-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCommunicating the Results of Clinical Research to Participants: Attitudes, Practices, and Future Directions
article has not abstract
Published in the journal: . PLoS Med 5(5): e91. doi:10.1371/journal.pmed.0050091
Category: Guidelines and Guidance
doi: https://doi.org/10.1371/journal.pmed.0050091Summary
article has not abstract
Recent commentaries advocate routinely offering study results to research participants [1,2]. However, debate continues over the scope and limits of investigators' responsibilities in this regard. A 2006 review identified 30 national and international policies and guidelines concerning the duty to return research results [3], of which 21 were published in the last decade. Worldwide interest in this complex issue will likely continue to rise in light of the increasing relevance of the results of biomedical research to participants' health and well-being.
Unfortunately, many policies and commentaries on communication of results either do not adequately take into account relevant available data, or fail to recognize the lack thereof. For example, existing data on participant desire and investigator support for communication of research results have not been synthesized, nor have the data on potential positive and negative consequences that communication of results may have for both participants and researchers.
The results of clinical research may be classified as either aggregate study results, representing synthesized data and conclusions drawn from groups of research participants, or individual results, representing distinct items of data collected from or about individual participants. In this article, we present a narrative review of available data on the effects of communicating aggregate and individual research results on participants, investigators, and the research enterprise. We also present available data on disclosure practices and the attitudes of investigators and participants towards communication of research results. Our aim is not to provide definitive analyses of any of these domains; rather, it is to highlight trends in the literature as well as areas that require further investigation.
Data on Communicating Research Results
A literature search revealed 28 empirical studies concerning communication of research results (Text S1). Because these studies encompass many different participant populations and research settings, we did not pool quantitative data or conduct a formal quality assessment of studies. Of the 28 studies summarized below, 22 are primarily quantitative and six are primarily qualitative [4–9]. Sample size ranged from 13 [7] to 8,941 [8]. Twelve studies involved either cancer research or the attitudes of patients with cancer [7,10–20]; seven studies involved genetics research [7,9,11,20–23]. Ten studies were conducted in the United States [9,10,16–21,24,25], nine in the United Kingdom [4–8,11,26–28], four in Canada [14,15,29,30], one in France [23], and one in Sweden [22]. Three studies enrolled a multinational participant population [12,13,31].
Participant attitudes towards disclosure.
Eighteen studies provided empirical data on participants' desire to receive study results [5–11,14–16,18–23,27,32]. Nine studies involved aggregate study results, eight involved individual results, and one involved both (Table 1). Of studies that reported desire to receive results as a percentage of respondents, a median of 90% (range 20%–100%) wished to receive study results. In studies not reporting percentages, mothers of pediatric patients with cancer rated the importance of aggregate study results at a mean of 4.5 of a maximum importance of 5 [10], parents of infants in a randomized controlled trial of extracorporeal membrane oxygenation (ECMO) “felt strongly they should be sent the trial results” [5], and relatives of deceased patients with prostate cancer stated that they “had a right to know information that they could use to make personal risk management decisions” [7]. When asked an open-ended question about their experience in a study allowing them to access their individual obstetric records, 99 of 247 pregnant women volunteered a desire to receive aggregate study results without being prompted [6].
Tab. 1. Participant Desire for Study Results 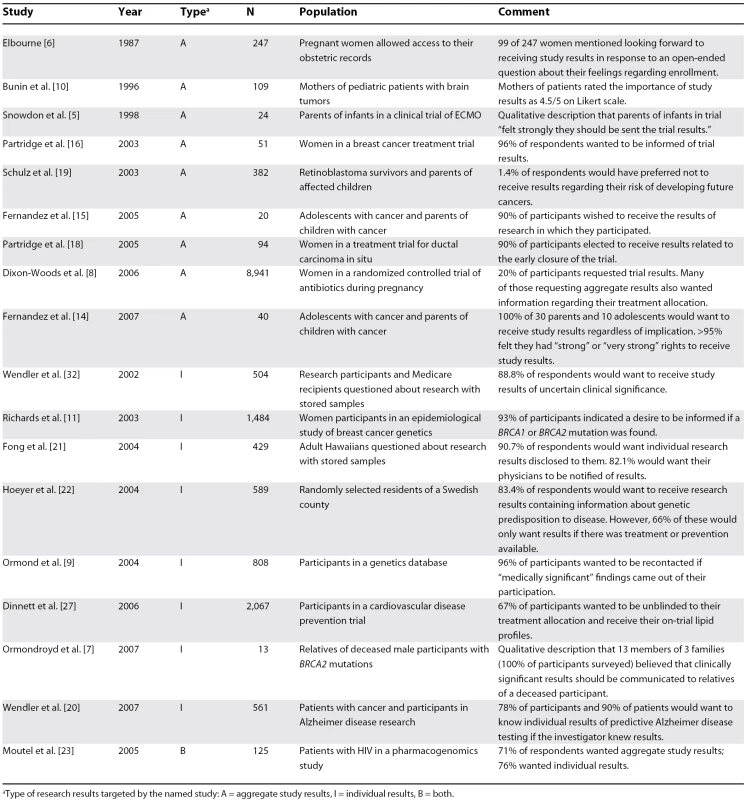
aType of research results targeted by the named study: A = aggregate study results, I = individual results, B = both. Nine studies assessed participants' reasons for wanting aggregate or individual study results. Participants in these studies cited clinical significance (e.g., treatment, prevention, or understanding of a disease) for self or relatives [7–11,23], respect for participants in research or a “right” to receive results [5,11,14], and raising public awareness of research [14]. Interestingly, Wendler et al. reported that the mere fact that investigators have information about participants that participants lack contributes to the desire to receive individual research results [20]. In a study by Fernandez et al., participants suggested that the possibility of causing distress to families of deceased participants or situations in which participants were harmed or not helped by the research could be reasons for not disclosing research results [14]. However, Partridge et al. reported that participants wanted to be contacted with aggregate results even if they were not helped by the research [16].
Ten studies [5,8–11,14–16,18,19] assessed participants' preferences for the method of receiving research results. Two prospective studies of adolescent patients with cancer and parents of children with cancer by Fernandez et al. report that (1) 60% of participants felt communication of results by mail would be satisfactory, though 50% stated that they would prefer face-to-face contact [15]; and (2) approximately 75% of participants would prefer to receive results with positive or neutral implications by letter or e-mail, while approximately 53% of participants would prefer to have results with negative implications communicated in person [14]. Participants in a longitudinal genetic database were “relatively open with regards to how they would prefer to be recontacted” with individual results [9], and participants in a clinical treatment trial for breast cancer preferred that their physicians communicate the aggregate results of clinical trials in which they participated [16].
Participants were asked to retrospectively evaluate written communication of research results in five studies [5,8,10,18,19]. Mothers of child participants in an epidemiological study of brain tumors found written aggregate study results understandable and important; however, 40% wanted a phone number to call with questions [10]. Written notification of aggregate study results with contact numbers was also the preferred method of communication for retinoblastoma survivors and participants in a trial of antibiotics during pregnancy [8,19]. In addition, 74% of women informed by mail of the early stoppage of a phase II trial of breast excision for ductal carcinoma in situ felt comfortable with this method of notification [18]. However, parents of infants who had taken part in a clinical trial of ECMO reported mixed experiences with receiving written communication about aggregate study results, as many felt the information was either too complex or too simplistic [5].
Investigator attitudes and practices regarding disclosure.
Five studies assessed investigators' support for communicating research results to participants [4,12,17,26,31]. Four studies involved aggregate study results and one involved individual results (Table 2). A substantial majority of investigators surveyed in four studies supported communicating study results to participants [12,17,26,31]. In the remaining study, only one-third of six midwives and ten physicians surveyed favored the communication of clinical trial results regarding fetal heart monitoring techniques [4].
Tab. 2. Investigators' Support for Communicating Research Results 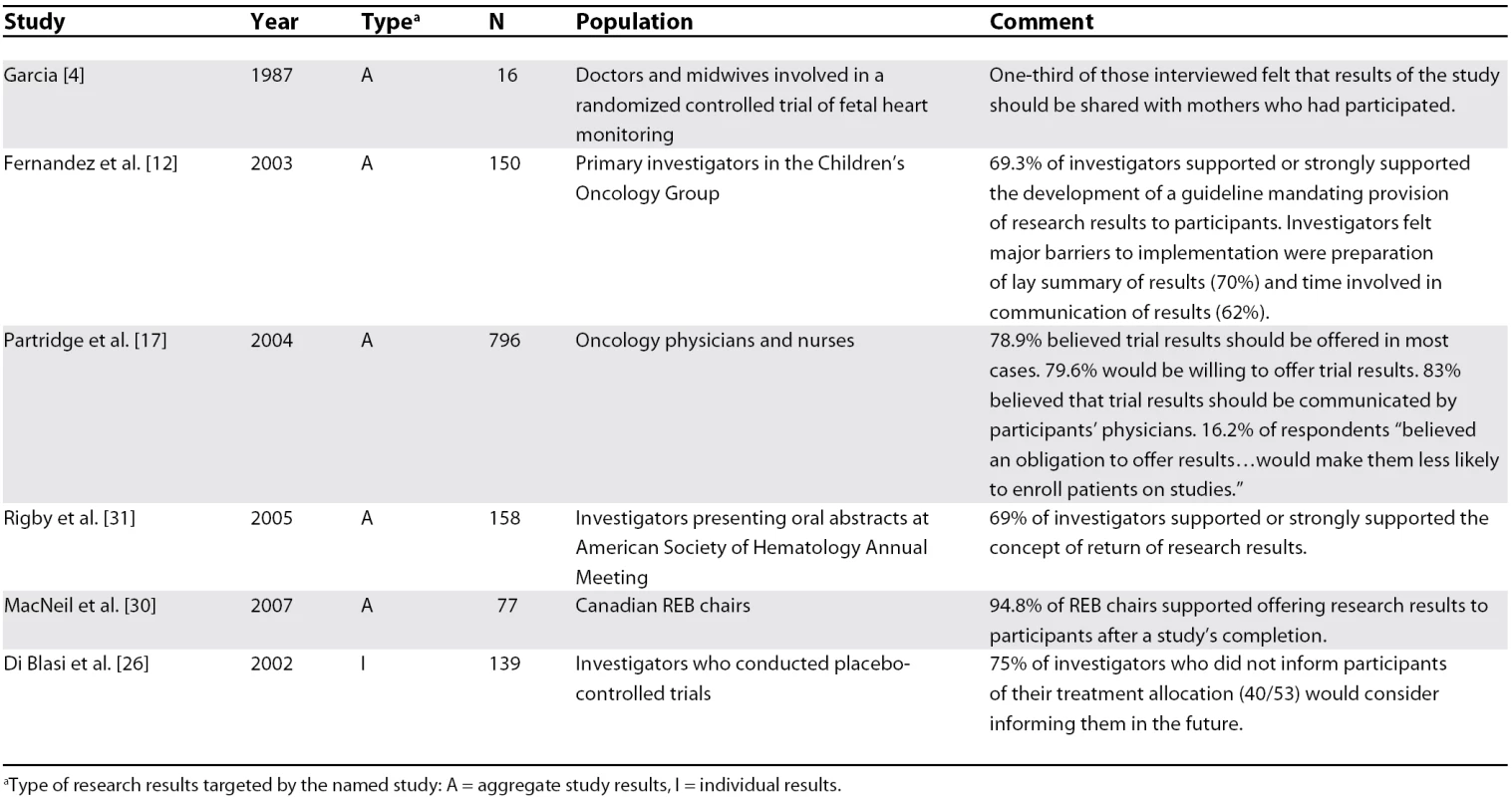
aType of research results targeted by the named study: A = aggregate study results, I = individual results. Cancer investigators identified the cost and time involved in preparing a lay summary as well as difficulty in contacting participants as major barriers to communicating aggregate study results [12]. Clinical investigators also identified possible biasing of study follow-ups and cost as major barriers to communicating individual results [26]. In both cases, a minority of investigators identified negative psychological consequences as a perceived barrier to communication of research results [12,26]. Cancer clinicians in another study expressed a reluctance to inform participants of negative study conclusions owing to a desire to protect participants from harmful psychological consequences [17].
The chairs of Canadian research ethics boards (REBs) overwhelmingly supported offering research results after the conclusion of the study [30], and the monitoring committee of a pharmacogenetics study involving HIV-infected patients strongly favored communicating results of “direct benefit” to the participant, but expressed doubts regarding the communication of other results [23].
Six studies assessed the practices and policies of investigators and institutions concerning the communication of research results to participants [12,13,17,26,29,31]. Four studies involved aggregate study results, one study involved individual results, and one involved both (Table 3). Communicating research results seems to represent the exception to practice. For example, only five of 150 institutions surveyed by Fernandez et al. had a formal mechanism for returning research results to participants [12], and only 3% of 180 consent forms for leukemia clinical trials indicated that participants could receive study results [13]. Furthermore, only nine of 22 Canadian REBs surveyed had policies addressing communication of results or required investigators to address the issue themselves [29].
Tab. 3. Research Results Communication Practices 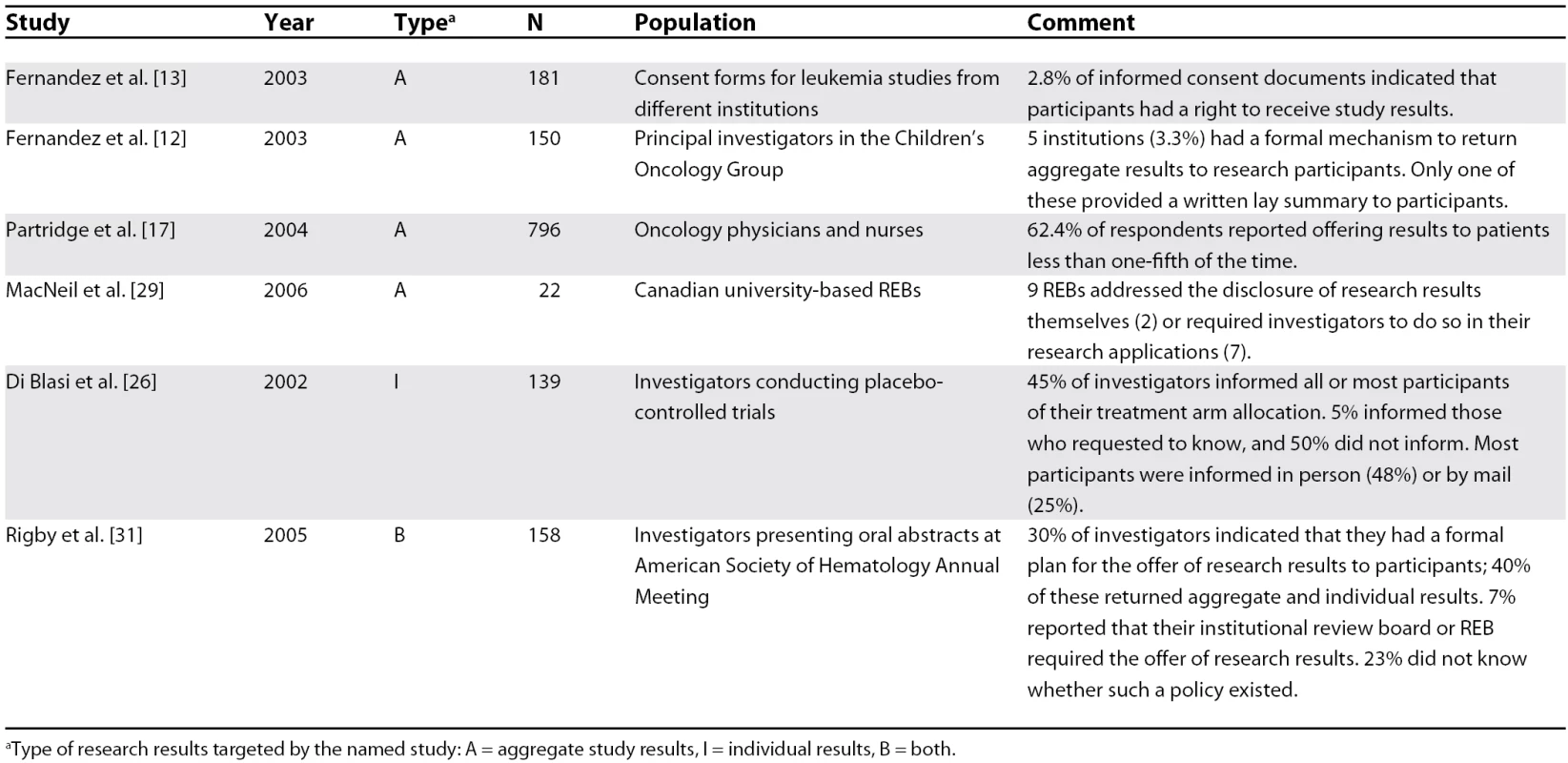
aType of research results targeted by the named study: A = aggregate study results, I = individual results, B = both. Impact of disclosure on participants.
Eight studies empirically assessed the impact of receiving study results, all of which involved aggregate results [5,7,8,10,11,18,19,24] (Table 4). These studies used various methods of assessing participants' reactions to receiving study information, including interviews [5,7,8,11], Likert scales of possible psychological reactions [10,19], the revised Impact of Event Scale [18], and the McMaster Health Index Questionnaire [24]. All but one study [24] reported some incidence of negative psychological consequences for participants, including increased anxiety, anger, guilt, or upset. However, six studies [5,7,8,10,19,24] reported psychological benefits for participants from receiving research results, including pleasure, satisfaction, and relief.
Tab. 4. Impact on Participants of Receiving Research Results 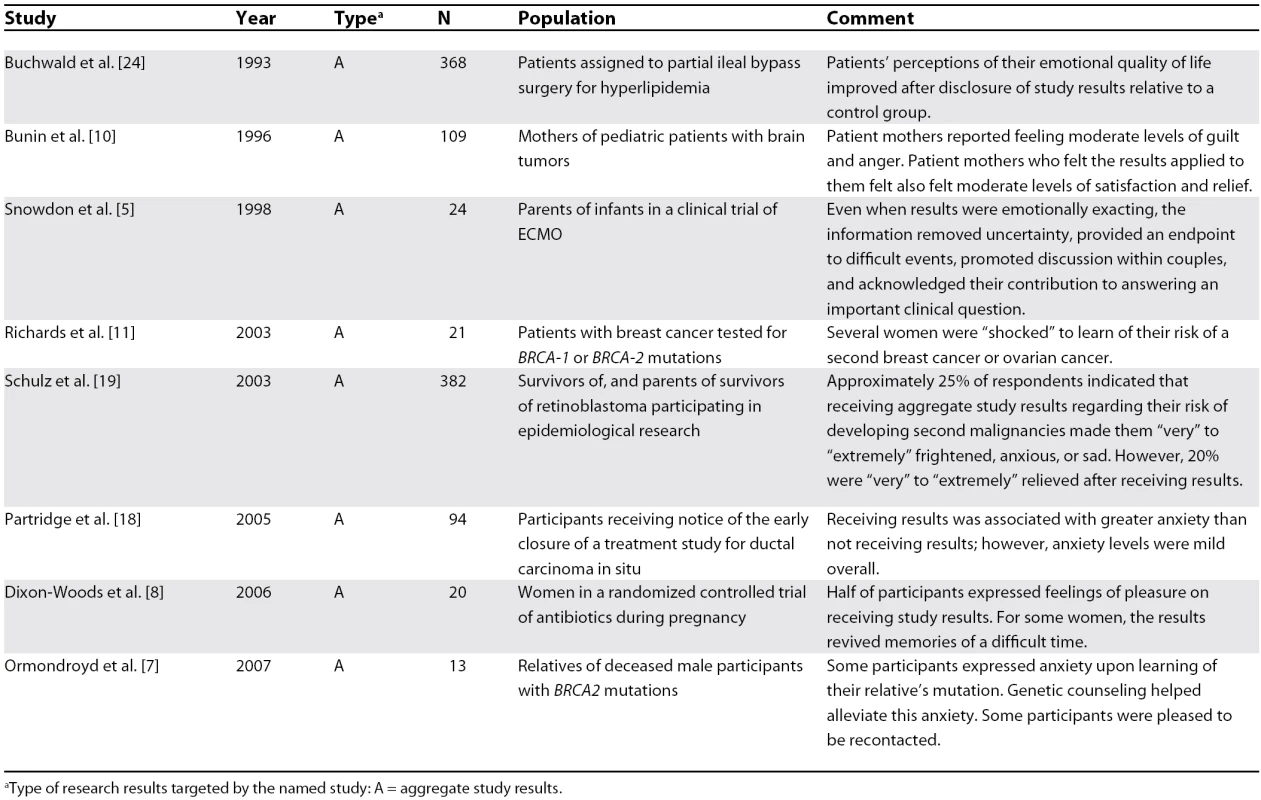
aType of research results targeted by the named study: A = aggregate study results. Importantly, the vast majority of participants reported feeling that it was important to receive study results, despite potentially negative emotional impact (Table 1). For example, Schulz et al. [19] reported that approximately a quarter of retinoblastoma survivors or parents of children with retinoblastoma indicated that receiving aggregate study results regarding their risk of developing second malignancies made them “very” to “extremely” frightened, anxious, or sad. However, 20% of participants were “very” to “extremely” relieved by the results, and only 1.4% of participants in this study would have preferred not to receive results.
Three studies examined correlations between education and psychological impact of disclosure: one found that participants with less than a college education were significantly more sad, angry, overwhelmed, or frightened than those with a college education [19], one found the opposite [18], and one found no correlation between educational level and psychological impact of disclosure [10].
Only the study by Schulz et al. addressed clinical follow-up after receiving research data [19]. The study authors found that despite their recommendations, only 18% of participants spoke with their physicians about the disclosed results, 16% of survivors attended cancer screening check-ups, and 12% of parents of child retinoblastoma survivors took their children to be screened. The authors hypothesized that the impersonal nature of written communication may have led to poor utilization of clinically significant information.
Impact of disclosure on investigators.
One study addressed the costs of communicating research results. Dinnett et al. reported that expenses associated with communicating treatment allocation and lipid profiles to participants included preparation, printing, and distribution of letters as well as additional salary support for existing staff. The authors also report receiving only 21 phone calls after unblinding 1,391 participants [28].
Impact of disclosure on the research enterprise.
Buchwald et al. show trends (p-values between 0.09 and 0.13) indicating that after communication of aggregate study results, 726 participants were more likely to be (1) satisfied with their decision to enroll, (2) satisfied with randomization allocation, and (3) disposed to advise others to join a research study after communication of aggregate study results [24]. Snowdon et al. report that of 31 parents notified of their infant's allocation to the ECMO or standard care groups in a completed clinical trial, few said that this information affected their view of the trial, randomization, or their doctor [5].
Discussion
As we have conducted a narrative review of studies concerning communicating research results to participants, rather than a systematic review, definitive conclusions about findings and their ethical import cannot be drawn. Nevertheless, the data reviewed here suggest several important implications. Available data consistently indicate that research participants want aggregate and clinically significant individual study results made available to them. Participants' desires do not necessarily determine policy, but respect for participants requires taking their preferences seriously. Though investigators appear to support the communication of aggregate study results, less is known about investigators' attitudes towards communicating individual research results or about the costs and time required to do so. Future research should focus on these issues, including ways to facilitate communication of results by addressing investigators' concerns.
It may be helpful to consider the significant body of literature from the 1990s concerning worker notification of research showing increased occupational health risk [10]. Employers initially resisted notifying workers of risk, citing concerns of causing unduly negative psychological reactions as well as affecting workers' insurability, employability, and credit rating [33–36]. Subsequent research showed little evidence that notification of occupational health risks leads to significant long - or short-term psychological consequences [37–39]. One study also reported that after 3,189 notification letters were mailed containing a toll-free number for additional information, only 40 calls were received, suggesting that cost of follow-up after communication of results may not be especially burdensome [40]. Authors in the occupational health literature have carefully assessed the method, timing, and content of risk-notification procedures; clinical investigators considering how to approach communication of research results may find these analyses and the lessons learned useful [34,40–46].
Both the clinical research and occupational health literature demonstrate that some participants will show positive and/or negative psychological changes after receiving research results. However, studies of the psychological impact of genetic testing demonstrate that individuals tend to exhibit less emotional distress than anticipated by clinicians, and show strong coping skills in dealing with undesired results [47–50]. Likewise, the balance of evidence suggests that false reassurance is not a significant problem for the communication of research results [51–55]. Three studies that identify false reassurance suggest that inadequacies in explaining results may be to blame, and recommend that, in the case of genetic testing, the implications of positive and negative results be emphasized equally [56–58]. The impact of communicated study results may also vary by study type and characteristics of the participant population, including diagnoses, health status, education, and health literacy. Investigators should therefore tailor their communication practices with respect to the situation and needs of their intended audience. Future research should assess the effect of various modes of communicating research results on psychological sequelae, health behaviors, and understanding of results by participants. Importantly, despite the potential for negative psychological consequences, participants want the opportunity to receive research results. These data suggest that fear of psychological harm should not be used as a reason not to offer research results, without clear evidence of a threat to participants' safety.
The costs of communicating research results to a study population remain unknown. However, in the absence of data, two points should be considered. First, concerns over cost of disclosure often stem from the assumption that research results must be communicated in person, and in the case of genetics research, by trained genetic counselors. Though in some cases, in-person disclosure may encourage follow-through on clinical recommendations and discourage false reassurance, participants often prefer to receive results in writing with contact information. Second, although there will undoubtedly be some expenditure associated with communicating research results, cost should not be used as an argument against routine offering of study results to participants unless communicating results would substantially compromise the feasibility of the research. Future research should therefore assess the actual cost of various communication strategies.
It remains unclear whether communicating research results to participants will significantly affect participants' perception of investigators and biomedical research, or influence their likelihood of enrolling in future studies. More empirical data will be needed to resolve this issue; however, participants tend to be grateful when they receive study results, suggesting that such communication may bolster public opinion of investigators and the research they conduct.
Our analysis of the empirical literature on disclosure of research results to participants also revealed that the impact of presenting aggregate results to participants may equal that of presenting individual results. For example, while post-trial disclosure of treatment randomization or communication of individual genotypes may be clear examples of information relevant to individual participants, aggregate results of epidemiological studies may be just as individually relevant if participants know their own risk factors. Furthermore, many studies we found did not explicitly identify a focus on aggregate study results, individual results, or both. As communicating these two types of results may pose different practical and ethical challenges, researchers should specify the type(s) of results under consideration.
The literature on communication of research results is limited by a lack of commonly used and well-validated measures for most outcomes of interest. This limitation may be especially problematic given that framing effects can result in widely differing estimates of preferences, attitudes, and impact. Additionally, detailed data extraction is often impossible when the impact of disclosure on participants is reported either qualitatively or using a Likert scale. It will therefore be necessary to employ rigorous study designs (e.g., controlled trials and longitudinal studies) to assess the effects of communicating research results. Finally, 16 of the 28 studies we identified involved either cancer or genetics research. Future research should consider issues specific to other clinical research settings as well as to sociobehavioral research.
As discussion and research move forward, we recommend that investigators include their planned approach to communicating aggregate and individual results in study protocols and address disclosure in informed consent documents. Research ethics committees should review the appropriateness of investigators' plans for communicating results. Much still rests, however, on careful examination of the ethical issues involved in determining investigators' responsibilities for communicating research results to participants. Policies should incorporate available empirical data into an ethical framework that (1) respects and supports the collaborative relationship between investigators and research participants and (2) enhances trust in, and trustworthiness of, research and researchers.
Supporting Information
Zdroje
1. FernandezCVKodishEWeijerC
2003
Informing study participants of research results: An ethical imperative.
IRB
25
12
9
2. ShalowitzDIMillerFG
2005
Disclosing individual results of clinical research: Implications of respect for participants.
JAMA
294
737
740
3. KnoppersBMJolyYSimardJDurocherF
2006
The emergence of an ethical duty to disclose genetic research results: International perspectives.
Eur J Hum Genet
14
1170
1178
4. GarciaJ
1987
Sharing research results with patients: The views of care-givers involved in a randomized controlled trial.
J Reprod Infant Psychol
5
9
13
5. SnowdonCGarciaJElbourneD
1998
Reactions of participants to the results of a randomised controlled trial: Exploratory study.
BMJ
317
21
26
6. ElbourneD
1987
Subjects' views about participation in a randomized controlled trial.
J Reprod Infant Psychol
5
3
8
7. OrmondroydEMoynihanCWatsonMFosterCDavollsS
2007
Disclosure of genetics research results after the death of the patient participant: A qualitative study of the impact on relatives.
J Genet Couns
16
527
538
8. Dixon-WoodsMJacksonCWindridgeKCKenyonS
2006
Receiving a summary of the results of a trial: Qualitative study of participants' views.
BMJ
332
206
210
9. OrmondKESmithMECirinoALChisholmRLWolfWA
2004
“Duty” to recontact participants in a population based genetic database: The NUgene experience.
American College of Medical Genetics Annual Conference; 6 March 2004; Kissimmee, Florida, United States
10. BuninGRKazakAEMitelmanO
1996
Informing subjects of epidemiologic study results. Children's Cancer Group.
Pediatrics
97
486
491
11. RichardsMPPonderMPharoahPEverestSMackayJ
2003
Issues of consent and feedback in a genetic epidemiological study of women with breast cancer.
J Med Ethics
29
93
96
12. FernandezCVKodishEShurinSWeijerC
2003
Offering to return results to research participants: Attitudes and needs of principal investigators in the Children's Oncology Group.
J Pediatr Hematol Oncol
25
704
708
13. FernandezCVKodishETaweelSShurinSWeijerC
2003
Disclosure of the right of research participants to receive research results: An analysis of consent forms in the Children's Oncology Group.
Cancer
97
2904
2909
14. FernandezCVSantorDWeijerCStrahlendorfCMoghrabiA
2007
The return of research results to participants: Pilot questionnaire of adolescents and parents of children with cancer.
Pediatr Blood Cancer
48
441
446
15. FernandezCVTaweelSKodishEWeijerC
2005
Disclosure of research results to research participants: A pilot study of the needs and attitudes of adolescents and parents.
Paediatr Child Health
10
332
334
16. PartridgeAHBursteinHJGelmanRSMarcomPKWinerEP
2003
Do patients participating in clinical trials want to know study results.
J Natl Cancer Inst
95
491
492
17. PartridgeAHHackettNBloodEGelmanRJoffeS
2004
Oncology physician and nurse practices and attitudes regarding offering clinical trial results to study participants.
J Natl Cancer Inst
96
629
632
18. PartridgeAHWongJSKnudsenKGelmanRSampsonE
2005
Offering participants results of a clinical trial: Sharing results of a negative study.
Lancet
365
963
964
19. SchulzCJRiddleMPValdimirsdottirHBAbramsonDHSklarCA
2003
Impact on survivors of retinoblastoma when informed of study results on risk of second cancers.
Med Pediatr Oncol
41
36
43
20. WendlerDPentzR
2007
How does the collection of genetic test results affect research participants.
Am J Med Genet A
143
1733
1738
21. FongMBraunKLChangRM
2004
Native Hawaiian preferences for informed consent and disclosure of results from research using stored biological specimens.
Pac Health Dialog
11
154
159
22. HoeyerKOlofssonBOMjorndalTLynoeN
2004
Informed consent and biobanks: A population-based study of attitudes towards tissue donation for genetic research.
Scand J Public Health
32
224
229
23. MoutelGDuchangeNRaffiFShararaLITheodorouI
2005
Communication of pharmacogenetic research results to HIV-infected treated patients: Standpoints of professionals and patients.
Eur J Hum Genet
13
1055
1062
24. BuchwaldHFitchLLMattsJPJohnsonJWHansenBJ
1993
Perception of quality of life before and after disclosure of trial results: A report from the Program on the Surgical Control of the Hyperlipidemias (POSCH).
Control Clin Trials
14
500
510
25. WendlerDPrasadKWilfondB
2002
Does the current consent process minimize the risks of genetics research.
Am J Med Genet
113
258
262
26. Di BlasiZKaptchukTJWeinmanJKleijnenJ
2002
Informing participants of allocation to placebo at trial closure: Postal survey.
BMJ
325
1329
27. DinnettEMMungallMMGordonCRonaldESGawA
2006
Offering results to research participants.
BMJ
332
549
550
28. DinnettEMMungallMMKentJARonaldESMcIntyreKE
2005
Unblinding of trial participants to their treatment allocation: Lessons from the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER).
Clin Trials
2
254
259
29. MacneilSDFernandezCV
2006
Informing research participants of research results: Analysis of Canadian university based research ethics board policies.
J Med Ethics
32
49
54
30. MacNeilSDFernandezCV
2007
Attitudes of research ethics board chairs towards disclosure of research results to participants: Results of a national survey.
J Med Ethics
33
549
553
31. RigbyHFernandezCV
2005
Providing research results to study participants: Support versus practice of researchers presenting at the American Society of Hematology annual meeting.
Blood
106
1199
1202
32. WendlerDEmanuelE
2002
The debate over research on stored biological samples: What do sources think.
Arch Intern Med
162
1457
1462
33. BayerR
1986
Notifying workers at risk: The politics of the right-to-know.
Am J Public Health
76
1352
1356
34. SchultePABoalWLFriedlandJMWalkerJTConnallyLB
1993
Methodologic issues in risk communications to workers.
Am J Ind Med
23
3
9
35. RingenK
1989
The case for worker notification.
Ann N Y Acad Sci
572
133
141
discussion 142–143
36. WoodFB
1990
Letter submitted to NIOSH by Westinghouse Electric Corporation as part of a panel of experts' analysis regarding interim report.
HETA
89
116
37. HornsbyJLSappingtonJTMonganPGullenWHBonoSF
1985
Risk for bladder cancer. Psychological impact of notification.
JAMA
253
1899
1902
38. HoutsPSMcDougallV
1988
Effects of informing workers of their health risks from exposure to toxic materials.
Am J Ind Med
13
271
279
39. MeyerowitzBESullivanCDPremeauCL
1989
Reactions of asbestos-exposed workers to notification and screening.
Am J Ind Med
15
463
475
40. MazzuckelliLFSchultePA
1993
Notification of workers about an excess of malignant melanoma: A case study.
Am J Ind Med
23
85
91
41. KumekawaESLashAABeckerCE
1992
Challenges of worker notification in neurotoxic studies of the central nervous system.
Environ Res
59
125
131
42. LashAAKumekawaESBeckerCE
1993
Evaluating the clarity of research reports written for research subjects.
Am J Ind Med
23
211
219
43. MeyerowitzBE
1993
Assessing quality of life when planning and evaluating worker notification programs: Two case examples.
Am J Ind Med
23
221
227
44. NeedlemanC
1993
Social aspects of high-risk notification among chromium-exposed workers.
Am J Ind Med
23
113
123
45. NeedlemanC
1993
Worker notification: Lessons from the past.
Am J Ind Med
23
11
23
46. RudolphL
1993
Issues in notification: Reflections of a public health worker.
Am J Ind Med
23
53
59
47. BroadstockMMichieSMarteauT
2000
Psychological consequences of predictive genetic testing: A systematic review.
Eur J Hum Genet
8
731
738
48. LermanCCroyleRTTercyakKPHamannH
2002
Genetic testing: Psychological aspects and implications.
J Consult Clin Psychol
70
784
797
49. SmithCOLipeHPBirdTD
2004
Impact of presymptomatic genetic testing for hereditary ataxia and neuromuscular disorders.
Arch Neurol
61
875
880
50. SteinbartEJSmithCOPoorkajPBirdTD
2001
Impact of DNA testing for early-onset familial Alzheimer disease and frontotemporal dementia.
Arch Neurol
58
1828
1831
51. DorvalMGauthierGMaunsellEDugasMJRouleauI
2005
No evidence of false reassurance among women with an inconclusive BRCA1/2 genetic test result.
Cancer Epidemiol Biomarkers Prev
14
2862
2867
52. DrossaertCHBoerHSeydelER
2001
Does mammographic screening and a negative result affect attitudes towards future breast screening.
J Med Screen
8
204
212
53. MarteauTMKinmonthALThompsonSPykeS
1996
The psychological impact of cardiovascular screening and intervention in primary care: A problem of false reassurance? British Family Heart Study Group.
Br J Gen Pract
46
577
582
54. MarteauTMRobertsSLaRusseSGreenRC
2005
Predictive genetic testing for Alzheimer's disease: Impact upon risk perception.
Risk Anal
25
397
404
55. van DijkSOttenWTimmermansDRvan AsperenCJMeijers-HeijboerH
2005
What's the message? Interpretation of an uninformative BRCA1/2 test result for women at risk of familial breast cancer.
Genet Med
7
239
245
56. JohnsonKATrimbathJDPetersenGMGriffinCAGiardielloFM
2002
Impact of genetic counseling and testing on colorectal cancer screening behavior.
Genet Test
6
303
306
57. LermanCMarshallJAudrainJGomez-CamineroA
1996
Genetic testing for colon cancer susceptibility: Anticipated reactions of patients and challenges to providers.
Int J Cancer
69
58
61
58. ClausenHBrandtNJSchwartzMFlemmingS
1996
Psychological impact of carrier screening for cystic fibrosis among pregnant women.
Eur J Hum Genet
4
120
123
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2008 Číslo 5- Léčba bolesti u seniorů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Flexofytol® – přírodní revoluce v boji proti osteoartróze kloubů
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
-
Všechny články tohoto čísla
- Expectations for Recovery Important in the Prognosis of Whiplash Injuries
- Disease Mongering Is Now Part of the Global Health Debate
- Is HIV Screening in the Labor and Delivery Unit Feasible and Acceptable in Low-Income Settings?
- Retail Sales of Alcohol and the Risk of Being a Victim of Assault
- Regulatory RNAs: Have mRNA Untranslated Regions Joined the Party?
- A Paradigm Shift to Prevent HIV Drug Resistance
- A User's Guide to the NINDS rt-PA Stroke Trial Database
- False Hopes, Unwarranted Fears: The Trouble with Medical News Stories
- Communicating the Results of Clinical Research to Participants: Attitudes, Practices, and Future Directions
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Disease Mongering Is Now Part of the Global Health Debate
- Communicating the Results of Clinical Research to Participants: Attitudes, Practices, and Future Directions
- A User's Guide to the NINDS rt-PA Stroke Trial Database
- Expectations for Recovery Important in the Prognosis of Whiplash Injuries
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



