-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaThe Majority of Primate-Specific Regulatory Sequences Are Derived from Transposable Elements
Although emerging evidence suggests that transposable elements (TEs) have contributed novel regulatory elements to the human genome, their global impact on transcriptional networks remains largely uncharacterized. Here we show that TEs have contributed to the human genome nearly half of its active elements. Using DNase I hypersensitivity data sets from ENCODE in normal, embryonic, and cancer cells, we found that 44% of open chromatin regions were in TEs and that this proportion reached 63% for primate-specific regions. We also showed that distinct subfamilies of endogenous retroviruses (ERVs) contributed significantly more accessible regions than expected by chance, with up to 80% of their instances in open chromatin. Based on these results, we further characterized 2,150 TE subfamily–transcription factor pairs that were bound in vivo or enriched for specific binding motifs, and observed that TEs contributing to open chromatin had higher levels of sequence conservation. We also showed that thousands of ERV–derived sequences were activated in a cell type–specific manner, especially in embryonic and cancer cells, and we demonstrated that this activity was associated with cell type–specific expression of neighboring genes. Taken together, these results demonstrate that TEs, and in particular ERVs, have contributed hundreds of thousands of novel regulatory elements to the primate lineage and reshaped the human transcriptional landscape.
Published in the journal: . PLoS Genet 9(5): e32767. doi:10.1371/journal.pgen.1003504
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003504Summary
Although emerging evidence suggests that transposable elements (TEs) have contributed novel regulatory elements to the human genome, their global impact on transcriptional networks remains largely uncharacterized. Here we show that TEs have contributed to the human genome nearly half of its active elements. Using DNase I hypersensitivity data sets from ENCODE in normal, embryonic, and cancer cells, we found that 44% of open chromatin regions were in TEs and that this proportion reached 63% for primate-specific regions. We also showed that distinct subfamilies of endogenous retroviruses (ERVs) contributed significantly more accessible regions than expected by chance, with up to 80% of their instances in open chromatin. Based on these results, we further characterized 2,150 TE subfamily–transcription factor pairs that were bound in vivo or enriched for specific binding motifs, and observed that TEs contributing to open chromatin had higher levels of sequence conservation. We also showed that thousands of ERV–derived sequences were activated in a cell type–specific manner, especially in embryonic and cancer cells, and we demonstrated that this activity was associated with cell type–specific expression of neighboring genes. Taken together, these results demonstrate that TEs, and in particular ERVs, have contributed hundreds of thousands of novel regulatory elements to the primate lineage and reshaped the human transcriptional landscape.
Introduction
Nearly half of human DNA is derived from sequences known as TEs that have successfully replicated in the genome during the evolution of our species [1]. Although the parasitic behavior of TEs was initially put forward as a sufficient explanation for their maintenance within genomes [2], [3] there is growing evidence to support the alternative view that TEs have facilitated genomic innovations [4], [5] and contributed critical regulatory elements to their host [6]. Indeed, a number of studies have shown recently that TEs have been the source of binding sites for various mammalian transcription factors (TFs) [7]–[9] and that they have rewired different developmental regulatory networks [10]–[12]. However, given that previous studies were limited either by the number of TFs they surveyed [7]–[10], [13], [14] or by the cell types they explored [10], [11], [15], a key question that remains is to what extent have TEs globally contributed to human transcriptional networks in undifferentiated and differentiated cells. The importance of characterizing the functional role of TEs and other repetitive regions in the human genome is accentuated by the facts that these sequences constitute most of the sequence diversity between mammalian species [16] and are a significant source of human polymorphisms [17] and of somatic mutations in healthy and disease tissues [18]–[20].
To systematically survey the contribution of TEs to human regulatory networks across a range of cell types, we made use of DNase I hypersensitive sites (DHS) data generated at the University of Washington (UW) and Duke as part of ENCODE [21], [22]. The benefit of using these chromatin accessibility maps is that they highlight active DNA sequences [23], [24] independently of a selected set of TFs. Although accessibility does not equate regulatory function, we build upon these data sets to measure the global activity profile of all classes of transposon-derived sequences and systematically characterize the impact that ancient and recent TEs expansions have had on the human chromatin landscape.
Results
TEs have contributed a large fraction of accessible regions in human cells
Starting from 106 DHS data sets we performed extensive quality control and retained 75 data sets defining a total of 11,848,530 regions of open chromatin in 41 distinct human cell types derived from normal, embryonic, and cancer tissues (Table 1 and Table S1, see Materials and Methods). These DHS data were further grouped across cell types into 1,643,643 distinct regions of open chromatin. By measuring the overlap with repeat elements, we found that 725,610 (44.1%) DHS regions overlapped instances of the 4 major classes of TEs (ERV, also known as LTR, DNA, LINE and SINE). Notably, by partitioning the DHS regions based on the presence or absence of homologous sequences at orthologous loci in other species, we also found that this proportion reached 63.1% for elements embedded in primate-specific sequences (Figure 1A, see Materials and Methods). A large fraction of these primate-specific DHS regions were observed in repeat subfamilies that were themselves specific to the primate lineage as estimated from the divergence of the repeat instances from their consensus (Figure S1, see Materials and Methods).
Fig. 1. TEs have contributed a large fraction of accessible regions in human cells. 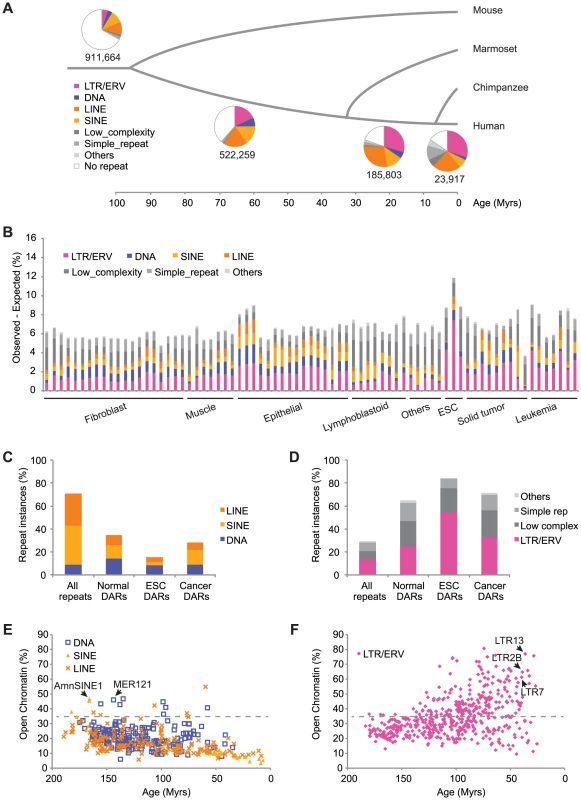
(A) Proportion of human DHS regions overlapping different classes of repeats based on the age of the sequence in which they are embedded. (B) Specific repeat subfamilies, called DHS-associated repeats (DARs), are over-represented and their cumulative relative contribution (Observed-Expected) is shown as a percentage of all DHS data. (C–D) Proportion of all repeat instances in the genome (All repeats) and for DAR instances in three classes of cells (Normal, ESC and Cancer). (E–F) Fraction of repeat subfamily instances that is contributing to open chromatin in at least one data set. The estimated age is in millions of years (Myrs). Tab. 1. The 75 DNase I data sets used in this study were grouped in 8 tissues. 
Cell lines marked with a “†” or “††” had two or three biological replicates respectively. Given that repeats are ubiquitous in the genome, we wanted to compare the proportion of DHS regions observed in TEs relative to what would be expected by chance. Using annotation-matched random distributions we found that specific repeat subfamilies were significantly over-represented in individual DHS data sets (see Materials and Methods). For example, we observed that 1237 of the 2337 (52.9%) LTR7 repeat instances (a subfamily of the LTR/ERV class) were contributing to open chromatin in the human embryonic stem cell (ESC) line H7 when we would have only expected 60.5 (2.6%). This corresponds to a 20-fold enrichment and is highly significant (p<1.0E-100). We call such repeat subfamilies DHS-associated repeats (DARs) and, using a stringent cutoff (p<1.0E-05), we identified 8937 DARs enriched in various cell types (Table S2). These DARs provided on average 6.7% and up to 11.9% more open chromatin regions than expected by chance in the data sets surveyed (Figure 1B).
We were interested in characterizing further the families of TEs that were contributing to regions of open chromatin. By combining the DAR instances across the various cell types and comparing them to the number of repeat instances of each family across the genome, we found that LINE and SINE repeats were depleted while DNA repeats were observed at levels expected (Figure 1C). In contrast, we found that LTR/ERV, Low complexity, Simple repeats and Others repeat classes were enriched (Figure 1D). For example, although LTR/ERV repeats constitute 13.5% of the repeat instances in the genome, they represent 25.0%, 54.6%, and 33.0% of the DAR instances in normal, embryonic, and cancer cells, respectively. The over-representation of LTR/ERVs in DHS corroborates an observation made previously [22] and did not appear to be a consequence of intrinsic properties of the repeat subfamilies including mappability (Figures S2 and S3, see Materials and Methods). Low complexity, Simple repeats and Others repeat classes were excluded from most downstream analyses because of their extreme GC content (Figure S3) potentially affected by sequencing biases [25].
Next, we looked at the fraction of instances in all repeat subfamilies that were contributing to open chromatin in relation to their estimated age (see Materials and Methods). We observed that for SINE, LINE, and DNA repeats, older subfamilies tended to contribute more often to open chromatin (Figure 1E). Two of the subfamilies contributing the most were AmnSINE1 and MER121, both previously suggested to have acquired functionality in the host [26], [27]. Intriguingly, we observed the reverse pattern for the LTR/ERV repeats with many of the young subfamilies contributing to open chromatin at very high levels (e.g. LTR13 with 379 instances contributing to open chromatin out of 492 (77.0%), LTR2B with 215 out of 332 (64.8%), and LTR7 with 1432 out of 2337 (61.3%)) (Figure 1F). This pattern although dampened was also visible if we restricted the analysis to the data sets derived from normal differentiated tissues (Figure S4).
DARs are enriched for binding motifs, occupied by TFs, and show sequence conservation
We noted that DHS overlapping repeats were enriched in chromatin states corresponding to promoters, enhancers, and insulators as defined previously using histone marks profiles [28] (Figure S5). To understand why specific TEs were contributing to open chromatin, we wanted to integrate the DARs with other more targeted functional genomics data sets. For example, it was shown previously that the pluripotency TF OCT4 was bound on LTR9B repeats [10] and it was interesting to see the same repeat subfamily as a DAR in ESCs (Table S2). When we looked in LTR9B for the binding motifs of OCT4 and SOX2, another pluripotency TF, we found them to be specifically over-represented in the repeat instances contributing to open chromatin (p = 6.8E-64 and 3.6E-38 respectively, see Materials and Methods). Notably, the peaks in the aggregate read density profiles of the DHS data in ESCs were also correlating with the localization of the motifs within the repeat instances (Figure 2A).
Fig. 2. DARs are enriched for binding motifs, occupied by TFs, and show sequence conservation. 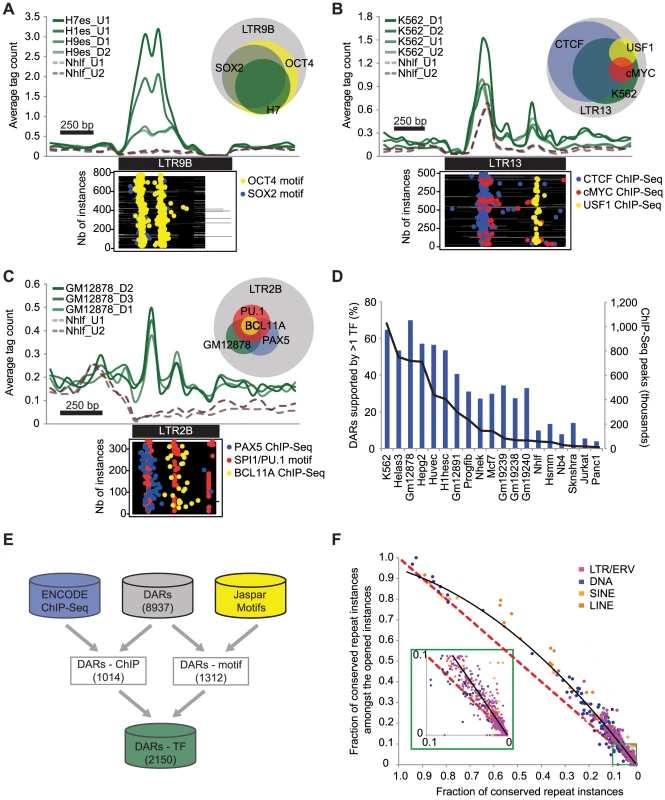
Aggregate profiles of DNase I tags (green) over the instances of different DARs: (A) LTR9B in ESC, (B) LTR13 in K562 and (C) LTR2B in GM12878. The profiles over another cell type (Nhlf) are shown as a control (dashed brown lines). The plots underneath the profiles represent the localization of regulatory motifs or ChIP-Seq peaks in the same cell lines (yellow, blue, red points). The Venn diagrams represent the proportion of repeat instances (grey) containing DHS and regulatory motifs or ChIP-Seq peaks using the same color code. (D) Proportion of DARs with at least one enriched TF (blue bars) and the total number of binding sites reported (black line) for each cell line. (E) Diagram showing the number of DARs supported by at least one TF based on ChIP-Seq or motif enrichment. (F) Scatterplot showing for each repeat subfamily, the fraction of conserved repeat instances amongst the opened instances. The black line represents a polynomial trend line of order 2. The red dashed line is the expected distribution. To characterize more systematically the role of repeat instances in the host genome and to identify putative functional factors associated with the DARs, we used a collection of TF binding sites determined by ENCODE using ChIP-Seq. In the 19 cell types where both DHS and ChIP-Seq data were available, we found that 1014 of the 2784 DARs (36.4%) were statistically enriched for at least one TF (Table S3). This relied on two statistical tests: one that showed that the TF was enriched in the same repeat subfamily and in the same cell type, and one that showed that the number of instances with both DHS and ChIP-Seq signal was also significant (see Materials and Methods). Using this strategy, we found for example that 82.9% of the 210 LTR13 instances that were contributing to open chromatin in K562 were also bound by CTCF (p = 1.1E-13, Figure 2B). Additional DARs supported by specific TFs such as PU.1, BCL11A, and PAX5 in LTR2B are shown in Figure 2C and Figure S6. Predictably, we found that a larger fraction of DARs can be explained by the binding of specific TFs in cell lines where more ChIP-Seq data sets were available (Figure 2D).
To improve on the limited ChIP-Seq coverage in some cell types and in order to characterize the DARs more comprehensively, we developed a classifier to predict TF-repeat associations using Jaspar TF binding motifs (see Materials and Methods). Using this classifier we were able to suggest 3073 high-confidence motif-repeat subfamily associations for 1312 DARs (Table S4). By combining both methods, we were able to predict a total of 2150 unique TF-repeat subfamily associations, which suggest potential functional candidates for 24.1% of the DARs (Figure 2E).
Finally, to further confirm the functional importance of DARs, we also used the annotated conserved non-exonic elements (CNEEs) [29] and assessed the overall sequence conservation of the TEs that were contributing to open chromatin. In total, while only 5.5% of all repeat instances were conserved, we found that 9.0% of the repeats contributing to open chromatin were conserved, a difference that is highly significant (p<1.0E-100, Figure S7). Notably, for almost all repeat subfamilies, we found that the subset of instances contributing to open chromatin was more conserved than expected by chance (Figure 2F).
Thousands of LTR/ERV sequences are activated in a cell type–specific manner especially in ESCs
Next, we were interested in the contribution of repeats to cell type-specific DHS. When we calculated the number of cell types contributing to individual DHS regions, we found that 76.0% of the loci were open in 4 cell types or less (Figure S8, see Materials and Methods). We also observed that regions contributed by few cell types were found more frequently in repetitive sequences (Figure 3A). To determine the cell type-specificity of each repeat subfamily we used the median number of open instances in all DHS data sets as the denominator and calculated the fold enrichment for each repeat subfamily in each cell type (see Materials and Methods). A total of 770 DARs showed a cell type-specific fold enrichment greater than 3 (Figure S8 and Table S2). Notably, we observed that LTR/ERV repeat subfamilies were over-represented in the cell type-specific DARs (Figure 3B) and that on average a higher number of cell type-specific DARs were found in ESCs and cancer data sets (Figure 3C). These patterns were also recapitulated in the top 100 repeat subfamilies with the greatest cell type-specific enrichment (Figure 3D). For example, in the case of the LTR7 repeat subfamily, we observed a remarkable enrichment of 131.6 - and 88.7-fold in the ESC lines H7 and H1 respectively. While most cell type-specific DARs were found in ESCs and cancer cell lines, we also found examples, such as the LTR2B and MER121 subfamilies, which had most of their instances in open chromatin from normal differentiated cells (Figure 3E). Additional examples of cell type-specific DARs are shown in Figure S9. We also found that the cell type-specificity of various subfamilies of TEs was supported by the chromatin states previously described [28]. For example, more than 40% of the LTR2B instances were annotated as enhancers in GM12878 while only 10% were annotated as such in H1. In contrast, more than 40% of the LTR7 instances were annotated as enhancers in H1 while only 2.2% of them were annotated as such in GM12878 (Figure S10).
Fig. 3. Thousands of LTR/ERV–derived sequences are activated in a cell type–specific manner, especially in ESCs. 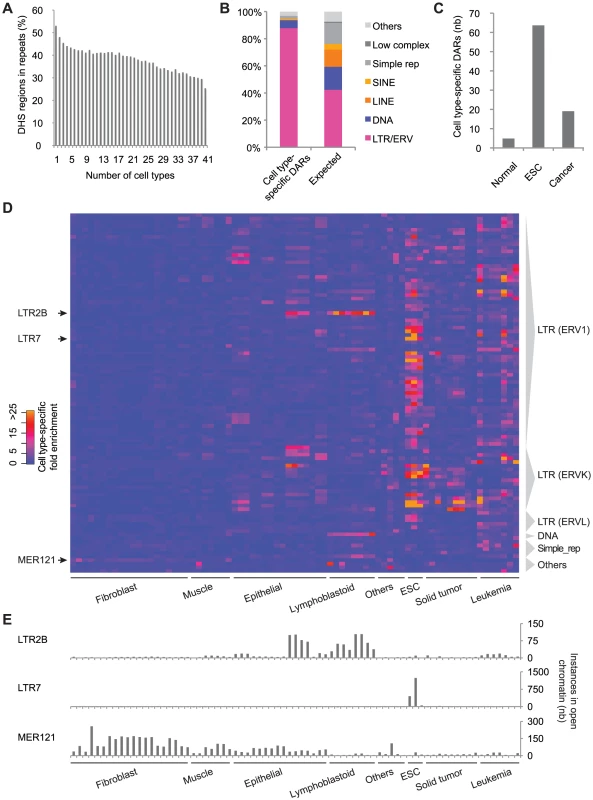
(A) Proportion of DHS clusters that overlap repeats based on the number of distinct cell types in which they are observed. (B) Proportion of cell type-specific DARs and of all repeat subfamilies (Expected) by repeat class. (C) Average number of cell type-specific DARs per data set in normal, embryonic and cancer cell lines. (D) Heatmap showing the cell type-specific fold enrichment for the top 100 repeat subfamilies. (E) Number of instances contributing to open chromatin for the LTR2B, LTR7 and MER121 repeat subfamilies. Finally, using a collection of TF binding motifs including novel motifs identified in DNase I footprints [30], we identified tissue-specific motifs enriched in these cell type-specific DARs (Table S5, see Materials and Methods). In particular, we observed that many ESC-specific DARs were supported by ESC-specific motifs that were not enriched in normal - or cancer-specific DARs (Figure S11). The top three ESC-specific motifs found in this way were OCT4, SOX2 and KLF4.
DARs are associated with cell type–specific expression and over-represented in dsQTLs
To evaluate the impact of DARs on gene regulation, we used 43 gene expression exon-array data sets from ENCODE and calculated the number of genes in proximity to DAR instances that were up-regulated in the relevant cell type relative to the others (see Materials and Methods). We identified 783 DARs with more proximal up-regulated genes than expected by chance (Table S6). For example, we identified 11 genes in proximity to LTR2B instances that were up-regulated in GM12865 while we would have only expected 4.27 (Figure 4A). Examples of cell type-specific LTR2B associated genes in GM12865 include NAPSB and CLECL1 (Figure 4B and Figures S12, S13), two genes that have been shown to play a role in lymphoblastoid cells [31], [32]. Moreover, we observed that the expression of the DAR-associated genes were frequently highest in the cell type where the DAR had been identified (Figure 4C and Figure S14). We also found that DARs with a higher cell type-specificity score had a higher chance of being associated with cell type-specific expression (Figure 4D). Similar results were obtained using ENCODE RNA-Seq data sets generated by Caltech (Figure S15).
Fig. 4. Cell type–specific expression of DAR–associated genes. 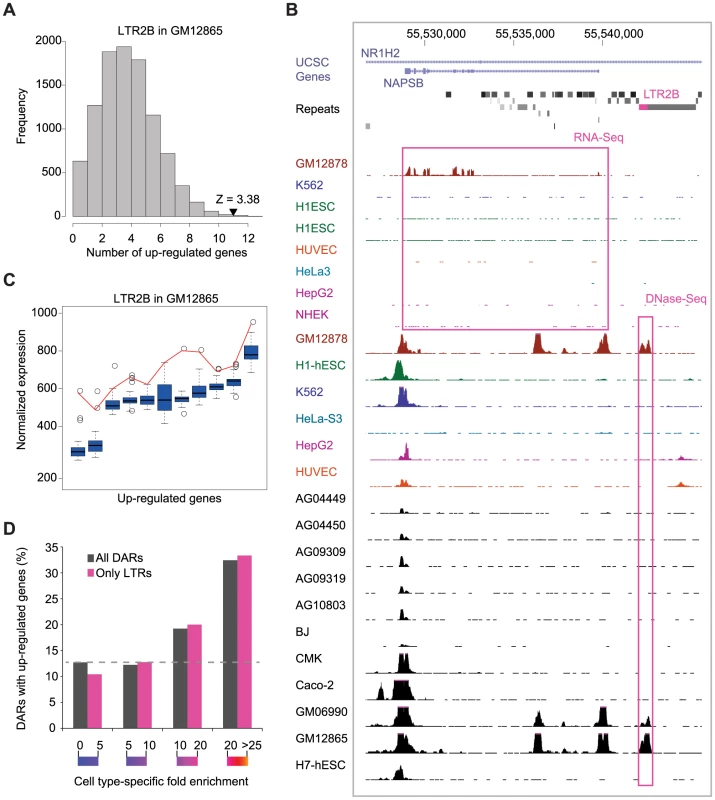
(A) Distribution of the expected number of up-regulated genes in proximity to the LTR2B DAR instances in GM18265. Actual number of up-regulated genes is shown using an arrowhead. (B) UCSC genome browser view of the NAPSB gene with selected RNA-Seq and DHS ENCODE tracks (y-axis maximum set to 20 and 100 respectively). The LTR2B repeat is highlighted in pink along with its cell type-specific open chromatin and expression profiles. (C) Boxplots showing the expression values across cell types for the DAR-associated genes that are up-regulated. Red lines are connecting the expression values observed in GM18265. (D) Cell type-specific DARs have more cell type-specific expression. DARs were binned according to their cell type-specific fold enrichment and the proportion of them having a Z-score of cell type-specificity expression above 3 is shown. Finally, a recent study combining genotypes with DHS data in 70 lymphoblastoid cell lines has shown that a significant proportion of open chromatin regions, known as dsQTLs, can be influenced by polymorphisms [33]. Having demonstrated that DARs exhibit features associated with regulatory elements, we wanted to test if they also showed this variation across individuals. We found that 36.8% of the reported dsQTLs overlapped repeat instances and that these were contributed by DAR instances in lymphoblastoid cells more than expected by chance (p = 1.1E-6, see Materials and Methods).
Discussion
In summary, we found that TEs have contributed nearly half of the open chromatin regions of the human genome and the majority of primate-specific elements. This estimate is a lower bound that is likely to grow given that better strategies using longer and paired-end reads will be needed to measure the contribution of young repeat subfamilies and polymorphic sites (Figure S3). An example is the L1PA2 repeat subfamily where, despite the fact that the mappability ratio is 0.08, 117 and 257 of the 4904 L1PA2 instances contributed to the H1 and H7 DHSs respectively. This finding is consistent with previous observations [7], [15], [34], [35] but greatly expands on our understanding of the repeat families contributing to open chromatin in the human genome.
To better understand the regulatory functions that could have been retained in exapted TEs beyond the ones that have already been studied (e.g. [8]–[11], [14]), we predicted a total of 2150 TF-repeat subfamily associations and confirmed that a broad range of functional proteins are targeting these regions (Figure 2 and Tables S3, S4). This resource will be useful to provide insights into the regulation of some of the TE-derived loci that have already been implicated in disease [36]. There is an important distinction between biochemical activity and functional relevance to the host. To help confirm the importance of these regions, we also showed that repeat instances contributing to open chromatin were more conserved than expected by chance (Figure 2F).
Next, we demonstrated that LTR/ERV repeats have contributed a disproportionate fraction of cell type-specific accessible chromatin regions especially in embryonic and cancer cell lines (Figure 3). This is interesting given that network rewiring using ERV elements has already been described in ESCs [10]–[12] and that it has been shown that stem cell potency fluctuates with endogenous retrovirus activity in mouse [37]. The level of activity observed in ERV sequences is likely a consequence of the permissive chromatin state found in ESCs that it sometimes reinstated in cancer [38]. There is fine balance between the successful replication of endogenous retroviruses, from which these repeats are derived, and retrotransposition control in the host [39]. One intriguing possibility is that the manipulations that were initially exerted by the ancestral viruses on their host to by-pass these control mechanisms have also facilitated co-option [40].
Finally, we also reported that repeat subfamilies activated in a cell type-specific manner were also frequently associated with higher expression of neighboring genes. This result corroborates the fact that at the level of expression, TE-derived transcripts, including lincRNAs [41], are also usually tissue-specific [42]. Interestingly, this pattern was observed not only in ESCs but also in differentiated and cancer cells (Figure 4 and Table S6).
Taken together, these results demonstrate that TEs, and in particular endogenous retroviruses, have considerably transformed the transcriptional landscape during primate evolution.
Materials and Methods
DNase I hypersensitive sites datasets
We retrieved 106 ENCODE DHS data sets available from the October 2010 freeze which included replicates for 50 different cell types from a variety of normal differentiated cell types, human ESCs and cancer cell types using the UCSC ENCODE portal (http://genome.ucsc.edu/ENCODE/). These data had been generated from performing DNase I digestion of intact nuclei, isolating DNase I digested fragments and direct sequencing of fragment ends [23], [24]. We discarded 3 data sets involving treatments and performed extensive quality control of the data sets. Specifically, for each peaks file generated by UW corresponds one tagAlign file such that we calculated the average GC content of the tags and removed data sets with GC bias (>55% or <45%). We also used FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) on these files to remove data sets that failed the per sequence quality criteria (when the most frequently observed mean quality is below 20) or where the total number of overrepresented sequences was above 1 million. Multiple tagAlign files generated by Duke had been combined to call the peaks and we analyzed the individual tagAlign files as described above. The quality control summary is presented in Table S1. In the end, we retained 75 data sets that did not have any unusual deviation based on our quality metrics: 51 produced by UW and 24 by Duke, covering 41 different cell types grouped in 8 “tissues” (Table 1). Most of the UW data sets had a read length of 36 bp while the Duke data sets had a read length of 20 bp. For the downstream analyses, we used the narrow peak files that were generated from the uniquely mapped reads and provided by ENCODE.
Overlap between DHS regions and repeats
The peak regions from the 75 data sets were combined and those within less than 100 bp were grouped in 1,643,643 distinct DHS regions. These DHS regions were resized to 200 bp using their middle point before intersecting with the 5,269,366 repeat instances of RepeatMasker [43] from the UCSC Genome Browser [44] (Figure 1A). In order to calculate the proportion of DHS regions overlapping different classes of repeats based on the age of the sequence in which they are embedded, we sequentially used the liftOver utility from UCSC using default parameters and minMatch set to 0.5. The DHS regions (hg18) were first converted to the mouse genome (mm9), those not converted were then mapped to the marmoset genome (CalJac3), and again those not converted were mapped to the chimp genome (PanTro2) to identify the 23,917 human-specific DHS regions. Primate species divergence time taken from [45]. We also obtained an alignment-free estimate of the age of the repeat subfamilies using the average divergence between the instances and the ancestral repeat consensus (milliDiv value reported by RepBase) and applying the Jukes Cantor method with a substitution rate of 2.2×10-9 per site per year [7]. The ages obtained using this method were largely consistent with previous estimates [16], [46]. Repeat subfamilies with an estimated age <95 Myrs were said to be primate-specific.
Annotation-matched random distributions and DAR identification
For each DHS data set, we calculated the number of overlaps within each repeat subfamily using a 200bp window surrounding the center of the DHS peaks. Next, following a strategy developed on ChIP-Seq data sets [7], [10], we annotated each DHS with respect to its nearest RefSeq genes and we binned the DHS into six categories according to the peak location: TSS (within 1 kbp of a TSS), promoter (up to 5 kbp upstream of TSS), intragenic (within the RefSeq gene boundary), proximal (up to 10 kbp away from the gene boundaries), distal (up to 100 kbp away from the gene boundaries) and desert (more than 100 kbp away from any RefSeq genes). We then generated for each DHS data set a random set of 200,000 regions with the same annotation distribution as the true regions and intersected with the RepeatMasker track to obtain the expected number of overlaps for each repeat subfamily. We used a onesided binomial test to compare the observed number of repeats intersecting the true DHS with the expected numbers from the annotationmatched background. We identified repeat subfamilies with statistically significant contribution to open chromatin (p<1E-5) as DHS-associated repeats (DARs).
Properties of repeat subfamilies and mappability ratio
We verified that various properties of the repeat subfamilies were comparable across repeat classes. Specifically, we checked for an association between the fraction of repeat instance contributing to open chromatin and the number of instances in a given subfamily, average size and GC content (Figure S2). We only detected an association between open chromatin contribution and GC content affecting Low complexity, Simple repeats and Others repeat classes, which we excluded from most analyses. The majority of the ENCODE processed data sets, such as the narrow peak files that we used in the current study, rely on uniquely mapped reads [47], [48]. To test the impact of such a criteria on the detection of TEs regulatory activity, we extracted 50 million 20 bp and 36 bp sequences (to mimic Duke and UW read lengths respectively) from random location on the human and re-mapped these artificial reads using the Bowtie program [49] and allowing 1 and 2 mismatches respectively. The goal of these simulations was to compute a mappability ratio for each repeat subfamily that is: the ratio between the number of uniquely mapped tags in a particular subfamily and the number of tags that were extracted from this subfamily (Figure S3).
Chromatin states analyses
The DHS from the six cell lines (some with two replicates for a total of eight DHS data sets) for which the chromatin states (CS) were available [28] were used for this analysis. The DHS were associated to one of the 15 CS (overlap >50%) using intersectBed from BEDtools [50], then grouped whether they overlap any repeat instance or not. For each data set, the proportion of DHS annotated as each CS was computed, and similar CS were combined together (ex: strong and weak enhancers grouped as enhancers). Similarly, random DHS (using shuffleBed) were generated and overlapped with the CS. Figure S5 is showing the average and standard deviation over the eight data sets. Repeat instances from specific DARs (LTR7, LTR2B and LTR13) were intersected with the CS using a similar strategy (Figure S10).
ChIP–Seq analysis
We used 183 distinct ENCODE ChIP-Seq data sets generated by the Broad Institute, Duke, HudsonAlpha and Yale, covering 87 different TFs from 19 cell lines for which we also used DHS. For each of these data sets we applied the same procedure as to identify the DARs and identified a total of 9367 TF-repeat subfamily pairs. These pairs were then intersected with the DARs and for each combination from the same cell type and the same repeat subfamily we applied a hypergeometric to test the significance of the number of instances with ChIP-Seq peak and DHS. Using a stringent cutoff (p-value<0.001), we identified 2800 statistically significant combinations of DAR-ChIP Seq for 1014 distinct DARs (Table S3).
Motif analysis
Using ChIP-Seq data sets obtained previously [51], we trained a classifier that uses the over-representation of TF binding motifs and other features of repeat subfamilies to predict TF-repeat associations. Briefly, five features of repeat subfamilies were used: 1) fraction of repeat instances with motif, 2) fraction of motifs contained within repeat subfamily, 3) motif score ratio between bound and unbound repeat instances, 4) enrichment test for binding motifs within repeat subfamilies, 5) simulations looking at the potential of repeat sequences to generate binding motifs. By combining these individual features using a weighted rank average we were able to achieve an Area-Under-the-Curve (AUC) of 0.81 for this classifier (Jeyakani et al., in preparation). Using 103 JASPAR TF binding motifs derived from human or mouse [52], we applied our classifier to the list of putative motif-repeat subfamily pairs and using a stringent cutoff (top 10%) we identified 2337 potential associations. These motif-repeat subfamily pairs were then intersected with the DARs and for each combination from the same repeat subfamily we applied a hypergeometric to test the significance of the number of instances with motifs and DHS. Using a stringent cutoff (p-value<0.001), we identified 3857 statistically significant combinations of DAR-motif from 1312 distinct DARs (Table S4).
Cell type specificity and motif enrichment
In order to calculate the cell type-specific enrichment for each repeat subfamily, we determined the median number of repeats bound across the DHS data sets (these numbers were further normalized to the total number of sites in each data sets). The median value was computed independently for the UW and the Duke data sets because of the expected differences in mappability given the differences in read lengths. Next, we calculated a cell type-specific fold enrichment for each repeat subfamily in a given cell type by dividing the observed number of repeats contributing to open chromatin in this particular DHS data set by the median number of repeats contributing to open chromatin for this subfamily. This was done for all DARs and non-DARs (Figure S8). Next, we scanned the 56,837 DHS from the 770 cell type-specific DARs for motifs using the FIMO software tool with a maximum p-value threshold of 1×10−5 as was done in [30]. We provided motif templates from Jaspar [52], TransFac [53], Uniprobe [54] and novel de novo motifs identified previously in DNAse I footprints [30]. For each DAR, we then identified the motifs present in >25 repeat instances and in >20% of the instances contributing DHS (Table S5). We also calculated the proportion of DARs from ESC, Cancer and differentiated normal cells with support of at least one of the 28 ESC-specific motif identified in [30] (Figure S11).
Expression analyses
From the 70 ENCODE UW Affy All-Exon Arrays expression data sets, only those from the cell types with DHS were selected. These data sets were clustered showing that a few replicates were inconsistent and therefore removed of the downstream analyses, leaving 43 expression data sets (most of them in duplicate). Note that we tried to combine these data sets with the ENCODE Duke Affy All-Exon Arrays data sets but found that the platform correlation was higher than the biological correlation between biological replicates so we therefore decided to only use the UW data (data not shown). Expression data was available in the cell type for 6054 of the 8937 DARs. A gene was called up-regulated in a cell type if it had a Z-score >2 in at least one of the data set compared to the other data sets. For each DAR, the Z-score of cell type-specific expression based on the number of up-regulated genes was computed on permutation tests by randomly picking 10,000 times the same number of genes that associated with the DAR from the set of 35,865 different gene names covered by the arrays. For example, from the 2337 instances of LTR7 in the genome, 788 were contributing to open chromatin in H7 and those were associated to a total of 561 distinct genes. The fact that 85 of these genes were up-regulated in the ESC cell type while only 19.6 (+/−4.3) were expected based the permutation test gives a Z-score of 15.1 (Figure S13 and Table S6).
Similarly, 13 RNA-Seq data sets generated by Caltech from 7 distinct cell types were used to calculate the association of DARs with up-regulation of expression. A 50 kb window centered in the middle of each repeat instance was used and, to estimate the background, the genome was independently segmented in non-overlapping 50 kb windows. For each RNA-Seq data set, the average tag density was calculated in each window. For each window, the mean and SD of the average tag density was then calculated across the 13 RNA-Seq data sets in order to identify up-regulated windows defined as a Z-score >2 in one of the data set of the same cell type compared to the other data sets. For the 2124 DARs for which expression data was available, the Z-score of cell type-specificity expression was computed using permutation tests by randomly picking 10,000 times the same number of windows than the number or repeat instances contributing to open chromatin in this cell type from the background genomic segments (Figure S14).
dsQTLs analysis
The 1,034,427 DHS from the 8 lymphoblastoid DHS data sets were first grouped into 430,159 clusters as described above. Using intersectBed, we found as expected that most (4891 of 6070 (80.6%)) short dsQTLs from [33] were overlapping these DHS lymphoblastoid clusters. We also found that 2234 of 6070 (36.8%) short dsQTLs were overlapping a repeat instance. Considering that 995 of the 4891 (20.3%) short dsQTLs overlap one of the 77,135 DHS lymphoblastoid clusters contributed by DAR instances in lymphoblastoid cells (17.9% of all lymphoblastoid clusters), this overlap is highly significant (hypergeometric p = 1.11E-6). Doing the same for the DHS lymphoblastoid clusters that were overlapping DAR instances from the other cell types gave a more marginal enrichment (hypergeometric p = 1.09E-2).
Supporting Information
Zdroje
1. Craig N, R C, M G, AM L (2002) Mobile DNA II. Washington, DC: ASM Press.
2. OrgelLE, CrickFH (1980) Selfish DNA: the ultimate parasite. Nature 284 : 604–607.
3. DoolittleWF, SapienzaC (1980) Selfish genes, the phenotype paradigm and genome evolution. Nature 284 : 601–603.
4. McClintockB (1984) The significance of responses of the genome to challenge. Science 226 : 792–801.
5. DavidsonEH, BrittenRJ (1979) Regulation of gene expression: possible role of repetitive sequences. Science 204 : 1052–1059.
6. FeschotteC (2008) Transposable elements and the evolution of regulatory networks. Nature reviews Genetics 9 : 397–405.
7. BourqueG, LeongB, VegaVB, ChenX, LeeYL, et al. (2008) Evolution of the mammalian transcription factor binding repertoire via transposable elements. Genome Res 18 : 1752–1762.
8. WangT, ZengJ, LoweCB, SellersRG, SalamaSR, et al. (2007) Species-specific endogenous retroviruses shape the transcriptional network of the human tumor suppressor protein p53. Proc Natl Acad Sci U S A 104 : 18613–18618.
9. SchmidtD, SchwaliePC, WilsonMD, BallesterB, GoncalvesA, et al. (2012) Waves of retrotransposon expansion remodel genome organization and CTCF binding in multiple mammalian lineages. Cell 148 : 335–348.
10. KunarsoG, ChiaNY, JeyakaniJ, HwangC, LuX, et al. (2010) Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat Genet 42 : 631–634.
11. LynchVJ, LeclercRD, MayG, WagnerGP (2011) Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat Genet 43 : 1154–1159.
12. ChuongEB, RumiMA, SoaresMJ, BakerJC (2013) Endogenous retroviruses function as species-specific enhancer elements in the placenta. Nat Genet 45 : 325–329.
13. WangJ, ZhuangJ, IyerS, LinX, WhitfieldTW, et al. (2012) Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res 22 : 1798–1812.
14. TestoriA, CaizziL, CutrupiS, FriardO, De BortoliM, et al. (2012) The role of Transposable Elements in shaping the combinatorial interaction of Transcription Factors. BMC Genomics 13 : 400.
15. Marino-RamirezL, JordanIK (2006) Transposable element derived DNaseI-hypersensitive sites in the human genome. Biology direct 1 : 20.
16. WaterstonRH, Lindblad-TohK, BirneyE, RogersJ, AbrilJF, et al. (2002) Initial sequencing and comparative analysis of the mouse genome. Nature 420 : 520–562.
17. EwingAD, KazazianHHJr (2010) High-throughput sequencing reveals extensive variation in human-specific L1 content in individual human genomes. Genome Res 20 : 1262–1270.
18. CoufalNG, Garcia-PerezJL, PengGE, YeoGW, MuY, et al. (2009) L1 retrotransposition in human neural progenitor cells. Nature 460 : 1127–1131.
19. IskowRC, McCabeMT, MillsRE, ToreneS, PittardWS, et al. (2010) Natural mutagenesis of human genomes by endogenous retrotransposons. Cell 141 : 1253–1261.
20. BaillieJK, BarnettMW, UptonKR, GerhardtDJ, RichmondTA, et al. (2011) Somatic retrotransposition alters the genetic landscape of the human brain. Nature 479 : 534–537.
21. BernsteinBE, BirneyE, DunhamI, GreenED, GunterC, et al. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489 : 57–74.
22. ThurmanRE, RynesE, HumbertR, VierstraJ, MauranoMT, et al. (2012) The accessible chromatin landscape of the human genome. Nature 489 : 75–82.
23. CrawfordGE, DavisS, ScacheriPC, RenaudG, HalawiMJ, et al. (2006) DNase-chip: a high-resolution method to identify DNase I hypersensitive sites using tiled microarrays. Nature methods 3 : 503–509.
24. SaboPJ, KuehnMS, ThurmanR, JohnsonBE, JohnsonEM, et al. (2006) Genome-scale mapping of DNase I sensitivity in vivo using tiling DNA microarrays. Nature methods 3 : 511–518.
25. DohmJC, LottazC, BorodinaT, HimmelbauerH (2008) Substantial biases in ultra-short read data sets from high-throughput DNA sequencing. Nucleic Acids Res 36: e105.
26. NishiharaH, SmitAF, OkadaN (2006) Functional noncoding sequences derived from SINEs in the mammalian genome. Genome Res 16 : 864–874.
27. KamalM, XieX, LanderES (2006) A large family of ancient repeat elements in the human genome is under strong selection. Proc Natl Acad Sci U S A 103 : 2740–2745.
28. ErnstJ, KheradpourP, MikkelsenTS, ShoreshN, WardLD, et al. (2011) Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473 : 43–49.
29. LoweCB, KellisM, SiepelA, RaneyBJ, ClampM, et al. (2011) Three periods of regulatory innovation during vertebrate evolution. Science 333 : 1019–1024.
30. NephS, VierstraJ, StergachisAB, ReynoldsAP, HaugenE, et al. (2012) An expansive human regulatory lexicon encoded in transcription factor footprints. Nature 489 : 83–90.
31. CookM, BuhlingF, AnsorgeS, TatnellPJ, KayJ (2002) Pronapsin A and B gene expression in normal and malignant human lung and mononuclear blood cells. Biochimica et biophysica acta 1577 : 10–16.
32. RyanEJ, MarshallAJ, MagalettiD, FloydH, DravesKE, et al. (2002) Dendritic cell-associated lectin-1: a novel dendritic cell-associated, C-type lectin-like molecule enhances T cell secretion of IL-4. Journal of immunology 169 : 5638–5648.
33. DegnerJF, PaiAA, Pique-RegiR, VeyrierasJB, GaffneyDJ, et al. (2012) DNase I sensitivity QTLs are a major determinant of human expression variation. Nature 482 : 390–394.
34. Marino-RamirezL, LewisKC, LandsmanD, JordanIK (2005) Transposable elements donate lineage-specific regulatory sequences to host genomes. Cytogenetic and genome research 110 : 333–341.
35. van de LagemaatLN, LandryJR, MagerDL, MedstrandP (2003) Transposable elements in mammals promote regulatory variation and diversification of genes with specialized functions. Trends in genetics : TIG 19 : 530–536.
36. LamprechtB, WalterK, KreherS, KumarR, HummelM, et al. (2010) Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nature medicine 16 : 571–579, 571p following 579.
37. MacfarlanTS, GiffordWD, DriscollS, LettieriK, RoweHM, et al. (2012) Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487 : 57–63.
38. JonesPA, BaylinSB (2007) The epigenomics of cancer. Cell 128 : 683–692.
39. RoweHM, TronoD (2011) Dynamic control of endogenous retroviruses during development. Virology 411 : 273–287.
40. FeschotteC, GilbertC (2012) Endogenous viruses: insights into viral evolution and impact on host biology. Nature reviews Genetics 13 : 283–296.
41. KelleyD, RinnJ (2012) Transposable elements reveal a stem cell-specific class of long noncoding RNAs. Genome biology 13: R107.
42. FaulknerGJ, KimuraY, DaubCO, WaniS, PlessyC, et al. (2009) The regulated retrotransposon transcriptome of mammalian cells. Nat Genet 41 : 563–571.
43. Smit A, Hubley, R, Green, P (1996–2012) RepeatMasker Open - 3.0, version 3.2.7. Available: http://www.repeatmasker.org/.
44. KentWJ, SugnetCW, FureyTS, RoskinKM, PringleTH, et al. (2002) The human genome browser at UCSC. Genome Res 12 : 996–1006.
45. GlazkoGV, NeiM (2003) Estimation of divergence times for major lineages of primate species. Mol Biol Evol 20 : 424–434.
46. GiordanoJ, GeY, GelfandY, AbrusanG, BensonG, et al. (2007) Evolutionary history of mammalian transposons determined by genome-wide defragmentation. PLoS Comput Biol 3: e137 doi:10.1371/journal.pcbi.0030137.
47. BirneyE, StamatoyannopoulosJA, DuttaA, GuigoR, GingerasTR, et al. (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 : 799–816.
48. RoyS, ErnstJ, KharchenkoPV, KheradpourP, NegreN, et al. (2010) Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science 330 : 1787–1797.
49. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology 10: R25.
50. QuinlanAR, HallIM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26 : 841–842.
51. ChenX, XuH, YuanP, FangF, HussM, et al. (2008) Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133 : 1106–1117.
52. SandelinA, AlkemaW, EngstromP, WassermanWW, LenhardB (2004) JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res 32: D91–94.
53. MatysV, Kel-MargoulisOV, FrickeE, LiebichI, LandS, et al. (2006) TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res 34: D108–110.
54. RobaskyK, BulykML (2011) UniPROBE, update 2011: expanded content and search tools in the online database of protein-binding microarray data on protein-DNA interactions. Nucleic Acids Res 39: D124–128.
Štítky
Genetika Reprodukční medicína
Článek Attachment Site Selection and Identity in Bxb1 Serine Integrase-Mediated Site-Specific RecombinationČlánek Bck2 Acts through the MADS Box Protein Mcm1 to Activate Cell-Cycle-Regulated Genes in Budding YeastČlánek High-Resolution Transcriptome Maps Reveal Strain-Specific Regulatory Features of Multiple IsolatesČlánek Neuropeptides Function in a Homeostatic Manner to Modulate Excitation-Inhibition Imbalance inČlánek Implicates Tyrosine-Sulfated Peptide Signaling in Susceptibility and Resistance to Root Infection
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 5
-
Všechny články tohoto čísla
- Functional Elements Are Embedded in Structurally Constrained Sequences
- RNA–Mediated Epigenetic Heredity Requires the Cytosine Methyltransferase Dnmt2
- Loss of Expression and Promoter Methylation of SLIT2 Are Associated with Sessile Serrated Adenoma Formation
- Attachment Site Selection and Identity in Bxb1 Serine Integrase-Mediated Site-Specific Recombination
- Human Genetics in Rheumatoid Arthritis Guides a High-Throughput Drug Screen of the CD40 Signaling Pathway
- Genome-Wide Analysis in German Shepherd Dogs Reveals Association of a Locus on CFA 27 with Atopic Dermatitis
- Liver X Receptors Protect from Development of Prostatic Intra-Epithelial Neoplasia in Mice
- Chromosomal Organization and Segregation in
- A Statistical Framework for Joint eQTL Analysis in Multiple Tissues
- Cell Polarity and Patterning by PIN Trafficking through Early Endosomal Compartments in
- Bck2 Acts through the MADS Box Protein Mcm1 to Activate Cell-Cycle-Regulated Genes in Budding Yeast
- High-Resolution Transcriptome Maps Reveal Strain-Specific Regulatory Features of Multiple Isolates
- Neuropeptides Function in a Homeostatic Manner to Modulate Excitation-Inhibition Imbalance in
- A Compendium of Nucleosome and Transcript Profiles Reveals Determinants of Chromatin Architecture and Transcription
- Wnt Signaling Regulates the Lineage Differentiation Potential of Mouse Embryonic Stem Cells through Tcf3 Down-Regulation
- Filamin and Phospholipase C-ε Are Required for Calcium Signaling in the Spermatheca
- The Specificity and Flexibility of L1 Reverse Transcription Priming at Imperfect T-Tracts
- Imputation-Based Meta-Analysis of Severe Malaria in Three African Populations
- Implicates Tyrosine-Sulfated Peptide Signaling in Susceptibility and Resistance to Root Infection
- Clathrin and AP2 Are Required for Phagocytic Receptor-Mediated Apoptotic Cell Clearance in
- Encodes CDF Transporters That Excrete Zinc from Intestinal Cells of and Act in a Parallel Negative Feedback Circuit That Promotes Homeostasis
- Global Properties and Functional Complexity of Human Gene Regulatory Variation
- DNA Binding of the Cell Cycle Transcriptional Regulator GcrA Depends on N6-Adenosine Methylation in and Other
- Side Effects: Substantial Non-Neutral Evolution Flanking Regulatory Sites
- From Paramutation to Paradigm
- From Mouse to Human: Evolutionary Genomics Analysis of Human Orthologs of Essential Genes
- Distinct Translational Control in CD4 T Cell Subsets
- Female Bias in and Regulation by the Histone Demethylase KDM6A
- ATM–Dependent MiR-335 Targets CtIP and Modulates the DNA Damage Response
- HDAC7 Is a Repressor of Myeloid Genes Whose Downregulation Is Required for Transdifferentiation of Pre-B Cells into Macrophages
- The Majority of Primate-Specific Regulatory Sequences Are Derived from Transposable Elements
- Identification of Meiotic Cyclins Reveals Functional Diversification among Plant Cyclin Genes
- EGL-13/SoxD Specifies Distinct O and CO Sensory Neuron Fates in
- Congruence of Additive and Non-Additive Effects on Gene Expression Estimated from Pedigree and SNP Data
- Using Extended Genealogy to Estimate Components of Heritability for 23 Quantitative and Dichotomous Traits
- Ikbkap/Elp1 Deficiency Causes Male Infertility by Disrupting Meiotic Progression
- Analysis of the Genetic Basis of Disease in the Context of Worldwide Human Relationships and Migration
- Duplication and Retention Biases of Essential and Non-Essential Genes Revealed by Systematic Knockdown Analyses
- Strong Purifying Selection at Synonymous Sites in
- , a Susceptibility Gene for Type 1 and Type 2 Diabetes, Modulates Pancreatic Beta Cell Apoptosis via Regulation of a Splice Variant of the BH3-Only Protein
- Chromosome Movements Promoted by the Mitochondrial Protein SPD-3 Are Required for Homology Search during Meiosis
- The Secretory Pathway Calcium ATPase PMR-1/SPCA1 Has Essential Roles in Cell Migration during Embryonic Development
- The Genomic Signature of Crop-Wild Introgression in Maize
- CDK4 T172 Phosphorylation Is Central in a CDK7-Dependent Bidirectional CDK4/CDK2 Interplay Mediated by p21 Phosphorylation at the Restriction Point
- Genome-Wide Identification of Regulatory RNAs in the Human Pathogen
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Using Extended Genealogy to Estimate Components of Heritability for 23 Quantitative and Dichotomous Traits
- HDAC7 Is a Repressor of Myeloid Genes Whose Downregulation Is Required for Transdifferentiation of Pre-B Cells into Macrophages
- Female Bias in and Regulation by the Histone Demethylase KDM6A
- ATM–Dependent MiR-335 Targets CtIP and Modulates the DNA Damage Response
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



