-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaHepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
In a forward genetic screen for regulators of pancreas development in zebrafish, we identified donuts908, a mutant which exhibits failed outgrowth of the exocrine pancreas. The s908 mutation leads to a leucine to arginine substitution in the ectodomain of the hepatocyte growth factor (HGF) tyrosine kinase receptor, Met. This missense mutation impedes the proteolytic maturation of the receptor, its trafficking to the plasma membrane, and diminishes the phospho-activation of its kinase domain. Interestingly, during pancreatogenesis, met and its hgf ligands are expressed in pancreatic epithelia and mesenchyme, respectively. Although Met signaling elicits mitogenic and migratory responses in varied contexts, normal proliferation rates in donut mutant pancreata together with dysmorphic, mislocalized ductal cells suggest that met primarily functions motogenically in pancreatic tail formation. Treatment with PI3K and STAT3 inhibitors, but not with MAPK inhibitors, phenocopies the donut pancreatic defect, further indicating that Met signals through migratory pathways during pancreas development. Chimera analyses showed that Met-deficient cells were excluded from the duct, but not acinar, compartment in the pancreatic tail. Conversely, wild-type intrapancreatic duct and “tip cells” at the leading edge of the growing pancreas rescued the donut phenotype. Altogether, these results reveal a novel and essential role for HGF signaling in the intrapancreatic ducts during exocrine morphogenesis.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003650
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003650Summary
In a forward genetic screen for regulators of pancreas development in zebrafish, we identified donuts908, a mutant which exhibits failed outgrowth of the exocrine pancreas. The s908 mutation leads to a leucine to arginine substitution in the ectodomain of the hepatocyte growth factor (HGF) tyrosine kinase receptor, Met. This missense mutation impedes the proteolytic maturation of the receptor, its trafficking to the plasma membrane, and diminishes the phospho-activation of its kinase domain. Interestingly, during pancreatogenesis, met and its hgf ligands are expressed in pancreatic epithelia and mesenchyme, respectively. Although Met signaling elicits mitogenic and migratory responses in varied contexts, normal proliferation rates in donut mutant pancreata together with dysmorphic, mislocalized ductal cells suggest that met primarily functions motogenically in pancreatic tail formation. Treatment with PI3K and STAT3 inhibitors, but not with MAPK inhibitors, phenocopies the donut pancreatic defect, further indicating that Met signals through migratory pathways during pancreas development. Chimera analyses showed that Met-deficient cells were excluded from the duct, but not acinar, compartment in the pancreatic tail. Conversely, wild-type intrapancreatic duct and “tip cells” at the leading edge of the growing pancreas rescued the donut phenotype. Altogether, these results reveal a novel and essential role for HGF signaling in the intrapancreatic ducts during exocrine morphogenesis.
Introduction
The vertebrate pancreas is an endodermal organ that is part endocrine, releasing hormones that regulate glucose metabolism, and part exocrine, releasing pancreatic juices that aid in digestion. Pancreatic endocrine and exocrine developmental dysmorphogenesis and dysregulation, including diabetes mellitus and pancreatic adenocarcinoma, can result in human diseases with high morbidity and mortality. Thus, a more sophisticated understanding of molecular mechanisms mediating pancreatic development and homeostasis will certainly refine the treatment of these diseases.
In zebrafish as in mammals, all pancreatic endocrine and exocrine tissues derive from the fusion of a dorsal and ventral bud arising at the level of somites 2–9 [1], [2], [3]. In zebrafish, the dorsal bud generates the principal islet by 24 hours post fertilization (hpf), and fuses with the emerging ventral bud between 40–44 hpf [4], [5]. Around 52 hpf, acinar and ductal cells start to expand caudally to form the tail of the pancreas [5], [6], [7]. The pancreatic mesenchyme is essential for the induction, growth, branching, and cytodifferentiation of the pancreatic epithelium [8]. While several mesenchymal signals mediating pancreatic induction have been identified (reviewed in [9]), our knowledge of how the mesenchymal/epithelial signaling pathways regulate pancreatic growth and branching is more limited [8].
Hepatocyte Growth Factor (HGF) is a stromally-produced ligand which binds Met, a receptor tyrosine kinase that is predominantly expressed in epithelia. Upon receptor dimerization and autophosphorylation, Met activates a bevy of cellular processes including motogenesis, tubulogenesis, mitosis, chemotaxis, and cell survival [10]. During organogenesis, HGF/Met signaling has been shown to be involved in liver and placenta formation, as well as in the migration of hypaxial muscle precursors into limbs [11], [12], [13], [14]. However, the role of HGF/Met signaling in vertebrate pancreas development remains unclear. Both HGF and Met are expressed in the developing rodent pancreas [15], [16], but pancreatic phenotypes have not been characterized in global knockout mice. Studies have been mostly focused on the role of HGF/Met signaling in pancreatic tumorigenesis and beta-cell survival. Indeed, pancreas-specific Met knockout mice are euglycemic and morphologically unaffected at maturity, but show impaired beta-cell homeostasis during pregnancy [17] and following STZ-induced islet inflammation [18]. Even though HGF/Met signaling has been shown to activate the PI3K/Akt and ERK pathways in acinar cells [19], its biological role during exocrine pancreas development remains undetermined.
Results/Discussion
Identification and genetic mapping of donuts908 mutants
To find novel regulators of endodermal organ morphogenesis and differentiation, we conducted a forward genetic screen utilizing doubly transgenic (2CLIP: 2-color liver, insulin, exocrine pancreas) zebrafish with EGFP-expressing pancreatic acinar cells and DsRed-expressing pancreatic beta-cells and hepatocytes [20]. Using this approach, we identified donuts908 mutants, which show shortened (type 1) or absent (type 2) exocrine pancreatic (xp) tails. Tg(ptf1a:GFP)jh1 [21] was crossed into the donuts908 background to reveal the short exocrine pancreas phenotype at 3 days post fertilization (dpf; Fig. 1A–E), prior to the terminal differentiation of acinar cells. Differentiation of the principal islet (beta-cells, β), acinar and ductal cells, and liver (li) appeared unaffected in donut mutants (Fig. 1B,C; and see below). Nearly 25% of progeny from heterozygous intercrosses showed the donut phenotype, evenly split between types 1 and 2 (Fig. 1F). The variable expressivity suggested either a hypomorphic mutation in donut, and/or a genetic interaction with other loci.
Fig. 1. donuts908 is a hypomorphic allele of met. 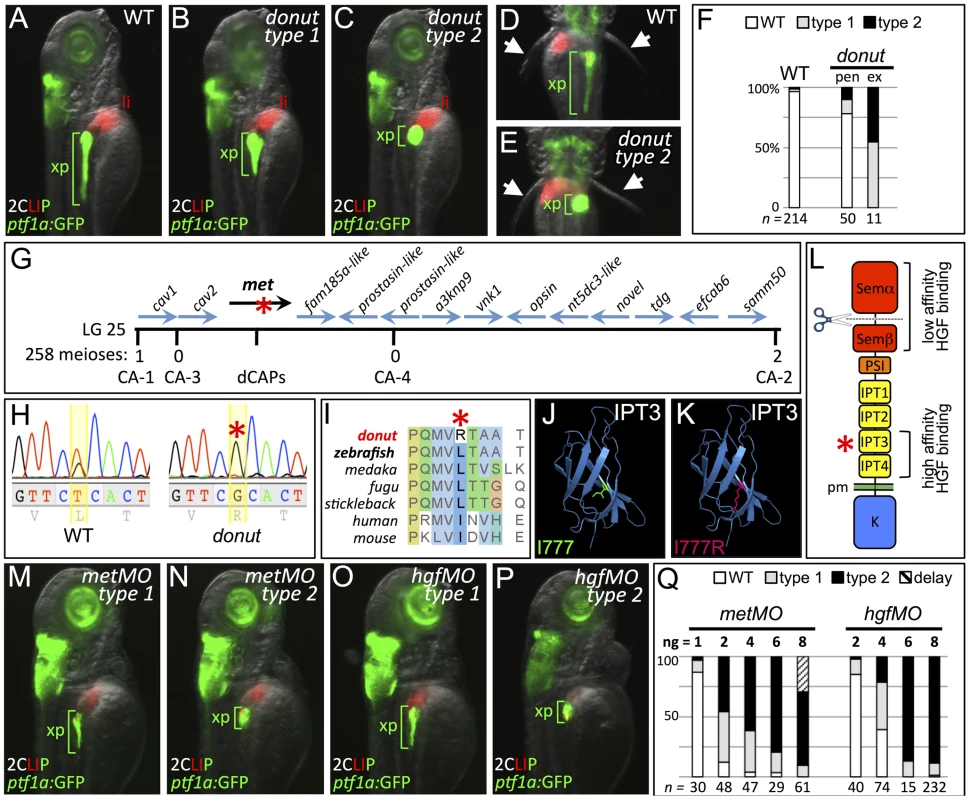
(A–E) Exocrine pancreas (xp) structure marked by ptf1a:GFP expression in 3 dpf WT (A,D), and in type 1 (B) and type 2 (C,E) donut mutants. The pectoral fins (arrowheads) of donut mutants lack muscle tone, often asymmetrically, leading to their “open wing” appearance (right arrow, E). (F) Penetrance (pen) and expressivity (ex) of the donut phenotype. A small fraction of WT embryos shows donut-like pancreatic phenotypes, while 22% of clutchmates from heterozygous intercrosses exhibited either a spherical (55%) or an intermediately shortened (45%) pancreas. n below bars represents the number of embryos examined from WT clutches (right), heterozygote in-crossed clutches (center), and embryos exhibiting donut-like pancreatic phenotypes (right). (G–I) donut mutants have a lesion in met. donut was mapped to a critical interval on Chr. 25 containing 14 annotated genes (G); met showed a T2324G variant (H) causing an L775R amino acid substitution (I). (J,K) Model structures of the human MET IPT3 domain with isoleucine 777 (analogous to zebrafish residue L775) marked green (J) or substituted arginine marked red (K). (L) Diagram of Met showing the I777R substitution localized to the high affinity HGF binding site in IPT3, and the furin cleavage site in the semaphorin-like domain. (M–P) Morpholino-mediated knockdown of met (M,N) or hgfa/b (O,P) resulted in phenocopy of the donut mutation. (Q) Dose dependence of morpholino-induced phenotypes. At 8 ng, metMO exhibited some non-specific developmental delay, suggesting toxicity. pen, penetrance; ex, expressivity; li, liver; pm, plasma membrane. To isolate the molecular lesion responsible for the donut phenotype, we used bulk segregant analysis and localized donut on linkage group 25; we then used z-markers and customized CA-repeat markers to define a critical interval containing 14 candidate genes (Fig. 1G). In sequencing these candidates, we identified a T2324G variant in the coding sequence of the met gene, resulting in an L775R missense substitution (Fig. 1H). The MetL775R substitution in donuts908 mutants represents a significant shift in amino acid charge and polarity at a residue that is conserved in vertebrates as a leucine or an isoleucine (Fig. 1I). Importantly, L775 is localized to the IPT3 (immunoglobulin-like regions in plexins and transcription factors 3) domain in the Met ectodomain, which together with IPT4 comprises a high affinity HGF binding site (Fig. 1L) [22], [23]. Moreover, protein domain modeling based on human MET IPT3 indicates that the analogous residue, I777, resides in a solvent inaccessible region of IPT3, and that its substitution with arginine may be sterically unfavorable (Fig. 1J,K), suggesting that Met folding may be compromised in zebrafish donut mutants.
We hypothesized that the donuts908 mutation would result in a Met loss of function. To test this hypothesis, we injected zygotes with translation-blocking met morpholinos (metMO). We found that even low doses of metMO (2 ng) could reproduce the shortened pancreatic tail phenotype observed in donut mutants with high penetrance (Fig. 1M,N). Importantly, when donuts908+/− embryos were injected with a near-threshold (1 ng) dose of MO, 53.4% displayed a short pancreas phenotype, as compared to 29.1% of injected wild-type embryos (Fig. S1). Since HGF is the only demonstrated ligand for Met, we reasoned that knockdown of both zebrafish hgf paralogs, hgfa and hgfb, should also mimic donut mutant phenotypes. Similar to donut mutants, we found that embryos injected with a mixture of MOs directed against hgfa and hgfb (hgfMO) also developed shortened pancreata (Fig. 1O,P). We observed a positive correlation between the injected quantity of each morpholino and the penetrance and expressivity of the pancreatic tail phenotypes (Fig. 1Q), with expressivity variation resolving towards the more severe type 2 phenotype at higher doses. Finally, donut mutants frequently lack muscle tone in the pectoral fins, suggesting a failed morphogenesis of hypaxial muscle (Fig. 1D,E, arrowheads; Fig. S2), which corroborates previous studies of Met function in myogenic precursor cells in mouse and zebrafish [11], [12]. In sum, these data are consistent with the interpretation that the phenotypic spectrum observed in donuts908 mutants is due to a hypomorphic effect of the L775R substitution.
To clarify how HGF signaling is implemented in pancreatogenesis, we examined the expression of met, hgfa and hgfb during several stages of pancreas formation. We also analyzed the expression of both furin genes, furina and furinb, which encode the proteases that cleave Met into a mature form. At 34 hpf, which is prior to pancreatic bud fusion, met is expressed throughout the endodermal organ forming region, including pancreas, liver, and intestine anlagen (Fig. 2A). Additionally, met expression is detected in pancreatic endoderm before (44 hpf), during (52 hpf), and after its caudal extension (80 hpf; Fig. 2A). We found that hgfa and hgfb were expressed adjacent to the dorsal pancreatic bud at 34 hpf, and that their expression remains in register with the leading edge of the growing pancreatic tail (red arrowhead) between 52 and 80 hpf (Fig. 2B,C). Importantly, both furina and furinb are expressed in endodermal tissue throughout pancreatogenesis, with furina present in liver and pancreas at 75 hpf (Fig. S3), indicating that the process of Met maturation is active in these organs. To determine which exocrine tissues express met during pancreatic tail morphogenesis, we performed fluorescent in situ hybridization in the Tg(nkx2.2a(-3.5 kb):GFP) line (hereafter duct:GFP) which highlights the intrapancreatic ducts (IPDs) [6]. We found that within the pancreatic tail, met was expressed in both IPD and acinar cells at 60 hpf, a time of active pancreatic tail extension (Fig. 2D–F). A role for Met signaling in pancreatic exocrine morphogenesis has not been reported in mammalian model systems. Hgf and Met knockout mice die during organogenesis precluding analysis [11], [13], [14], and a pancreas specific knockout (PancMet KO) showed no clear morphological defect of the adult pancreas [18]. However, as pancreas development was not described in PancMet KO, it is possible that a transient phenotype or developmental delay could have been overlooked. Furthermore, it is likely that early mosaic recombinase activity inherent to the Pdx1:Cre line that was utilized in these experiments leaves a population of wild-type cells in these mice. These cells may be competent to effect normal morphogenesis, and would mirror what we observed in chimera studies (see below). Alternately, residual Met protein, which is expressed in endoderm prior to the onset of Pdx1 expression, or a parallel/redundant mechanism could drive exocrine outgrowth in mammals. Additional targeted studies are needed in mice to determine whether the role of Met in exocrine pancreas growth is conserved.
Fig. 2. Dynamic expression of met and hgf during pancreas morphogenesis. 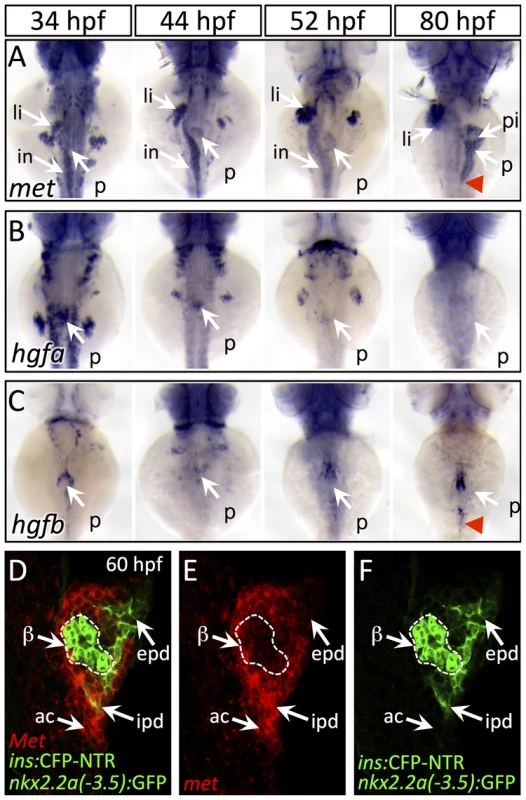
(A) met is expressed strongly in the pancreatic bud (p), liver bud (li), and intestinal bulb (in) from 34–52 hpf. At 80 hpf, met expression is found throughout the exocrine pancreas and is diminished in the principal islet (pi). (B,C) hgfa (B) and hgfb (C) are expressed at 34–44 hpf near the dorsal pancreatic bud prior to pancreatic tail formation. At 52 hpf, at the onset of pancreatic tail formation, both hgfa and hgfb are expressed near the distal tip of the pancreatic bud, with hgfb expression persisting until the completion of pancreatic tail outgrowth (red arrowhead). (D–F) One-micron confocal plane images of duct:GFP;ins:CFP-NTR embryos stained for met mRNA using fluorescent in situ hybridization shows met expression (red) in both acinar cells (ac) and intrapancreatic ducts (ipd; green), but not in the principal islet (green outlined in white). Dorsal views, anterior to the top. beta-cells, β; epd, extrapancreatic duct. MetL775R shows impaired plasma membrane targeting and activation
To model how the donut mutation affects Met function, we generated an I776R point mutation in murine Met, an analogous lesion to the donuts908 mutation, and then transfected either MetWT or MetI776R into TOV112D cells. This cell line lacks endogenous Met activity, but expresses the intracellular components of the Met signaling pathway [22], [24]. Met is translated as a single polypeptide chain which is cleaved by furin into alpha and beta chains [25]. In TOV112D cells transfected with MetWT, most of the nascent polypeptide was cleaved to the mature form (150 kDa+30 kDa), while a small proportion of the receptor remained unprocessed (190 kDa). Since the I776R lesion lies far from the furin cleavage site (residues 302–307) [26], we were surprised to find that the MetI776R precursor polypeptide failed to be cleaved (Fig. 3A, bottom panels). Since cleavage-incompetent Met mutants (i.e. MetR306A) can signal normally [27], we next assessed the signaling efficacy of MetWT and MetI776R using specific anti-phosphorylated Met antibodies directed against several key tyrosine residues. Upon HGF binding, Met dimerizes and its cytoplasmic catalytic region is activated by trans-phosphorylation of tyrosines Y1234 and Y1235. Subsequently, residues Y1349 and Y1356 are phosphorylated in the Met multifunctional docking site, which connects Met to multiple downstream branches, including Ras/MAPK and PI3K, through several adapter proteins. In addition, phosphorylation of tyrosine Y1003 negatively regulates Met signaling by promoting receptor turnover. Compared to MetWT, phosphorylation of Y1234-5, Y1349, and Y1003 were significantly diminished in MetI776R (Fig. 3A,B). Finally, since cleavage by Furin occurs in the Golgi apparatus [28], we hypothesized that intracellular trafficking of MetI776R from the endoplasmic reticulum to the plasma membrane was impaired. To investigate this hypothesis, we next examined the presentation of Met at the plasma membrane using an antibody that binds specifically to the Met ectodomain (α-Met ab1). Antibody staining was evident at the membrane of unfixed, unpermeabilized HEK293T cells expressing MetWT or cleavage incompetent MetR306A, but not MetI776R (Fig. 3C–E). However, all of these Met variants were detected in fixed and detergent-permeabilized cells using an antibody that recognizes an intracellular epitope (α-Met ab2), thereby indicating similar transfection efficiencies of the three constructs (Fig. 3F–H). Thus, these data suggest that MetI776R is retained in an intracellular compartment, and that the lack of MetI776R cleavage per se is not causing this retention. To further test this hypothesis, RNA encoding zebrafish MetWT or MetL775R fused to mCherry were injected in zebrafish embryos and the localization of the proteins analyzed at blastula stages (Fig. 3I–J). As observed in cell culture, MetL775R was not detected at the plasma membrane in vivo. This defect is not simply a delay of membrane targeting, as similar results were observed at later time points (data not shown). Together, these data support the hypothesis that deficient signaling through MetL775R causes the hypomorphic effect observed in donuts908 mutants. Likely, reduced Met signaling is due to (1) localization of MetL775R away from the plasma membrane, (2) impaired binding of HGF to the high-affinity binding site in IPT3-4, and/or (3) impaired assembly of Met co-receptor moieties, that would then lead to defective phospho-activation of Met.
Fig. 3. Orthologous murine donut mutation, I776R, impairs HGF signaling and receptor trafficking. 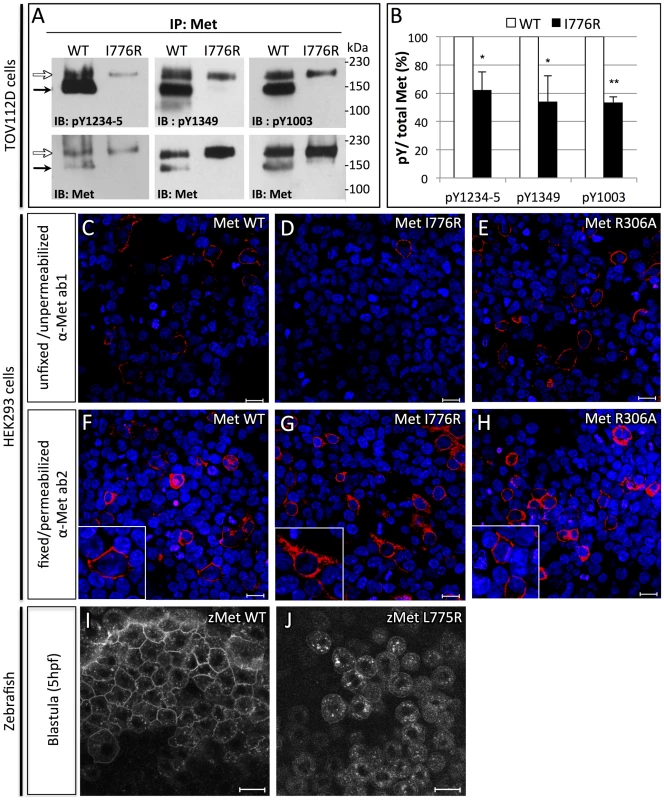
(A) Immunoblots of Met protein prepared from lysates of TOV112D cells transfected with murine MetWT or MetI776R. Upper panels blotted with anti-pY show diminished phosphorylation at tyrosines critical for signaling and lower panels blotted with anti-Met show loading controls. Precursor (white arrows) and mature (black arrows) forms of MetWT are both detected, but MetI776R is only present in the precursor form. (B) Quantification by densitometry of phosphorylated Met versus total Met in panel A (t test, * p<0,05; **p<0,01). (C–H) Immunofluorescence staining of HEK293 cells transfected with MetWT (C,F), MetI776R (D,G), or furin cleavage-incompetent MetR306A (E,H). Unfixed, unpermeabilized cells were stained with anti-Met ab1, which binds an extracellular epitope (C–E), and fixed, permeabilized cells were stained with ab2, which binds an intracellular epitope (F–H). MetWT and MetR306A are localized to the plasma membrane (C, E, insets in F, H), but MetI776R is not (D), and is mostly retained in the cytoplasm (G, inset). Intracellular staining of Met demonstrates similar transfection efficiency. DAPI staining (blue) marks nuclei. (I–J) Live imaging of zebrafish blastulae injected with zebrafish MetWT-mCherry (I) and MetL775R-mCherry (J) at 5 hpf. Zebrafish MetL775R-mCherry largely fails to localize to the plasma membrane as compared to MetWT. Scale bars, 20 µm. Cell migration but not proliferation is impaired by the donuts908 mutation
Growth and elongation of the pancreatic tail involves both proliferation and directed migration of exocrine cells [6], [7]. To analyze which mechanism is impaired in donut mutants, we first assayed cell proliferation using 30 min incorporation of the thymidine analog EdU at two time points during pancreatic tail growth: 56 and 75 hpf (Fig. 4A–D). Quantification of labeled cells showed no significant difference in exocrine cell proliferation between WT and donut mutant animals (Fig. 4E). To determine whether acinar or ductal cell migration was affected in donut mutants, we next examined the distribution of acinar and ductal cells using ptf1a:GFP and duct:GFP, respectively, in 84 hpf wild-type (WT) and metMO-injected (Fig. 4F–I) larvae. Although ptf1a:GFP+ acinar cells were always confined to the pancreas, duct:GFP+ cells were clearly localized in the hepatic region in both type 1 and type 2 mutants. Furthermore, while IPD cells were elongated and spindle-shaped in WT, they showed a more rounded morphology in metMO-injected larvae (Fig. 4H,I, insets). We marked hepatocytes and biliary ducts with Prox1 and Alcam antibodies, respectively, and found that duct:GFP+ pancreatic cells had extensively invaded the liver, tracking along the biliary duct network (Fig. 4J,K). Taken together, our data show that HGF/Met signaling promotes invasive cell behavior during pancreatic tail morphogenesis, rather than the proliferation of exocrine cells.
Fig. 4. Cell migration, but not proliferation, is dysregulated in donuts908 mutants. 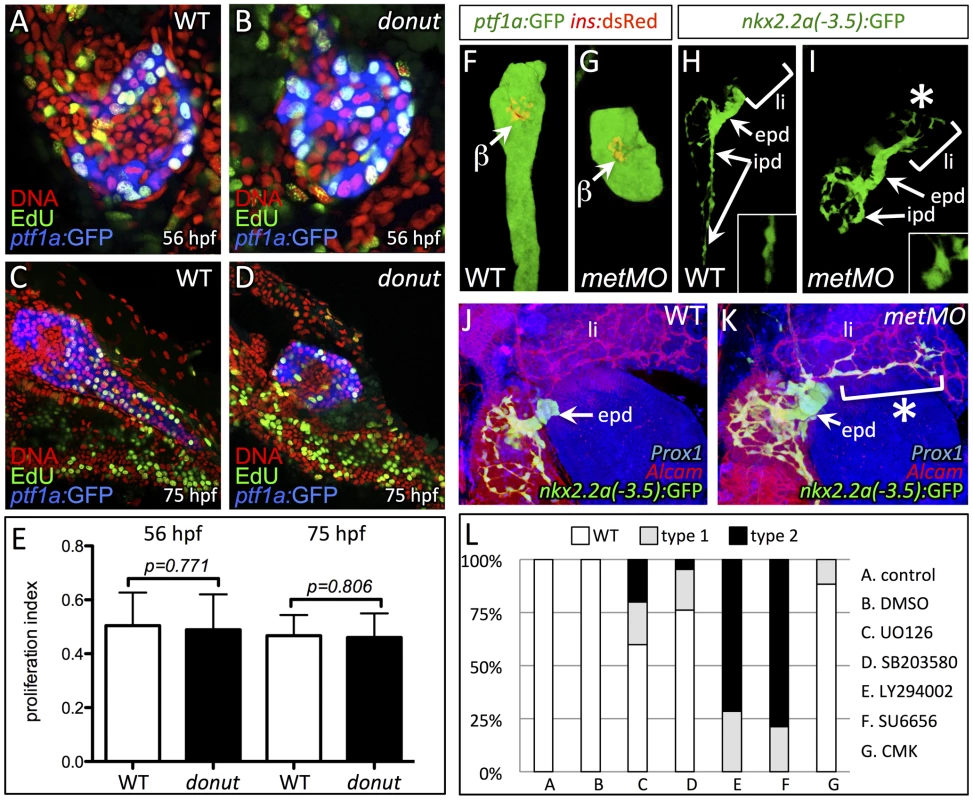
(A–D) EdU incorporation assay in WT (A,C) and donuts908 mutant (B,D) animals at 56 (A,B) and 75 (C,D) hpf. (E) Quantification of proliferation assay data shows no significant change in acinar cell proliferation at early or late stages of pancreatic tail formation. (F–I) Morphology of exocrine tissues at 84 hpf in WT (F,H) and metMO-injected (G,I) larvae revealed by Tg(ptf1a:GFP) (acinar; F,G) or Tg(nkx2.2a(-3.5 kb):GFP) (duct; H,I) expression. In metMO-injected embryos, acinar and ductal cells fail to migrate caudally, and remain near the principal islet (β). In metMO larvae, ductal cells exhibit a more rounded morphology in the exocrine pancreas (insets) and are also observed in the liver region (asterisk). (J,K) 84 hpf WT (J) and metMO-injected (K) Tg(duct:GFP) larvae stained with Prox1 (blue) and Alcam (red). Pancreatic ductal cells are ectopically localized in the liver (li) along tracts of biliary ducts. (L) Quantification of pancreatic tail defects in small molecule treated larvae; inhibitors of the MAPK pathway (UO126, SB203580) and furin function (CMK) had little effect on pancreatic tail outgrowth, while inhibitors of PI3K (LY294002) and STAT3 (SU6656) function mimicked the type 1 and type 2 donut phenotypes. epd, extrapancreatic duct. The invasive cell behaviors activated by HGF/Met signaling, such as motility and proliferation, work through multiple parallel downstream branches of Met signaling. Three key branches involve the PI3K, ERK, and STAT3 pathways, which may be activated by direct interaction with Met, or through adapter proteins: the PI3K/Akt pathway elicits cell migration via Rac1, as well as cell survival; the ERK branch promotes proliferation and transformation; and STAT3 signaling mediates branching morphogenesis and proliferation (reviewed in [10]). To dissect which specific branches might be critical for Met-mediated exocrine morphogenesis, we treated larvae during the formation of the pancreatic tail with specific inhibitors (Fig. 4L). Inhibition of ERK signaling with the MEK inhibitor UO126 or the p38 MAPK inhibitor SB203580 resulted in a mild phenocopy of donut type 1 and type 2 mutants, ranging from 25–40%. However, treatment with the potent PI3K inhibitor LY294002 or the STAT3/Src family kinase inhibitor SU6656 generated a nearly perfect phenocopy of type 1 and 2 donut mutants. These data confirm our previous observations that cell proliferation via MAPK/MEK is not the primary mechanism driving pancreatic tail outgrowth. The furin protease inhibitor CMK caused only a minor effect on pancreatic tail formation confirming that failure of proteolytic cleavage of Met may not cause the reduced signal transduction capacity of MetL775R.
Met signaling is autonomously required in IPD cells and “tip cells” for pancreatic tail elongation
The differentiating exocrine pancreas is highly organized at the onset of pancreatic tail elongation [6], [7]. Acinar and ductal cells are the only two cell types found in the growing pancreatic tail, and they are always closely associated. Even though met is expressed in both cell types, we hypothesized that it was required primarily in ductal cells, since these cells were mislocalized and dysmorphic in metMO-injected larvae. To test this hypothesis, we performed cell transplantation experiments to generate chimeras and determine the autonomy of Met function in the pancreas (Fig. 5A). First, blastomeres isolated from control or metMO-injected Tg(hs:mCherry) (see Methods) embryos were directed toward an endodermal fate by cas mRNA coinjection [29], [30], and were transplanted into WT hosts. Incorporated donor cells ubiquitously express mCherry RFP upon heat shock induction, and were quantified based on their contribution to ptf1a:GFP+ acinar or ptf1a:GFP− ductal cell compartments in the distal or proximal portion of the exocrine pancreas (Fig. 5B). In contrast to WT cells that contributed evenly to both acinar and ductal compartments, metMO-injected cells were strongly biased toward the acinar cell and proximal ductal compartments, and were largely excluded from the distal ductal compartment (Fig. 5C–E; Tables 1,2). We observed similar results when we transplanted endodermal cells from donut mutant embryos into Tg(hs:mCherry) hosts (data not shown). Next, we assessed the rescue of exocrine pancreatic tail outgrowth by transplanting varying numbers of WT Tg(hs:mCherry) cells into the endoderm of metMO-injected hosts (Tables 3,4). metMO-injected larvae lacking WT contribution showed a type 1 donut phenotype (Fig. 5F), whereas a high contribution of WT endoderm cells led to a complete rescue of the pancreatic tail outgrowth (Fig. 5G). Notably, the tip of the rescued pancreatic tail appeared to be composed exclusively of WT donor cells (Fig. 5G, inset). In larvae with fewer integrated WT cells, the pancreatic tail was also rescued, with the WT cells being preferentially found in the IPD (Fig. 5H, inset). Finally, we confirmed that WT endoderm could not similarly rescue the short exocrine pancreas phenotype observed in hgfMO-injected embryos, consistent with a mesenchyme-specific role for hgfa and hgfb (Fig. S4). These data indicate that endodermal Met signaling, probably preferentially in distal IPD cells, directs the caudal extension of the pancreatic tail.
Fig. 5. HGF/Met signaling is required in intrapancreatic ducts for pancreatic tail morphogenesis. 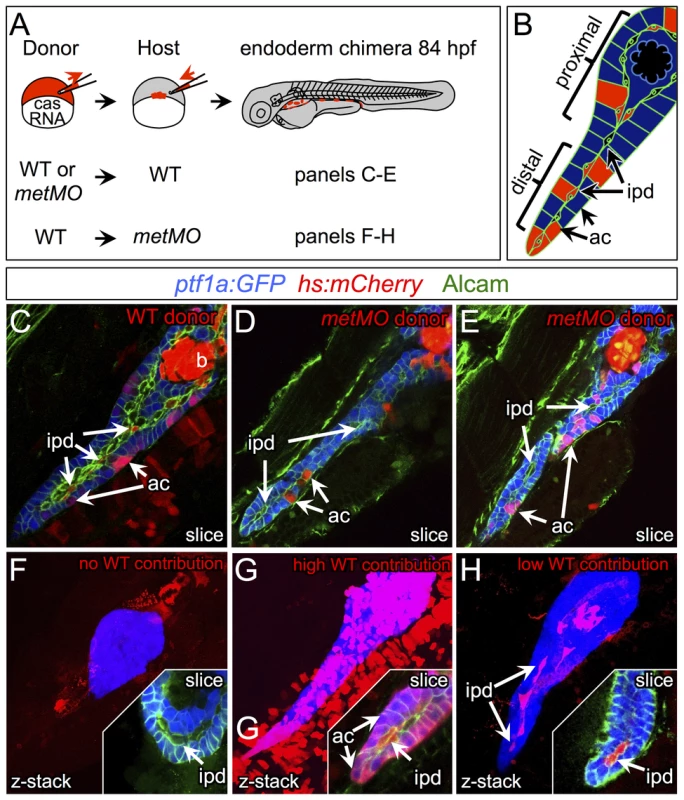
(A) Schematic of cell transplantation experiments. WT or metMO-injected donor cells were transplanted into WT hosts (top), and WT donor cells were transplanted to metMO-injected hosts (bottom); cas mRNA injection biased donor cells toward endodermal differentiation. (B) Scheme used to identify and quantify contribution of transplanted cells in chimeras. Donor cells are marked by Tg(hs:mCherry) (red), acinar cells are marked by Tg(ptf:GFP)jh1 (blue), and Alcam immunostaining (green) delineates the ducts. (C–E) Single plane confocal images of WT (C) and metMO (D,E) donor endoderm transplanted into WT hosts: WT donor cells contributed to both intrapancreatic ductal (ipd) and acinar (ac) compartments, but metMO donor cells were excluded from the ducts. (F–H) WT≫metMO hosts: With no WT contribution to the pancreas, metMO morphants show donut-like phenotypes (E); with high contribution of WT donor cells, the pancreatic tail growth is rescued (G), and the tip of the tail is almost entirely comprised of WT donor cells (inset of single confocal slice); with moderate contribution of WT donor cells, the pancreatic tail morphology can be rescued with only WT ductal cells (H), and no WT acinar cells are observed in the tip (inset of single confocal slice). Tab. 1. Endodermal contributions in WT>WT chimeric embryos. 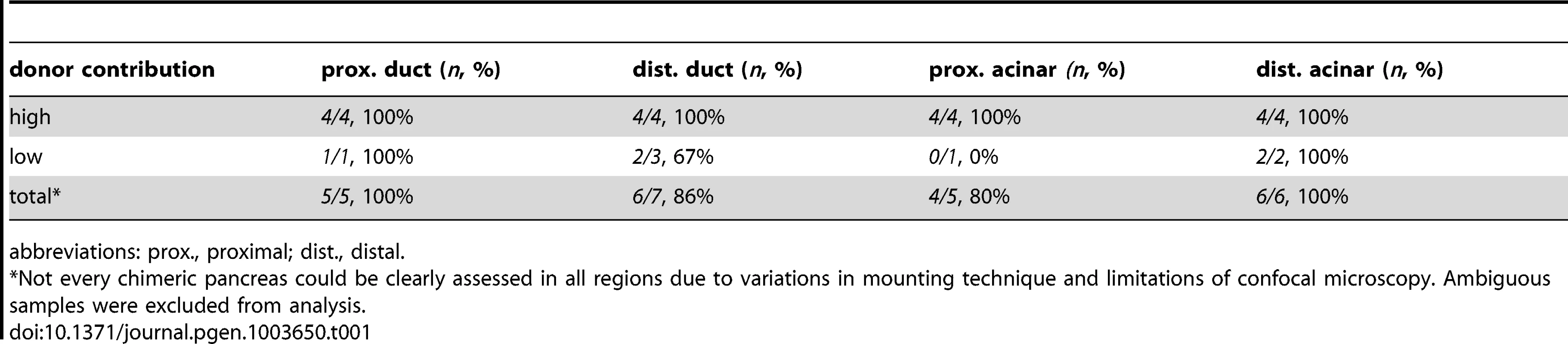
abbreviations: prox., proximal; dist., distal. Tab. 2. Endodermal contributions in <i>metMO</i>>WT chimeric embryos. 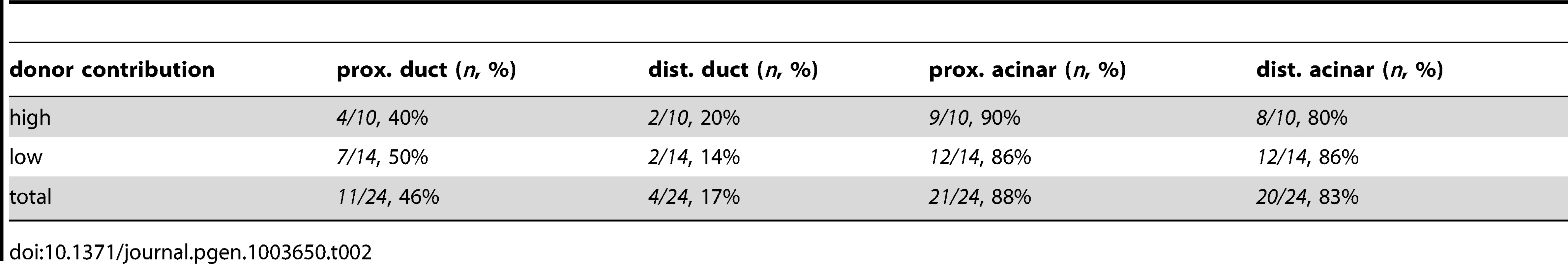
Tab. 3. Endodermal contributions in WT><i>metMO</i>-injected chimeric embryos. 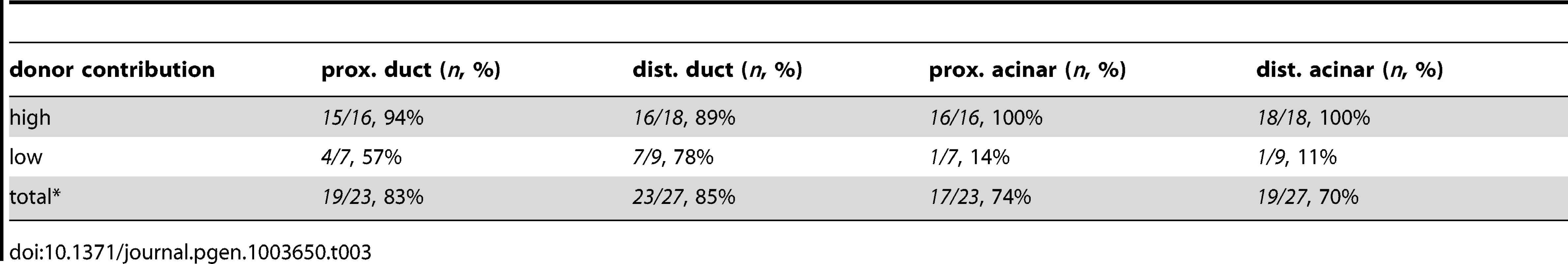
Tab. 4. Breakdown of rescued embryos by WT tissue contribution. 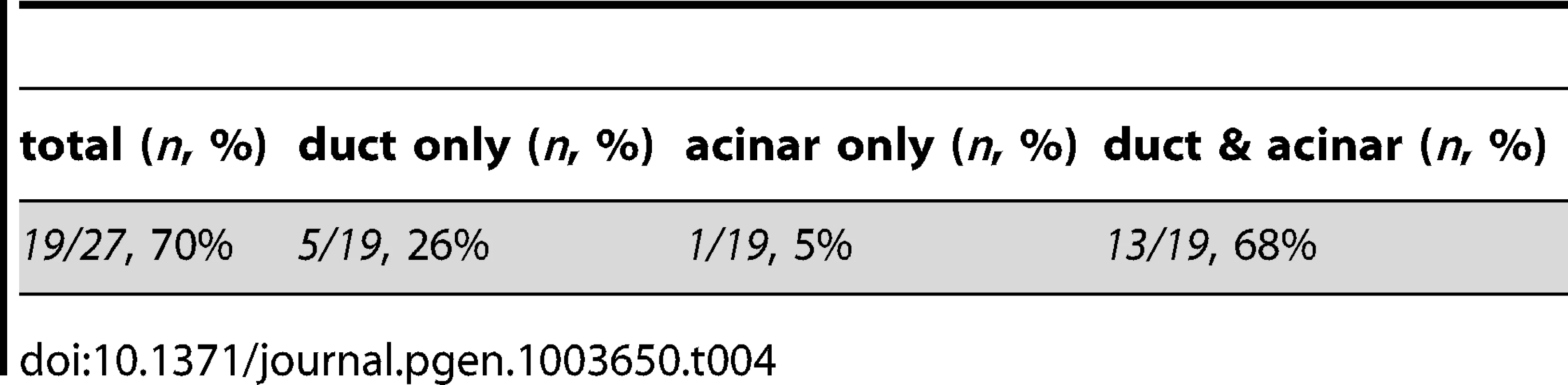
We thus propose a model for pancreatic tail outgrowth in which ductal cells would respond to HGF secreted from the mesenchyme adjacent to the pancreatic primordium and initiate migration caudally. The HGF signal may constitute a chemotactic signal for the ductal cells, or simply a motogenic signal, with directionality imparted by distinct signaling pathways. Even though Met signaling appears to be required in IPD cells for pancreatic outgrowth, Met signaling may also be active in a small population of “tip” cells that are mature or differentiating acinar cells. From the analysis of a specific subset of chimeric embryos, ductal cells may thus be directing the migration of the pancreatic tail from a trailing position, possibly exhibiting cellular extensions, such as cytonemes or filipodia, that were not resolved in our studies.
Hence, our data expand the understanding of the role of HGF/Met signaling in mitogenic, motogenic and morphogenetic events required for the development, homeostasis and regeneration of different tissues. In addition, they provide a developmental framework to dissect the role of Met in pancreatic cancer stem cells [31], [32].
Materials and Methods
Zebrafish strains
Fish were raised and maintained under standard conditions [33]. Pigmentation was inhibited with 0.02 mM phenlythiourea (Sigma). We used the following published strains: Tg(fabp10:RFP, ela3l:EGFP)gz12 [34], Tg(ins:CFP-NTR)s892 [35], Tg(ins:dsRed)m1018 [36], Tg(nkx2.2a(-3.5 kb):GFP)ia3 (a.k.a. “duct:GFP”) [6], and Tg(ptf1a:eGFP)jh1 [21]. To generate the Tg(hsp70l:loxP-mCherry-stop-loxP-NICD-P2A-Emerald) line (a.k.a. Tg(hs:mCherry)), H2B-GFP was replaced in the plasmid hsp70l:loxP-mCherry-loxP-H2B-GFP-cryaa:Cerulean [37] by an in-frame PCR fusion product of NICD [38], P2A [39], and Emerald GFP (Invitrogen, Carlsbad, CA). Transgenesis was achieved as described [40].
Genetic screen and mapping
An ENU mutagenesis screen was executed as described [20]. Bulk segregant analysis was performed using pooled DNA extracted from 20 WT or donut mutant embryos. donut mutants were subsequently genotyped using a dCAPS strategy: a PCR product amplified by the primer set: 5′-TCCAG CCCAA ACATT CTTTC and 5′ - CGTTT GTGTG GGTTG TATAG ACTCA CCACT TGGAA GAGTT TGCCC TCAGT GGCGG CAGcG was digested with HhaI.
Protein modeling of donut/I777R
Pymol software (Schrödinger) was used to manipulate the PDB model of human IPT1-4 [41].
Immunohistochemistry and immunofluorescence
Whole mount antibody staining, in situ hybridization, and proliferation analysis with EdU were performed as described [20]. Proliferation index was calculated using the number of EdU+ cells divided by the total number of exocrine pancreas nuclei, per section; >5 slices were counted per animal. Significance was assessed using student's t test. Fluorescent in situ hybridizations were performed as described [42]. Templates for antisense RNA probes were amplified from embryonic cDNA with the following primers: met: CGGAG AGAGA GGGAG GAAG and TAATA CGACT CACTA TAGGG AGACA TTGAT GTCCG TGATG GAG; hgfa: TGTGT GCTTG AGAAA GAGAG AGA and TAATA CGACT CACTA TAGGG AGATC GACAA ATTGC CACGA TAA; hgfb: AGCCA CTGCA GGGAG ACTAC and TAATA CGACT CACTA TAGGG AGAGG GGTAC CTTTT AGGGT GGA; furina: GTGTC GGAGT GGCCT ACAAT and TAATA CGACT CACTA TAGGG AGGGT CTTCA TCCCA GGAGT; furinb: TGACC TGGAG AGACA TGCAG and TAATA CGACT CACTA TAGGG AATGC TGGGG GATTT TCTCT. For cytoimmunofluorescence, human embryonic kidney cells (HEK293T) were maintained in DMEM supplemented with 10% FBS. HEK293T cells were plated on polylysine-coated coverslips and transfected with lipofectamine with either murine pBabe-puro-MetWT (Addgene) or site directed mutagenesis-generated pBabe-puro-MetI776R. Cells were transfected 48 h before immunostaining: unfixed cells, as well as 4% paraformaldehyde-fixed and 0.1% Triton-permeabilized cells were incubated with indicated antibodies for 2 hours prior to incubation with appropriate fluorescent secondary antibodies.
Immunoprecipitation and Western blot analysis
TOV112D human ovarian carcinoma cells were obtained from ATCC (Manassas, VA) and cultured using a 1∶1 mixture of MCDB 105 medium and medium 199 supplemented with 15% FBS. MetWT and MetI776R were transfected into TOV112D cells with lipofectamine. Coimmunoprecipitations and protein blot analyses were performed as previously described [43]. The antibodies used for immunoprecipitation, protein blot and immunofluorescence are: anti-Met (25H2) and anti-phosphoMet (Cell Signaling), goat anti-Met (extracellular epitope, R&D Systems), rabbit anti-Met (SP260 – intracellular epitope, Santa Cruz).
Embryo treatment with pathway specific inhibitors
Tg(nkx2.2a(-3.5 kb):GFP)ia3; Tg(ptf1a:dsRed)jh1 embryos, distributed in 12-well plates (20 embryos per well), were incubated with different chemicals from 60 to 76 hpf, during the growth of the pancreatic tail. Chemicals were specific inhibitors of MEK (UO126, at 100 µM), p38MAPK (SB203580, at 100 µM), PI3K (LY294002, at 30 µM), Src kinase (SU6656, at 50 µM) and furin (CMK, at 65 µM).
Embryo microinjection and cell transplantation
The morpholinos (Gene Tools) used for gene knockdown were described in [12], [44]. For experiments at the blastula stage, mRNAs encoding zebrafish wild-type or L775R Met fused to mCherry were synthesized in vitro, by kit (Ambion), and 150 pg of mRNA was injected. Live imaging of embryos was performed 5 hours post-injection. For transplantation, capped cas/sox32 [29] mRNA was synthesized in vitro, and donor Tg(hsp70l:loxP-mCherry-stop-loxP-NICD-P2A-Emerald) embryos, which exhibit strong, ubiquitous RFP expression in the absence of Cre recombinase, were injected with 200 pg of cas mRNA alone or with 4 ng of met morpholino. Cell transplantations were performed as described [42]. When cas-injected putative met mutant cells were transplanted, donors were genotyped after transplantation.
Supporting Information
Zdroje
1. FieldHA, OberEA, RoeserT, StainierDY (2003) Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev Biol 253 : 279–290.
2. NiemannHH, GherardiE, BleymullerWM, HeinzDW (2012) Engineered variants of InlB with an additional leucine-rich repeat discriminate between physiologically relevant and packing contacts in crystal structures of the InlB:MET complex. Protein Sci 21 : 1528–1539.
3. WardAB, WargaRM, PrinceVE (2007) Origin of the zebrafish endocrine and exocrine pancreas. Dev Dyn 236 : 1558–1569.
4. BiemarF, ArgentonF, SchmidtkeR, EpperleinS, PeersB, et al. (2001) Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol 230 : 189–203.
5. FieldHA, DongPD, BeisD, StainierDY (2003) Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol 261 : 197–208.
6. PaulsS, ZecchinE, TisoN, BortolussiM, ArgentonF (2007) Function and regulation of zebrafish nkx2.2a during development of pancreatic islet and ducts. Dev Biol 304 : 875–890.
7. YeeNS, LorentK, PackM (2005) Exocrine pancreas development in zebrafish. Dev Biol 284 : 84–101.
8. LandsmanL, NijagalA, WhitchurchTJ, VanderlaanRL, ZimmerWE, et al. (2011) Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS Biol 9: e1001143.
9. SerupP (2012) Signaling pathways regulating murine pancreatic development. Semin Cell Dev Biol 23 : 663–672.
10. GherardiE, BirchmeierW, BirchmeierC, Vande WoudeG (2012) Targeting MET in cancer: rationale and progress. Nat Rev Cancer 12 : 89–103.
11. BladtF, RiethmacherD, IsenmannS, AguzziA, BirchmeierC (1995) Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376 : 768–771.
12. HainesL, NeytC, GautierP, KeenanDG, Bryson-RichardsonRJ, et al. (2004) Met and Hgf signaling controls hypaxial muscle and lateral line development in the zebrafish. Development 131 : 4857–4869.
13. SchmidtC, BladtF, GoedeckeS, BrinkmannV, ZschiescheW, et al. (1995) Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373 : 699–702.
14. UeharaY, MinowaO, MoriC, ShiotaK, KunoJ, et al. (1995) Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 373 : 702–705.
15. JohanssonM, MattssonG, AnderssonA, JanssonL, CarlssonPO (2006) Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology 147 : 2315–2324.
16. SonnenbergE, MeyerD, WeidnerKM, BirchmeierC (1993) Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 123 : 223–235.
17. DemirciC, ErnstS, Alvarez-PerezJC, RosaT, ValleS, et al. (2012) Loss of HGF/c-Met signaling in pancreatic beta-cells leads to incomplete maternal beta-cell adaptation and gestational diabetes mellitus. Diabetes 61 : 1143–1152.
18. Mellado-GilJ, RosaTC, DemirciC, Gonzalez-PertusaJA, Velazquez-GarciaS, et al. (2011) Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic beta-cell death and accelerates the onset of diabetes. Diabetes 60 : 525–536.
19. AparicioIM, Garcia-MarinLJ, AndreolottiAG, BodegaG, JensenRT, et al. (2003) Hepatocyte growth factor activates several transduction pathways in rat pancreatic acini. Biochim Biophys Acta 1643 : 37–46.
20. AndersonRM, BoschJA, GollMG, HesselsonD, DongPD, et al. (2009) Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev Biol 334 : 213–223.
21. GodinhoL, MummJS, WilliamsPR, SchroeterEH, KoerberA, et al. (2005) Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development 132 : 5069–5079.
22. BasilicoC, ArnesanoA, GalluzzoM, ComoglioPM, MichieliP (2008) A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J Biol Chem 283 : 21267–21277.
23. GherardiE, YoulesME, MiguelRN, BlundellTL, IameleL, et al. (2003) Functional map and domain structure of MET, the product of the c-met protooncogene and receptor for hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A 100 : 12039–12044.
24. BirchmeierC, BirchmeierW, GherardiE, Vande WoudeGF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4 : 915–925.
25. KomadaM, KitamuraN (1993) The cell dissociation and motility triggered by scatter factor/hepatocyte growth factor are mediated through the cytoplasmic domain of the c-Met receptor. Oncogene 8 : 2381–2390.
26. MarkMR, LokkerNA, ZioncheckTF, LuisEA, GodowskiPJ (1992) Expression and characterization of hepatocyte growth factor receptor-IgG fusion proteins. Effects of mutations in the potential proteolytic cleavage site on processing and ligand binding. J Biol Chem 267 : 26166–26171.
27. KomadaM, HatsuzawaK, ShibamotoS, ItoF, NakayamaK, et al. (1993) Proteolytic processing of the hepatocyte growth factor/scatter factor receptor by furin. FEBS Lett 328 : 25–29.
28. ShapiroJ, SciakyN, LeeJ, BosshartH, AngelettiRH, et al. (1997) Localization of endogenous furin in cultured cell lines. J Histochem Cytochem 45 : 3–12.
29. KikuchiY, AgathonA, AlexanderJ, ThisseC, WaldronS, et al. (2001) casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev 15 : 1493–1505.
30. StaffordD, WhiteRJ, KinkelMD, LinvilleA, SchillingTF, et al. (2006) Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development 133 : 949–956.
31. HidalgoM (2012) New insights into pancreatic cancer biology. Ann Oncol 23 Suppl 10: x135–138.
32. LloydRV, HardinH, Montemayor-GarciaC, RotondoF, SyroLV, et al. (2013) Stem cells and cancer stem-like cells in endocrine tissues. Endocr Pathol 24 : 1–10.
33. Westerfield M, editor (2000) The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). Eugene: Univ. of Oregon Press.
34. FarooqM, SulochanaKN, PanX, ToJ, ShengD, et al. (2008) Histone deacetylase 3 (hdac3) is specifically required for liver development in zebrafish. Dev Biol 317 : 336–353.
35. CuradoS, AndersonRM, JungblutB, MummJ, SchroeterE, et al. (2007) Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn 236 : 1025–1035.
36. ShinCH, ChungWS, HongSK, OberEA, VerkadeH, et al. (2008) Multiple roles for Med12 in vertebrate endoderm development. Dev Biol 317 : 467–479.
37. HesselsonD, AndersonRM, BeinatM, StainierDY (2009) Distinct populations of quiescent and proliferative pancreatic beta-cells identified by HOTcre mediated labeling. Proc Natl Acad Sci U S A 106 : 14896–14901.
38. KikuchiY, VerkadeH, ReiterJF, KimCH, ChitnisAB, et al. (2004) Notch signaling can regulate endoderm formation in zebrafish. Dev Dyn 229 : 756–762.
39. KimJH, LeeSR, LiLH, ParkHJ, ParkJH, et al. (2011) High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6: e18556.
40. ThermesV, GrabherC, RistoratoreF, BourratF, ChoulikaA, et al. (2002) I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev 118 : 91–98.
41. GherardiE, SandinS, PetoukhovMV, FinchJ, YoulesME, et al. (2006) Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc Natl Acad Sci U S A 103 : 4046–4051.
42. ChungWS, StainierDY (2008) Intra-endodermal interactions are required for pancreatic beta cell induction. Dev Cell 14 : 582–593.
43. DelousM, HellmanNE, GaudeHM, SilbermannF, Le BivicA, et al. (2009) Nephrocystin-1 and nephrocystin-4 are required for epithelial morphogenesis and associate with PALS1/PATJ and Par6. Hum Mol Genet 18 : 4711–4723.
44. ElsenGE, ChoiLY, PrinceVE, HoRK (2009) The autism susceptibility gene met regulates zebrafish cerebellar development and facial motor neuron migration. Dev Biol 335 : 78–92.
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



