-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaPregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
Shinya Ito and colleagues systematically review the literature to identify research reporting medications reporting pregnancy-associated changes in pharmacokinetics.
Published in the journal: . PLoS Med 13(11): e32767. doi:10.1371/journal.pmed.1002160
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002160Summary
Shinya Ito and colleagues systematically review the literature to identify research reporting medications reporting pregnancy-associated changes in pharmacokinetics.
Introduction
Women frequently take a variety of medications during pregnancy, including prescription, over-the-counter (OTC), and herbal agents [1,2]. During the last three decades the average number of medications (prescription and nonprescription) used per woman in North America during the first trimester increased by 60% from 1.6 to 2.6 [3]. More recently, from 2006 to 2008, over 80% of women reported using at least one medication during the first trimester, and over 90% reported using at least one medication at any point during their pregnancy [3]. Other studies have demonstrated increased rates of use of various OTC medications in the first, second, or third trimester of pregnancy compared to the prepregnancy period [4]. While some studies have found that the proportion of women receiving at least one prescription medicine increases from the first to third trimester of pregnancy [5,6], others have found that rates of prescription drug use are highest in the first trimester of pregnancy [1,7]. The most common medications used in pregnancy are nonprescription or OTC medications [4]. A longitudinal study aimed at identifying the medications that are most often consumed during pregnancy demonstrated that 95.8% of participants took prescription medications, 92.6% self-medicated with OTC medications, and 45.2% used herbal medications [2].
Most organ systems are affected by substantial anatomical and physiological changes during pregnancy. Such pregnancy-related changes are observed in decreased gastrointestinal motility and increased gastric pH (impacting absorption), increased total body water and plasma volume and decreased concentrations of drug-binding proteins (affecting the apparent volume of distribution and, in some cases, clearance rates), increased glomerular filtration rate (increasing renal clearance), and altered activity of drug-metabolizing enzymes in the liver (affecting hepatic clearance). Overall, these changes in physiological indices take place progressively during gestation (reviewed in [8] and [9]). The increases in cardiac output, total body water, fat compartment, and glomerular filtration rate, together with the decrease in plasma albumin concentration and altered activity of drug-metabolizing enzymes, are all reported to peak during the third trimester (reviewed in [8] and [10]). Table 1 presents typical pregnancy-related changes in organ function leading to altered pharmacokinetics (PK) [10–16]. Changes during pregnancy in drug metabolism by cytochrome P450 isoenzymes (i.e., CYP3A4, CYP2D6, CYP2C9, CYP1A2, and CYP2C19) and by uridine 5′-diphospho-glucuronosyltransferase (UGT) isoenzymes (i.e., UGT1A4 and UGT2B7) have also been demonstrated (Table 2) [10,17–20].
Tab. 1. Physiological changes during pregnancy: effects on drug disposition [10–16]. ![Physiological changes during pregnancy: effects on drug disposition [<em class="ref">10</em>–<em class="ref">16</em>].](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/b2492a2222f6b6a30d0f0242a27bf4ce.png)
UGT, uridine diphosphate glucuronosyltransferase; Vd, volume of distribution. Tab. 2. Reported effects of pregnancy on hepatic enzyme activity. 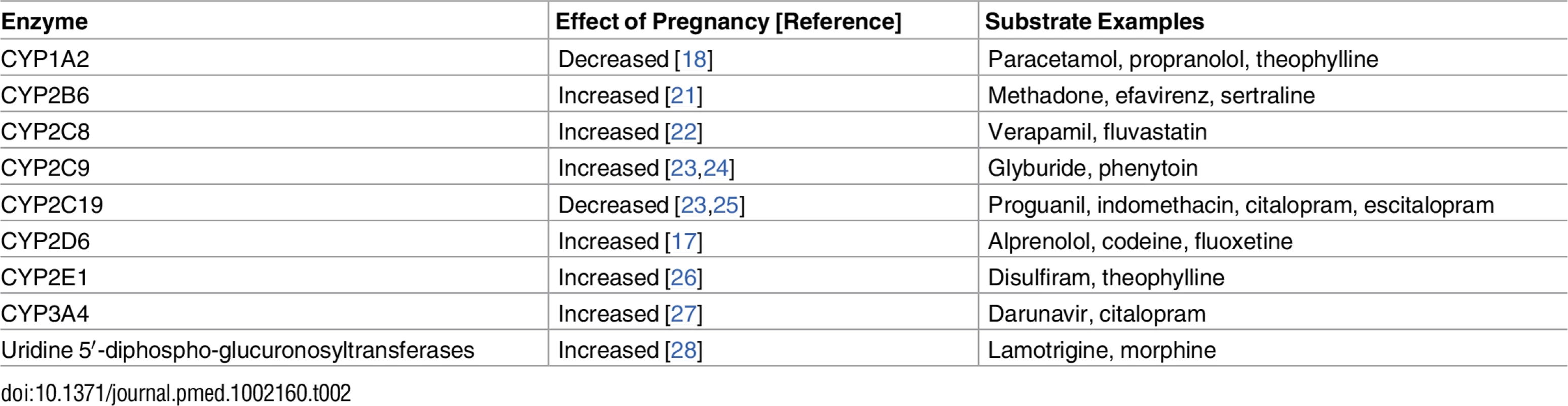
For some drug classes, a large number of PK clinical trials during pregnancy are available in the literature [29–34]. A recent review noted that, since 2008, about a third of these trials investigated drugs used in the treatment of acute labor and delivery issues, another third investigated drugs used in infectious disease treatment during pregnancy, and the remaining third investigated drugs used for various antepartum indications [35]. However, for the large majority of drugs used during pregnancy, there is little or no information available regarding PK changes or dosage requirements during pregnancy [35]. Moreover, it is often unclear if observed PK changes lead to alterations in drug efficacy and/or adverse effect profiles. Given the complexity of the field, the lack of clear understanding of the clinical significance of PK changes, and renewed recognition of the need to rationalize drug therapy for pregnant and lactating women, it is imperative to systematically examine existing data on PK changes in pregnancy and their potential clinical impact.
The objective of this study was to systematically identify all existing evidence of PK changes during pregnancy in the context of clinical significance. We hypothesized that known physiological changes occurring during pregnancy and associated PK alterations could consequently be translated into changes in dosing guidelines.
Methods
This research involved a structured review of the literature, according to the PRISMA guidelines [36] (S1 Checklist).
Search Strategy
Searches were conducted in MEDLINE (Ovid), Embase (Ovid), Cochrane Central Register of Controlled Trials (Ovid), and Web of Science (Thomson Reuters) from database inception to August 31, 2015 (S1 Table). An update of the search from September 1, 2015, to May 20, 2016, was performed, and relevant data were retrieved and added to the review (S2 Table). Text words and, where applicable, database subject heading fields (e.g., MeSH) were used for the following concepts: pregnancy AND pharmacokinetics OR dosing OR clearance OR distribution OR absorption OR metabolism OR excretion OR Cmax OR Tmax OR Ctrough OR AUC OR Vd OR t1/2 OR protein binding AND specific study types (randomized controlled trial, non-randomized controlled clinical trial, cohort study, case–control study, or case series). Truncation symbols were used with the text words, when appropriate, to capture variations in spelling and word endings. Subsequently, we reviewed the identified studies and examined their references to identify further potential articles. Information available from relevant conferences was also reviewed. No publication date, language, or location restrictions were applied.
Study Selection
In order to locate all published literature, we established a set of criteria to define types of studies to be reviewed. Inclusion criteria were as follows: (1) the study reported dosing data or at least one PK parameter of interest in pregnant women; (2) a comparison of the dosing data or PK parameter between pregnant and nonpregnant women was done; and (3) the data are described in the form of a peer-reviewed randomized controlled trial, non-randomized controlled clinical trial, cohort study, case–control study, or case series. The review did not cover animal studies, case reports, or studies containing no original research or data. Retrieved articles were inspected by two independent reviewers (G. P. and T. L.) to determine whether they met the inclusion criteria. In cases where the eligibility of the study was unclear, it was reviewed by a third independent reviewer (G. K.). The full texts were retrieved and read in full.
Data Extraction
The data extractors (G. P. and T. L.) reviewed each of the included studies independently and extracted data according to the predetermined guidelines, using a predesigned data extraction form. When needed, authors of the included studies were contacted for missing data; however, none of the authors who were contacted for more information responded. Data from studies presented in multiple publications were identified to avoid duplications and were reported as a single study, with all other relevant publications listed.
Data Presentation and Analysis
Results of the literature search
The results from each step of the review process are documented in a PRISMA flow diagram (Fig 1), with an overall summary of the number and types of articles included in the review.
Fig. 1. Flow diagram of numbers of studies screened, assessed for eligibility, and included in the review. 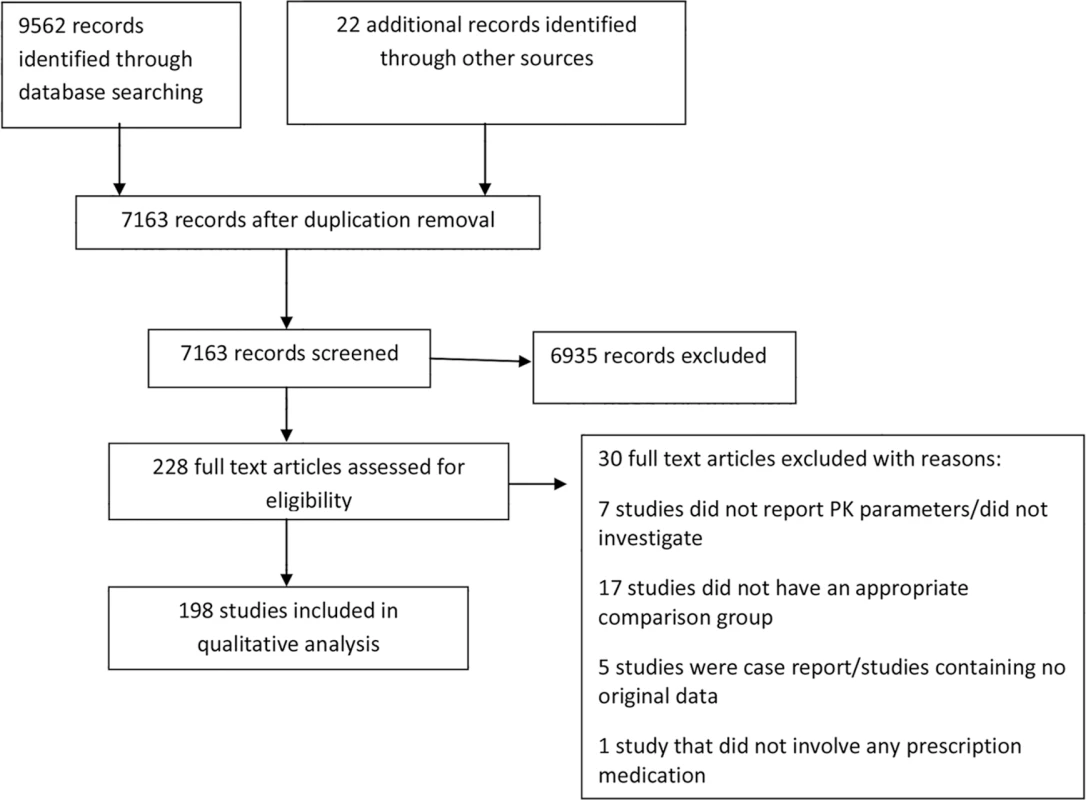
PK, pharmacokinetics. When more than one study reported the same PK parameter(s) for the same drug, these parameters were examined for consistency in the change direction (i.e., decrease, increase, or no change). When study data were presented by trimester, the PK parameters obtained during the third trimester were selected for this study because the majority of the pregnancy-associated physiological changes peak during the third trimester.
Drugs were divided into two major categories according to between-study agreement of directions of statistically significant changes in PK parameters. If statistically significant pregnancy-associated changes in PK parameters were in the same direction (e.g., increase in clearance and decrease in volume of distribution) among the studies for all reported PK parameters, we categorized the drug as “consistent.” On the other hand, a drug was categorized as “inconsistent” if at least one study reported a statistically significant change in a PK parameter in the opposite direction (e.g., increased Cl in one study and decreased Cl in the other). The potential source of inconsistency is speculated on and addressed in the Discussion. Note that the definition of the categories described above is based on statistically significant changes of PK parameters, but statistically non-significant changes are also presented, for completeness. In addition, if only one study showed a statistically significant PK parameter change for a drug, the drug was included in the “consistent” category for simplicity of the data presentation, even though the PK parameters were reported in only one study.
Quality assessment
The quality of each accepted article was assessed using the ClinPK checklist [37] for assessing methodological quality in clinical PK studies (Table 3).
Tab. 3. ClinPK checklist for assessing methodological quality in clinical pharmacokinetic studies [37]. ![ClinPK checklist for assessing methodological quality in clinical pharmacokinetic studies [<em class="ref">37</em>].](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/2f04c24145445e05e9cfbf10ad471dc3.png)
All the items presented in the table correspond to the original checklist as published in [37]. No discrepancies exist between the original protocol and the final data analyses.
Results
Literature Retrieval
The search strategy for the comprehensive systematic review retrieved 9,562 articles, and after removing duplicates, the first screen on title and abstract was performed on 7,163 articles (Fig 1). For 6,935 of these, the title or abstract clearly indicated that the topic of the article was not relevant to the review question or did not satisfy one of the inclusion criteria. The remaining 228 articles were screened using the full text, applying the full set of eligibility criteria. After applying the eligibility criteria, 202 articles containing comparisons of PK parameters of different drugs between pregnant and nonpregnant women were eligible for inclusion. Twenty-six studies were excluded because they didn’t report PK parameters, didn’t include a comparison group, or were either review papers or case reports (S3 Table). Following review, four further articles were excluded because they duplicated the same outcome domain, in the same cohort, as another article. The remaining 198 articles were included in the data extraction for the comprehensive systematic review. Twenty-two additional articles were identified using a monthly update search between September 1, 2015, and May 20, 2016. Hence, this review article summarizes the results of a total of 198 studies, involving 121 different medications, reporting comparisons of different PK parameters and dosing data between pregnant and nonpregnant cohorts.
Reviewed studies were found to vary widely in both design and quality (S4 Table). There were some differences in the stages of pregnancy in which the women were investigated; while most of the studies provided third trimester results, others reported results from both the second and the third trimesters together [38–42] or separately [43–46], and a few reported results from all trimesters together [47] or separately [48]. Two studies reported only first trimester results [49,50].
Studies Comparing Pregnant and Nonpregnant Women for Each Drug Class
Certain drug classes were far more commonly investigated during pregnancy than others (Fig 2). Approximately one-half of the studies (48%) addressed medications given chronically during pregnancy. Of the studies of chronic medications, 54 studies focused on drugs for HIV treatment and vertical transmission prevention, 27 studies focused on antiepileptic drugs, 17 studies focused on drugs related to cardiovascular disorders, and nine studies focused on drugs for endocrine disorders. An additional eight studies investigated antidepressants and anxiolytic drugs, five other studies focused on drugs involved in addiction management, and two studies described drugs treating immunological conditions. In comparison, 84 studies addressed drugs used in the treatment of acute issues during pregnancy; among them, 23 studies addressed antibiotics, 22 studies addressed antimalarial medications, 13 studies addressed analgesics or anesthetic drugs, and eight studies addressed antithrombotic drugs in pregnancy. Fifty-one studies investigated more than one drug. Among the antiretroviral class, all studies but one presented women living with HIV infection who were treated with more than one antiretroviral medication. Eleven of 22 studies investigating antimalarial drugs described more than one drug given to the same patient population. Four of 27 studies investigating antiepileptic drugs described more than one drug given to the same patient population. Other drug classes that reported results of pregnant women taking more than one drug included antibiotics (four studies), anesthesia and analgesia drugs (one study), and antiemetics (one study).
Fig. 2. Number of studies comparing pregnant and nonpregnant women for each drug class. 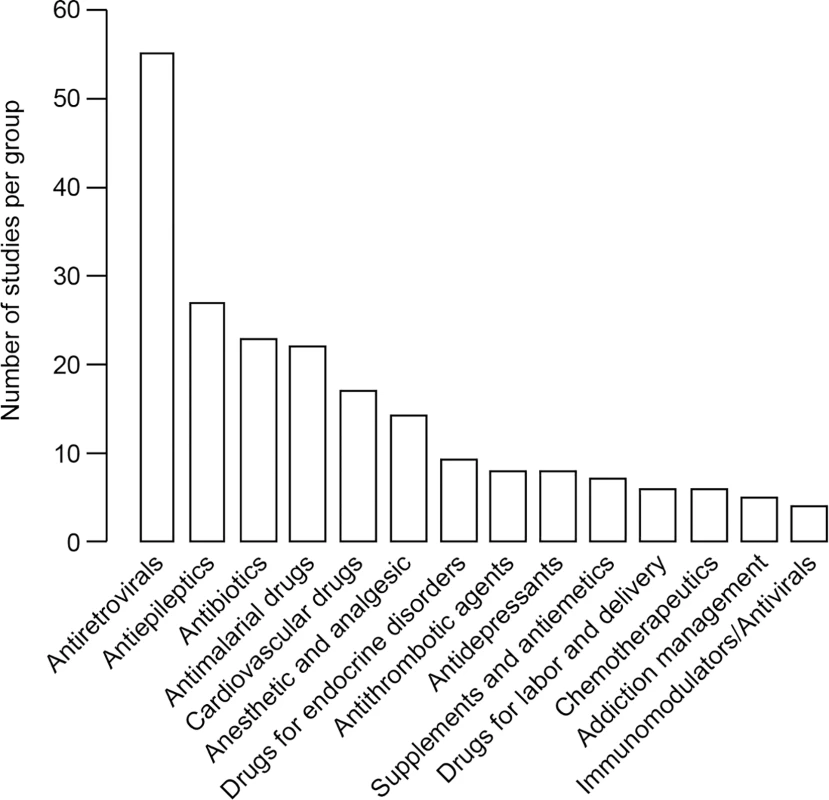
Reported Pharmacokinetics Parameters
PK parameters of interest as defined by our search terms were the following: elimination half-life (t1/2), clearance (Cl), Cmax, Ctrough, concentration-to-dose ratio (C/D ratio), area under the curve (AUC), volume of distribution (Vd), and protein binding (i.e., free fraction). The majority of the studies reported on various combinations of some of these PK parameters of interest (Table 4). The most frequently reported PK parameter was Cl, followed by AUC, t1/2, and Cmax with 116, 103, 88, and 87 counts, respectively. In most of the studies that focused on the free fraction of a drug in plasma, the free fraction was the only PK parameter reported in the study. While Cl and AUC were the most frequently reported parameters, both parameters were reported for only 46% of the drugs. Whereas more than half of the drugs (53%) were described with both the Cl and the t1/2, only 16% of the drugs included Ctrough, Cmax, and AUC. The latter group mostly consisted of antiretroviral drugs. Cmax and AUC were described together for 30% of the drugs.
Tab. 4. Pharmacokinetics parameters—data count. 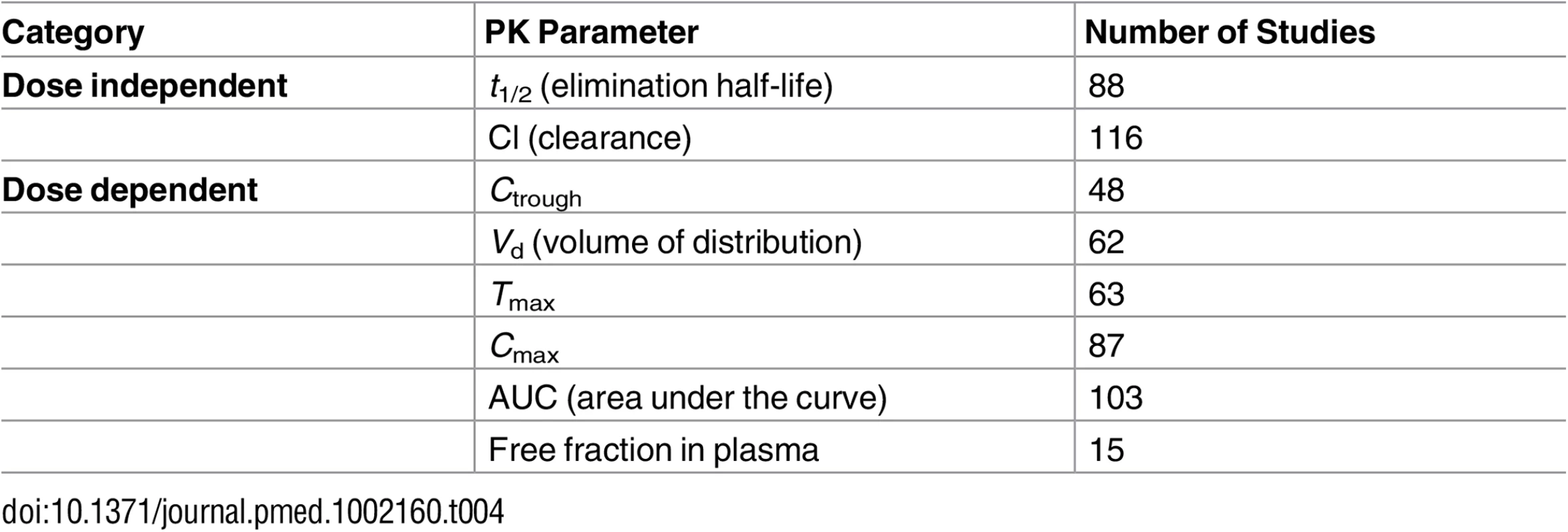
Pharmacokinetics Parameters That Are Vital for Dosing Decision Support in Pregnancy
We clustered the different PK parameters into three groups. (1) Distribution parameters are Vd and percent of free fraction. Vd defines how widely the drug is spread in the body. Larger Vd causes lower peak plasma concentration (Cmax) and also longer elimination half-life. Percent free fraction represents the fraction (percent) of the drug in plasma that is unbound to plasma proteins and, therefore, likely to be pharmacologically active. (2) Exposure parameters are Cmax, Ctrough, AUC, C/D ratio. These represent indices of plasma drug concentrations. Cmax and Ctrough are the highest and lowest levels within a dosing interval, respectively. AUC is literally the area bounded by the drug concentration–time curve and the x-axis, equivalent to an average drug concentration over time. C/D ratio is the dose-standardized drug concentration in plasma or serum at a given time. By and large, these parameters signify drug exposure levels at a given time point or on average, thereby potentially serving as a surrogate for drug effects. (3) Elimination parameters are t1/2 and clearance. Half-life is related to the velocity of a drug’s disappearance from plasma/serum. Clearance is an index of drug elimination capacity: higher clearance results in a smaller AUC and a shorter elimination half-life, reducing drug exposure levels.
Tables 5–18 provide information regarding changes in PK parameters (weight-standardized values, if available) during pregnancy compared to the nonpregnant state, assorted by drug classes and the data agreement definitions provided above. In these tables, non-significant results are shown together with statistically significant results (in bold). When a certain PK parameter was reported by several studies, the median value and the range in parentheses are provided. The quality column represents the quality score that was assigned to the study, according to the ClinPK Statement checklist. If the drug was investigated in more than one study, the quality column presents the average quality score of all the studies. Among the frequently investigated drug classes (antibiotics, antidepressants, antiepileptics, cardiovascular drugs, antiretrovirals, and antimalarials), studies have demonstrated enhanced elimination together with a decrease in exposure in pregnancy, indicating decreased availability of the drugs in pregnant women compared to nonpregnant women so far as total drug levels (bound plus unbound) are concerned.
Tab. 5. Antibiotics: consistent/single studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 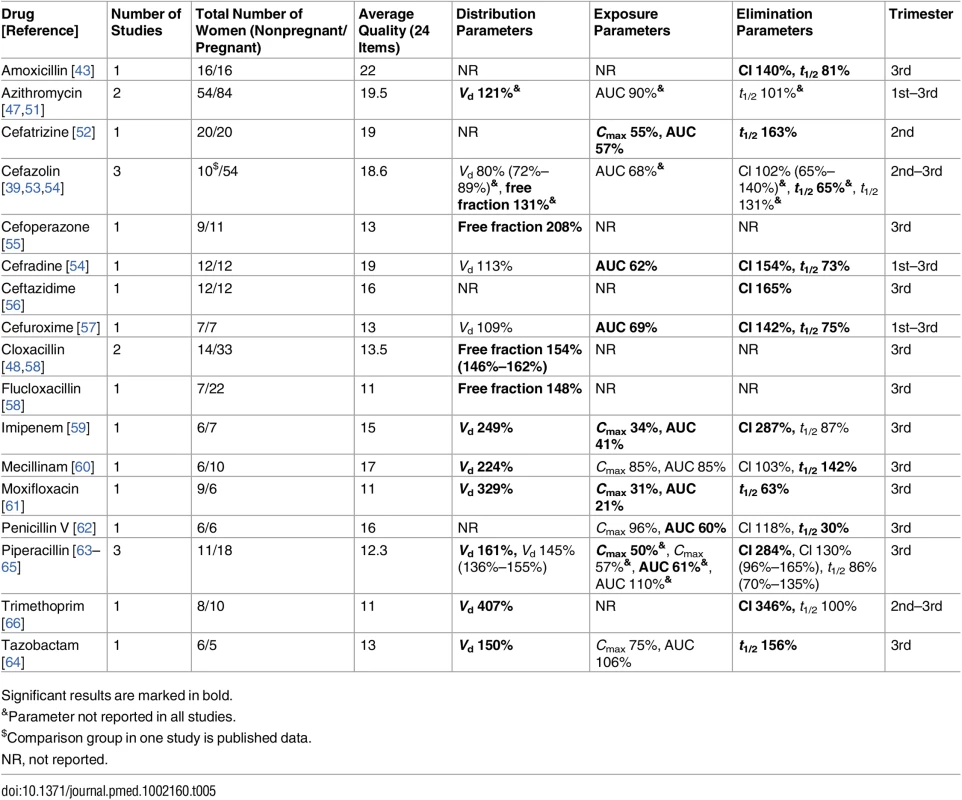
Significant results are marked in bold. Tab. 6. Antibiotics: inconsistent studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/non-pregnant values). 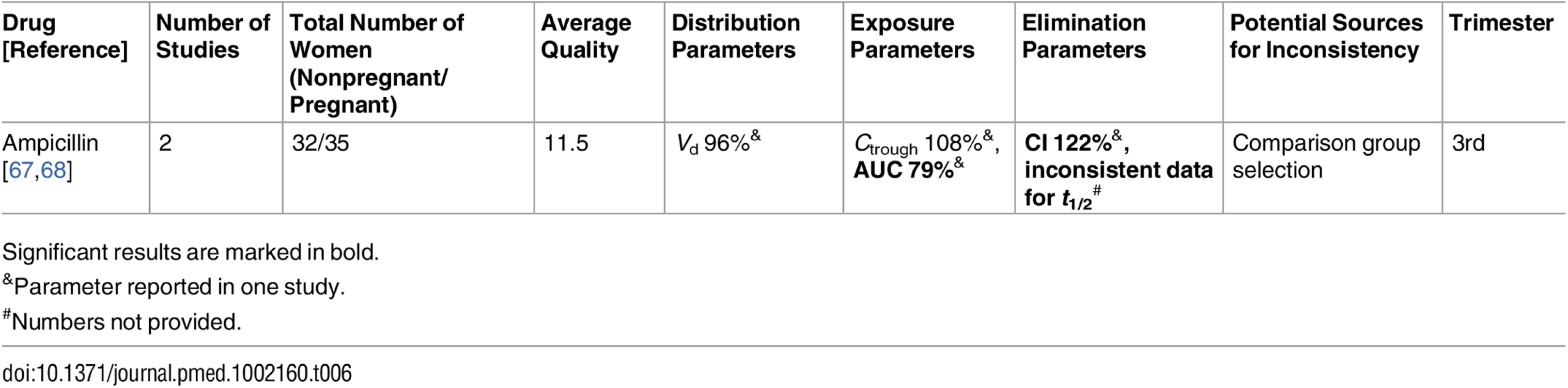
Significant results are marked in bold. Tab. 7. Antidepressant/anxiolytic drugs: consistent/single studies of pregnancy associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 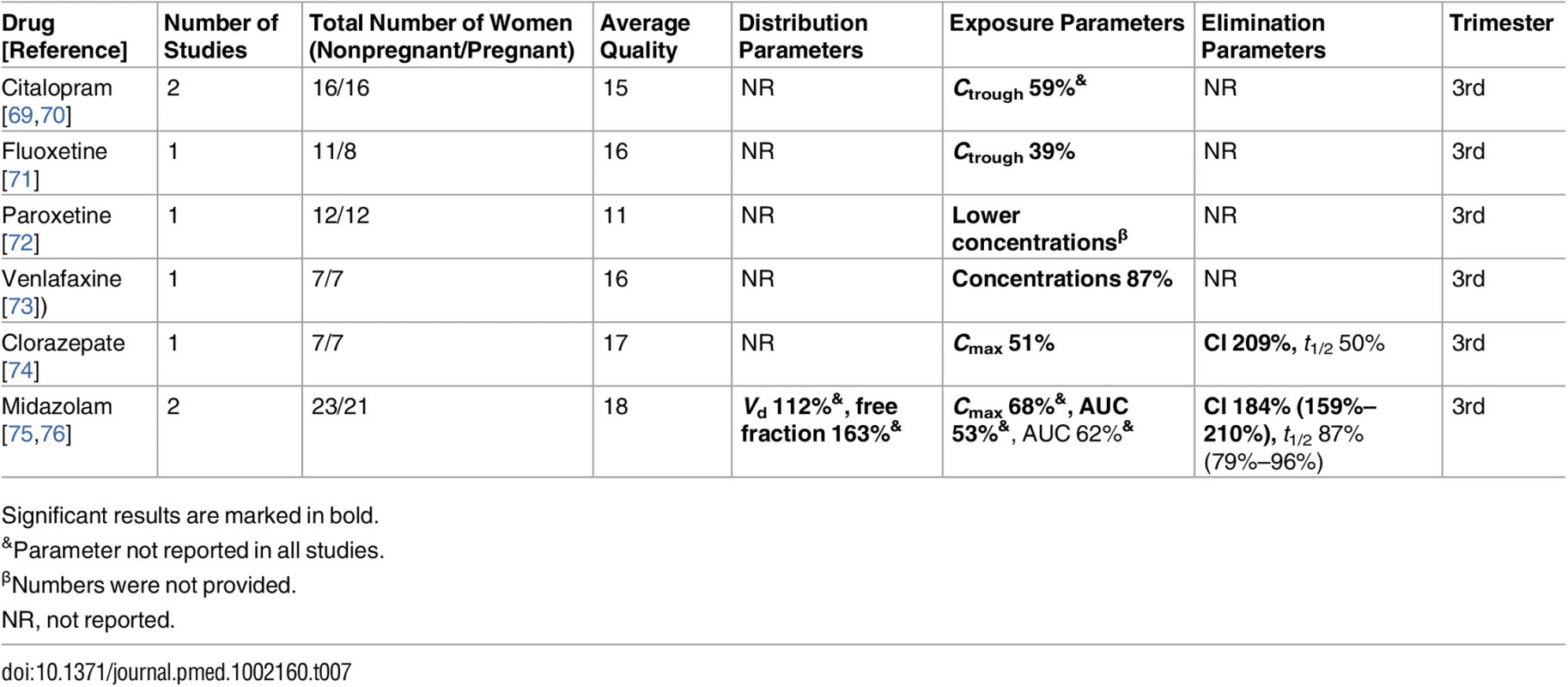
Significant results are marked in bold. Tab. 8. Antiepileptic drugs: consistent/single studies of pregnancy associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 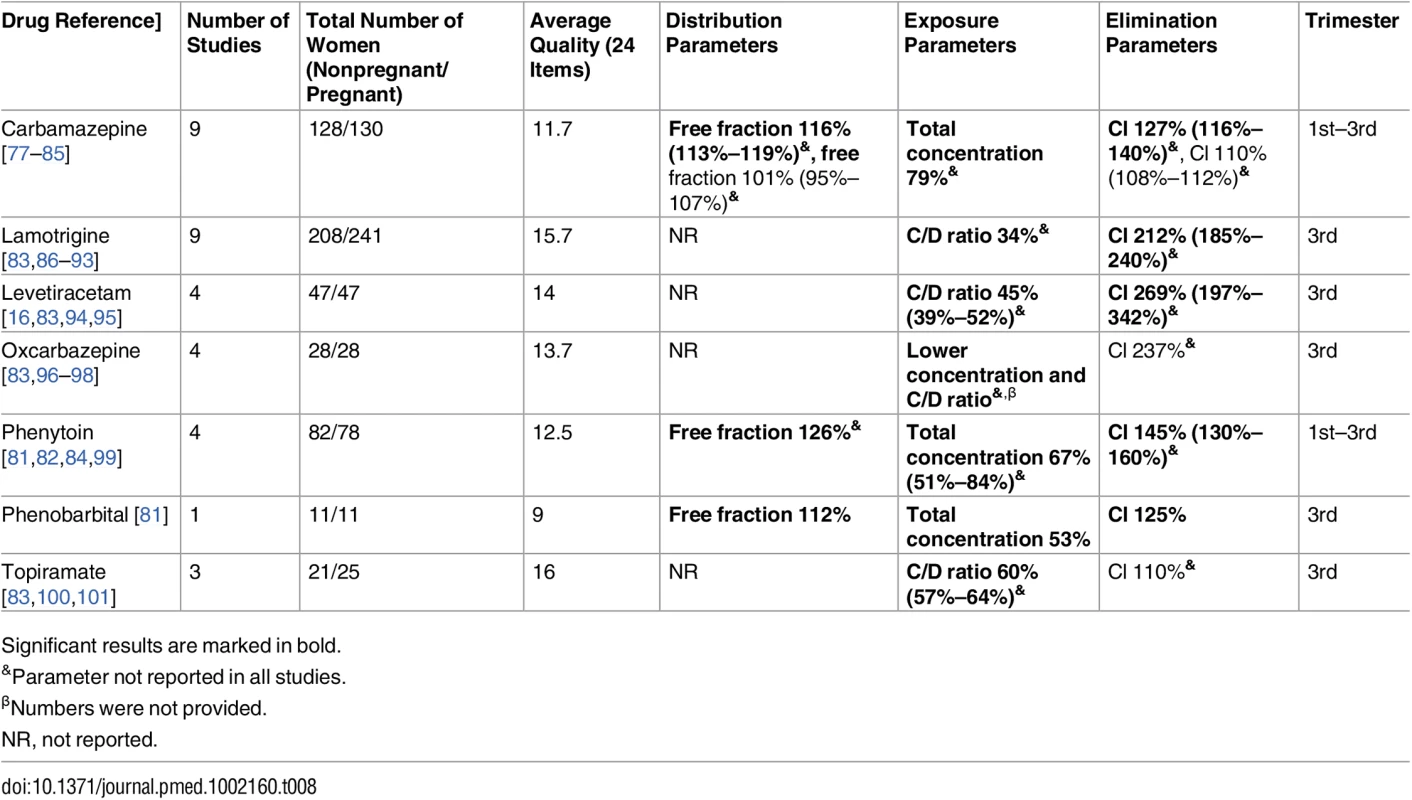
Significant results are marked in bold. Tab. 9. Drugs for analgesia and anesthesia: consistent/single studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 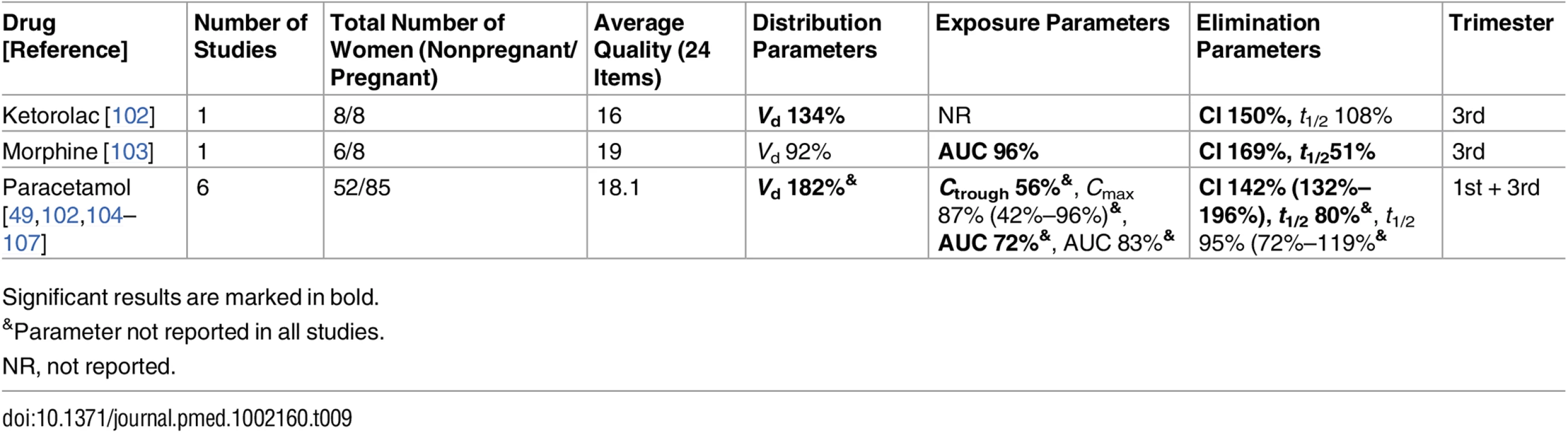
Significant results are marked in bold. Tab. 10. Drugs for analgesia and anesthesia: inconsistent studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 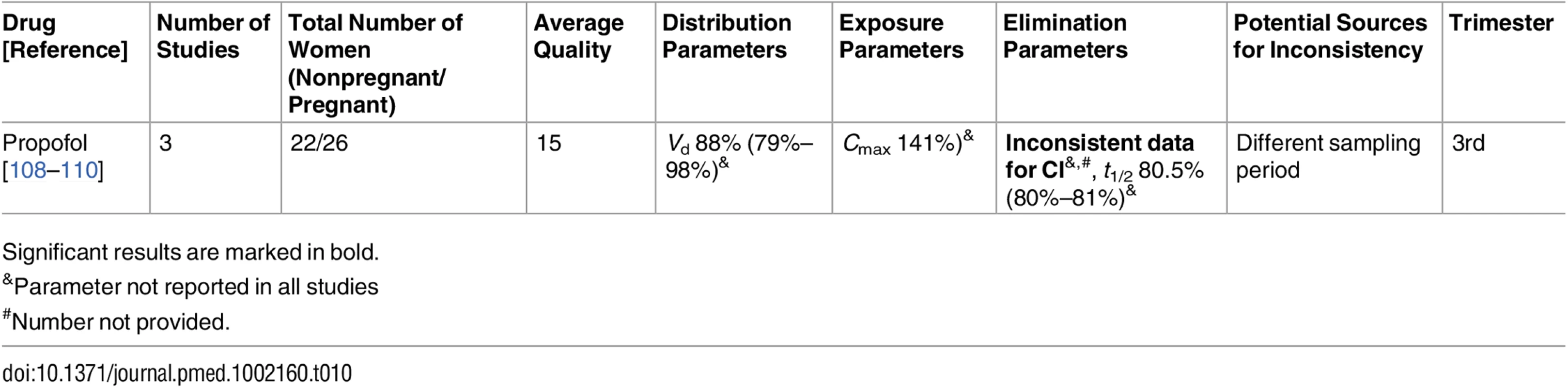
Significant results are marked in bold. Tab. 11. Antithrombotic drugs: consistent/single studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 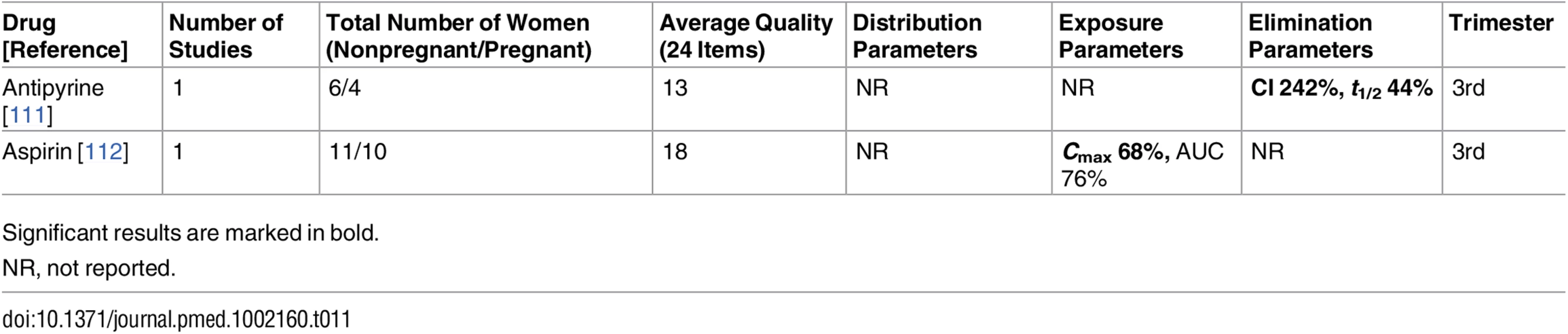
Significant results are marked in bold. Tab. 12. Antithrombotic drugs: inconsistent studies of pregnancy-associated pharmacokinetic changes. 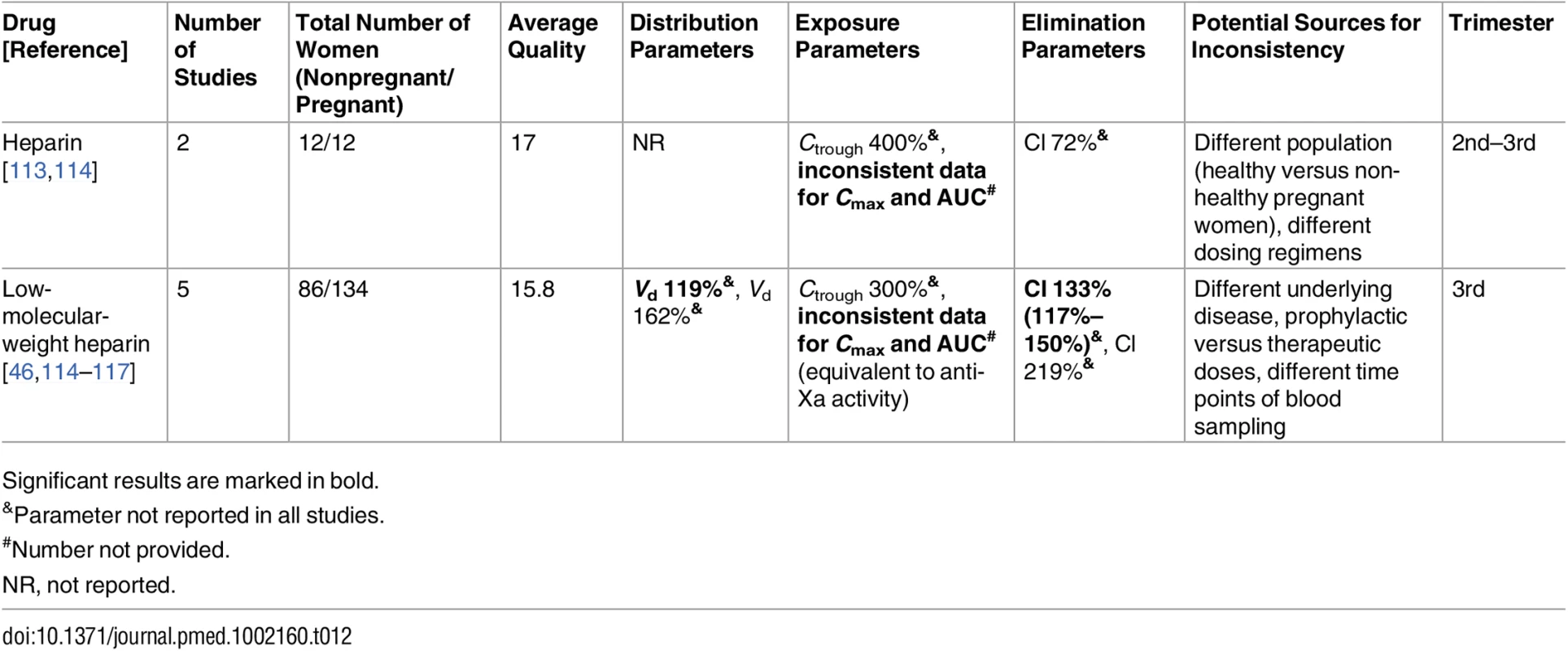
Significant results are marked in bold. Tab. 13. Cardiovascular drugs: consistent/single studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 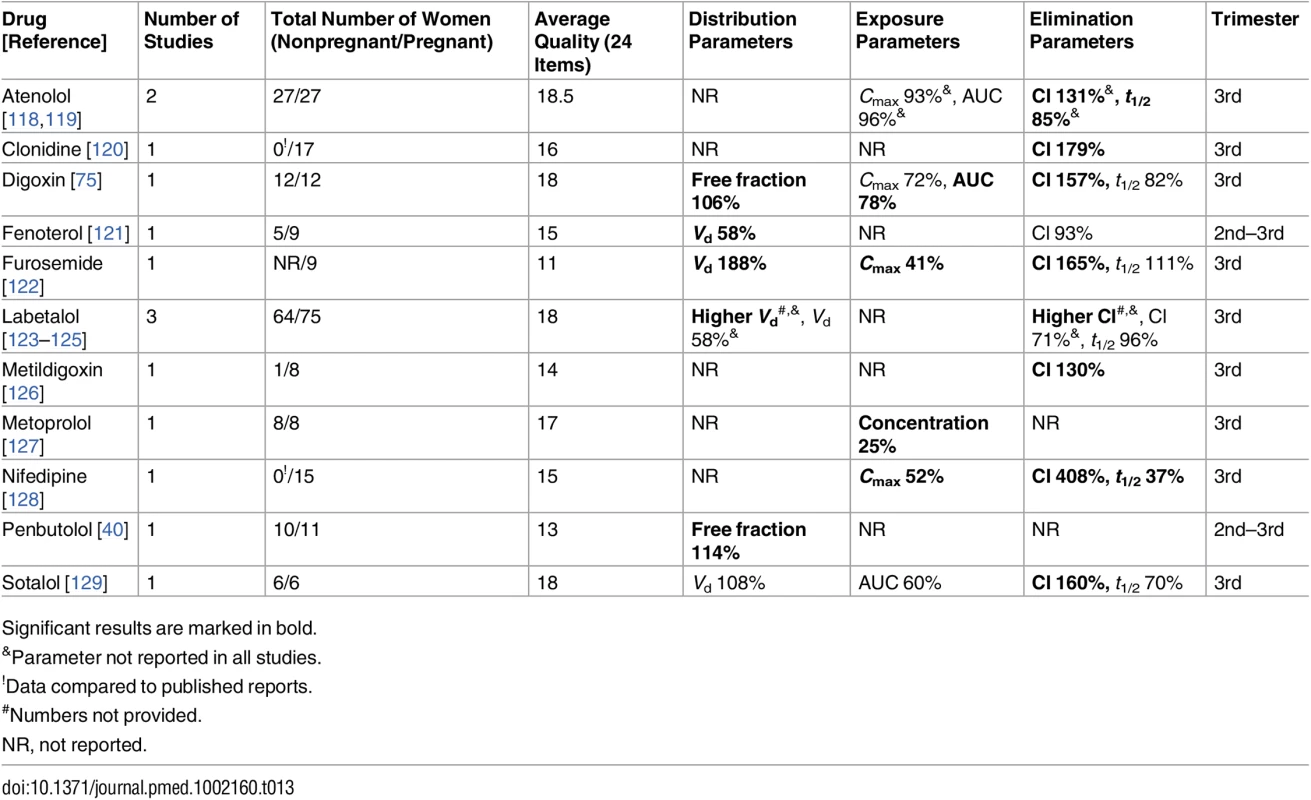
Significant results are marked in bold. Tab. 14. Antiretroviral drugs: consistent/single studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 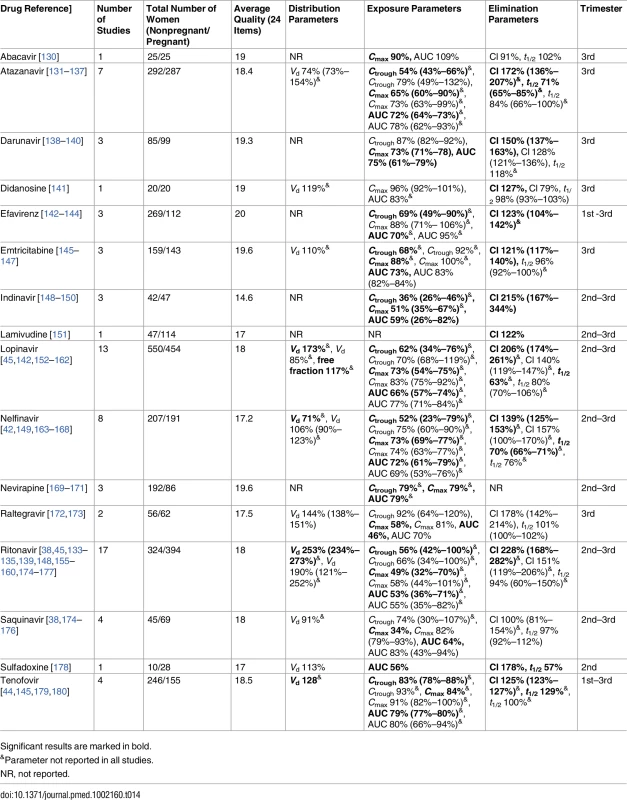
Significant results are marked in bold. Tab. 15. Antimalarial drugs: consistent/single studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 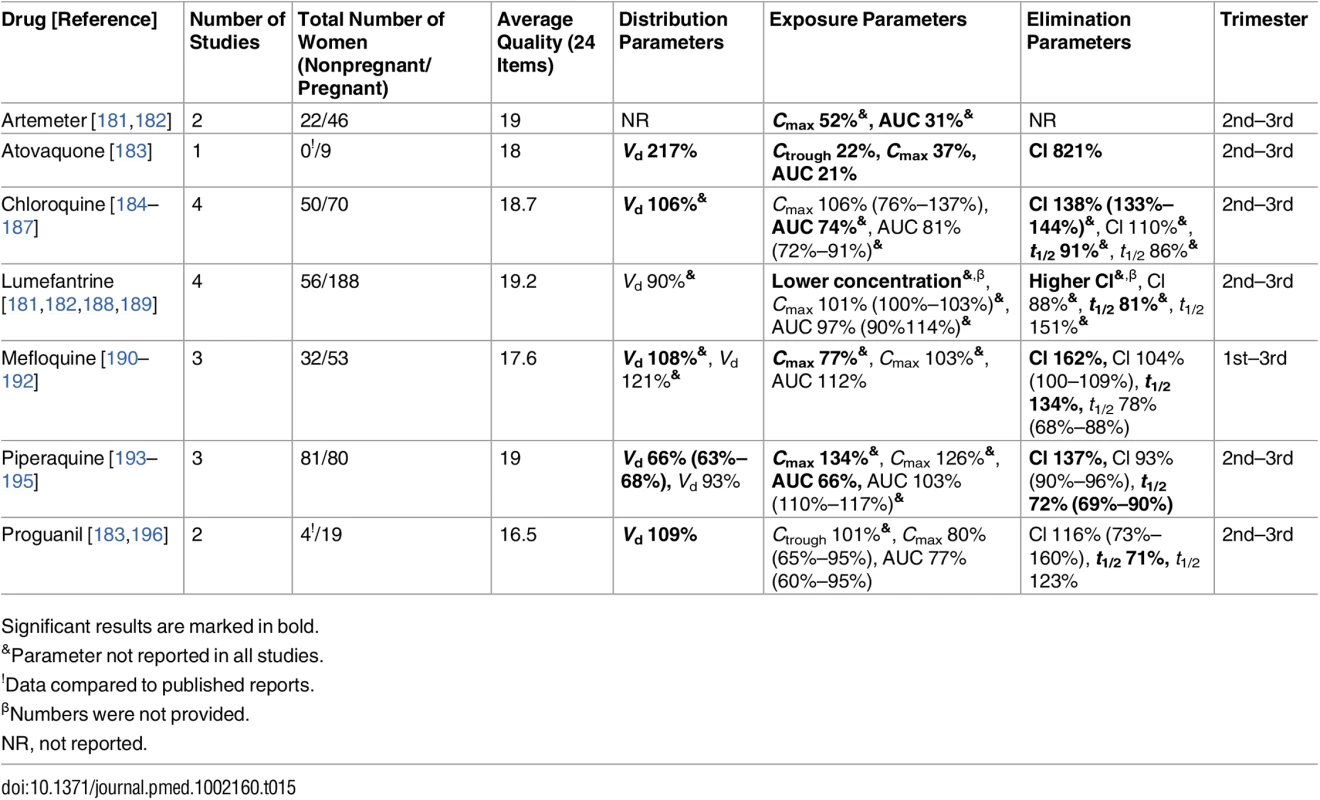
Significant results are marked in bold. Tab. 16. Antimalarial drugs: inconsistent studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 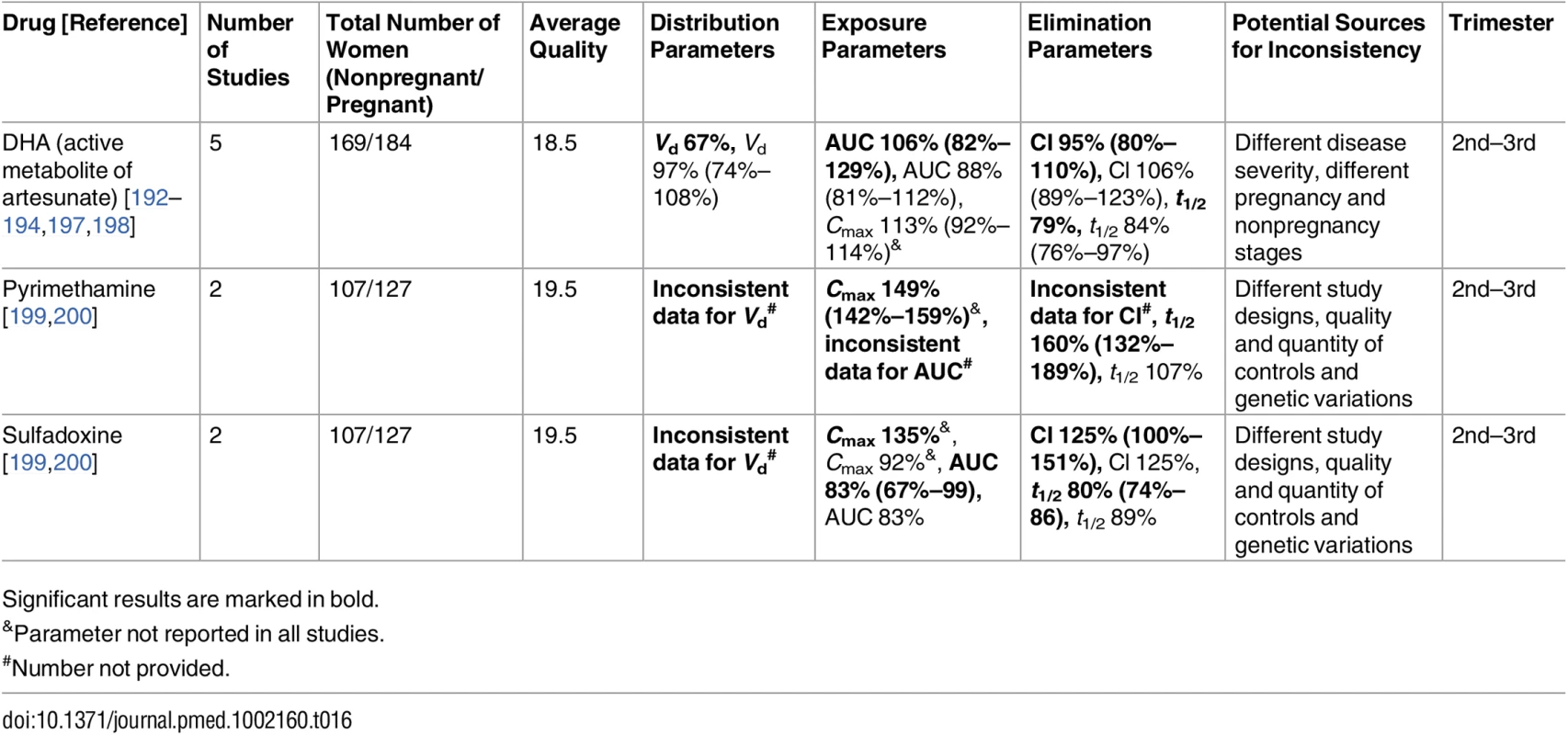
Significant results are marked in bold. Tab. 17. Miscellaneous classes: consistent/single studies of pregnancy associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 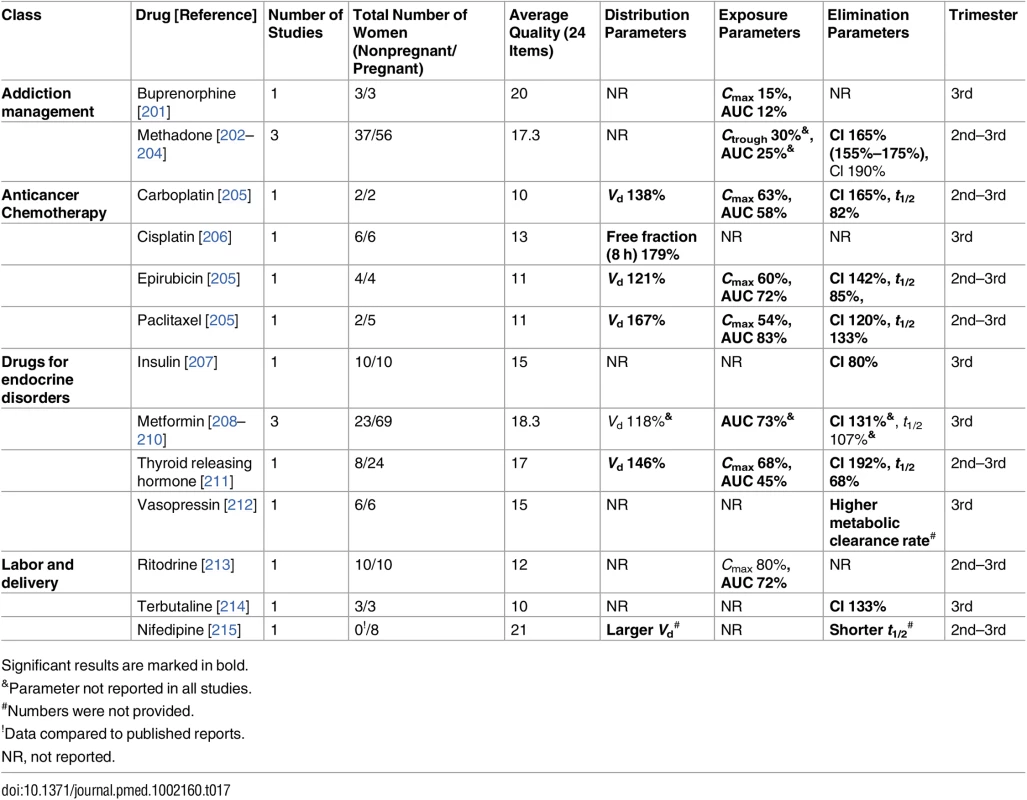
Significant results are marked in bold. Tab. 18. Miscellaneous classes: inconsistent studies of pregnancy-associated pharmacokinetic changes (percent calculated as pregnant/nonpregnant values). 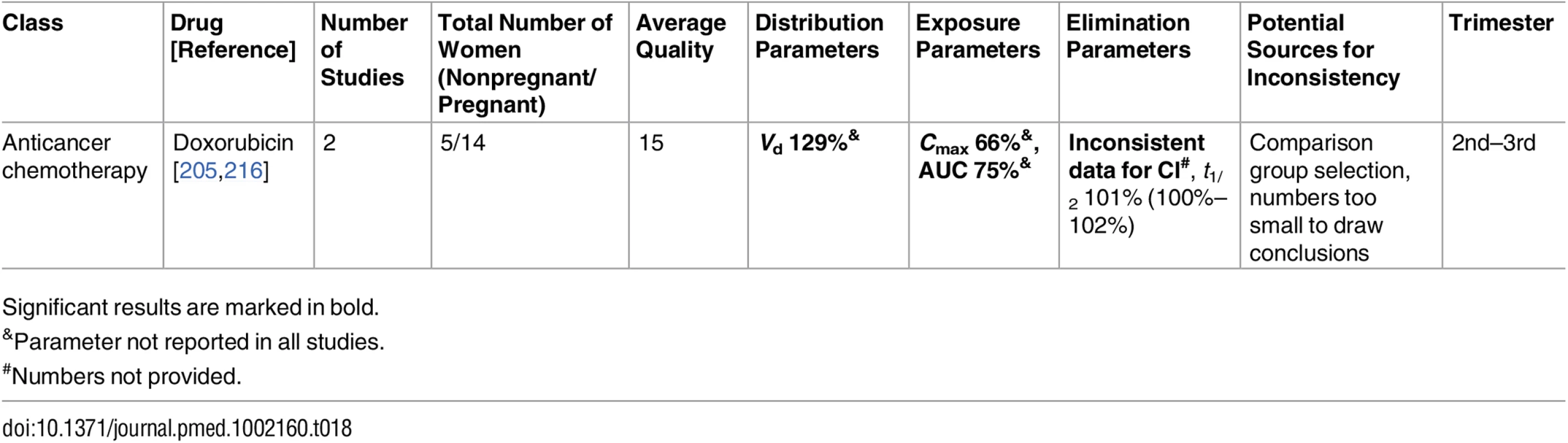
Significant results are marked in bold. Table 19 shows drugs for which all the studies (36) reported no statistically significant PK differences between pregnant and nonpregnant women. Most of the drugs presented in Table 19 were only investigated in one study, while sertraline, propranolol, quinine folic acid and vitamin D3 were each presented in two publications. For sertraline, statistically non-significant decreases in the exposure parameters were reported [70,217]. In the case of propranolol, mean elimination half-life in pregnancy was shorter in both studies, but the exposure parameter (AUC) changes were not consistent; non-significant increase in the AUC [218] versus non-significant decrease in AUC [219]. Consistent but non-significant increase in Cl was reported for quinine [189,220–222]. Plasma folate concentrations showed no statistically significant changes [221,222], but conflicting change directions were seen in the mean values, depending on the dose [222]. Similarly, vitamin D3 showed conflicting change directions in exposure parameters, which were statistically non-significant [223,224].
Tab. 19. Non-significant pharmacokinetic differences between pregnant and nonpregnant women. 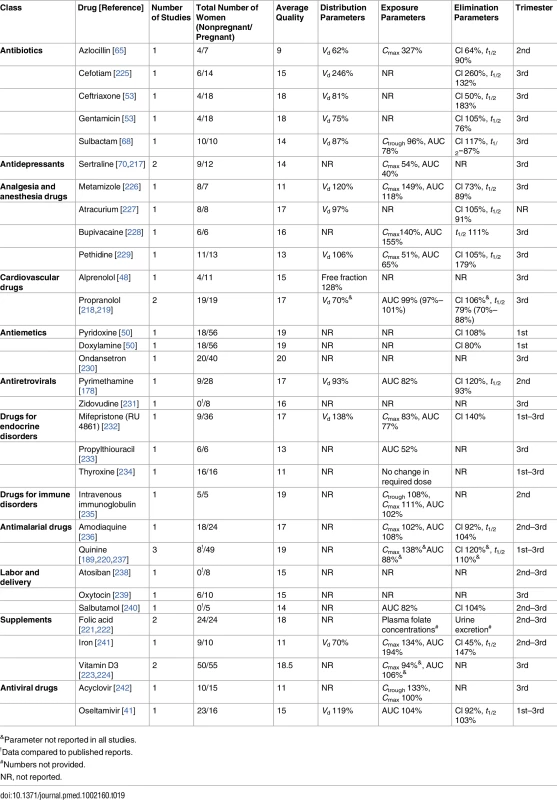
&Parameter not reported in all studies. Sixty of the total 218 PK observations (27.5%) reported changes in either the elimination parameters or exposure parameters. Seven PK observations (3.2%) did not report either exposure or elimination parameters. Among the 116 PK observations reporting changes in both elimination and exposure, 79.3% (92) demonstrated increased elimination together with decreased exposure in pregnant women compared to the nonpregnant population.
Discussion
In this first systematic review, to our knowledge, of pregnancy-associated PK changes, we were able to obtain a clear overview of the landscape of the field. Now that trends of pregnancy PK change have been mapped in major drug categories and responsible metabolism or transport pathways, existing knowledge gaps critical for patient management can be addressed by the combined efforts of regulatory agencies, academia, and industry. As many women presently delay childbearing to an older age [243] and the frequency of medical conditions seen during pregnancy among older women is dramatically greater than that of younger women [244], the results of this review raise the question of whether there are sufficient data to manage these health issues appropriately during pregnancy.
Recently, the most commonly used medications in the first trimester were reported [245]. Results from 5,381 mothers identified 54 different medications used in the first trimester by at least 0.5% of pregnant women. The most commonly used prescription medications reported fell into the categories of antibiotics, analgesics, antiemetics, antidiabetic medications, and antidepressants. Among those 54 most commonly used medications, only a few had adequate data available to assess PK characteristics and dosing recommendation during pregnancy, as demonstrated by our present study results.
Although our study strived to identify all available studies describing PK changes occurring in pregnancy, the total number of these studies was relatively small. Widespread exclusion of pregnant women from clinical studies is most probably the major reason for this limitation.
Changes such as increased clearance, reduced half-life, and reduced AUC in pregnancy have been described for many drugs. These PK alterations generally lead to lower drug concentrations in plasma, decreasing maternal target exposure to drug molecules. However, whether these PK changes compromise efficacy is not necessarily certain. Indeed, the total (unbound plus bound fractions) serum concentration of a drug does not necessarily reflect its activity, as lowered plasma albumin concentration during pregnancy may increase free “active” drug concentrations, depending on the PK characteristics of the drug. Moreover, the impact of maternal dose modifications on fetal exposure requires careful planning.
Published data were inconsistent for several medications, preventing this review from defining a certain direction in PK changes. These conflicting results were seen among the antimalarial drugs (pyrimethamine [199,200], sulfadoxine [199,200], and dihydroartemisinin (DHA) [192–194,197,198]), antithrombotic drugs (unfractionated heparin [113,114] and low-molecular-weight heparin [46,114–117]), and other drugs (ampicillin [67,68] and doxorubicin [205,216]). We will discuss these drugs in detail in the following section. Also, we confirmed that the current understanding of pregnancy-associated decrease in CYP1A2 and CYP2C19 activities is not based on large studies. These findings require further validation before making clinical recommendations.
For patients who are indicated to undergo routine therapeutic drug monitoring for clinical decision making and dose titration, pregnancy may be a challenging period in which serum drug levels may decrease below the target value despite adequate adherence by patients to their regimen. As we discussed above, decrease in drug exposure levels (e.g., reduction in serum concentrations and AUC) in pregnancy may not necessarily alter clinical outcomes. The decision to change dosing schedules in patients based on therapeutic drug monitoring and/or knowledge of PK changes in pregnancy should be associated with critical assessment of the risks of therapeutic failure and adverse effects.
Fifty-one studies included in our review investigated more than one drug. Among the antiretroviral class, all studies but one presented women with HIV infection who were treated with more than one antiretroviral medication. The only study that examined a single antiretroviral drug is also the earliest study from this class, investigating zidovudine during pregnancy (published in 1993) [231]. The authors noted that in those 51 studies, no drug that interfered with absorption, elimination, distribution, etc., was included. In addition, as per Health Canada, the US Centers for Disease Control and Prevention, and the World Health Organization, antiretroviral therapy, when indicated, includes at least three agents. Therefore, it is most natural to have multiple drugs on board when conducting a PK study in HIV-positive cohorts.
Clinical Outcome Data
The focus of the present systematic review is on PK data in pregnancy as a first step toward improving drug therapy in this orphan population. Although clinical outcomes were not reported in many of these PK studies, we identified several studies with such information.
For lamotrigine and indinavir, pregnancy-related changes in the clinical endpoints were in agreement with the observed PK changes [88,148]. Others have found significant PK changes and yet no clinical correlation was demonstrated (emtricitabine [145], levetiracetam [16], and topiramate [101]). Interestingly, while the PK-clinical correlation of some drugs was consistent among different studies (e.g., lamotrigine [86,88,91]), this was not the case for others (e.g., oxcarbazepine [96,97]). The scope of studies to investigate both PK and clinical outcome data seems to be dependent on drug class. For example, none of the studies that investigated antibiotics [47,52,53] or anesthetic and analgesic drugs [102] provided data on clinical outcomes. On the other hand, studies of addiction management drugs and antidepressant drugs reported clinical data, showing a positive correlation between decreased drug exposure and diminished clinical effects in pregnancy [70,202]. A study investigating cardiovascular drugs that reported clinical outcomes did not demonstrate significant positive clinical correlations [127]. The three drug groups that provided the richest evidence regarding clinical correlation were the antiretrovirals, antimalarials, and antiepileptics. In the case of antiretrovirals, all studies had showed decreased drug exposure in pregnancy due to PK changes. While most of these studies reported adequate viral suppression and no mother-to-child HIV transmission [132,135,138], one study reported an increased viral load during pregnancy, with a few cases of neonatal transmission of the virus [150]. Conflicting clinical results were also reported for antimalarial drugs: while some studies reported equal parasite clearance time or no increase in treatment failure in spite of decreased exposure [182], others demonstrated a positive correlation between the decreased exposure and poor clinical outcome, reporting an increase in treatment failure or a decrease in post-treatment prophylactic effect [181,195].
Our review has highlighted those medications that have relatively consistent PK change directions in pregnancy. This collection of PK data could prove to be a decision support base for future attempts to tailor medication prescription for pregnant women to achieve target serum concentrations; however, one must take into account that many studies often report undiminished drug efficacy despite the aforementioned pregnancy-associated PK changes [132,135,138,145,146,163,172,177].
Drugs with a Consistent Pharmacokinetics Change Direction
For the vast majority of drugs (114), data gathered in this review are consistent among studies. Although not all studies presented a full set of PK parameters, the evidence exists to support the notion that in pregnancy, drug exposure levels per given dose are decreased for most medications. In addition, lower plasma protein binding (higher free drug level) is a consistent finding. This tandem trending of higher Cl rate, higher Vd, and higher free fraction is observed for most drugs except for those metabolized by CYP1A2 and CYP2C19, which show a trend toward decreased metabolism during pregnancy.
Drugs with Variable Pharmacokinetic Change Directions
Studies of seven drugs were found to yield conflicting PK results among studies in pregnancy. Three of these drugs are part of the antimalarial drug group (pyrimethamine [199,200], sulfadoxine [199,200], and DHA [192–194,197,198]), two are antithrombotic drugs (unfractionated heparin [113,114] and low-molecular-weight heparin [46,114–117]), one is an antibiotic (ampicillin [67,68]), and the last is an anticancer chemotherapeutic drug (doxorubicin [205,216]). The average quality score of the consistent antibiotic and antithrombotic studies tended to be higher than the quality score of the inconsistent studies from the same group (14.4 versus 11.5, p < 0.05, and 16.4 versus 15.5, p = 0.119, for the antibiotic and antithrombotic drugs, respectively). Nevertheless, the average quality score of the consistent studies was not higher than that of the inconsistent studies for both the antimalarial drugs (18.2 versus 19.1, p = 0.62) and the anticancer chemotherapeutics (11.5 versus 14.5: averages). Thus, variability of quality scores cannot account for the inconsistent PK directions that were demonstrated.
Ampicillin [67,68]
The pregnancy PK of ampicillin had been reported in two studies [67,68]. Both studies presented PK parameters during delivery and demonstrated conflicting results regarding the half-life of elimination. While the elimination half-life presented in one study [67] was longer among pregnant women compared to the control group (58.3 min versus 44.8 min, respectively), the other study [68] demonstrated a difference in the opposite direction (52.4 min versus 69.9 min, respectively). We believe that one of the potential sources for these conflicting results is the choice of control group: while the control group in the former [67] comprised healthy nonpregnant individuals, the post-pregnant women (who may be still under some influence of pregnancy-associated physiological changes) served as their own control in the latter study [68].
Pyrimethamine and sulfadoxine [199,200]
The pregnancy PK of this antimalarial drug combination had been studied in Papua New Guinea [199] and in four African countries (Mozambique, Sudan, Zambia, and Mali) [200]. These two publications present conflicting results. Concerning pyrimethamine, the Papua New Guinea time-concentration plots showed average pregnancy levels to be lower at most time points than the nonpregnant comparison, while data from the African countries indicated the opposite (measurements in pregnancy were higher). This same phenomenon was also evident in some, but not all, data reported on sulfadoxine.
Appraising the methodologies used by these two research groups, we have identified a potential source for this conflict regarding the raw data. In both studies, pregnancy was associated with significant anemia, and both papers (Table 1) reported an average reduction of ~20% in hemoglobin values during pregnancy. However, while the Papua New Guinea study used plasma for drug assays, the African study used whole blood from dried blood spots, with no correction for hematocrit values. This limitation of the dried blood spot method may have caused an overestimation of drug levels per blood spot area in pregnant women in the African study, as a result of a relative abundance of plasma per blood spot due to severe anemia [246]. Although there are likely to be other factors contributing to the discrepancies between the two studies, we speculate that the difference in the sample matrix is the major cause, and that pyrimethamine and sulfadoxine apparent clearance is higher during pregnancy. This also highlights the importance of methodological standardization in PK studies, including sample analysis procedures.
Dihydroartemisinin [192–194,197,198]
Five studies met the inclusion criteria that investigated the effect of pregnancy on the PK of DHA, the active metabolite of artesunate, for severe malaria (Table 16). Inconsistencies in PK parameter changes exist in the AUC and clearance of DHA; a statistically significant reduction in AUC (decreased exposure) and an increase in oral clearance in pregnancy were observed in one study [197], while the change directions were opposite in the other [198]. However, this can be explained by increased disease severity at PK sampling in the latter [198], as systemic exposure of DHA is higher in infected patients with a severe course of malaria than in those with a mild course [198,247]. The increased DHA exposure in acute malaria during pregnancy after oral artesunate is probably a result of increased bioavailability due to decreased presystemic elimination through glucuronidation in the intestine [198]. Hepatic metabolism of DHA occurs through enzymes such as CYP2B6, UGT1A9, and UGT2B7, but data on these isoenzymes in pregnant women with acute infection are still limited.
Low-molecular-weight heparin [46,114–117] and heparin [113,114]
Six studies investigated the PK of heparin and low-molecular-weight heparin by using factor anti-Xa activity as a surrogate marker of enoxaparin (n = 2), dalteparin (n = 3), and unfractionated heparin (n = 2) in pregnant women (Table 12). The statistically significant discrepancies in the pharmacokinetic parameters can be mainly attributed to the different study designs, dosing regimens, and indications for heparin in the study population (therapeutic versus prophylactic administration). However, the most important parameter in these studies is the Cmax (2–4 h after administration) of the factor anti-Xa activity because it determines whether the woman is properly controlled for thromboembolic events. Studies with a dose increase design had an increase in the Cmax of anti-Xa activity [114,116]. The remaining studies revealed lower Cmax values during pregnancy, even with higher doses [46,113,115,117]. Those studies [46,114,117] showed higher clearance during pregnancy, which was statistically significant in two of them [46,117]. The recommended therapeutic range of 0.6–1.0 IU/ml [248] was achieved in only half of the population in one of the two studies [117]. It should be noted that the Barbour et al. [116] study compared women in the third trimester to women in early pregnancy (as the control group). Peak levels of anti-Xa activity (equivalent to Cmax) were 0.63 IU/ml in early pregnancy versus 0.69 IU/ml in the third trimester. These control values were somewhat higher than the Cmax values reported for the other nonpregnant populations in the other studies [46,114,115].
Study Limitations
Most studies that demonstrated significant PK changes had relatively small sample sizes. The mixture of small sample sizes with different pharmacological/research methodologies poses substantial challenges to comparing and summarizing their study results. Another limitation stems from the fact that, for many drugs, pregnancy-related PK changes were considered to be significant on the basis of a single study, often of low quality, with small numbers of women and a small subset of PK parameters. Although we show single studies with statistically significant results in the “consistent” category for simplicity of presentation, single studies do not inform on the consistency of the changes. Further replication studies are required. The quality assessment of the studies included in this review was performed using the ClinPK checklist for assessing methodological quality in clinical PK studies. This checklist provides meticulous guidelines for quality assessment, but having been only recently published, it will need refinement and external validation.
We are acutely aware of the fact that by excluding studies lacking a comparison group of nonpregnant women we may miss a significant amount of PK data. However, in the context of our research question, we find it imperative to not only document certain kinetic patterns but also provide quantitative or semiquantitative estimates of the extent and directionality of those pregnancy-associated PK changes. Comparing cohort data for pregnant women to normal population averages would expose our study to a multitude of biases, mainly due to the fact that the most dominant contributors to the “normal population” PK parameter values, in textbooks and seminal papers, are healthy men (Lexicomp and Micromedex databases, for example, report “adult” data with no gender, yet the citation lists are rich with male volunteer publications). Moreover, in the majority of studies included in this systematic review, pregnant women served as their own controls (in the prepregnancy or postpartum state), which isolates the pregnancy as the most dominant factor in the assessment.
Lastly, trimester-specific PK changes were difficult to summarize. While most of the studies provided third trimester results, others reported separate results from the second and third trimesters, and few reported separate results from all trimesters. Physiological changes in pregnancy take place progressively during gestation (reviewed by Costantine [8] and Loebstein et al. [9]). As such, we hypothesized that this would lead to trimester-specific differences in drug disposition. Unfortunately, however, many studies in this review did not report trimester-specific changes, which could possibly have contributed to the conflicting PK results in some studies described above.
Conclusions
Our systematic analyses confirmed that many drugs are subject to pregnancy-associated PK changes, which may alter plasma/serum drug concentration profiles. However, we have also found a paucity of clinically useful data on whether dose adjustment is necessary for these PK changes. Where such PK studies were done, generally only a few PK parameters were estimated, sample sizes were small, and maternal and/or fetal outcomes were not examined. Further studies that address these limitations are needed to optimize drug therapy for pregnant women.
Supporting Information
Zdroje
1. Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191(2):398–407. doi: 10.1016/j.ajog.2004.04.025 15343213
2. Glover DD, Amonkar M, Rybeck BF, Tracy TS. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol. 2003;188(4):1039–45. 12712107
3. Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):51 e1–8.
4. Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193(3 Pt 1):771–7.
5. Bakker MK, Jentink J, Vroom F, Van Den Berg PB, De Walle HE, De Jong-Van Den Berg LT. Drug prescription patterns before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. BJOG. 2006;113(5):559–68. doi: 10.1111/j.1471-0528.2006.00927.x 16637899
6. Gagne JJ, Maio V, Berghella V, Louis DZ, Gonnella JS. Prescription drug use during pregnancy: a population-based study in Regione Emilia-Romagna, Italy. Eur J Clin Pharmacol. 2008;64(11):1125–32. doi: 10.1007/s00228-008-0546-y 18685836
7. Olesen C, Steffensen FH, Nielsen GL, de Jong-van den Berg L, Olsen J, Sorensen HT. Drug use in first pregnancy and lactation: a population-based survey among Danish women. The EUROMAP group. Eur J Clin Pharmacol. 1999;55(2):139–44. 10335909
8. Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5 : 65. doi: 10.3389/fphar.2014.00065 24772083
9. Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33(5):328–43. doi: 10.2165/00003088-199733050-00002 9391746
10. Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44(10):989–1008. doi: 10.2165/00003088-200544100-00001 16176115
11. Anderson GD. Using pharmacokinetics to predict the effects of pregnancy and maternal-infant transfer of drugs during lactation. Expert Opin Drug Metab Toxicol. 2006;2(6):947–60. doi: 10.1517/17425255.2.6.947 17125410
12. DeVane CL, Stowe ZN, Donovan JL, Newport DJ, Pennell PB, Ritchie JC, et al. Therapeutic drug monitoring of psychoactive drugs during pregnancy in the genomic era: challenges and opportunities. J Psychopharmacol. 2006;20(4 Suppl):54–9. doi: 10.1177/1359786806066054 16785271
13. Leppik IE, Rask CA. Pharmacokinetics of antiepileptic drugs during pregnancy. Semin Neurol. 1988;8(3):240–6. doi: 10.1055/s-2008-1041385 3057557
14. McAuley JW, Anderson GD. Treatment of epilepsy in women of reproductive age: pharmacokinetic considerations. Clin Pharmacokinet. 2002;41(8):559–79. doi: 10.2165/00003088-200241080-00002 12102641
15. Pennell PB. Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology. 2003;61(6 Suppl 2):S35–42.
16. Tomson T, Palm R, Kallen K, Ben-Menachem E, Soderfeldt B, Danielsson B, et al. Pharmacokinetics of levetiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia. 2007;48(6):1111–6. doi: 10.1111/j.1528-1167.2007.01032.x 17381438
17. Wadelius M, Darj E, Frenne G, Rane A. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther. 1997;62(4):400–7. doi: 10.1016/S0009-9236(97)90118-1 9357391
18. Tsutsumi K, Kotegawa T, Matsuki S, Tanaka Y, Ishii Y, Kodama Y, et al. The effect of pregnancy on cytochrome P4501A2, xanthine oxidase, and N-acetyltransferase activities in humans. Clin Pharmacol Ther. 2001;70(2):121–5. doi: 10.1067/mcp.2001.116495 11503005
19. Koren G. Pharmacokinetics in pregnancy; clinical significance. J Popul Ther Clin Pharmacol. 2011;18(3):e523–7. 22113390
20. Anger GJ, Piquette-Miller M. Pharmacokinetic studies in pregnant women. Clin Pharmacol Ther. 2008;83(1):184–7. doi: 10.1038/sj.clpt.6100377 17882157
21. Dickmann LJ, Isoherranen N. Quantitative prediction of CYP2B6 induction by estradiol during pregnancy: potential explanation for increased methadone clearance during pregnancy. Drug Metab Dispos. 2013;41(2):270–4. doi: 10.1124/dmd.112.047118 22815312
22. Choi SY, Koh KH, Jeong H. Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab Dispos. 2013;41(2):263–9. doi: 10.1124/dmd.112.046276 22837389
23. Ke AB, Nallani SC, Zhao P, Rostami-Hodjegan A, Unadkat JD. Expansion of a PBPK model to predict disposition in pregnant women of drugs cleared via multiple CYP enzymes, including CYP2B6, CYP2C9 and CYP2C19. Br J Clin Pharmacol. 2014;77(3):554–70. doi: 10.1111/bcp.12207 23834474
24. Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Disposition of carbamazepine and phenytoin in pregnancy. Epilepsia. 1994;35(1):131–5. 8112235
25. McGready R, Stepniewska K, Seaton E, Cho T, Cho D, Ginsberg A, et al. Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur J Clin Pharmacol. 2003;59(7):553–7. doi: 10.1007/s00228-003-0651-x 12955370
26. Lee JK, Chung HJ, Fischer L, Fischer J, Gonzalez FJ, Jeong H. Human placental lactogen induces CYP2E1 expression via PI 3-kinase pathway in female human hepatocytes. Drug Metab Dispos. 2014;42(4):492–9. doi: 10.1124/dmd.113.055384 24408518
27. Aweeka FT, Hu C, Huang L, Best BM, Stek A, Lizak P, et al. Alteration in cytochrome P450 3A4 activity as measured by a urine cortisol assay in HIV-1-infected pregnant women and relationship to antiretroviral pharmacokinetics. HIV Med. 2015;16(3):176–83. doi: 10.1111/hiv.12195 25407158
28. Chen H, Yang K, Choi S, Fischer JH, Jeong H. Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17beta-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos. 2009;37(9):1841–7. doi: 10.1124/dmd.109.026609 19546240
29. Roustit M, Jlaiel M, Leclercq P, Stanke-Labesque F. Pharmacokinetics and therapeutic drug monitoring of antiretrovirals in pregnant women. Br J Clin Pharmacol. 2008;66(2):179–95. doi: 10.1111/j.1365-2125.2008.03220.x 18537960
30. Pennell PB. Antiepileptic drugs during pregnancy: what is known and which AEDs seem to be safest? Epilepsia. 2008;49(Suppl 9):43–55. doi: 10.1111/j.1528-1167.2008.01926.x 19087117
31. Anderson GD, Carr DB. Effect of pregnancy on the pharmacokinetics of antihypertensive drugs. Clin Pharmacokinet. 2009;48(3):159–68. doi: 10.2165/00003088-200948030-00002 19385709
32. Clark CT, Klein AM, Perel JM, Helsel J, Wisner KL. Lamotrigine dosing for pregnant patients with bipolar disorder. Am J Psychiatry. 2013;170(11):1240–7. doi: 10.1176/appi.ajp.2013.13010006 24185239
33. Klieger C, Pollex E, Kazmin A, Koren G. Hypoglycemics: pharmacokinetic considerations during pregnancy. Ther Drug Monit. 2009;31(5):533–41. doi: 10.1097/FTD.0b013e3181b385ba 19730277
34. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244–55. doi: 10.1097/JCP.0000000000000087 24525634
35. McCormack SA, Best BM. Obstetric pharmacokinetic dosing studies are urgently needed. Front Pediatr. 2014;2 : 9. doi: 10.3389/fped.2014.00009 24575394
36. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 19621072
37. Kanji S, Hayes M, Ling A, Shamseer L, Chant C, Edwards DJ, et al. Reporting guidelines for clinical pharmacokinetic studies: the ClinPK Statement. Clin Pharmacokinet. 2015;54(7):783–95. doi: 10.1007/s40262-015-0236-8 25637173
38. Acosta EP, Bardeguez A, Zorrilla CD, Van Dyke R, Hughes MD, Huang S, et al. Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women? Antimicrob Agents Chemother. 2004;48(2):430–6. doi: 10.1128/AAC.48.2.430-436.2004 14742191
39. Allegaert K, Van Mieghem T, Verbesselt R, Vanhole C, Devlieger R, Cossey V, et al. Cefazolin plasma protein binding saturability during pregnancy. Methods Find Exp Clin Pharmacol. 2009;31(1):25–8. doi: 10.1358/mf.2009.31.1.1338413 19357795
40. Aquirre C, Rodriguez-Sasiain JM, Navajas P, Calvo R. Plasma protein binding of penbutolol in pregnancy. Eur J Drug Metab Pharmacokinet. 1988;13(1):23–6. 3396610
41. Beigi RH, Han K, Venkataramanan R, Hankins GD, Clark S, Hebert MF, et al. Pharmacokinetics of oseltamivir among pregnant and nonpregnant women. Am J Obstet Gynecol. 2011;204(6 Suppl):S84–8. doi: 10.1016/j.ajog.2011.03.002 21492826
42. Bryson YJ, Mirochnick M, Stek A, Mofenson LM, Connor J, Capparelli E, et al. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: Pediatric AIDS Clinical Trials Group (PACTG) Protocol 353. HIV Clin Trials. 2008;9(2):115–25. doi: 10.1310/hct0902-115 18474496
43. Andrew MA, Easterling TR, Carr DB, Shen D, Buchanan ML, Rutherford T, et al. Amoxicillin pharmacokinetics in pregnant women: Modeling and simulations of dosage strategies. Clin Pharmacol Ther. 2007;81(4):547–56. doi: 10.1038/sj.clpt.6100126 17329990
44. Best BM, Burchett S, Li H, Stek A, Hu C, Wang J, et al. Pharmacokinetics of tenofovir during pregnancy and postpartum. HIV Med. 2015;16(8):502–11. doi: 10.1111/hiv.12252 25959631
45. Best BM, Stek AM, Mirochnick M, Hu C, Li H, Burchett SK, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquire Immune Defic Syndr. 2010;54(4):381–8.
46. Casele HL, Laifer SA, Woelkers DA, Venkataramanan R. Changes in the pharmacokinetics of the low-molecular-weight heparin enoxaparin sodium during pregnancy. Am J Obstet Gynecol. 1999;181(5 Pt 1):1113–7.
47. Fischer JH, Sarto GE, Habibi M, Kilpatrick SJ, Tuomala RE, Shier JM, et al. Influence of body weight, ethnicity, oral contraceptives, and pregnancy on the pharmacokinetics of azithromycin in women of childbearing age. Antimicrob Agents Chemother. 2012;56(2):715–24. doi: 10.1128/AAC.00717-11 22106226
48. Herngren L, Ehrnebo M, Boreus LO. Drug binding to plasma proteins during human pregnancy and in the perinatal period. Studies on cloxacillin and alprenolol. Dev Pharmacol Ther. 1983;6(2):110–24. 6861596
49. Beaulac-Baillargeon L, Rocheleau S. Paracetamol pharmacokinetics during the first trimester. Eur J Clin Pharmacol. 1994;46(5):451–4. 7957542
50. Matok I, Clark S, Caritis S, Miodovnik M, Umans J, Hankins G, et al. Comparing the pharmacokinetics of doxylamine/pyridoxine delayed-release combination in nonpregnant women of reproductive age and women in the first trimester of pregnancy. J Clin Pharmacol. 2013;53(3):334–8. doi: 10.1177/0091270012445207 23444286
51. Salman S, Rogerson SJ, Kose K, Griffin S, Gomorai S, Baiwog F, et al. Pharmacokinetic properties of azithromycin in pregnancy. Antimicrob Agents Chemother. 2010;54(1):360–6. doi: 10.1128/AAC.00771-09 19858250
52. Papantoniou N, Ismailos G, Daskalakis G, Karabinas C, Mesogitis S, Papapanagiotou A, et al. Pharmacokinetics of oral cefatrizine in pregnant and non-pregnant women with reference to fetal distribution. Fetal Diagn Ther. 2007;22(2):100–6. doi: 10.1159/000097105 17135753
53. Popovic J, Grujic Z, Sabo A. Influence of pregnancy on ceftriaxone, cefazolin and gentamicin pharmacokinetics in caesarean vs. non-pregnant sectioned women. J Clin Pharm Ther. 2007;32(6):595–602. doi: 10.1111/j.1365-2710.2007.00864.x 18021337
54. Philipson A, Stiernstedt G, Ehrnebo M. Comparison of the pharmacokinetics of cephradine and cefazolin in pregnant and non-pregnant women. Clin Pharmacokinet. 1987;12(2):136–44. doi: 10.2165/00003088-198712020-00004 3829560
55. Gonik B, Feldman S, Pickering LK, Doughtie CG. Pharmacokinetics of cefoperazone in the parturient. Antimicrob Agents Chemother. 1986;30(6):874–6. 3813513
56. Nathorst-Boos J, Philipson A, Hedman A, Arvisson A. Renal elimination of ceftazidime during pregnancy. Am J Obstet Gynecol. 1995;172(1):163–6. 7847529
57. Philipson A, Stiernstedt G. Pharmacokinetics of cefuroxime in pregnancy. Am J Obstet Gynecol. 1982;142(7):823–8. 7065060
58. Herngren L, Ehrnebo M, Boreus LO. Drug distribution in whole blood of mothers and their newborn infants: studies of cloxacillin and flucloxacillin. Eur J Clin Pharmacol. 1982;22(4):351–8. 7106171
59. Heikkila A, Renkonen OV, Erkkola R. Pharmacokinetics and transplacental passage of imipenem during pregnancy. Antimicrob Agents Chemother. 1992;36(12):2652–5. 1482132
60. Heikkila A, Pyykko K, Erkkola R, Iisalo E. The pharmacokinetics of mecillinam and pivmecillinam in pregnant and non-pregnant women. Br J Clin Pharmacol. 1992;33(6):629–33. 1389936
61. Nemutlu E, Kir S, Eroglu H, Katlan D, Ozek A, Ozyuncu O, et al. Comparison of pharmacokinetic profiles of moxifloxacin in Caesarean versus non-pregnant sectioned women by fully validated HPLC with fluorescence detection. Comb Chem High Throughput Screen. 2010;13(6):502–9. 20426751
62. Heikkila AM, Erkkola RU. The need for adjustment of dosage regimen of penicillin V during pregnancy. Obstet Gynecol. 1993;81(6):919–21. 8497356
63. Heikkila A, Erkkola R. Pharmacokinetics of piperacillin during pregnancy. J Antimicrob Chemother. 1991;28(3):419–23. 1960122
64. Bourget P, Sertin A, Lesne-Hulin A, Fernandez H, Ville Y, Van Peborgh P. Influence of pregnancy on the pharmacokinetic behaviour and the transplacental transfer of the piperacillin-tazobactam combination. Eur J Obstet Gynecol Reprod Biol. 1998;76(1):21–7. 9481541
65. Voigt R, Schroder S, Peiker G. Pharmacokinetic studies of azlocillin and piperacillin during late pregnancy. Chemotherapy. 1985;31(6):417–24. 3908007
66. Peiker G, Traeger A, Pischke U, Schroder S, Muller B, Noschel H. [Studies on the pharmacokinetics of the compound preparation sulfamerazine/trimethoprim (Berlocombin-200) in pregnancy]. Pharmazie. 1982;37(8):578–83. 7146066
67. Voigt R, Schroder S, Meinhold P, Zenner I, Noschel H. [Clinical studies on the effect of pregnancy and labor on the pharmacokinetics of ampicillin]. Zentralbl Gynakol. 1978;100(11):701–5. 356490
68. Chamberlain A, White S, Bawdon R, Thomas S, Larsen B. Pharmacokinetics of ampicillin and sulbactam in pregnancy. Am J Obstet Gynecol. 1993;168(2):667–73. 8438948
69. Heikkinen T, Ekblad U, Kero P, Ekblad S, Laine K. Citalopram in pregnancy and lactation. Clin Pharmacol Ther. 2002;72(2):184–91. doi: 10.1067/mcp.2002.126181 12189365
70. Sit DK, Perel JM, Helsel JC, Wisner KL. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69(4):652–8. 18426260
71. Heikkinen T, Ekblad U, Palo P, Laine K. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Ther. 2003;73(4):330–7. 12709723
72. Brogtrop J, Zwarts P, Holleboom CAG, Van Der Linden PD, Touw DJ. [Optimisation of pharmacotherapy for depression during pregnancy. Pilot study for evaluation of existing paroxetine therapy]. Pharmaceutisch Weekblad. 2007;142(11):34–7.
73. Ter Horst PGJ, Larmene-Beld KHM, Bosman J, Van Der Veen EL, Wieringa A, Smit JP. Concentrations of venlafaxine and its main metabolite O-desmethylvenlafaxine during pregnancy. J Clin Pharm Ther. 2014;39(5):541–4. doi: 10.1111/jcpt.12188 24989434
74. Rey E, d’Athis P, Giraux P, de Lauture D, Turquais JM, Chavinie J, et al. Pharmacokinetics of clorazepate in pregnant and non-pregnant women. Eur J Clin Pharmacol. 1979;15(3):175–80. 37089
75. Hebert MF, Easterling TR, Kirby B, Carr DB, Buchanan ML, Rutherford T, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84(2):248–53. doi: 10.1038/clpt.2008.1 18288078
76. Wilson CM, Dundee JW, Moore J, Howard PJ, Collier PS. A comparison of the early pharmacokinetics of midazolam in pregnant and nonpregnant women. Anaesthesia. 1987;42(10):1057–62. 3688386
77. Battino D, Binelli S, Bossi L, Canger R, Croci D, Cusi C, et al. Plasma concentrations of carbamazepine and carbamazepine 10,11-epoxide during pregnancy and after delivery. Clin Pharmacokinet. 1985;10(3):279–84. doi: 10.2165/00003088-198510030-00007 4017398
78. Bernus I, Hooper WD, Dickinson RG, Eadie MJ. Metabolism of carbamazepine and co-administered anticonvulsants during pregnancy. Epilepsy Res. 1995;21(1):65–75. 7641678
79. Johnson EL, Stowe ZN, Ritchie JC, Newport DJ, Newman ML, Knight B, et al. Carbamazepine clearance and seizure stability during pregnancy. Epilepsy Behav. 2014;33 : 49–53. doi: 10.1016/j.yebeh.2014.02.011 24632353
80. Yerby MS, Friel PN, Miller DQ. Carbamazepine protein binding and disposition in pregnancy. Ther Drug Monit. 1985;7(3):269–73. 4049462
81. Yerby MS, Friel PN, McCormick K, Koerner M, Van Allen M, Leavitt AM, et al. Pharmacokinetics of anticonvulsants in pregnancy: alterations in plasma protein binding. Epilepsy Res. 1990;5(3):223–8. 2384078
82. Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Epilepsy and pregnancy: a prospective study of seizure control in relation to free and total plasma concentrations of carbamazepine and phenytoin. Epilepsia. 1994;35(1):122–30. 8112234
83. Reisinger TL, Newman M, Loring DW, Pennell PB, Meador KJ. Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav. 2013;29(1):13–8. doi: 10.1016/j.yebeh.2013.06.026 23911354
84. Bernus I, Hooper WD, Dickinson RG, Eadie MJ. Effects of pregnancy on various pathways of human antiepileptic drug metabolism. Clin Neuropharmacol. 1997;20(1):13–21. 9037569
85. Kuhnz W, Steldinger R, Nau H. Protein binding of carbamazepine and its epoxide in maternal and fetal plasma at delivery: comparison to other anticonvulsants. Dev Pharmacol Ther. 1984;7(1):61–72. 6697871
86. Pennell PB, Newport DJ, Stowe ZN, Helmers SL, Montgomery JQ, Henry TR. The impact of pregnancy and childbirth on the metabolism of lamotrigine. Neurology. 2004;62(2):292–5. 14745072
87. Pennell PB, Peng L, Newport DJ, Ritchie JC, Koganti A, Holley DK, et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology. 2008;70(22 Pt 2):2130–6.
88. de Haan GJ, Edelbroek P, Segers J, Engelsman M, Lindhout D, Devile-Notschaele M, et al. Gestation-induced changes in lamotrigine pharmacokinetics: a monotherapy study. Neurology. 2004;63(3):571–3. 15304599
89. Fotopoulou C, Kretz R, Bauer S, Schefold JC, Schmitz B, Dudenhausen JW, et al. Prospectively assessed changes in lamotrigine-concentration in women with epilepsy during pregnancy, lactation and the neonatal period. Epilepsy Res. 2009;85(1):60–4. doi: 10.1016/j.eplepsyres.2009.02.011 19272754
90. Ohman I, Beck O, Vitols S, Tomson T. Plasma concentrations of lamotrigine and its 2-N-glucuronide metabolite during pregnancy in women with epilepsy. Epilepsia. 2008;49(6):1075–80. doi: 10.1111/j.1528-1167.2007.01471.x 18076642
91. Petrenaite V, Sabers A, Hansen-Schwartz J. Individual changes in lamotrigine plasma concentrations during pregnancy. Epilepsy Res. 2005;65(3):185–8. doi: 10.1016/j.eplepsyres.2005.06.004 16084694
92. Polepally AR, Pennell PB, Brundage RC, Stowe ZN, Newport DJ, Viguera AC, et al. Model-based lamotrigine clearance changes during pregnancy: clinical implication. Ann Clin Transl Neurol. 2014;1(2):99–106. doi: 10.1002/acn3.29 24883336
93. Tran TA, Leppik IE, Blesi K, Sathanandan ST, Remmel R. Lamotrigine clearance during pregnancy. Neurology. 2002;59(2):251–5. 12136066
94. Lopez-Fraile IP, Cid AO, Juste AO, Modrego PJ. Levetiracetam plasma level monitoring during pregnancy, delivery, and postpartum: clinical and outcome implications. Epilepsy Behav. 2009;15(3):372–5. doi: 10.1016/j.yebeh.2009.04.006 19362602
95. Westin AA, Reimers A, Helde G, Nakken KO, Brodtkorb E. Serum concentration/dose ratio of levetiracetam before, during and after pregnancy. Seizure. 2008;17(2):192–8. doi: 10.1016/j.seizure.2007.11.027 18180176
96. Christensen J, Sabers A, Sidenius P. Oxcarbazepine concentrations during pregnancy: a retrospective study in patients with epilepsy. Neurology. 2006;67(8):1497–9. doi: 10.1212/01.wnl.0000240047.11166.0e 17060586
97. Petrenaite V, Sabers A, Hansen-Schwartz J. Seizure deterioration in women treated with oxcarbazepine during pregnancy. Epilepsy Res. 2009;84(2–3):245–9. doi: 10.1016/j.eplepsyres.2009.01.011 19231139
98. Mazzucchelli I, Onat FY, Ozkara C, Atakli D, Specchio LM, Neve AL, et al. Changes in the disposition of oxcarbazepine and its metabolites during pregnancy and the puerperium. Epilepsia. 2006;47(3):504–9. doi: 10.1111/j.1528-1167.2006.00459.x 16529613
99. Appleton MP, Kuehl TJ, Raebel MA, Adams HR, Knight AB, Gold WR. Magnesium sulfate versus phenytoin for seizure prophylaxis in pregnancy-induced hypertension. Am J Obstet Gynecol. 1991;165(4 Pt 1):907–13.
100. Ohman I, Sabers A, de Flon P, Luef G, Tomson T. Pharmacokinetics of topiramate during pregnancy. Epilepsy Research. 2009;87(2–3):124–9. doi: 10.1016/j.eplepsyres.2009.08.004 19740626
101. Westin AA, Nakken KO, Johannessen SI, Reimers A, Lillestolen KM, Brodtkorb E. Serum concentration/dose ratio of topiramate during pregnancy. Epilepsia. 2009;50(3):480–5. doi: 10.1111/j.1528-1167.2008.01776.x 19178558
102. Kulo A, van Calsteren K, Verbesselt R, Smits A, Devlieger R, de Hoon J, et al. The impact of caesarean delivery on paracetamol and ketorolac pharmacokinetics: a paired analysis. J Biomed Biotechnol. 2012;2012 : 437639. doi: 10.1155/2012/437639 22675252
103. Gerdin E, Salmonson T, Lindberg B, Rane A. Maternal kinetics of morphine during labour. J Perinat Med. 1990;18(6):479–87. 2097341
104. Rayburn W, Shukla U, Stetson P, Piehl E. Acetaminophen pharmacokinetics: comparison between pregnant and nonpregnant women. Am J Obstet Gynecol. 1986;155(6):1353–6. 3789044
105. Miners JO, Robson RA, Birkett DJ. Paracetamol metabolism in pregnancy. Br J Clin Pharmacol. 1986;22(3):359–62. 3768250
106. Kulo A, Peeters MY, Allegaert K, Smits A, de Hoon J, Verbesselt R, et al. Pharmacokinetics of paracetamol and its metabolites in women at delivery and post-partum. Br J Clin Pharmacol. 2013;75(3):850–60. doi: 10.1111/j.1365-2125.2012.04402.x 22845052
107. Allegaert K, Peeters MY, Beleyn B, Smits A, Kulo A, van Calsteren K, et al. Paracetamol pharmacokinetics and metabolism in young women. BMC Anesthesiol. 2015;15 : 163. doi: 10.1186/s12871-015-0144-3 26566962
108. Gin T, Yau G, Jong W, Tan P, Leung RK, Chan K. Disposition of propofol at caesarean section and in the postpartum period. Br J Anaesth. 1991;67(1):49–53. 1859759
109. Gin T, Gregory MA, Chan K, Buckley T, Oh TE. Pharmacokinetics of propofol in women undergoing elective caesarean section. Br J Anaesth. 1990;64(2):148–53. 2138487
110. Cortambert F, Marti-Flich J, Dasset MP, Mullet C. [The pharmacokinetics of propofol used in cesarean section; a preliminary study in the newborn infant]. Cah Anesthesiol. 1989;37(1):33–7. 2784338
111. Chiba K, Ishizaki T, Tabuchi T, Wagatsuma T, Nakazawa Y. Antipyrine disposition in relation to lowered anticonvulsant plasma level during pregnancy. Obstet Gynecol. 1982;60(5):620–6. 6815599
112. Rymark P, Berntorp E, Nordsjo P, Liedholm H, Melander A, Gennser G. Low-dose aspirin to pregnant women: single dose pharmacokinetics and influence of short term treatment on bleeding time. J Perinat Med. 1994;22(3):205–11. 7823260
113. Brancazio LR, Roperti KA, Stierer R, Laifer SA. Pharmacokinetics and pharmacodynamics of subcutaneous heparin during the early third trimester of pregnancy. Am J Obstet Gynecol. 1995;173(4):1240–5. 7485329
114. Ensom MHH, Stephenson MD. Pharmacokinetics of low molecular weight heparin and unfractionated heparin in pregnancy. J Soc Gynecol Investig. 2004;11(6):377–83. doi: 10.1016/j.jsgi.2004.02.007 15350250
115. Sephton V, Farquharson RG, Topping J, Quenby SM, Cowan C, Back DJ, et al. A longitudinal study of maternal dose response to low molecular weight heparin in pregnancy. Obstet Gynecol. 2003;101(6):1307–11. 12798541
116. Barbour LA, Oja JL, Schultz LK. A prospective trial that demonstrates that dalteparin requirements increase in pregnancy to maintain therapeutic levels of anticoagulation. Am J Obstet Gynecol. 2004;191(3):1024–9. doi: 10.1016/j.ajog.2004.05.050 15467584
117. Lebaudy C, Hulot JS, Amoura Z, Costedoat-Chalumeau N, Serreau R, Ankri A, et al. Changes in enoxaparin pharmacokinetics during pregnancy and implications for antithrombotic therapeutic strategy. Clin Pharmacol Ther. 2008;84(3):370–7. doi: 10.1038/clpt.2008.73 18431408
118. Hebert MF, Carr DB, Anderson GD, Blough D, Green GE, Brateng DA, et al. Pharmacokinetics and pharmacodynamics of atenolol during pregnancy and postpartum. J Clin Pharmacol. 2005;45(1):25–33. doi: 10.1177/0091270004269704 15601802
119. Hurst AK, Shotan A, Hoffman K, Johnson J, Goodwin TM, Koda R, et al. Pharmacokinetic and pharmacodynamic evaluation of atenolol during and after pregnancy. Pharmacotherapy. 1998;18(4):840–6. 9692658
120. Buchanan ML, Easterling TR, Carr DB, Shen DD, Risler LJ, Nelson WL, et al. Short communication clonidine pharmacokinetics in pregnancy. Drug Metab Dispos. 2009;37(4):702–5. doi: 10.1124/dmd.108.024984 19116263
121. Hildebrandt R, Weitzel H, Warnke K, Gundert-Remy U. Pharmacokinetics of fenoterol in pregnant and nonpregnant women. Eur J Clin Pharmacol. 1993;45(3):275–7. 8276054
122. Peiker G, Traeger A, Stiller K, Muller B. [Pharmacokinetics and electrolyte balance following administration of furosemide in pregnant women with E gestosis]. Pharmazie. 1984;39(5):336–9. 6473496
123. Fischer JH, Sarto GE, Hardman J, Endres L, Jenkins TM, Kilpatrick SJ, et al. Influence of gestational age and body weight on the pharmacokinetics of labetalol in pregnancy. Clin Pharmacokinet. 2014;53(4):373–83. doi: 10.1007/s40262-013-0123-0 24297680
124. Rubin PC, Butters L, Kelman AW, Fitzsimons C, Reid JL. Labetalol disposition and concentration-effect relationships during pregnancy. Br J Clin Pharmacol. 1983;15(4):465–70. 6849783
125. Rogers RC, Sibai BM, Whybrew WD. Labetalol pharmacokinetics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1990;162(2):362–6. 2309815
126. Gonser M, Stoll P, Kahle P. Clearance prediction and drug dosage in pregnancy: a clinical study on metildigoxin, and application to other drugs with predominant penal elimination. Clin Drug Investig. 1995;9(4):197–205.
127. Hogstedt S, Rane A. Plasma concentration-effect relationship of metoprolol during and after pregnancy. Eur J Clin Pharmacol. 1993;44(3):243–6. 8491238
128. Prevost RR, Akl SA, Whybrew WD, Sibai BM. Oral nifedipine pharmacokinetics in pregnancy-induced hypertension. Pharmacotherapy. 1992;12(3):174–7. 1608848
129. O’Hare MF, Leahey W, Murnaghan GA, McDevitt DG. Pharmacokinetics of sotalol during pregnancy. Eur J Clin Pharmacol. 1983;24(4):521–4. 6861867
130. Best BM, Mirochnick M, Capparelli EV, Stek A, Burchett SK, Holland DT, et al. Impact of pregnancy on abacavir pharmacokinetics. AIDS. 2006;20(4):553–60. doi: 10.1097/01.aids.0000210609.52836.d1 16470119
131. Conradie F, Zorrilla C, Josipovic D, Botes M, Osiyemi O, Vandeloise E, et al. Safety and exposure of once-daily ritonavir-boosted atazanavir in HIV-infected pregnant women. HIV Med. 2011;12(9):570–9. doi: 10.1111/j.1468-1293.2011.00927.x 21569187
132. Ripamonti D, Cattaneo D, Maggiolo F, Airoldi M, Frigerio L, Bertuletti P, et al. Atazanavir plus low-dose ritonavir in pregnancy: pharmacokinetics and placental transfer. AIDS. 2007;21(18):2409–15. doi: 10.1097/QAD.0b013e32825a69d1 18025877
133. Kreitchmann R, Best BM, Wang J, Stek A, Caparelli E, Watts DH, et al. Pharmacokinetics of an increased atazanavir dose with and without tenofovir during the third trimester of pregnancy. J Acquire Immune Defic Syndr. 2013;63(1):59–66.
134. Mirochnick M, Best BM, Stek AM, Capparelli EV, Hu C, Burchett SK, et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquire Immune Defic Syndr. 2011;56(5):412–9.
135. Else LJ, Jackson V, Brennan M, Back DJ, Khoo SH, Coulter-Smith S, et al. Therapeutic drug monitoring of atazanavir/ritonavir in pregnancy. HIV Med. 2014;15(10):604–10. doi: 10.1111/hiv.12164 24825070
136. Colbers A, Hawkins D, Hidalgo-Tenorio C, van der Ende M, Gingelmaier A, Weizsacker K, et al. Atazanavir exposure is effective during pregnancy regardless of tenofovir use. Antivir Ther. 2015;20(1):57–64. doi: 10.3851/IMP2820 24992294
137. Le MP, Mandelbrot L, Descamps D, Soulie C, Ichou H, Bourgeois-Moine A, et al. Pharmacokinetics, safety and efficacy of ritonavir-boosted atazanavir (300/100 mg once daily) in HIV-1-infected pregnant women. Antivir Ther. 2015;20(5):507–13. doi: 10.3851/IMP2936 25599649
138. Colbers A, Molto J, Ivanovic J, Kabeya K, Hawkins D, Gingelmaier A, et al. Pharmacokinetics of total and unbound darunavir in HIV-1-infected pregnant women. J Antimicrob Chemother. 2015;70(2):534–42. doi: 10.1093/jac/dku400 25326090
139. Zorrilla CD, Wright R, Osiyemi O, Yasin S, Baugh B, Brown K, et al. Total and unbound darunavir pharmacokinetics in pregnant women infected with HIV-1: results of a study of darunavir/ritonavir 600/100mg administered twice daily. HIV Med. 2014;15(1):50–6. doi: 10.1111/hiv.12047 23731450
140. Stek A, Best BM, Wang J, Capparelli EV, Burchett SK, Kreitchmann R, et al. Pharmacokinetics of once versus twice daily darunavir in pregnant HIV-infected women. J Acquire Immune Defic Syndr. 2015;70(1):33–41.
141. Wang Y, Livingston E, Patil S, McKinney RE, Bardeguez AD, Gandia J, et al. Pharmacokinetics of didanosine in antepartum and postpartum human immunodeficiency virus—infected pregnant women and their neonates: an AIDS clinical trials group study. J Infect Dis. 1999;180(5):1536–41. doi: 10.1086/315067 10515813
142. Bartelink IH, Savic RM, Mwesigwa J, Achan J, Clark T, Plenty A, et al. Pharmacokinetics of lopinavir/ritonavir and efavirenz in food insecure HIV-infected pregnant and breastfeeding women in Tororo, Uganda. J Clin Pharmacol. 2014;54(2):121–32. doi: 10.1002/jcph.167 24038035
143. Cressey TR, Stek A, Capparelli E, Bowonwatanuwong C, Prommas S, Sirivatanapa P, et al. Efavirenz pharmacokinetics during the third trimester of pregnancy and postpartum. J Acquire Immune Defic Syndr. 2012;59(3):245–52.
144. Olagunju A, Bolaji O, Amara A, Else L, Okafor O, Adejuyigbe E, et al. Pharmacogenetics of pregnancy-induced changes in efavirenz pharmacokinetics. Clin Pharmacol Ther. 2015;97(3):298–306. doi: 10.1002/cpt.43 25669165
145. Colbers APH, Hawkins DA, Gingelmaier A, Kabeya K, Rockstroh JK, Wyen C, et al. The pharmacokinetics, safety and efficacy of tenofovir and emtricitabine in HIV-1-infected pregnant women. AIDS. 2013;27(5):739–48. doi: 10.1097/QAD.0b013e32835c208b 23169329
146. Stek AM, Best BM, Luo W, Capparelli E, Burchett S, Hu C, et al. Effect of pregnancy on emtricitabine pharmacokinetics. HIV Med. 2012;13(4):226–35. doi: 10.1111/j.1468-1293.2011.00965.x 22129166
147. Valade E, Treluyer JM, Dabis F, Arrive E, Pannier E, Benaboud S, et al. Modified renal function in pregnancy: impact on emtricitabine pharmacokinetics. Br J Clin Pharmacol. 2014;78(6):1378–86. doi: 10.1111/bcp.12457 24995851
148. Cressey TR, Best BM, Achalapong J, Stek A, Wang J, Chotivanich N, et al. Reduced indinavir exposure during pregnancy. Br J Clin Pharmacol. 2013;76(3):475–83. doi: 10.1111/bcp.12078 23305215
149. Kosel BW, Beckerman KP, Hayashi S, Homma M, Aweeka FT. Pharmacokinetics of nelfinavir and indinavir in HIV-1-infected pregnant women. AIDS. 2003;17(8):1195–9. doi: 10.1097/01.aids.0000060367.78202.cb 12819521
150. Unadkat JD, Wara DW, Hughes MD, Mathias AA, Holland DT, Paul ME, et al. Pharmacokinetics and safety of indinavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2007;51(2):783–6. doi: 10.1128/AAC.00420-06 17158945
151. Benaboud S, Treluyer JM, Urien S, Blanche S, Bouazza N, Chappuy H, et al. Pregnancy-related effects on lamivudine pharmacokinetics in a population study with 228 women. Antimicrob Agents Chemother. 2012;56(2):776–82. doi: 10.1128/AAC.00370-11 22106227
152. Bouillon-Pichault M, Jullien V, Azria E, Pannier E, Firtion G, Krivine A, et al. Population analysis of the pregnancy-related modifications in lopinavir pharmacokinetics and their possible consequences for dose adjustment. J Antimicrob Chemother. 2009;63(6):1223–32. doi: 10.1093/jac/dkp123 19389715
153. Calza L, Manfredi R, Trapani F, Salvadori C, Colangeli V, Borderi M, et al. Lopinavir/ritonavir trough concentrations with the tablet formulation in HIV-1-infected women during the third trimester of pregnancy. Scand J Infect Dis. 2012;44(5):381–7. doi: 10.3109/00365548.2011.642306 22263609
154. Else LJ, Douglas M, Dickinson L, Back DJ, Khoo SH, Taylor GP. Improved oral bioavailability of lopinavir in melt-extruded tablet formulation reduces impact of third trimester on lopinavir plasma concentrations. Antimicrob Agents Chemother. 2012;56(2):816–24. doi: 10.1128/AAC.05186-11 22106215
155. Lambert JS, Else LJ, Jackson V, Breiden J, Gibbons S, Dickinson L, et al. Therapeutic drug monitoring of lopinavir/ritonavir in pregnancy. HIV Med. 2011;12(3):166–73. doi: 10.1111/j.1468-1293.2010.00865.x 20726906
156. Mirochnick M, Best BM, Stek AM, Capparelli E, Hu C, Burchett SK, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquire Immune Defic Syndr. 2008;49(5):485–91.
157. Ramautarsing RA, Van Der Lugt J, Gorowara M, Kerr SJ, Burger D, Ruxrungtham K, et al. Thai HIV-1-infected women do not require a dose increase of lopinavir/ritonavir during the third trimester of pregnancy. AIDS. 2011;25(10):1299–303. doi: 10.1097/QAD.0b013e328347f7e9 21516029
158. Santini-Oliveira M, Estrela Rde C, Veloso VG, Cattani VB, Yanavich C, Velasque L, et al. Randomized clinical trial comparing the pharmacokinetics of standard - and increased-dosage lopinavir-ritonavir coformulation tablets in HIV-positive pregnant women. Antimicrob Agents Chemother. 2014;58(5):2884–93. doi: 10.1128/AAC.02599-13 24614377
159. Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20(15):1931–9. doi: 10.1097/01.aids.0000247114.43714.90 16988514
160. Patterson KB, Dumond JB, Prince HA, Jenkins AJ, Scarsi KK, Wang R, et al. Protein binding of lopinavir and ritonavir during 4 phases of pregnancy: implications for treatment guidelines. J Acquire Immune Defic Syndr. 2013;63(1):51–8.
161. Aweeka FT, Stek A, Best BM, Hu C, Holland D, Hermes A, et al. Lopinavir protein binding in HIV-1-infected pregnant women. HIV Med. 2010;11(4):232–8. doi: 10.1111/j.1468-1293.2009.00767.x 20002783
162. Fauchet F, Treluyer JM, Illamola SM, Pressiat C, Lui G, Valade E, et al. Population approach to analyze the pharmacokinetics of free and total lopinavir in HIV-infected pregnant women and consequences for dose adjustment. Antimicrob Agents Chemother. 2015;59(9):5727–35. doi: 10.1128/AAC.00863-15 26149996
163. Fang A, Valluri SR, O’Sullivan MJ, Maupin R, Jones T, Delke I, et al. Safety and pharmacokinetics of nelfinavir during the second and third trimesters of pregnancy and postpartum. HIV Clin Trials. 2012;13(1):46–59. doi: 10.1310/hct1301-046 22306587
164. Hirt D, Treluyer JM, Jullien V, Firtion G, Chappuy H, Rey E, et al. Pregnancy-related effects on nelfinavir-M8 pharmacokinetics: a population study with 133 women. Antimicrob Agents Chemother. 2006;50(6):2079–86. doi: 10.1128/AAC.01596-05 16723569
165. Nellen JFJB, Schillevoort I, Wit FWNM, Bergshoeff AS, Godfried MH, Boer K, et al. Nelfinavir plasma concentrations are low during pregnancy. Clin Infect Dis. 2004;39(5):736–40. doi: 10.1086/422719 15356791
166. Read JS, Best BM, Stek AM, Hu C, Capparelli EV, Holland DT, et al. Pharmacokinetics of new 625mg nelfinavir formulation during pregnancy and postpartum. HIV Med. 2008;9(10):875–82. doi: 10.1111/j.1468-1293.2008.00640.x 18795962
167. Villani P, Floridia M, Pirillo MF, Cusato M, Tamburrini E, Cavaliere AF, et al. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and nonpregnant women. Br J Clin Pharmacol. 2006;62(3):309–15. doi: 10.1111/j.1365-2125.2006.02669.x 16934047
168. Van Heeswijk RPG, Khaliq Y, Gallicano KD, Bourbeau M, Seguin I, Phillips EJ, et al. The pharmacokinetics of nelfinavir and M8 during pregnancy and post partum. Clin Pharmacol Ther. 2004;76(6):588–97. doi: 10.1016/j.clpt.2004.08.011 15592330
169. Capparelli EV, Aweeka F, Hitti J, Stek A, Hu C, Burchett SK, et al. Chronic administration of nevirapine during pregnancy: impact of pregnancy on pharmacokinetics. HIV Med. 2008;9(4):214–20. doi: 10.1111/j.1468-1293.2008.00553.x 18366444
170. Nellen JFJB, Damming M, Godfried MH, Boer K, Van Der Ende ME, Burger DM, et al. Steady-state nevirapine plasma concentrations are influenced by pregnancy. HIV Med. 2008;9(4):234–8. doi: 10.1111/j.1468-1293.2008.00551.x 18366447
171. Lamorde M, Byakika-Kibwika P, Okaba-Kayom V, Flaherty JP, Boffito M, Namakula R, et al. Suboptimal nevirapine steady-state pharmacokinetics during intrapartum compared with postpartum in HIV-1-seropositive Ugandan women. J Acquire Immune Defic Syndr. 2010;55(3):345–50.
172. Blonk M, Colbers A, Hidalgo-Tenorio C, Weizsacker K, Molto J, Hawkins D, et al. A comparison of the pharmacokinetics of raltegravir during pregnancy and postpartum. Top Antivir Med. 2014;22 (e-1):465.
173. Watts DH, Stek A, Best BM, Wang J, Capparelli EV, Cressey TR, et al. Raltegravir pharmacokinetics during pregnancy. J Acquir Immune Defic Syndr. 2014;67(4):375–81. doi: 10.1097/QAI.0000000000000318 25162818
174. Martinez-Rebollar M, Lonca M, Perez I, Soy D, Brunet M, Martin R, et al. Pharmacokinetic study of saquinavir 500 mg plus ritonavir (1000/100 mg twice a day) in HIV-positive pregnant women. Ther Drug Monit. 2011;33(6):772–7. doi: 10.1097/FTD.0b013e318236376d 22105596
175. van der Lug J, Colbers A, Molto J, Hawkins D, van der Ende M, Vogel M, et al. The pharmacokinetics, safety and efficacy of boosted saquinavir tablets in HIV type-1-infected pregnant women. Antivir Ther. 2009;14(3):443–50. 19474478
176. von Hentig N, Nisius G, Lennemann T, Khaykin P, Stephan C, Babacan E, et al. Pharmacokinetics, safety and efficacy of saquinavir/ritonavir 1,000/100 mg twice daily as HIV type-1 therapy and transmission prophylaxis in pregnancy. Antivir Ther. 2008;13(8):1039–46. 19195329
177. Cespedes MS, Castor D, Ford SL, Lee D, Lou Y, Pakes GE, et al. Steady-state pharmacokinetics, cord blood concentrations, and safety of ritonavir-boosted fosamprenavir in pregnancy. J Acquire Immune Defic Syndr. 2013;62(5):550–4.
178. Green MD, Van Eijk AM, Van Ter Kuile FO, Ayisi JG, Parise ME, Kager PA, et al. Pharmacokinetics of sulfadoxine-pyrimethamine in HIV-infected and uninfected pregnant women in western Kenya. J Infect Dis. 2007;196(9):1403–8. doi: 10.1086/522632 17922406
179. Benaboud S, Hirt D, Launay O, Pannier E, Firtion G, Rey E, et al. Pregnancy-related effects on tenofovir pharmacokinetics: A population study with 186 women. Antimicrob Agents Chemother. 2012;56(2):857–62. doi: 10.1128/AAC.05244-11 22123690
180. Hirt D, Urien S, Ekouevi DK, Rey E, Arrive E, Blanche S, et al. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109). Clin Pharmacol Ther. 2009;85(2):182–9. doi: 10.1038/clpt.2008.201 18987623
181. Mosha D, Guidi M, Mwingira F, Abdulla S, Mercier T, Decosterd LA, et al. Population pharmacokinetics and clinical response for artemether-lumefantrine in pregnant and nonpregnant women with uncomplicated Plasmodium falciparum malaria in Tanzania. Antimicrob Agents Chemother. 2014;58(8):4583–92. doi: 10.1128/AAC.02595-14 24867986
182. McGready R, Stepniewska K, Lindegardh N, Ashley EA, La Y, Singhasivanon P, et al. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006;62(12):1021–31. doi: 10.1007/s00228-006-0199-7 17053895
183. McGready R, Stepniewska K, Edstein MD, Cho T, Gilveray G, Looareesuwan S, et al. The pharmacokinetics of atovaquone and proguanil in pregnant women with acute falciparum malaria. Eur J Clin Pharmacol. 2003;59(7):545–52. doi: 10.1007/s00228-003-0652-9 12955371
184. Chukwuani MC, Bolaji OO, Onyeji CO, Makinde ON, Ogunbona FA. Evidence for increased metabolism of chloroquine during the early third trimester of human pregnancy. Trop Med Int Health. 2004;9(5):601–5. doi: 10.1111/j.1365-3156.2004.01227.x 15117305
185. Karunajeewa HA, Salman S, Mueller I, Baiwog F, Gomorrai S, Law I, et al. Pharmacokinetics of chloroquine and monodesethylchloroquine in pregnancy. Antimicrob Agents Chemother. 2010;54(3):1186–92. doi: 10.1128/AAC.01269-09 20086162
186. Lee SJ, McGready R, Fernandez C, Stepniewska K, Paw MK, Viladpai-Nguen SJ, et al. Chloroquine pharmacokinetics in pregnant and nonpregnant women with vivax malaria. Eur J Clin Pharmacol. 2008;64(10):987–92. doi: 10.1007/s00228-008-0500-z 18594802
187. Massele AY, Kilewo C, Aden Abdi Y, Tomson G, Diwan VK, Ericsson O, et al. Chloroquine blood concentrations and malaria prophylaxis in Tanzanian women during the second and third trimesters of pregnancy. Eur J Clin Pharmacol. 1997;52(4):299–305. 9248769
188. Kloprogge F, Piola P, Dhorda M, Muwanga S, Turyakira E, Apinan S, et al. Population pharmacokinetics of lumefantrine in pregnant and nonpregnant women with uncomplicated plasmodium falciparum malaria in Uganda. CPT Pharmacometrics Syst Pharmacol. 2013;2(11):e83.
189. Tarning J, Kloprogge F, Dhorda M, Jullien V, Nosten F, White NJ, et al. Pharmacokinetic properties of artemether, dihydroartemisinin, lumefantrine, and quinine in pregnant women with uncomplicated plasmodium falciparum malaria in Uganda. Antimicrob Agents Chemother. 2013;57(10):5096–103. doi: 10.1128/AAC.00683-13 23917320
190. Nosten F, Karbwang J, White NJ, Honeymoon, Na Bangchang K, Bunnag D, et al. Mefloquine antimalarial prophylaxis in pregnancy: dose finding and pharmacokinetic study. Br J Clin Pharmacol. 1990;30(1):79–85. 2390434
191. Bangchang KN, Davis TME, Looareesuwan S, White NJ, Bunnag D, Karbwang J. Mefloquine pharmacokinetics in pregnant women with acute falciparum malaria. Trans R Soc Trop Med Hyg. 1994;88(3):321–3. 7974678
192. Valea I, Tinto H, Coulibaly MT, Toe LC, Lindegardh N, Tarning J, et al. Pharmacokinetics of co-formulated mefloquine and artesunate in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum infection in Burkina Faso. J Antimicrob Chemother. 2014;69(9):2499–507. doi: 10.1093/jac/dku154 24891429
193. Rijken MJ, McGready R, Phyo AP, Lindegardh N, Tarning J, Laochan N, et al. Pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and nonpregnant women with uncomplicated falciparum malaria. Antimicrob Agents Chemother. 2011;55(12):5500–6. doi: 10.1128/AAC.05067-11 21947392
194. Benjamin JM, Moore BR, Salman S, Page-Sharp M, Tawat S, Yadi G, et al. Population pharmacokinetics, tolerability, and safety of dihydroartemisinin-piperaquine and sulfadoxine-pyrimethamine-piperaquine in pregnant and nonpregnant Papua New Guinean women. Antimicrob Agents Chemother. 2015;59(7):4260–71. doi: 10.1128/AAC.00326-15 25963981
195. Tarning J, Rijken MJ, McGready R, Phyo AP, Hanpithakpong W, Day NPJ, et al. Population pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and nonpregnant women with uncomplicated malaria. Antimicrob Agents Chemother. 2012;56(4):1997–2007. doi: 10.1128/AAC.05756-11 22252822
196. Wangboonskul J, White NJ, Nosten F, Ter Kuile F, Moody RR, Taylor RB. Single dose pharmacokinetics of proguanil and its metabolites in pregnancy. Eur J Clin Pharmacol. 1993;44(3):247–51. 8491239
197. Onyamboko MA, Meshnick SR, Fleckenstein L, Koch MA, Atibu J, Lokomba V, et al. Pharmacokinetics and pharmacodynamics of artesunate and dihydroartemisinin following oral treatment in pregnant women with asymptomatic Plasmodium falciparum infections in Kinshasa DRC. Malar J. 2011;10(49).
198. McGready R, Phyo AP, Rijken MJ, Tarning J, Lindegardh N, Hanpithakpon W, et al. Artesunate/dihydroartemisinin pharmacokinetics in acute falciparum malaria in pregnancy: absorption, bioavailability, disposition and disease effects. Br J Clin Pharmacol. 2012;73(3):467–77. doi: 10.1111/j.1365-2125.2011.04103.x 21950338
199. Karunajeewa HA, Salman S, Mueller I, Baiwog F, Gomorrai S, Law I, et al. Pharmacokinetic properties of sulfadoxine-pyrimethamine in pregnant women. Antimicrob Agents Chemother. 2009;53(10):4368–76. doi: 10.1128/AAC.00335-09 19620325
200. Nyunt MM, Adam I, Kayentao K, van Dijk J, Thuma P, Mauff K, et al. Pharmacokinetics of sulfadoxine and pyrimethamine in intermittent preventive treatment of malaria in pregnancy. Clin Pharmacol Ther. 2010;87(2):226–34. doi: 10.1038/clpt.2009.177 19776738
201. Concheiro M, Jones HE, Johnson RE, Choo R, Huestis MA. Preliminary buprenorphine sublingual tablet pharmacokinetic data in plasma, oral fluid, and sweat during treatment of opioid-dependent pregnant women. Ther Drug Monit. 2011;33(5):619–26. doi: 10.1097/FTD.0b013e318228bb2a 21860340
202. Bogen DL, Perel JM, Helsel JC, Hanusa BH, Romkes M, Nukui T, et al. Pharmacologic evidence to support clinical decision making for peripartum methadone treatment. Psychopharmacology. 2013;225(2):441–51. doi: 10.1007/s00213-012-2833-7 22926004
203. Jarvis MAE, Wu-Pong S, Kniseley JS, Schnoll SH. Alterations in methadone metabolism during late pregnancy. J Addict Dis. 1999;18(4):51–61. doi: 10.1300/J069v18n04_05 10631963
204. Wolff K, Boys A, Rostami-Hodjegan A, Hay A, Raistrick D. Changes to methadone clearance during pregnancy. Eur J Clin Pharmacol. 2005;61(10):763–8. doi: 10.1007/s00228-005-0035-5 16261362
205. Van Calsteren K, Verbesselt R, Ottevanger N, Halaska M, Heyns L, Van Bree R, et al. Pharmacokinetics of chemotherapeutic agents in pregnancy: a preclinical and clinical study. Acta Obstet Gynecol Scand. 2010;89(10):1338–45. doi: 10.3109/00016349.2010.512070 20846067
206. Zemlickis D, Klein J, Moselhy G, Koren G. Cisplatin protein binding in pregnancy and the neonatal period. Med Pediatr Oncol. 1994;23(6):476–9. 7935173
207. Bjorklund AO, Adamson UKC, Lins PES, Westgren LMR. Diminished insulin clearance during late pregnancy in patients with Type I diabetes mellitus. Clin Sci. 1998;95(3):317–23. 9730851
208. Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit. 2006;28(1):67–72. 16418696
209. Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GDV, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38(5):833–40. doi: 10.1124/dmd.109.031245 20118196
210. Hughes RCE, Gardiner SJ, Begg EJ, Zhang M. Effect of pregnancy on the pharmacokinetics of metformin. Diabet Med. 2006;23(3):323–6. doi: 10.1111/j.1464-5491.2005.01769.x 16492218
211. Bajoria R, Oteng-Ntim E, Peek MJ, Fisk NM. Pharmacokinetics and pharmacodynamics of TRH during pregnancy. Obstet Gynecol. 1997;90(2):176–82. doi: 10.1016/S0029-7844(97)00221-4 9241288
212. Davison JM, Sheills EA, Philips PR, Barron WM, Lindheimer MD. Metabolic clearance of vasopressin and an analogue resistant to vasopressinase in human pregnancy. Am J Physiol Renal Physiol. 1993;264(2 Pt 2):F348–53.
213. Caritis SN, Venkataramanan R, Cotroneo M, Chiao JP. Pharmacokinetics of orally administered ritodrine. Am J Obstet Gynecol. 1989;161(1):32–5. 2641984
214. Berg G, Lindberg C, Ryden G. Terbutaline in the treatment of preterm labour. Eur J Respir Dis Suppl. 1984;134 : 219–30. 6586483
215. ter Laak MA, Roos C, Touw DJ, van Hattum PR, Kwee A, Lotgering FK, et al. Pharmacokinetics of nifedipine slow-release during sustained tocolysis. Int J Clin Pharmacol Ther. 2015;53(1):84–91. doi: 10.5414/CP202215 25407260
216. Ryu RJ, Eyal S, Kaplan HG, Akbarzadeh A, Hays K, Puhl K, et al. Pharmacokinetics of doxorubicin in pregnant women. Cancer Chemother Pharmacol. 2014;73(4):789–97. doi: 10.1007/s00280-014-2406-z 24531558
217. Freeman MP, Nolan PE Jr, Davis MF, Anthony M, Fried K, Fankhauser M, et al. Pharmacokinetics of sertraline across pregnancy and postpartum. J Clin Psychopharmacol. 2008;28(6):646–53. doi: 10.1097/JCP.0b013e31818d2048 19011433
218. O’Hare MF, Kinney CD, Murnaghan GA, McDevitt DG. Pharmacokinetics of propranolol during pregnancy. Eur J Clin Pharmacol. 1984;27(5):583–7. 6519163
219. Smith MT, Livingstone I, Eadie MJ, Hooper WD, Triggs EJ. Chronic propranolol administration during pregnancy. Maternal pharmacokinetics. Eur J Clin Pharmacol. 1983;25(4):481–90. 6653642
220. Abdelrahim II, Adam I, Elghazali G, Gustafsson LL, Elbashir MI, Mirghani RA. Pharmacokinetics of quinine and its metabolites in pregnant Sudanese women with uncomplicated Plasmodium falciparum malaria. J Clin Pharm Ther. 2007;32(1):15–9. doi: 10.1111/j.1365-2710.2007.00788.x 17286785
221. Shere M, Nguyen P, Tam C, Stern S, Kapur B, O’Connor D, et al. Optimizing periconceptional folic acid supplementation: steady-state folate pharmacokinetics in pregnancy. FASEB J. 2014;28:LB416.
222. Gregory IJF, Caudill MA, Jeffery Opalko F, Bailey LB. Kinetics of folate turnover in pregnant women (second trimester) and nonpregnant controls during folic acid supplementation: stable-isotopic labeling of plasma folate, urinary folate and folate catabolites shows subtle effects of pregnancy on turnover of folate pools. J Nutr. 2001;131(7):1928–37. 11435509
223. Roth DE, Al Mahmud A, Raqib R, Akhtar E, Black RE, Baqui AH. Pharmacokinetics of high-dose weekly oral vitamin D3 supplementation during the third trimester of pregnancy in Dhaka, Bangladesh. Nutrients. 2013;5(3):788–810. doi: 10.3390/nu5030788 23482056
224. Roth DE, Al Mahmud A, Raqib R, Black RE, Baqui AH. Pharmacokinetics of a single oral dose of vitamin D3 (70,000 IU) in pregnant and non-pregnant women. Nutr J. 2012;11 : 114. doi: 10.1186/1475-2891-11-114 23268736
225. Voigt R, Schroder S, Bierwisch I, Michels W, Spencker FB. [Pharmacokinetic studies of cefotiam in late pregnancy]. Z Geburtshilfe Perinatol. 1989;193(4):185–7. 2800661
226. Peiker G, Muller B, Voigt G, Noschel H. [Blood level kinetics of sodium noramidopyrine methansulphonate (Analgin) in late pregnancy]. Pharmazie. 1983;38(6):406–8. 6611643
227. Guay J, Beaudry B, Lortie L, Varin F. Pharmacokinetics of atracurium in pregnant women. Clin Drug Investig. 1996;11(3):167–73.
228. Pihlajamaki K, Kanto J, Lindberg R, Karanko M, Kiilholma P. Extradural administration of bupivacaine: pharmacokinetics and metabolism in pregnant and non-pregnant women. Br J Anaesth. 1990;64(5):556–62. 2354094
229. Peiker G, Mueller B, Ihn W, Noeschel H. [The pharmacokinetics of pethidin (Dolcontral) of parturients and their new-borns]. Pharmazie. 1981;36(8):560–3. 7291290
230. Elkomy MH, Sultan P, Carvalho B, Peltz G, Wu M, Clavijo C, et al. Ondansetron pharmacokinetics in pregnant women and neonates: towards a new treatment for neonatal abstinence syndrome. Clin Pharmacol Ther. 2015;97(2):167–76. doi: 10.1002/cpt.5 25670522
231. O’Sullivan MJ, Boyer PJJ, Scott GB, Parks WP, Weller S, Blum R, et al. The pharmacokinetics and safety of zidovudine in the third trimester of pregnancy for women infected with human immunodeficiency virus and their infants: phase I Acquired Immunodeficiency Syndrome Clinical Trials Group study (protocol 082). Am J Obstet Gynecol. 1993;168(5):1510–6. 8098905
232. Shi YE, Ye ZH, He CH, Zhang GQ, Xu JQ, Van Look PFA, et al. Pharmacokinetic study of RU 486 and its metabolites after oral administration of single doses to pregnant and non-pregnant women. Contraception. 1993;48(2):133–49. 8403910
233. Gardner DF, Cruikshank DP, Hays PM, Cooper DS. Pharmacology of propylthiouracil (PTU) in pregnant hyperthyroid women: correlation of maternal PTU concentrations with cord serum thyroid function tests. J Clin Endocrinol Metab. 1986;62(1):217–20. doi: 10.1210/jcem-62-1-217 3940267
234. Chopra IJ, Baber K. Treatment of primary hypothyroidism during pregnancy: is there an increase in thyroxine dose requirement in pregnancy? Metabolism. 2003;52(1):122–8. doi: 10.1053/meta.2003.50019 12524672
235. Ensom MHH, Stephenson MD. A two-center study on the pharmacokinetics of intravenous immunoglobulin before and during pregnancy in healthy women with poor obstetrical histories. Hum Reprod. 2011;26(9):2283–8. doi: 10.1093/humrep/der227 21771770
236. Rijken MJ, McGready R, Jullien V, Tarning J, Lindegardh N, Phyo AP, et al. Pharmacokinetics of amodiaquine and desethylamodiaquine in pregnant and postpartum women with Plasmodium vivax malaria. Antimicrob Agents Chemother. 2011;55(9):4338–42. doi: 10.1128/AAC.00154-11 21709098
237. Kloprogge F, Jullien V, Piola P, Dhorda M, Muwanga S, Nosten F, et al. Population pharmacokinetics of quinine in pregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. J Antimicrob Chemother. 2014;69(11):3033–40. doi: 10.1093/jac/dku228 24970740
238. Goodwin TM, Millar L, North L, Abrams LS, Weglein RC, Holland ML. The pharmacokinetics of the oxytocin antagonist atosiban in pregnant-women with preterm uterine contractions. Am J Obstet Gynecol. 1995;173(3):913–7. 7573268
239. Leake RD, Weitzman RE, Fisher DA. Pharmacokinetics of oxytocin in the human subject. Obstet Gynecol. 1980;56(6):701–4. 7443113
240. Hutchings MJ, Paull JD, Wilson-Evered E, Morgan DJ. Pharmacokinetics and metabolism of salbutamol in premature labour. Br J Clin Pharmacol. 1987;24(1):69–75. 3620288
241. Peiker G, Muller B, Dawczynski H, Erdmann M, Scharke U. [Comparison of the pharmacokinetics of iron in pregnant and nonpregnant women after oral administration of Vitaferro]. Pharmazie. 1988;43(5):335–6. 3174811
242. Frenkel LM, Brown ZA, Bryson YJ, Corey L, Unadkat JD, Hensleigh PA, et al. Pharmacokinetics of acyclovir in the term human pregnancy and neonate. Am J Obstet Gynecol. 1991;164(2):569–76. 1847004
243. Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep. 2012;60(7):1–21. 22970648
244. Yogev Y, Melamed N, Bardin R, Tenenbaum-Gavish K, Ben-Shitrit G, Ben-Haroush A. Pregnancy outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2010;203(6):558.e1–7.
245. Thorpe PG, Gilboa SM, Hernandez-Diaz S, Lind J, Cragan JD, Briggs G, et al. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf. 2013;22(9):1013–8. doi: 10.1002/pds.3495 23893932
246. McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899–925. 18232218
247. Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, Fleckenstein L. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar J. 2011;10 : 263. doi: 10.1186/1475-2875-10-263 21914160
248. Chan WS, Rey E, Kent NE, VTE in Pregnancy Guideline Working Group. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36(6):527–53. 24927193
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 11- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Nutraceutikum Armolipid Plus podle klinických důkazů zlepšuje lipidový profil − metaanalýza
- Snižuje terapie betablokátory kardiovaskulární benefit aerobního cvičení u pacientů s arteriální hypertenzí?
-
Všechny články tohoto čísla
- Effectiveness of Seasonal Malaria Chemoprevention in Children under Ten Years of Age in Senegal: A Stepped-Wedge Cluster-Randomised Trial
- Lifestyle Advice Combined with Personalized Estimates of Genetic or Phenotypic Risk of Type 2 Diabetes, and Objectively Measured Physical Activity: A Randomized Controlled Trial
- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Projected Impact of Mexico’s Sugar-Sweetened Beverage Tax Policy on Diabetes and Cardiovascular Disease: A Modeling Study
- Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050?
- Educational Outreach with an Integrated Clinical Tool for Nurse-Led Non-communicable Chronic Disease Management in Primary Care in South Africa: A Pragmatic Cluster Randomised Controlled Trial
- Measures of Malaria Burden after Long-Lasting Insecticidal Net Distribution and Indoor Residual Spraying at Three Sites in Uganda: A Prospective Observational Study
- Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults
- Under-prescribing of Prevention Drugs and Primary Prevention of Stroke and Transient Ischaemic Attack in UK General Practice: A Retrospective Analysis
- The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
- Improving the Pipeline for Developing and Testing Pharmacological Treatments in Pregnancy
- Seasonal Malaria Chemoprevention: An Evolving Research Paradigm
- Exposure Patterns Driving Ebola Transmission in West Africa: A Retrospective Observational Study
- Minimally Invasive Autopsy: A New Paradigm for Understanding Global Health?
- Towards Equity in Health: Researchers Take Stock
- Willingness to Know the Cause of Death and Hypothetical Acceptability of the Minimally Invasive Autopsy in Six Diverse African and Asian Settings: A Mixed Methods Socio-Behavioural Study
- Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels
- Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis
- Validity of a Minimally Invasive Autopsy for Cause of Death Determination in Adults in Mozambique: An Observational Study
- The Dengue Vaccine Dilemma: Balancing the Individual and Population Risks and Benefits
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review
- Three Steps to Improve Management of Noncommunicable Diseases in Humanitarian Crises
- Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis
- A Core Outcome Set for the Benefits and Adverse Events of Bariatric and Metabolic Surgery: The BARIACT Project
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



