-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEspA Acts as a Critical Mediator of ESX1-Dependent Virulence in by Affecting Bacterial Cell Wall Integrity
Mycobacterium tuberculosis (Mtb) requires the ESX1 specialized protein secretion system for virulence, for triggering cytosolic immune surveillance pathways, and for priming an optimal CD8+ T cell response. This suggests that ESX1 might act primarily by destabilizing the phagosomal membrane that surrounds the bacterium. However, identifying the primary function of the ESX1 system has been difficult because deletion of any substrate inhibits the secretion of all known substrates, thereby abolishing all ESX1 activity. Here we demonstrate that the ESX1 substrate EspA forms a disulfide bonded homodimer after secretion. By disrupting EspA disulfide bond formation, we have dissociated virulence from other known ESX1-mediated activities. Inhibition of EspA disulfide bond formation does not inhibit ESX1 secretion, ESX1-dependent stimulation of the cytosolic pattern receptors in the infected macrophage or the ability of Mtb to prime an adaptive immune response to ESX1 substrates. However, blocking EspA disulfide bond formation severely attenuates the ability of Mtb to survive and cause disease in mice. Strikingly, we show that inhibition of EspA disulfide bond formation also significantly compromises the stability of the mycobacterial cell wall, as does deletion of the ESX1 locus or individual components of the ESX1 system. Thus, we demonstrate that EspA is a major determinant of ESX1-mediated virulence independent of its function in ESX1 secretion. We propose that ESX1 and EspA play central roles in the virulence of Mtb in vivo because they alter the integrity of the mycobacterial cell wall.
Published in the journal: . PLoS Pathog 6(6): e32767. doi:10.1371/journal.ppat.1000957
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000957Summary
Mycobacterium tuberculosis (Mtb) requires the ESX1 specialized protein secretion system for virulence, for triggering cytosolic immune surveillance pathways, and for priming an optimal CD8+ T cell response. This suggests that ESX1 might act primarily by destabilizing the phagosomal membrane that surrounds the bacterium. However, identifying the primary function of the ESX1 system has been difficult because deletion of any substrate inhibits the secretion of all known substrates, thereby abolishing all ESX1 activity. Here we demonstrate that the ESX1 substrate EspA forms a disulfide bonded homodimer after secretion. By disrupting EspA disulfide bond formation, we have dissociated virulence from other known ESX1-mediated activities. Inhibition of EspA disulfide bond formation does not inhibit ESX1 secretion, ESX1-dependent stimulation of the cytosolic pattern receptors in the infected macrophage or the ability of Mtb to prime an adaptive immune response to ESX1 substrates. However, blocking EspA disulfide bond formation severely attenuates the ability of Mtb to survive and cause disease in mice. Strikingly, we show that inhibition of EspA disulfide bond formation also significantly compromises the stability of the mycobacterial cell wall, as does deletion of the ESX1 locus or individual components of the ESX1 system. Thus, we demonstrate that EspA is a major determinant of ESX1-mediated virulence independent of its function in ESX1 secretion. We propose that ESX1 and EspA play central roles in the virulence of Mtb in vivo because they alter the integrity of the mycobacterial cell wall.
Introduction
Mycobacterium tuberculosis (Mtb) is a devastating pathogen that causes epidemic disease and latently infects much of the world's population. However, the molecular details of its pathogenesis are poorly understood. Many lines of evidence underscore the importance of an alternative protein secretion system, ESX1, to Mtb survival in the macrophage and virulence in animals. The primary attenuating deletion in the vaccine strain, Mycobacterium bovis BCG is the loss of nine genes from the ESX1 locus [1]–[4]. Deletion of the ESX1 locus from virulent Mtb significantly attenuates the bacterium for growth in macrophages and animals [5]–[6]. ESX1 has been implicated in the ability of the bacterium to trigger macrophage production of IFN-β [7]–[8], activate the inflammasome [9], modulate macrophage cytokine production and signaling [5], and escape from the phagolysosome [10]–[11]. The ESX1 substrate proteins are also important targets of the adaptive immune response and are recognized by both CD4+ and CD8+ T cells in a majority of infected individuals [12].
The primary function of ESX1 activity in mediating virulence is unknown, however. There are data demonstrating that ESX1 is required for Mtb to damage the host cell membranes but it is less clear whether this is a direct function of the ESX1 locus. Mtb induces IFN-β production during macrophage infection by activation of the cytosolic pattern receptors [8], [13]. ESX1 dependent escape from phagolysosomes [10]–[11], [14] could similarly result from ESX1-mediated membrane damage. This has been hypothesized to be the direct effect of one of the ESX1 substrates, EsxA (Esat6) which has been found to be capable of forming pores in a variety of membrane systems [2], [15]–[16].
The pore-forming function of EsxA is controversial, however, in part because the ESX1 locus and EsxA are highly conserved in nonpathogenic gram positive organisms that lack obvious pore-forming ability [17]–[18]. In non-pathogenic organisms, ESX1 function has been associated with intrinsic bacterial processes including conjugative DNA transfer [19] and phage susceptibility [20] although the molecular basis for this is unclear. Interestingly, in pathogenic mycobacteria, loss of ESX1 function has also been associated with changes in colony morphology. Both M. bovis BCG and H37Ra, which are spontaneous mutants of virulent mycobacteria that were attenuated through loss of ESX1 function [21]–[23], were initially isolated from populations of virulent organisms because of changes in their colony morphology. When BCG was complemented with a wildtype copy of the ESX1 locus, the colony morphology reverted to that of virulent Mtb [3]. These data have suggested that ESX1 activity modifies Mtb cell wall composition although the basis for these observations is also unclear.
None of the ESX1 substrates has predicted cell wall modifying activity. In addition to EsxA, four other substrates of the ESX1 locus have been reported in Mtb. EsxB (Cfp10) heterodimerizes with EsxA and appears to direct its secretion [24]–[25]. ESX1 secretes a transcriptional regulator, EspR (Rv3849) [26] and two proteins of unknown function, EspA (Rv3616c) [27] and EspB (Rv3881) [28]–[29]. Identifying a unique function for any ESX1 substrate has been complicated by the fact that EsxA, EsxB, EspA and EspB require each other for secretion [27]–[28]. Thus, it has not been possible to use a loss-of-function approach to define the distinct activities of the individual substrate proteins.
In this study, we designed a novel strategy to determine whether the EspA has an independent role in virulence beyond its role in codependent secretion, using a structure-function approach to examine determinants of EspA's post-secretory activity. We demonstrate that EspA forms disulfide bonded homodimers after secretion and that abrogation of EspA disulfide bond formation does not alter protein secretion, the ability of Mtb to trigger the IFN-β response, or to stimulate robust CD4+ and CD8+ T cell responses. However, blocking EspA disulfide bond formation significantly attenuates the virulence of Mtb in animals and this attenuation correlates with a loss of cell wall integrity. Taken together, these data suggest that ESX1 is required for Mtb to survive and cause disease in animals in part because the full activity of at least one of its substrates, EspA, is required to maintain the structural integrity of the mycobacterial cell wall.
Results
Secreted EspA forms disulfide bonded homodimers
We sought to define unique functions of EspA that are independent of its role in the secretion of other ESX1 substrates. To do this we reasoned that EspA might participate in unique protein-protein interactions after secretion that could be targeted to disrupt EspA's post-secretory function. Indeed, when we analyzed culture filtrates from wildtype Mtb using SDS-PAGE in the absence of reducing agent, secreted EspA predominantly migrated with an apparent molecular mass of 80 kDa though smaller forms were detected (Figure 1A). Upon reduction, these forms of EspA resolved to a single species with an apparent molecular weight of 38 kDa, close to the predicted molecular weight of the monomer. As EspA contains a single cysteine at position 138, we hypothesized that after secretion, EspA dimerizes either with itself or with another protein via intermolecular disulfide bond formation. Of note, small amounts of the higher molecular weight forms of EspA were also detectable in the cell pellets (Figure 1A). We hypothesize that these represent secreted EspA that remains associated with the mycobacterial cell wall or perhaps is retained in the functional periplasmic space of the bacterium [30].
Fig. 1. EspA forms disulfide dependent homodimers in wildtype Mtb. 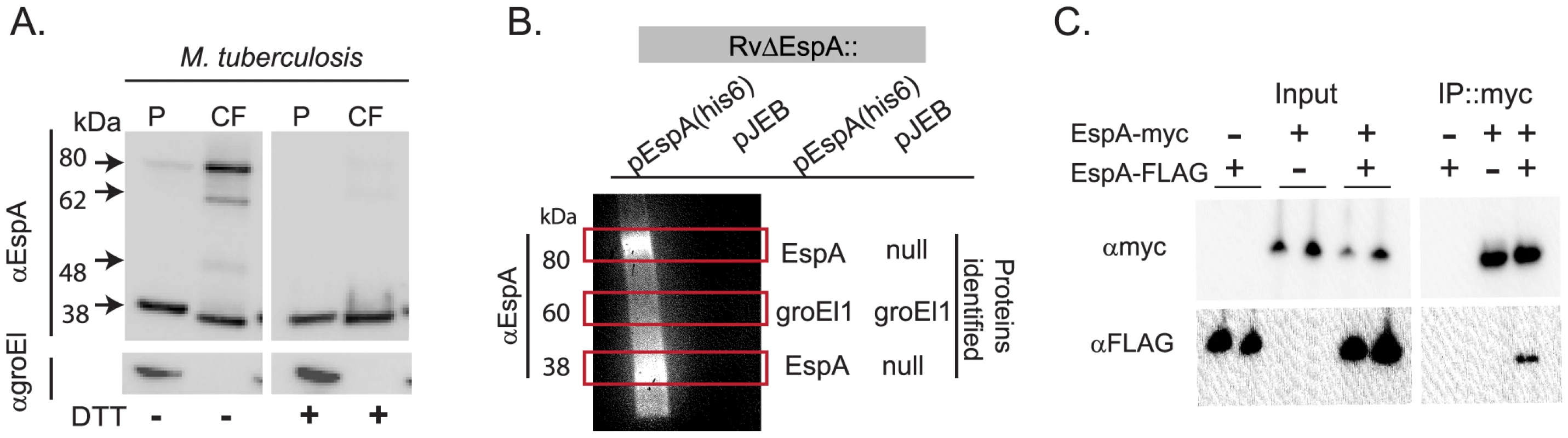
A. Whole cell pellets (P) and short term culture filtrates (CF) from wildtype Mtb (H37Rv) grown in N-salt media were analyzed by SDS-PAGE under nonreducing conditions and Western blot for EspA and groEl1, a lysis control. Results are representative of three independent experiments. B. Proteins were affinity purified with nickel agarose from RvΔespA complemented with the indicated vectors. Purified proteins were analyzed for the presence of EspA by Western blot analysis. Regions of the corresponding gel with bands visible by Coomassie staining are indicated with boxes. Protein composition of these bands was determined by LCQ-MS/MS (38 kDa band) or LTQ-FT-MS/MS (60 and 80 kDa bands). C. Immunoprecipitation of the EspA homodimer from M. smegmatis coexpressing the indicated forms of espA and analyzed by SDS-PAGE under reducing conditions and Western blot analysis. To identify the proteins that were disulfide-bonded to EspA, we affinity purified complexes associated with a C-terminally-tagged EspA allele, which we previously showed fully complements an EspA deletion mutant [27]. As a negative control we evaluated a strain carrying a deletion of the espA gene complemented with an empty vector in parallel. When affinity-purified proteins were resolved by SDS-PAGE and visualized by Coomassie staining, we identified bands specific to the EspA-his6 expressing strain only at 38kDa and 80 kDa. Western blot analysis indicated that both bands contained EspA (Figure 1B). Using tandem mass spectrometry, we identified only multiple unique peptides from EspA in both bands (Figure 1B, Table S1). A nonspecific 60 kDa band isolated from both strains was identified as Mtb GroEl1, a protein that contains a naturally occurring polyhistidine motif [31] and thus, would be expected to copurify. These data suggested that the 80 kDa species represents a homodimer of EspA which is covalently linked via an intermolecular disulfide bond.
To further test the model that EspA homodimerizes, we co-expressed EspA tagged with a FLAG epitope and EspA tagged with a Myc epitope in M. smegmatis. When heterologously expressed in M. smegmatis, EspA is found in both the 38 kDa and 80 kDa forms that are observed in M. tuberculosis (data not shown). As predicted, when EspA-Myc was affinity purified with an anti-Myc antibody from bacteria expressing EspA-Myc and EspA-FLAG, both the Myc - and FLAG - tagged forms of the protein were isolated, but EspA-FLAG was not isolated from the control strain which did not express EspA-Myc (Figure 1C).
Taken together, these data demonstrate that EspA homodimerizes and that a subset of these homodimers are covalently linked through intermolecular disulfide bond formation. Further analysis of secreted EspA suggested that the intermediate forms of EspA that migrate between the EspA dimer and monomer (Figure 1A) represent cleavage products of the EspA dimer (Figures S1A–C).
Mutation of EspA cysteine 138 does not inhibit ESX1 secretion
Because disulfide bond formation occurs rarely in the reducing cytosolic environment [32], we reasoned that disulfide bond formation in the EspA dimer occurs after secretion and could, therefore, be targeted to disrupt EspA function but not interfere with ESX1 secretion. To test this prediction, we mutated the unique cysteine in EspA, at position 138, to alanine (espAC138A). We find that EspA is significantly more abundant when expressed in the context of the other genes in its operon, espC and espD (data not shown). To test the effect of the espAC138A mutation, we therefore generated an unmarked deletion of espACD and complemented this mutant with the wildtype espACD genes under the control of a tetracycline inducible promoter [33], a similar construct expressing espAC138ACD or an empty vector as a negative control.
We confirmed that when EspA was expressed in RvΔespACD::pACD EspA was secreted and formed the same high molecular weight complexes that are observed in the culture filtrates of wildtype Mtb (Figure 2A). In contrast, EspAC138A was secreted but did not form the SDS-stable dimer. Thus, mutation of the sole cysteine in EspA inhibits disulfide-bonding of the EspA dimer but does not inhibit EspA secretion.
Fig. 2. Inhibition of EspA disulfide bond formation does not abrogate ESX1 secretion. 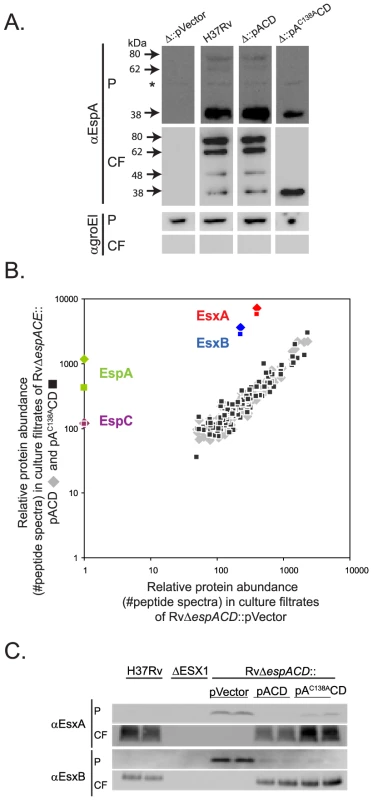
A. Western blot analysis of whole cell pellets (P) and short term culture filtrates (CF) of the indicated strains grown in N-salt media analyzed under non-reducing conditions. Arrows mark bands specific for EspA. The asterix marks a nonspecific background band. Results are representative of three independent experiments. Samples were assessed for GroEl1 to control for baterial autolysis. B. The relative abundance of proteins in culture filtrates collected in N-salt media from RvΔespACD complemented with a control vector was compared to the abundance of proteins in RvΔespACD::pACD (diamonds) or RvΔespACD::pAC138ACD (squares). Protein abundance was determined by spectral count analysis. The lack of EspA and EspC in the culture filtrates from the deletion strain validates the quantitative method. Peptide counts are found in Table S2. Where no spectra were identified, an arbitrary value of 1 was assigned. Data were obtained from four biologically independent samples processed in duplicate and are representative of at least two fully independent experiments. C. Whole cell pellets (P) and short term culture filtrates (CF) from wildtype H37Rv, an ESX1 deletion mutant, and the RvΔespACD complemented with the indicated constructs grown in Sauton's media. Samples were analyzed by SDS-PAGE under reducing conditions and Western blot analysis. Biologically independent duplicates were analyzed in each panel and these data are representative of at least 3 fully independent experiments. To comprehensively determine whether espAC138A alters Mtb protein secretion we used quantitative tandem mass spectrometry to analyze the culture filtrate proteins of RvΔespACD::pVector, RvΔespACD::pACD and RvΔespACD::pAC138ACD. To determine relative protein abundance, we made use of the fact that, using appropriate data acquisition parameters, the number of peptide spectra observed from a given protein directly reflects its overall abundance. Thus, we could estimate the relative abundance of each protein by quantifying the protein's spectral counts [34]–[35]. For robust quantitation, we focused on the 150 most abundant culture filtrate proteins, each of which was quantifiable by 75 or more spectra (Figure 2B and Table S2).
To validate the method, we assessed how the presence or absence of the espACD affected Mtb protein secretion. As previously shown [27], [36], we found that optimal EsxA and EsxB secretion requires the presence of the espACD operon (Figure 2C). By proteomic analysis, EsxA and EsxB secretion was ∼20 fold less efficient in the absence of espACD than presence of wildtype genes; however, EsxA and EsxB could still be identified in the culture filtrates of this strain (Figure 2B). By quantitative western blot analysis, we estimated that there was ∼100 fold less EsxA in the culture filtrates of Mtb lacking espACD, consistent with the proteomic data but suggesting that the quantitative dynamic range of the proteomic method is compressed. Interestingly, secreted isoforms of EsxA are found in the culture filtrates of the espACD deletion mutant () although we and others have not found them in culture filtrates from ESX1 mutants lacking core components of the ESX1 apparatus such as the FtsK-like ATPases, Rv3870 and Rv3871 (A. Garces and T. Ramsdel, unpublished data, and as previously shown in [6]), suggesting that optimal EsxA and EsxB secretion requires espACD but that residual EsxA and EsxB secretion occurs in the absence of these genes.
We then assessed the effect of inhibiting EspA disulfide bond formation on Mtb protein secretion. Loss of EspA disulfide bond formation did not substantially alter the global protein secretion profile of Mtb (Figure 2B & Table S2). Importantly, inhibition of EspA disulfide bond formation did not affect EsxA and EsxB secretion, which we confirmed by western blot analysis (Figure 2C). In Mtb expressing espAC138ACD, the proteomic analysis suggested that EspA secretion was intact though somewhat reduced relative to wildtype. EspC was also identified by mass spectrometry in the culture filtrates, as has been predicted by recently published studies in M. marinum [37], and the total secretion of EspC was not altered in bacteria expressing espAC138ACD. Thus, the proteomic data indicate that inhibition of EspA disulfide bond formation does not globally alter protein secretion in Mtb and is not required for ESX1 secretion of EsxA and EsxB.
EspA disulfide bond formation is required for virulence of Mtb
We hypothesized that inhibition of EspA disulfide bond formation would allow us to specifically identify aspects of ESX1 mediated virulence that require EspA function and dissociate them from those that require ESX1 secretion of EsxA and EsxB. To do this, we assessed the effect of the espAC138A mutation on the virulence of Mtb. In a SCID mouse model of infection, which was chosen in order to assess the virulence of the Mtb strains independent of the effects of adaptive immunity, animals infected with wildtype Mtb succumbed to infection after roughly 35 days (Figure 3A). The espACD deletion mutant was significantly attenuated for virulence; mice infected with this strain survived for an average of 127 days. Wildtype espACD significantly but incompletely complemented the deletion mutant for virulence. It is possible that the failure to fully complement the virulence defect is due to the fact that the espACD genes were ectopically expressed from an episomal vector under the control of an inducible promoter. Unlike the ESX1 locus, the espACD genes have been shown to be under the control of multiple regulators including EspR [26] and PhoPR [23]. Thus, it is not surprising that ectopic expression of the locus via an inducible promoter does not fully recapitulate the appropriate amount and timing of secretion during infection of an animal.
Fig. 3. Inhibition of EspA disulfide bond formation significantly attenuates Mtb for virulence. 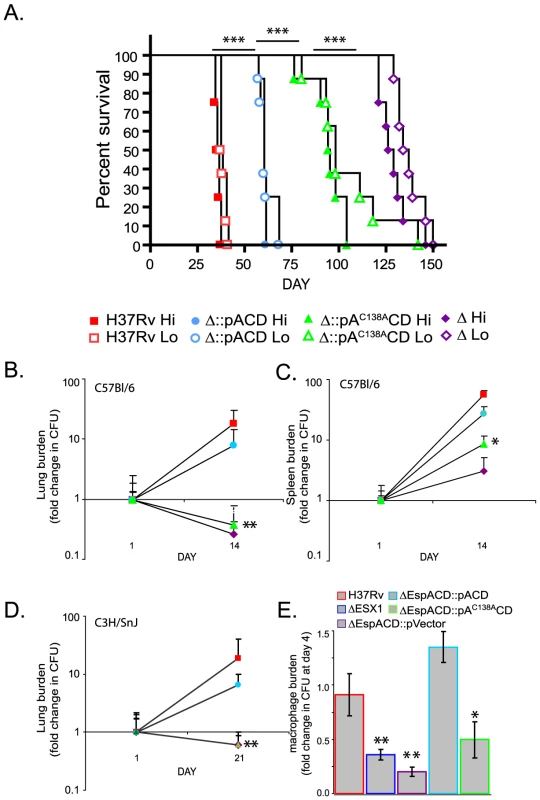
A. SCID mice were infected intravenously with the indicated strains. RvΔespACD::pVector is indicated as “Δ”. The goal “Hi” innoculum was 1×105 organisms; the working stock of “Hi” innoculum was diluted 3 fold to obtain the “Lo” innoculum. Dosing, as confirmed by sacrificing 2 mice from the Hi group 24 hours after infection and plating for CFU, was H37Rv-6.2×104, RvΔespACD::pACD-9.5×104, RvΔespACD::pAC138ACD-6.6×104, RvΔespACD::pVector-22×104. The differences in survival between groups of animals infected with the different strains were highly statistically significant by Chi Square test; differences between Hi groups are shown (***p<0.001). B–D. C57Bl/6 and C3H/HeSnJ mice were infected intravenously with the indicated strains. Organ burden is expressed as fold change from the organ burden at 24 hours. Data points represent mean+/− standard deviation of bacterial numbers from 4 mice/group. The organ burdens of H37Rv and RvΔespACD::pACD were significantly greater than RvΔespACD::pAC138ACD or RvΔespACD::pVector by T-test as shown (*p<0.05, **p<0.01). E. Murine bone marrow derived macrophages were infected with the indicated strains of Mtb. Bacterial survival at day 4 relative to day 1 is plotted and represents the mean+/−standard deviation of 4 biologic replicates. The relative survival of the ESX1 deletion mutant, RvΔespACD::pVector and RvΔespACD::pAC138ACD was significantly less than that of H37Rv or RvΔespACD::pACD by T-test as shown (*p<0.05, **p<0.01). However, in comparison to the strain expressing wild type espACD, the strain of Mtb expressing espAC138ACD was significantly attenuated (Figure 3A). Mice infected with the strain expressing espAC138ACD survived 95 days on average, about 35 days longer than mice infected with bacteria expressing the wildtype espACD. We obtained very similar survival times in mice infected in parallel with a three fold dilution of each innocula, demonstrating that the differences in survival reflect marked differences in the virulence of the infecting strains rather than small differences in infecting doses (Figure 3A).
We also found that virulence depended critically on C138 of EspA in immunocompetent mice. In both C57Bl/6 and C3H/HeSnJ mice, whose MHC haplotype also allows us to simultaneously measure EsxA and EsxB specific T cell responses as described below, the espACD mutant complemented with espAC138ACD was attenuated to nearly the same extent as the deletion mutant complemented with an empty vector while complementation with wildtype espACD largely restored Mtb growth in lungs and spleen (Figures 3B–D). Loss of ESX1 has also been shown to attenuate Mtb for growth in macrophages [6]. We therefore assessed the ability of Mtb expressing espAC138ACD to survive in murine bone marrow derived macrophages. Like the ESX1 deletion mutant, Mtb lacking espACD or expressing espAC138ACD were attenuated for survival in macrophages (Figure 3E). Thus, we find that inhibition of EspA disulfide bond formation significantly attenuates the virulence of Mtb in animals and in macrophages despite apparently normal secretion of EsxA and EsxB.
EspA disulfide bond formation is not required for ESX1-dependent activation of the innate and adaptive immune responses
Strains expressing mutant EspA could be attenuated because they elicit different host responses or host damage. Because EsxA has been postulated to disrupt host cell membranes, we sought to determine whether Mtb expressing espAC138ACD retain the ability of perturb host cell membranes. To test this we took advantage the fact that ESX1 is required for the rapid induction of IFN-β transcription upon M. tuberculosis infection [7]. We have shown that maximal IFN-β expression depends on activation of the NOD2 pathway which is triggered by bacterial peptidoglycan in the host cell cytosol [13]. We therefore assessed the ability of Mtb expressing wildtype or mutant EspA to induce secretion of IFN-β after macrophage infection. As previously shown, wildtype Mtb activates IFN-β expression and secretion in an ESX1, esxA and espA dependent fashion Figure 4A–B) while loss of these virulence determinants did not affect induction of TNF-α (Figure 4C). Complementation of the espACD deletion mutant with espAC138ACD restored the ability of the cells to activate IFN-β production to the same extent as complementation with the wildtype genes. Thus, inhibition of EspA disulfide bond formation does not perturb the bacterium's ability to activate the cytosolic pattern receptors.
Fig. 4. EspA disulfide bond formation is not required for innate or adaptive immune responses to Mtb. 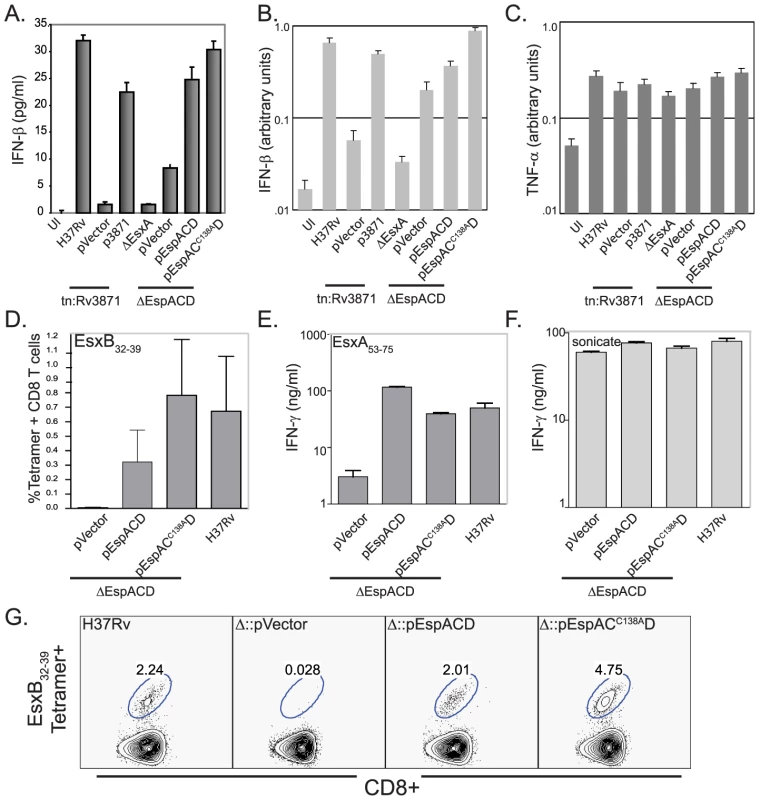
A. IFN-β secreted by RAW-264.7 cells infected with the indicated strains. IFN-β production was assessed by ELISA 24 hours after infection. Bars represent mean± standard deviation of three biologic replicates. Data are representative of at least 3 independent experiments. B, C. IFN-β and TNF-α expression by RAW-264.7 cells infected with the indicated strains 24 hours after infection. IFN-β and TNF-α expression was assessed by QT-PCR analysis and values were normalized to GAPDH expression. Bars represent mean± standard deviation of three biologic replicates and are representative of at least three independent experiments. D. Percent of CD8+ T cells which stain with H2-Kk/EsxB32–39 tetramer. CD90+ T cells from the spleens of mice (n = 4) 3 weeks post-infection were analyzed individually and bar represent indicate mean values± standard deviation. There was a statistically significant difference in CD8+ T cell responses between mice infected with RvΔespACD::pVector vs. H37Rv and RvΔespACD::pAC138ACD (p<0.05 by T-test). E, F. Splenic CD90+ T cells isolated from infected mice (n = 4, pooled) 3 weeks post-infection with the indicated strains were restimulated in vitro with the CD4+ antigenic peptide, EsxA53–75 or H37Rv sonicate. Bars indicate means+/−standard deviations. The CD4+ responses to EsxA53–75 were significantly lower in animals infected with RvΔespACD::pVector than in animals infected with the other strains (all p<0.01 by T-test). G. H2-Kk/CFP1032–39 tetramer staining of CD90+ T cells pooled from the lungs of 4 mice 3 weeks post-infection with the given strains. We extended these observations by assessing whether the espAC138A mutant's ability to stimulate the IFN-β response correlated with its ability to prime a CD8+ T cell response. EsxB is an important CD8+ T cell antigen in both mice and humans [38]–[39]. The path by which Mtb antigens reach the class I MHC processing pathway has not been well established. However, we have previously shown that ESX1 secretion is required in order to prime a CD8+ T cell response to EsxB [40]. We reasoned that the ESX1 substrates might strongly induce CD8+ T cell responses because they can gain access to the host cell cytosol and thus are readily sampled by the cytosolic class I MHC processing and presentation pathway. Consequently, we assessed the EsxB-specific CD8+ T cell response elicited by Mtb expressing espAC138ACD. We found a robust CD8+ T cell response to EsxB in the spleens and lungs of animals infected with RvΔespACD::pAC138ACD (Figures 4D and 4G). These findings are consistent with the data showing this strain is also capable of secreting EsxB and inducing IFN-β production. As anticipated from previously published results [4], the CD4+ T cell response to EsxA is abrogated in the absence of espACD (Figure 4E). However, it is intact in animals infected with espAC138ACD (Figure 4E), providing evidence that EsxA is secreted in vivo as well as in vitro in the absence of EspA disulfide bond formation. T-cells from mice infected with the various mycobacterial mutants produced similar amounts of IFN-γ in response to mycobacterial whole cell lysate, indicating that the global T-cell response to Mtb was not affected by EspA disulfide bond formation (Figure 4F).
Inhibition of EspA disulfide bond formation alters mycobacterial cell wall integrity
Spontaneous loss of ESX1 function during the laboratory evolution of both M. bovis BCG and H37Ra was associated with marked changes in colony morphology [21]–[22]. Complementation of BCG with a wildtype copy of the ESX1 genes resulted in colonies that again appeared similar to colonies of virulent Mtb [3]. More recent expression studies have also indicated that the espACD genes are highly transcriptionally regulated by cell wall stress [41]–[43], suggesting a link between these ESX1 substrates and cell wall structure. Based on these observations, we hypothesized that inhibition of EspA disulfide bond formation might alter the virulence of Mtb because it compromises the integrity of the cell wall.
Colony morphology is a subjective measure of cell wall structure and we have found it difficult to reproducibly and quantitatively score for ESX1 associated changes in colony morphology. Therefore, we sought more objective assays to assess cell wall integrity in our mutants. We found no evidence that loss of ESX1 or espACD altered bacterial resistance to reactive oxygen or nitrogen species (data not shown). However, we found that Mtb strains lacking the ESX1 locus, an FtsK-family ATPase in the ESX1 locus (Rv3871), or espACD were significantly more susceptible than wild type to a direct cell wall stress, SDS treatment (Figures 5A and 5B). Deletion of the ESX1 locus had a quantitatively greater effect on cell wall integrity than loss of espACD. The cell wall defect could be complemented by introduction of the wildtype genes (Figures 5A and 5B). We then assessed whether EspA disulfide bond formation was required for EspA's contribution to the cell wall integrity of Mtb. Strikingly, we found that bacteria expressing espAC138ACD show a similar susceptibility to SDS-induced stress as strains lacking the espACD locus entirely (Figure 5B). We tested other cell wall stressors and found that Mtb lacking ESX1, espACD or Mtb expressing espAC138ACD were also susceptible to other detergent stresses including n-dodecyl beta-D-maltoside and TritonX-100 (Figure 5C and Figure S3). Thus, ESX1 activity was required for the functional integrity of the mycobacterial cell wall and this effect requires EspA disulfide bond formation.
Fig. 5. ESX1 and EspA activity are required for the ability of Mtb to survive cell wall stress. 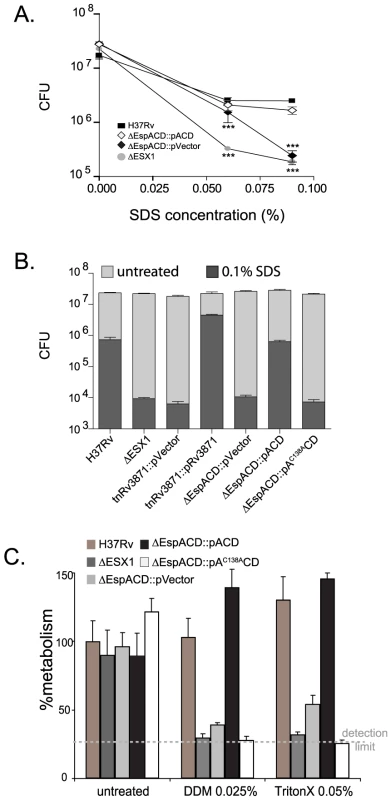
A. Bacterial survival after SDS stress. The indicated strains were exposed to increasing concentrations of SDS for 6 hours and then plated for CFU. Untreated bacteria were plated as a control and survival is shown. The mean+/−standard deviations of three independent biologic replicates are expressed. Strains lacking ESX1 and espACD were significantly more susceptible to SDS stress than H37Rv as assessed by T-test (***p<0.005). B. The indicated strains were exposed to treatment medium containing 0.1% SDS or control medium for 6 hours and then plated for CFU. Strains carrying tetracycline inducible vector and paired control strained were cultured in AT at 100 ng/ml overnight prior to and during SDS treatment. The mean+/−standard deviations of three independent biologic replicates are expressed. Data are representative of at least three fully independent experiments. Strains lacking ESX1 function, espACD and EspA disulfide bond formation were significantly more susceptible to SDS stress than H37Rv as determined by T-test (for these comparisons, all p<0.01). C. Bacterial survival after n-dodecyl beta-D-maltoside or TritonX-100 exposure. Bacterial metabolism as measured by Alamar blue after treatment with n-dodecyl beta-D-maltoside (DDM) orTritonX-100 expressed relative to the metabolism of untreated H37Rv. The mean+/−standard deviations of three independent biologic replicates are expressed. Strains lacking ESX1 function, espACD and EspA disulfide bond formation were significantly more susceptible to DDM and TritonX-100 as compared to H37Rv as determined by T-test (for these comparisons, all p<0.005). Discussion
The ESX1 secretion system is critically required for the virulence of Mtb yet little is understood about its mechanism of action. One hypothesis is that EsxA is the primary mediator of ESX1-associated virulence, acting as a pore-forming molecule that allows the bacterium access to the host cell cytosol [11],[16],[44]. Alternatively, the ESX1 system might function like a type IV secretion system, secreting effector proteins directly into the host cell cytosol [45]. In both of these models, the ESX1-dependent stimulation of cytosolic immune pathways and CD8+ T cell responses has been used as evidence that the ESX1 system targets host cell membranes. These models of ESX1 function do not address the experimental observations that suggest that the ESX1 locus affects the composition of the mycobacterial cell wall.
In this work, we have dissociated ESX1 secretion and the effects of the ESX1 apparatus on the innate and adaptive immune systems from ESX1 dependent cell wall effects and virulence. Disruption of EspA disulfide bond formation does not perturb ESX1 secretion or ESX1 dependent interactions between Mtb and the innate and adaptive immune systems. It does, however, alter the functional integrity of the mycobacterial cell wall and dramatically attenuate the bacterium for virulence in vivo. These data suggest that ESX1 is required for Mtb to survive and cause disease in vivo at least in part because of its effects on the cell wall. Perturbation of the cell wall structure may attenuate the organism for growth in vivo because it broadly disrupts the bacterial interface with the host cell, undermining specific virulence functions, or because the organism is more susceptible to host antimicrobial defenses.
The most parsimonious explanation for our findings is that EspA acts directly on the mycobacterial cell wall. We and others have shown that EspA is secreted in standard mycobacterial growth media, which includes low concentrations of nonionic detergent. However, we have found that this protein remains associated with the cell wall when Mtb was grown in the absence of detergent (data not shown), in keeping with recently published microscopy data demonstrating that several ESX1 substrates are associated with the mycobacterial capsule in minimally disturbed bacterial cultures [46]. Thus, EspA could reasonably be engaged in modifying the cell wall and perhaps the capsule more specifically. For example, EspA may be directly required for the transport of cell wall components or may regulate the activity of other cell wall acting proteins. Alternatively, EspA could also have indirect effects that alter gene expression, although there is little evidence of ESX1 dependent changes in transcription [47].
We show that Mtb strains lacking ESX1 or EspA function have a marked defect in cell wall integrity as measured by detergent susceptibility. However, we have found that ESX1 function does not affect other measures of cell wall permeability or structure such as susceptibility to the hydrophobic antibiotic, rifampin, the cell wall acting antibiotics, isoniazid and meropenem, or lysozyme (data not shown). Our findings are consistent with studies of other cell wall mutants which have found that different mutants in cell wall biosynthesis have variable defects in permeability and susceptibility assays. In some cases, susceptibility can be easily predicted by gene function. For example, disrupting ponA, which acts on peptidoglycan, causes hypersusceptibility to lysozyme [48]. In many cases, however, the link between the genetic lesion and susceptibility to different cell wall stressors is not obvious [48]–[50], reflecting our limited insight into cell wall assembly in Mtb. In the case of ESX1, further biochemical analysis will be required to determine the specific cell wall defect caused by loss of activity.
Our model does not exclude the possibility that other ESX1 substrates, such as EsxA, have a direct activity on the macrophage as we find that activation of the host cytosolic surveillance systems occurs independently of EspA disulfide bond formation but requires ESX1 activity. The espACD deletion mutant is less virulent in SCID mice than Mtb lacking EspA disulfide bond formation, suggesting that isolated EsxA secretion may make an independent contribution to virulence in animals. However, EsxA, like the rest of the ESX1 locus, is highly conserved in both pathogenic and nonpathogenic gram positive bacteria [18], suggesting that this protein has an important biologic function in the bacterium that is a prerequisite for the virulence of Mtb but that it does not directly mediate virulence. Indeed, the data presented here suggest that the primary target of the ESX1 system is the bacterial cell wall.
Materials and Methods
Culture of Mtb and preparation of culture filtrates and cell lysates
Mtb and M. smegmatis strains were maintained as previously published [27], [51]. The EsxA deletion mutant and Rv3871 transposon mutant have been previously described [6]. For analysis of protein expression and secretion, bacteria from cultures normalized to the same growth phase were washed and resuspended in designated medium at an O.D.∼0.3 for 72 hours at 37°C. Where indicated, bacteria were cultured in N salt media (100 mM Bis/Tris HCl, 5 mM KCL, 7.5 mM (NH4)2SO4, 0.5 mM K2HSO4, 1 mM KH2PO4, 10 mM MgCl2, 38 mM glycerol, pH 7.0). N salt media is a minimal medium that allows titration of the divalent cation concentration which we have historically used when collecting samples for proteomic analysis [27]. Cell pellets and culture filtrates were collected and processed as described previously [27] except that culture filtrates were concentrated by precipitation with 10% trichloroacetic acid unless otherwise noted.
Generation of mutant strains and complementing constructs
The genes encoding espACD were deleted from wildtype H37Rv through homologous recombination using a suicide vector approach. Deletion was confirmed by PCR analysis. As described in detail in Text S1, espACD was amplified from H37Rv genomic DNA and the espAC138A mutation was introduced via PCR mutagenesis and cloning of an internal gene fragment. The PCR products were recombined into a Gateway donor vector (Invitrogen, Carlsbad, CA) and transferred to an episomal expression vector (pTET) that was constructed to express genes under the control of a tetracycline inducible mycobacterial promoter [33]. All constructs were confirmed by sequencing. The constructs or an empty vector were transformed into RvΔespACD to generate the designated strains. Rv3871 was amplified (Forward primer: GGCTAAGAAGGAGATATACATATGACTGCTGAACCGGAAGTA; Reverse primer: CTTGTCGTCGTCGTCCTTGTAGTCACCGGCGCTTGGGGGTGC) and the PCR product was similarly recombined into pTET. This construct or an empty vector was transformed into the Rv3871 transposon insertion mutant. Gene expression was induced from the tetracycline inducible promoter with 100 ng/ml of anhydrotetracycline (AT) (Spectrum Chemicals, Gardena, CA) for 24 hours prior to beginning culture filtrate collections.
Protein analysis
Samples were analyzed via SDS-PAGE and western blotting as previously published [27]. Where noted, samples were reduced with 10 mM dithiothreitol (DTT) for 30 minutes at 37°C prior to gel electrophoresis. Antibodies to EsxA, EsxB and EspA were published previously [27]. The antibody to poly-histidine (#NB600-318), which was used to detect GroEl1, was obtained from Novus Biologicals (Littleton, CO) as were antibodies to the Myc - and FLAG - epitopes. Antibodies were used according to the manufacturer's directions. In addition, where indicated, relevant gel slices were excised and analyzed by tandem mass spectrometry (MS/MS) as using published methods [52]–[54] and as described in Text S1. Affinity purification of EspA(his6) from RvΔespA::pEspA(6his) and EspA-FLAG and EspA-myc from M. smegmatis were performed as described in Text S1.
Infection of mice and assessment of CD8+ T cell responses
BALB/c-SCID, C57Bl/6 and C3H/HeSnJ mice were purchased from Jackson Laboratory (Bar Harbor, ME). 24 hours prior to infection, mycobacterial strains were cultured overnight in media containing 100 ng/ml AT and mice were started on chow containing 2000 ppm tetracycline (Research Diets, New Brunswick, NJ). Mice were maintained on tet-chow through the course of the experiment. Mice were infected by intravenous tail vein injection and doses were confirmed by plating the innocula. At the indicated times, 4 mice/group were sacrificed and bacterial organ burden was determined by plating for CFU. Organs from C57Bl/6 mice were plated on medium in the presence and absence of hygromycin to assess for loss of the episomal plasmid over the course of the experiment. No significant vector loss was detected. Mice with organ burdens that differed by more than 5 fold from other animals in the group were considered missed injections and these data were discarded. CD8+ and CD4+ T cell responses were assessed as previously published [40]. In order to ensure that the infected mice had equivalent bacterial burdens at the time of T cell analysis, the infecting doses of RvΔespACD::pVector and RvΔespACD::pAC138ACD were ten fold higher than that of RvΔespACD::pACD or H37Rv.
Macrophage infections and cytokine responses
Bacterial strains were prepared and induced as described for murine infections. Murine bone marrow derived macrophages were prepared from C57Bl/6 mice according to previously published protocols [55]. After 7 days of culture, differentiated macrophages were frozen for future use. For infections, bone marrow derived macrophages were thawed and plated at a density of 2.5×104 cells per well of a 96 well tissue culture treated plate and allowed to adhere overnight. Monolayers were washed, and infected with the indicated strains at an MOI of 10 to produce a final infection of roughly 1 bacterium/macrophage. Bacteria were spun onto the macrophage monolayer and infection was allowed to proceed for 3 hours. Monolayers were washed three times and fresh medium was added containing 100 ng/ml AT. At the indicated times after infection, monolayers were lysed with PBS-0.1%TritonX-100 and bacteria in the well were enumerated by plating serial dilutions.
For cytokine assays, RAW-264.7 macrophages were infected with the indicated strains at an MOI of 1 bacterium/macrophage as previously described [56]. At the indicated times, culture filtrates were removed and IFN-β secretion was assessed by ELISA for IFN-β (R&D Systems, Minneapolis, MN). In addition, RNA was isolated from infected macrophages as previously described [56]. 2 µg of RNA was transcribed into DNA using random hexamers with Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative PCR assays were performed with TaqMan Gene Expression IFN-β, TNF-α and GAPDH assays (Applied Biosystems, Foster City, CA). For these assays, standard curves were generated using serial dilutions of pooled cDNA from macrophages 4 hours after LPS stimulation.
Detergent susceptibility
Mtb strains were grown to early log phase (∼0.2 O.D. at 600nm) in Sauton's medium supplemented with 0.05% Tween-80. Strains complemented with tetracycline inducible constructs, pEmpty, pACD, pAC138ACD and pRv3871, were cultured overnight in Sauton's containing 100 ng/ml AT. Cells were pelleted, washed once and resuspended at a density of 1.2×108 cells/ml in 7H9 medium containing the indicated concentrations of SDS, DDM (n-dodecyl β-D-maltoside) and Triton-X-100. DDM and Triton-X-100 were purchased from Sigma-Aldrich (St. Louis, MO). In studies of strains expressing the tetracycline inducible constructs the medium also contained AT at 100 ng/ml. Bacteria were incubated in SDS for 6h at 37°C with shaking, washed twice and then plated for CFU on 7H10 agar plates containing 10% OADC. For susceptibility to DDM and Triton-X-100, bacteria were incubated overnight with detergent on a shaker at 37°C, washed twice and resuspended in 7H9 media supplemented with 10% OADC and 0.05% Tween-80. Cultures were serially diluted (10-fold) onto 96 wells plate and their viability determined by adding 20 µl of 10× Alamar Blue dye (AbD Serotec, Raleigh, NC). After incubating at 37°C for 2 days, cells were fixed for 1 h with 2% paraformaldehyde and absorbance measured at 570 and 600 nm on a Versamax microplate reader using Softmax Pro version 5.3 (Molecular Devices, CA).
Bioinformatics
Proteomics data analysis was performed as described in Text S1 according to published methods. Statistical analyses and graphing were otherwise performed with GraphPad Prism.
Ethics
All animal experimentation was conducted following the National Institutes of Health guidelines for housing and care of laboratory animals and performed in accordance with Institutional regulations after protocol review and approval by the Harvard Medical Area Standing Committee on Animals.
Supporting Information
Zdroje
1. LewisKN
LiaoR
GuinnKM
HickeyMJ
SmithS
2003 Deletion of RD1 from Mycobacterium tuberculosis Mimics Bacille Calmette-Guerin Attenuation. J Infect Dis 187 117 123
2. HsuT
Hingley-WilsonSM
ChenB
ChenM
DaiAZ
2003 The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A 100 12420 12425
3. PymAS
BrodinP
BroschR
HuerreM
ColeST
2002 Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 46 709 717
4. PymAS
BrodinP
MajlessiL
BroschR
DemangelC
2003 Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 9 533 539
5. StanleySA
RaghavanS
HwangWW
CoxJS
2003 Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci U S A 100 13001 13006
6. GuinnKM
HickeyMJ
MathurSK
ZakelKL
GrotzkeJE
2004 Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol 51 359 370
7. StanleySA
JohndrowJE
ManzanilloP
CoxJS
2007 The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol 178 3143 3152
8. LeberJH
CrimminsGT
RaghavanS
Meyer-MorseNP
CoxJS
2008 Distinct TLR - and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog 4 e6 doi:10.1371/journal.ppat.0040006
9. KooIC
WangC
RaghavanS
MorisakiJH
CoxJS
2008 ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol 10 1866 1878
10. GaoLY
GuoS
McLaughlinB
MorisakiH
EngelJN
2004 A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol 53 1677 1693
11. van der WelN
HavaD
HoubenD
FluitsmaD
van ZonM
2007 M. tuberculosis and M. leprae translocate from the phagolysosome into the cytosol in myeloid cells. Cell 129 1287 1298
12. BrodinP
RosenkrandsI
AndersenP
ColeST
BroschR
2004 ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol 12 500 508
13. PandeyAK
YangY
JiangZ
FortuneSM
CoulombeF
2009 NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog 5 e1000500 doi:10.1371/journal.ppat.1000500
14. StammLM
MorisakiJH
GaoLY
JengRL
McDonaldKL
2003 Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J Exp Med 198 1361 1368
15. de JongeMI
Pehau-ArnaudetG
FretzMM
RomainF
BottaiD
2007 ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol 189 6028 34
16. SmithJ
ManoranjanJ
PanM
BohsaliA
XuJ
Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from vacuole. Infect Immun 76 5478 5487
17. CorosA
CallahanB
BattaglioliE
DerbyshireKM
2008 The specialized secretory apparatus ESX-1 is essential for DNA transfer in Mycobacterium smegmatis. Mol Microbiol 69 794 808
18. PallenMJ
2002 The ESAT-6/WXG100 superfamily – and a new Gram-positive secretion system? Trends Microbiol 10 209 212
19. FlintJL
KowalskiJC
KarnatiPK
DerbyshireKM
2004 The RD1 virulence locus of Mycobacterium tuberculosis regulates DNA transfer in Mycobacterium smegmatis. Proc Natl Acad Sci U S A 101 12598 12603
20. São-JoséC
BaptistaC
SantosMA
2004 Bacillus subtilis operon encoding a membrane receptor for bacteriophage SPP1. J Bacteriol 186 8337 8346
21. CalmetteA
1927 La Vacinne Preventive Contre la Tuberculose Paris Masson et cie. 250
22. SteenkenW
1938 Spontaneous lysis of tubercle bacilli on artificial culture media. Am Rev Tuberc 38 777 790
23. FriguiW
BottaiD
MajlessiL
MonotM
JosselinE
2008 Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog 4 e33 doi:10.1371/journal.ppat.0040033
24. ChampionPA
StanleySA
ChampionMM
BrownEJ
CoxJS
2006 C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 313 1632 1636
25. RenshawPS
LightbodyKL
VeverkaV
MuskettFW
KellyG
2005 Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J 24 2491 2498
26. RaghavanS
ManzanilloP
ChanK
DoveyC
CoxJS
2008 Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature 454 717 721
27. FortuneSM
JaegerA
SarracinoDA
ChaseMR
SassettiCM
2005 Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci U S A 102 10676 10681
28. XuJ
LaineO
MasciocchiM
ManoranjanJ
SmithJ
2007 A unique Mycobacterium ESX-1 protein co-secretes with CFP-10/ESAT-6 and is necessary for inhibiting phagosome maturation. Mol Microbiol 66 787 800
29. McLaughlinB
ChonJS
MacGurnJA
CarlssonF
ChengTL
2007 A mycobacterium ESX-1-secreted virulence factor with unique requirements for export. PLoS Pathog 3 e105 doi:10.1371/journal.ppat.0030105
30. HoffmannC
LeisA
NiederweisM
PlitzkoJM
EngelhardtH
2008 Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci U S A 105 3963 3967
31. OjhaA
AnandM
BhattA
KremerL
JacobsWRJr
2005 GroEL1: a dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell 123 861 873
32. KadokuraH
KatzenF
BeckwithJ
2003 Protein disulfide bond formation in prokaryotes. Annu Rev Biochem 72 111 135
33. EhrtS
GuoXV
HickeyCM
RyouM
MonteleoneM
2005 Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res 33 e21
34. LiuH
SadygovRG
YatesJR3rd
2004 A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem 76 4193 4201
35. OldWM
Meyer-ArendtK
Aveline-WolfL
PierceKG
MendozaA
2005 Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics 4 1487 1502
36. MacGurnJA
RaghavanS
StanleySA
CoxJS
2005 A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol Microbiol 57 1653 1663
37. DiGiuseppe ChampionPA
ChampionMM
ManzanilloP
CoxJS
2009 ESX-1 secreted virulence factors are recognized by multiple cytosolic AAA ATPases in pathogenic mycobacteria. Mol Microbio 73 950 962
38. KamathAB
WoodworthJ
XiongX
TaylorC
WengY
2004 Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J Exp Med 200 1479 1489
39. LewinsohnDM
ZhuL
MadisonVJ
DillonDC
FlingSP
2001 Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J Immunol 166 439 446
40. WoodworthJS
FortuneSM
BeharSM
2008 Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect Immun 76 4199 4205
41. PangX
VuP
ByrdTF
GhannyS
SoteropoulosP
2007 Evidence for complex interactions of stress-associated regulons in an mprAB deletion mutant of Mycobacterium tuberculosis. Microbiology 153(Pt 4) 1229 1242
42. HeH
ZahrtTC
2005 Identification and characterization of a regulatory sequence recognized by Mycobacterium tuberculosis persistence regulator MprA. J Bacteriol 187 202 212
43. FisherMA
PlikaytisBB
ShinnickTM
2002 Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J Bacteriol 184 4025 4032
44. PathakSK
BasuS
BasuKK
BanerjeeA
PathakS
2007 Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol 8 610 618
45. AbdallahAM
Gey van PittiusNC
ChampionPA
CoxJ
LuirinkJ
2007 Type VII secretion–mycobacteria show the way. Nat Rev Microbiol 5 883 891
46. SaniM
HoubenEN
GeurtsenJ
PiersonJ
de PunderK
2010 Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog 6 e1000794 doi:10.1371/journal.ppat.1000794
47. MostowyS
CletoC
ShermanDR
BehrMA
2004 The Mycobacterium tuberculosis complex transcriptome of attenuation. Tuberculosis (Edinb) 84 197 204
48. VandalOH
RobertsJA
OdairaT
SchnappingerD
NathanCF
2009 Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J Bacteriol 191 625 631
49. CosmaCL
KleinK
KimR
BeeryD
RamakrishnanL
2006 Mycobacterium marinum Erp is a virulence determinant required for cell wall integrity and intracellular survival. Infect Immun 74 3125 3133
50. BanaeiN
KincaidEZ
LinSY
DesmondE
JacobsWRJr
2009 Lipoprotein processing is essential for resistance of Mycobacterium tuberculosis to malachite green. Antimicrob Agents Chemother 53 3799 3802
51. SiegristMS
UnnikrishnanM
McConnellMJ
BorowskyM
ChengTY
2009 Mycobacterial Esx-3 is required for mycobactin-mediated iron acquisition. Proc Natl Acad Sci U S A 106 18792 18797
52. EliasJE
GygiSP
2007 Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4 207 214
53. NesvizhskiiAI
KellerA
KolkerE
AebersoldR
2003 A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75 4646 4658
54. ZhangB
VerBerkmoesNC
LangstonMA
UberbacherE
HettichRL
2006 Detecting differential and correlated protein expression in label-free shotgun proteomics. J Proteome Res 5 2909 2918
55. RengarajanJ
BloomBR
RubinEJ
2005 Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A 102 8327 8332
56. FortuneSM
SolacheA
JaegerA
HillPJ
BelisleJT
2004 Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J Immunol 172 6272 6280
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin NeutralizationČlánek Serum-Dependent Selective Expression of EhTMKB1-9, a Member of B1 Family of Transmembrane KinasesČlánek Host Cell Invasion and Virulence in Sepsis Is Facilitated by the Multiple Repeats within FnBPA
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Cognitive Dysfunction Is Sustained after Rescue Therapy in Experimental Cerebral Malaria, and Is Reduced by Additive Antioxidant Therapy
- DNA Watermarking of Infectious Agents: Progress and Prospects
- Self-Protection against Gliotoxin—A Component of the Gliotoxin Biosynthetic Cluster, GliT, Completely Protects Against Exogenous Gliotoxin
- Modifies the Tsetse Salivary Composition, Altering the Fly Feeding Behavior That Favors Parasite Transmission
- Requirement of NOX2 and Reactive Oxygen Species for Efficient RIG-I-Mediated Antiviral Response through Regulation of MAVS Expression
- The Enteropathogenic Effector EspF Targets and Disrupts the Nucleolus by a Process Regulated by Mitochondrial Dysfunction
- Epithelial p38α Controls Immune Cell Recruitment in the Colonic Mucosa
- Coexpression of PD-1, 2B4, CD160 and KLRG1 on Exhausted HCV-Specific CD8+ T Cells Is Linked to Antigen Recognition and T Cell Differentiation
- Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin Neutralization
- EspA Acts as a Critical Mediator of ESX1-Dependent Virulence in by Affecting Bacterial Cell Wall Integrity
- An RNA Element at the 5′-End of the Poliovirus Genome Functions as a General Promoter for RNA Synthesis
- Tetherin Restricts Productive HIV-1 Cell-to-Cell Transmission
- Epstein-Barr Virus-Encoded LMP2A Induces an Epithelial–Mesenchymal Transition and Increases the Number of Side Population Stem-like Cancer Cells in Nasopharyngeal Carcinoma
- Protein Kinase A Dependent Phosphorylation of Apical Membrane Antigen 1 Plays an Important Role in Erythrocyte Invasion by the Malaria Parasite
- Requirement for Ergosterol in V-ATPase Function Underlies Antifungal Activity of Azole Drugs
- The SOCS-Box of HIV-1 Vif Interacts with ElonginBC by Induced-Folding to Recruit Its Cul5-Containing Ubiquitin Ligase Complex
- A Crucial Role for Infected-Cell/Antibody Immune Complexes in the Enhancement of Endogenous Antiviral Immunity by Short Passive Immunotherapy
- Modulation of the Arginase Pathway in the Context of Microbial Pathogenesis: A Metabolic Enzyme Moonlighting as an Immune Modulator
- Role of Abl Kinase and the Wave2 Signaling Complex in HIV-1 Entry at a Post-Hemifusion Step
- Serum-Dependent Selective Expression of EhTMKB1-9, a Member of B1 Family of Transmembrane Kinases
- Epigenetic Repression of by Latent Epstein-Barr Virus Requires the Interaction of EBNA3A and EBNA3C with CtBP
- Host Cell Invasion and Virulence in Sepsis Is Facilitated by the Multiple Repeats within FnBPA
- Role of PKR and Type I IFNs in Viral Control during Primary and Secondary Infection
- A Kinome RNAi Screen Identified AMPK as Promoting Poxvirus Entry through the Control of Actin Dynamics
- Cryptococcal Cell Morphology Affects Host Cell Interactions and Pathogenicity
- NleG Type 3 Effectors from Enterohaemorrhagic Are U-Box E3 Ubiquitin Ligases
- Human Cytomegalovirus UL29/28 Protein Interacts with Components of the NuRD Complex Which Promote Accumulation of Immediate-Early RNA
- Complement Receptor 1 Is a Sialic Acid-Independent Erythrocyte Receptor of
- A Viral microRNA Down-Regulates Multiple Cell Cycle Genes through mRNA 5′UTRs
- Immunotoxin Complementation of HAART to Deplete Persisting HIV-Infected Cell Reservoirs
- Protein Expression Redirects Vesicular Stomatitis Virus RNA Synthesis to Cytoplasmic Inclusions
- Entry and Fusion of Emerging Paramyxoviruses
- Paramyxovirus Entry and Targeted Vectors for Cancer Therapy
- Suppressing Glucose Transporter Gene Expression in Schistosomes Impairs Parasite Feeding and Decreases Survival in the Mammalian Host
- Formation of Complexes at Plasmodesmata for Potyvirus Intercellular Movement Is Mediated by the Viral Protein P3N-PIPO
- Fungal Cell Gigantism during Mammalian Infection
- Two Novel Point Mutations in Clinical Reduce Linezolid Susceptibility and Switch on the Stringent Response to Promote Persistent Infection
- Rotavirus Structural Proteins and dsRNA Are Required for the Human Primary Plasmacytoid Dendritic Cell IFNα Response
- The Epigenetic Landscape of Latent Kaposi Sarcoma-Associated Herpesvirus Genomes
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requirement of NOX2 and Reactive Oxygen Species for Efficient RIG-I-Mediated Antiviral Response through Regulation of MAVS Expression
- Formation of Complexes at Plasmodesmata for Potyvirus Intercellular Movement Is Mediated by the Viral Protein P3N-PIPO
- Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin Neutralization
- Two Novel Point Mutations in Clinical Reduce Linezolid Susceptibility and Switch on the Stringent Response to Promote Persistent Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



