-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaDisease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
Coronary heart disease (CHD) is the leading cause of mortality in both developed and developing countries worldwide. Genome-wide association studies (GWAS) have now identified 46 independent susceptibility loci for CHD, however, the biological and disease-relevant mechanisms for these associations remain elusive. The large-scale meta-analysis of GWAS recently identified in Caucasians a CHD-associated locus at chromosome 6q23.2, a region containing the transcription factor TCF21 gene. TCF21 (Capsulin/Pod1/Epicardin) is a member of the basic-helix-loop-helix (bHLH) transcription factor family, and regulates cell fate decisions and differentiation in the developing coronary vasculature. Herein, we characterize a cis-regulatory mechanism by which the lead polymorphism rs12190287 disrupts an atypical activator protein 1 (AP-1) element, as demonstrated by allele-specific transcriptional regulation, transcription factor binding, and chromatin organization, leading to altered TCF21 expression. Further, this element is shown to mediate signaling through platelet-derived growth factor receptor beta (PDGFR-β) and Wilms tumor 1 (WT1) pathways. A second disease allele identified in East Asians also appears to disrupt an AP-1-like element. Thus, both disease-related growth factor and embryonic signaling pathways may regulate CHD risk through two independent alleles at TCF21.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003652
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003652Summary
Coronary heart disease (CHD) is the leading cause of mortality in both developed and developing countries worldwide. Genome-wide association studies (GWAS) have now identified 46 independent susceptibility loci for CHD, however, the biological and disease-relevant mechanisms for these associations remain elusive. The large-scale meta-analysis of GWAS recently identified in Caucasians a CHD-associated locus at chromosome 6q23.2, a region containing the transcription factor TCF21 gene. TCF21 (Capsulin/Pod1/Epicardin) is a member of the basic-helix-loop-helix (bHLH) transcription factor family, and regulates cell fate decisions and differentiation in the developing coronary vasculature. Herein, we characterize a cis-regulatory mechanism by which the lead polymorphism rs12190287 disrupts an atypical activator protein 1 (AP-1) element, as demonstrated by allele-specific transcriptional regulation, transcription factor binding, and chromatin organization, leading to altered TCF21 expression. Further, this element is shown to mediate signaling through platelet-derived growth factor receptor beta (PDGFR-β) and Wilms tumor 1 (WT1) pathways. A second disease allele identified in East Asians also appears to disrupt an AP-1-like element. Thus, both disease-related growth factor and embryonic signaling pathways may regulate CHD risk through two independent alleles at TCF21.
Introduction
A recent meta-analysis of 14 Genome-wide association studies (GWAS) for CHD, Coronary ARtery DIsease Genome-wide Replication And Meta-analysis (CARDIoGRAM), including 22,233 cases and 64,762 controls in Europeans, elucidated 13 novel susceptibility loci [1]. One of these novel loci includes a variant, rs12190287 at 6q23.2, located within the 3′ untranslated region (3′UTR) of TCF21 [1]. This lead SNP at 6q23.2 had the lowest P value (P<4.6×10−11) of the novel loci in the meta-analysis and was also highly associated in the combined meta-analysis (P<1.1×10−12). rs12190287 was also identified as an expression quantitative trait locus (eQTL) through correlation with increased TCF21 gene expression in both liver and adipose tissue [1], [2]. Importantly, the TCF21 locus was recently replicated in another GWAS for CHD in a Han Chinese population (15,460 cases and 11,472 controls), however a second variant (rs12524865) that is poorly correlated with rs12190287 and located 14 kb upstream of TCF21 was the lead SNP in this racial ethnic group [3].
TCF21 is a member of the basic helix-loop-helix (bHLH) transcription factor (TF) family and regulates cell differentiation and cell fate decisions during development of the coronary vasculature, lung, kidney, and spleen [4], [5]. Tcf21 is expressed in mesodermal cells in the proepicardial organ (PEO) as early as E9.5 in mice, and later in mesenchymal cells forming the pericardial layer [4]. Recent elegant studies employing knockout mice have established a specific role for this factor in the origin of coronary artery smooth muscle cells and cardiac fibroblasts [6], [7]. Loss of Tcf21 expression in mouse results in increased expression of SMC markers in cells on the heart surface consistent with premature SMC differentiation [7], and a dramatic failure of cardiac fibroblast development [6], [7]. These data are most consistent with a role for Tcf21 in a bipotential precursor cell for SMC and cardiac fibroblast lineages, with loss of Tcf21 expression being essential for SMC development, and persistent Tcf21 expression being required for cardiac fibroblast development [6], [7].
In studies described here we examine the function of a regulatory element at the lead variant rs12190287 though allele-specific reporter assays, gel mobility shift assays, and haplotype specific chromatin immunoprecipitation (haploChIP). We further demonstrate allele-specific regulation of TCF21 gene expression, though modulating this regulatory element, via platelet derived growth factor receptor beta (PDGFR-β) and Wilms tumor 1 (WT1) mediated signaling. Lastly, we identify a conserved AP-1 dependent mechanism acting upstream of TCF21 at rs12524865, which was recently associated with CHD in East Asians [3]. Taken together, these studies elucidate both disease-related and embryonic pathways upstream of TCF21, at two independent risk alleles, thus providing further pathophysiological insight into the common heritable risk of CHD.
Results
Haplotype and regulatory analysis of TCF21 locus at 6q23.2
The CARDIoGRAM meta-analysis in Caucasians identified rs12190287 as the lead CHD association at 6q23.2 (P<4.64×10−11), which was 3 orders of magnitude more significant than other SNPs in this region (Figure 1A) [1]. We then set out to identify potential causal risk-associated mechanisms at 6q23.2 using an integrated workflow (Figure 1B). We examined the overall linkage disequilibrium (LD) plot around the TCF21 locus at 6q23.2 using 1568 individuals of European descent genotyped on the fine-mapping Metabochip array [8] (Illumina), which contains 196,726 polymorphisms [8], [9], of which approximately 280 markers were contained in the 170 kb block between Chr6 : 134,171,000–134,341,000. We identified regions of high LD surrounding the lead SNP, rs12190287, which is located in the 3′UTR of the non-coding exon of the long variant 1 of TCF21. Two haplotypes contained the high-risk allele at the lead SNP rs12190287, with one containing 3 out of the 5 additional eSNPs, while the haplotype with frequency 0.366 contained all of the eSNPS in the TCF21 locus (Figure 1C and 1D). We examined the LD plot containing the eSNPs for TCF21 (Figure 1C) and found that none of these variants had r2 values >0.8 with the lead SNP rs12190287, suggesting that if a single variant is responsible for the association observed by CARDIoGRAM, it is most likely to be rs12190287. This LD pattern is also consistent with the observation that rs12190287 was 3 orders of magnitude more significant than other SNPs in the region [1]. We then mapped regulatory chromatin regions surrounding rs12190287 using the ENCylopedia Of DNA Elements (ENCODE) Integrated Regulation data from 7 cell lines (Figure 1E), which demonstrated enrichment of the enhancer mark H3K4me1. Enrichment for histone modifications H3K4me3 (marks promoters), and H3K27ac (marks active regulatory elements) were also observed to a lesser extent. We also found regions of DNaseI hypersensitivity for open chromatin and overlapping RNA-seq peaks for transcriptional activity in the region containing rs12190287. We validated these histone modification and DNaseI ENCODE data with our own ChIP-seq and FAIRE-seq (Formaldehyde-Assisted Isolation of Regulatory Elements) experiments in HCASMC (Figure S1), demonstrating consistent regulation at rs12190287 and relevance to CHD. We also mapped the transcription factor binding sites (TFBS) using ENCODE data, which identified enrichment of an activator protein 1 (AP-1) component, JUND in a human embryonic stem cell line (Figure 1E).
Fig. 1. Haplotype and regulatory analysis of TCF21 locus at 6q23.2. 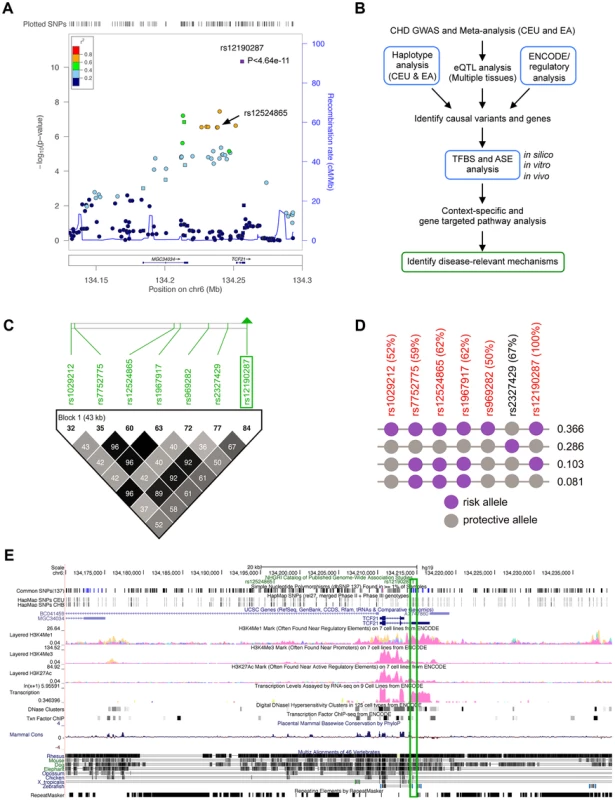
(a) Regional association plot of TCF21 locus showing results from CARDIoGRAM meta-analysis in Caucasians, identifying rs12190287, and also depicting rs12524865. (b) Integrated workflow to identify CHD risk-associated mechanisms, with emphasis of approaches used in the study (blue boxes). CEU: Caucasians of European descent from UT, USA; EA: East Asians; eQTL: Expression quantitative trait loci; ASE: Allele-specific expression, TFBS: Transcription factor binding site. (c) Linkage disequilibrium (LD) plot of the TCF21 locus at 6q23.2 from 1568 Metabochip genotyped Europeans (ADVANCE replication cohort) with imputation from HapMap Phase II and III CEU, showing eSNPs for TCF21, and rs2327429. White to black squares represent increasing r2 values. (d) The risk haplotype block containing all of the eSNPS (in red) at the TCF21 locus has a combined frequency of 0.366. r2 values in LD with rs12190287 shown as a percentage in parentheses. Risk and protective alleles determined from CARDIoGRAM meta-analysis phenotypic data. (e) ENCODE ChIP-seq active chromatin histone modification data from 7 cell lines including promoter and enhancer marks, H3K4me1, H3K4me3, and H3K27ac. ENCODE tracks for DNase hypersensitivity, RNA-seq, and TF binding ChIP-seq data. Green box surrounds peaks overlapping rs12190287. Allele-specific transcriptional regulatory activity at rs12190287 in vitro
Given that rs12192087 appeared to be the most likely causal variant at 6q23.2, we proceeded with in vitro functional studies to identify the risk-associated mechanisms through this variant. First, we mapped the putative TFBS in silico using various bioinformatics tools, including TRANSFAC, PROMO, MatInspector, and TFSearch (Table 1). Interestingly, multiple AP-1 TFs were predicted to preferentially bind to the major risk C allele, containing the binding motif, TGACTTCA (Figure S2A). Luciferase reporters containing the putative binding site for the risk and protective alleles were then transfected into various cell lines, including HepG2, HEK, and A7r5, as well as primary human coronary artery smooth muscle cells (HCASM), and rat aortic smooth muscle cells (RASM). We observed approximately 150–200 fold increase in activity with the rs12192087-C (C-Luc) reporter relative to the empty vector reporter, and this relative activity of the C-luc reporter was ∼20-fold greater than the rs12190287-G reporter (G-Luc) (Figure 2A). Similar results were observed in primary HCASM and RASM, suggesting that the G allele disrupts TF binding, leading to reduced TCF21 transcription. Given the ubiquitous expression of AP-1 factors in various cell types, it is not surprising that cell-specific activity was not observed. Interestingly, the allele-specific difference in transcription was lost when we mutated the 8-mer binding site to create a classical AP-1 7-mer (closely resembling a TPA element), but not when we mutated to another atypical AP-1 8-mer (Figure 2B). This is consistent with predicted binding of either c-Jun homodimers or c-Jun/ATF heterodimers, rather than classical c-Jun/c-Fos AP-1 complex [10], to confer allele-specific transcriptional regulation. In order to measure relative TF protein binding we performed electrophoretic mobility shift assays (EMSA). We observed binding to both radiolabeled alleles containing a single putative binding site in nuclear extracts from various cell types (Figure 2C). Greater binding to the C risk allele was observed, while competition with excess cold probe was more effective at the G allele. These results are consistent with the reporter assays, suggesting the G allele has weaker transcriptional regulatory activity due to decreased TF binding.
Fig. 2. Allele-specific transcriptional activity at rs12190287. 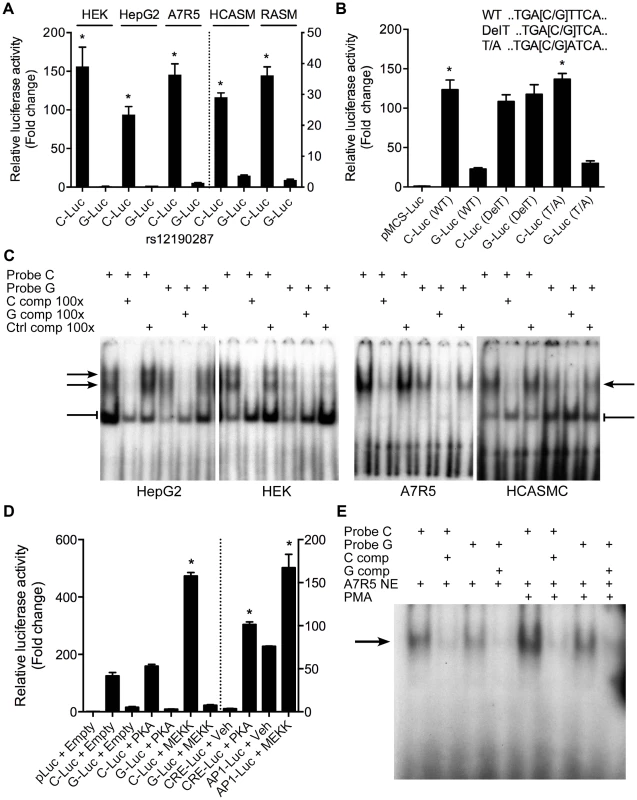
(a) Transcriptional activity of rs12190287-C and G variants were determined in heterozygous primary human coronary artery smooth muscle (HCASM), rat aortic smooth muscle cells (RASM) and HEK, HepG2, and A7r5 cell lines. pLuc-MCS vector containing the putative enhancer region for each rs12190287 variant (C-Luc and G-Luc) was transfected for 24 hours and the ratio of firefly and Renilla luciferase activities were normalized to empty reporter (pLuc). *P<0.01 versus G-Luc for each condition. (b) Dual-luciferase assay of wildtype rs12190287 enhancer or mutants, DelT or T/A (as shown), transfected in A7r5 as described above. *P<0.001 versus G-Luc for each condition. (c) Electrophoretic mobility shift assays (EMSA) showing protein binding to [γ32P]ATP-labeled rs12190287 C/G probes incubated with nuclear extract (NE) from various cell types, along with 100× excess C, G, or negative control (C, G, or Ctrl comp) unlabeled probe as competitor. Arrows and bar-headed lines represent specific and non-specific shifted complexes, respectively. (d) Dual-luciferase assay of rs12190287 C/G enhancer co-transfected with empty vector (Empty) or constitutively active protein kinase A (PKA) or mitogen-activated protein kinase (MEKK) in A7r5 using consensus CRE and AP-1 reporters as positive controls. *P<0.001 versus C-Luc+Empty or CRE-Luc+Veh or AP1-Luc+Veh where indicated. (e) EMSA of rs12190287 C/G with respective competition showing protein binding using control A7r5 or PMA treated A7r5 NE. Values are mean ± SD from triplicates. Similar results were observed from three independent experiments. Tab. 1. In silico allele-specific transcription factor binding to rs12190287. 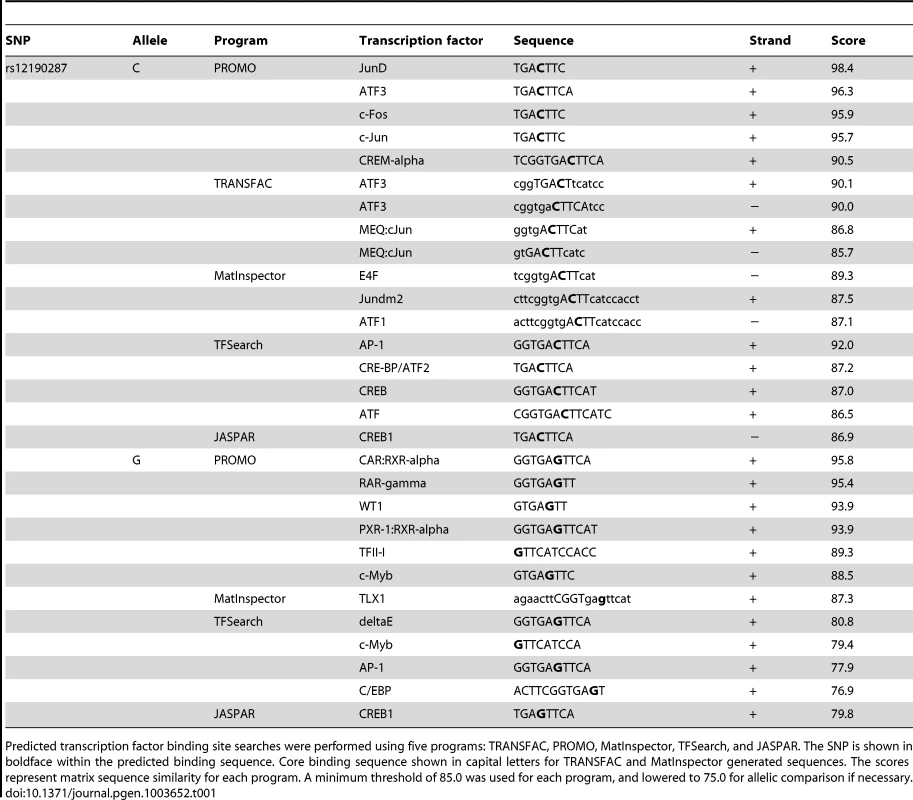
Predicted transcription factor binding site searches were performed using five programs: TRANSFAC, PROMO, MatInspector, TFSearch, and JASPAR. The SNP is shown in boldface within the predicted binding sequence. Core binding sequence shown in capital letters for TRANSFAC and MatInspector generated sequences. The scores represent matrix sequence similarity for each program. A minimum threshold of 85.0 was used for each program, and lowered to 75.0 for allelic comparison if necessary. AP-1-like transcription factor binding to rs12190287 in vitro
Given that the putative binding site closely resembles an AP-1 or CRE element, we measured the effects of activating protein kinase C (PKC) via phorbol-12-myristate-13-acetate (PMA) or adenylyl cyclase (AC) via forksolin (fsk) on the transcriptional activity at rs12190287 (Figure S2B). Surprisingly, neither PMA nor forskolin altered C or G-Luc reporter activity, while both activated the consensus AP-1 and CRE reporters, respectively. This may indicate the element at rs12190287 can be activated in normal growth media, which contains growth factors upstream of AP-1. Overexpression of constitutively active MEKK (preferentially upstream of AP-1 elements), but not active protein kinase A (PKA) (preferentially upstream of CRE elements) led to greater transactivation of the C allele (Figure 2D). We observed an increase in the bound TF complex upon PMA stimulation, which was greater at the C allele, suggesting binding of an AP-1-like element (Figure 2E). This is further supported by the gel shift observation of a similar higher molecular weight complex bound to C and G alleles, compared to the consensus AP-1 probe (Figure S2C). Interestingly, a second lower molecular weight complex bound to the consensus AP-1 probe was not observed with C and G probes, suggesting some differences in TFs binding to these distinct elements.
AP-1 dependent transcriptional regulation at rs12190287 in vitro
We then sought to determine the specific identity of the TFs predicted to bind rs12192087 in vitro. Using allele specific reporters for rs12190287, we measured the regulatory effects of overexpression of AP-1 related factors meeting a predicted in silico binding threshold of >0.85, which included c-Jun, JunD, and ATF3 (Figure 3A). c-JUN overexpression elicited robust activation of the C allele, similar to the activation of consensus AP-1-luc. Less overall activity was observed with the G allele and minor effects were observed upon JUND and ATF3 overexpression. Prior reports also demonstrate that c-Jun predominately activates AP-1 elements in vitro, whereas JunD and ATF3 alone often result in transrepression [11]. We also measured the transcriptional regulation at rs12192087 via loss-of-function experiments. The dominant negative mutant ΔJun (TAM67), which lacks the transactivation domain of c-Jun, resulted in blunted transcriptional activity at C and G alleles (Figure 3B). Similar results were observed with ΔATF3, whereas ΔCREB led to slightly increased activity. Transfection of siRNAs against c-JUN, JUND, and ATF3 also led to reduced transcriptional activity at the C and G alleles, specifically implicating these factors in mediating the activity at rs12190287 (Figure 3C). siRNA-mediated protein knockdown for each AP-1 TF was confirmed by immunoblotting (Figure S3). Using EMSA we also observed super-shifted complexes upon incubation with antibodies against c-Jun and JunD (Figure 3D). Together these results implicate the AP-1 factors c-Jun, JunD, as well as ATF3 in regulating putative enhancer activity at rs12190287.
Fig. 3. AP-1 dependent transcriptional regulation at rs12190287 in vitro. 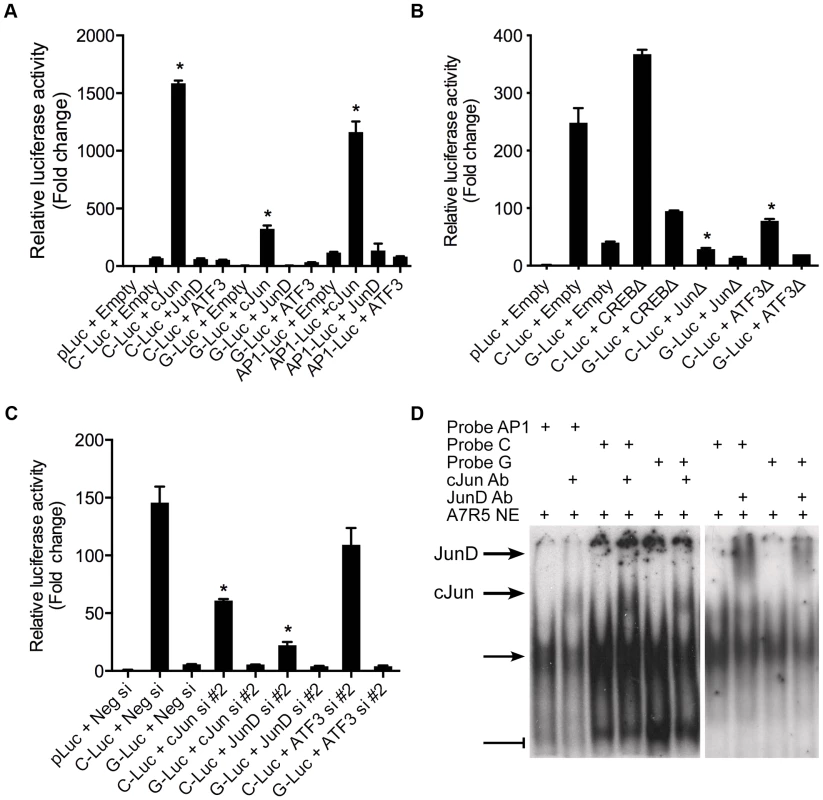
(a) Dual-luciferase assay of rs12190287-C and G variants determined in A7r5 cells transfected with rs12190287 enhancer (C-Luc and G-Luc), consensus AP-1 reporter (AP1-Luc) or empty reporter (pLuc), along with human c-JUN, JUND, or ATF3 expression vectors and measured after 24 hours. *P<0.0001 versus C-Luc, G-Luc or −1-Luc+Empty where indicated. (b) Dual-luciferase assay of rs12190287 enhancer in A7r5 cells transfected with empty vector (Empty) or dominant negative expression constructs for human CREB (CREBΔ), c-JUN (JunΔ), or ATF3 (ATF3Δ). *P<0.01 versus C-Luc+Empty. (c) Dual-luciferase assay of rs12192087 enhancer in heterozygous HCASMC transfected with small interfering RNA duplexes (siRNA) against c-JUN, JUND, ATF3 or a negative control (Neg si). *P<0.01 versus C-Luc or G-Luc+Neg si. (d) EMSA of rs12190287 C/G enhancer showing specific shifted complexes (arrow) and JunD and c-Jun super-shifted complexes (bold arrows). Bar-head line denotes non-specific shifted complexes. Values are normalized mean ± SD from triplicates. Similar results were observed from three independent experiments. PDGFRβ is upstream of TCF21 at rs12190287 in HCASMC
To ascertain the functional implications of allelic variation at rs12190287, we measured the effects of relevant upstream stimuli on TCF21 expression in HCASMC. Platelet-derived growth factor (PDGF) is a potent growth factor ligand responsible for activation of AP-1-dependent gene expression in SMCs, leading to synthetic phenotypic properties such as increased proliferation, survival, and migration [12]. Further, signaling through PDGFRβ is required for epithelial-mesenchymal transition (EMT) and epicardial fate determination in developing CASMC [13]. Transforming growth factor beta (TGF-β1) is also critically involved in both EMT and adult SMC phenotypic modulation. Interestingly, PDGF-BB treatment resulted in a time-dependent increase in TCF21, whereas transforming growth factor beta (TGF-β1) and PMA led to slightly reduced or unaltered TCF21 levels (Figure 4A). Western blots demonstrated changes in TCF21 protein levels were consistent with changes in TCF21 mRNA levels in response to PMA and PDGF-BB (Figure S4). Concordantly, c-JUN and ATF3 were upregulated by PDGF-BB, while JUND levels were unchanged (Figure 4B). We then assessed the effects of PDGF-BB and TGF-β1 treatment on allele-specific expression (ASE) of TCF21 in HCASMC using TaqMan allelic discrimination (Figure 4C, Figure S5A, B). Using heterozygous CG HCASMC, we observed that PDGF-BB treated samples had greater normalized C/G ratios, which peaked around 6 hours, while TGF-β1 treated samples had lower C/G ratios (Figure 4C). The phasic allelic imbalance observed with PDGF-BB may be partially dependent on activation of AP-1 to regulate TCF21 gene expression.
Fig. 4. PDGFRβ is upstream of TCF21 at rs12190287 in HCASMC. 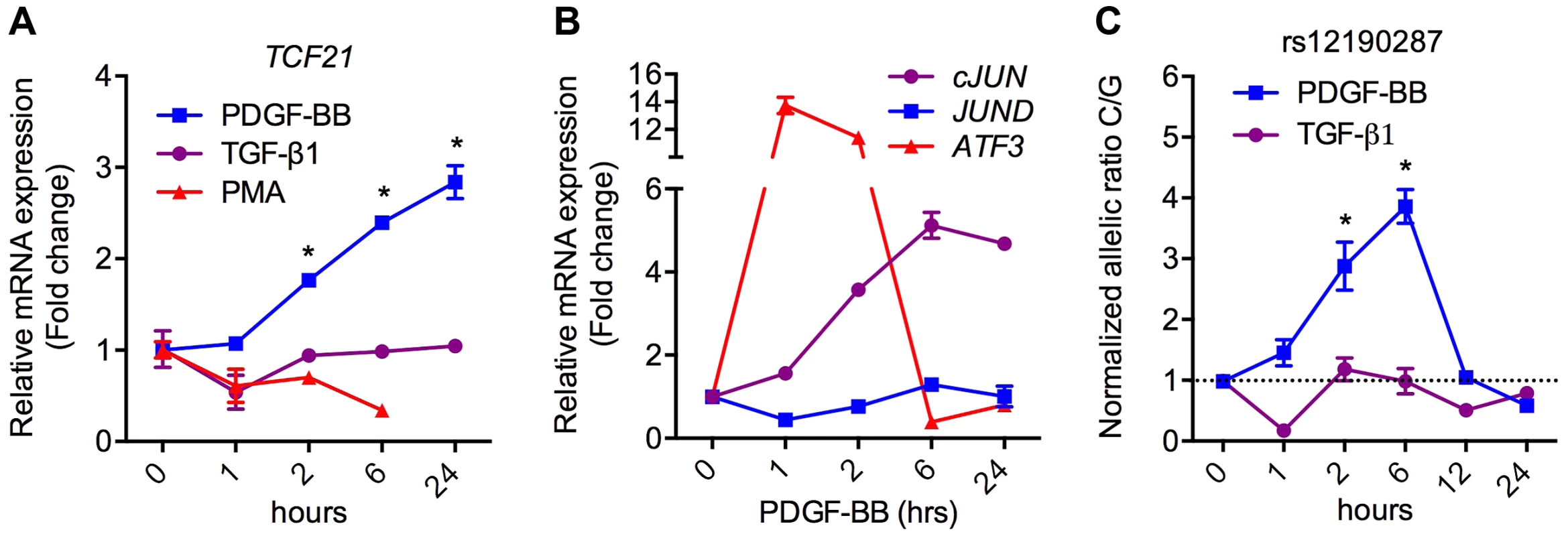
(a) TaqMan based qRT-PCR results showing relative mRNA expression levels of total TCF21 in heterozygous HCASMC treated with TGF-β1, PDGF-BB or PMA for indicated times. Expression levels were normalized to 18S and expressed as fold change from 0 h. *P<0.0001 versus 0 h. (b) TaqMan qRT-PCR expression results of c-JUN, JUND, and ATF3 in heterozygous HCASMC treated TGF-β1 or PDGF-BB for the indicated times. (c) Allele-specific TaqMan qRT-PCR based expression of TCF21 at rs12190287 shown as normalized allelic ratio C/G in heterozygous HCASMC treated with either TGF-β1 or PDGF-BB for the indicated times. *P<0.01 versus 0 h. Values are mean ± SD of triplicates. Similar results were observed from three independent experiments. AP-1 mediated regulation at rs12190287 in vivo
Transcriptional regulation of gene expression is tightly controlled by the native chromatin architecture in vivo. Therefore, we interrogated allele-specific AP-1 occupancy at rs12190827 using chromatin immunoprecipitation (ChIP) and haplotype specific ChIP (haploChIP). In HCASMC treated with PDGF-BB we observed a significant increase in enrichment at rs12190287 by c-Jun and ATF3 (Figure 5A). JunD enrichment, while significantly above IgG in control samples, was unchanged with PDGF-BB. We then measured allele-specific enrichment in heterozygous HCASMC treated with PDGF-BB, as done previously for haploChIP [14]. Interestingly, c-Jun was predominately enriched at the C allele under control treatment, indicated by greater C/G ratios (Figure 5B). Both c-Jun and ATF3 were more enriched at the C allele upon PDGF-BB treatment, and JunD enrichment was unchanged. Similar observations were made using pyrosequencing-based allelic discrimination (Figure S5C). ChIP products were also amplified at FOSB and MYOG promoters, as AP-1 positive and negative control regions, respectively (Figure 5C, D). We then measured putative enhancer activity at rs12190287 via post-transcriptional histone modification. PDGF-BB treatment led to significantly increased enrichment of H3K4me1 (marks active/poised promoters) and H3K27ac (marks active enhancers) and H3K27me1 (marks active/poised promoters) (Figure 5E). We also observed increased relative enrichment of active histone modifications at the C allele, which was further potentiated with PDGF-BB stimulation in HCASMC (Figure 5F). These data indicate that the AP-1 complex positively regulates the rs12190287 risk allele in the native and active chromatin state.
Fig. 5. AP-1 mediated regulation at rs12190287 in vivo. 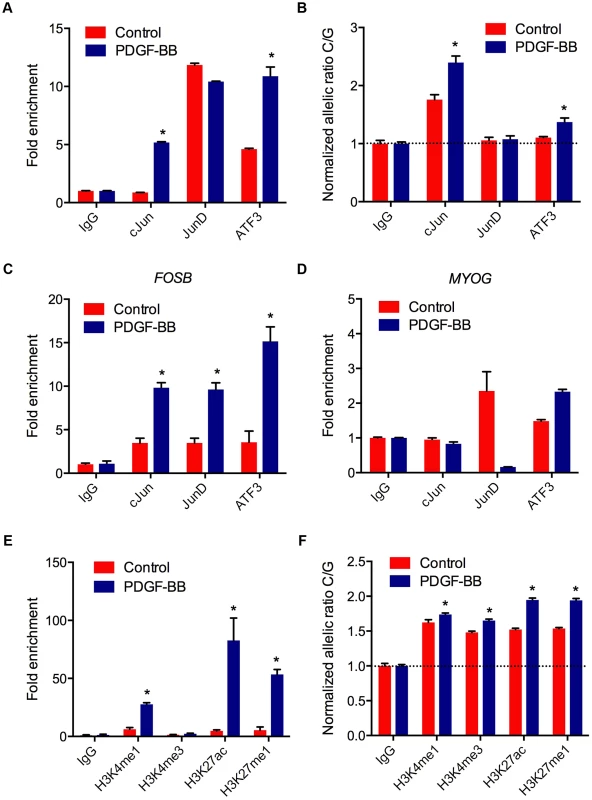
(a) Total enrichment of c-Jun, JunD, and ATF3 at rs12190287 determined by chromatin immunoprecipitation (ChIP) in heterozygous HCASMC treated with PDGF-BB (20 ng/ml) or vehicle (Control) for 6 hrs. (b) Allele-specific enrichment of c-Jun, JunD and ATF3 at rs12190287 determined by HaploChIP in heterozygous HCASMC treated with PDGF-BB, shown as normalized allelic-ratio C/G. (c–d) AP-1 positive and negative control regions for c-Jun, JunD, and ATF3 enrichment at FOSB (c) and MYOG (d) promoters, respectively. (e) Total enrichment of histone modifications H3K4me1, H3K4me3, H3K27ac, and H3K27me1 at rs12190287 determined by ChIP in heterozygous HCASMC treated with PDGF-BB. (f) Allele-specific enrichment of H3K4me1, H3K4me3, H3K27ac, and H3K27me1 at rs12190287 determined by HaploChIP in heterozygous HCASMC treated with PDGF-BB, shown as normalized allelic ratio C/G. *P<0.001 versus Control for each condition. Values are mean ± SD from triplicates. Similar results were observed from 3–4 independent experiments. For HaploChIP experiments, separate heterozygous individual HCASMCs were used for each replicate experiment. WT1 regulation of rs12190287 in vitro and in vivo
We also investigated the potential functional effects of non-AP-1 TFs predicted to bind rs12190287 (Table 1). Wilms tumor 1 (WT1) was of particular interest given its known role in CASMC development [15], and evidence in developmental models indicating wt1 directly regulates tcf21 [16]. The G allele resides in a WT1-like binding element [17] (WTE; 5′-GCGTGGGAGT-3′), which was previously implicated in the regulation of the human thromboxane A2 receptor [18]. WT1-D (+KTS amino acid insertion) binds DNA with reduced affinity compared to WT1-B (−KTS) [19], and the ratio of the two alternatively spliced isoforms has been implicated in Frasier syndrome [20]. We observed that expression of WT1-B (−KTS) and WT1-D (+KTS) led to similar transrepression of both C and G alleles at rs12190287 (Figure 6A), consistent with the role of WT1 as a transcriptional repressor. As a tumor suppressor gene, WT1 often represses AP-1 mediated transcription [21], [22] and WT1-B and WT1-D also repressed c-Jun-mediated activation of rs12190287 in vitro (Figure 6B). Similar regulation was observed at both C and G alleles suggesting WT1-mediated regulation may not be the rate-limiting step altered by rs12190287. Consistently, WT1 siRNA mediated knockdown led to increased activity of C and G alleles (Figure 6C), with protein knockdown verified by immunoblotting (Figure S3D). Next, we assessed the expression changes of WT1 upon TGF-β1 or PDGF-BB treatment of HCASMC (Figure 6D). Interestingly, PDGF-BB led to a rapid decline in WT1, which recovered by 24 hours. TGF-β1 led to a slower yet persistent reduction of WT1. We also observed decreased enrichment of WT1 at rs12190287 upon PDGF-BB stimulation, consistent with effects observed at the FOSB promoter (AP-1 positive control region) (Figure 6E). Less reduction was observed at the MYOG promoter (AP-1 negative control region). Surprisingly we observed WT1 preferentially enriched at the C allele, which was increased upon PDGF-BB stimulation (Figure 6F). Given that WT1 negatively regulates transcription at rs12190287 and is downregulated by PDGF-BB, may suggest that the WT1 cofactor preferentially associates with the C risk allele to temporally fine-tune AP-1 mediated transcription upon growth factor stimulation.
Fig. 6. WT1 regulation of rs12190287 in vitro and in vivo. 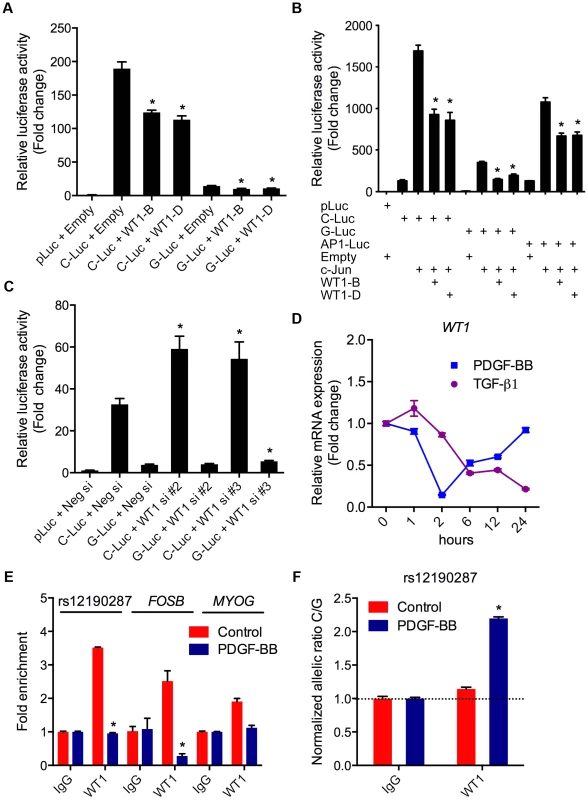
(a) Dual-luciferase assay of rs12190287 C/G enhancer transfected with human WT1-B (−KTS), WT1-D (+KTS) expression constructs in A7r5 cells, measured after 24 hours. *P<0.01 versus C-Luc or G-Luc+Empty. (b) Dual-luciferase assay of rs12190287 C/G enhancer co-transfected with human c-JUN and WT1-B or WT1-D expression constructs in A7r5 cells. AP1-Luc reporter was used as a positive control. *P<0.05 versus C-Luc or G-Luc+cJun. (c) Dual-luciferase assay of rs12190287 C/G enhancer transfected in heterozygous HCASMC with siRNA against WT1 (−/+KTS) compared to negative control (Neg si). *P<0.05 versus C-Luc or G-luc+Neg si. (d) TaqMan based qRT-PCR results showing relative human WT1 mRNA expression levels in heterozygous HCASMC treated with TGF-β1 or PDGF-BB for the indicated times. (e) Total enrichment of WT1 at rs12190287 enhancer, FOSB or MYOG promoter regions determined by chromatin immunoprecipitation (ChIP) in heterozygous HCASMC treated with PDGF-BB for 6 hrs. Values represent fold change relative to enrichment with IgG control. *P<0.01 versus Control WT1. (f) Allele-specific enrichment of WT1 at rs12190287 determined by HaploChIP in heterozygous HCASMC treated with PDGF-BB, shown as normalized allelic-ratio C/G. *P<0.0005 versus Control WT1. Values are mean ± SD from triplicates. Similar results were observed from three independent experiments. AP-1 regulation at rs12524865 and haplotype structure in East Asians
TCF21 was one of three Caucasian CAD associated loci that was recently replicated in a Han Chinese population [3]. While association at rs12190287 did not reach genome-wide significance at the TCF21 locus, rs12524865 (Figure 1A) represented the lead SNP in this racial ethnic group and showed consistent association in the discovery and replication stages [3]. We examined the haplotype structure at 6q23.2 in ∼2400 Han Chinese from the HALST study in Taiwan who were also genotyped with the Metabochip, and augmented genotype data in this region through imputation using data from the HapMap II and III Han Chinese (CHB) samples. We identified regions of high LD surrounding rs12190287, however much less LD surrounding rs12524865 compared to the European samples, and we found that rs12524865 is located in a distinct haplotype block from the lead SNP in Europeans, rs12190287 (Figure 7A). Further, the risk haplotype containing rs12524865 and four other TCF21 eSNPs occurs at similar frequency (0.361) in this population (Figure S6A). rs12524865 is in perfect LD with other eSNPs for TCF21 in one haplotype block, including rs1967917 and rs7752775. However, the haplotype block for rs12190287 does not contain other alleles in LD (Figure 7A). We then mapped the putative TFBS at rs12524865 using multiple prediction tools (Table 2). Interestingly, rs12524865 is also located within an AP-1/CREB-like element, TAA[C/A]GTCA, which closely resembles the consensus ATF/CREB binding site, TGACGTCA (Figure S6B). As expected, the C allele (also major, risk allele) is predicted to bind AP-1 and CREB family members, whereas predicted binding is disrupted by the minor, protective allele. Using luciferase reporters containing this putative enhancer we observed robust transcriptional activity with the risk allele, which was absent with the protective allele (Figure 7B). Forskolin but not PMA stimulation potentiated this activity, suggestive of a cAMP-responsive ATF/CREB element (Figure 7B). Dominant negative mutants of CREB, Jun, and ATF3 reduced this activity (Figure S6C). We then measured occupancy of AP-1 and active chromatin histone modifications at rs12524865. Interestingly, we observed increased c-Jun and ATF3 enrichment with PDGF-BB treatment, with much greater enrichment by ATF3 (Figure 7C). Enrichment of active histone modifications, H3K4me1 and H3K27ac also suggest a functionally active chromatin state at rs12524865 (Figure 7D). Together these results implicate both rs12190287 and rs12524865 risk alleles in a shared AP-1-dependent mechanism for regulating TCF21 in HCASMC, thus further defining the genetic risk mechanisms of CHD which have been conserved across racial ethnic groups during evolution (Figure 8).
Fig. 7. AP-1 regulation at rs12524865 and haplotype structure in East Asians. 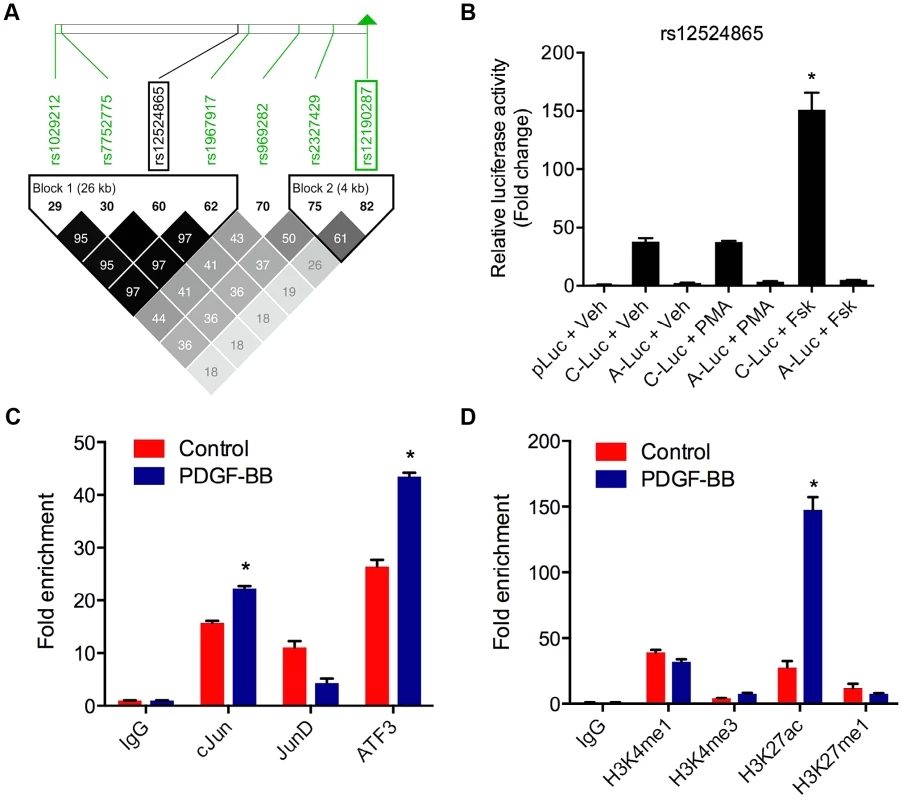
(a) Linkage disequilibrium (LD) plot from ∼2400 Metabochip genotyped East Asian samples from the TAICHI study (HALST cohort) with imputation from HapMap Phase II and III Han Chinese (CHB), showing eSNPs for TCF21 and distinct haplotype blocks containing rs12524865 and rs12190287. White to black squares represent increasing r2 values, also shown in blocks. (b) Dual-luciferase assay of rs12524865 C/A enhancer transfected in A7r5 and treated with adenylyl cyclase activator, forskolin (Fsk) or PKC activator, phorbol-12-myristate-13-acetate (PMA) for 4 hours. Relative luciferase activities measured after 24 hours. *P<0.01 versus C-Luc+Veh. (c) Total enrichment of c-Jun, JunB, JunD, and ATF3 at rs12524865 determined by chromatin immunoprecipitation (ChIP) in HCASMC treated with PDGF-BB or vehicle (Control) for 6 hours. *P<0.005 versus Control for each condition. (d) Total enrichment of histone modifications H3K4me1, H3K4me3, H3K27ac, and H3K27me1 at rs12524865 determined by ChIP in HCASMC treated with PDGF-BB. *P<0.001 versus Control for each condition. Values are mean ± SD from triplicates. Similar results were observed from three independent experiments. Fig. 8. Predicted model of AP-1 dependent regulation of TCF21 at rs12190287 and rs12524865. 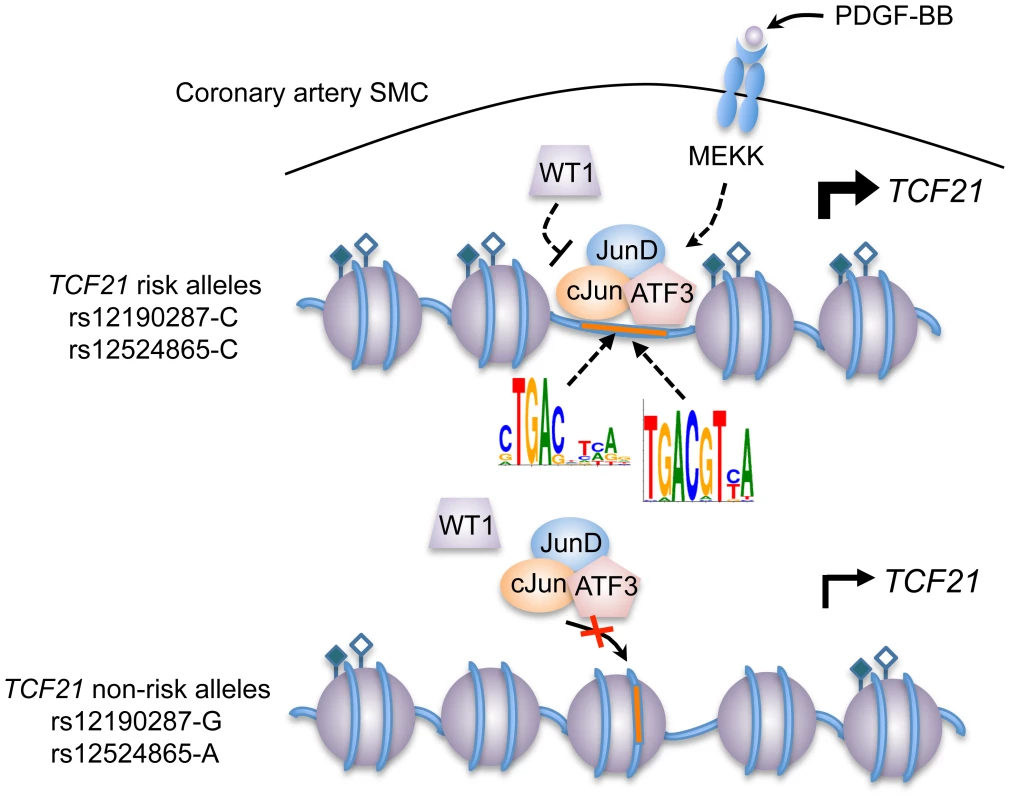
Individuals carrying risk alleles for rs12190287 or rs12524865 at 6q23.2 are expected to have increased TCF21 expression upon stimulation of PDGFR-β by PDGF-BB in coronary artery smooth muscle cells, due to increased enrichment of active histone modifications (represented by closed and open diamonds) leading to an open chromatin conformation, allowing binding of an active AP-1 TF complex containing various combinations of c-Jun, JunD, and ATF3. WT1 functions as a transrepressor of this active complex at rs12190287, whereby WT1 may fine-tune the spatial and temporal activation of TCF21 expression. Multiple kinases other than mitogen-activated kinase (MEKK) may be involved in the activation and recruitment of AP-1 complexes to these binding sites. Tab. 2. In silico allele-specific transcription factor binding to rs12524865. 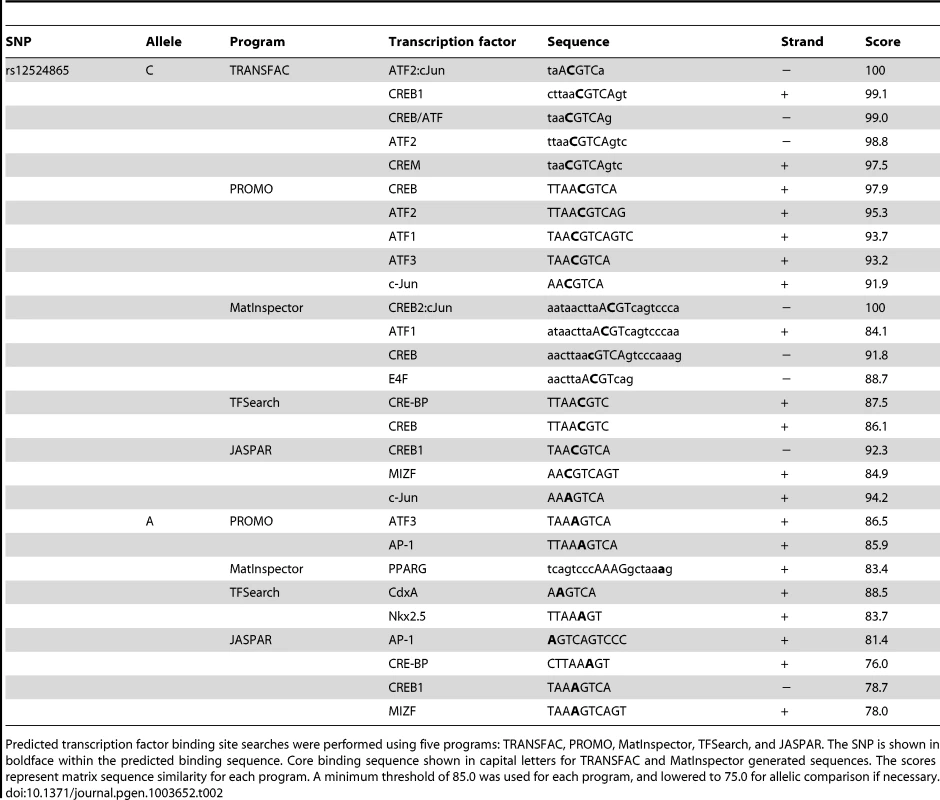
Predicted transcription factor binding site searches were performed using five programs: TRANSFAC, PROMO, MatInspector, TFSearch, and JASPAR. The SNP is shown in boldface within the predicted binding sequence. Core binding sequence shown in capital letters for TRANSFAC and MatInspector generated sequences. The scores represent matrix sequence similarity for each program. A minimum threshold of 85.0 was used for each program, and lowered to 75.0 for allelic comparison if necessary. Discussion
Therapeutic targeting of traditional CHD risk factors has reduced overall mortality rates, however there are currently no therapies that directly target disease processes of the vessel wall. Recent GWAS have identified 46 independent risk-associated loci for CHD/MI, and 104 independent loci associated at a false discovery rate <5% [8], [23]. Many of the genes identified are implicated in the regulation of SMC plasticity during atherosclerosis, including PDGFD, COL4A1/2, CDKN2B and CDKN2A/p19ARF [24]–[26]. However, the molecular mechanisms and relevant pathways underlying these risk associations are relatively underexplored. Here, using an integrated beyond GWAS strategy (Figure 1B), we reveal the interplay of both developmental and disease-related pathways, which coordinate the regulation of TCF21 at independent CHD susceptibility alleles in humans. Individuals carrying the risk haplotype for rs12190287 and rs12524865 are predicted to have greater TCF21 expression due to increased binding of AP-1 complexes to a cis-regulatory element. Our studies further reveal a potential PDGFRβ-dependent mechanism for the CHD risk association in human coronary artery smooth muscle cells both in vitro and in vivo.
Of the 13 novel loci identified in the initial CARDIoGRAM meta-analysis, TCF21 was particularly attractive as a missing link to CHD. Tcf21 (Pod-1/Capsulin/Epicardin) was initially cloned in our laboratory and two others [4], [27], and is expressed in epicardial progenitor cells that give rise to developing CASMC [4]. Studies of TCF21 function in the adult have been hampered since Tcf21 null mice die postnatally due to pulmonary hypoplasia and respiratory failure [5]. TCF21 has been identified as a candidate tumor suppressor gene and is frequently epigenetically silenced in various human cancers [28], [29]. These studies have implicated loss of TCF21 expression as an early-stage biomarker for increased cancer risk. Based on our findings, we can reason that aberrant upregulation of TCF21 in coronary SMC may increase CHD risk through alteration of the SMC response to injury in the vessel wall.
Identification of cis-regulatory elements altered by disease related variants is critical for post-GWAS functional characterization studies [30]–[32]. Here, we observed that PDGF-BB mediates binding of an AP-1 complex, likely containing c-Jun, and JunD or ATF3 heterodimers to the risk allele at rs12190287, which is preferentially in an active chromatin state. The activator protein-1 (AP-1) family of TFs have been implicated in growth factor-dependent SMC activation following vessel injury [33]. The prototypical basic region-leucine zipper (bZip) protein, c-JUN is expressed in human atherosclerotic plaques and promotes SMC proliferation and neointima formation in vivo [34]. ATF3 is another stress-inducible gene upregulated in many cancers [35] and also in SMCs within injured mouse femoral arteries, to promote SMC migration and ECM synthesis [36]. It has been shown that c-Jun readily forms heterodimers with ATF2 and ATF3, which have distinct DNA binding affinities to CRE and AP-1 elements [37]. Interestingly, genes encoding extracellular matrix (ECM) proteins are often the targets of c-Jun/ATF enhancer elements [10].
A challenge in prioritization of regulatory SNPs is elucidating the biologically relevant upstream pathways driving these associations [38], [39]. Platelet-derived growth factor (PDGF) is a critical growth factor involved in vascular development. It has been shown in mice that PDGFR-β is required for development of mural cells, CASMC and pericytes, involving epithelial-to-mesenchymal transition (EMT) in the epicardium [13], [40]. PDGF-BB is also a potent inducer of the synthetic SMC phenotype, increasing migration, lipid uptake, and ECM synthesis, both in vitro and in vivo during vascular injury and atherosclerosis [12]. A recent GWAS for CHD in Europeans and South Asians identified the PDGFD gene, and this PDGF family member has been shown to have many of the same disease-related actions as related PDGFs [41]. In contrast, TGF-β is a pleiotropic cytokine mostly responsible for maintaining SMC differentiation, through activation of Smad, SRF, and RhoA signaling pathways [42]. Indeed, VSMC differentiation during aortic development likely depends on TGF-β rather than PDGF-BB/PDGFR-β [43]. Our observations that TCF21 was selectively induced by PDGF-BB rather than TGF-β in HCASMC is consistent with the notion that TCF21 inhibits coronary artery SMC differentiation while inducing SMC phenotypic modulation.
Similar to Tcf21, Wilms tumor 1 (Wt1) is expressed in the early proepicardium, epicardium and mesenchyme during development of the heart and other mesoderm-derived tissues [15], [44], [45]. In fact, previous studies in zebrafish have demonstrated that tcf21 expression in the proepicardial organ is dependent on wt1 [46]. Wt1 expression is also induced in the coronary vasculature in regions of ischemia and hypoxia after MI in mice [47]. As a tumor suppressor gene, WT1 was previously shown to repress PMA induced transcription [22]. WT1 binding to the thrombospondin-1 promoter leads to repression upon c-Jun overexpression in ECs [48] and fibroblasts [21]. Here we identify WT1 upstream of TCF21 to repress the enhancer at rs12190287. While in silico analyses predicted preferential binding to the G allele, haploChIP data suggest that WT1 preferentially associates with the C allele. This is consistent with greater c-Jun enrichment at the C allele. The orthogonal regulation of WT1 expression by PDGF-BB compared to TCF21, c-JUN, JUND, and ATF3, also may imply that WT1 acts to spatially and temporally fine-tune TCF21 expression, rather than cause repression.
CHD involving atherosclerosis continues to burden both developed and developing countries, largely due to urbanization and westernization of diet and lifestyle. Compared to developed countries, CHD related deaths are predicted to rise more than 3-fold in China and India, for instance [49]. While most GWAS for CHD have focused on individuals of European ancestry, large-scale studies of non-European populations may allow further understanding of the risk-associated mechanisms driving CHD. A recent meta-analysis of GWAS for CHD in a Han Chinese population (15,460 cases and 11,472 controls) replicated the TCF21 association in Europeans [3]. The combined discovery and replication stages identified a near genome-wide significant association at rs12524865, upstream of TCF21 at 6q23.2 (P = 1.87×10−7). The discovery stage identified primarily rs12524865 (P = 3.40×10−3), although rs12190287 showed a trend and directionality as a reporter for Caucasian cohorts (P = 3.03×10−2). The linkage disequilibrium r2 values between these two eQTLs for TCF21 is 0.62 in Europeans and only 0.18 in Han Chinese, and these variants are found in separate haplotype blocks in Han Chinese. Further, we demonstrated rs12524865 disrupts a binding site for CREB/ATF in silico and measured enrichment for c-Jun and ATF3 at rs12524865 in HCASMC. These studies highlight the value of multi-ethnic post-GWAS validation of causal variants to assess both the functional impact and heritable risk of common variants in complex diseases.
The compelling promise of the new disease associated genes and pathways afforded by GWAS methodology is that they will provide biological insights and targets for the development of new therapeutic approaches, and this is particularly compelling for CHD where there are no therapies directed at the blood vessel wall. TCF21 and its downstream targets provide one such pathway. eQTL data have suggested that the disease-associated major allele shows increased TCF21 expression, and this is consistent with the functional studies described herein, where the major risk C alleles at rs12190287 and rs12524865 confer greater transcriptional activity compared to the minor protective G and A alleles, respectively. The embryonic function of TCF21 in the developing coronary circulation seems most consistent with a role aimed at inhibiting differentiation of SMC progenitors, and thus it is likely that TCF21 function might interfere with the SMC response to vascular injury in the disease setting. It is now generally accepted that vascular SMC provide a stabilization of the atherosclerotic plaque, and thus therapeutic inhibition of the TCF21 pathway would be expected to decrease the risk for coronary events. However, such an approach might also put the patient at increased risk for head and neck and lung cancer, as TCF21 is a potent tumor suppressor gene that is frequently mutated or silenced in cells of these tumors. This situation contrasts with the emerging information related to the risk mechanisms at 9p21.3, where the function of one likely causal gene CDKN2B is associated with decreased risk for vascular disease [25], [50] as well as a broad range of human cancers [51]–[53]. Therapeutic activation of expression of this gene would be expected to decrease risk for both types of disease. While it is still early days for such extrapolations, follow-up of vascular wall GWAS genes is expected to provide insights into disease-related pathways to better inform therapeutic manipulation.
Materials and Methods
Cell culture
Primary human coronary artery smooth muscle cells (HCASMC) were purchased from three different manufacturers, Lonza, PromoCell and Cell Applications and were cultured in complete smooth muscle basal media (Lonza) according to the manufacturer's instructions. All experiments were performed on HCASMC between passages 4–7, using lots identified as heterozygous at rs12190287 as indicated. Primary rat aortic smooth muscle cells (RASM) were obtained from Dr. Phil Tsao (Stanford University) and cultured in Dulbecco's' Modified Eagle Media (DMEM) low glucose with 10% fetal bovine serum (FBS). The rat aortic smooth muscle cell line, A7r5 was purchased from ATCC and also obtained from Dr. Joe Miano (University of Rochester) and were maintained in DMEM low glucose with 10% FBS. HepG2 and HEK cells were purchased from ATCC and maintained in DMEM low glucose with 10% FBS.
siRNA transfection
Pre-designed Silencer Select siRNA duplexes against human c-JUN, JUND, ATF3, and WT1 were purchased from Ambion/Life Technologies. At least two individual siRNAs were tested for each. Briefly, HCASMC were plated in 12-well (dual-luciferase assay) or 6-well plates (qPCR) in complete media. Approximately 24 hours after plating, and between 40–60% confluence, cells were transiently transfected with negative control or TF specific siRNAs (50 nM) using RNAiMAX reagent (Life Technologies) according to the manufacturer's instructions. Cells were incubated for 48 hours prior to performing dual-luciferase assays, harvesting total RNA using miRNeasy Mini kit (Qiagen) for TaqMan based qPCR assays, or nuclear protein extraction for Western blotting.
Dual-luciferase assay
Oligonucleotides containing the putative enhancer elements for rs12190287 C/G and rs12524865 C/A (Table S1) were annealed at 95 degrees for 10 minutes in annealing buffer and allowed to cool to room temperature. Double-stranded DNA fragments were then subcloned into the MCS of the minimal promoter containing pLuc-MCS vector (Agilent). Constructs were validated by Sanger sequencing. Empty vector (pLuc-MCS), rs12190287-C or rs12190287-G and Renilla luciferase constructs were transfected into HCASMC, RASMC, A7r5, HepG2, and HEK using Lipofectamine 2000. Media was changed after 6 hours, and dual luciferase activity was measured after 24 hours using a SpectraMax L luminometer (Molecular Devices). Relative luciferase activity (firefly/Renilla luciferase ratio) is expressed as the fold change of the empty vector control (pLuc-MCS).
Electrophoretic mobility shift assay
Double stranded oligonucleotides for rs12190287-C/G, AP-1, CREB, rs12190287 mixed were obtained by annealing single stranded oligos (Table S1), as previously described [54]. Annealed oligos were then labeled with [γ32P]-ATP (Perkin Elmer) using T4 polynucleotide kinase (NEB) for 30 minutes at room temperature and then purified through Sephadex G-50 Quick Spin columns (Roche). After measuring radioactivity, reactions were assembled with 1× EMSA binding buffer, 1 µg poly-dIdC, 10 µg nuclear extract, 100× unlabeled probe (for competitions), 2 µg polyclonal antibody (for super-shifts), [γ32P]-ATP labeled probe, and incubated at room temperature for 30 min prior to protein separation on a 4% TBE gel. Gels were dried on Whatman paper using a heated vacuum drier and proteins were detected on radiographic film.
TaqMan qPCR gene expression
Primary human coronary artery smooth muscle cells (HCASMC) were cultured in normal growth media until approximately 75% confluent, then cultured in the absence of serum and supplements for 24 hours, prior to stimulation with 50 ng/ml human recombinant PDGF-BB (R&D Systems), 5 ng/ml human recombinant TGF-β1 (R&D Systems), 100 nM PMA (Sigma) or vehicle for indicated times. Total RNA was prepared using miRNeasy Mini kit (Qiagen) and total cDNA was prepared from 0.5 µg of RNA using the TaqMan High Capacity cDNA synthesis kit (Life Technologies). TaqMan gene expression probes (Table S1) were used to amplify human TCF21, c-JUN, JUND, ATF3, and WT1, which were normalized to human 18S levels.
Western blotting
Nuclear extracts were generated from HCASMC harvested at indicated time points. Protein concentrations were determined using a BCA assay (Pierce) and 50–100 µg nuclear protein for each condition was loaded on a pre-cast NuPAGE 4–12% Bis-Tris polyacrylamide gel (Invitrogen/Life Technologies), with gel run at 150 v for 1 h using MES buffer (Invitrogen/Life Technologies), and transferred to PVDF membrane at 35 v for 1 h. Membranes were blocked in 5% non-fat dry milk in 1× TBST for 1 h and incubated overnight with rabbit polyclonal antibodies against TCF21 (Sigma; 0.25 µg/ml), cJUN (Santa Cruz; 1.0 µg/ml), JUND (Santa Cruz; 1.0 µg/ml), ATF3 (Santa Cruz; 1.0 µg/ml), or WT1 (Santa Cruz; 1.0 µg/ml), followed by incubation in a secondary anti-rabbit HRP-conjugated antibody (Invitrogen/Life Technologies; 0.2 µg/ml) and detection by standard ECL (Pierce). Blots were reprobed with a mouse monoclonal antibody against GAPDH as a loading control.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed according to the Millipore EZ-ChIP protocol with slight modifications. HCASMC were cultured as described above and treated with PDGF-BB or vehicle. Cells were fixed in 1% formaldehyde to cross-link chromatin, followed by quenching with glycine. 2×107 cells per condition were collected, and nuclear lysates were prepared as previously described [55]. Cross-linked chromatin nuclear extracts were sheared into ∼500 bp fragments using a Bioruptor (Diagenode) and clarified via centrifugation. 1×106 nuclei per condition were precleared with 20 ul Protein G Dynabeads (Invitrogen) for 1 hour, followed by incubation with 2 ug Rabbit IgG or anti-c-Jun, JunB, JunD, ATF3, WT1 (Santa Cruz or Active Motif), H3K4Me1, H3K4Me3, H3K27Ac, H3K27Me1 (Diagenode or Abcam) overnight at 4C. Immunoprecipitated chromatin samples were incubated with 20 µl Protein G Dynabeads for 1 hour at 4C to capture the protein-DNA complexes. Complexes were washed and eluted as described. Protein-DNA cross-links were reversed, treated with RNase A and proteinase K and free DNA was purified using Qiagen PCR purification kits. Total enrichment was measured using rs12190287 or rs12524865 specific primers, or a known AP-1 regulatory region, or a negative control region using the primers listed (Table S1). Semi-quantitative PCR was used to verify ChIP products via gel electrophoresis. Quantitative real-time PCR (ViiA 7, Life Technologies) was performed using SYBR Green (Applied Biosystems) assays and fold enrichment was calculated by measuring the delta Ct – delta Ct IgG. Melting curve analysis was also performed for each ChIP primer pair.
ChIP-sequencing
1×107 HCASMCs per condition were processed as previously described [56], using anti-H3K4me1 (pAb-037-050, Diagenode), anti-H3K4me3 (pAb-003-050, Diagenode), anti-H3K27me3 (pAb-069-050, Diagenode), or anti-rabbit IgG (X 0903, DAKO). ChIP-seq library generation, cluster formation and next-generation sequencing was performed at the Stanford Functional Genomics Facility, Stanford University, Stanford CA, USA, on an Illumina MiSeq instrument. 36 bp single reads from next-generation sequencing of ChIP libraries were then mapped to the reference genome using Burrows-Wheeler Aligner (BWA). BigWig files were created using the R/Bioconductor environment.
FAIRE-sequencing
1×107 HCASMCs were mainly processed similar to the ChIP-seq samples. However, instead of preclearing and immunoprecipitation, protein-depleted DNA was extracted from cross-linked nuclear lysates by phenol-chloroform extraction. After DNA precipitation, purification and reverse cross-linking, samples were sequenced and further processed as described above.
PCR fragment-based genotyping
Genomic DNA was isolated from >106 HCASMC cultured in complete media for approximately 48 hours, using the Blood and Tissue DNA isolation Kit (Qiagen). 50 ng of gDNA template was amplified using primers flanking rs12190287 to generate 250 bp fragments. Fragments were then sequenced via Sanger sequencing using an internal forward sequencing primer, and genotypes were determined from chromatograms using Sequence Analyzer (Applied Biosystems).
Allele-specific expression using TaqMan qPCR
Heterozygous genotypes were determined by Sanger sequencing, and RNA and cDNA prepared as described above. Allele-specific expression of TCF21 at rs12190287 was determined using a pre-designed TaqMan SNP genotyping assay for rs12190287 (Table S1). Calibration of the SNP genotyping assay was determined by mixing 10 ng of HCASMC gDNA or cDNA, homozygous for each allele at the following ratios: 8∶1, 4∶1, 2∶1 1∶1, 1∶2, 1∶4, 1∶8. The Log2 ratio of the VIC/FAM intensity at cycle 40 was then plotted against the Log ratio of the two alleles to generate a linear regression standard curve. The Log ratio of the intensity of the two alleles from cDNA samples was fitted to the standard curve. These values were then normalized to the ratio of gDNA for each allele to obtain the normalized allelic ratio.
Allele-specific ChIP-qPCR
Heterozygous genotypes were determined as described above. Briefly, heterozygous HCASMC were cultured for the indicated timepoints, and chromatin cross-linked, sheared and immunoprecipitated as described above. Purified DNA was then amplified using TaqMan SNP genotyping assay probes for rs12190287. The Log2 ratio of VIC/FAM intensity at cycle 40 was then fitted to the standard curve and normalized to gDNA ratio, with the normalized allelic ratio of IgG control enrichment arbitrarily set to 1.
Allele-specific ChIP using pyrosequencing
Pyrosequencing assay for rs12190287 was generated using PyroMark Assay Design software (Qiagen). Forward rs12190287 PCR primer: 5′ - and biotinylated reverse PCR primer, and forward pyrosequencing primers were synthesized by the Protein And Nucleic acid (PAN) facility (Stanford). Approximately 20 ng ChIP DNA was amplified using forward and reverse pyrosequencing primers under the following conditions: 94 C 4 min, (94 C 30 s, 60 C 30 s, 72 C 45 s) ×45, 72 C 6 min. Pyrosequencing reaction was performed on a PyroMark Q24 according to manufacturer's instructions. Allelic quantitation was obtained automatically from the mean allele frequencies derived from the peak heights using PyroMark Q24 software.
In silico bioinformatics
Transcription factor binding site (TFBS) prediction was determined using the following online bioinformatics tools: TRANSFAC (BIOBASE), PROMO, MatInspector, JASPAR, and TFSearch. Sequences from dbSNP for each allele were scanned for TFBS in vertebrates meeting a minimum similarity score of 0.85. Regional association plot was generated from CARDIoGRAM meta-analysis dataset at TCF21 using LocusZoom [57]. Linkage disequilibrium plots and haplotype frequencies were generated from Europeans in the ADVANCE cohort (Stanford) from the CARDIoGRAM consortium, and East Asians from the HALST cohort within the TAICHI consortium. Briefly, genotyping data was extracted for each region of interest using PLINK [58] and transposed files were imported into Haploview [59].
Statistical analysis
Experiments were performed using at least three independent preparations with individual treatments/conditions performed in triplicate. Data is presented as mean ± standard deviation (SD) of replicates. GraphPad Prism 6.0 was used for statistical analysis. Comparisons between two groups were performed using paired two-tailed t-test. P values <0.05 were considered statistically significant. For multiple comparison testing, two-way analysis of variance (ANOVA) accompanied by Tukey's post-hoc test were used as appropriate.
Ethics statement
All samples reported in this study were obtained under written informed consent for participation in the Atherosclerotic Disease, VAscular functioN, and genetiC Epidemiology (ADVANCE) and Healthy Aging Longitudinal Study in Taiwan (HALST) studies with the approval of the Institutional Review Boards of Stanford University and National Health Research Institutes, respectively.
Supporting Information
Zdroje
1. SchunkertH, KonigIR, KathiresanS, ReillyMP, AssimesTL, et al. (2011) Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 43 : 333–338.
2. ZhongH, BeaulaurierJ, LumPY, MolonyC, YangX, et al. (2010) Liver and adipose expression associated SNPs are enriched for association to type 2 diabetes. PLoS Genet 6: e1000932.
3. LuX, WangL, ChenS, HeL, YangX, et al. (2012) Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat Genet 44 : 890–894.
4. HidaiH, BardalesR, GoodwinR, QuertermousT, QuertermousEE (1998) Cloning of capsulin, a basic helix-loop-helix factor expressed in progenitor cells of the pericardium and the coronary arteries. Mech Dev 73 : 33–43.
5. QuagginSE, SchwartzL, CuiS, IgarashiP, DeimlingJ, et al. (1999) The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development 126 : 5771–5783.
6. AcharyaA, BaekST, HuangG, EskiocakB, GoetschS, et al. (2012) The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139 : 2139–2149.
7. BraitschCM, CombsMD, QuagginSE, YutzeyKE (2012) Pod1/Tcf21 is regulated by retinoic acid signaling and inhibits differentiation of epicardium-derived cells into smooth muscle in the developing heart. Dev Biol 368 : 345–357.
8. DeloukasP, KanoniS, WillenborgC, FarrallM, AssimesTL, et al. (2012) Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 45 : 25–33.
9. VoightBF, KangHM, DingJ, PalmerCD, SidoreC, et al. (2012) The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet 8: e1002793.
10. van DamH, CastellazziM (2001) Distinct roles of Jun : Fos and Jun : ATF dimers in oncogenesis. Oncogene 20 : 2453–2464.
11. KarinM, LiuZ, ZandiE (1997) AP-1 function and regulation. Curr Opin Cell Biol 9 : 240–246.
12. RainesEW, RossR (1993) Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J 69: S30–37.
13. SmithCL, BaekST, SungCY, TallquistMD (2011) Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circulation research 108: e15–26.
14. KnightJC, KeatingBJ, RockettKA, KwiatkowskiDP (2003) In vivo characterization of regulatory polymorphisms by allele-specific quantification of RNA polymerase loading. Nat Genet 33 : 469–475.
15. Martinez-EstradaOM, LetticeLA, EssafiA, GuadixJA, SlightJ, et al. (2010) Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet 42 : 89–93.
16. WhiteJT, ZhangB, CerqueiraDM, TranU, WesselyO (2010) Notch signaling, wt1 and foxc2 are key regulators of the podocyte gene regulatory network in Xenopus. Development 137 : 1863–1873.
17. NakagamaH, HeinrichG, PelletierJ, HousmanDE (1995) Sequence and structural requirements for high-affinity DNA binding by the WT1 gene product. Mol Cell Biol 15 : 1489–1498.
18. GannonAM, KinsellaBT (2009) The Wilms' tumour suppressor protein WT1 acts as a key transcriptional repressor of the human thromboxane A2 receptor gene in megakaryocytes. J Cell Mol Med 13 : 4571–4586.
19. LaityJH, DysonHJ, WrightPE (2000) Molecular basis for modulation of biological function by alternate splicing of the Wilms' tumor suppressor protein. Proc Natl Acad Sci U S A 97 : 11932–11935.
20. BarbauxS, NiaudetP, GublerMC, GrunfeldJP, JaubertF, et al. (1997) Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet 17 : 467–470.
21. DejongV, DegeorgesA, FilleurS, Ait-Si-AliS, MettouchiA, et al. (1999) The Wilms' tumor gene product represses the transcription of thrombospondin 1 in response to overexpression of c-Jun. Oncogene 18 : 3143–3151.
22. McCoyC, McGeeSB, CornwellMM (1999) The Wilms' tumor suppressor, WT1, inhibits 12-O-tetradecanoylphorbol-13-acetate activation of the multidrug resistance-1 promoter. Cell Growth Differ 10 : 377–386.
23. HindorffLA, SethupathyP, JunkinsHA, RamosEM, MehtaJP, et al. (2009) Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A 106 : 9362–9367.
24. Gonzalez-NavarroH, Abu NabahYN, VinueA, Andres-ManzanoMJ, ColladoM, et al. (2010) p19(ARF) deficiency reduces macrophage and vascular smooth muscle cell apoptosis and aggravates atherosclerosis. J Am Coll Cardiol 55 : 2258–2268.
25. LeeperNJ, RaiesdanaA, KojimaY, KunduRK, ChengH, et al. (2013) Loss of CDKN2B promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation. Arterioscler Thromb Vasc Biol 33: e1–e10.
26. JarinovaO, StewartAF, RobertsR, WellsG, LauP, et al. (2009) Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler Thromb Vasc Biol 29 : 1671–1677.
27. QuagginSE, Vanden HeuvelGB, IgarashiP (1998) Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech Dev 71 : 37–48.
28. SmithLT, LinM, BrenaRM, LangJC, SchullerDE, et al. (2006) Epigenetic regulation of the tumor suppressor gene TCF21 on 6q23-q24 in lung and head and neck cancer. Proc Natl Acad Sci U S A 103 : 982–987.
29. RichardsKL, ZhangB, SunM, DongW, ChurchillJ, et al. (2011) Methylation of the candidate biomarker TCF21 is very frequent across a spectrum of early-stage nonsmall cell lung cancers. Cancer 117 : 606–617.
30. MusunuruK, StrongA, Frank-KamenetskyM, LeeNE, AhfeldtT, et al. (2010) From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 466 : 714–719.
31. TuupanenS, TurunenM, LehtonenR, HallikasO, VanharantaS, et al. (2009) The common colorectal cancer predisposition SNP rs6983267 at chromosome 8q24 confers potential to enhanced Wnt signaling. Nat Genet 41 : 885–890.
32. Cowper-Sal lariR, ZhangX, WrightJB, BaileySD, ColeMD, et al. (2012) Breast cancer risk-associated SNPs modulate the affinity of chromatin for FOXA1 and alter gene expression. Nat Genet 44 : 1191–1198.
33. MianoJM, VlasicN, TotaRR, StemermanMB (1993) Localization of Fos and Jun proteins in rat aortic smooth muscle cells after vascular injury. Am J Pathol 142 : 715–724.
34. KhachigianLM, FahmyRG, ZhangG, BobryshevYV, KaniarosA (2002) c-Jun regulates vascular smooth muscle cell growth and neointima formation after arterial injury. Inhibition by a novel DNA enzyme targeting c-Jun. J Biol Chem 277 : 22985–22991.
35. BuganimY, MadarS, RaisY, PomeraniecL, HarelE, et al. (2011) Transcriptional activity of ATF3 in the stromal compartment of tumors promotes cancer progression. Carcinogenesis 32 : 1749–1757.
36. LvD, MengD, ZouFF, FanL, ZhangP, et al. (2011) Activating transcription factor 3 regulates survivability and migration of vascular smooth muscle cells. IUBMB Life 63 : 62–69.
37. HaiT, CurranT (1991) Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A 88 : 3720–3724.
38. MaoucheS, SchunkertH (2012) Strategies beyond genome-wide association studies for atherosclerosis. Arterioscler Thromb Vasc Biol 32 : 170–181.
39. FreedmanML, MonteiroAN, GaytherSA, CoetzeeGA, RischA, et al. (2011) Principles for the post-GWAS functional characterization of cancer risk loci. Nat Genet 43 : 513–518.
40. MellgrenAM, SmithCL, OlsenGS, EskiocakB, ZhouB, et al. (2008) Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circulation research 103 : 1393–1401.
41. ThomasJA, DeatonRA, HastingsNE, ShangY, MoehleCW, et al. (2009) PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerosis-prone flow patterns. Am J Physiol Heart Circ Physiol 296: H442–452.
42. MackCP (2011) Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol 31 : 1495–1505.
43. ArmulikA, AbramssonA, BetsholtzC (2005) Endothelial/pericyte interactions. Circulation research 97 : 512–523.
44. MooreAW, McInnesL, KreidbergJ, HastieND, SchedlA (1999) YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 126 : 1845–1857.
45. TurnbullC, PerdeauxER, PernetD, NaranjoA, RenwickA, et al. (2012) A genome-wide association study identifies susceptibility loci for Wilms tumor. Nat Genet 44 : 681–684.
46. SerlucaFC (2008) Development of the proepicardial organ in the zebrafish. Dev Biol 315 : 18–27.
47. WagnerKD, WagnerN, BondkeA, NafzB, FlemmingB, et al. (2002) The Wilms' tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction. FASEB J 16 : 1117–1119.
48. DardikR, LoscalzoJ, EskaraevR, InbalA (2005) Molecular mechanisms underlying the proangiogenic effect of factor XIII. Arterioscler Thromb Vasc Biol 25 : 526–532.
49. GershBJ, SliwaK, MayosiBM, YusufS (2010) Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J 31 : 642–648.
50. McPhersonR, PertsemlidisA, KavaslarN, StewartA, RobertsR, et al. (2007) A common allele on chromosome 9 associated with coronary heart disease. Science 316 : 1488–1491.
51. SheteS, HoskingFJ, RobertsonLB, DobbinsSE, SansonM, et al. (2009) Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet 41 : 899–904.
52. StaceySN, SulemP, MassonG, GudjonssonSA, ThorleifssonG, et al. (2009) New common variants affecting susceptibility to basal cell carcinoma. Nat Genet 41 : 909–914.
53. TimofeevaMN, HungRJ, RafnarT, ChristianiDC, FieldJK, et al. (2012) Influence of common genetic variation on lung cancer risk: meta-analysis of 14 900 cases and 29 485 controls. Hum Mol Genet 21 : 4980–4995.
54. BuX, QuertermousT (1997) Identification of an endothelial cell-specific regulatory region in the murine endothelin-1 gene. J Biol Chem 272 : 32613–32622.
55. RobertsonG, HirstM, BainbridgeM, BilenkyM, ZhaoY, et al. (2007) Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods 4 : 651–657.
56. NurnbergST, RendonA, SmethurstPA, PaulDS, VossK, et al. (2012) A GWAS sequence variant for platelet volume marks an alternative DNM3 promoter in megakaryocytes near a MEIS1 binding site. Blood 120 : 4859–4868.
57. PruimRJ, WelchRP, SannaS, TeslovichTM, ChinesPS, et al. (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26 : 2336–2337.
58. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
59. BarrettJC, FryB, MallerJ, DalyMJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 : 263–265.
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



