-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPhysical Activity Attenuates the Genetic Predisposition to Obesity in 20,000 Men and Women from EPIC-Norfolk Prospective Population Study
Background:
We have previously shown that multiple genetic loci identified by genome-wide association studies (GWAS) increase the susceptibility to obesity in a cumulative manner. It is, however, not known whether and to what extent this genetic susceptibility may be attenuated by a physically active lifestyle. We aimed to assess the influence of a physically active lifestyle on the genetic predisposition to obesity in a large population-based study.Methods and Findings:
We genotyped 12 SNPs in obesity-susceptibility loci in a population-based sample of 20,430 individuals (aged 39–79 y) from the European Prospective Investigation of Cancer (EPIC)-Norfolk cohort with an average follow-up period of 3.6 y. A genetic predisposition score was calculated for each individual by adding the body mass index (BMI)-increasing alleles across the 12 SNPs. Physical activity was assessed using a self-administered questionnaire. Linear and logistic regression models were used to examine main effects of the genetic predisposition score and its interaction with physical activity on BMI/obesity risk and BMI change over time, assuming an additive effect for each additional BMI-increasing allele carried. Each additional BMI-increasing allele was associated with 0.154 (standard error [SE] 0.012) kg/m2 (p = 6.73×10−37) increase in BMI (equivalent to 445 g in body weight for a person 1.70 m tall). This association was significantly (pinteraction = 0.005) more pronounced in inactive people (0.205 [SE 0.024] kg/m2 [p = 3.62×10−18; 592 g in weight]) than in active people (0.131 [SE 0.014] kg/m2 [p = 7.97×10−21; 379 g in weight]). Similarly, each additional BMI-increasing allele increased the risk of obesity 1.116-fold (95% confidence interval [CI] 1.093–1.139, p = 3.37×10−26) in the whole population, but significantly (pinteraction = 0.015) more in inactive individuals (odds ratio [OR] = 1.158 [95% CI 1.118–1.199; p = 1.93×10−16]) than in active individuals (OR = 1.095 (95% CI 1.068–1.123; p = 1.15×10−12]). Consistent with the cross-sectional observations, physical activity modified the association between the genetic predisposition score and change in BMI during follow-up (pinteraction = 0.028).Conclusions:
Our study shows that living a physically active lifestyle is associated with a 40% reduction in the genetic predisposition to common obesity, as estimated by the number of risk alleles carried for any of the 12 recently GWAS-identified loci.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(8): e32767. doi:10.1371/journal.pmed.1000332
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000332Summary
Background:
We have previously shown that multiple genetic loci identified by genome-wide association studies (GWAS) increase the susceptibility to obesity in a cumulative manner. It is, however, not known whether and to what extent this genetic susceptibility may be attenuated by a physically active lifestyle. We aimed to assess the influence of a physically active lifestyle on the genetic predisposition to obesity in a large population-based study.Methods and Findings:
We genotyped 12 SNPs in obesity-susceptibility loci in a population-based sample of 20,430 individuals (aged 39–79 y) from the European Prospective Investigation of Cancer (EPIC)-Norfolk cohort with an average follow-up period of 3.6 y. A genetic predisposition score was calculated for each individual by adding the body mass index (BMI)-increasing alleles across the 12 SNPs. Physical activity was assessed using a self-administered questionnaire. Linear and logistic regression models were used to examine main effects of the genetic predisposition score and its interaction with physical activity on BMI/obesity risk and BMI change over time, assuming an additive effect for each additional BMI-increasing allele carried. Each additional BMI-increasing allele was associated with 0.154 (standard error [SE] 0.012) kg/m2 (p = 6.73×10−37) increase in BMI (equivalent to 445 g in body weight for a person 1.70 m tall). This association was significantly (pinteraction = 0.005) more pronounced in inactive people (0.205 [SE 0.024] kg/m2 [p = 3.62×10−18; 592 g in weight]) than in active people (0.131 [SE 0.014] kg/m2 [p = 7.97×10−21; 379 g in weight]). Similarly, each additional BMI-increasing allele increased the risk of obesity 1.116-fold (95% confidence interval [CI] 1.093–1.139, p = 3.37×10−26) in the whole population, but significantly (pinteraction = 0.015) more in inactive individuals (odds ratio [OR] = 1.158 [95% CI 1.118–1.199; p = 1.93×10−16]) than in active individuals (OR = 1.095 (95% CI 1.068–1.123; p = 1.15×10−12]). Consistent with the cross-sectional observations, physical activity modified the association between the genetic predisposition score and change in BMI during follow-up (pinteraction = 0.028).Conclusions:
Our study shows that living a physically active lifestyle is associated with a 40% reduction in the genetic predisposition to common obesity, as estimated by the number of risk alleles carried for any of the 12 recently GWAS-identified loci.
: Please see later in the article for the Editors' SummaryIntroduction
Changes in our lifestyle, including increased energy intake and lack of physical activity, have been the driving force behind the dramatic increase in obesity prevalence over the past few decades [1]–[3], and increasing physical activity levels have been associated with reduced body fatness and metabolic risk [4]. However, genetic epidemiological studies have firmly established that genetic factors also play a critical role in the development of obesity [5]. Although in theory, genetically predisposed individuals may be more susceptible to obesity in an obesogenic environment, there has been no previous convincing evidence of genotype–lifestyle interactions.
Recent genome-wide association studies (GWAS) have identified 12 loci robustly associated with increased body mass index (BMI) [6]–[10]. We have shown that these loci have a cumulative effect on BMI and on the risk of obesity and that, collectively, these loci can be used to estimate an individual's genetic predisposition to obesity [11]. Although the associations between this set of loci and BMI and risk of obesity were convincing, the variance in BMI explained by these variants is still very small (less than 1%) [11], despite previous observations that BMI has an estimated heritability of 40%–70% [5]. Gene–lifestyle interactions may partly account for the unexplained heritability of BMI [12].
In the current study, we examined whether the genetic predisposition to increased BMI and obesity risk as assessed by a genetic predisposition score, based on the 12 susceptibility loci that were recently identified through GWAS, was modified by self-reported daily physical activity in a large population-based sample from the European Prospective Investigation of Cancer (EPIC)-Norfolk study.
Methods
Study Sample
The EPIC-Norfolk study is a population-based cohort study of 25,631 people living in the city of Norwich, UK and its nearby areas. Participants were 39 to 79 y old during the health check between 1993 and 1997. From January 1998, participants were invited for a second health examination, which was attended by 15,786 individuals by October 2000. Full details of the study cohort have been described previously [13],[14]. In brief, trained nurses measured height in centimetres and weight in kilograms and BMI was calculated as weight in kilogram divided by height in meter squared.
DNA of 21,631 individuals, all of white European descent, was available for genotyping. Individuals with prevalent type 2 diabetes (n = 522), those with missing values for any of the phenotypes under study (n = 617), and those with an absolute annual change of BMI greater than 2 kg/m2 or of waist circumference greater than 7 cm (n = 62) during a follow-up period of 3–4 y were excluded. In total, 20,430 individuals had baseline data available, of which 11,936 had BMI data at the second health check (Table 1). Those who participated in the second health check-up were leaner (p = 1.06×10−33) and more physically active (p = 3.85×10−36). Proportionally more women than men participated in the second health compared to baseline participation (p = 0.0004) (Table S1).
Tab. 1. Characteristics of the study samples at baseline and follow-up by sex. 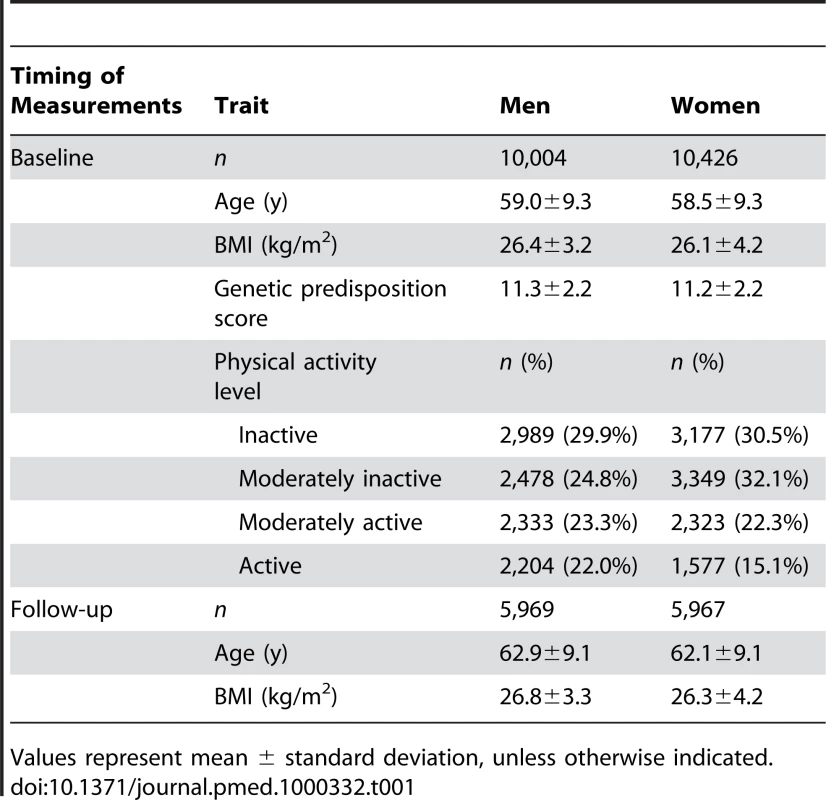
Values represent mean ± standard deviation, unless otherwise indicated. The Norfolk, UK, Local Research Ethics Committee approved the study and all participants gave their informed written consent.
Physical Activity Assessment
Both occupational (sedentary, standing, physical work, heavy manual work) and leisure-time (cycling, exercise) activities were assessed with a validated self-administered questionnaire [15]. Leisure-time physical activity (hours/week) for both summer and winter was recorded. On the basis of this information, average daily physical activity was calculated as total hours of physical activity per week divided by 7, and this was used to categorise physical activity levels into four groups: inactive (sedentary job, no recreational activity), moderately inactive (sedentary job, <0.5 h/d recreational activity or standing job, no recreational activity), moderately active (sedentary job, 0.5–1.0 h/d recreational activity or standing job, <0.5 h/d recreational activity or physical job, no recreational activity), and active (sedentary job, >1 h/d recreational activity or standing job, >1 h/d recreational activity or physical job with some recreational activity or heavy manual job). This categorization of physical activity levels was predefined and validated against objective measurements of physical activity by means of repeated individually calibrated minute-by-minute heart rate monitoring as described previously [15].
Genotyping
We genotyped rs3101336, rs10913469, rs6548238, rs7647305, rs10938397, rs925946, rs10838738, rs7132908, rs7498665, rs1121980, rs17782313, and rs368794, representing the obesity susceptibility loci near or in NEGR1, SEC16B, TMEM18, ETV5, GNPDA2, BDNF, MTCH2, FAIM2, SH2B1, FTO, MC4R, and KCTD15 genes, respectively. These loci have been identified through recent GWAS for BMI [6]–[10]. Genotype information and genotyping methods for the 12 variants have been reported previously in detail (Table S2) [11]. All variants met the quality control criteria (call rate >95%, blind duplicate concordance >97%, and Hardy-Weinberg equilibrium p>0.05).
Statistical Analyses
Individual SNPs were recoded as 0, 1, and 2 according to the number of BMI-increasing alleles for that particular SNP. The BMI-increasing alleles were defined on the basis of the robust associations of the SNPs with BMI observed in the recent GWAS [6]–[10].
A genetic predisposition score was calculated for each individual by adding up the BMI-increasing alleles of all 12 variants. For individuals with missing genotype data for three or fewer SNPs (97.3% of the total sample), missing genotypes were substituted by the average count of risk alleles for the respective SNP for the purpose of calculating the genetic predisposition score. This resulted in a total number of 19,878 individuals at baseline with a genetic predisposition score of whom 12,201 had full genotyped data for all SNPs and 7,677 individuals had substituted genotypes for 3 or fewer SNPs. Of the 19,878 individuals, 11,651 had data from the second health check. The genetic predisposition score was not different between individuals who did participate in the follow-up and those who did not participate in the follow-up (p = 0.606). Sensitivity analyses showed that the results of data with and without substitution of missing genotypes were similar. Here, we only present the results based on the predisposition score with substitution. The genetic predisposition score was normally distributed.
First, we analysed the baseline data cross-sectionally. General linear models (GLMs) were used to test the association of individual SNPs and of the genetic predisposition score with BMI. Logistic regression models were used to examine associations with risk of obesity (18.5≤ BMI <25 kg/m2 versus BMI ≥30 kg/m2) or overweight (18.5≤ BMI <25 kg/m2 versus BMI ≥25 kg/m2). Data were adjusted for age, age2, sex, and physical activity, and we assumed an additive effect of the BMI-increasing alleles. Interactions between individual SNPs or the genetic predisposition score and physical activity on BMI or risk of obesity or of overweight were examined by including a SNP (or score)-physical activity interaction term in the respective model with the main effects included in the model as well. Analyses were also stratified by physical activity level. We examined the explained variance (R-square) of BMI by the genetic predisposition score using GLMs. Furthermore, we examined the predictive value of the genetic predisposition score on obesity risk, stratified by physical activity level by using the area under the receiver operating characteristic (ROC) curve produced by a logistic regression model. We also divided the sample into a “genetically susceptible” group, i.e., those with a genetic predisposition score >11 (median of the genetic predisposition score) and a “genetically nonsusceptible” group, i.e., those with a genetic predisposition score of 11 or less to show interactions between the genetic predisposition and physical activity levels on BMI and obesity risk.
Next, we analysed the data longitudinally with the annual BMI change between the first and second health check as the outcome. GLMs were used to examine the interaction between the genetic predisposition score and physical activity on the annual BMI change, adjusting for age, age2, sex, and baseline BMI. All analyses were performed using SAS version 9.1 (SAS Institute Inc.).
Results
At baseline, each additional BMI-increasing allele in the genetic predisposition score was associated with a 0.154 (standard error [SE] 0.012) kg/m2 (p = 6.73×10−37) increase in BMI, which corresponds to a 445 g increase in body weight for a person 1.70 m tall, but was not associated with physical activity levels (p = 0.49). Each increase in physical activity level was associated with a reduction of 0.313 kg/m2 (SE 0.025; p = 1.2×10−36) in baseline BMI, which corresponds to a 904 g decrease in body weight for a person 1.70 m tall.
Physical activity significantly (pinteraction = 0.016) modified the effect of the genetic predisposition score on BMI (Table 2). Each additional BMI-increasing allele was associated with an increase of 0.205 (SE 0.024) kg/m2 in BMI (p = 3.62×10−18, equivalent to 592 g in weight) in the inactive group, but the effect was much less in the active individuals (0.126 [SE 0.025] kg/m2, p = 6.04×10−7; 364 g in weight). The effect in moderately active and moderately inactive individuals was intermediate, but more similar to that in the active group. In the combined active group (i.e., the three “active groups” considered together), each additional risk allele increased the BMI with 0.131 (SE 0.014) kg/m2 (p = 7.97×10−21, 379 g in weight), which was significantly less pronounced (pinteraction = 0.005) than the effect observed in the inactive group (Figure 1). The interaction term remained significant after inverse normal transformation of BMI, suggesting that interaction effects between the genetic predisposition score and physical activity on BMI were not due to unequal variance in different physical activity groups. Similar trends for interaction were observed after further exclusion of individuals with cardiovascular disease (n = 1,128) and cancer (n = 4,534) (pinteraction = 0.09 and pinteraction = 0.05, for using four and two groups of physical activity, respectively).
Fig. 1. BMI with different genetic predisposition scores in inactive versus active individuals. 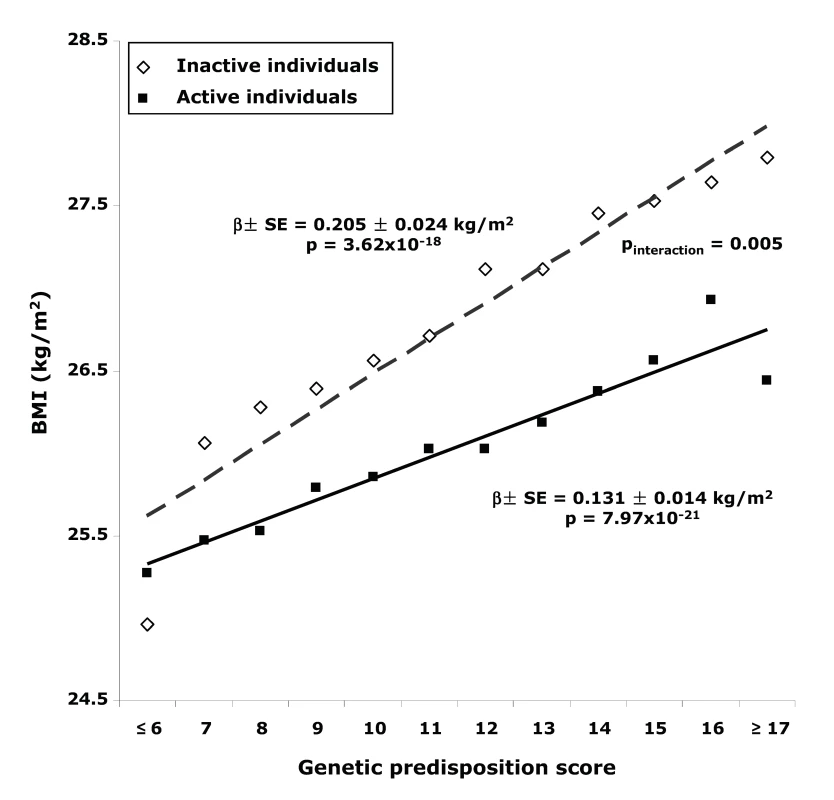
Tab. 2. Associations of the genetic predisposition score with BMI and risk of obesity in the total population and stratified by physical activity level. 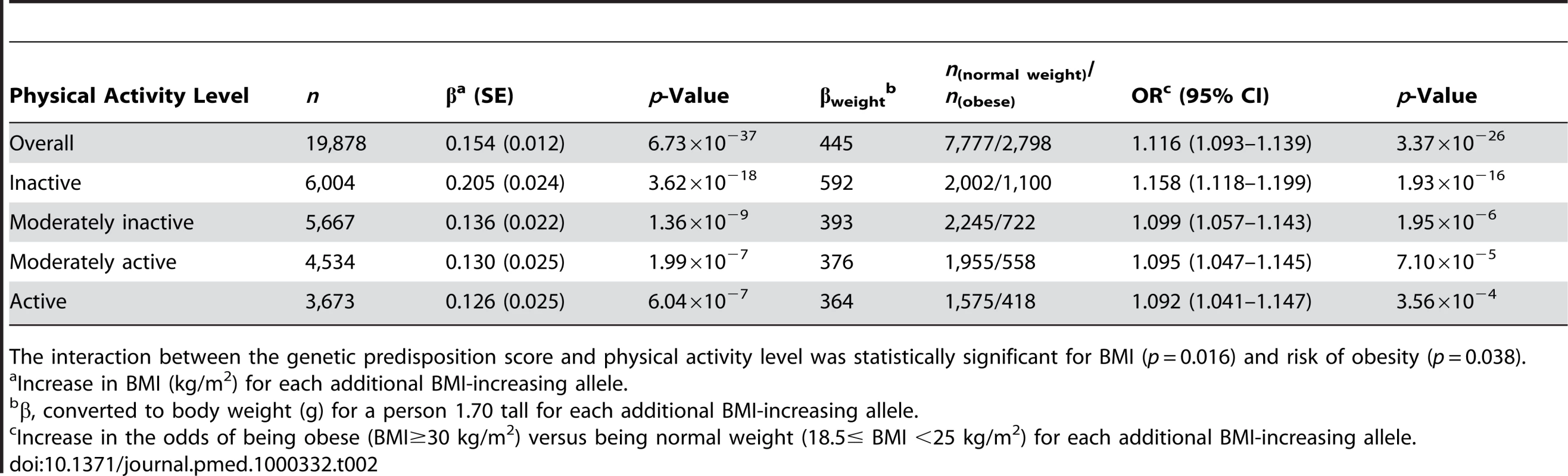
The interaction between the genetic predisposition score and physical activity level was statistically significant for BMI (p = 0.016) and risk of obesity (p = 0.038). A similar interaction pattern between the genetic predisposition score and physical activity on obesity risk was observed. Each additional BMI-increasing allele was associated with an odds ratio (OR) of 1.116 (95% confidence interval [CI] 1.093–1.139; p = 3.37×10−26) in the total sample. In the inactive group, each additional BMI-increasing allele was associated with an OR of 1.158 (95% CI 1.118–1.199; p = 1.93×10−16), which was significantly (pinteraction = 0.038) greater than the ORs observed for the other physical activity groups (Table 2). In the combined active group, each additional BMI-increasing allele was associated with an OR of 1.095 (95% CI 1.068–1.123; p = 1.15×10−12) (pinteraction = 0.015, compared to the inactive group). We observed similar trends for risk of being overweight (pinteraction = 0.064 for four levels of physical activity; pinteraction = 0.043 for the active versus the inactive group).
In the inactive group, the difference in BMI between individuals with a high genetic predisposition score (>11 BMI-increasing alleles) and those with a low genetic predisposition score (≤11 BMI-increasing alleles) amounted to 0.739 (SE 0.103) kg/m2 (or 2,136 g in weight) (p<8.07×10−13), whereas this difference was only 0.407 (SE 0.061) kg/m2 (or 1,176 g higher weight) (p<2.23×10−11) in the active group (pinteraction = 0.004, Figure 2). Similarly, in the inactive group, the odds of obesity were 1.722-fold (95% CI 1.486–1.996; p = 2.22×10−16) higher in those with a high genetic susceptibility as compared to those with a low genetic susceptibility, while this difference was much smaller (OR 1.287 [95% CI 1.156–1.433; p = 1.15×10−12]) in the active group (pinteraction = 0.007).
Fig. 2. Difference in least square means of BMI between the high (>11 BMI-increasing alleles) and the low (≤11 BMI-increasing alleles) genetic susceptibility groups in the combined active group and the inactive group. 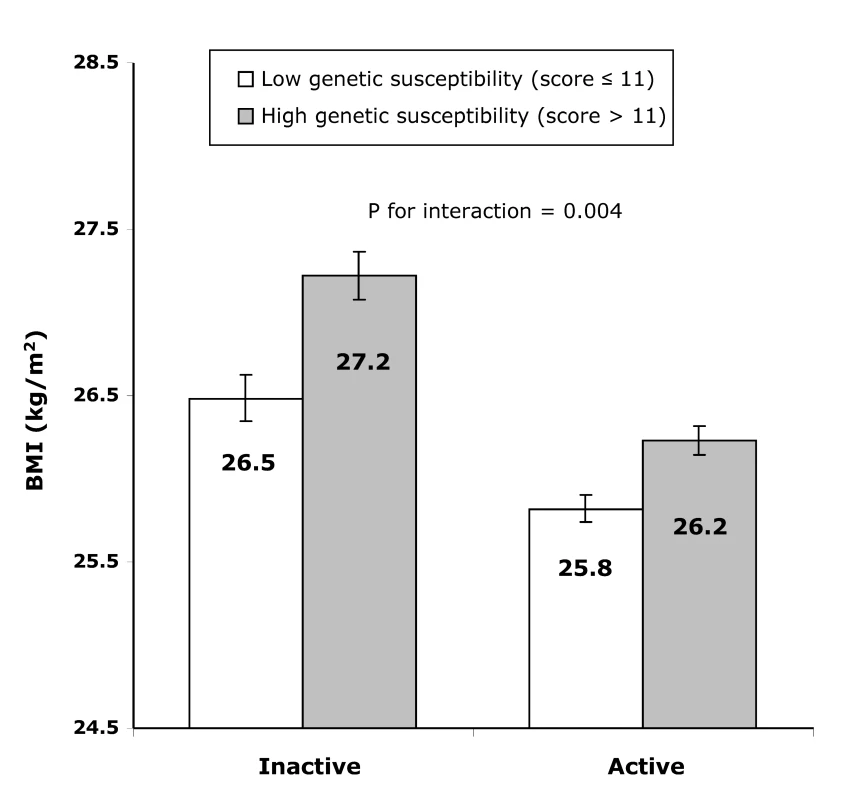
Error bars show 95% CIs. The genetic predisposition score explained 1.2% of the variation in BMI in the inactive group and 0.6% in the active group. Furthermore, the ROC curves for the prediction of obesity based on the genetic predisposition score together with age, age2, sex, showed that the prediction was significantly (p<1.00×10−30) better in the inactive group (area under the ROC curve, 0.614 [95% CI 0.594–0.635]) than that in the combined active group (0.576 [95% CI 0.561–0.591]).
Of the individual SNP analyses, only rs6548238 near TMEM18, rs10838738 in MTCH2, and rs7498665 near SH2B1 showed nominally significant interactions with physical activity level on BMI or obesity risk (Tables S3 and S4), but none survived adjustment for multiple comparisons.
Consistent with the cross-sectional observations, physical activity modified the association between the genetic predisposition score and annual change in BMI during follow-up (pinteraction = 0.028, Figure 3). While overall the genetic predisposition score was not associated with the annual BMI change during follow-up (p = 0.95), the genetic predisposition score tended to be associated with an increase in annual BMI in physically inactive individuals, whereas the trend was opposite in physically active individuals (pinteraction = 0.028; Figure 3).
Fig. 3. Effect of the genetic predisposition score on the annual change in BMI during follow-up by physical activity level at baseline. 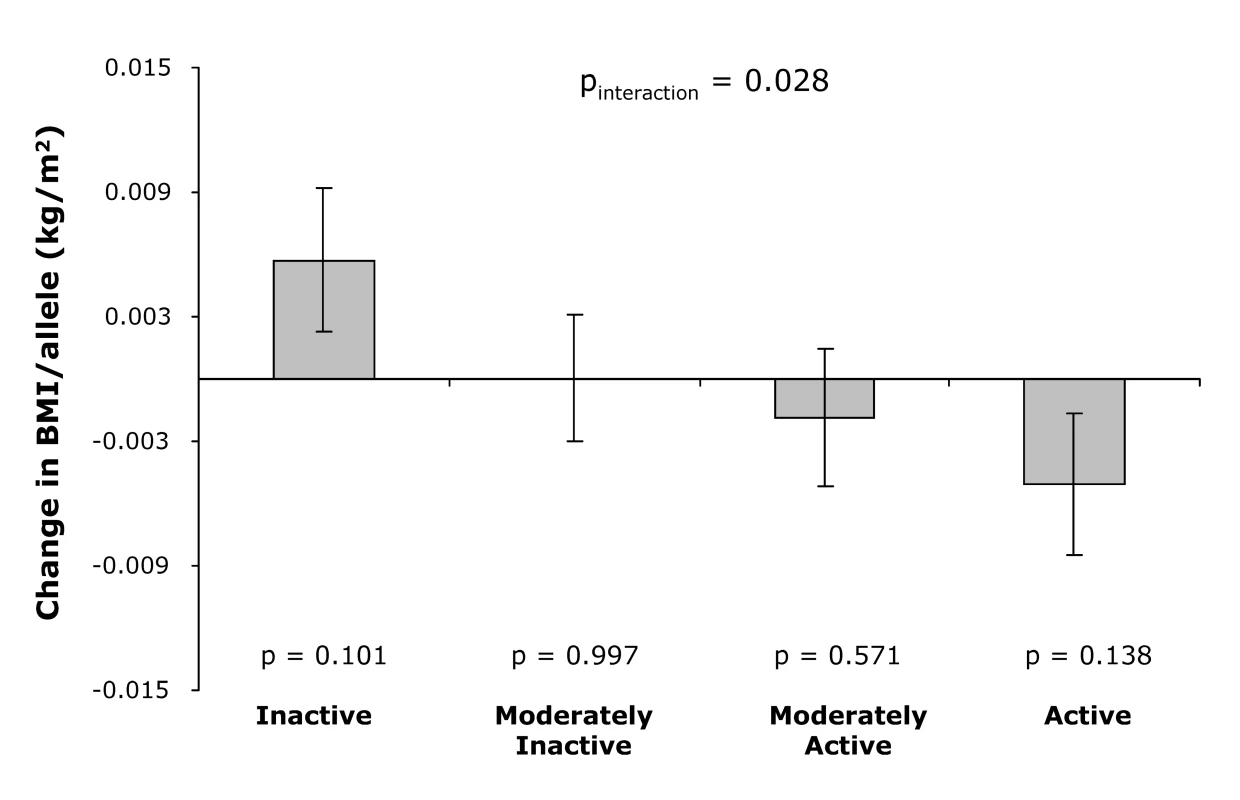
Error bars show standard error, and p-value at each physical activity level represents the significance of the association between the genetic predisposition score and annual change in BMI. Discussion
In this analysis of a large-scale population-based study, we show that a physically active lifestyle can modify the genetic predisposition to obesity. On average, each additional obesity-susceptibility allele is associated with an increase in body weight of 445 g. However, in individuals who have a physically active lifestyle, this difference is only 379 g/allele or 36% lower than in physically inactive individuals in whom the difference is 592 g/allele. Consistently, in the total sample each additional obesity-susceptibility allele increases the odds of obesity by 1.116-fold. However, the increased odds per allele for obesity risk are 40% lower in physically active individuals (OR = 1.095) compared to physically inactive individuals (OR = 1.158). We observed the attenuation of the genetic predisposition to obesity already at the lowest levels of physical activity, equivalent to a standing job or a sedentary job with <0.5 h of recreational activity. Importantly, our longitudinal analysis corroborate these cross-sectional observations showing that physical activity significantly (pinteraction = 0.028) modifies the effect of the genetic predisposition score on the annual BMI change during follow-up. Our findings further emphasise the importance of physical activity in the prevention of obesity.
Preliminary evidence for gene-lifestyle interaction has come from studies on the FTO locus, the firstly GWA-identified obesity-susceptibility locus with the largest influence on BMI and obesity risk to date [6],[7],[16]. Several studies have reported that the effect of common FTO variants is attenuated in active individuals in different populations [17]–[21]. In some studies, the effect size of FTO variants is up to 80% lower in physically active individuals compared to inactive individuals [17],[18],[20]. However, not all studies have been able to demonstrate an FTO–physical activity interaction [21]–[25]. This failure to detect an interaction in some studies may reflect the influence of population-specific characteristics such as high overall physical activity levels in the study population [22], small sample size [23],[25], or the effects of age [21]. In our study, the genetic predisposition was estimated by the multiple well-established obesity variants rather than a single locus. While this approach is less informative at a biological level, the greater genetic variation explained by the allele risk score explains why this approach may be preferable in terms of demonstrating an interaction between genetic susceptibility and physical activity.
Our study also showed that variance explained by the genetic predisposition score in the inactive group was 1.2% or twice that observed in the active group (0.6%). This finding is consistent with the increased effect size of the genetic predisposition score on BMI and risk of obesity in the inactive group, and consistent with most of the previous twin studies showing that the genetic contribution to the variation of obesity-related traits, is reduced by increased physical activity levels [26]–[29]. Our finding suggests that gene–environment interactions contribute to the unexplained variance in obesity traits. It also indicates that future GWAS of obesity-related traits may benefit from studying physically inactive individuals because the effect sizes of genetic variants may be more pronounced and therefore easier to identify.
Our data show that increased physical activity levels are associated with lower BMI in the population overall, but that in particular individuals who are genetically predisposed to obesity would benefit more from increased physical activity levels than individuals who are genetically protected. Interventions that target the genetically predisposed may be more effective, a hypothesis to be confirmed in future studies.
The predictive value of the genetic predisposition score for obesity is higher in inactive people, compared to that in the active people. However, even in physically inactive individuals, the extra predictive value provided by the genetic predisposition score beyond information from age and sex is still limited, suggesting that more genetic variants including other forms of variation such as copy number variants and rarer variants remain to be identified. Interactions between these variants and lifestyle factors other than physical activity also need to be examined in future studies.
The strengths of our study include a large sample size, a population-based, prospective study design, and a comprehensive estimation of the genetic predisposition to increased obesity traits based on multiple obesity-susceptibility variants. Previously, we have shown that the identification of convincing gene–environment interactions requires large sample sizes and accurate measurement of genes and environment [30]–[32]. In our study, we combined the strength of a large sample size with a more accurate estimation of the genetic predisposition to obesity. Our results are further strengthened by the longitudinal analysis of BMI change over time. A limitation of our study is that physical activity was measured by a self-administered physical activity questionnaire, which is less accurate than other objective instruments. However, the questionnaire used has been validated and shown to perform well in categorising physical activity levels in this population [15]. Furthermore, we have shown that physical activity assessed by this questionnaire is associated with mortality [33],[34]. Nondifferential measurement error might have attenuated the true strength of the gene–physical activity interaction. We recognise that our longitudinal analysis was limited to a group of individuals who had a lower BMI and were more physically active than the rest of the participants at baseline. However, as the genetic predisposition score was not associated with either physical activity or follow-up status, the selection bias may be limited.
In conclusion, the genetic predisposition to increased BMI and obesity is attenuated by a physically active lifestyle. This attenuation of the genetic predisposition was already observed at low levels of physical activity. Our finding that living a physically active lifestyle is associated with a 40% reduction in the genetic predisposition to common obesity is an important observation for public health. Promoting physical activity, particularly in those who are genetically predisposed, may be an important approach to controlling the current increasing obesity epidemic.
Supporting Information
Zdroje
1. PapasMA
AlbergAJ
EwingR
HelzlsouerKJ
GaryTL
2007
The built environment and obesity.
Epidemiol Rev
29
129
143
2. BouchardC
2008
Gene-environment interactions in the etiology of obesity: defining the fundamentals.
Obesity (Silver Spring)
16
Suppl 3
S5
S10
3. HillJO
2006
Understanding and addressing the epidemic of obesity: an energy balance perspective.
Endocr Rev
27
750
761
4. EkelundU
FranksPW
SharpS
BrageS
WarehamNJ
2007
Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness.
Diabetes Care
30
2101
2106
5. MaesHH
NealeMC
EavesLJ
1997
Genetic and environmental factors in relative body weight and human adiposity.
Behav Genet
27
325
351
6. FraylingTM
TimpsonNJ
WeedonMN
ZegginiE
FreathyRM
2007
A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity.
Science
316
889
894
7. ScuteriA
SannaS
ChenWM
UdaM
AlbaiG
2007
Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits.
PLoS Genet
3
e115
doi:10.1371/journal.pgen.0030115
8. LoosRJ
LindgrenCM
LiS
WheelerE
ZhaoJH
2008
Common variants near MC4R are associated with fat mass, weight and risk of obesity.
Nat Genet
40
768
775
9. WillerCJ
SpeliotesEK
LoosRJ
LiS
LindgrenCM
2009
Six new loci associated with body mass index highlight a neuronal influence on body weight regulation.
Nat Genet
41
25
34
10. ThorleifssonG
WaltersGB
GudbjartssonDF
SteinthorsdottirV
SulemP
2009
Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity.
Nat Genet
41
18
24
11. LiS
ZhaoJH
LuanJ
LubenRN
RodwellSA
2010
Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies.
Am J Clin Nutr
91
184
190
12. ManolioTA
CollinsFS
CoxNJ
GoldsteinDB
HindorffLA
2009
Finding the missing heritability of complex diseases.
Nature
461
747
753
13. DayN
OakesS
LubenR
KhawKT
BinghamS
1999
EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer.
Br J Cancer
80
Suppl 1
95
103
14. RiboliE
KaaksR
1997
The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition.
Int J Epidemiol
26
Suppl 1
S6
14
15. WarehamNJ
JakesRW
RennieKL
SchuitJ
MitchellJ
2003
Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study.
Public Health Nutr
6
407
413
16. HinneyA
NguyenTT
ScheragA
FriedelS
BronnerG
2007
Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants.
PLoS ONE
2
e1361
17. VimaleswaranKS
LiS
ZhaoJH
LuanJ
2009
Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene.
Am J Clin Nutr
90
425
428
18. RampersaudE
MitchellBD
PollinTI
FuM
ShenH
2008
Physical activity and the association of common FTO gene variants with body mass index and obesity.
Arch Intern Med
168
1791
1797
19. SonestedtE
RoosC
GullbergB
EricsonU
WirfaltE
2009
Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity.
Am J Clin Nutr
90
1418
1425
20. AndreasenCH
Stender-PetersenKL
MogensenMS
TorekovSS
WegnerL
2008
Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation.
Diabetes
57
95
101
21. CauchiS
StutzmannF
Cavalcanti-ProencaC
DurandE
PoutaA
2009
Combined effects of MC4R and FTO common genetic variants on obesity in European general populations.
J Mol Med
87
537
546
22. JonssonA
RenstromF
LyssenkoV
BritoEC
IsomaaB
2009
Assessing the effect of interaction between an FTO variant (rs9939609) and physical activity on obesity in 15,925 Swedish and 2,511 Finnish adults.
Diabetologia
52
1334
1338
23. HakanenM
RaitakariOT
LehtimakiT
PeltonenN
PahkalaK
2009
FTO genotype is associated with body mass index after the age of seven years but not with energy intake or leisure-time physical activity.
J Clin Endocrinol Metab
94
1281
1287
24. TanJT
DorajooR
SeielstadM
SimXL
OngRT
2008
FTO variants are associated with obesity in the Chinese and Malay populations in Singapore.
Diabetes
57
2851
2857
25. LappalainenTJ
TolppanenAM
KolehmainenM
SchwabU
LindstromJ
2009
The common variant in the FTO gene did not modify the effect of lifestyle changes on body weight: the Finnish Diabetes Prevention Study.
Obesity (Silver Spring)
17
832
836
26. McCafferyJM
PapandonatosGD
BondDS
LyonsMJ
WingRR
2009
Gene X environment interaction of vigorous exercise and body mass index among male Vietnam-era twins.
Am J Clin Nutr
89
1011
1018
27. MustelinL
SilventoinenK
PietilainenK
RissanenA
KaprioJ
2009
Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins.
Int J Obes (Lond)
33
29
36
28. SilventoinenK
HasselbalchAL
LallukkaT
BoglL
PietilainenKH
2009
Modification effects of physical activity and protein intake on heritability of body size and composition.
Am J Clin Nutr
90
1096
1103
29. KarnehedN
TyneliusP
HeitmannBL
RasmussenF
2006
Physical activity, diet and gene-environment interactions in relation to body mass index and waist circumference: the Swedish young male twins study.
Public Health Nutr
9
851
858
30. LuanJA
WongMY
DayNE
WarehamNJ
2001
Sample size determination for studies of gene-environment interaction.
Int J Epidemiol
30
1035
1040
31. WongMY
DayNE
LuanJA
ChanKP
WarehamNJ
2003
The detection of gene-environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement?
Int J Epidemiol
32
51
57
32. WongMY
DayNE
LuanJA
WarehamNJ
2004
Estimation of magnitude in gene-environment interactions in the presence of measurement error.
Stat Med
23
987
998
33. KhawKT
JakesR
BinghamS
WelchA
LubenR
2006
Work and leisure time physical activity assessed using a simple, pragmatic, validated questionnaire and incident cardiovascular disease and all-cause mortality in men and women: The European Prospective Investigation into Cancer in Norfolk prospective population study.
Int J Epidemiol
35
1034
1043
34. BessonH
EkelundU
BrageS
LubenR
BinghamS
2008
Relationship between subdomains of total physical activity and mortality.
Med Sci Sports Exerc
40
1909
1915
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 8- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
- Léčba bolesti u seniorů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Flexofytol® – přírodní revoluce v boji proti osteoartróze kloubů
-
Všechny články tohoto čísla
- Reducing Malaria Transmission in Africa: A Model-Based Evaluation of Intervention Strategies
- Quantifying the Impoverishing Effects of Purchasing Medicines: A Cross-Country Comparison of the Affordability of Medicines in the Developing World
- Physical Activity Attenuates the Genetic Predisposition to Obesity in 20,000 Men and Women from EPIC-Norfolk Prospective Population Study
- Using Touchscreen Electronic Medical Record Systems to Support and Monitor National Scale-Up of Antiretroviral Therapy in Malawi
- Social Relationships Are Key to Health, and to Health Policy
- Assessing Strategy and Equity in the Elimination of Malaria
- Challenges in Developing Evidence-Based Recommendations Using the GRADE Approach: The Case of Mental, Neurological, and Substance Use Disorders
- Will Cardiovascular Disease Prevention Widen Health Inequalities?
- Moving from Data on Deaths to Public Health Policy in Agincourt, South Africa: Approaches to Analysing and Understanding Verbal Autopsy Findings
- Rapid Scaling Up of Insecticide-Treated Bed Net Coverage in Africa and Its Relationship with Development Assistance for Health: A Systematic Synthesis of Supply, Distribution, and Household Survey Data
- Ecology: A Prerequisite for Malaria Elimination and Eradication
- An Intervention to Reduce HIV Risk Behavior of Substance-Using Men Who Have Sex with Men: A Two-Group Randomized Trial with a Nonrandomized Third Group
- Impact of Community-Based Maternal Health Workers on Coverage of Essential Maternal Health Interventions among Internally Displaced Communities in Eastern Burma: The MOM Project
- The Costs and Underappreciated Consequences of Research Misconduct: A Case Study
- The Effect of Raltegravir Intensification on Low-level Residual Viremia in HIV-Infected Patients on Antiretroviral Therapy: A Randomized Controlled Trial
- Harnessing Health IT for Improved Cardiovascular Risk Management
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Using Touchscreen Electronic Medical Record Systems to Support and Monitor National Scale-Up of Antiretroviral Therapy in Malawi
- Physical Activity Attenuates the Genetic Predisposition to Obesity in 20,000 Men and Women from EPIC-Norfolk Prospective Population Study
- Challenges in Developing Evidence-Based Recommendations Using the GRADE Approach: The Case of Mental, Neurological, and Substance Use Disorders
- Reducing Malaria Transmission in Africa: A Model-Based Evaluation of Intervention Strategies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



