-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaScreening for HIV-Associated Tuberculosis and Rifampicin Resistance before Antiretroviral Therapy Using the Xpert MTB/RIF Assay: A Prospective Study
Background:
The World Health Organization has endorsed the Xpert MTB/RIF assay for investigation of patients suspected of having tuberculosis (TB). However, its utility for routine TB screening and detection of rifampicin resistance among HIV-infected patients with advanced immunodeficiency enrolling in antiretroviral therapy (ART) services is unknown.Methods and Findings:
Consecutive adult HIV-infected patients with no current TB diagnosis enrolling in an ART clinic in a South African township were recruited regardless of symptoms. They were clinically characterised and invited to provide two sputum samples at a single visit. The accuracy of the Xpert MTB/RIF assay for diagnosing TB and drug resistance was assessed in comparison with other tests, including fluorescence smear microscopy and automated liquid culture (gold standard) and drug susceptibility testing. Of 515 patients enrolled, 468 patients (median CD4 cell count, 171 cells/µl; interquartile range, 102–236) produced at least one sputum sample, yielding complete sets of results from 839 samples. Mycobacterium tuberculosis was cultured from 81 patients (TB prevalence, 17.3%). The overall sensitivity of the Xpert MTB/RIF assay for culture-positive TB was 73.3% (specificity, 99.2%) compared to 28.0% (specificity, 100%) using smear microscopy. All smear-positive, culture-positive disease was detected by Xpert MTB/RIF from a single sample (sensitivity, 100%), whereas the sensitivity for smear-negative, culture-positive TB was 43.4% from one sputum sample and 62.3% from two samples. Xpert correctly identified rifampicin resistance in all four cases of multidrug-resistant TB but incorrectly identified resistance in three other patients whose disease was confirmed to be drug sensitive by gene sequencing (specificity, 94.1%; positive predictive value, 57%).Conclusions:
In this population of individuals at high risk of TB, intensive screening using the Xpert MTB/RIF assay increased case detection by 45% compared with smear microscopy, strongly supporting replacement of microscopy for this indication. However, despite the ability of the assay to rapidly detect rifampicin-resistant disease, the specificity for drug-resistant TB was sub-optimal.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(7): e32767. doi:10.1371/journal.pmed.1001067
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001067Summary
Background:
The World Health Organization has endorsed the Xpert MTB/RIF assay for investigation of patients suspected of having tuberculosis (TB). However, its utility for routine TB screening and detection of rifampicin resistance among HIV-infected patients with advanced immunodeficiency enrolling in antiretroviral therapy (ART) services is unknown.Methods and Findings:
Consecutive adult HIV-infected patients with no current TB diagnosis enrolling in an ART clinic in a South African township were recruited regardless of symptoms. They were clinically characterised and invited to provide two sputum samples at a single visit. The accuracy of the Xpert MTB/RIF assay for diagnosing TB and drug resistance was assessed in comparison with other tests, including fluorescence smear microscopy and automated liquid culture (gold standard) and drug susceptibility testing. Of 515 patients enrolled, 468 patients (median CD4 cell count, 171 cells/µl; interquartile range, 102–236) produced at least one sputum sample, yielding complete sets of results from 839 samples. Mycobacterium tuberculosis was cultured from 81 patients (TB prevalence, 17.3%). The overall sensitivity of the Xpert MTB/RIF assay for culture-positive TB was 73.3% (specificity, 99.2%) compared to 28.0% (specificity, 100%) using smear microscopy. All smear-positive, culture-positive disease was detected by Xpert MTB/RIF from a single sample (sensitivity, 100%), whereas the sensitivity for smear-negative, culture-positive TB was 43.4% from one sputum sample and 62.3% from two samples. Xpert correctly identified rifampicin resistance in all four cases of multidrug-resistant TB but incorrectly identified resistance in three other patients whose disease was confirmed to be drug sensitive by gene sequencing (specificity, 94.1%; positive predictive value, 57%).Conclusions:
In this population of individuals at high risk of TB, intensive screening using the Xpert MTB/RIF assay increased case detection by 45% compared with smear microscopy, strongly supporting replacement of microscopy for this indication. However, despite the ability of the assay to rapidly detect rifampicin-resistant disease, the specificity for drug-resistant TB was sub-optimal.
: Please see later in the article for the Editors' SummaryIntroduction
Tuberculosis is a major challenge for antiretroviral therapy (ART) services in resource-limited settings where patients typically enrol with advanced immunodeficiency [1]. Many patients referred for ART have a current TB diagnosis, and an additional large burden of disease is detected during pre-treatment screening [2]–[4]. Tuberculosis in this population is a major cause of morbidity and mortality [1],[5]–[7] and presents a substantial hazard of nosocomial disease transmission to other patients and health care workers [8]. These risks are heightened when patients have multidrug-resistant TB (MDR-TB) [9]–[11]. To address these challenges, there is a critical need in such settings for rapid, effective screening for TB and detection of drug resistance [1],[12].
Screening for TB in this patient population is difficult, however [12]. The World Health Organization's (WHO) intensified case finding symptom screen has low specificity and misses approximately 10%–20% of cases [13],[14]. Sputum smear microscopy, the mainstay of TB diagnosis in resource-limited settings, detects as few as one in five cases when used as a screening tool pre-ART [4],[12],[15]. Chest radiography is costly and not widely available; interpretation is difficult, and up to one-third of culture-confirmed cases of pulmonary TB diagnosed during screening have a normal radiograph [12],[16]. Availability of culture-based diagnosis is also extremely limited in resource-limited settings because of high cost and technical complexity, and this approach often provides a diagnosis only after several weeks [15],[17]. These challenges are further compounded by the extremely limited laboratory capacity to detect drug resistance [18]. The threat posed by MDR-TB to efforts to control TB worldwide [19] requires urgent improvements in diagnostic capacity.
Following a large multi-country evaluation [20], the WHO, in December 2010, endorsed the roll-out of a novel rapid test for the investigation of patients suspected of having TB, especially in settings with a high prevalence of HIV-associated disease and/or MDR-TB [21]. The Xpert MTB/RIF assay (Cepheid) is a fully automated molecular assay in which real-time polymerase chain reaction technology is used to simultaneously detect Mycobacterium tuberculosis and rifampicin resistance mutations in the rpoB gene [22],[23]. The cartridge-based system dispenses with the need for prior sputum processing and requires minimal laboratory expertise, and results are available in less than 2 h, permitting a specific TB diagnosis and rapid detection of rifampicin resistance. Excellent performance characteristics were observed among symptomatic adults with suspected TB in a large multi-country evaluation [20]. These findings have been confirmed in a subsequent multi-country implementation study [24] and in several laboratory-based studies [25]–[29]. The assay has sensitivities of 98%–100% for smear-positive pulmonary TB, 57%–78% for smear-negative pulmonary TB, and 53%–81% for extrapulmonary TB when testing a variety of clinical samples [20],[24]–[29].
Further studies are needed to examine the performance of the assay in different clinical settings, including use as a routine screening test to increase TB case detection in HIV-infected patients. We evaluated the diagnostic accuracy of the Xpert MTB/RIF assay among consecutive patients with advanced immunodeficiency being screened for TB (regardless of symptoms) prior to starting ART in a South African township with a very high burden of TB.
Methods
Setting
The ART cohort was based in Gugulethu township, Cape Town, where the prevalence of HIV and the TB notification rate are both extremely high [5]. Several studies reporting the burden, diagnosis, and complications of TB in this cohort have previously been published [3],[5],[15],[16],[30],[31]. National TB programme guidelines recommend investigating symptomatic adults with suspected pulmonary TB using smear microscopy of two sputum samples; in suspected “retreatment TB” cases only, culture of one sputum sample may be requested in addition [32]. In accordance with the national ART programme guidelines, ART was provided for all patients with WHO stage 4 disease and/or blood CD4 cell counts <200 cells/µl and for pregnant women and patients with TB with CD4 cell counts <350 cells/µl. All patients gave written informed consent, and this study was approved by the human research ethics committees of the University of Cape Town and the London School of Hygiene and Tropical Medicine. This study conforms to the STARD initiative guidelines (http://www.stard-statement.org/) (Text S1) for reporting of studies of diagnostic accuracy.
Patients and Investigations
Patients eligible for the study were ART-naive, were aged ≥18 y, and did not have a current TB diagnosis. Consecutive patients referred to the clinic were prospectively recruited and investigated at their first visit. Demographic details were recorded, and a standardised symptom-screening questionnaire was completed. Data collected included the WHO symptom screen (one or more of the following symptoms: current cough, fever, night sweats, or weight loss) [14]. Two sputum samples were requested from each patient; a spot specimen was first obtained, followed by a second sample that was induced using nebulised 3% hypertonic saline. If necessary, both specimens were induced. Chest radiographs were obtained on all patients except pregnant women and were evaluated by an experienced reader certified in the use of the chest radiograph reading and recording system [16],[33]. Radiographs were scored with regard to the presence of any radiographic abnormality consistent with a diagnosis of TB. Blood CD4 cell counts and plasma viral load were measured on all patients via the routine laboratory services. For patients subsequently found to have false-positive Xpert MTB/RIF assays, all clinical records at baseline and follow-up were reviewed to determine the clinical course and ascertain any further evidence to support or refute a TB diagnosis.
Laboratory Procedures
Sputum specimens were processed using standardised protocols and quality assurance procedures by a centralised accredited laboratory that participated in the previous multi-country evaluation of the Xpert MTB/RIF assay [20]. Following decontamination with N-acetyl-L-cysteine and sodium hydroxide, centrifuged sputum deposits underwent microscopy, and following resuspension in phosphate buffer, equal volumes were tested by liquid culture and the Xpert MTB/RIF assay. The results of all tests were read by technologists blinded to the outcomes of the other assays. The length of time between sample collection and results being issued to the clinic was also recorded.
Smears stained with auramine O fluorescent stain were examined using fluorescence microscopy. Bacillary density was graded as scanty, 1+, 2+, and 3+, and all such smears were defined as “smear-positive”. Sputum pellets were also tested by trained technologists using the Xpert MTB/RIF assay as previously described [20],[22],[23]. Sample reagent (1.5 ml) was added to 0.5 ml of the resuspended sputum pellet and manually agitated twice at room temperature during a 15-min period. The inactivated material was then transferred to the test cartridge and inserted into the automated test platform, and the results were recorded.
Mycobacterial growth indicator tubes (MGITs, BD) were also inoculated and incubated for up to 6 wk. The time to automated growth detection was recorded. Culture isolates positive for acid-fast bacilli were identified as M. tuberculosis complex and assessed for genotypic resistance using the MTBDRplus assay (Hain Lifescience). Isolates also underwent phenotypic resistance testing for rifampicin and isoniazid by automated liquid MGIT culture (using the modified proportion method and standard protocols). For isolates found to have discrepant rifampicin susceptibility results using different assays, the rpoB region was sequenced using standard methods as previously described [20].
Definitions and Analyses
Patients with M. tuberculosis cultured from one or more sputum samples were defined as cases of TB. Resistance to rifampicin and isoniazid was defined by phenotypic resistance typing using MGIT cultures wherever available; the remainder were defined by MTBDRplus assay testing of the culture isolate.
The study population was characterised using simple descriptive statistics, and patients with and without TB were compared using the Wilcoxon rank-sum test, t-test, chi-square test, or Fisher's exact test as appropriate. Disease prevalence was determined, and binomial regression analysis was used to identify factors associated with TB risk. Results of MGIT culture were compared with the results of the three other laboratory tests in a per-patient analysis. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the assays with 95% confidence intervals (95% CIs) were determined using Stata software. All statistical tests were two-sided at α = 0.05.
Results
Patients and TB Diagnoses
Between 12 March 2010 and 9 February 2011, 515 of 517 consecutively invited patients agreed to participate in this study. A total of 908 samples were collected: two samples from 440 patients, one sample from 28 patients, and no sample from 47 patients (Figure 1). The vast majority of first sputum samples (89%) were obtained by spontaneous expectoration, and the remainder of first samples and all second samples were induced using hypertonic saline. From the 908 sputum samples obtained, 28 (3.1%) cultures were contaminated and were excluded (Figure 1). M. tuberculosis was cultured from a total of 137 samples, resulting in TB diagnoses in 81 patients. Of these, 67 (82.7%) were diagnosed from a first sputum sample, and an additional 14 (17.3%) cases from a second sample. Twenty five (30.9%) were sputum smear-positive cases for which the highest smear grades were scanty (n = 8), 1+ (n = 6), 2+ (n = 8), and 3+ (n = 3). The median time to positivity of MGIT cultures was 16 d (interquartile range [IQR], 11–20) overall (10 d for smear-positive disease; 18 d for smear-negative disease).
Fig. 1. Flow diagram showing the numbers of patients enrolled, losses, numbers of sputum samples analysed, and numbers of results obtained. 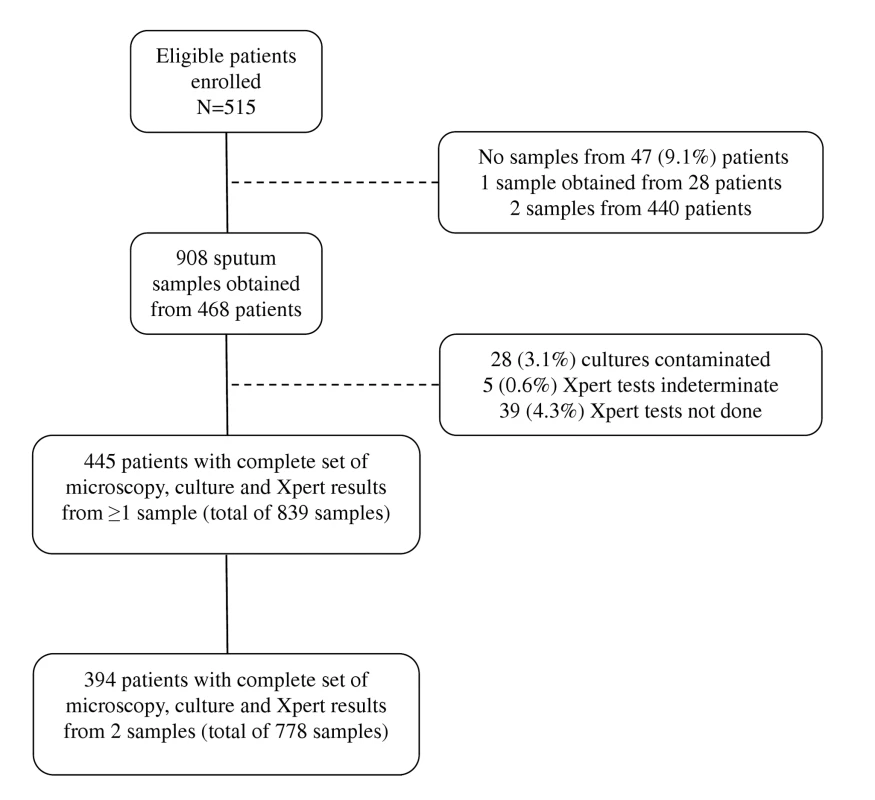
The characteristics of the patient population are shown in Table 1. Patients typically had advanced immunodeficiency (median CD4 cell count, 171 cells/µl), and 26.5% of patients had previously had TB. Compared to patients in whom no TB diagnosis was made, TB patients had a lower body mass index, lower CD4 cell counts, higher plasma viral loads, and more advanced WHO stage of disease at enrolment (Table 1). A positive WHO symptom screen was observed in 84% of TB patients (92% for smear-positive disease and 76% for smear-negative disease) compared to 67% of patients who were TB-free (p<0.01). However, TB patients were not significantly more likely to report chronic cough lasting ≥2 wk. Although radiological abnormalities were more common among patients with culture-confirmed TB, 28.9% of confirmed TB patients had a normal chest radiograph (Table 1). Conversely, 33.9% of patients who did not have TB had an abnormal radiograph.
Tab. 1. Characteristics of all patients (n = 468) for whom results of sputum cultures were available from one or more sputum samples. 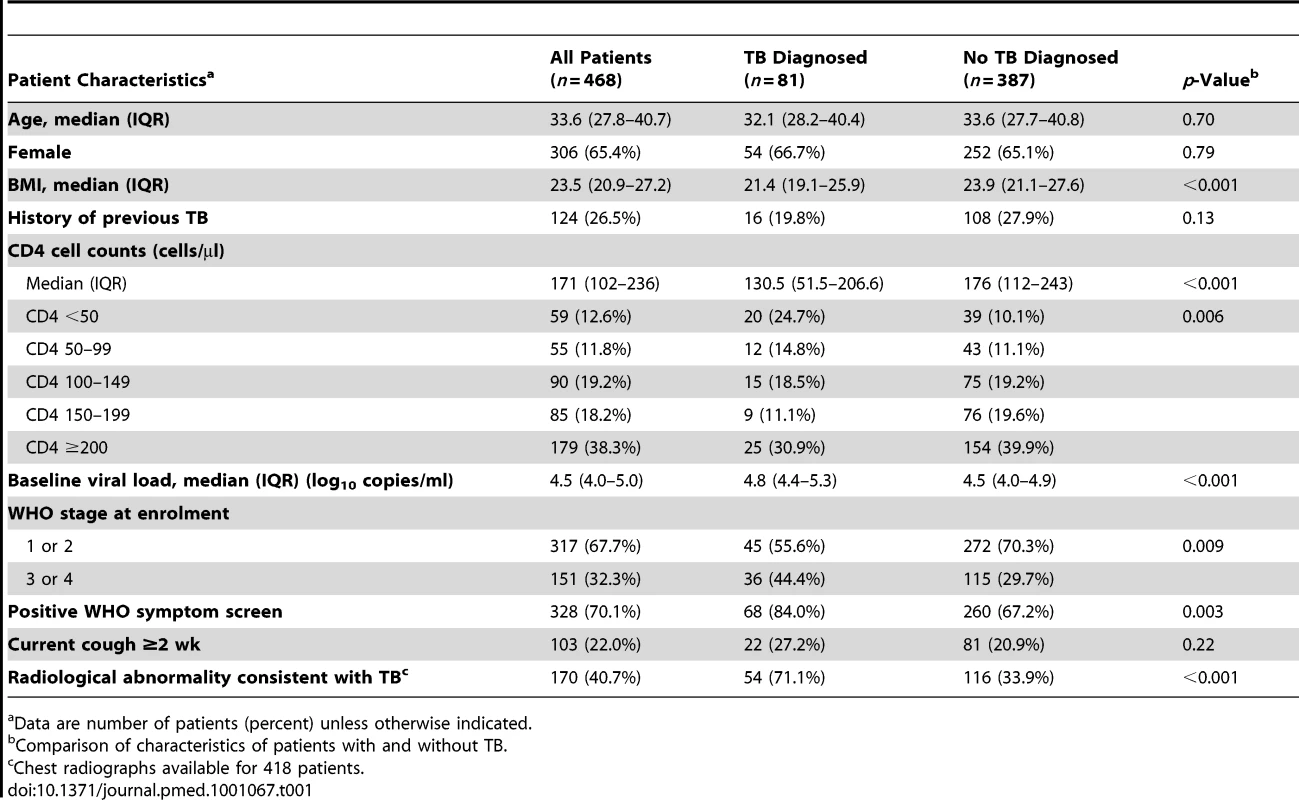
Data are number of patients (percent) unless otherwise indicated. TB Prevalence and Risk Factors
The prevalence of culture-proven TB was 17.3% (95% CI, 13.9–20.7) among those from whom sputum could be obtained. The prevalence rates of sputum smear-positive and smear-negative disease were 5.3% and 12.0%, respectively. TB prevalence was strongly associated with baseline CD4 cell count. Prevalence rates among those with CD4 cell counts of <100 cells/µl, <200 cells/µl, and >200 cells/µl were 28.1% (95% CI, 19.7–36.4), 19.4% (95% CI, 14.7–24.0), and 13.8% (95% CI, 10.2–17.5), respectively. In binomial regression analysis (Table 2), risk of TB was independently associated with low CD4 cell count, low body mass index, high viral load, not previously having received TB treatment, and having a positive WHO symptom screen. However, risk of TB was not associated with chronic cough of ≥2 wk duration.
Tab. 2. Binomial regression analysis showing crude and adjusted risk ratios for the associations between risk of sputum culture-positive tuberculosis and patient characteristics. 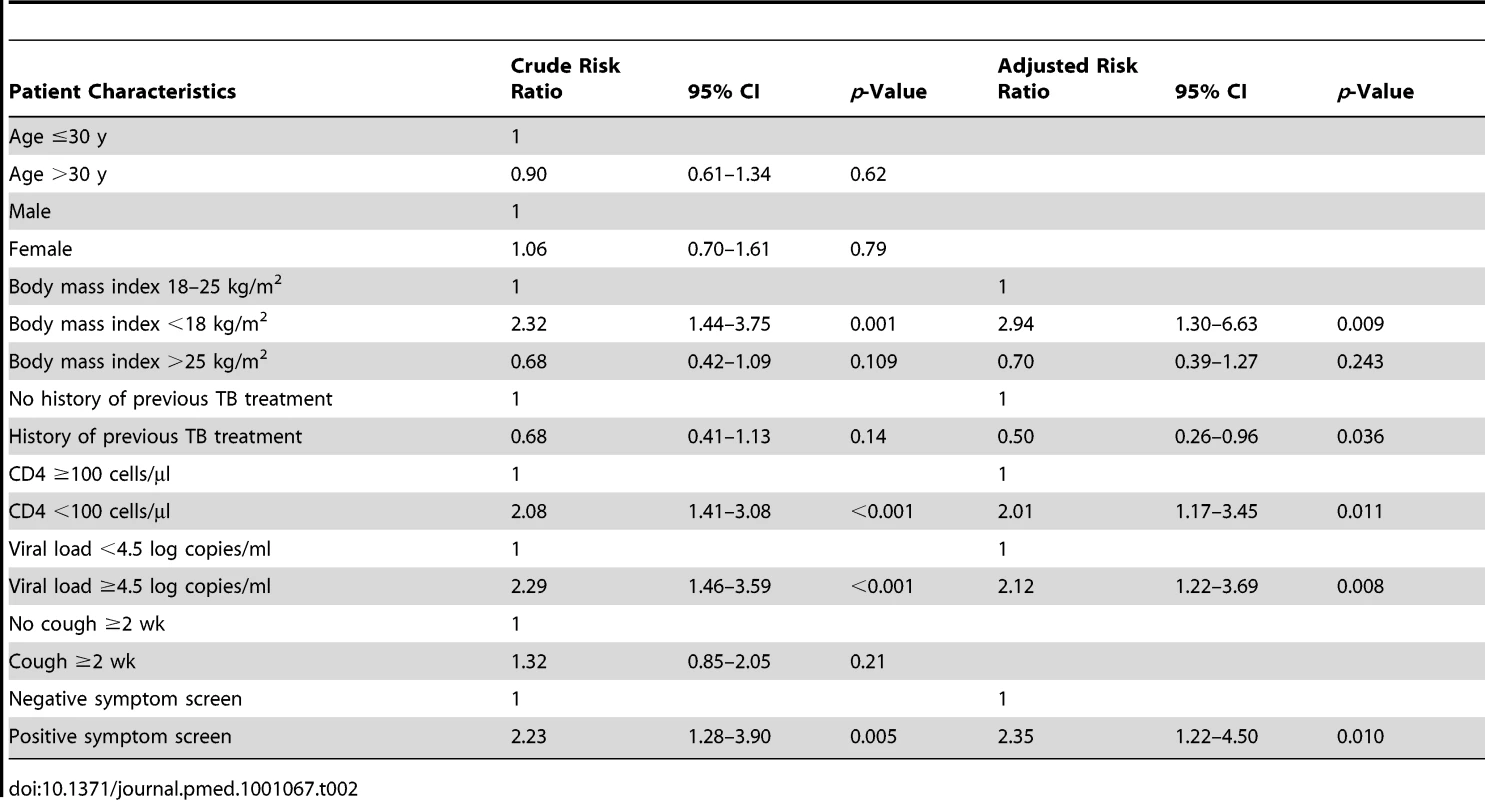
Diagnostic Accuracy of Xpert MTB/RIF for Tuberculosis
Xpert MTB/RIF assay results were obtained from 864 samples. Tests were not done on 39 samples because of a laboratory clerical error that was not in any way related to sputum culture outcomes or patient status. Xpert MTB/RIF assays also gave indeterminate results for five (0.6%) samples, which were excluded from subsequent analyses. Of these five samples, three were culture-positive for M. tuberculosis. A second sputum sample was available for two of these, and Xpert MTB/RIF assays were positive in both. Non-tuberculous mycobacteria were cultured from a total of ten (1.1%) sputum samples from eight patients, but none of these samples was associated with a positive Xpert MTB/RIF test.
In analyses to determine the diagnostic accuracy of Xpert MTB/RIF for TB diagnosis, we used data from the 839 samples from 445 patients for which results of smear microscopy, MGIT culture, and Xpert MTB/RIF assays were all complete (Figure 1). Analyses were first done for all patients (n = 445) with results from either one or two samples. Just over one-quarter of TB cases (28.0%) were diagnosed using fluorescence microscopy, with 100% specificity (Table 3). In contrast, overall, 73.3% of culture-confirmed TB cases were diagnosed using the Xpert MTB/RIF assay, increasing case detection by 45.3% (95% CI, 32.7–57.9) compared to smear microscopy. The Xpert MTB/RIF assay detected all smear-positive cases (100% sensitivity) and just under two-thirds (63%) of smear-negative cases, with high specificity (Table 3). The PPV and NPV of the Xpert MTB/RIF assay were both 94.8% (Table 3).
Tab. 3. Per-patient analysis of data showing the sensitivity and specificity of the Xpert MTB/RIF assay for tuberculosis diagnosis compared to sputum smear microscopy, using sputum liquid culture as the gold standard. 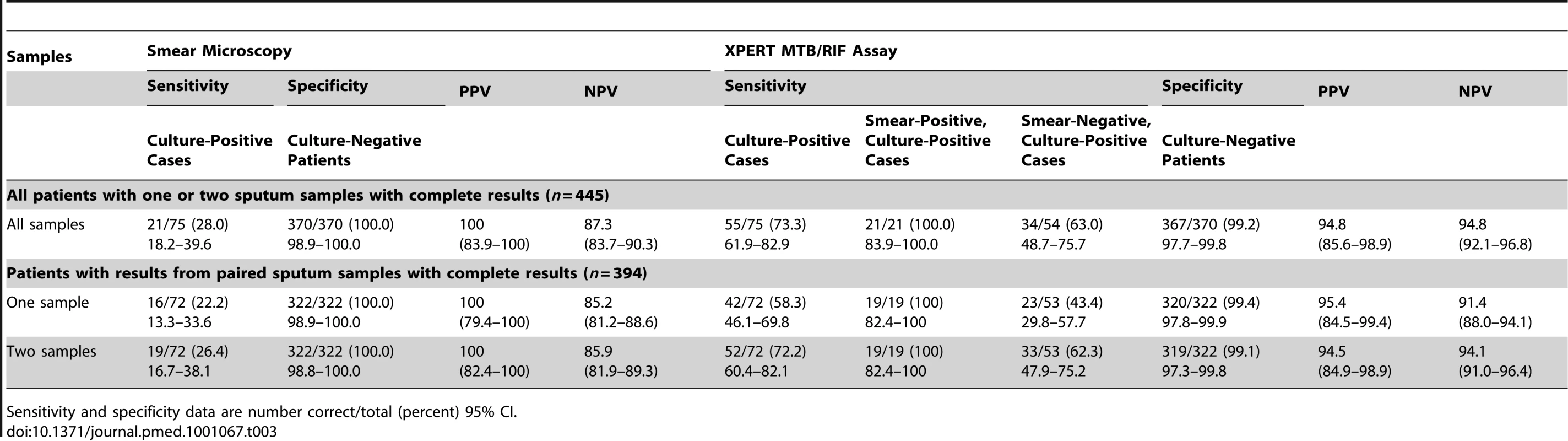
Sensitivity and specificity data are number correct/total (percent) 95% CI. A second analysis was restricted to patients with complete data from two sputum samples (778 samples from 394 patients). Analysis of this restricted set of data also showed that smear microscopy performed poorly, with one and two samples yielding just 22.2% and 26.4% of TB diagnoses, respectively, compared to 58.3% and 72.2% using the Xpert MTB/RIF assay (Table 3). The incremental yields of using Xpert on one and two sputum samples were 36.1% (95% CI, 23.6–48.6) and 45.8% (95% CI, 32.9–58.7), respectively. The Xpert MTB/RIF assay also identified all cases of smear-positive TB from a single sputum sample. Compared to the gold standard of MGIT cultures of two samples, the diagnostic yields of a single MGIT culture for all culture-positive, smear-positive, and smear-negative cases were 80.6% (95% CI, 69.5–88.9), 89.5% (95% CI, 66.9–98.7), and 77.4% (95% CI, 63.8–87.7), respectively.
The sensitivity of the Xpert MTB/RIF assay for smear-negative TB was substantially lower than for smear-positive disease and was dependent on the number of sputum samples, with sensitivities of 43.4% and 62.3% from one and two samples, respectively. In further analyses, factors associated with the sensitivity of the Xpert MTB/RIF assay for smear-negative disease were explored. Sensitivity was 100% for those with cough duration of >2 wk compared to 56.5% (95% CI, 41.6–71.4) among those with either no cough or cough of shorter duration (p = 0.018). Moreover, sensitivity was substantially greater in patients for whom the time to positivity of sputum samples was less than the median of 16 d (85.7%; 95% CI, 69.4–100) than in those with longer times to positivity (48.5%; 95% CI, 30.4–66.5) (p = 0.005). There was also a weak association between sensitivity and CD4 cell counts: sensitivity was 78.9% (95% CI, 58.8–99.1) in those with CD4 cell counts <100 cells/µl compared to 54.3% (95% CI, 36.9–71.6) in those with higher CD4 cell counts (p = 0.075). However, there was no association with radiographic abnormalities or with a positive WHO symptom screen.
There were three patients with apparent false-positive Xpert MTB-RIF assays, giving an assay specificity of over 99.0% in each of the different analyses (Table 3). Review of the study and clinical records of these patients revealed that two of these patients had overt pulmonary and systemic symptoms suggestive of TB, and both had chest radiographs revealing parenchymal consolidation and marked hilar and paratracheal lymphadenopathy highly suggestive of TB. One of these patients was reinvestigated during routine clinical follow-up and had two positive sputum smears (2+ and 3+). Both patients received standard treatment for TB and made excellent clinical responses. The third patient had symptoms and an abnormal chest radiograph but was lost to follow-up.
Use of Xpert MTB/RIF in Screening Algorithms
To further explore the utility of the Xpert MTB/RIF assay, we considered clinical populations with a TB prevalence of 20%, 15%, 10%, or 5%. With an overall sensitivity of 73.3% and specificity of 99.2% (Table 3), the PPVs at these TB prevalence rates would be 95.8%, 94.2%, 91.0%, and 82.8%, respectively, and the NPVs would be 93.7%, 95.5%, 97.1%, and 98.6%, respectively.
We next considered the utility of incorporating the Xpert MTB/RIF assay into different screening algorithms, examining the use of smear microscopy, symptom screening, one Xpert assay, two Xpert assays (Xpert done on a second sample if the first was negative), and sequential smear microscopy and Xpert testing (Xpert tests done if smear microscopy was negative). This was simulated for a hypothetical cohort of 1,000 patients with a TB prevalence of 20%, 15%, 10%, or 5% and assuming that 30% of cases were smear-positive. Symptom frequencies and the sensitivity and specificity of the Xpert assay as reported above were used.
The yield of TB cases, the number of missed cases, and the number of Xpert tests done for each correct TB diagnosis were compared between these different screening strategies and clinical populations (Table 4). Compared to a base case scenario of smear microscopy of two sputum samples in patients with a positive WHO symptom screen, the sensitivity of algorithms incorporating the Xpert MTB/RIF assay was much greater and the corresponding number of missed diagnoses was far fewer. However, at a TB prevalence of 5%, the number of Xpert tests done per case diagnosed was high (Table 4). A strategy of sequential smear microscopy and then Xpert testing of smear-negative patients yielded the same number of diagnoses, but did not substantially reduce the number of Xpert tests per case diagnosed.
Tab. 4. Utility of the Xpert MTB/RIF assay for tuberculosis diagnosis when incorporated into different screening algorithms and when used in hypothetical patient cohorts with a tuberculosis prevalences of 20%, 15% 10%, or 5%. 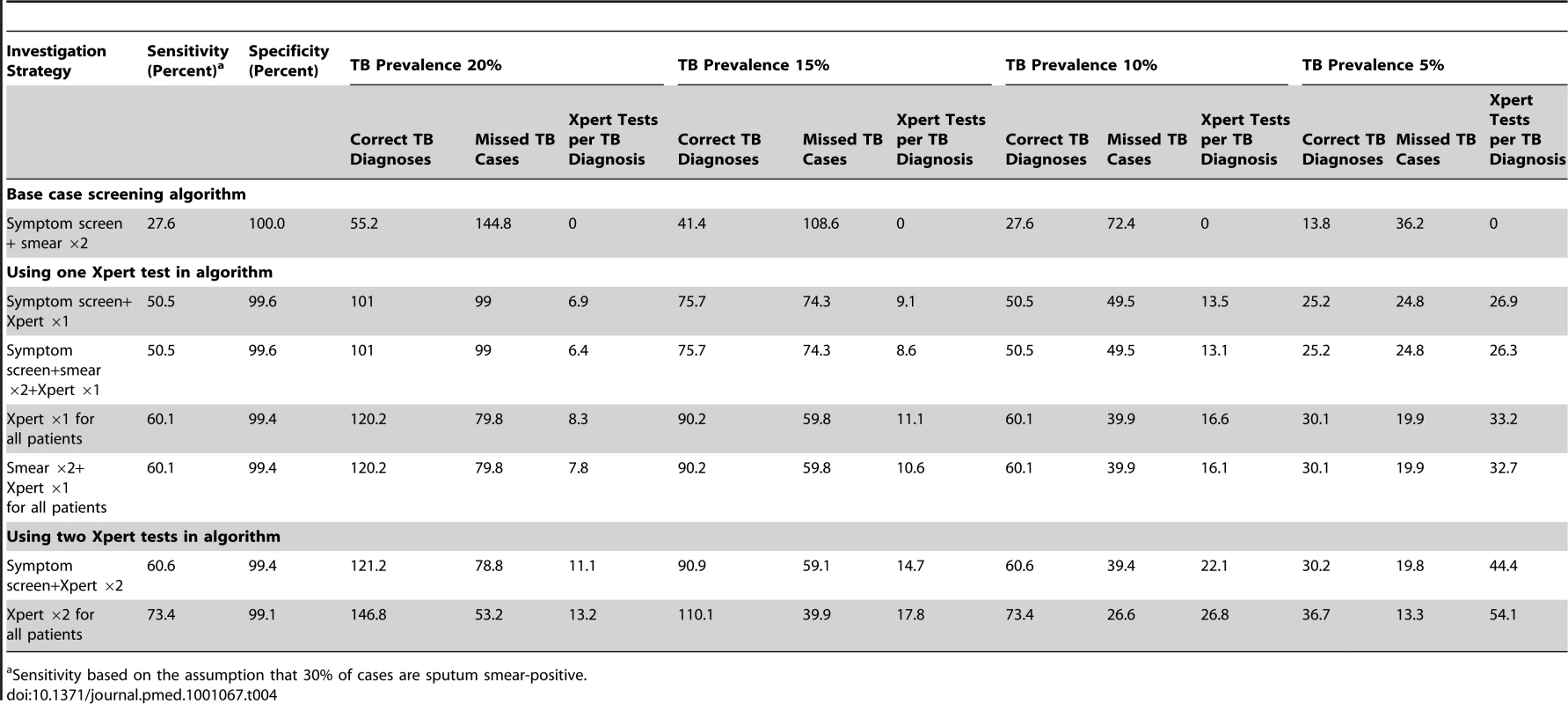
Sensitivity based on the assumption that 30% of cases are sputum smear-positive. Use of symptom pre-screening limited the sensitivity of TB detection. In populations with high TB prevalence, Xpert testing of all patients regardless of symptoms increased sensitivity without substantially increasing the number of Xpert tests done per TB case diagnosed (Table 4). Compared to the strategy of doing an Xpert assay on one sputum sample from patients with a positive symptom screen, a strategy of doing two Xpert tests on all patients was associated with 22.9% higher sensitivity for TB and the fewest missed cases. Although the latter strategy would require a large absolute number of tests, at a TB prevalence of 20%, one extra TB case would be diagnosed for every additional 6.3 tests done.
Detection of Rifampicin Resistance
Among 81 cases of TB diagnosed, four cases had isolates resistant to rifampicin because of MDR-TB (prevalence, 4.9%; 95% CI, 1.4–12.2). Among the 445 patients (839 samples) with results of culture, drug susceptibility testing, and Xpert MTB/RIF assays all available, there were 84 isolates from 55 patients (including all four cases of MDR-TB) in which rifampicin susceptibility could be compared. Rifampicin resistance was correctly identified in all four cases of MDR-TB by the Xpert MTB/RIF assay (100% sensitivity) (Table 5). However, the Xpert MTB/RIF assay also reported rifampicin resistance in three samples from three further patients in which the isolates were reported as rifampicin susceptible using comparator assays (Table 5). A paired sputum sample was available from two of these patients and rifampicin-susceptible M. tuberculosis was reported by Xpert MTB/RIF assay in both. To resolve these discrepancies, the rpoB regions of all five isolates from these three patients were sequenced. All were found to be wild-type, confirming absence of genotypic rifampicin resistance and indicating that the three Xpert MTB/RIF assay results were false positives. All remaining patients with susceptible isolates were correctly identified as such by the assay. Thus, in a per-patient analysis, the PPV of the Xpert MTB/RIF assay for detecting rifampicin resistance was 4/7 (57%) and the specificity was 48/51 (94.1%; 95% CI, 84.8–98.8).
Tab. 5. Comparison of results regarding drug susceptibility testing for rifampicin among paired samples from patients (n = 6) in whom rifampicin resistance was detected using one or more assays. 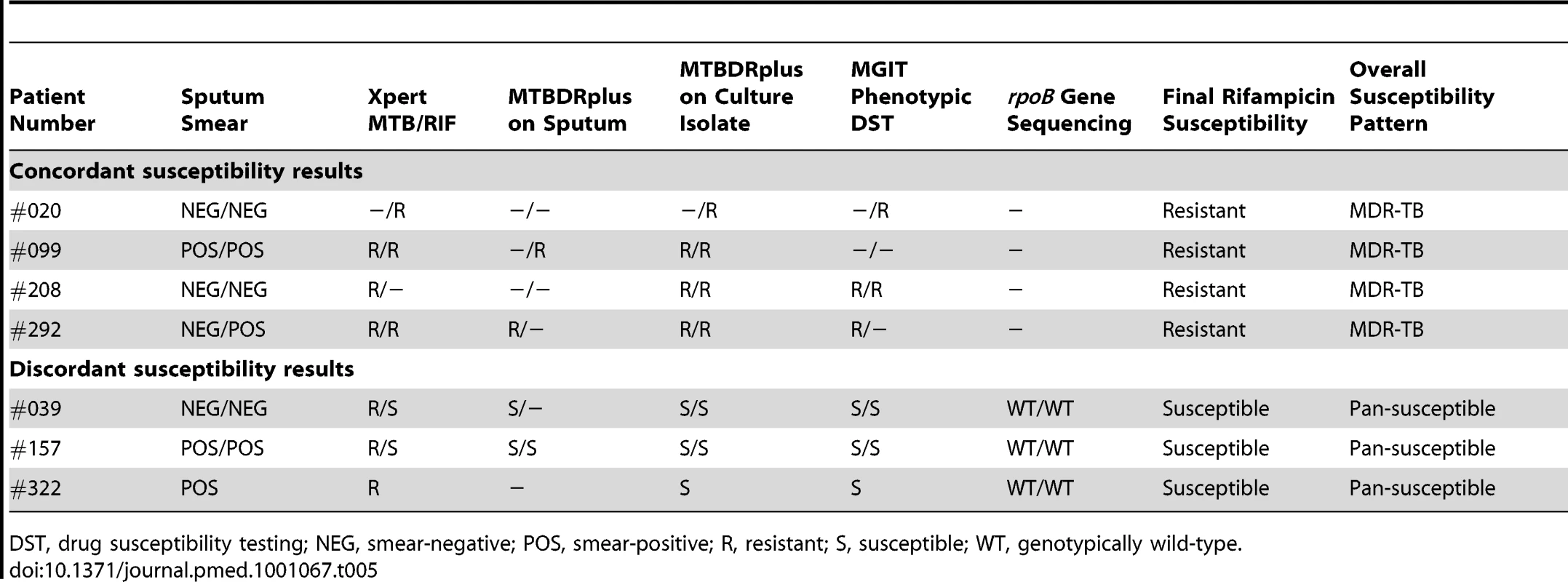
DST, drug susceptibility testing; NEG, smear-negative; POS, smear-positive; R, resistant; S, susceptible; WT, genotypically wild-type. Time to Diagnosis
The median delays between sputum collection and results being available to the clinic for smear microscopy and Xpert MTB/RIF assays and positive liquid cultures were 3 d (IQR, 2–5) and 4 d (IQR, 3–6), respectively. The median delays for culture results were 12 d (IQR, 10–14) and 20 d (IQR, 17–27) for smear-positive and smear-negative disease, respectively. Cultures were incubated for 42 d before being declared negative for M. tuberculosis, with a median time to reporting of 43 d (IQR, 43–45). For the patients with confirmed MDR-TB (n = 4), the mean time to TB diagnosis and detection of rifampicin resistance was 2 d using Xpert MTB/RIF assay, 21 d using the MTBDRplus assay on a positive culture isolate, and 40 d using phenotypic drug susceptibility testing in liquid culture.
Discussion
A high prevalence (17.3%) of culture-proven pulmonary TB was diagnosed in this patient population, but conventional diagnostic tools widely used in resource-limited settings performed poorly. Smear microscopy detected just 28% of cases, and chest radiology was of low discriminatory value. Even using automated liquid culture as the diagnostic gold standard, diagnosis was slow, with a median delay of almost 3 wk among those with smear-negative disease. In contrast, the Xpert MTB/RIF assay was able to diagnose with extremely high specificity all cases of smear-positive TB and almost two-thirds of smear-negative cases and three-quarters of cases overall when testing two samples. Only 0.6% of test results were indeterminate. The assay also rapidly detected rifampicin resistance in all four cases of confirmed MDR-TB. However, false-positive rifampicin resistance results were also observed.
The TB prevalence and associated risk factors detected in this clinical setting were similar to those previously reported from this and another ART clinic in South Africa [3],[4],[15]. Almost 30% of patients with CD4 cell counts <100 cells/µl had culture-proven TB, and rapid diagnosis is needed since such patients have high mortality risk [5],[34]. Only one-quarter of all TB patients reported a cough lasting ≥2 wk—a symptom screen widely used for many years to define suspected TB cases. Use of the new WHO symptom screening tool [13],[14] had higher sensitivity but still would have missed 13 of the 81 TB diagnoses made in this study, suggesting the need for routine microbiological screening of all patients in this setting.
We evaluated the utility of the Xpert MTB/RIF assay as a screening tool in consecutive HIV-infected adult patients enrolling for ART, excluding those who already had a TB diagnosis (approximately one-third of referrals to this cohort [35]). Since patients were screened regardless of the presence or absence of symptoms, our study is likely to have diagnosed TB cases at an earlier stage in the disease course than studies in which symptomatic patients were tested. In contrast, the previous Foundation for Innovative New Diagnostics multi-country evaluation [20] enrolled only patients with overt TB symptoms; all had a chronic cough of at least 2 wk duration and were able to produce three 1.5-ml sputum specimens. Early disease in our study would tend to be associated with lower bacillary numbers in sputum samples, as indicated by the observations that almost 70% of cases were sputum smear-negative and the prolonged median time to positivity of liquid cultures. This patient population therefore represents a major challenge for any diagnostic assay [17]. The limits of detection of the Xpert MTB/RIF assay (95% sensitivity) defined by in vitro experiments is 131 bacilli/ml of sputum, which approaches than that of liquid culture, which falls within the range 10–100 bacilli/ml [17],[23]. In contrast, smear microscopy is able to detect only samples with more than approximately 10,000 organisms per millilitre [17],[23].
Testing a single sputum sample using Xpert MTB/RIF allowed diagnosis of all smear-positive cases regardless of smear grade; these cases pose the greatest infectious hazard within the community and health care settings. As anticipated [17], the sensitivity for smear-negative disease was lower than that reported in the previous multi-country evaluation [20] (43.3% versus 72.5% using one sputum sample; 63.3% versus 85.1% using two samples). Presence of cough of ≥2 wk was associated with much higher sensitivity for smear-negative TB, as was shorter time to culture positivity. The latter observation suggests that sensitivity was likely to have been limited by very low numbers of bacilli in sputum samples.
Three patients had false-positive TB diagnoses using Xpert MTB/RIF compared to the predefined laboratory gold standard of liquid culture. However, the clinical and radiological features in these cases were highly suggestive of TB; one was confirmed as having smear-positive TB on reinvestigation, two exhibited excellent responses to TB treatment, and the third patient was lost to follow-up. These follow-up data suggest that some or all of these false-positive Xpert MTB/RIF assays may actually have been correct. The proportion of cultures lost to contamination was very low (3.1%), highlighting possible over-decontamination in the laboratory and loss of sensitivity in the culture gold standard. If this was the case, the PPV of the assay would be higher, which would increase assay utility, especially in clinical populations with lower disease prevalence. Few Xpert MTB/RIF assays were indeterminate, but the observation that three out of five of these were in culture-positive cases suggests that indeterminate results should be followed up by a repeat test.
Despite only moderate sensitivity for smear-negative disease, Xpert MTB/RIF nevertheless increased overall case detection by 36% when testing one sample and by 45% when testing two samples, compared to smear microscopy. Used for baseline screening evaluation of patients enrolling in this ART service, Xpert MTB/RIF testing of a single sputum sample would detect TB in approximately 10% of the cohort, and testing two samples would detect TB in 12.5%. Thus, the assay would detect approximately one TB case for every eight patients screened, compared to one in 18 patients screened using sputum microscopy.
We explored the potential impact of incorporating the assay in several screening algorithms applied to clinical populations with a range of TB prevalence rates. The likely benefits (increased TB yield) and assay costs (tests done per case diagnosed) were highly dependent on TB prevalence, and at a prevalence rate of 5%, the number of tests done per case diagnosed was high (4-fold higher than for a population with a prevalence of 20%). A strategy of screening with sputum microscopy and then testing smear-negative samples with Xpert MTB/RIF assay would result in minimal savings with regard to the number of Xpert tests done but would also result in failure to diagnose MDR-TB in highly infectious smear-positive cases. Symptom pre-screening restricted sensitivity and, at higher TB prevalence rates, did not substantially reduce the number of Xpert MTB/RIF tests done to identify one case of TB when compared to a strategy of testing all patients regardless of symptoms. Screening two samples with Xpert MTB/RIF would substantially increase the absolute number of tests done, but at high TB prevalence rates the high incremental yield may justify this approach. The number of Xpert MTB/RIF assays done might logically be stratified by CD4 cell count since this is a strong predictor of TB prevalence. For example, in high-burden settings such as South Africa, two tests might be done for those with CD4 cell count <200 cells/µl and just one test for those with higher counts. These strategies need to be evaluated by detailed cost-effectiveness analyses that take into account not simply the costs of testing but also the downstream impact on clinical outcomes and associated costs.
Since the Xpert MTB/RIF instrument was based in a centralised laboratory service, with results reported via the routine laboratory system, the median time to diagnosis was similar to that of smear microscopy (4 d versus 3 d, respectively). The time to diagnosis of smear-negative disease, however, was shortened by a median of 2 wk compared to culture. Time to diagnosis and treatment would potentially be further shortened by location of the instrument in the ART clinic [24]. The assay also has the potential to shorten the time to exclude a diagnosis of TB; this normally takes 6 wk or more via negative cultures and may lead to inappropriate delays in ART initiation. In view of the high NPV of the Xpert MTB/RIF assay in this cohort (94.8%), a negative result at baseline evaluation could provide a useful indication of a low probability of TB, increasing clinical confidence to start ART without undue delay. In cohorts with a lower prevalence of TB, the NPV would be higher, further increasing its utility in this regard.
HIV-associated MDR-TB carries a high mortality risk, and nosocomial outbreaks in HIV care and treatment centres pose a grave threat to patients accessing these services [9],[10],[36]. Many patients with HIV-associated MDR-TB die before a diagnosis can be made [9],[36]. In this study, the Xpert MTB/RIF assay identified four patients with rifampicin-resistant isolates who had MDR-TB, greatly reducing the mean time to detection (2 d) compared to using conventional culture-based susceptibility testing (40 d) or using line probe assays on culture isolates (20 d). By accelerating diagnosis, the Xpert MTB/RIF assay has the potential to substantially reduce the risks of nosocomial transmission of MDR-TB and improve the prognosis of affected individuals.
The Xpert MTB/RIF assay reported three false-positive rifampicin resistance results. The finding of discordant rifampicin susceptibility results from paired samples using the Xpert MTB/RIF assay suggests that specificity might be increased by requiring confirmation of resistance in more than one sample. While such false positives were not found in the initial multi-country evaluation [20], another ongoing field study sponsored by the Foundation for Innovative New Diagnostics has also detected cases, leading the manufacturer to modify the instrument software and cartridge specifications [24],[37]. With WHO approval of roll-out of this assay in December 2010, confirmation of successful reconfiguration of the test platform is urgently required.
Strengths of the study include the use of a quality-assured laboratory that participated in the previous multi-country evaluation [20]. Whereas all previously published studies have evaluated use of the assay among individuals with suspected TB [20],[24]–[29], this study evaluated the assay as a screening tool in unselected consecutive patients regardless of symptoms in a high-burden setting. The TB status of all patients was clearly defined based on a rigorous laboratory gold standard. Weaknesses include the fact that a small number of tests were not done because of a laboratory clerical error and that there were few cases of MDR-TB. While a similar burden of disease has been reported from an ART clinic elsewhere in South Africa [4], the prevalence of TB may differ in other countries, and we therefore explored utility at a range of prevalence rates. The impact of the sputum concentration procedure and of dividing the sputum pellet between three assays rather than testing unprocessed sputum was not investigated in this study, but these methods were not found to impact assay sensitivity in a previous large-scale multi-country evaluation [20]. The usefulness of the assay as a point-of-care test was not evaluated. Further studies are needed to assess the impact of Xpert MTB/RIF screening on subsequent patient outcomes, the operational feasibility of using the assay within the clinic, and cost-effectiveness.
In conclusion, when used as a routine screening test among patients with advanced immunodeficiency and high TB risk, rapid screening using the Xpert MTB/RIF assay substantially increased case detection, supporting replacement of microscopy as the initial diagnostic tool. The assay also greatly decreased the time to diagnosis of MDR-TB. Use of Xpert MTB/RIF as a screening tool might effectively reduce the risk of nosocomial MDR-TB outbreaks in HIV care and treatment settings and improve the prognosis of affected patients. However, the specificity of the assay for detecting rifampicin resistance needs to be improved to prevent overdiagnosis of rifampicin-resistant disease.
Supporting Information
Zdroje
1. LawnSDWoodR 2011 Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis In press
2. KranzerKHoubenRMGlynnJRBekkerLGWoodR 2010 Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis 10 93 102
3. LawnSDKranzerKEdwardsDJMcNallyMBekkerLG 2010 Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS 24 1323 1328
4. BassettIVWangBChettySGiddyJLosinaE 2010 Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis 51 823 829
5. LawnSDMyerLBekkerLGWoodR 2006 Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS 20 1605 1612
6. MooreDLiechtyCEkwaruPWereWMwimaG 2007 Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS 21 713 719
7. LawnSDHarriesADAnglaretXMyerLWoodR 2008 Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 22 1897 1908
8. BockNNJensenPAMillerBNardellE 2007 Tuberculosis infection control in resource-limited settings in the era of expanding HIV care and treatment. J Infect Dis 196 Suppl 1 S108 S113
9. GandhiNRMollASturmAWPawinskiRGovenderT 2006 Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368 1575 1580
10. WellsCDCegielskiJPNelsonLJLasersonKFHoltzTH 2007 HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis 196 Suppl 1 S86 S107
11. O'DonnellMRJarandJLovedayMPadayatchiNZelnickJ 2010 High incidence of hospital admissions with multidrug-resistant and extensively drug-resistant tuberculosis among South African health care workers. Ann Intern Med 153 516 522
12. ReidMJShahNS 2009 Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis 9 173 184
13. GetahunHKittikraisakWHeiligCMCorbettELAylesH 2011 Development of a standardized screening rule for tuberculosis in people livign with HIV in resource-contrained settings: individual participant data meta-analysis of observational studies. PLoS Med 8 e1000391 doi:10.1371/journal.pmed.1000391
14. World Health Organization 2010 Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings Geneva World Health Organization Available: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. Accessed 7 December 2010
15. LawnSDEdwardsDJKranzerKVogtMBekkerLG 2009 Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS 23 1875 1880
16. DawsonRMasukaPEdwardsDJBatemanEDBekkerLG 2010 Chest radiograph reading and recording system: evaluation for tuberculosis screening in patients with advanced HIV. Int J Tuberc Lung Dis 14 52 58
17. LawnSDWoodR 2011 Tuberculosis screening in patients starting antiretroviral therapy: stretching diagnostics to the limits. Clin Infect Dis 52 276 277
18. World Health Organization 2010 Multidrug and extensively drug-resistant TB (M/XDR-TB). 2010 global report on surveillance and response. WHO/HTM/TB/2010.3 Geneva World Health Organization Available: http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf. Accessed 18 August 2010
19. GandhiNRNunnPDhedaKSchaafHSZignolM 2010 Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375 1830 1843
20. BoehmeCCNabetaPHillemannDNicolMPShenaiS 2010 Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363 1005 1015
21. World Health Organization 2010 December 6 Roadmap for rolling out Xpert MTB/RIF for rapid diagnosis of TB and MDR-TB Geneva World Health Organization Available: http://www.who.int/tb/laboratory/roadmap_xpert_mtb_rif_rev23dec2010.pdf. Accessed 8 December 2010
22. BlakemoreRStoryEHelbDKopJBanadaP 2010 Evaluation of the analytical performance of the Xpert(R) MTB/RIF assay. J Clin Microbiol 48 2495 2501
23. HelbDJonesMStoryEBoehmeCWallaceE 2010 Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 48 229 237
24. BoehmeCCNicolMPNabetaPMichaelJSGotuzzoE 2011 Feasibility and impact of decentralised use of Xpert MTB/RIF for the diagnosis of tuberculosis and multi-drug resistance—results from a multi-center implementation study. Lancet 377 1495 1505
25. ArmandSVanhulsPDelcroixGCourcolRLemaitreN 2011 Comparison of the Xpert MTB/RIF test with an IS6110-TaqMan real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol 49 1772 1776
26. MarloweEMNovak-WeekleySMCumpioJSharpSEMomenyMA 2011 Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol 49 1621 1623
27. MoureRMunozLTorresMSantinMMartinR 2011 Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol 49 1137 1139
28. HillemannDRusch-GerdesSBoehmeCRichterE 2011 Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol 49 1202 1205
29. VadwaiVBoehmeCNabetaPShettyAAllandD 2011 Xpert MTB/RIF, a new pillar in the diagnosis of extrapulmonary tuberculosis? J Clin Microbiol E-pub ahead of print. doi:10.1128/JCM.02319-10
30. LawnSDMyerLEdwardsDBekkerLGWoodR 2009 Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 23 1717 1725
31. LawnSDMyerLBekkerLGWoodR 2007 Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS 21 335 341
32. South Africa Department of Health 2004 The South African National Tuberculosis Control Programme: practical guidelines Pretoria South Africa Department of Health Available: http://www.kznhealth.gov.za/chrp/documents/Guidelines/Guidelines%20National/Tuberculosis/SA%20TB%20Guidelines%202004.pdf. Accessed 15 April 2010
33. Den BoonSBatemanEDEnarsonDABorgdorffMWVerverS 2005 Development and evaluation of a new chest radiograph reading and recording system for epidemiological surveys of tuberculosis and lung disease. Int J Tuberc Lung Dis 9 1088 1096
34. LawnSDMyerLOrrellCBekkerLGWoodR 2005 Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS 19 2141 2148
35. LawnSDFraenzelAKranzerKCaldwellJBekkerLG 2011 Improving access to antiretroviral therapy for patients with HIV-associated tuberculosis in South Africa. S Afr Med J In press
36. GandhiNRShahNSAndrewsJRVellaVMollAP 2010 HIV coinfection in multidrug - and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med 181 80 86
37. NicolM 2010 Algorithms for referral to rapid diagnostics for MDR-TB: preliminary outcomes [presentation]. International Union Against Tuberculosis and Lung Disease 41st Union World Conference on Lung Health; November 2010; Berlin, Germany. Available: http://uwclh.conference2web.com/content/227. Accessed 2 February 2011
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 7- Léčba bolesti u seniorů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Flexofytol® – přírodní revoluce v boji proti osteoartróze kloubů
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
-
Všechny články tohoto čísla
- Retention in HIV Care between Testing and Treatment in Sub-Saharan Africa: A Systematic Review
- Health Care Systems and Conflict: A Fragile State of Affairs
- Simplified ART Delivery Models Are Needed for the Next Phase of Scale Up
- Individualized Cost-Effectiveness Analysis
- Is Scale-Up Worth It? Challenges in Economic Analysis of Diagnostic Tests for Tuberculosis
- Treatment Outcomes and Cost-Effectiveness of Shifting Management of Stable ART Patients to Nurses in South Africa: An Observational Cohort
- Risk Factors for Severe Outcomes following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis
- Predicting the Epidemic Sizes of Influenza A/H1N1, A/H3N2, and B: A Statistical Method
- GeneXpert—A Game-Changer for Tuberculosis Control?
- LED Fluorescence Microscopy for the Diagnosis of Pulmonary Tuberculosis: A Multi-Country Cross-Sectional Evaluation
- Configuring Balanced Scorecards for Measuring Health System Performance: Evidence from 5 Years' Evaluation in Afghanistan
- A Multi-Country Non-Inferiority Cluster Randomized Trial of Frontloaded Smear Microscopy for the Diagnosis of Pulmonary Tuberculosis
- Comparison of Xpert MTB/RIF with Other Nucleic Acid Technologies for Diagnosing Pulmonary Tuberculosis in a High HIV Prevalence Setting: A Prospective Study
- Global Pharmacovigilance for Antiretroviral Drugs: Overcoming Contrasting Priorities
- Evidence-Based African First Aid Guidelines and Training Materials
- Screening for HIV-Associated Tuberculosis and Rifampicin Resistance before Antiretroviral Therapy Using the Xpert MTB/RIF Assay: A Prospective Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Individualized Cost-Effectiveness Analysis
- GeneXpert—A Game-Changer for Tuberculosis Control?
- Screening for HIV-Associated Tuberculosis and Rifampicin Resistance before Antiretroviral Therapy Using the Xpert MTB/RIF Assay: A Prospective Study
- Treatment Outcomes and Cost-Effectiveness of Shifting Management of Stable ART Patients to Nurses in South Africa: An Observational Cohort
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



