-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaRisk Factors for Severe Outcomes following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis
Background:
Since the start of the 2009 influenza A pandemic (H1N1pdm), the World Health Organization and its member states have gathered information to characterize the clinical severity of H1N1pdm infection and to assist policy makers to determine risk groups for targeted control measures.Methods and Findings:
Data were collected on approximately 70,000 laboratory-confirmed hospitalized H1N1pdm patients, 9,700 patients admitted to intensive care units (ICUs), and 2,500 deaths reported between 1 April 2009 and 1 January 2010 from 19 countries or administrative regions—Argentina, Australia, Canada, Chile, China, France, Germany, Hong Kong SAR, Japan, Madagascar, Mexico, the Netherlands, New Zealand, Singapore, South Africa, Spain, Thailand, the United States, and the United Kingdom—to characterize and compare the distribution of risk factors among H1N1pdm patients at three levels of severity: hospitalizations, ICU admissions, and deaths. The median age of patients increased with severity of disease. The highest per capita risk of hospitalization was among patients <5 y and 5–14 y (relative risk [RR] = 3.3 and 3.2, respectively, compared to the general population), whereas the highest risk of death per capita was in the age groups 50–64 y and ≥65 y (RR = 1.5 and 1.6, respectively, compared to the general population). Similarly, the ratio of H1N1pdm deaths to hospitalizations increased with age and was the highest in the ≥65-y-old age group, indicating that while infection rates have been observed to be very low in the oldest age group, risk of death in those over the age of 64 y who became infected was higher than in younger groups. The proportion of H1N1pdm patients with one or more reported chronic conditions increased with severity (median = 31.1%, 52.3%, and 61.8% of hospitalized, ICU-admitted, and fatal H1N1pdm cases, respectively). With the exception of the risk factors asthma, pregnancy, and obesity, the proportion of patients with each risk factor increased with severity level. For all levels of severity, pregnant women in their third trimester consistently accounted for the majority of the total of pregnant women. Our findings suggest that morbid obesity might be a risk factor for ICU admission and fatal outcome (RR = 36.3).Conclusions:
Our results demonstrate that risk factors for severe H1N1pdm infection are similar to those for seasonal influenza, with some notable differences, such as younger age groups and obesity, and reinforce the need to identify and protect groups at highest risk of severe outcomes.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(7): e32767. doi:10.1371/journal.pmed.1001053
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001053Summary
Background:
Since the start of the 2009 influenza A pandemic (H1N1pdm), the World Health Organization and its member states have gathered information to characterize the clinical severity of H1N1pdm infection and to assist policy makers to determine risk groups for targeted control measures.Methods and Findings:
Data were collected on approximately 70,000 laboratory-confirmed hospitalized H1N1pdm patients, 9,700 patients admitted to intensive care units (ICUs), and 2,500 deaths reported between 1 April 2009 and 1 January 2010 from 19 countries or administrative regions—Argentina, Australia, Canada, Chile, China, France, Germany, Hong Kong SAR, Japan, Madagascar, Mexico, the Netherlands, New Zealand, Singapore, South Africa, Spain, Thailand, the United States, and the United Kingdom—to characterize and compare the distribution of risk factors among H1N1pdm patients at three levels of severity: hospitalizations, ICU admissions, and deaths. The median age of patients increased with severity of disease. The highest per capita risk of hospitalization was among patients <5 y and 5–14 y (relative risk [RR] = 3.3 and 3.2, respectively, compared to the general population), whereas the highest risk of death per capita was in the age groups 50–64 y and ≥65 y (RR = 1.5 and 1.6, respectively, compared to the general population). Similarly, the ratio of H1N1pdm deaths to hospitalizations increased with age and was the highest in the ≥65-y-old age group, indicating that while infection rates have been observed to be very low in the oldest age group, risk of death in those over the age of 64 y who became infected was higher than in younger groups. The proportion of H1N1pdm patients with one or more reported chronic conditions increased with severity (median = 31.1%, 52.3%, and 61.8% of hospitalized, ICU-admitted, and fatal H1N1pdm cases, respectively). With the exception of the risk factors asthma, pregnancy, and obesity, the proportion of patients with each risk factor increased with severity level. For all levels of severity, pregnant women in their third trimester consistently accounted for the majority of the total of pregnant women. Our findings suggest that morbid obesity might be a risk factor for ICU admission and fatal outcome (RR = 36.3).Conclusions:
Our results demonstrate that risk factors for severe H1N1pdm infection are similar to those for seasonal influenza, with some notable differences, such as younger age groups and obesity, and reinforce the need to identify and protect groups at highest risk of severe outcomes.
: Please see later in the article for the Editors' SummaryIntroduction
In late April 2009, a novel strain of influenza A H1N1 was identified in Mexico and the United States. This virus quickly spread globally, and on June 11, 2009, the World Health Organization (WHO) declared a pandemic alert phase 6, indicating that the first influenza pandemic of the 21st century had begun [1]–[3]. Many Northern hemisphere temperate countries experienced their first wave of infection during the spring and summer months of 2009, followed by an early 2009 fall influenza season. Southern hemisphere temperate countries experienced the first wave of infection during their winter of 2009, and at the time of writing are finishing their winter 2010 season. By the end of 2009, the peak of the local influenza epidemic had passed in most countries around the world [4].
Since the start of the pandemic, WHO and member states have been gathering information to characterize the clinical picture and patterns of risk associated with the 2009 pandemic influenza A H1N1 (H1N1pdm) virus infection to assist public health policy makers in targeting of vaccination strategies, antiviral use, and other control measures. Risk factors for severe disease following seasonal influenza infection have been well documented in many countries, and include chronic medical conditions such as pulmonary, cardiovascular, renal, hepatic, neuromuscular, hematologic, and metabolic disorders, some cognitive conditions, and immunodeficiency [5]–[7]. The risk associated with seasonal influenza during pregnancy is less well documented but in previous pandemics, pregnant women were identified as being at increased risk of adverse outcomes, and many countries include healthy pregnant women among the seasonal influenza high risk groups as well [8]–[11]. However, early in the 2009 H1N1 pandemic, risk factors for severe disease following infection were largely unknown. Following a series of teleconferences organized by WHO with clinicians treating H1N1pdm patients around the world, it appeared that the most common risk factors for severe H1N1pdm disease were similar to those for seasonal influenza infection; however, several new factors (e.g., obesity and tuberculosis [TB]) were also observed with high frequency in some countries. It was also noted that members of indigenous/aboriginal communities in some countries appeared to be overrepresented among severe cases [12].
While many countries have recently reported data on the association between severe H1N1pdm influenza and the presence of a variety of underlying risk factors (e.g., [13]–[26]), these data are presented in different formats, making direct comparisons across countries difficult, and no clear consensus has emerged for some conditions. This paper presents data from approximately 70,000 lab-confirmed hospitalized and 2,500 fatal cases of H1N1pdm infection in 19 countries or administrative regions—Argentina, Australia, Canada, Chile, China, France, Germany, Hong Kong SAR, Japan, Madagascar, Mexico, the Netherlands, New Zealand, Singapore, South Africa, Spain, Thailand, the United States, and the United Kingdom—in order to characterize and compare the distribution of underlying risk factors among H1N1pdm confirmed patients who were hospitalized, admitted to an intensive care unit (ICU), or died, and to assess the frequency and distribution of known and new potential risk factors for severe H1N1pdm infection.
Methods
This study compares data primarily obtained from surveillance programs of the Ministries of Health or National Public Health Institutes of 19 countries or administrative regions covering the period 1 April 2009 to 1 January 2010. Countries were asked to provide risk factor data on laboratory-confirmed cases using a standardized format for this analysis. The data were collected in the course of routine surveillance, methods of which varied from country to country [27]–[49], and were reported anonymously and as aggregate data; hence, no ethics approval was required. It should be noted that considerable effort was put into negotiating permission for these data to be presented in these formats. As many countries would not be willing to have their country-specific data published in direct comparisons with others, we are taking the approach of publishing data from a wide range of countries and showing the variability observed, so that results from specific studies can be compared with the international results reported here.
Potential risk factors were grouped into four categories: age, chronic medical illnesses, pregnancy (by trimester), and “other,” which included conditions that were not previously considered as risk factors for severe influenza outcomes, such as obesity, membership in a vulnerable social or ethnic group, and TB. Details of the standardized format and definitions of each of the conditions are provided in Text S1.
Risk factor information was collected separately for three levels of severity of illness in laboratory-confirmed patients: hospitalizations, admissions to ICU, and fatalities by country. Details of the available data from countries by risk factor and severity level are provided in . For each risk factor, except for pregnancy, the percentage of patients who were hospitalized, were admitted to ICU, and died was calculated using the total number of cases reported in each severity category. To evaluate the risk associated with pregnancy, the ratio of pregnant women to all women of childbearing age (age 15–49 y) in each level of severity was used to describe the differences between levels. The overall median and interquartile ranges (IQRs) were calculated for each risk factor using all available data. In addition, where available, countries provided baseline comparison data for prevalence of the risk factor in the general population (details and sources provided in Text S1). Data on age were provided by age groups (<5, 5–14, 15–24, 25–49, 50–64, and ≥65 y).
Risk of Severe Disease
Where data were available, we calculated the risk for severe H1N1pdm outcomes (hospitalization, admission to ICU, and death) compared to the prevalence of risk factors in the general population (relative risk [RR] of hospitalization [RRhosp], RR of ICU admission [RRICU], and RR of death [RRdeath]) by country. See Text S1 for more information and formulae.
For pregnancy, we first calculated the proportion of women of childbearing age who were pregnant in each severity category by dividing the number of pregnant women in that category by the number of women of childbearing age in that category. As individual case data were not available, we calculated the number of fertile women in each level of severity using the numbers of patients in each level of severity in the age range between 15 and 49 y multiplied by the percentage for that severity level that was female. Unless provided by the country, the point prevalence of pregnant women in the general population (the denominator of the RR calculation) was calculated using crude birth rate and 2010 United Nations population estimates [50] to derive the annual number of pregnancies, multiplied by 40/52 and without adjusting for seasonality of pregnancies, abortions, miscarriages, early deliveries, or multiple births. We also calculated the country-specific odds ratios (ORs) and 95% confidence intervals (CIs) for death given hospitalization separately for each risk factor (i.e., the odds of death given hospitalization and a specific risk factor), thereby comparing the odds of death in one group (e.g., among hospitalized patients with asthma) with the odds of death in all other hospitalized patients combined (e.g., among hospitalized patients without asthma) (individual country ORs not shown). We then used the I2 statistic to quantify the percentage of variation across countries that is due to true underlying heterogeneity in the ORs rather than chance variability [51]. The I2 statistics for all examined risk factors indicated that there was substantial true underlying variation between ORs from different countries. We undertook meta-analyses with and without random effects in parallel to describe the distribution of the OR estimates across the countries for which data were available for analysis. As expected, given the heterogeneity observed between countries, the random effects meta-analysis yielded wider CIs. We conservatively report the pooled estimates from the random effects meta-analysis to describe the distribution of the OR estimates across the countries for which data were available for analysis. Underlying the random effects approach is the assumption that, although the individual countries give rise to different OR estimates, these estimates arise from a distribution with a central value, the estimate of which is referred to as the “pooled OR,” and normally distributed variability around this value. However, because of the limited number of countries in each analysis, the number of random effects is too small for diagnostics such as quantile–quantile plots to demonstrate whether the assumption of a normal distribution is valid.
Finally, with a meta-analysis of data from such diverse countries as those included in this study, reasons for heterogeneity were sought through exploratory meta-regression analyses. However, because of the limited number of countries included in the meta-regression models, and with country being the unit of analysis, the meta-regression results were not considered to be robust and so are not presented or discussed further [52].
All meta-analyses and meta-regression techniques were performed using Stata version 10 (StataCorp).
Results
Data were collected on approximately 70,000 patients requiring hospitalization, 9,700 patients admitted to ICU, and 2,500 fatalities from 19 countries and administrative regions across the Americas, Asia, Europe, and Africa.
Age and Gender
Approximately half of all patients included in this analysis in each level of severity were female (49.8%, 47.0%, and 44.7% of all hospitalized, ICU-admitted, and fatal H1N1pdm cases, respectively). This proportion did not vary significantly by country (Table 1). Age was associated with increased risk of poor outcome, as indicated by several different parameters. The median age of patients increased with increasing levels of severity (Table 1). Among hospitalized patients, the median age within each country ranged from 7 y in Japan to 38 y in Spain, with a median reported value among all countries that provided data (n = 14) of 19 y (IQR 14.8–27.5); among patients admitted to ICU, the median age within each country ranged from 28 y in China to 49.5 y in Hong Kong SAR, with a median value among all countries that provided data (n = 9) of 42 y (IQR 35.0–45.0); and among fatal cases, median age within each country ranged from 30 y in China to 56 y in Hong Kong SAR, with a median value among all countries that provided data (n = 13) of 46 y (IQR 37.0–42.0). When the age distribution of the proportion of patients in each level of severity was compared to the distribution in the general population, the RR was highest in the age groups <5 y and 5–14 y (RRhosp = 3.3 and 3.2, respectively) but the RR of death was highest in the age groups 50–64 y and ≥65 y (RRdeath = 1.6 and 1.7, respectively) (Figure 1). The ratio of H1N1pdm deaths to hospitalizations increased with age and was the highest in the ≥65-y-old age group in all countries for which data were available (Figure 2).
Fig. 1. Relative risk of hospitalization, ICU admission, and death by age group compared to the general population. 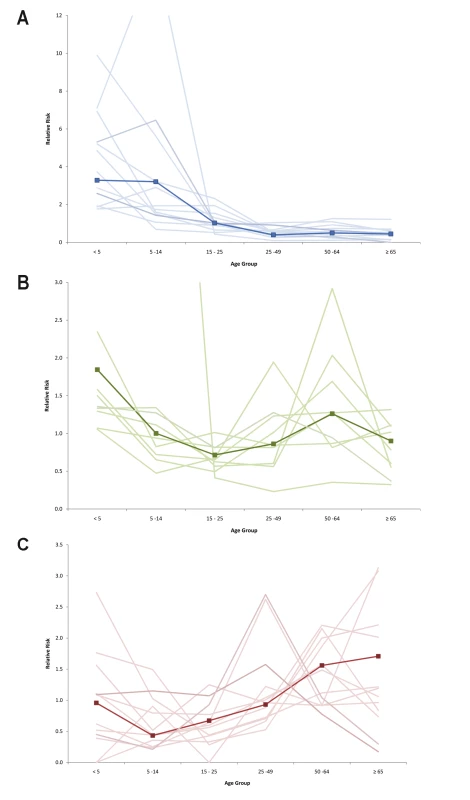
Countries included in hospitalization (A) and mortality (C) RRs: Japan, Hong Kong SAR, China, Singapore, Thailand, Chile, Germany, the Netherlands, Spain, New Zealand, Canada, US, Madagascar (hospitalizations only), and France (deaths only). Countries included in ICU admission RR (B): Japan, Hong Kong SAR, China, Singapore, Canada, Spain, the Netherlands, US, New Zealand, and South Africa. Dark line represents pooled RR; shaded lines are individual country RR. Fig. 2. Ratio of confirmed H1N1pdm deaths to hospitalizations for selected countries. 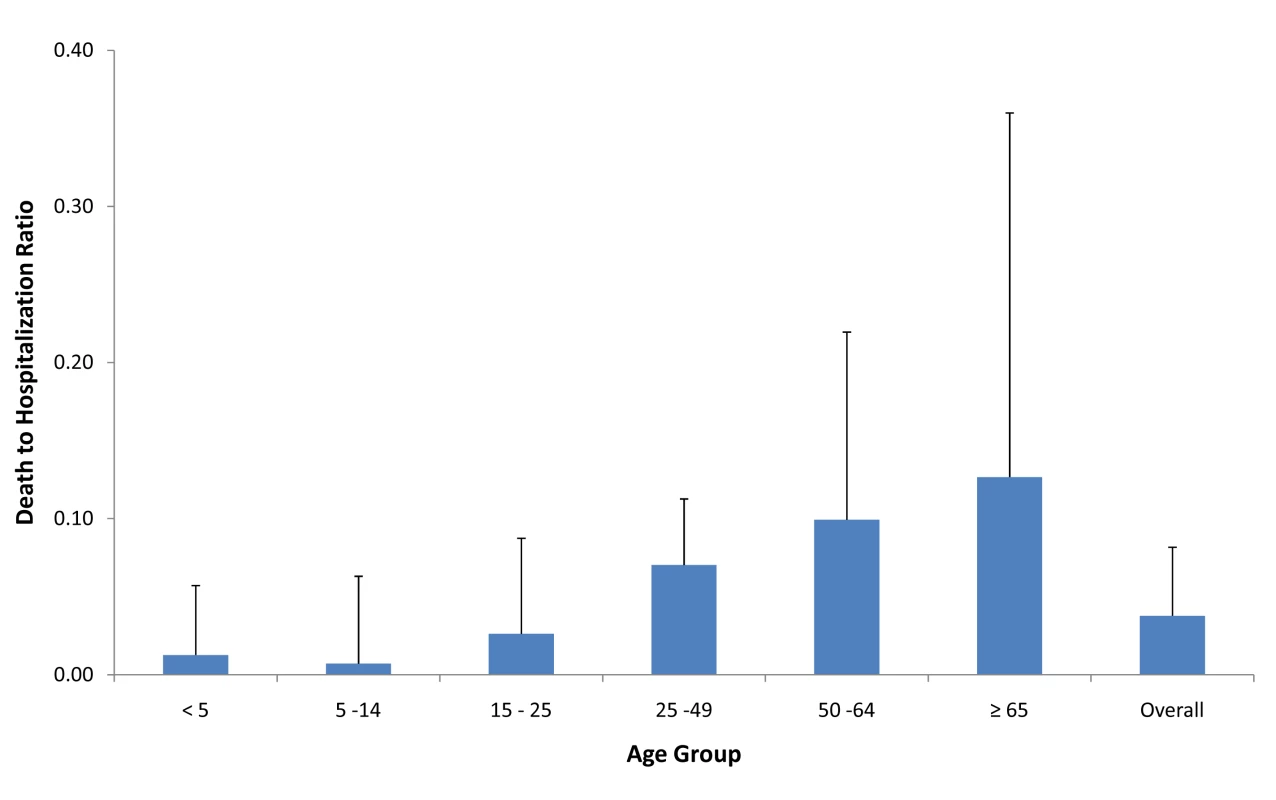
Countries included in figure: Spain, Singapore, China, Hong Kong SAR, Canada, the Netherlands, Thailand, Chile, Germany, Japan, US, and New Zealand. Bars represent maximum country ratio. Tab. 1. Risk factors by severity level for select countries and risk of severe disease. 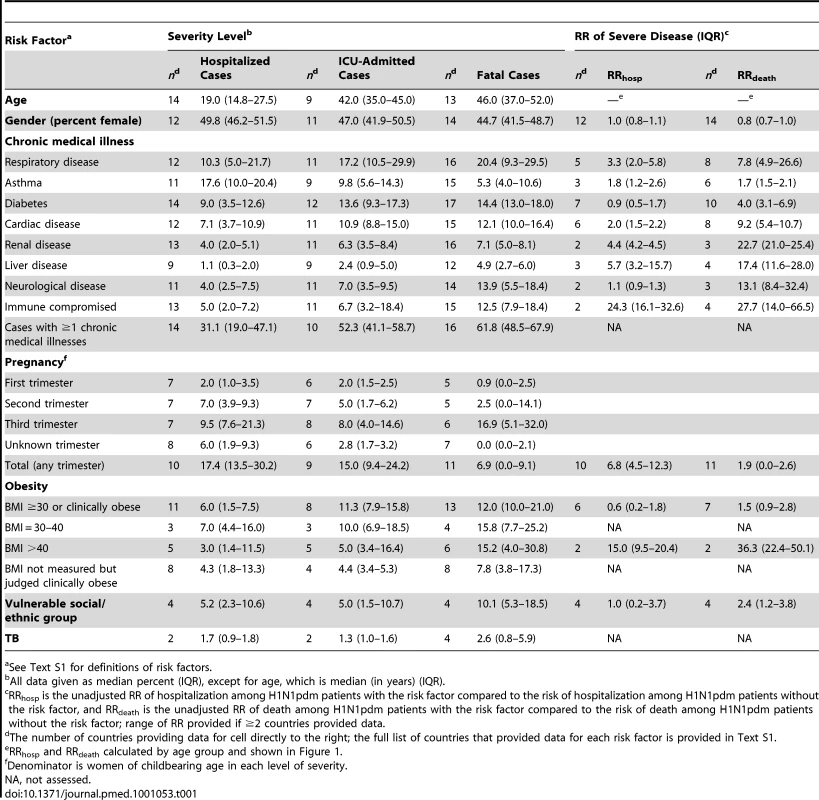
See Text S1 for definitions of risk factors. Chronic Illness
The proportion of H1N1pdm patients with at least one chronic medical condition generally increased with severity (median among all countries that provided data was 31.1% [n = 14], 52.3% [n = 10], and 61.8% [n = 16] of hospitalized, ICU-admitted, and fatal H1N1pdm cases, respectively (Table 1). This pattern was observed for most countries (individual country data not shown). For nearly every individual risk factor under study, the prevalence increased significantly with severity level. Chronic respiratory conditions excluding asthma (median = 10.3%, 17.2%, and 20.4%, respectively) and asthma (median = 17.6%, 9.8%, and 5.3%, respectively) were the risk factors most often reported among severe cases, followed closely by diabetes (median = 9.0%, 13.6%, and 14.4%, respectively) and chronic cardiac conditions (median = 7.1%, 10.9%, and 12.1%, respectively). The pooled OR for death given hospitalization was significantly above one for each risk factor listed, with the exception of asthma, and was highest for chronic liver disease and immunocompromised patients (Figure 3).
Fig. 3. Pooled odds ratio and 95% CIs of risk of death given hospitalization for selected countries. 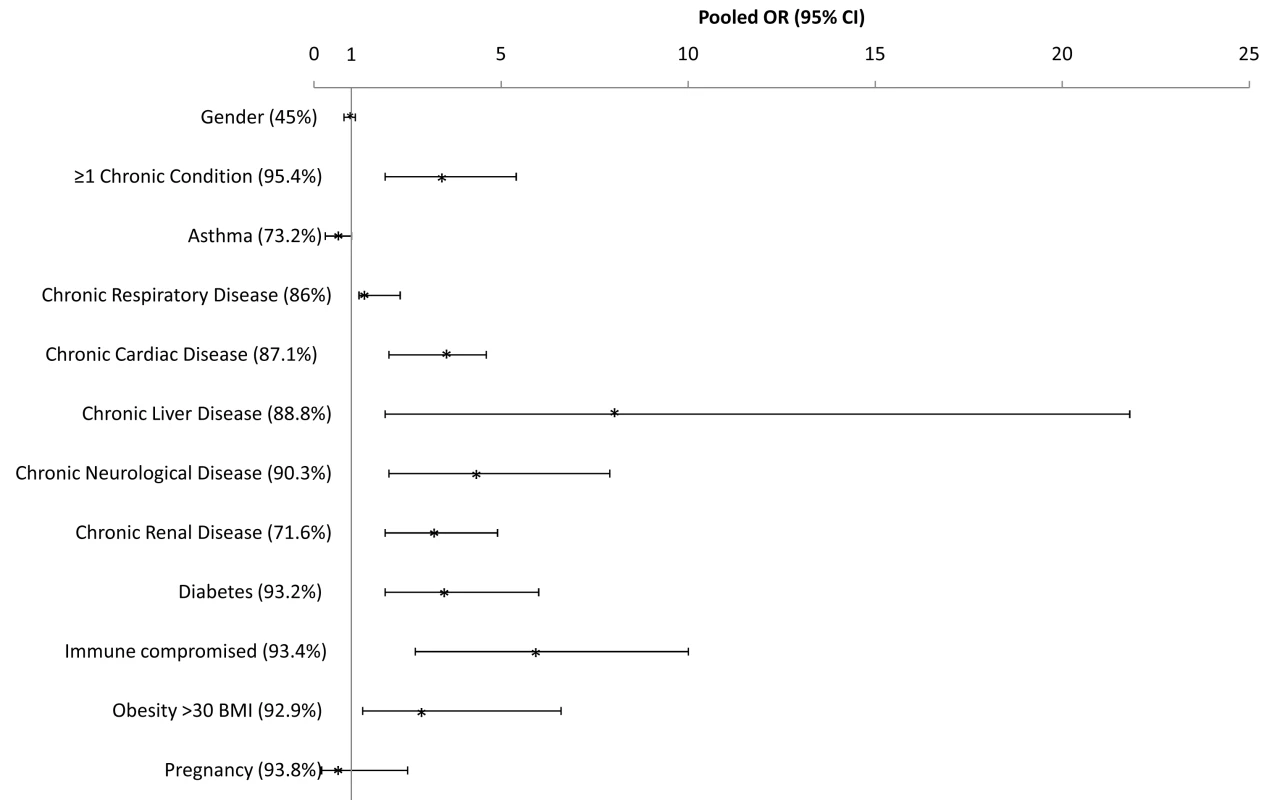
See Text S1 for countries included in the pooled risk factor ORs. The risk of severe disease due to H1N1 infection, including hospitalization and death, was elevated for every chronic condition for which data were available (Table 1). Notably, the RR for fatal disease due to H1N1pdm infection was elevated for asthma (median RRdeath = 1.7 [IQR 1.5–2.1]) and not markedly different from the RR associated with hospitalizations (median RRhosp = 1.8 [IQR 1.2–2.6]). Data on chronic illness rates in the general population were not available from enough countries to permit an assessment of the relative magnitude of risk associated with various conditions with certainty.
Pregnancy
The proportions of women of childbearing age who were hospitalized with H1N1 and were pregnant as part of all hospitalizations (median of all country data = 17.4% [IQR 13.5–30.2]), who were admitted to ICU (median of all country data = 15.0% [IQR 9.4–24.2]), and who died (median of all country data = 6.9% [0.0–9.1]) varied within each country. Pregnant women in their third trimester consistently accounted for more than half of all pregnant women among hospitalized, ICU-admitted, and fatal cases. However, with the exception of China, Thailand, and the US, the proportion of pregnant women decreased with increasing level of severity, and the pooled OR for death given hospitalization during pregnancy was below 1 (pooled OR = 0.6, 95% CI 0.2–2.5).
Pregnant women with H1N1pdm infection were at higher risk of hospitalization than women of childbearing age in the general population without H1N1pdm infection, with an unadjusted RR of hospitalization ranging from 3.5 in Germany to 25.3 in France (median RRhosp = 6.8, n = 10 countries). The unadjusted RR of death, while elevated compared to non-pregnant women in more than half of countries, was generally lower than that for hospitalization, with a median RRdeath of 1.9 (n = 11 countries). Four areas (Japan, the Netherlands, Hong Kong SAR, and Singapore) had a RRdeath of zero.
Other Risk Factors
The proportion of patients with obesity (body mass index [BMI] ≥30 or clinically judged as obese) increased with increasing disease severity and represented a median of 6%, 11.3%, and 12.0% of all hospitalized, ICU-admitted, and fatal H1N1pdm cases, respectively, and this pattern was also observed for morbid obesity (BMI >40), with 3.0%, 5.0%, and 15.2%, respectively (Table 1). However, this pattern was not consistently reported in each country. For example, France, Thailand, and China observed similar proportions of obese patients among ICU-admitted and fatal cases, while Hong Kong SAR reported a lower prevalence of obesity among fatal cases than among ICU admissions. Using data from all countries, the pooled OR for death given hospitalization for obesity (BMI ≥30 or clinically judged as obese) was 2.9 (95% CI 1.3–6.6; Figure 3). Compared to the general population in the two countries for which data were available, the risk of death associated with morbid obesity was increased (mean RRdeath = 36.3 [IQR 22.4–50.1], n = 2).
Canada, Australia, and New Zealand reported significant disparities in the burden of severe H1N1pdm disease across different ethnic groups. In these three countries, indigenous population groups were overrepresented among severe H1N1pdm cases requiring hospitalization and among fatal cases. In contrast, in Thailand and Mexico, minority groups were underrepresented among severe H1N1pdm cases. Taken together, the unadjusted median RR of hospitalization for H1N1pdm patients among minority groups was 1.0 (IQR 0.2–3.7) and the median RR of death was 2.4 (IQR 1.2–3.8). TB data were reported from three countries, and the incidence increased slightly with level of severity. The disease was reported in a median of 1.7%, 1.3%, and 2.6% of hospitalized, ICU-admitted, and fatal H1N1pdm cases, respectively. We were not specifically able to evaluate HIV incidence because of a paucity of data on HIV in H1N1pdm patients.
Discussion
Our analysis represents to our knowledge the first comprehensive assessment of the frequency and distribution of risk factors for severe H1N1pdm infection from a global perspective, with data from approximately 70,000 patients requiring hospitalization, 9,700 patients admitted to ICU, and 2,500 fatalities from 19 countries and administrative regions around the world. Consistent with other published data, our results reaffirm that the age distribution of severe H1N1pdm cases significantly differs from that of seasonal influenza [53]–[56]. The highest rates of hospitalization per capita were in children <15 y, but the highest rates of mortality per capita were in persons over 64 y. The low apparent attack rate in the oldest age group, evidenced by low rates of hospitalization, and the high odds associated with age in the fatal group compared to hospitalized cases seems to indicate that although older adults may have a lower risk of infection, they have a significantly higher risk of death if they are infected [54],[57]–[61]. It is likely that increasing prevalence of chronic risk conditions in the oldest age group contributes to this effect, but our data do not allow for quantification of this association.
Our results demonstrate that in a significant portion of severe and fatal cases, patients had preexisting chronic illness, and that the presence of chronic illness increased the likelihood of death. It was notable, however, that approximately 2/3 of hospitalized cases and 40% of fatal cases did not have any identified preexisting chronic illness. It is unknown how many of these cases had other risk factors, such as pregnancy, obesity, and substance abuse (including smoking and alcohol), for which we had insufficient information in this study. These figures are also dependent on the completeness of available data for recorded risk factors. As with seasonal influenza, the most common underlying chronic conditions among hospitalized patients were respiratory disease, asthma, cardiac disease, and diabetes. Interestingly, we found that although asthma was frequently associated with both hospitalization and death in most countries, with an increased RR for both, the OR for death given hospitalization suggested that a higher proportion of hospitalized cases survived compared to patients with other conditions. This may represent the occurrence of manageable influenza-induced exacerbations of asthma prompting admission that do not progress to viral pneumonia or other fatal complications, and may also reflect the fact that asthma tends to occur in younger age groups [62].
Early data suggested that pregnancy might be an important risk factor for severe disease with H1N1pdm [21],[25],[63],[64]. Our analysis is consistent with these reports and more recent studies [47],[65], which found an overall trend that pregnant women, mainly in their third trimester, have a higher incidence of hospitalization than the general population. Several published studies have also shown that pregnancy is associated with a higher risk of ICU admission and fatal outcome [54],[58],[66],[67]. In our analysis, the risk associated with pregnancy was elevated for both hospitalization and fatality compared to women of childbearing age, though the latter association was not consistently observed in every country. As with asthma, the proportion of pregnant women generally decreased with severity level for most of the countries. Our results suggest that pregnant women with H1N1pdm are approximately seven times more likely to be hospitalized and two times more likely to die than non-pregnant women with H1N1pdm. The greater risk for hospitalization than for death with H1N1pdm influenza infection during pregnancy may have resulted from a lower threshold for admitting infected pregnant women to hospital and/or a more aggressive approach to antiviral or other treatment for pregnant women. In addition, the occurrence of non-respiratory complications of pregnancy, such as hypertension, pre-eclampsia, and premature labor, provoked by H1N1pdm infection may have increased the risk of hospitalization while not resulting in death [68]. This would be consistent with published reports of case series of pregnant patients that list complications of pregnancy as a common cause of admission [63],[69],[70]. The dataset did not allow us to adjust for underlying conditions in pregnant women, and thus to distinguish between risks for healthy pregnant women, and pregnant women with underlying medical conditions; however, we believe that the results support an approach of early intervention with pregnant women who develop influenza.
Early in the 2009 pandemic, clinicians from the US reported a surprisingly high prevalence of morbid obesity, a risk factor not previously associated with severe outcomes for seasonal influenza infection, in patients with severe complications of H1N1pdm infection [71]. Subsequent studies in several countries, including the US, Mexico, Canada, Spain, Greece, France, Australia, and New Zealand, reported high proportions of obesity among ICU admissions and fatal cases [13],[20],[58],[64],[72]–[77]. Our results provide supportive evidence that obesity may be a risk factor for severe disease, as seen in the increasing proportion of morbidly obese patients with severity level and the associated elevated OR. Our findings also suggest that morbidly obese patients with H1N1pdm are more likely to die if hospitalized; however, the results in our analysis were not consistent across all countries. The association between obesity (or morbid obesity) and severe outcomes may reflect direct causation (e.g., due to greater respiratory strain of infection on obese individuals), causation through other known risk factors (e.g., obesity causes diabetes and heart disease, which pose an increased risk for severe outcome [36]), or a noncausal association, if some other factor (e.g., genetic or dietary) caused both morbid obesity and increased risk of severe outcome. Unfortunately, our dataset did not allow us to distinguish among these nonexclusive alternatives.
Indigenous populations and ethnic minorities have been reported to experience a disproportionately high burden of severe H1N1pdm infection, particularly in the Americas [14],[21],[23],[36],[64],[75],[78]–[80] and the Australasia-Pacific region [43],[80]–[82], similar to reports during the 1918 influenza pandemic [83]–[85]. Our analysis of Australian, New Zealand, and Canadian data concur with these published reports, and while compelling, were not universal. Neither Thailand nor Mexico observed a significantly increased burden of severe H1N1pdm disease among indigenous or minority populations. Our data are not sufficient to explain the observed differences in the reported risk of severe disease among minority groups, but several hypotheses have been proposed, including a higher prevalence of chronic medical conditions known to increase risk of severe influenza, delayed or reduced access to healthcare, cultural differences in healthcare-seeking behavior and approaches to health, potential differences in genetic susceptibility, and social inequalities [23],[78],[80]. More research is needed to better understand and quantify the increased risk of severe H1N1pdm disease among these groups. However, an imperfect understanding of the mechanisms of health disparities related to severe H1N1pdm disease should not impede the public health community in undertaking actions to mitigate this risk by disseminating appropriate public information, targeting outreach and prevention programs, and involving at-risk population groups in pandemic planning.
Our analysis has a number of limitations, not least of which is the wide differences in surveillance systems, case management policies, and antiviral use in the countries studied. The criteria and indications for hospital and ICU admission for certain conditions (e.g., pregnancy and asthma) and by age (e.g., pediatric patients) varied significantly by country, and may have been somewhat dependent on capacity for admission, which likely varied over time. Risk factors are also dependent on the completeness and quality of data on risk factors reported and classification of death in the absence of complete testing. These variables could lead to a bias in the estimate of these conditions among severe cases and could make direct comparisons across countries difficult. Second, our data do not consider multiple risk factors for individual H1N1pdm patients. A lack of individual-level data on underlying medical conditions of H1N1pdm patients precludes our ability to sufficiently control for confounding and therefore identify the independent contribution of individual risk factors for severe disease and death. The differences observed in risk factors for hospitalization and death among H1N1pdm patients compared to among seasonal influenza patients, and the wide range of RR values between countries may be explained by differences in age structure in the general population. Several studies have identified important differences in the proportions of underlying conditions by age among hospitalized and fatal cases, including, but not limited to, the UK [15],[53], the US [36], Canada [47], and Singapore [39],[86].
A third limitation is related to our imperfect calculation of the point prevalence of pregnancy among women of childbearing age in the general population. However, we believe that our findings of the range of RR values for hospitalization and death is valid, but may be very slightly inflated because of undercounting in the denominator. The inflationary effect of undercounting is likely greatest for pregnant women in the first trimester, as we didn't adjust for common first trimester events such as miscarriages or abortions, and in this group there is likely substantial undercounting in the numerator as well because of women not knowing they are pregnant in that period. Fourth, the data used in our analysis relied on hospital records, which were not standardized, and were likely to be incomplete or vary in quality between hospitals or countries. This poses a problem in the direct comparativeness between settings.
Despite these limitations, this analysis is the first to our knowledge to compare risk factors across a variety of countries using data from a very large number of patients, and we found a great deal of consistency for much of the data. Clearly, cardiac disease, chronic respiratory disease, and diabetes are important risk factors for severe disease that will be especially relevant for countries with high rates of these illnesses. We provide evidence to support the concern regarding obesity, particularly morbid obesity, as a risk factor, though this needs more study. We found large between-country variations for some important risk factors, most notably pregnancy, and the reasons for these differences need more study. There is evidence to suggest that the differences observed for pregnancy might represent differences in case management practices, and we believe that the available evidence supports vaccination and early intervention for pregnant women. Our study reinforces the need to identify and target high-risk groups for interventions, such as immunization, information, early medical advice, and use of antiviral medications. Experience with the 2009 H1N1 pandemic and the differences observed between countries have highlighted the need for country-specific surveillance data and global standardization of case definitions and data collection, and the usefulness of data sharing to aid policy makers in critical decision making for global influenza epidemics.
Supporting Information
Zdroje
1. Centers for Disease Control and Prevention 2009 Update: swine influenza A (H1N1) infections—California and Texas, April 2009 MMWR Morb Mortal Wkly Rep 58 435 437 Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5816a7.htm. Accessed 22 January 2010
2. World Health Organization 2009 Swine influenza—update 3. Available: http://www.who.int/csr/don/2009_04_27/en/index.html. Accessed 22 January 2010
3. ChanM 2009 April 25 Swine influenza: statement by WHO Director-General, Dr Margaret Chan. Available: http://www.who.int/mediacentre/news/statements/2009/h1n1_20090425/en/index.html. Accessed 21 January 21 2010
4. World Health Organization 2010 Evolution of a pandemic: A(H1N1) 2009, April 2009–March 2010 Geneva World Health Organization
5. National Center for Immunization and Respiratory Diseases 2009 Use of influenza A (H1N1) 2009 monovalent vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 58 RR-10 1 8
6. FioreAShayDBroderKUyekiTMootreyG 2009 Prevention and control of influenza, recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 57 RR-7 1 60
7. MereckieneJCotterSNicollALevy-BruhlDFerroA 2008 National seasonal influenza vaccination survey in Europe, 2008. Euro Surveill 13 pii: 19017
8. DoddLMcNeilSAFellDBAllenVMCoombsA 2007 Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ 176 463 468
9. FreemanDWBarnoA 1959 Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol 78 1172 1175
10. HarrisJW 1919 Influenza occurring in pregnant women. JAMA 72 978 980
11. NeuzilKMReedGWMitcheEFSimonsenLGriffinMR 1998 Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 148 1094 1102
12. World Health Organization 2009 Clinical features of severe cases of pandemic influenza. Pandemic (H1N1) 2009 briefing note 13. Available: http://www.who.int/csr/disease/swineflu/notes/h1n1_clinical_features_20091016/en/index.html. Accessed 19 May 2011
13. The ANZIC Influenza Investigators 2009 Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 361 1925 1934 Available: http://www.nejm.org/doi/full/10.1056/NEJMoa0908481#t=article. Accessed 19 May 2011
14. Centers for Disease Control and Prevention 2009 Deaths related to 2009 pandemic influenza A (H1N1) among American Indian/Alaska Natives—12 states, 2009. MMWR Morb Mortal Weekly Rep 58 1341 1344
15. DonaldsonLJRutterPDEllisBMGreavesFECMyttonOT 2009 Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ 339 b5213
16. BinSaeedA 2010 Characteristics of pandemic influenza A (H1N1) infection in patients presenting to a university hospital in Riyadh, Saudi Arabia. Ann Saudi Med 30 59 62
17. Centers for Disease Control and Prevention 2009 Novel influenza A (H1N1) virus infections in three pregnant women—United States, April–May 2009. MMWR Morb Mortal Weekly Rep 58 497 500
18. Centers for Disease Control and Prevention 2009 Hospitalized patients with novel influenza A (H1N1) virus infection—California, April–May, 2009. MMWR Morb Mortal Weekly Rep 58 536 541
19. PalaciosGHornigMCisternaDSavjiNBussettiA 2009 Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS ONE 4 e8540 doi:10.1371/journal.pone.0008540
20. FuhrmanCBonmarinIPatyADuportNChironE 2010 Severe hospitalised 2009 pandemic influenza A(H1N1) cases in France, 1 July–15 November 2009. Euro Surveill 15 pii: 19463 Available: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19463. Accessed 19 May 2011
21. LouieJKAcostaMWinterKJeanCGavaliS 2009 Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 302 1896 1902
22. ChienYSuCTsaiHHuangSChuangJ 2009 The first 100 hospitalized severe complicated influenza cases caused by 2009 pandemic influenza A (H1N1) in Taiwan. Taiwan Epidemiology Bulletin. Available: http://teb.cdc.gov.tw/public/Attachment/23552_25-10-%E8%8B%B12-692-707.pdf. Accessed 19 May 2011
23. ZarychanskiRStuartTLKumarADoucetteSElliottL 2010 Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ 182 257 264
24. CullenGMartinJO'DonnellJBolandMCannyM 2009 Surveillance of the first 205 confirmed hospitalised cases of pandemic H1N1 influenza in Ireland, 28 April–3 October 2009. Euro Surveill 14 pii: 19389
25. DenholmJGordonCJohnsonPHewagamaSStuartR 2010 Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. MJA 192 84 86
26. YangPDengYPangXShiWLiX 2010 Severe, critical and fatal cases of 2009 H1N1 influenza in China. J Infect 61 277 283 doi:10.1016/j.jinf.2010.07.010
27. ShimadaTGuYKamiyaHKomiyaNOdairaF 2010 Epidemiology of influenza A(H1N1)v virus infection in Japan, May–June 2009. Euro Surveill 14 pii: 19244 Available: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19244. Accessed 19 May 2011
28. PoggenseeGGilsdorfABudaSEckmannsTClausH 2010 The first wave of pandemic influenza (H1N1) 2009 in Germany: from initiation to acceleration. BMC Infect Dis 10 155 doi:110.1186/1471-2334-1110-1155
29. van 't KloosterTWieldersCDonkerTIskenLMeijerA 2010 Surveillance of hospitalisations for 2009 pandemic influenza A(H1N1) in the Netherlands, 5 June–31 December 2009. Euro Surveill 15 pii: 19461
30. HahnéSDonkerTMeijerATimenAvan SteenbergenJ 2009 Epidemiology and control of influenza A(H1N1)v in the Netherlands: the first 115 cases. Euro Surveill 14 pii: 19267 Available: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19267. Accessed 19 May 2011
31. VriendHJHahnéSJMDonkerTMeijerATimenA 2009 De nieuwe influenza A(H1N1)-epidemie in Nederland. Epidemiologische gegevens over de periode 30 april–14 augustus 2009. Ned Tijdschr Geneeskd 153 A969
32. van der SandeMABvan der HoekWHooiveldMDonkerGAvan SteenbergenJE 2009 Bestrijding van de nieuwe influenza A (H1N1). II. Epidemiologie en niet-medicamenteuze maatregelen. Ned Tijdschr Geneeskd 153 A771
33. DijkstraFvan 't KloosterTMBrandsemaPvan Gageldonk-LafeberABMeijerA 2010 Jaarrapportage surveillance respiratoire infectieziekten 2009. Bilthoven: Rijksinstituut voor Volksgezondheid en Milieu, 2010. RIVM RIVM-briefrapportnummer: 210231006. Available: http://www.rivm.nl/bibliotheek/rapporten/210231006.pdf. Accessed 19 May 2011
34. Institut de Veille Sanitaire 2009 December 16 Surveillance de la grippe A (H1N1) 2009 en France: outils et méthodes. Available: http://www.invs.sante.fr/surveillance/grippe_dossier/docs_professionnels/methodo_surveillance_grippe_161209.pdf. Accessed 19 May 2011
35. SkarbinskiJJainSBramleyALeeEJHuangJ 2011 Hospitalized patients with 2009 pandemic influenza A (H1N1) virus infection in the United States—September–October 2009. Clin Infect Dis 52 Suppl 1 S50 S59
36. JainSKamimotoLBramleyAMSchmitzAMBenoitSR 2009 Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 361 1935 1944
37. UK Health Protection Agency 2010 Sources of UK flu data: influenza surveillance in the United Kingdom. Available: http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1195733821514?p=1191942171484. Accessed 19 May 2011
38. RandrianasoloLRaoelinaYRatsitorahinaMRavolomananaLAndriamandimbyS 2010 Sentinel surveillance system for early outbreak detection in Madagascar. BMC Public Health 10 31
39. CutterJAngLWLaiFYSubramonyHMaS 2010 Outbreak of pandemic influenza A (H1N1-2009) in Singapore, May to September 2009. Ann Acad Med Singapore 39 273 282
40. Sierra MorosMJVázquez TorresMSanta-Olalla PeraltaPLimia SánchezACortes GarcíaM 2010 Epidemiological surveillance activities during the 2009 influenza pandemic in Spain. Lessons learnt one year after. Rev Esp Salud Publica 84 463 480
41. Santa-Olalla PeraltaPCortes-GarciaMVicente-HerreroMCastrillo-VillamandosCArias-BohigasP 2010 Risk factors for disease severity among hospitalised patients with 2009 pandemic influenza A (H1N1) in Spain, April–December 2009. Euro Surveill 15 pii: 19667
42. Santa-Olalla PeraltaPCortes GarcíaMLimia SánchezAAndrés PradoJPachón del AmoI 2010 Critically ill patients with 2009 pandemic influenza A (H1N1) infection in Spain: factors associated with death, April 2009–January 2010. Rev Esp Salud Publica 84 547 568
43. BakerMGWilsonNHuangQSPaineSLopezL 2009 Pandemic influenza A(H1N1)v in New Zealand: the experience from April to August 2009. Euro Surveill 14 pii: 19319
44. HuangQSBandaranayakeDLopezLDPirieRPeaceyM 2009 Surveillance for the 2009 pandemic influenza A (H1N1) virus and seasonal influenza viruses - New Zealand, 2009. MMWR Morb Mortal Wkly Rep 58 918 921
45. YuHLiaoQYuanYZhouLXiangN 2010 Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ 341 c4779
46. YuHFengZUjekiTMLiaoQZhouL 2011 Risk factors for severe illness with 2009 pandemic influenza A(H1N1) virus infection in China. Clin Infect Dis 52 457 465
47. CampbellARodinRKroppRMaoYHongZ 2010 Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ 182 349 355
48. HelfertyMVachonJTarasukJRodinRSpikaJ 2011 Canadian pandemic H1N1 cases: a description of the changing epidemiology of hospitalizations and deaths in the first and second waves. CMAJ In press
49. KongWM 2000 A review on the Hong Kong influenza surveillance system. Public Health Epidemiology Bull 9
50. United Nations Population Division 2009 World population prospects: the 2008 revision population database. http://esa.un.org/unpd/wpp/unpp/panel_population.htm
51. HigginsJThompsonSG 2002 Quantifying heterogeneity in a meta-analysis. Stat Med 21 1539 1558
52. SimmondsMCHigginsJP 2007 Covariate heterogeneity in meta-analysis: criteria for deciding between meta-regression and individual patient data. Stat Med 26 2982 2999
53. PebodyRGMcLeanEZhaoHClearyPBracebridgeS 2010 Pandemic influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveill 15 pii: 19571
54. VaillantLLa RucheGTarantolaABarbozaP epidemic intelligence team at InVS 2009 Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 14 pii: 19309
55. BettingerJASauvéLJScheifeleDWMooreDVaudryW 2010 Pandemic influenza in Canadian children: A summary of hospitalized pediatric cases. Vaccine 28 3180 3184
56. LemaitreMCarratF 2010 Comparative age distribution of influenza morbidity and mortality during seasonal influenza epidemics and the 2009 H1N1 pandemic. BMC Infect Dis 10 162
57. KamigakiTOshitaniH 2009 Epidemiological characteristics and low case fatality rate of pandemic (H1N1) 2009 in Japan. PLoS Curr 1 RRN1139
58. HanslikTBoellePFlahaultA 2010 Preliminary estimation of risk factors for admission to intensive care units and for death in patients infected with A(H1N1)2009 influenza virus, France, 2009–2010. PLoS Curr 2 RRN1150
59. WuJTMaESLeeCKChuDKHoPL 2010 The infection attack rate and severity of 2009 pandemic H1N1 influenza in Hong Kong. Clin Infect Dis 51 1184 1191
60. MillerEHoschlerKHardelidPStanfordEAndrewsN 2010 Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 375 1100 1108
61. PresanisAMDe AngelisDHagyAReedC The New York City Swine Flu Investigation Team 2009 The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med 6 e1000207 doi:10.1371/journal.pmed.1000207
62. WatsonLTurkFJamesPHolgateST 2007 Factors associated with mortality after an asthma admission: A national United Kingdom database analysis. Respir Med 101 1659 1664
63. JamiesonDJHoneinMARasmussenSAWilliamsJLSwerdlowDL 2009 H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 374 451 458
64. KumarAZarychanskiRPintoRCookDJMarshallJ 2009 Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 302 1872 1879
65. CreangaAAJohnsonTFGraitcerSBHartmanLKAl-SamarraiT 2010 severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol 115 717 726
66. The ANZIC Influenza Investigators and Australasian Maternity Outcomes Surveillance System 2010 Critical illness due to2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ 340 c1279 doi:1210.1136/bmj.c1279
67. ArcherBCohenCNaidooDThomasJMakungaC 2009 Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: epidemiology and factors associated with fatal cases. Euro Surveill 14 pii: 19369
68. SistonAMRasmussenSAHoneinMAFryAMSeibK 2010 Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 303 1517 1525
69. JamiesonDTheilerRRasmussenS 2006 Emerging infections and pregnancy. Emerg Infect Dis 12 1638 1643
70. GoodnightWSoperD 2005 Pneumonia in pregnancy. Crit Care Med 33 S390 S397
71. Centers for Disease Control and Prevention 2009 Intensive-care patients with severe novel influenza A (H1N1) virus infection—Michigan, June 2009. MMWR Morb Mortal Weekly Rep 58 749 752
72. Dominguez-CheritGLapinskySEMaciasAEPintoREspinosa-PerezL 2009 Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 302 1880 1887
73. RelloJRodríguezAIbañezPSociasLCebrianJ 2009 Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care 13 R148
74. RodríguezASocíasLGuerreroJFigueiraJGonzálezN 2010 Gripe A pandémica en una unidad de cuidados intensivos: experiencia en España y Latinoamérica (Grupo Español de Trabajo de Gripe A Grave/Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias). Med Intensiva 34 87 94
75. Centers for Disease Control and Prevention 2009 Patients hospitalized with 2009 pandemic influenza A (H1N1)—New York City, May 2009. MMWR Morb Mortal Weekly Rep 58 1436 1440
76. PlessaEDiakakisPGardelisJThiriosAKoletsiP 2010 Clinical features, risk factors, and complications among pediatric patients with pandemic influenza A (H1N1). Clin Pediatr (Phila) 49 777 781
77. MorganOBramleyAFowlkesAFreedmanDTaylorTH 2010 Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS ONE 5 e9694 doi:9610.1371/journal.pone.0009694
78. Arizona Department of Health Services 2010 Arizona—Supplemental influenza summary MMWR Week 9 (2/28/10–3/6/10) Phoenix (Arizona) Arizona Department of Health Services
79. Fundação Nacional de Saúde 2009 Saúde indígena—informe técnico sobre influenza A H1N1. Influenza A (H1N1) em populações indígenas do Brasil. Informe Técnico n°06/2009. Available: http://www.funasa.gov.br/internet/desai/arquivos/informeTecnicoInfluenzaA_6.pdf. Accessed 30 May 2011
80. La RucheGTarantolaABarbozaPVaillantLGueguenJ 2009 The 2009 pandemic H1N1 influenza and indigenous populations of the Americas and the Pacific. Euro Surveill 14 pii: 19366
81. Australian Government Department of Health and Aging 2010 Australian influenza surveillance report no. 8, 2010. Available: http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/EB136394E79CA5E2CA2576A50010783A/File/ozflu-no8-2010.pdf. Accessed 19 May 2011
82. WebbSAPettilaVSeppeltIBellomoRBaileyM 2009 Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 361 1925 1934
83. WilsonNBakerM 2008 Ninety years on: what we still need to learn from “Black November” 1918 about pandemic influenza. N Z Med J 121 136 138
84. GroomAVJimCLaroqueMMasonCMcLaughlinJ 2009 Pandemic influenza preparedness and vulnerable populations in tribal communities. Am J Public Health 99 Suppl 2 S271 S278
85. JohnsonNPMuellerJ 2002 Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med 76 105 115
86. SubramonyHLaiFYAngLWCutterJLimPL 2010 An epidemiological study of 1348 cases of pandemic H1N1 influenza admitted to Singapore hospitals from July to September 2009. Ann Acad Med Singapore 39 283 290
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 7- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Nutraceutikum Armolipid Plus podle klinických důkazů zlepšuje lipidový profil − metaanalýza
- Snižuje terapie betablokátory kardiovaskulární benefit aerobního cvičení u pacientů s arteriální hypertenzí?
-
Všechny články tohoto čísla
- Retention in HIV Care between Testing and Treatment in Sub-Saharan Africa: A Systematic Review
- Health Care Systems and Conflict: A Fragile State of Affairs
- Simplified ART Delivery Models Are Needed for the Next Phase of Scale Up
- Individualized Cost-Effectiveness Analysis
- Is Scale-Up Worth It? Challenges in Economic Analysis of Diagnostic Tests for Tuberculosis
- Treatment Outcomes and Cost-Effectiveness of Shifting Management of Stable ART Patients to Nurses in South Africa: An Observational Cohort
- Risk Factors for Severe Outcomes following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis
- Predicting the Epidemic Sizes of Influenza A/H1N1, A/H3N2, and B: A Statistical Method
- GeneXpert—A Game-Changer for Tuberculosis Control?
- LED Fluorescence Microscopy for the Diagnosis of Pulmonary Tuberculosis: A Multi-Country Cross-Sectional Evaluation
- Configuring Balanced Scorecards for Measuring Health System Performance: Evidence from 5 Years' Evaluation in Afghanistan
- A Multi-Country Non-Inferiority Cluster Randomized Trial of Frontloaded Smear Microscopy for the Diagnosis of Pulmonary Tuberculosis
- Comparison of Xpert MTB/RIF with Other Nucleic Acid Technologies for Diagnosing Pulmonary Tuberculosis in a High HIV Prevalence Setting: A Prospective Study
- Global Pharmacovigilance for Antiretroviral Drugs: Overcoming Contrasting Priorities
- Evidence-Based African First Aid Guidelines and Training Materials
- Screening for HIV-Associated Tuberculosis and Rifampicin Resistance before Antiretroviral Therapy Using the Xpert MTB/RIF Assay: A Prospective Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Individualized Cost-Effectiveness Analysis
- GeneXpert—A Game-Changer for Tuberculosis Control?
- Screening for HIV-Associated Tuberculosis and Rifampicin Resistance before Antiretroviral Therapy Using the Xpert MTB/RIF Assay: A Prospective Study
- Treatment Outcomes and Cost-Effectiveness of Shifting Management of Stable ART Patients to Nurses in South Africa: An Observational Cohort
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



