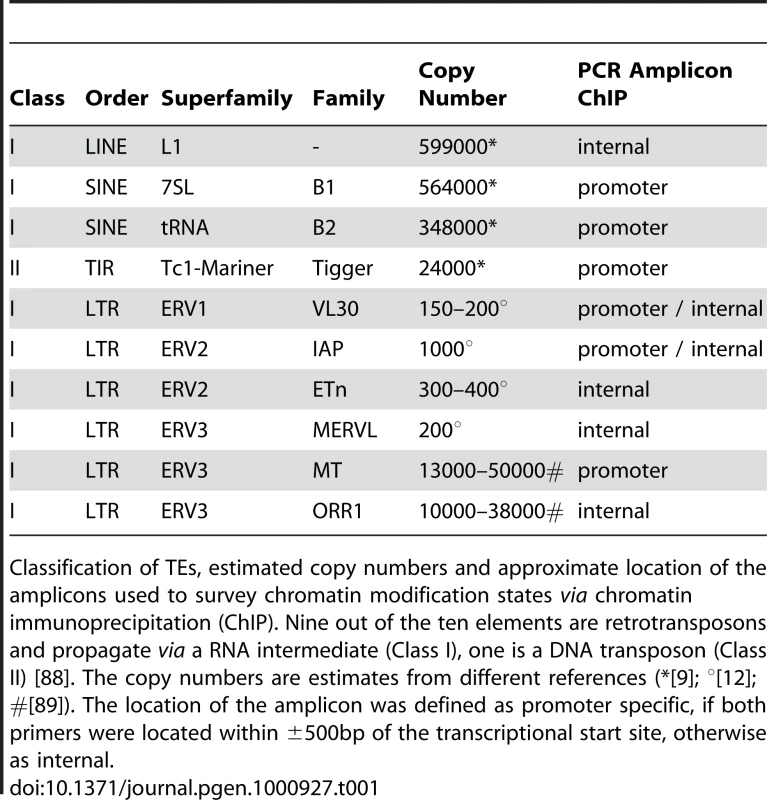
Classification of TEs, estimated copy numbers and approximate location of the amplicons used to survey chromatin modification states via chromatin immunoprecipitation (ChIP). Nine out of the ten elements are retrotransposons and propagate via a RNA intermediate (Class I), one is a DNA transposon (Class II) [88]. The copy numbers are estimates from different references (*[9]; °[12]; #[89]). The location of the amplicon was defined as promoter specific, if both primers were located within ±500bp of the transcriptional start site, otherwise as internal. Next, we analysed histone acetylation levels upon loss of DNA methylation. Incubation of cells with Aza-dC increased H3 acetylation at SINE B1 and VL30 elements (Figure 2B, upper panel), although to a lesser extent than TSA. Furthermore, IAP element promoter sequences exhibited slightly increased H3 acetylation levels in response to Aza-dC. Finally, Aza-dC treatment resulted in elevated H4 acetylation levels of SINEs, VL30 and IAP elements (Figure 2B; lower panel).
Transcriptional activation of transposable elements
Since hyperacetylation of histones in general correlates with transcriptional activation, we tested whether increased histone acetylation levels were mirrored by changes in transcriptional activity of TEs. Therefore we monitored mRNA levels of the ten mouse transposons, listed in Table 1, in untreated wildtype fibroblasts (HDAC1+/+), TSA or Aza-dC treated wildtype cells, and HDAC1-deficient fibroblasts (HDAC1−/−) by real-time PCR analyses. We also analysed the expression of another LTR element, MuLV, which comprises endogenous as well as exogenous family members [36] (Figure S1). As shown in Figure 3, upper panel and Figure S1, loss of HDAC1 did not influence TE expression. In contrast, general HDAC inhibition via TSA strongly increased VL30 element expression in this fibroblast cell line (Figure 3, upper panel), as well as in other commonly used fibroblast cell lines such as Swiss 3T3 and NIH/3T3 (data not shown). Enhanced activity of VL30 elements correlated with hyperacetylation of VL30 chromatin upon TSA treatment. This suggests that modulation of histone acetylation regulates VL30 element activity. Expression of SINE B1, the second element showing H3 and H4 hyperacetylation after TSA treatment, was modestly increased. Besides IAPs showing a moderate two-fold increase, all other TE candidates investigated did not exhibit major changes in transcriptional activity. This expression pattern mirrors the unchanged local acetylation state of histone H3, but not the one of histone H4 acetylation, which was elevated upon TSA treatment. Therefore, H3 acetylation of the transposons analysed correlates with transcriptional activity, whereas H4 hyperacetylation is not sufficient to trigger transcriptional activation.
Fig. 3. Effect of TSA and Aza-dC treatment on the expression of transposable elements. 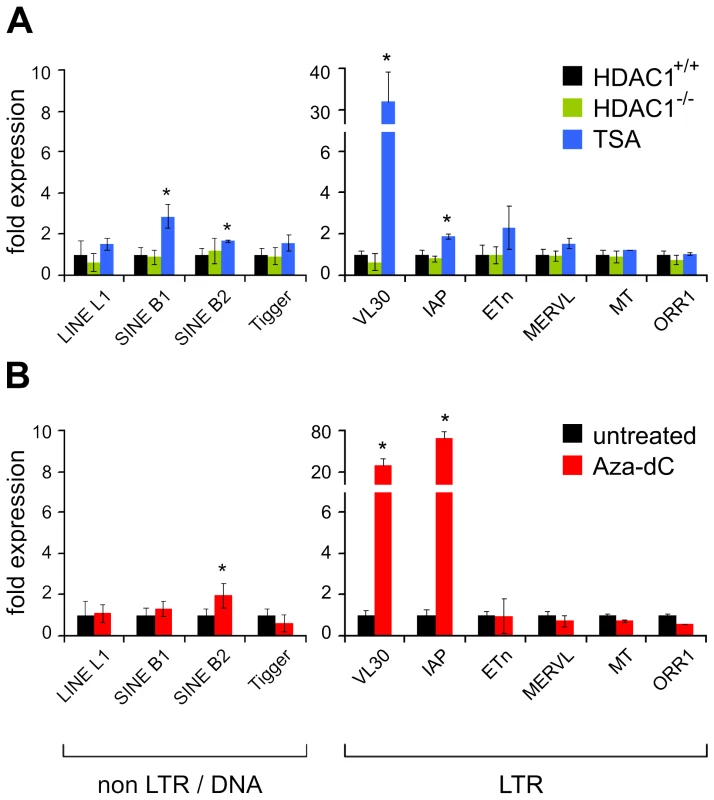
(A) Logarithmically growing fibroblasts show increased mRNA expression levels of VL30 LTR elements when treated with TSA. Expression levels were determined with qRT-PCR. Values are normalised to HPRT expression and are shown relative to the expression in untreated HDAC1+/+ fibroblasts. Treatment as in Figure 2. N = 3; ± SD *p<0.05. (B) Aza-dC causes high level induction of VL30 and IAP LTR element expression. N = 3; ± SD *p<0.05. The role of DNA methylation in transposon silencing is well established and it has been shown earlier that loss of DNA methylation in somatic cells correlates with VL30, IAP and MuLV expression [37]–[39]. Indeed, Aza-dC treatment in fibroblasts caused highly elevated VL30, IAP and MuLV mRNA levels, but had no effect on other TEs tested (Figure 3, lower panel and Figure S1). However, it was surprising that the majority of murine elements tested could not be reactivated with Aza-dC. This finding suggests that those transposons are not permissive for transcriptional activation in our fibroblast system, either because they lack regulatory features necessary for efficient transcriptional activation or additional mechanisms contribute to their silencing [40]. ChIP analyses of TE-associated chromatin showed that two elements that were highly expressed upon Aza-dC treatment (VL30 and IAP elements) also gained hyperacetylation after DNMT inhibition (Figure 3, lower panel). SINE B1 and SINE B2 elements, both showing increased acetylation upon Aza-dC treatment, were not or only modestly activated.
In summary, we could establish a correlation between the transcriptional activity of TEs and the acetylation state of associated chromatin. In particular histone H3 acetylation correlated well with increased expression of certain TEs. Furthermore, we identified VL30 elements as the single candidate transposon efficiently regulated via acetylation of associated chromatin.
Transposition frequency
Retrotransposition is a complex process depending on multiple steps, each of which can be targeted by regulatory mechanisms [11]. Alterations in RNA stability, translation efficiency, nuclear shuttling, editing of template RNA, or integration efficiency can have severe impact on the rate of successful transposition.
Treatment of cells with the HDAC inhibitor TSA has strong effects on the chromatin state. Since chromatin constitutes the substrate for the last step of transposition, i.e. integration, we tested whether histone modification patterns might influence transposition efficiency, independent of TE transcription. Genetic screens in yeast have uncovered numerous host factors either restricting or enhancing transposon activity [41]–[45]. Strikingly, a substantial fraction of the proteins identified has a role in chromatin biology; for instance, SIN3 and RPD3, both major components of yeast HDAC complexes, were found to restrict Ty1 activity and modulate target site selection. Interestingly, SIN3 and RPD3 do not act on the transcriptional level, as they do not alter Ty1 mRNA levels [45].
To test whether changed histone acetylation levels induced by TSA treatment might have similar effects in mammalian cells, we determined transposition levels of four mammalian retroelements in untreated and TSA treated cells. To this end we took advantage of an experimental system described earlier [46]–[48], in which episomally delivered retrotransposons are marked with a neo-transgene, which gets activated only after its successful retrotransposition (Figure S2A). Following this approach we quantified the transposition efficiency of murine IAP and MusD LTR retrotransposons, and murine and human L1 elements in untreated and TSA treated cells. In order to avoid potential interference with endogenously transcribed IAP, MusD and L1 elements, we performed the experiments in the human U2OS osteosarcoma cell line. In addition, we monitored transcript levels of episomally delivered retroelements via real-time PCR (Figure S2B). As presented in Figure S2C, TSA treatment led to a 2 - to 4-fold increase in transposition rates, a moderate increase compared to findings reported elsewhere, where mutation of TE restricting factors in yeast led to an increase between 5 and several hundred times [45]. In our system, enhanced transposition was accompanied by a 2 - to 3-fold increase in TE mRNA abundance. These data suggest that TSA treatment led to enhanced expression of marked elements, which in turn resulted in increased transposition rates. Therefore, under our experimental conditions, inhibition of deacetylases by TSA was not sufficient to influence TE transposition rates independent of TE expression, suggesting transcription as the rate-limiting factor for transposition.
Chromosomal origin and structural features of reactivated transposable elements
In contrast to single copy genes, TEs exist in multiple copies dispersed throughout the genome. Individual elements within a TE family are different in respect of genomic environment, influencing chromatin status and transcriptional potential. Furthermore, transposons are under high selective pressure and mutations mediated by host and/or transposon-intrinsic factors lead to the generation of pools of elements with individual sequence specificities within given TE families. Therefore, a family of elements is a collection of similar, but heterogeneous individuals, differing in external and internal features.
Upon external signals increased transcriptional activity could originate from the upregulation of all TEs within a family, of a subgroup of elements exhibiting specific features, or even a single copy with unique intrinsic properties. In order to distinguish between these different scenarios, we aimed to determine the chromosomal origin of VL30 and IAP derived transcripts detected in untreated, TSA and Aza-dC treated wildtype fibroblasts. RNA from three independent biological samples each (untreated, TSA, Aza-dC) was collected, reversely transcribed, and subjected to PCR amplification. To amplify IAP derived transcripts, primers annealing in the highly conserved gag region were used (Figure S3A). For VL30 transcript amplification primers within the 3′ UTR and 3′LTR R region were chosen (Figure 4A). To exclude a bias due to individual PCR reactions, six independent PCR reactions for each condition were pooled, PCR products were cloned and sequenced. As control, the same procedure was also performed with genomic DNA as template. This approach allowed us to amplify chromosomal copies of both candidate elements and to estimate the diversity of IAP and VL30 elements in our cell system.
Fig. 4. Characterisation of transcribed VL30 elements reveals preferential expression of defined VL30 elements upon stimulation. 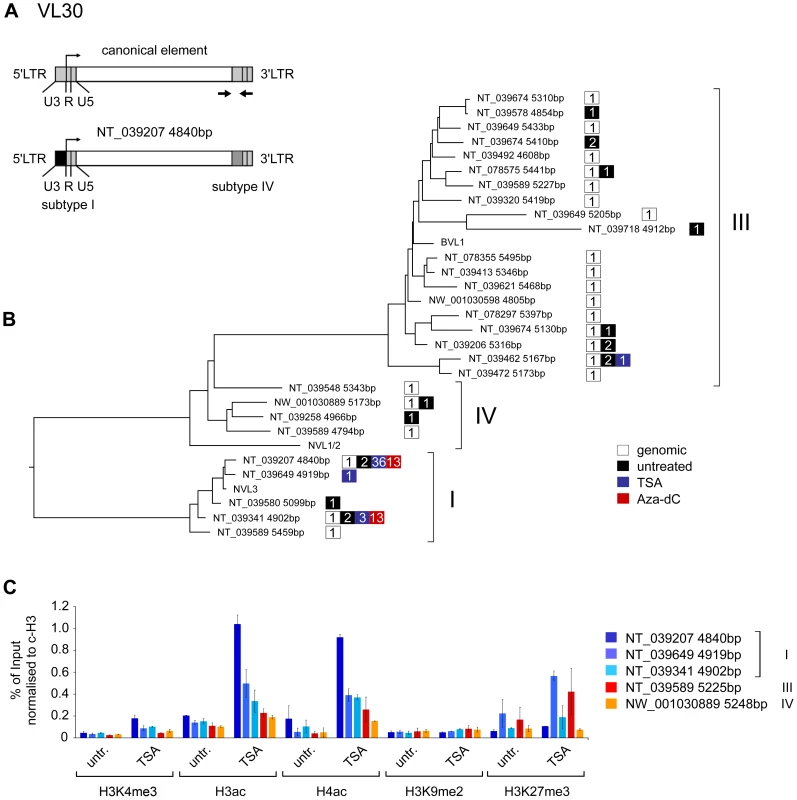
(A) Schematic view of a canonical VL30 retrotransposon. Two similar LTRs flank an internal region without functional open reading frame (upper part). Some elements have a hybrid structure and are flanked by LTRs belonging to different subtypes (lower part). Primers used to amplify fragments of genomic and transcribed VL30 elements from untreated, TSA and Aza-dC treated fibroblasts are indicated as black arrows. (B) To characterise the genomic VL30 elements identified as bona fide transcriptional sources, their 5′LTR sequences were aligned (using the Megalign program with the following settings: Clustal W, gap penalty 15, gap length penalty 6.66, delay divergent seqs 30%, DNA transition weight 0.5, DNA weight matrix IUB) and a phylogenetic tree was generated. The LTR sequences fall into three clades. The affiliation to one of the clades is determined by the structure of the highly polymorphic U3 region. While many VL30 elements identified in untreated fibroblasts are associated with a subtype III U3 region, TSA and Aza-dC treatment leads to preferential expression of elements with a type I U3 region. Coloured squares indicate the source, where the VL30 element has been isolated from (white, genomic DNA; black, untreated fibroblasts; blue, TSA treated fibroblasts (166nM; 24h); red, Aza-dC treated fibroblasts (1µM; 24h; recover 24h), numbers indicate the transcriptional activity of the respective elements (i.e. number of clones mapping best to this genomic locus). NVL3, BVL1, NVL1/2 serve as reference elements with known U3 regions and are described elsewhere [85]–[87]. (C) To survey the chromatin signature of individual VL30 elements, ChIP experiments in logarithmically growing fibroblasts untreated or treated for 12h with TSA (166nM) were performed. H3K4me3, H3ac and H4ac antibodies were used to survey the enrichment of active histone marks, H3K9me2 and K3K27me3 antibodies to measure levels of repressive marks. Values were normalised to local histone occupancy as determined with ChIP experiments using the c-H3 antibody and are given as percentage of Input. Primers targeting the upstream regions and 5′LTRs of individual elements allowed determining histone modification levels of five specific VL30 elements. NT_039207 4840bp is an element highly expressed upon TSA treatment. NT_039649 4919bp and NT_039341 4902bp are two additional elements found expressed in TSA treated fibroblasts. Those three elements are associated with a U3 subtype I LTR region. NT_039589 5224bp and NW_001030889 5248bp are equipped with subtype III and IV U3 regions, respectively. N = 2; ± SD. Each sequenced clone corresponding to an individual transcribed (or genomic) TE was then analysed as follows: to identify genomic elements serving as potential sources of transcription, sequences were blasted against the annotated murine genome (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Genomic sequences exhibiting at least 98% identity to the cloned sequences were considered to show sufficient homology to serve as potential sources of transcription and were analysed further. If more than one genomic sequence showed identity >98%, the sequence with the highest E-value was chosen. That way ∼90% of the cloned sequences were assigned to distinct genomic elements (IAP: 115 of 127 clones; VL30 : 111 of 122 clones). Individual characteristics, such as chromosomal localisation and length were examined for each element as summarised in Table S1. Next, we defined whether the transcribed IAP and VL30 elements were derived from multiple genomic sources, a small number of elements, or even unique elements. Therefore, we analysed the diversity of the identified genomic TEs, constituting bona fide sources of transposon transcription. Following this approach, each IAP transcript could be assigned to a different genomic element, indicating that IAP transcripts originated from multiple loci throughout the genome (see chromosomal origins and individual features of elements listed in Table S1).
In untreated cells, VL30 transcripts were of heterogeneous origin, with a broad level of sequence diversity similar to the chromosomal VL30 elements that were amplified from genomic DNA. In contrast, upon TSA and Aza-dC treatment, VL30 transcripts could be assigned to only four and two genomic loci, pointing towards a limited number of VL30 elements serving as source of expression. TSA treatment led to the accumulation of transcripts related to the genomic loci NT_039207 4840bp on chromosome 2 (36 of 41 sequences), NT_039341 4902bp on chromosome 6 (3 of 41), NT_039462 5167bp on chromosome 8 (1 of 41), and NT_039649 4919bp on chromosome 17 (1 of 41). Aza-dC treatment resulted in transcripts similar to NT_039207 4840bp on chromosome 2 (13 of 26 sequences) and NT_039341 4902bp on chromosome 6 (13 of 26) (Table S1), Alignment of VL30 sequences directly obtained after cloning and sequencing further showed that transcripts assigned to the genomic locus on chromosome 2 (NT_039207 4840bp) were almost identical, indicating a single locus as transcriptional source. In contrast, transcripts assigned to the locus on chromosome 6 (NT_39341 4902bp) were slightly divergent, demonstrating that they originate from a group of related VL30 elements, of which only one copy is annotated in the published mouse genome (data not shown).
The long terminal repeat regions of LTR elements are enriched with regulatory sequences important for transcriptional regulation. Therefore, we determined the structure of the LTR regions associated with expressed IAP and VL30 elements in more detail. First, we analysed the LTRs associated with transcribed IAP elements, which revealed a marked length polymorphism between different LTRs: the shortest LTR, identified with an IAP element expressed in untreated fibroblasts, comprised 322bp, whereas the longest LTR, found in an IAP element expressed after Aza-dC treatment, was 456bp long. Alignment of IAP LTR sequences disclosed that this difference was due to the presence or absence of nucleotides in a highly variable CT-dinucleotide-rich stretch within the R region of the LTR (Table S1 and Figure S3B). The region encoded a varying number of tandem repeat sequences containing a 13bp core sequence (TCTCTCTTGCTTC), previously described as polymorphic marker for different IAP subtypes [49]. Interestingly, the average length of this stretch was significantly longer in LTRs associated with IAPs expressed upon Aza-dC treatment (Figure S3C), indicating that upon loss of DNA methylation an increased number of tandem repeats might be favourable for expression.
As shown in earlier studies the structure of the VL30 LTR region is crucial for stimulus mediated activation of VL30 expression (reviewed in [27]). Based on their highly divergent U3 regions, VL30 LTRs have been classified into four distinct subtypes: I, II, III and IV [50]. To allocate the VL30 elements isolated in our transcript analysis to the four subtypes, we isolated the 5′LTR sequences from the genomic sequences, aligned them together with VL30 LTR reference sequences (NVL3: subclass I, BVL1: subclass III, NVL1/2: subclass IV) and generated a phylogenetic tree. As depicted in Figure 4B, all elements including the references were located in three distinct clades corresponding to subclass I, III and IV elements. Elements expressed in untreated cells mainly comprehend subtype III containing LTRs, which also associate with most genomic elements (Figure 4B and Table S1). As described above, transcripts arising upon TSA and Aza-dC treatment, mainly originate from two types of loci: one VL30 insertion on chromosome 2 (NT_039207 4840bp) and several other loci showing high similarity to another element on chromosome 6 (NT_039341 4902bp). Interestingly, whereas the VL30 element on chromosome 6 is flanked by LTRs containing subtype I U3 regions at both ends, the element located on chromosome 2 exhibits an unusual structural feature: it is associated with two different LTRs, a 5′ LTR containing a U3 region belonging to subtype I, but a 3′ LTR containing a U3 region of subtype IV (Figure 4A, lower part). Transposon transcription depends on the 5′ LTR, therefore we conclude that expression of this VL30 element is controlled by LTR sequences comprising a subtype I U3 region. 40 out of 41 transcripts isolated from TSA treated fibroblasts were associated with a LTR containing a subtype I U3 region, suggesting that this LTR design might be favourable for VL30 expression upon TSA treatment. However, 36 of those 40 were derived from a single genomic source. Therefore we wanted to clarify if increased VL30 transcription upon TSA treatment is exclusively caused by activation of this particular locus mirroring a locus specific phenomenon or if it reflects a general feature of subtype I elements. We surveyed the chromatin signature at the 5′LTRs of five genomic VL30 elements via ChIP using flanking primers: the highly responsive hybrid element on chromosome 2 (NT_039207 4840bp), two other subtype I elements (NT_039649 4919bp, NT_039341 4902bp), one subtype III (NT039589 5225bp) and one subtype IV element (MW_001030889 5248bp) (Figure 4C). Interestingly, we detected increased levels of H3K4me3 (a mark for active promoters) upon TSA treatment at all five VL30 elements, with the highest levels at the hybrid element, followed by the two other subclass I elements (Figure 4C). The subclass III and IV element showed the lowest response. A similar pattern could be observed for H3ac and H4ac. In addition we monitored the repressive histone mark H3K9me2, which was low in all elements and remained unchanged upon TSA treatment. Another repressive mark, H3K27me3 showed variable levels with no clear correlation with VL30 subtypes. Together these data suggest an increased responsiveness to activation of the hybrid element, followed by other subclass I elements and finally subclass III and IV elements.
Regulation of VL30 elements by a dual histone modification mark
Based on the finding that VL30 elements respond to histone acetylation, we examined the potential activation mechanism for VL30 elements in detail. In the recent past VL30 elements have been studied extensively and others showed that VL30 elements expression is highly sensitive to a plethora of internal and external stimuli [27], [39], [51]. Interestingly, many of those stimuli can be described as internal or external stress in the broadest sense, and a considerable number among them is able to trigger signalling cascades such as the ERK or p38 pathways. Signalling via these pathways ultimately leads to phosphorylation and activation of several transcription factors but also to phosphorylation of histone H3.
Phosphorylation of serine 10 at histone H3 (H3S10p) during interphase is a rare event that correlates with the activation of specific target genes [52], [53]. Importantly, the presence of the H3S10ph mark at promoter-associated chromatin coincides with acetylation of neighbouring lysines K9 and K14 leading to a dual histone modification termed phosphoacetylation [54]–[56] (Figure 5A). Histone H3 phosphoacetylation correlates with the activation of the so called “immediate early” genes c-fos and jun-B, as well as late inducible genes such as HDAC1 [57].
Fig. 5. VL30 expression correlates with the formation of a phosphoacetylation mark at chromatin associated with VL30 LTR sequences. 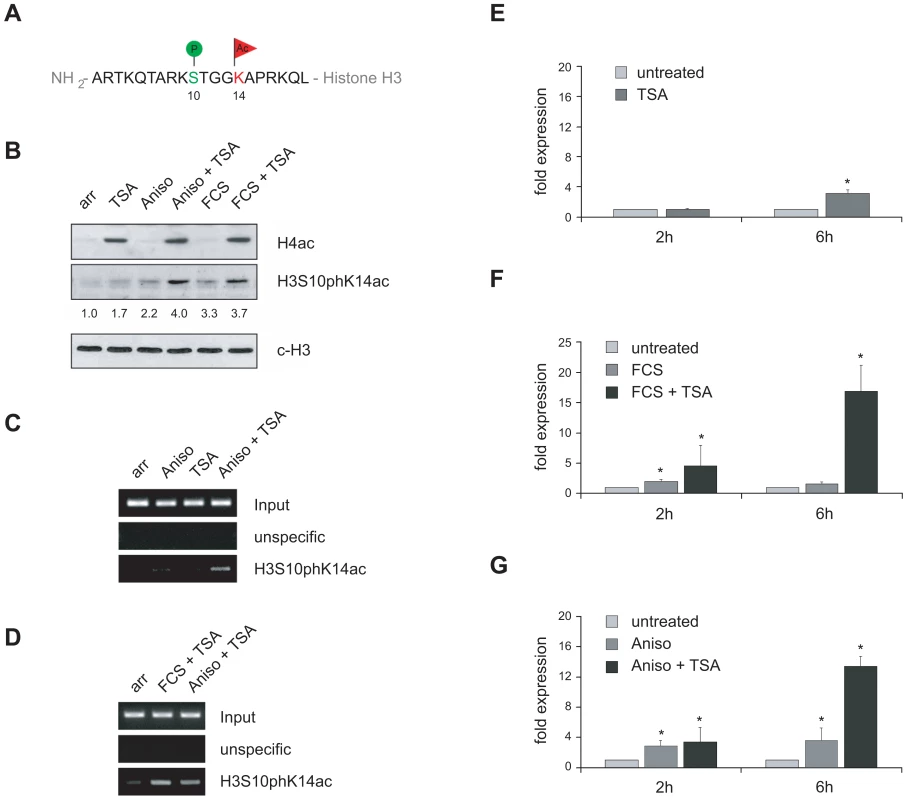
(A) Schematic view of a histone H3 tail carrying a so-called phosphoacetylation mark, i.e. concomitantly phosphorylated serine 10 (H3S10ph) and acetylated lysine 14 (H3K14ac). (B) Phosphoacetylation of histone H3 in response activation of the MAP kinase pathway and TSA treatment in quiescent Swiss 3T3 fibroblasts. Serum deprived Swiss 3T3 fibroblasts were left untreated (arr) or treated for 2 hours with 166nM TSA, 189nM anisomycin (Aniso) or 20% v/v fetal calf serum (FCS) alone or combinations of anisomycin and TSA or FCS and TSA. Western blot analysis was performed with antibodies directed against acetylated histone H4, H3S10pK14ac and an antibody against the C-terminus of histone H3 (c-H3) as loading control. H3S10pK14ac The H3S10pK14ac signals were determined by densitometric scanning, normalised to c-H3 signals and are shown relative to the value of untreated cells taken arbitrarily as 1. (C,D) The LTR region of VL30 elements is associated with histones carrying a phosphoacetylation mark only upon MAP kinase pathway activation together with HDAC inhibition. ChIP experiments using an H3S10pK14ac specific antibody reveals the presence of phosphoacetylated histones at VL30 LTR regions after anisomycin (189nM; 3h) or FCS (20%; 3h) and TSA (166nM; 3h) treatment (Aniso + TSA; FCS + TSA). (E–G) Combinatorial treatment efficiently activates VL30 element expression in quiescent Swiss 3T3 fibroblasts. Single treatment with TSA (166nM), FCS (20%) or anisomycin (189nM) (dark grey bars) leads to a modest induction compared to VL30 mRNA levels in untreated cells (light grey bars). In contrast, combinatorial treatment with anisomycin or FCS and TSA robustly increases VL30 expression after 6h (black bars). VL30 expression levels were determined with qRT-PCR; values are normalised to HPRT levels and given relative to VL30 levels in untreated cells. N = 3; ± SD *p<0.05. The finding that the HDAC inhibitor TSA was sufficient to highly induce VL30 expression in logarithmically growing fibroblasts, where ERK pathways are activated through the action of growth factors, prompted us to ask whether both stimuli, histone acetylation and histone phosphorylation, were required for full VL30 activation. To answer this question, we performed all following experiments with serum-arrested Swiss 3T3 fibroblasts. Serum withdrawal arrests cells in G0 and eliminates mitotic H3 phosphorylation, a modification that targets the majority of histone H3 molecules during mitosis and is associated with chromatin condensation. Furthermore, the absence of the serum-dependent activity of different kinase pathways allows to selectively activate specific signalling cascades, thereby triggering H3 phosphorylation. To induce the ERK pathway in fibroblasts, cells were arrested in G0 for 72h and then treated with foetal calf serum (FCS). Alternatively, anisomycin (Aniso) was used to activate the p38 stress pathway. Additionally cells were treated with TSA to induce hyperacetylation.
To analyse simultaneous phosphorylation and acetylation of histone H3 we used an antibody that recognises histone H3 only in the presence of the dual H3S10phK14ac mark (Figure 5A, Figure S4). Figure 5B shows the effect of different compounds on histone H4 acetylation and histone H3 phosphoacetylation in G0 arrested Swiss 3T3 fibroblasts. As expected, TSA led to the accumulation of hyperacetylated histones as detected by the H4ac antibody; H3 phosphoacetylation was low in untreated and TSA treated G0 cells and slightly induced upon Aniso or FCS treatment. Combined treatment with Aniso/TSA or FCS/TSA led to enhanced phosphoacetylation of histone H3.
It was shown previously that, even upon Aniso/TSA stimulation, the fraction of phosphoacetylated histone H3 is only minute [52], [58] and that the mark is localised to a restricted number of genomic loci in Aniso/TSA treated fibroblasts (Simboeck E., unpublished results). Hence we tested, if phosphoacetylated histones were associated with VL30 elements. To this end we isolated chromatin from G0 arrested fibroblasts that were untreated or treated with TSA, Aniso, or Aniso/TSA. We performed ChIP experiments using the H3S10ph/H3K14ac antibody or an unspecific control antibody and surveyed phosphoacetylation levels of the VL30 5′LTR regulatory region via semi-quantitative PCR. Strikingly, we could detect phosphoacetylated histones at VL30 elements at low levels when cells were treated with Aniso alone, and at higher levels upon Aniso/TSA treatment, but not in untreated or TSA-treated cells (Figure 5C). Additional ChIP experiments with resting cells activated with growth factors, containing serum in combination with TSA (FCS/TSA), demonstrated the presence of phosphoacetylation also upon activation of the ERK pathway, together with induction of hyperacetylation (Figure 5D). In summary, activation of the p38 or ERK pathway together with induction of hyperacetylation resulted in the establishment of a phosphoacetylation mark at VL30 LTRs. In agreement with these findings we detected an increased presence of the H3S10phK14ac mark at individual VL30 insertions in TSA-treated proliferating HDAC1+/+ fibroblasts suggesting a direct link between this dual histone modification and transcriptional induction of particular VL30 elements (Figure S5).
Next, we examined the effect of the different stimuli on VL30 expression. We isolated RNA from arrested 3T3 Swiss fibroblasts exposed to the aforementioned inducers, reversely transcribed them and quantified VL30 levels with Real Time PCR. Whereas single treatment of cells with TSA, Aniso, or FCS led to a modest increase in expression, combined treatment with Aniso/TSA or FCS/TSA resulted in robust induction, suggesting a synergistic effect between histone phosphorylation via p38 or ERK activation and histone acetylation (Figure 5E–5G). To directly test whether activation of the ERK and p38 pathways is linked to H3 phosphoacetylation and VL30 activation, we took advantage of the protein kinase inhibitor H89. This compound was used at a concentration (10µM), shown to efficiently block histone H3 phosphorylation without affecting transcription factor phosphorylation [53]. As presented in Figure 6A, inhibition of kinase activity via H89 abolished the formation of a phosphoacetylation mark upon Aniso/TSA treatment in arrested Swiss 3T3 fibroblasts. Equally important, H89 treatment also prevented transcriptional induction upon Aniso/TSA treatment (Figure 6B), indicating that H3S10p/H3K14ac does not only correlate with transcriptional induction mediated by Aniso/TSA treatment, but is necessary for proper VL30 expression.
Fig. 6. Inhibition of H3S10 phosphorylation with the protein kinase inhibitor H89 precludes transcriptional activation of VL30 elements. 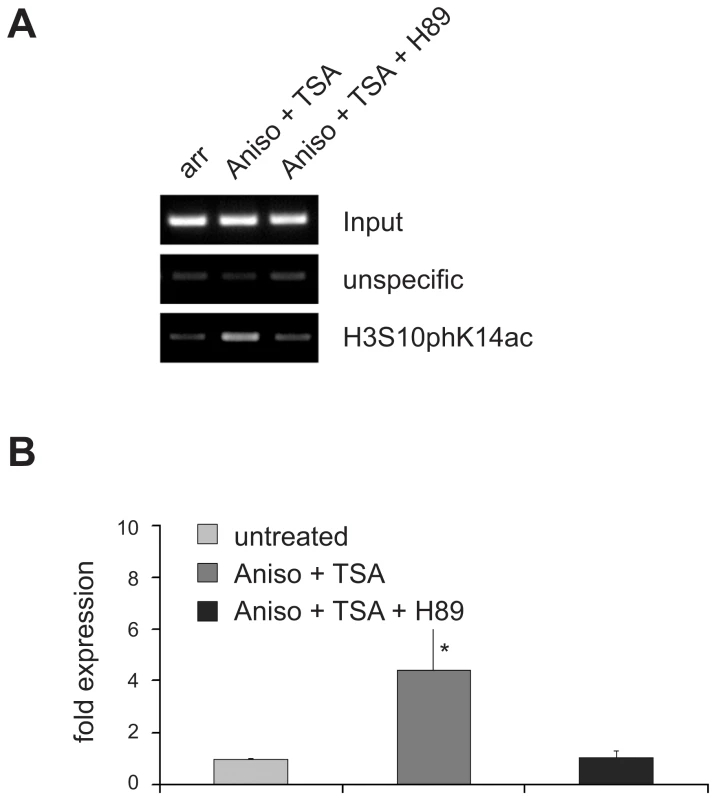
(A) Treatment of arrested Swiss 3T3 fibroblasts with the protein kinase inhibitor H89 (10µM), inhibiting H3S10 phosphorylation, prohibits the creation of a phosphoacetylation mark upon 1h of Aniso+TSA treatment. ChiP experiments were performed as in Figure 5C. (B) Precluding phosphoacetylation of VL30 elements with H89 also abolishes transcriptional induction upon Aniso + TSA treatment. Treatments as in (A); quantification of VL30 transcript levels were determined as in Figure 5E. N = 3; ± SD *p<0.05. Finally, we want to propose the following model for VL30 regulation, which integrates many of the previous findings about VL30 induction: external stimuli activate the p38 (stress) or the ERK (growth signal) pathway leading to activation of effector kinases such as MSK1/2. By local phosphorylation of H3S10 at responsive VL30 elements together with the induction of hyperacetylation, a phosphoacetylation mark is established leading to full transcriptional activation (Figure 7).
Fig. 7. Model of VL30 element activation. 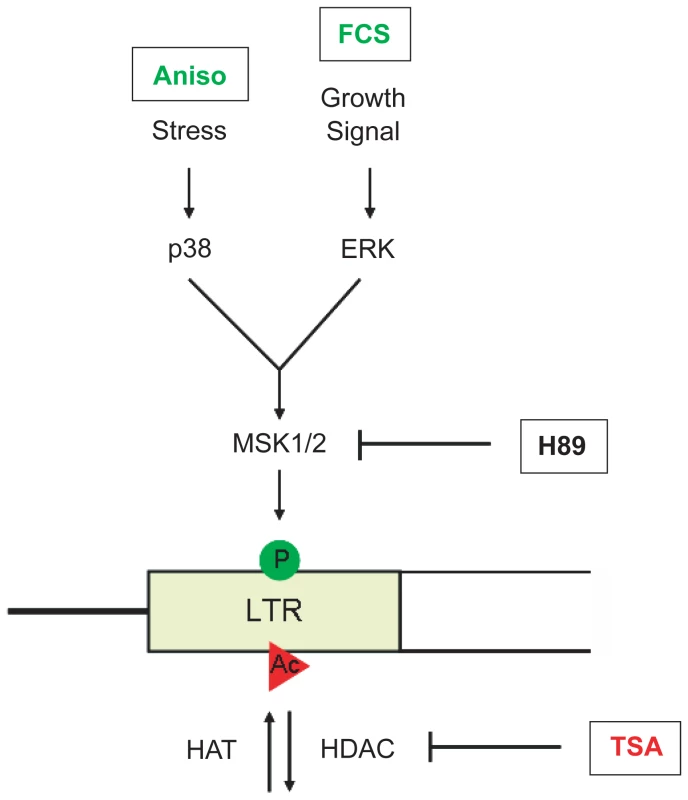
Full transcriptional activation of VL30 elements is triggered by a dual histone modification mark, i.e. phosphoacetylation of histone H3 at serine 10 and lysine 14. Phosphorylation of serine 10 (green circle) is conveyed by activation of the p38 stress (e.g. via anisomycin (Aniso)) or the ERK growth factor inducible MAPK pathway (e.g. via serum (FCS)) ultimately leading to the activation of the MSK1/2 kinase and H3S10 phosphorylation. Histone hyperacetylation (red circle) can be induced by HDAC inhibition (e.g. via TSA). Combined treatment results in the formation of phosphoacetylated histones at VL30 LTRs and full transcriptional activation. Discussion
It is well established that histone acetylation plays a fundamental role in the regulation of gene expression. This has been demonstrated on a genome wide level as well as for numerous individual genes [59]–[61]. The vast majority of work in mammals has been dedicated to gene regulation in different cancer cells [62], throughout development [6], [63] or to the silencing of retroviruses such as HIV [64], [65]. Also the role of histone acetylation in the reactivation of a silenced artificial transposon has been investigated [66], but to our knowledge no extensive study has been performed so far to specifically analyse the interrelation between histone acetylation and regulation of endogenous retroelements. This is surprising, since chemicals targeting histone deacetylases are considered as promising agents for the treatment of several human diseases and possible effects on the expression of endogenous retroelements remain largely unknown. Therefore we aimed to define the role of histone acetylation on TE activity in the mouse model system. We used an experimental system, in which cellular histone acetylation levels were shifted towards a hyperacetylated state. This was achieved using the broad HDAC inhibitor TSA. Depletion of the major histone deacetylase HDAC1 was not sufficient to induce global hyperacetylation. Interestingly, incubation of cells with the DNMT inhibitor Aza-dC also resulted in increased histone acetylation levels, once more underscoring the close link between DNA methylation and histone acetylation [7]. However, this relation seems to be asymmetric, since the maintenance of DNA methylation did not require normal histone acetylation levels.
TE expression and histone acetylation
Having monitored global changes mediated by TSA and Aza-dC treatment, we focused our interest on the interrelation between histone acetylation and TE regulation. We determined histone acetylation levels of a representative selection of TEs in HDAC1−/− background, upon TSA and Aza-dC treatment. Subsequently, we correlated these data with the expression levels of the corresponding TEs. This analysis revealed the following key findings: (1) only three out of eleven TEs tested were significantly activated by Aza-dC treatment. Inhibition of DNA methyltransferases in fibroblasts strongly enhanced the expression of IAP, VL30 and MuLV LTR elements. These data indicate that a block in DNA methylation was sufficient to activate transcription of some, but not all TEs in fibroblasts. Efficient transcriptional activation of other TEs might depend on activating cofactors absent in fibroblasts. This is consistent with the finding that many TEs exhibit tissue - or cell type-specific expression patterns [67]–[69]. Alternatively, additional layers of silencing mechanisms directed against specific TE families could be present in fibroblasts in the absence of DNA methylation.
(2) Enhanced expression roughly correlated with increased acetylation. This was obvious for VL30 elements, which were highly expressed upon TSA and Aza-dC treatment, and for IAP elements, which were induced after treatment with Aza-dC: both LTR elements exhibited hyperacetylation when activated. Remarkably, loss of DNA methylation at IAP elements was accompanied by increased acetylation, whereas HDAC inhibition by TSA did not result in IAP-associated histone hyperacetylation. This suggests a model, in which histone acetyltransferase (HAT) activity is tethered to IAPs only upon loss of DNA methylation, which otherwise might prohibit the binding of transcription factors recruiting HATs. Hence, HDAC inhibition in cells with intact DNA methylation was not sufficient to induce local hyperacetylation. The situation of VL30 element regulation was strikingly different. Inhibition of HDAC activity led to robust hyperacetylation, pointing towards a direct involvement of HDACs and HATs in the transcriptional control of VL30 elements. Finally, we observed that SINE elements gained hyperacetylation upon Aza-dC and TSA treatment, which was accompanied with mild effects on transcription. However, SINEs posses some peculiar features, making them distinct from other TEs: they are short, highly repetitive sequences, which tend to accumulate nearby and within genes and their regulatory regions. Therefore, it is difficult to unambiguously assign the increase in histone acetylation and RNA abundance to direct activation of SINE elements and not to the activation of Aza-dC - or TSA-responsive target genes containing SINE element sequences.
(3) TSA treatment led to hyperacetylation of histone H4 independent of transcriptional activity. Histone H4 specific HATs seemed to readily acetylate lysine residues of H4 when the dominant HDACs were inhibited, suggesting their regular presence at silent TEs. In contrast, acetylation of histone H3 seemed coupled with transcription, pointing towards a model, in which histone H3 acetylation might stimulate the recruitment of the transcription machinery. Alternatively, histone H3 specific HATs were recruited together with the transcription machinery. Interestingly, Koch and colleagues recently showed that although H4ac and H3ac were both enriched around the transcriptional start site, only H3ac could be used as a highly predictive marker for gene activity [60]. One might speculate that differential HAT recruitment reflects a functional difference between histone H3 and histone H4 acetylation. In addition to their role in transcription where both modifications act synergistically, H4 (de)acetylation could have other biological functions not described so far.
Preferential expression of defined TE subfamilies
Examining IAP and VL30 transcripts in untreated fibroblasts clearly showed that expressed TEs originate from multiple loci dispersed throughout the genome. However, upon treatment with different stimuli, certain transposon subfamilies were preferentially expressed. We could show that these inducible elements harboured characteristic DNA sequence features within the LTR, the regulatory region of the element. IAPs were more efficiently activated by Aza-dC, when they carried increasing numbers of DNA sequence repeats, containing putative binding sites for the transcription factor CCAAT/enhancer-binding protein alpha (C/EBPα) (identified with the Alibaba2.1 transcription factor binding site program using the TRANSFAC 4.0 database). Strikingly, Ishihara and colleagues, who characterised IAP elements active upon irradiation of mouse acute myeloid leukaemia (AML) cells, isolated IAP elements with especially long stretches of C/EBPα binding sites [70] (Table S1, AB099818). In contrast, IAP elements responsible for recent integration events in the mouse germline [12] harboured only one TF binding site (Table S1, X04120 and AxinFu). Therefore, the availability of the transcription factor might influence the choice of which IAP subtypes are expressed in different cellular contexts.
Treatment with TSA or Aza-dC led to preferential expression of one particular VL30 element associated with a U3 subtype I LTR. This individual element also showed particularly high levels of active histone marks (H3K4me3, H3ac, H4ac, H3S10phK14ac) upon TSA treatment. Other elements driven by a subtype I LTR giving rise to transcripts showed intermediate enrichment levels, whereas the analysed subclass III and IV elements, which were hardly expressed under these conditions, exhibited lowest levels of active chromatin marks. Nilsson and Bohm earlier proposed a model stating that the enhancer design of VL30 elements determines tissue specific expression and the response to external stimuli [50]. Our data support this model. Subtype I U3 promoters seem to be specifically susceptible to activation in this cell type. This could also be observed in arrested Swiss 3T3 fibroblasts. When treated with Anisomycin and TSA, 5 out of 14 (36%) transcripts were derived from an element with a subtype I U3 region; when left untreated we could only detect transcripts derived from subtype III elements (7 out of 7) (Table S1). Nevertheless, it remains unclear, which features distinguish the highly expressed hybrid element from other VL30 elements. Examination of the genomic environment did not reveal any obviously unusual architecture, the closest protein coding genes being located 764kb upstream and 16kb downstream of the element.
VL30—an unusual type of retrotransposon
Our finding that VL30 elements could be reactivated with Aza-dC was in accordance with DNA methylation as the dominant silencing mechanism for TEs and supported by previous reports [39]. However, it could not account for earlier findings illustrating that a multitude of other stimuli without link to DNA methylation was able to trigger VL30 expression [27], [39], [51], [71]. Therefore, DNA methylation might be only one of several independent silencing pathways. Alternatively, Aza-dC treatment could be the cause for indirect effects leading to VL30 upregulation independent of DNA methylation levels at VL30 elements. Interestingly, loss of Dnmt1 in mouse brain tissue is not sufficient to influence VL30 expression (S.L. and C.S., unpublished data), although other stimuli have been reported to efficiently trigger VL30 in neurons [71]. This observation supports a model that Aza-dC might be an indirect trigger for VL30 activation.
Other mammalian TEs also respond to external signals, e.g. SINE elements can be reactivated via stress as well as Adenovirus infection [24], [25], LINEs by benzo(a) pyrene [26]. In cancer cells, TE silencing is impaired probably due to altered DNA methylation patterns [72], [73], and several human diseases are associated with a relaxation of TE repression [74]. Nonetheless, the regulation of VL30 expression is unusual among mammalian retrotransposons; a diverse repertoire of stimuli is able to highly induce transcription in several cell systems such as fibroblasts, keratinocytes, haematopoietic, and neuronal cells [27], [39], [51]. In contrast to many other TEs, VL30 elements seem to exist in a default state, which is “poised for transcription”, without stringent silencing mechanisms. External stimuli can easily flip the switch to “active transcription”. There are several feasible explanations for this exceptional situation: (1) it may mirror the unusual life cycle of VL30 elements. They can be packed into MuLV (murine leukaemia virus) viral particles and get horizontally transferred, rather than vertically propagated as endogenous parasites [27]. For such a life cycle it is essential to be efficiently transcribed in somatic cells. Of special benefit could be a system that allows the elements to “sense” the physiological situation of the host. Stressful conditions might trigger high-level transcription and increase the chance to escape the host successfully via virus-mediated horizontal exit strategies. (2) Song and colleagues recently showed that VL30 RNA can interact with the DNA-binding protein PSF and modulate PSF-mediated repression of target genes [75], [76]. Therefore, VL30 elements could have acquired physiological functions in murine cells and might be necessary for the regulation of certain host genes (a process, called molecular domestication [77]).
VL30 expression is synergistically regulated by histone acetylation and phosphorylation
Another novel finding of our study is the positive impact of histone H3 phosphoacetylation on VL30 transcription. For several years, mechanistic links between histone acetylation and histone phosphorylation caused by different stimuli, including stress and growth factors, have been known. For example, immediate early genes such as c-myc, c-fos, c-jun, TFF1 [54]–[56], [78], but also others [57], depend on the induction of histone H3 phosphoacetylation.
In a series of experiments we could show that VL30 expression was synergistically activated by stimuli leading to histone phosphorylation (FCS, anisomycin) and histone acetylation (TSA); full transcriptional activity was correlated with the formation of phosphoacetylated histone proteins at the VL30 LTR. Inhibition of the nucleosomal response by H89 abolished VL30 phosphoacetylation and expression, indicating that the formation of this dual histone mark was necessary for activation caused by FCS/TSA or anisomycin/TSA and that MSK1/2 might be the responsible kinase responsible for phosphorylation. In summary, we collected data supporting a model that VL30 transposable elements are novel phosphoacetylation target genes. Still many details about the exact mechanism of VL30 regulation via dual histone modification are unclear, and it remains to be determined which are the responsible HAT(s), HDAC(s) and phosphatase(s) regulating dynamic acetylation and phosphorylation. In this context it will be interesting to investigate putative connections between enhancer design, transcription factor binding, and recruitment of histone modifiers dependent on the VL30 subtype.
Transposition rates of several TEs are not influenced by HDAC inhibition
The link between TE transcription and transposition is fundamental. Production of an adequate amount of mRNA is an essential premise for all subsequent steps in the lifecycle of a TE. Having monitored the influence of HDAC inhibition on transcription of several TEs, we also examined, if there is an influence on subsequent events resulting in altered transposition activity. We used an experimental system, which allowed us to survey transposition rates of episomally delivered LTR and LINE elements upon HDAC inhibition. We detected only minor differences in the frequency of retrotransposition events when cells were treated with TSA, i.e. transposition events were slightly increased upon TSA treatment. We also observed a comparable enrichment of the transcript. Therefore, we could not detect obvious effects on transposon activity exceeding effects coherent with enhanced transcription. Final answers on the issue of histone - and protein-acetylation, influencing the process of transposition in mammals, will certainly demand further and more detailed investigations.
Implications for HDAC inhibitor treatment of humans
HDAC inhibitors are promising anticancer drugs because they can change gene expression patterns in cancer cells, leading to cell cycle arrest or apoptosis [4]. More recently, their use for the treatment of non-cancer diseases has also been suggested [79], [80]. Our finding that HDAC inhibition can reactivate VL30 elements in mice might indicate that other TEs or non-coding elements could also respond to an epigenetic therapy in humans. Therefore, careful evaluation of potential effects beyond the deregulation of single copy genes will be an important issue for future studies. An interesting aspect – along with other dangers arising from increased TE activity – will be their emerging potential to modulate cellular regulatory networks. An exiting example for such a pathway conserved in mice and humans is the interaction of the PSF protein and VL30 RNA in mice and other non-coding TE-derived RNAs in human cells, respectively, affecting tumourigenesis [81].
Materials and Methods
Cell lines
Wildtype (HDAC1+/+) and corresponding HDAC1−/− fibroblasts cell lines were established from E13.5 embryos carrying two floxed alleles of the Hdac1 gene [82]. Primary fibroblasts (mixed 129SvOla/Bl6, more than 80% being of Bl6 origin) were isolated and immortalised following the 3T3 immortalisation protocol [83]. Subclones were either transfected with a control vector or a vector encoding the cre recombinase leading to the deletion of exon 6 encoding the catalytic core of HDAC1. Cells were maintained in DMEM supplemented with 10% foetal calf serum (FCS) and gentamycin. For growth arrest of cells, Swiss 3T3 fibroblasts were serum starved for 72h in DMEM containing 0.2% FCS.
Inhibitor treatments
The following inhibitors were used in this study: trichostatin A (TSA) (50 ng/ml [166 nM]; Wako Pure Chemical Industries) was applied for 3–24h; 5′Aza-2′-deoxycytidine (Aza-dC) (1µM) for 24h followed by additional incubation of cells for 24h without inhibitor; anisomycin (Aniso) (50 ng/ml [189 nM]; Sigma) for 1–6h, and H89 (10 µM; Alexis Biochemicals) for 1h 15min. H89 was added 15min prior to anisomycin.
Transposition assay
Plasmids carrying a tagged IAP, MusD, L1.Md or L1.Hs retrotransposon (described in [48]) were transfected into logarithmically growing U2OS cells maintained in DMEM with 10% FCS and gentamycin. Cells were split 24h prior to the transfection procedure and 1×105 cells were seeded on a 6-well plate. Transfection was performed with 1µg of plasmid DNA and Lipofectamine (Invitrogen) following the manufacturer's instructions. After 6h, the medium was replaced by DMEM supplemented with 10% FCS and gentamycin. If required, 66nM TSA was added for 24h after transfection. To monitor expression levels of the transfected constructs, cells were lysed 24h post transfection with TRIzol reagent (Invitrogen), RNA was isolated, DNase treated, reversely transcribed and subjected to quantitative RT-PCR as described above. The following primers were used for normalisation: GAPDH-for TCT TCT TTT GCG TCG CCA G, GAPDH-rev AGC CCC AGC CTT CTC CA and determination of episomal TE expression: Neo-for ATC AGA CAG CCG ATT GTC TG, Neo-rev CAG TTC CGC CCA TTC TCC G. For the analysis of transposition rates, cells were transferred to a 10cm dish 24h post transfection and cultured for 1 week, split and reseeded on 10cm dishes (5×105 per dish). Selection for neo-positive cells was started 24h later by adding 500µg/ml G418 to the growth medium. After 2 weeks, cell clones were fixed with 70% EtOH, stained with 0.1% methylene blue/0.5M Na-acetate solution and counted.
Western blot and dot blot analysis
Histone and total protein preparation for Western blot analysis was performed as previously described [57]. The following antibodies were used: HDAC1 polyclonal antibody (Millipore 06-720), HDAC2 3F3 monoclonal antibody (Millipore 05-814), HDAC3 (Abcam ab16047), DNMT1 (Abcam ab13537), β-actin (Sigma A5316), H3ac (Millipore 06-599), H4ac (Millipore 06-598), H3K4me3 (Millipore 07-473), H3S10pK14ac (Millipore 07-081 and a rabbit serum raised against the H3 peptide ARTKQTARKS(ph)TGGK(ac)APRKQL in collaboration with Eurogentec, Belgium) and c-H3 (Abcam ab1791). Densitometric quantification of Western blots was performed with the Imagequant software. For Dot blot analysis, 1 microliter of each peptide (0.2 mg/ml and 0.04 mg/ml in water) was spotted on a PVDF membrane, antibody incubation and detection was performed by standard Western blot methods.
DNA methylation analysis
To quantify DNA methylation levels at defined DNA sequences, we employed the MS-SNuPE technique as described elsewhere [32]. Primers for PCR: bs-IAP-rc-for GTT TTA GTA TAG GGG AAT GTT AGG GTT; bs-IAP-rc-rev ACC AAA AAA AAC ACC ACA AAC CAA AAT CTT CTA C. Primers for the SnuPE reaction: rcS789 GTA GTT AAT TAA GGA GTG ATA; rcS628 AGA TTT TTG TGT TGG GAG T; rcS894 GAA GAT GTA AGA ATA AAG TTT TGT.
Chromatin immunoprecipitation assay
Preparation of soluble chromatin and chromatin immunoprecipitation assays were carried out as described previously [57]. Antibodies for immunoprecipitation of modified histones were obtained from Millipore (H3ac 06-599, H4ac 06-598, H3S10p/H3K14ac 07-081) or generated in collaboration with Eurogentec, Belgium (H3S10p/H3K14ac), the antibody recognising C-terminal histone H3 was ordered from Abcam (c-H3 ab1791). H3K9me2 and H3K27me3 antibodies were a kind gift of T. Jenuwein, MPI Freiburg. As negative control unspecific IgGs from preimmunsera were used. Quantification of precipitated material was performed via quantitative Real-Time PCR employing an iCycler iQ system from Bio-Rad, SYBER-green (Molecular probes) and Taq DNA Polymerase (Fermentas) or via semi-quantitative PCR using a Biometra T3 thermocycler and the GoTaq PCR Master mix from Promega followed by visualisation of DNA fragments on an ethidium bromide stained 2% agarose-TAE gel. Primers have been described elsewhere [15], [84] or can be found in Table S2.
Expression analyses
RNA was extracted with TRIzol Reagent (Invitrogen), possible genomic DNA contamination was removed using Turbo DNase (Ambion) and cDNA was synthesised with the iScript cDNA Synthesis Kit (Bio-Rad). For quantification of transcripts we performed RT-PCR on an iCycler iQ system from Bio-Rad using SYBER-green (Molecular probes) and Taq DNA Polymerase (Fermentas). Primers sequences have been described previously [15], [84] and are given in Table S2.
Cloning of genomic and expressed transposable elements
To characterise TE transcripts, RNAs from three independent biological samples from untreated, TSA and Aza-dC treated fibroblasts were harvested, extracted with TRIzol Reagent (Invitrogen), DNase treated (Turbo DNase, Ambion), reversely transcribed (iScript, Bio-Rad) and used for PCR amplification in doublets (Biometra T3 thermocycler, GoTaq PCR Master mix from Promega; primers see Table S2). As control, PCR reactions were also performed with genomic DNA as template, amplifying genomic elements. The resulting six PCR reactions for each condition were pooled (to exclude a bias due to PCR reactions), loaded on an ethidium bromide stained agarose-TAE gel, eluted and purified using the Wizard Plus SV Gel and PCR Clean Up System (Promega). DNA fragments were cloned into the pGEM-T vector system (Promega) and transformed into E.coli (DH5α). Positive clones were grown in LB liquid medium, plasmid DNA was extracted using the Wizard Plus SV Miniprep DNA Purification System (Promega) and sequenced.
Supporting Information
Zdroje
1. StrahlBD
AllisCD
2000 The language of covalent histone modifications. Nature 403 41 45
2. YangXJ
SetoE
2008 The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol 9 206 218
3. WangZ
ZangC
CuiK
SchonesDE
BarskiA
2009 Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138 1019 1031
4. MarksP
RifkindRA
RichonVM
BreslowR
MillerT
2001 Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1 194 202
5. ShankarS
SrivastavaRK
2008 Histone deacetylase inhibitors: mechanisms and clinical significance in cancer: HDAC inhibitor-induced apoptosis. Adv Exp Med Biol 615 261 298
6. BrunmeirR
LaggerS
SeiserC
2009 Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol 53 275 289
7. IkegamiK
OhganeJ
TanakaS
YagiS
ShiotaK
2009 Interplay between DNA methylation, histone modification and chromatin remodeling in stem cells and during development. Int J Dev Biol 53 203 214
8. LanderES
LintonLM
BirrenB
NusbaumC
ZodyMC
2001 Initial sequencing and analysis of the human genome. Nature 409 860 921
9. WaterstonRH
Lindblad-TohK
BirneyE
RogersJ
AbrilJF
2002 Initial sequencing and comparative analysis of the mouse genome. Nature 420 520 562
10. PrakET
KazazianHHJr
2000 Mobile elements and the human genome. Nat Rev Genet 1 134 144
11. GoodierJL
KazazianHHJr
2008 Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell 135 23 35
12. MaksakovaIA
RomanishMT
GagnierL
DunnCA
van de LagemaatLN
2006 Retroviral elements and their hosts: insertional mutagenesis in the mouse germ line. PLoS Genet 2 e2 doi:10.1371/journal.pgen.0020002
13. MaksakovaIA
MagerDL
ReissD
2008 Keeping active endogenous retroviral-like elements in check: the epigenetic perspective. Cell Mol Life Sci 65 3329 3347
14. GoffSP
2004 Retrovirus restriction factors. Mol Cell 16 849 859
15. MartensJH
O'SullivanRJ
BraunschweigU
OpravilS
RadolfM
2005 The profile of repeat-associated histone lysine methylation states in the mouse epigenome. Embo J 24 800 812
16. MatsuiT
LeungD
MiyashitaH
MaksakovaI
MiyachiH
2010 Proviral silencing in embryonic stem cells requires the histone methyltransferase ESET. Nature advance online publication doi:10.1038/nature08858
17. SlotkinRK
MartienssenR
2007 Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet 8 272 285
18. CamHP
ChenES
GrewalSI
2009 Transcriptional scaffolds for heterochromatin assembly. Cell 136 610 614
19. MaloneCD
HannonGJ
2009 Small RNAs as guardians of the genome. Cell 136 656 668
20. ObbardDJ
FinneganDJ
2008 RNA interference: endogenous siRNAs derived from transposable elements. Curr Biol 18 R561 563
21. MatzkeM
KannoT
DaxingerL
HuettelB
MatzkeAJ
2009 RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol 21 367 376
22. McClintockB
1984 The significance of responses of the genome to challenge. Science 226 792 801
23. CapyP
GasperiG
BiemontC
BazinC
2000 Stress and transposable elements: co-evolution or useful parasites? Heredity 85 (Pt 2) 101 106
24. PanningB
SmileyJR
1993 Activation of RNA polymerase III transcription of human Alu repetitive elements by adenovirus type 5: requirement for the E1b 58-kilodalton protein and the products of E4 open reading frames 3 and 6. Mol Cell Biol 13 3231 3244
25. LiuWM
ChuWM
ChoudaryPV
SchmidCW
1995 Cell stress and translational inhibitors transiently increase the abundance of mammalian SINE transcripts. Nucleic Acids Res 23 1758 1765
26. StribinskisV
RamosKS
2006 Activation of human long interspersed nuclear element 1 retrotransposition by benzo(a)pyrene, an ubiquitous environmental carcinogen. Cancer Res 66 2616 2620
27. FrenchNS
NortonJD
1997 Structure and functional properties of mouse VL30 retrotransposons. Biochim Biophys Acta 1352 33 47
28. JanuchowskiR
DabrowskiM
OforiH
JagodzinskiPP
2007 Trichostatin A down-regulate DNA methyltransferase 1 in Jurkat T cells. Cancer Lett 246 313 317
29. YouJS
KangJK
LeeEK
LeeJC
LeeSH
2008 Histone deacetylase inhibitor apicidin downregulates DNA methyltransferase 1 expression and induces repressive histone modifications via recruitment of corepressor complex to promoter region in human cervix cancer cells. Oncogene 27 1376 1386
30. SchermellehL
SpadaF
EaswaranHP
ZolghadrK
MargotJB
2005 Trapped in action: direct visualization of DNA methyltransferase activity in living cells. Nat Methods 2 751 756
31. GhoshalK
DattaJ
MajumderS
BaiS
KutayH
2005 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal. Mol Cell Biol 25 4727 4741
32. GonzalgoML
JonesPA
1997 Rapid quantitation of methylation differences at specific sites using methylation-sensitive single nucleotide primer extension (Ms-SNuPE). Nucleic Acids Res 25 2529 2531
33. LaneN
DeanW
ErhardtS
HajkovaP
SuraniA
2003 Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis 35 88 93
34. LaggerG
O'CarrollD
RemboldM
KhierH
TischlerJ
2002 Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. Embo J 21 2672 2681
35. PeastonAE
EvsikovAV
GraberJH
de VriesWN
HolbrookAE
2004 Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev Cell 7 597 606
36. StockingC
KozakCA
2008 Murine endogenous retroviruses. Cell Mol Life Sci 65 3383 3398
37. DavisCM
ConstantinidesPG
van der RietF
van SchalkwykL
GeversW
1989 Activation and demethylation of the intracisternal A particle genes by 5-azacytidine. Cell Differ Dev 27 83 93
38. WalshCP
ChailletJR
BestorTH
1998 Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat Genet 20 116 117
39. TzavarasT
EftaxiaS
TavoulariS
HatziP
AngelidisC
2003 Factors influencing the expression of endogenous reverse transcriptases and viral-like 30 elements in mouse NIH3T3 cells. Int J Oncol 23 1237 1243
40. MaksakovaIA
MagerDL
2005 Transcriptional regulation of early transposon elements, an active family of mouse long terminal repeat retrotransposons. J Virol 79 13865 13874
41. ScholesDT
BanerjeeM
BowenB
CurcioMJ
2001 Multiple regulators of Ty1 transposition in Saccharomyces cerevisiae have conserved roles in genome maintenance. Genetics 159 1449 1465
42. GriffithJL
ColemanLE
RaymondAS
GoodsonSG
PittardWS
2003 Functional genomics reveals relationships between the retrovirus-like Ty1 element and its host Saccharomyces cerevisiae. Genetics 164 867 879
43. AyeM
IrwinB
Beliakova-BethellN
ChenE
GarrusJ
2004 Host factors that affect Ty3 retrotransposition in Saccharomyces cerevisiae. Genetics 168 1159 1176
44. IrwinB
AyeM
BaldiP
Beliakova-BethellN
ChengH
2005 Retroviruses and yeast retrotransposons use overlapping sets of host genes. Genome Res 15 641 654
45. NyswanerKM
CheckleyMA
YiM
StephensRM
GarfinkelDJ
2008 Chromatin-associated genes protect the yeast genome from Ty1 insertional mutagenesis. Genetics 178 197 214
46. HeidmannO
HeidmannT
1991 Retrotransposition of a mouse IAP sequence tagged with an indicator gene. Cell 64 159 170
47. MoranJV
HolmesSE
NaasTP
DeBerardinisRJ
BoekeJD
1996 High frequency retrotransposition in cultured mammalian cells. Cell 87 917 927
48. EsnaultC
HeidmannO
DelebecqueF
DewannieuxM
RibetD
2005 APOBEC3G cytidine deaminase inhibits retrotransposition of endogenous retroviruses. Nature 433 430 433
49. MietzJA
FewellJW
KuffEL
1992 Selective activation of a discrete family of endogenous proviral elements in normal BALB/c lymphocytes. Mol Cell Biol 12 220 228
50. NilssonM
BohmS
1994 Inducible and cell type-specific expression of VL30 U3 subgroups correlate with their enhancer design. J Virol 68 276 288
51. NoutsopoulosD
MarkopoulosG
KoliouM
DovaL
VartholomatosG
2007 Vanadium induces VL30 retrotransposition at an unusually high level: a possible carcinogenesis mechanism. J Mol Biol 374 80 90
52. MahadevanLC
WillisAC
BarrattMJ
1991 Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell 65 775 783
53. ThomsonS
ClaytonAL
HazzalinCA
RoseS
BarrattMJ
1999 The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. Embo J 18 4779 4793
54. CheungP
TannerKG
CheungWL
Sassone-CorsiP
DenuJM
2000 Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell 5 905 915
55. ClaytonAL
RoseS
BarrattMJ
MahadevanLC
2000 Phosphoacetylation of histone H3 on c-fos - and c-jun-associated nucleosomes upon gene activation. Embo J 19 3714 3726
56. LiJ
GorospeM
HutterD
BarnesJ
KeyseSM
2001 Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Mol Cell Biol 21 8213 8224
57. HauserC
SchuettengruberB
BartlS
LaggerG
SeiserC
2002 Activation of the mouse histone deacetylase 1 gene by cooperative histone phosphorylation and acetylation. Mol Cell Biol 22 7820 7830
58. WinterS
SimboeckE
FischleW
ZupkovitzG
DohnalI
2008 14-3-3 proteins recognize a histone code at histone H3 and are required for transcriptional activation. Embo J 27 88 99
59. KurdistaniSK
TavazoieS
GrunsteinM
2004 Mapping global histone acetylation patterns to gene expression. Cell 117 721 733
60. KochCM
AndrewsRM
FlicekP
DillonSC
KaraozU
2007 The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res 17 691 707
61. FischerJJ
ToedlingJ
KruegerT
SchuelerM
HuberW
2008 Combinatorial effects of four histone modifications in transcription and differentiation. Genomics 91 41 51
62. GlozakMA
SetoE
2007 Histone deacetylases and cancer. Oncogene 26 5420 5432
63. KalkhovenE
2004 CBP and p300: HATs for different occasions. Biochem Pharmacol 68 1145 1155
64. HeG
MargolisDM
2002 Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Mol Cell Biol 22 2965 2973
65. LusicM
MarcelloA
CeresetoA
GiaccaM
2003 Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter. Embo J 22 6550 6561
66. GarrisonBS
YantSR
MikkelsenJG
KayMA
2007 Postintegrative gene silencing within the Sleeping Beauty transposition system. Mol Cell Biol 27 8824 8833
67. BranciforteD
MartinSL
1994 Developmental and cell type specificity of LINE-1 expression in mouse testis: implications for transposition. Mol Cell Biol 14 2584 2592
68. DupressoirA
HeidmannT
1996 Germ line-specific expression of intracisternal A-particle retrotransposons in transgenic mice. Mol Cell Biol 16 4495 4503
69. SeifarthW
FrankO
ZeilfelderU
SpiessB
GreenwoodAD
2005 Comprehensive analysis of human endogenous retrovirus transcriptional activity in human tissues with a retrovirus-specific microarray. J Virol 79 341 352
70. IshiharaH
TanakaI
WanH
NojimaK
YoshidaK
2004 Retrotransposition of limited deletion type of intracisternal A-particle elements in the myeloid leukemia Clls of C3H/He mice. J Radiat Res (Tokyo) 45 25 32
71. CostainWJ
RasquinhaI
GraberT
LuebbertC
PrestonE
2006 Cerebral ischemia induces neuronal expression of novel VL30 mouse retrotransposons bound to polyribosomes. Brain Res 1094 24 37
72. RauchTA
ZhongX
WuX
WangM
KernstineKH
2008 High-resolution mapping of DNA hypermethylation and hypomethylation in lung cancer. Proc Natl Acad Sci U S A 105 252 257
73. WilsonAS
PowerBE
MolloyPL
2007 DNA hypomethylation and human diseases. Biochim Biophys Acta 1775 138 162
74. ColmegnaI
GarryRF
2006 Role of endogenous retroviruses in autoimmune diseases. Infect Dis Clin North Am 20 913 929
75. SongX
SuiA
GarenA
2004 Binding of mouse VL30 retrotransposon RNA to PSF protein induces genes repressed by PSF: effects on steroidogenesis and oncogenesis. Proc Natl Acad Sci U S A 101 621 626
76. SongX
SunY
GarenA
2005 Roles of PSF protein and VL30 RNA in reversible gene regulation. Proc Natl Acad Sci U S A 102 12189 12193
77. MillerWJ
McDonaldJF
NouaudD
AnxolabehereD
1999 Molecular domestication–more than a sporadic episode in evolution. Genetica 107 197 207
78. EspinoPS
LiL
HeS
YuJ
DavieJR
2006 Chromatin modification of the trefoil factor 1 gene in human breast cancer cells by the Ras/mitogen-activated protein kinase pathway. Cancer Res 66 4610 4616
79. WiechNL
FisherJF
HelquistP
WiestO
2009 Inhibition of histone deacetylases: a pharmacological approach to the treatment of non-cancer disorders. Curr Top Med Chem 9 257 271
80. RotiliD
SimonettiG
SavarinoA
PalamaraAT
MigliaccioAR
2009 Non-cancer uses of histone deacetylase inhibitors: effects on infectious diseases and beta-hemoglobinopathies. Curr Top Med Chem 9 272 291
81. LiL
FengT
LianY
ZhangG
GarenA
2009 Role of human noncoding RNAs in the control of tumorigenesis. Proc Natl Acad Sci U S A
82. YamaguchiT
CubizollesF
ZhangY
ReichertN
KohlerH
Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev 24 455 469
83. TodaroGJ
GreenH
1963 Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol 17 299 313
84. PuschendorfM
SteinP
OakeleyEJ
SchultzRM
PetersAH
2006 Abundant transcripts from retrotransposons are unstable in fully grown mouse oocytes. Biochem Biophys Res Commun 347 36 43
85. CarterAT
NortonJD
AveryRJ
1983 A novel approach to cloning transcriptionally active retrovirus-like genetic elements from mouse cells. Nucleic Acids Res 11 6243 6254
86. EatonL
NortonJD
1990 Independent regulation of mouse VL30 retrotransposon expression in response to serum and oncogenic cell transformation. Nucleic Acids Res 18 2069 2077
87. HodgsonCP
ElderPK
OnoT
FosterDN
GetzMJ
1983 Structure and expression of mouse VL30 genes. Mol Cell Biol 3 2221 2231
88. WickerT
SabotF
Hua-VanA
BennetzenJL
CapyP
2007 A unified classification system for eukaryotic transposable elements. Nat Rev Genet 8 973 982
89. SmitAF
1993 Identification of a new, abundant superfamily of mammalian LTR-transposons. Nucleic Acids Res 21 1863 1872
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 4
-
Všechny články tohoto čísla
- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
- Fragilities Caused by Dosage Imbalance in Regulation of the Budding Yeast Cell Cycle
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Activation of Estrogen-Responsive Genes Does Not Require Their Nuclear Co-Localization
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- A Pax3/Dmrt2/Myf5 Regulatory Cascade Functions at the Onset of Myogenesis
- Two New Loci for Body-Weight Regulation Identified in a Joint Analysis of Genome-Wide Association Studies for Early-Onset Extreme Obesity in French and German Study Groups
- Hypomethylation of a LINE-1 Promoter Activates an Alternate Transcript of the MET Oncogene in Bladders with Cancer
- Candidate Causal Regulatory Effects by Integration of Expression QTLs with Complex Trait Genetic Associations
- Combined Inactivation of pRB and Hippo Pathways Induces Dedifferentiation in the Retina
- Allele-Specific Virulence Attenuation of the HopZ1a Type III Effector via the ZAR1 Resistance Protein
- Down-Regulation of Honey Bee Gene Biases Behavior toward Food Rich in Protein
- A Microarray-Based Genetic Screen for Yeast Chronological Aging Factors
- Actin-Related Protein Arp6 Influences H2A.Z-Dependent and -Independent Gene Expression and Links Ribosomal Protein Genes to Nuclear Pores
- Phosphorylation of the Conserved Transcription Factor ATF-7 by PMK-1 p38 MAPK Regulates Innate Immunity in
- A -Regulatory Signature for Chordate Anterior Neuroectodermal Genes
- Genetic Analysis of Fin Development in Zebrafish Identifies Furin and Hemicentin1 as Potential Novel Fraser Syndrome Disease Genes
- Assembly of a 40 Mb Eukaryotic Genome from Short Sequence Reads: , a Model Organism for Fungal Morphogenesis
- Trait-Associated SNPs Are More Likely to Be eQTLs: Annotation to Enhance Discovery from GWAS
- Absence of Evidence for MHC–Dependent Mate Selection within HapMap Populations
- The TALE Class Homeobox Gene Defines the Anterior Compartment for Head Regeneration
- Cyclic Expression of Lhx2 Regulates Hair Formation
- Genetic Evidence for Hybrid Trait Speciation in Butterflies
- Epigenetic Regulation of a Murine Retrotransposon by a Dual Histone Modification Mark
- Chromosome 9p21 SNPs Associated with Multiple Disease Phenotypes Correlate with Expression
- S Phase Progression in Human Cells Is Dictated by the Genetic Continuity of DNA Foci
- The Next Generation Becomes the Now Generation
- Acts as a Tumor Suppressor in a Murine Retinoblastoma Model by Facilitating Tumor Cell Death
- Genome-Wide Association Study of Lp-PLA Activity and Mass in the Framingham Heart Study
- The Five Zinc Transporters Undergo Different Evolutionary Fates towards Adaptive Evolution to Zinc Tolerance in
- MicroRNA–Directed siRNA Biogenesis in
- Deletion of the WD40 Domain of LRRK2 in Zebrafish Causes Parkinsonism-Like Loss of Neurons and Locomotive Defect
- Incipient Balancing Selection through Adaptive Loss of Aquaporins in Natural Populations
- GTPase Activity Plays a Key Role in the Pathobiology of LRRK2
- Natural Single-Nucleosome Epi-Polymorphisms in Yeast
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Genome SNP Association in the Horse: Identification of a Deletion in Myosin Va Responsible for Lavender Foal Syndrome
- Admixture Mapping Scans Identify a Locus Affecting Retinal Vascular Caliber in Hypertensive African Americans: the Atherosclerosis Risk in Communities (ARIC) Study
- Genetic Tests for Ecological and Allopatric Speciation in Anoles on an Island Archipelago
- Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



