-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLaboratory Capacity Building in Asia for Infectious Disease Research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN)
article has not abstract
Published in the journal: . PLoS Med 7(4): e32767. doi:10.1371/journal.pmed.1000231
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.1000231Summary
article has not abstract
Summary Points
-
Enhancing laboratory capacity is essential for generating reliable and accurate data from clinical research, especially in resource-constrained settings.
-
Local well-trained laboratory experts and scientists are important to research, and must participate actively in scientific activities and continuing education programs.
-
Improving laboratory capacity is more than supplying new equipment and reagents; it also includes a long-term commitment to staff training, quality control, and biosafety.
-
Improved laboratory capacity optimizes responses to an epidemic or an outbreak of a novel virulent pathogens, and can support international agendas to reduce the impact of pandemic influenza viruses.
Introduction
The South East Asia Infectious Disease Clinical Research Network (SEAICRN) is a collaborative partnership of hospitals and institutions in Thailand, Vietnam, Indonesia, and Singapore. The network strives to advance scientific knowledge and clinical and laboratory management of infectious disease in general, and influenza in particular, through integrated, collaborative clinical research in the South East Asia region (see http://www.seaicrn.org/) [1]. The establishment of this network helps fulfill the declaration of the World Health Assembly in 2005 that urged its member states to “strengthen national laboratory capacity for human and zoonotic influenza,” and “to support an international research agenda to reduce the spread and impact of pandemic influenza viruses” [2],[3]. In addition it also supports the recently revised International Health Regulations (IHR 2005), which came into effect in 2007, by increasing surveillance and response capacity in strategic hospitals, national institutes, and referral laboratories and by promoting international collaboration and sharing of data [3].
SEAICRN's first intervention study was a multicenter randomized clinical trial in four Asian countries that compared the efficacy of high dose (150 mg bid) versus standard dose (75 mg bid) oseltamivir for severe influenza (Table 1). Subsequently the SEAICRN initiative developed a series of research protocols related to influenza and other infectious diseases being implemented in participating institutions. To make these studies possible, and to comply with international laboratory and clinical trial standards, the SEAICRN recently chose to enhance the capacity and quality of both the research and clinical laboratories at all participating hospitals and institutions in the region.
Tab. 1. List of approved protocols that will be or are being executed by SEAICRN. 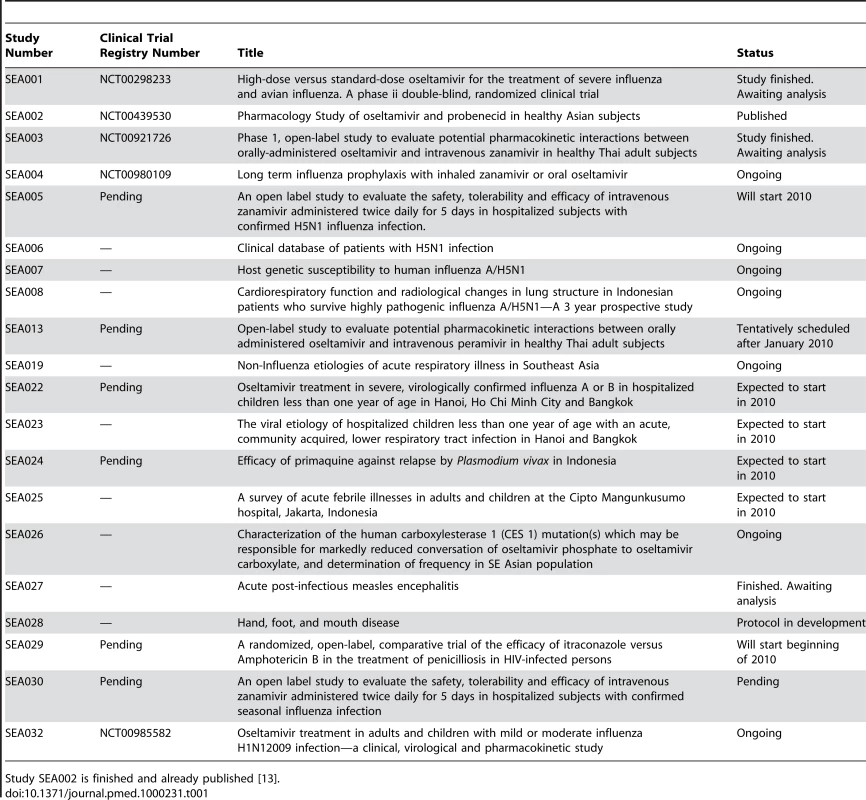
Study SEA002 is finished and already published [13]. The key objective of the laboratory strengthening program was to enhance laboratory facilities; ensure availability of necessary equipment; build human resource capacity by teaching, training, and mentoring; and ensure quality laboratory management and testing to comply with good clinical laboratory practice (GCLP) and other international standards such as the ISO 15189 [4],[5]. Here we present the experiences of the SEAICRN in setting up and conducting the laboratory capacity building program and discuss how this benefitted the institutions, the research studies, and the region. The US National Institutes of Health's (NIH) National Institute for Allergy and Infectious Disease (NIAID) and Wellcome Trust funding support for this program has been described previously [1].
Creating Laboratory Capacity
Given that SEAICRN laboratories had different levels of organization and expertise, it was considered important to create reference laboratories for different aspects of the research activities in each country (Figure 1). Due to the initial focus on influenza therapeutics, a SEAICRN clinical pharmacology laboratory was established at the Department of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Fig. 1. South East Asia Infectious Disease Clinical Research Network sites and laboratories. 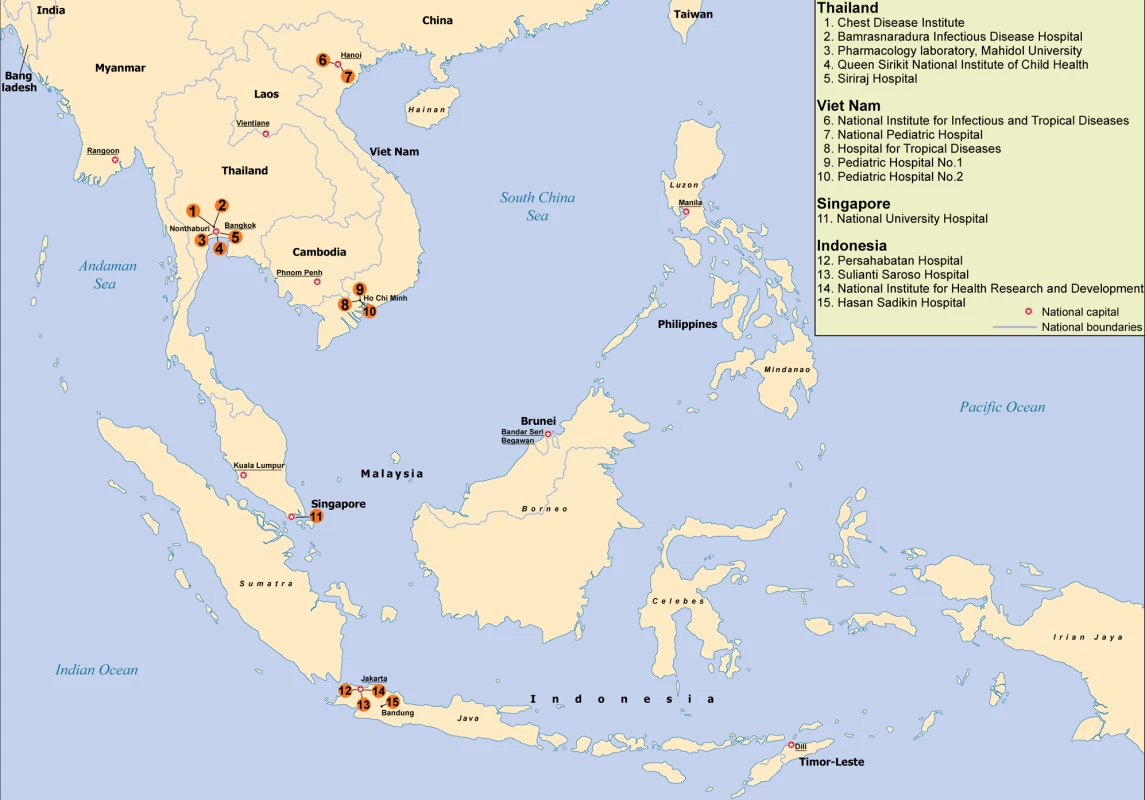
To enroll patients into the first clinical trial, participating sites needed to be able to screen patients by influenza reverse transcriptase polymerase chain reaction (RT-PCR) reliably, rapidly, and accurately. To be able to screen patients by RT-PCR, several laboratories were renovated to house, for the first time, a molecular diagnostic laboratory (MDL), with all the required infrastructure, equipment, reagents, and consumables. Physical separation of spaces for reagent preparation, nucleic acid extraction, and amplification was ensured in all laboratories to prevent PCR contamination. Also, a new biosafety level 3 laboratory facility was established at the Hospital for Tropical Diseases, Ho Chi Minh City, Viet Nam for isolation of H5N1 viruses and emerging (pandemic) influenza viruses. Additionally, in Viet Nam, (pyro)sequencing capacity was established to characterize viruses and detect mutations in the influenza virus genome that confer antiviral drug resistance.
Site staff from Bangkok, Jakarta, Bandung, Hanoi, and Ho Chi Minh City were trained in influenza real-time RT-PCR diagnostics and molecular diagnostics in general, including principles of unidirectional workflow and prevention of carry-over contamination. These trainings were performed on an ongoing basis at the reference laboratory in Ho Chi Minh City, followed by on-site training. To ensure quality, each MDL laboratory was enrolled in at least two different external quality assurance programs for molecular influenza diagnostics. Furthermore, proficiency testing for diagnosing influenza by RT-PCR was performed before sites were allowed to start screening patients for the studies.
Each hospital is encouraged to use the MDL for purposes other than SEAICRN-related research activities, such as HIV, hepatitis, dengue, meningitis, and encephalitis testing by molecular techniques. The philosophy is that it is important and highly beneficial to increase expertise in detecting infectious agents by molecular diagnostics and so stimulate the implementation of other relevant molecular diagnostic tests. For example, after implementation of a Streptococcus suis–specific molecular test in the MDL of the National Hospital of Tropical Diseases, Vietnam, it was found that S. suis is a common cause of bacterial meningitis in adults in Hanoi [6].
In preparation for the start of the clinical trials, dedicated laboratory trial staff members were appointed to become staff of the national institutions. They received training on processing, labeling, testing, storing, shipping, and documenting data from trial specimens according to protocol requirements. A centralized specimen labeling and database system was set up to track and trace all stored specimens for the SEAICRN studies. Currently the SEAICRN uses Freezerworks software (Dataworks Development; http://www.freezerworks.com/) to track study specimens and aliquots. Sites received deep freezers for adequate specimen storage and power back-up systems were installed where needed to ensure an uninterrupted power supply. Table 2 summarizes the required laboratory capacity and what was done to achieve this.
Tab. 2. Activities by SEAICRN to achieve required laboratory capacity for their research projects. 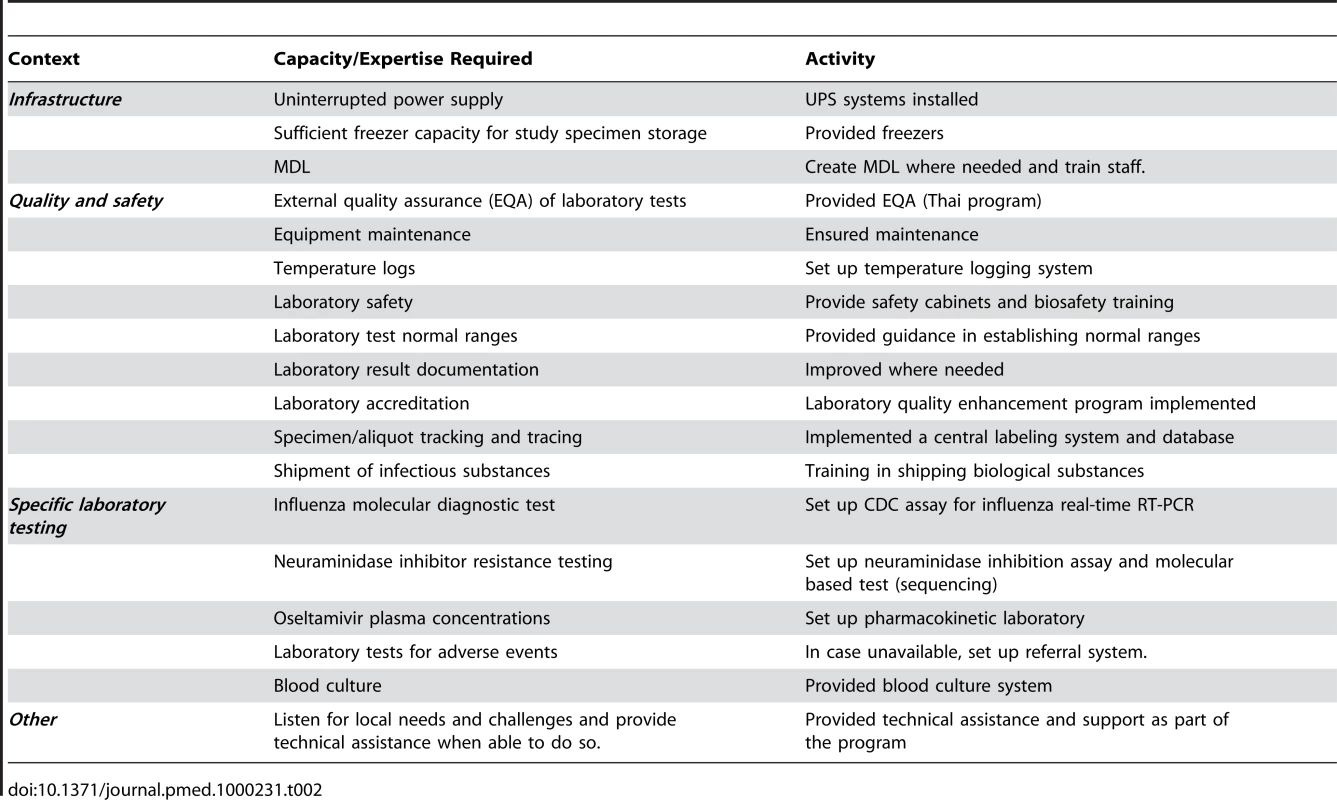
The laboratory capacity program of the SEAICRN enabled us to rapidly respond to changes in influenza epidemiology, such as the spread of naturally occurring oseltamivir resistance in seasonal H1N1 viruses and the recent emergence of the novel influenza A (H1N1) strain (pH1N1) in April 2009. In response to the increasing rates of oseltamivir resistance in seasonal H1N1 viruses, before the emergence of pH1N1, the laboratory committee decided to test Asian isolates and respiratory specimens from influenza patients in our studies [7],[8]. This step generated valuable resistance data from the region, which were instantly shared with the national and international medical and scientific community. In April 2009, when confronted with a pandemic threat of this novel influenza virus, we prepared collaborating laboratories by providing them with sequence information of this strain and primers and probes to enable them to detect it by RT-PCR. Furthermore, we were able to describe the first worldwide transmission of oseltamivir resistant pH1N1 in a community cluster [9].
Laboratory Quality Enhancement Program
In partnership with Family Health International (FHI), the SEAICRN initiated a clinical laboratory quality improvement program in the region. This included: (i) baseline assessment of each hospital clinical laboratory against international standards, ( ii) inventory, maintenance, and calibration of instruments, (iii) enrollment in external quality assurance program, (iv) assessment of training needs, (v) review of normal reference values used in the laboratory, and (vi) accreditation status of the laboratories by local and international accreditation bodies. The baseline assessments evaluated the overall quality of the clinical laboratory and helped to develop short - and long-term recommendations for improvement. Based on the assessment and recommendations, subsequent follow-up visits and technical support were provided to each hospital to implement those recommendations.
Laboratory management staff in each hospital were trained and encouraged to implement a quality management system. This included development and implementation of quality and technical manuals, standard operating procedures (SOPs), and a document control system. To help to improve laboratory quality it was necessary to appoint a senior staff member as a Quality Officer to oversee all aspects of laboratory quality and the quality enhancement program. Furthermore, each laboratory was supported with necessary instruments, and staff were encouraged to establish and monitor an equipment maintenance and calibration program. All staff from each laboratory were trained on standards developed by the technical committee of the International Organisation for Standardisation (ISO15189 : 2003 and ISO15190 : 2003, http://www.iso.org/) [10].
During the clinical trials it was noticed that occasionally reported biochemistry results were implausible. In response, a continuous improvement system was implemented to ensure that laboratory test results are reviewed by a qualified person to identify performance issues. In addition, advice was given on proper use of internal controls, how to monitor and investigate the possible cause(s) of controls not meeting acceptance criteria, and corrective and preventive action measures when performance issues are identified. Laboratories were encouraged to complement the capacity of each other by establishing a specimen testing referral linkage among them.
The overall activities of the SEAICRN created opportunities for laboratory staff to exchange experiences with senior laboratory personnel. In addition, a laboratory networking system and a platform for sharing experiences among laboratories was established through the SEAICRN annual meetings and regular teleconferences. Also, as part of the development of new clinical protocols there has been a strong interaction between laboratory experts, clinicians, and trial support staff. Having such systems in place have proved crucial to the ability of these clinical laboratories to respond quickly to the spread of oseltamivir-resistant influenza A/H1N1 and pH1N1. With the infrastructure in place it proved possible to respond immediately to the pH1N1 pandemic. It was also possible to implement a clinical research protocol in Viet Nam and start to make the data available in less than a month from the appearance of the first cases.
The ultimate goal of the program was to improve the quality of laboratory services, achieve compliance with GCLP standards, gain ISO 15189 accreditation, and facilitate better clinical care and collaborative research. Setting such goals makes the process more real and motivates laboratory staff to participate. Several laboratories in Thailand had already started working toward accreditation independently of the SEAICRN, and these efforts helped facilitate the SEAICRN program at those sites. Two laboratories in Thailand received “The Association of Medical Technologists of Thailand” accreditation, and one laboratory in Thailand and two in Vietnam have gained ISO15189 accreditation. In addition, another laboratory in Thailand and one in Indonesia are nearly ready for accreditation inspection.
Discussion
Although GCLP is the standard in developed countries, it can pose a disproportionate burden and significant challenge in resource-constrained settings (see Box 1). However, it was clear that laboratory staff were motivated to improve the quality of their testing and services when working toward a concrete endpoint, such as ISO15189 accreditation. A GCLP standard for resource-constrained settings that is simpler and less demanding on resources is urgently needed [5],[11].
Box 1. Key Challenges
-
Improving laboratory capacity requires investment in both time and money. It is essential that long term goals are set, as opposed to short term fixes, and that the finances are made available to enable and sustain continuous quality improvements.
-
Laboratories will be at different stages of implementing an international-level quality system. A capacity building program should be tailored to each laboratory taking cultural, technical, and financial differences into account. Working towards accreditation is a concrete and achievable goal and helps to motivate staff.
-
International accreditation (e.g. ISO15189 : 2003) may not always be desirable and sustainable in resource-constrained settings as the ongoing costs may be too high. It is worthwhile to explore alternate accreditation models in such settings, which can be used as a stepwise approach to full international accreditation.
-
Improving laboratory quality systems requires financial commitment and support from the hospital director and a time commitment from laboratory leadership and staff. All need to be convinced that improvement is essential for delivering good quality health care. Research activities should not be solely responsible for funding the running costs of quality systems as this would not be sustainable with the systems potentially ending when the research funding stops.
-
Not all countries have accreditation authorities that can accredit laboratories as qualified people are not available. The capacity for national or regional accreditation should be improved in the near future.
It is important that laboratory enhancement programs focus not only on the particular disease of research interest, so that the investments can also benefit other diseases and routine patient care [12]. Setting up the SEAICRN has served as a catalyst for laboratories to implement molecular diagnostic techniques as part of routine care, not only for influenza but for a range of other pathogens beyond direct research needs. The SEAICRN has provided tools, training (including short courses, Masters, and PhD programs), and protocols to facilitate these processes. Currently, six scientists from Asia are enlisted in a PhD program, nine in a Masters program, three in a Bachelor's program, and 295 in a short-term fellowship. A total of 2,146 individuals have participated in operational training activities organized or made possible by the SEAICRN.
The sharing of data has been a contentious issue over the last few years. The SEAICRN has a policy that all the protocols, case record forms, SOPs, and manuals of operations for all studies and for all processes are made available via the internet at the start of all studies (http://www.seaicrn.org/). During the pH1N1 pandemic, a number of non-Asian countries used these protocols, adapted them to their own local circumstances, including translation, and implemented them. SEAICRN is also committed to sharing all data with all institutions and national and international authorities, in line with the IHR 2005. However, it is also crucial for scientists and clinicians within countries and within regions to know each other, to have built sustainable relationships and networks in a manner that encourages and facilitates the exchange of experience and information within the regional clinical and scientific community.
Clearly, the success and sustainability of a research network depends on funding. The current support for the SEAICRN ends in September 2010, but investigators across the region are committed to continuing the network, and funding has been secured to ensure its sustainability beyond that date. There is a clear commitment to extend the network beyond the current centers in the four countries in Asia. Other centers have expressed an interest in joining or supporting such an expansion. The long-term aim is to build a sustainable clinical research network with its center of gravity firmly based in Asia.
Having a wider perspective has proven invaluable. Furthermore, the SEAICRN was able to respond immediately in assessing oseltamivir resistance in seasonal influenza A/H1N1 in 2008, providing vital information to the scientific and medical community. We believe this program has improved the clinical and research laboratory capacity and quality in the region. Besides enhancing research in Asia, the investments have also helped improve health care for all patients served at the participating hospitals. Our program addresses the issues raised by the World Health Assembly in 2005 and in the revised International Health Regulations, as it supports the region to be more prepared to deal with potential pandemic influenza viruses and other endemic and emerging infectious diseases, as we have recently experienced, and promotes international collaboration and sharing of data.
Zdroje
1. HiggsES
HaydenFG
ChotpitayasunondhT
WhitworthJ
FarrarJ
2008 The Southeast Asian Influenza Clinical Research Network: development and challenges for a new multilateral research endeavor. Antiviral Res 78 64 68
2. 2005 World Health Assembly. Strengthening pandemic-influenza preparedness and response:WHA58.5. May 2005. Available: http://wwwwhoint/gb/ebwha/pdf_files/WHA58/WHA58_5-enpdf Accessed 21 April 2009
3. PLoS Medicine Editors 2007 How is WHO responding to global public health threats? PLoS Med 4 e197 doi:10.1371/journal.pmed.0040197
4. EzzelleJ
Rodriguez-ChavezIR
DardenJM
StirewaltM
KunwarN
2008 Guidelines on good clinical laboratory practice: bridging operations between research and clinical research laboratories. J Pharm Biomed Anal 46 18 29
5. StevensW
2003 Good clinical laboratory practice (GCLP): the need for a hybrid of good laboratory practice and good clinical practice guidelines/standards for medical testing laboratories conducting clinical trials in developing countries. Qual Assur 10 83 89
6. WertheimHF
NguyenHN
TaylorW
LienTT
NgoHT
2009 Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One 4 e5973 doi:10.1371/journal.pone.0005973
7. SheuTG
DeydeVM
Okomo-AdhiamboM
GartenRJ
XuX
2008 Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother 52 3284 3292
8. BesselaarTG
NaidooD
BuysA
GregoryV
McAnerneyJ
2008 Widespread oseltamivir resistance in influenza A viruses (H1N1), South Africa. Emerg Infect Dis 14 1809 1810
9. LeQM
WertheimHF
TranND
van DoornHR
NguyenTH
HorbyP
2010 A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 362 86 87
10. BurnettD
BlairC
2001 Standards for the medical laboratory–harmonization and subsidiarity. Clin Chim Acta 309 137 145
11. KuepferI
BurriC
2009 Reflections on clinical research in sub-Saharan Africa. Int J Parasitol 39 947 954
12. KrukME
2008 Emergency preparedness and public health systems lessons for developing countries. Am J Prev Med 34 529 534
13. WattanagoonY
StepniewskaK
LindegardhN
PukrittayakameeS
SilachamroonU
2009 Pharmacokinetics of high-dose oseltamivir in healthy volunteers. Antimicrob Agents Chemother 53 945 952
Štítky
Interní lékařství
Článek Economic Appraisal of Ontario's Universal Influenza Immunization Program: A Cost-Utility AnalysisČlánek The Effect of Rural-to-Urban Migration on Obesity and Diabetes in India: A Cross-Sectional Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
-
Všechny články tohoto čísla
- Economic Appraisal of Ontario's Universal Influenza Immunization Program: A Cost-Utility Analysis
- The Effect of Rural-to-Urban Migration on Obesity and Diabetes in India: A Cross-Sectional Study
- Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages
- Association between the 2008–09 Seasonal Influenza Vaccine and Pandemic H1N1 Illness during Spring–Summer 2009: Four Observational Studies from Canada
- China's Engagement with Global Health Diplomacy: Was SARS a Watershed?
- Laboratory Capacity Building in Asia for Infectious Disease Research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN)
- Mortality Measurement Matters: Improving Data Collection and Estimation Methods for Child and Adult Mortality
- Brazil and the Framework Convention on Tobacco Control: Global Health Diplomacy as Soft Power
- Health Diplomacy and the Enduring Relevance of Foreign Policy Interests
- Measuring Under-Five Mortality: Validation of New Low-Cost Methods
- What Can We Conclude from Death Registration? Improved Methods for Evaluating Completeness
- Improving Newborn Survival in Low-Income Countries: Community-Based Approaches and Lessons from South Asia
- Does Seasonal Influenza Vaccination Increase the Risk of Illness with the 2009 A/H1N1 Pandemic Virus?
- Measuring Adult Mortality Using Sibling Survival: A New Analytical Method and New Results for 44 Countries, 1974–2006
- Early Emergence of Ethnic Differences in Type 2 Diabetes Precursors in the UK: The Child Heart and Health Study in England (CHASE Study)
- Comparative Effectiveness Research: Challenges for Medical Journals
- Alternative Strategies to Reduce Maternal Mortality in India: A Cost-Effectiveness Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages
- Economic Appraisal of Ontario's Universal Influenza Immunization Program: A Cost-Utility Analysis
- China's Engagement with Global Health Diplomacy: Was SARS a Watershed?
- Laboratory Capacity Building in Asia for Infectious Disease Research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



