-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRotavirus Structural Proteins and dsRNA Are Required for the Human Primary Plasmacytoid Dendritic Cell IFNα Response
Rotaviruses are the leading cause of severe dehydrating diarrhea in children worldwide. Rotavirus-induced immune responses, especially the T and B cell responses, have been extensively characterized; however, little is known about innate immune mechanisms involved in the control of rotavirus infection. Although increased levels of systemic type I interferon (IFNα and β) correlate with accelerated resolution of rotavirus disease, multiple rotavirus strains, including rhesus rotavirus (RRV), have been demonstrated to antagonize type I IFN production in a variety of epithelial and fibroblast cell types through several mechanisms, including degradation of multiple interferon regulatory factors by a viral nonstructural protein. This report demonstrates that stimulation of highly purified primary human peripheral plasmacytoid dendritic cells (pDCs) with either live or inactivated RRV induces substantial IFNα production by a subset of pDCs in which RRV does not replicate. Characterization of pDC responses to viral stimulus by flow cytometry and Luminex revealed that RRV replicates in a small subset of human primary pDCs and, in this RRV-permissive small subset, IFNα production is diminished. pDC activation and maturation were observed independently of viral replication and were enhanced in cells in which virus replicates. Production of IFNα by pDCs following RRV exposure required viral dsRNA and surface proteins, but neither viral replication nor activation by trypsin cleavage of VP4. These results demonstrate that a minor subset of purified primary human peripheral pDCs are permissive to RRV infection, and that pDCs retain functionality following RRV stimulus. Additionally, this study demonstrates trypsin-independent infection of primary peripheral cells by rotavirus, which may allow for the establishment of extraintestinal viremia and antigenemia. Importantly, these data provide the first evidence of IFNα induction in primary human pDCs by a dsRNA virus, while simultaneously demonstrating impaired IFNα production in primary human cells in which RRV replicates. Rotavirus infection of primary human pDCs provides a powerful experimental system for the study of mechanisms underlying pDC-mediated innate immunity to viral infection and reveals a potentially novel dsRNA-dependent pathway of IFNα induction.
Published in the journal: . PLoS Pathog 6(6): e32767. doi:10.1371/journal.ppat.1000931
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000931Summary
Rotaviruses are the leading cause of severe dehydrating diarrhea in children worldwide. Rotavirus-induced immune responses, especially the T and B cell responses, have been extensively characterized; however, little is known about innate immune mechanisms involved in the control of rotavirus infection. Although increased levels of systemic type I interferon (IFNα and β) correlate with accelerated resolution of rotavirus disease, multiple rotavirus strains, including rhesus rotavirus (RRV), have been demonstrated to antagonize type I IFN production in a variety of epithelial and fibroblast cell types through several mechanisms, including degradation of multiple interferon regulatory factors by a viral nonstructural protein. This report demonstrates that stimulation of highly purified primary human peripheral plasmacytoid dendritic cells (pDCs) with either live or inactivated RRV induces substantial IFNα production by a subset of pDCs in which RRV does not replicate. Characterization of pDC responses to viral stimulus by flow cytometry and Luminex revealed that RRV replicates in a small subset of human primary pDCs and, in this RRV-permissive small subset, IFNα production is diminished. pDC activation and maturation were observed independently of viral replication and were enhanced in cells in which virus replicates. Production of IFNα by pDCs following RRV exposure required viral dsRNA and surface proteins, but neither viral replication nor activation by trypsin cleavage of VP4. These results demonstrate that a minor subset of purified primary human peripheral pDCs are permissive to RRV infection, and that pDCs retain functionality following RRV stimulus. Additionally, this study demonstrates trypsin-independent infection of primary peripheral cells by rotavirus, which may allow for the establishment of extraintestinal viremia and antigenemia. Importantly, these data provide the first evidence of IFNα induction in primary human pDCs by a dsRNA virus, while simultaneously demonstrating impaired IFNα production in primary human cells in which RRV replicates. Rotavirus infection of primary human pDCs provides a powerful experimental system for the study of mechanisms underlying pDC-mediated innate immunity to viral infection and reveals a potentially novel dsRNA-dependent pathway of IFNα induction.
Introduction
Dendritic cells (DCs), a highly specialized subset of professional antigen-presenting cells, play a central role in the initiation of innate and adaptive immunity. There are two known major subsets of primary human and murine circulating DCs: myeloid DCs, which function principally in antigen presentation, and plasmacytoid DCs (pDCs), which secrete the type I interferons (IFN), IFNα and IFNβ [1], as well as a variety of other cytokines and chemokines. Viral induction of type I IFN expression has been well studied in recent years and has been shown to be mediated by multiple pattern recognition receptors (PRRs), including retinoic acid-inducible gene (RIG)-I, melanoma differentiation-associated gene (MDA)5, toll-like receptor (TLR)3, TLR7 and TLR9. PRR expression is restricted in pDCs, with only TLR7 and TLR9 implicated in viral-induced IFNα production through the recognition of single-stranded (ss)RNA or DNA, respectively [2], [3], [4], [5], [6], [7], [8], [9]. IFNα production by pDCs is also observed following TLR7/8 stimulus with synthetic resiquimods and imiquimods, or TLR9 antagonism by CpG oligodeoxynucleotides (ODN). pDCs are not generally thought to be able to respond to double-stranded (ds)RNA, as stimulation with poly I:C or long (500 base pairs) dsRNA molecules fail to elicit IFNα or upregulate activation or maturation markers, such as CD86 and CD83 [10], [11], [12]. However, low levels of IFNα production have been reported following pDC stimulation with poly A:U [13] or in vitro transcribed viral dsRNA [11], and a recent report indicates a role for RIG-I-like helicases in recognizing replicating virus in murine pDCs lacking the IFN receptor [14]. Short interfering dsRNAs have also been demonstrated to elicit an IFNα response through TLR7, although this appears dependent on a specific sequence, and not on the dsRNA structure [12].
It is well understood that pDCs activate natural killer cells [15], macrophages [15], [16] and T and B cells [17], [18], presumably, in part, through IFNα stimulus. Recent evidence suggests that pDCs may have additional antiviral effects, including the direct inhibition of viral replication in target cells secondary to the secretion of type I IFN [19], [20], [21]. pDCs are also implicated in restricting viral replication in vivo, as there is an inverse correlation between circulating pDC numbers and human immunodeficiency virus (HIV) or hepatitis C virus (HCV) viral load [22], [23], [24], [25].
Rotavirus, a dsRNA icosahedral virus in the Reoviridae family, is the leading cause of severe dehydrating diarrhea in young children worldwide, with 500,000 to 600,000 annual deaths attributed to rotavirus infections [26], [27], [28], [29]. Significant morbidity and economic impact are the main effects in the United States, accounting for approximately 50,000 to 60,000 hospitalizations a year and loss of time from work for caregivers [30].
Rotaviruses are characterized by a triple-layered protein capsid composed of four major structural proteins. Viral protein (VP)2 comprises the innermost layer, in which the dsRNA genome is contained, while the middle layer consists of VP6. The outer layer of the virion is composed of the VP7 glycoprotein and protease-sensitive VP4 spikes [31]. Although both triple - and double-layered particles (TLPs and DLPs, respectively) are generated during rotavirus replication, only TLPs are infectious, due to the requirements of VP4 and VP7 for cell binding, and trypsin cleavage of VP4 for viral entry and infectivity [32], [33], [34], [35], [36]. Formation of noninfectious empty TLPs and DLPs, which lack the viral genome, is also observed during infection. The nonstructural proteins (NSP1 through 5) are involved in viral replication, morphogenesis and assembly, but they are not expressed by nonreplicating virus and are not part of the infectious virion [31].
Rotavirus-induced immune responses, especially the T and B cell responses, have been extensively characterized; however, little is known about innate immune mechanisms required to control rotavirus infection. Rotavirus structural and nonstructural proteins have been detected in primary DCs from murine mesenteric lymph nodes and spleens [37], [38], and murine bone marrow-derived DCs have been shown to secrete type I IFN and tumor necrosis factor (TNF)α following rhesus (RRV) or bovine rotavirus (RF-81) challenge, respectively [39], [40]. Mature human monocyte-derived myeloid DCs (moDCs) have been demonstrated to be more susceptible to rotavirus infection than immature moDCs, although infection did not result in substantial cell death [41]. Additionally, infection did not induce moDC maturation, but instead promoted priming of Th1 cells [41]. A recent study of total human peripheral blood mononuclear cells (PBMCs) exposed to RRV or human rotavirus indicated that both myeloid DCs and pDCs are susceptible to infection, and that infection results in the secretion of IFNα, presumably from pDCs [42]. While increased levels of IFNα have also been correlated with a positive clinical outcome in infected children [43], [44], several rotaviruses, including RRV, have recently been demonstrated to antagonize the production of type I IFN through the degradation of interferon regulatory factors (IRF)3, IRF5, and IRF7, and the inhibition of NFκB activation, by a viral nonstructural protein, NSP1 [45], [46], [47], [48].
Here we report the effects of rotavirus infection on highly purified primary human pDCs. We demonstrate that although rotavirus initiates detectable transcription and translation in only a small percentage of pDCs, a significant percentage of pDCs activate and mature following exposure to virus. Importantly, we demonstrate that stimulation of pDCs with live or inactivated RRV effects secretion of IFNα and multiple proinflammatory cytokines and chemokines. This response is dependent on the presence of the viral dsRNA genome. As IFNα production by pDCs is classically triggered in response to ssRNA or DNA, the induction of this response by a replication-incompetent dsRNA virus indicates a potentially novel mechanism of viral sensing by pDCs.
Results
A subpopulation of human primary peripheral pDCs is permissive to RRV replication
To characterize the interaction between pDCs (phenotypically defined as viable lineage-HLA-DR+CD11c-CD123+ cells, Figure S1) and rotavirus, pDCs were purified from human blood by negative selection (mean purity ± standard error mean [SEM]: 86.48%±0.92) and exposed to RRV at a multiplicity of infection (moi) of 5 or 10, or to equivalent quantities of inactivated RRV (iRRV). Expression of NSP2, a viral nonstructural protein expressed only in cells in which rotavirus replicates, was detected by intracellular flow cytometry at 6 and 12 hours post infection (hpi). As illustrated in Figure 1, NSP2 was not detected in pDCs receiving MA104 supernatant (mock-treated; Figure 1A) or iRRV (data not shown), but was expressed in a small but significant percentage (mean ± SEM, moi 5 : 1.18%±0.23 6hpi, 1.43%±0.28 12hpi; moi 10 : 1.45%±0.25 6hpi, 3.02%±0.99 12hpi) of pDCs inoculated with live RRV (Figure 1B and 1D). Additionally, NSP2 was detected only after pDCs were permeabilized (data not shown). Two populations of NSP2+ cells, NSP2dim and NSP2bright, were frequently apparent in pDCs exposed to live RRV (Figure 1B and 1C); approximately 20% of donors had only a single NSP2dim population. While the total percent of pDCs expressing NSP2 following RRV challenge increased from 6 to 12hpi, this increase was not significant (moi 5: p = 0.77; moi 10: p = 0.30; Mann-Whitney) (Figure 1D). The percent of NSP2+ pDCs also increased with moi (6hpi: p = 0.26; 12hpi: p = 0.063; Mann-Whitney), but remained a minor proportion of the total pDC population (median: 2.01%, moi 10, 12hpi) (Figure 1D). Further increasing the moi to 25 or 100 modestly increased the NSP2+ population, but it remained relatively small (mean: 4.99%, median: 2.07%, moi 100, n = 4, data not shown). pDCs from multiple donors (n = 5, mean purity: 91.51%) were titered after overnight culture to determine whether rotavirus infection was productive. A significant increase in viral titer (approximately 20-fold, p≤0.03; Wilcoxon signed rank test) (data not shown) was observed in two of these donors, while the other three showed no significant change over time (p≥0.17; Wilcoxon signed rank test) (data not shown). While pDC viability was significantly decreased from 6 to 12hpi in cultures receiving mock, RRV (moi 5) or iRRV stimulus (p≤0.004; Mann-Whitney), viral exposure did not result in significant cell death compared to mock stimulus at a given time point (p>0.063; Wilcoxon signed rank test) (Figure 1E). The low frequency of NSP2+ pDCs shows that the majority of human pDCs are resistant to RRV replication, as defined by evidence of expression of a non-structural rotavirus protein.
Fig. 1. Infection of human peripheral pDCs with rhesus rotavirus. 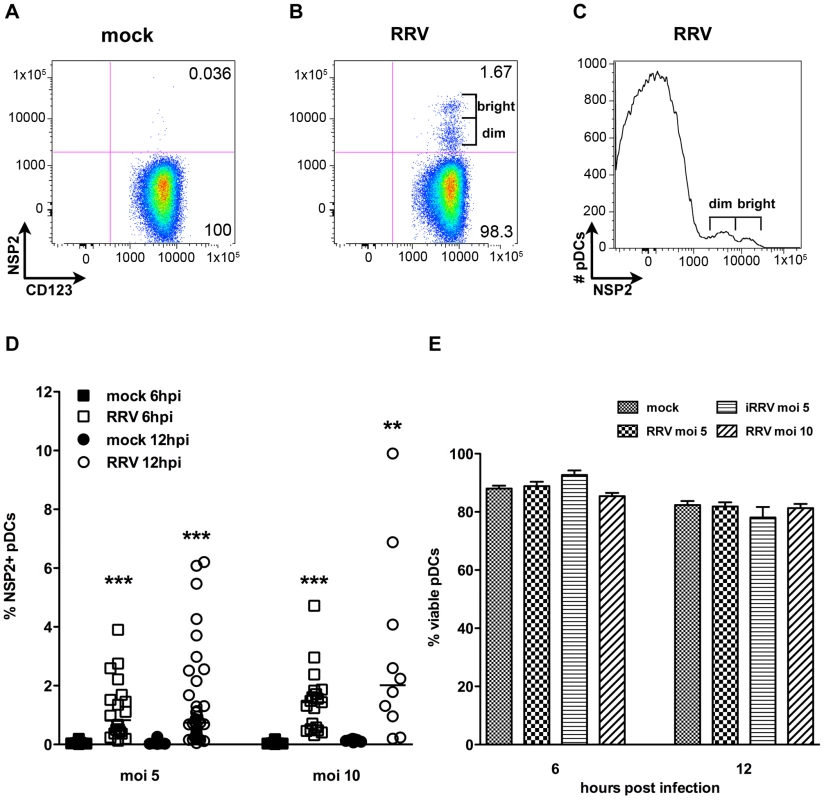
Replication of rhesus rotavirus (RRV) in pDCs was assessed by intracellular staining for NSP2. (A, B) Representative FACS plots of purified pDCs exposed to MA104 supernatant alone (A) or live RRV (moi 10, B) 6 hpi. (C) Representative histogram demonstrating NSP2dim and NSP2bright populations. (D) Percent NSP2+ pDCs from multiple healthy donors (n = 9–37) exposed to mock or RRV (moi 5 or 10) stimulus at 6 or 12 hpi. **: p = 0.0039; ***: p≤0.0002 by Wilcoxon signed rank test. (E) Percent viable pDCs 6 or 12 h following mock or rotavirus stimulus. p≥0.125 vs. mock; Wilcoxon signed rank test. pDCs activate and mature following RRV stimulation
To establish whether pDCs activate or mature following inoculation with RRV, the frequency of pDCs expressing markers for activation (CD86) or maturation (CD83) was determined by flow cytometry. pDC activation (Figure 2A and 2C) and maturation (Figure 2B and 2D) occurred at similar levels 12h after stimulation with either live or inactivated rotavirus (p≥0.125; Wilcoxon signed rank test), and were significantly increased compared to mock-stimulated cells (p≤0.03; Wilcoxon signed rank test). Stimulation with CpG ODN 2395 resulted in activation of a significantly greater percentage of the pDC population than that observed in pDCs exposed to RRV (p = 0.03; Wilcoxon signed rank test). The percent of pDCs matured by CpG ODN 2395 stimulus was also increased, but not significantly compared to those exposed to RRV (p = 0.094; Wilcoxon signed rank test). Viral replication did not inhibit maturation or activation, as pDCs positive for NSP2 expressed CD86 and CD83 at a significantly higher frequency (p≤0.008; Wilcoxon signed rank test) than bystander pDCs (Figure 2E-H). Expression of both molecules was significantly increased in both populations compared to pDCs receiving mock stimulus. Together, these data demonstrate that pDCs retain the ability to activate and mature after viral exposure, likely due to secondary effects, as the presence of replicative rotavirus is dispensable for induction of these phenotypes.
Fig. 2. Peripheral pDC activation and maturation following exposure to live or inactivated RRV. 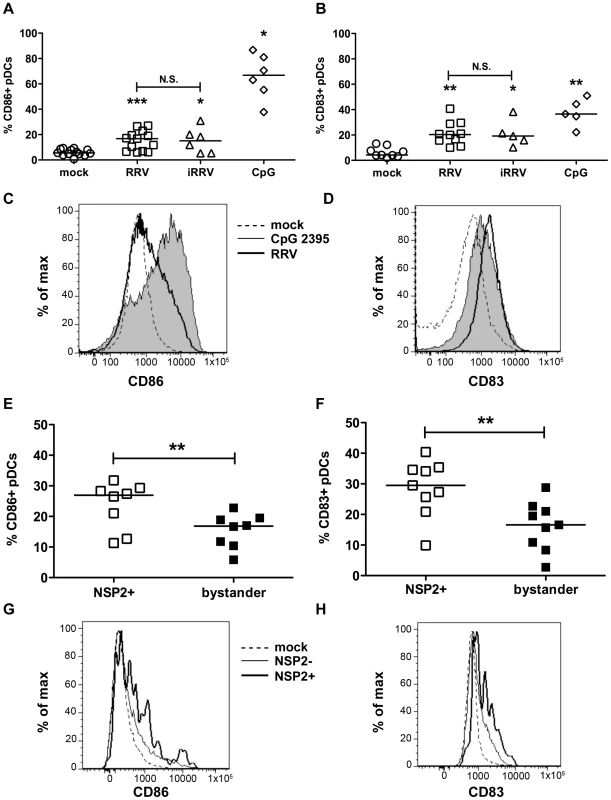
The ability of live or inactivated RRV (moi 5), or CpG 2395 (4 µg/ml), to stimulate pDCs was assessed by flow cytometry for expression of activation (A, C, CD86) or maturation (B, D, CD83) markers 12h following stimulus. (A, B) Summary of multiple donors (n = 5–13). *: p = 0.0313; ***: p≤0.0005; Wilcoxon signed rank test. (C, D) Representative histograms showing pDC activation (C) or maturation (D) following exposure to MA104 supernatant alone (dotted line), CpG 2395 (4 µg/ml; shaded) or RRV (moi 5; bold line). (E-H) To determine whether viral replication affected the pDC response, cells exposed to live RRV were gated based on NSP2 expression. The expression of CD86 (E, G) and CD83 (F, H) by NSP2+ and bystander (NSP2-) pDCs was subsequently assessed. (E, F) Summary of percent CD86+ (E) or CD83+ (F) pDCs in NSP2+ or bystander pDC populations from multiple donors 12hpi (n = 8–9); p≤0.0078; Wilcoxon signed rank test. (G, H) Representative histograms showing pDC activation (G) or maturation (H) of NSP2+ (bold) or NSP2- bystander (thin line) pDCs in cultures exposed to RRV (moi 5) or mock stimulus (dotted line). Cytokine production by human primary pDCs in response to rotavirus is independent of viral replication
Production of IFNα is an important component of the pDC-derived antiviral response. To assess this component of the pDC anti-rotaviral response, the frequency of cells expressing IFNα was determined by flow cytometry. Intracellular IFNα was detected within 4hpi of RRV stimulus, peaked at 6hpi and was sustained until at least 12hpi (Figure 3A and 3B). IFNα was detected intracellularly at similar frequencies in pDCs exposed to iRRV (which was both infectivity and transcriptionally negative) (p = 0.815; Mann-Whitney) (Figure 3A and data not shown), suggesting that replication or transcription of rotavirus is largely dispensable for IFNα induction in human pDCs. Intracellular IFNα was abrogated by the addition of RRV neutralizing VP7 or VP4 monoclonal antibodies (either mAb 159 or 2G4), indicating that the observed induction was virus-specific and likely dependent on an event occurring after virus binding and entry (Figure 3C) [49]. To identify and quantify IFNα secretion, and to detect other cytokines and chemokines secreted in response to RRV exposure, Luminex assays or ELISAs were performed on supernatants collected from inoculated pDCs at various times post infection. Only supernatants from pDC cultures ≥85% pure (mean: 90.84%±0.75; n = 3–18) were analyzed to minimize the contribution of contaminating cells to the cytokine milieu. A significant IFNα response, approaching 10,000 pg IFNα/ml, was detectable 6h following exposure to live or inactivated RRV (Figure 4A). In addition, significant quantities of IFNβ, TNFα, interleukin (IL)6, IL8, CXCL10 (IP10), CCL3 (MIP1α), CCL4 (MIP1β), CCL5 (RANTES) and TNFβ were detected at various times post infection (Figure 4B-I and data not shown). Together, these data suggest that live or inactivated RRV rapidly induce production of significant levels of IFNα and other proinflammatory cytokines and chemokines by pDCs.
Fig. 3. Intracellular production of IFNα by pDCs after exposure to live or inactivated RRV. 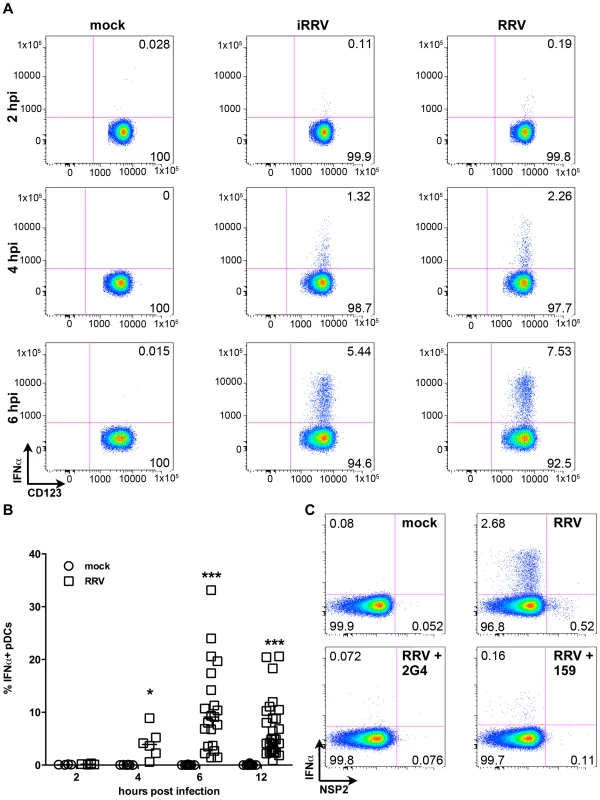
(A) Representative FACS plots of IFNα vs. CD123 expression by pDCs exposed to mock, iRRV or RRV stimulus 2-6hpi (moi 5). (B) Time course of IFNα induction in pDCs after mock or RRV (moi 5) stimulus (n = 3–30). *: p = 0.0313; ***: p≤0.0002; Wilcoxon signed rank test. (C) IFNα induction and NSP2 production in pDCs exposed to RRV in the presence or absence of neutralizing VP4 (2G4) or VP7 (159) mAb. Fig. 4. Cytokine secretion by pDCs following exposure to live or inactivated RRV. 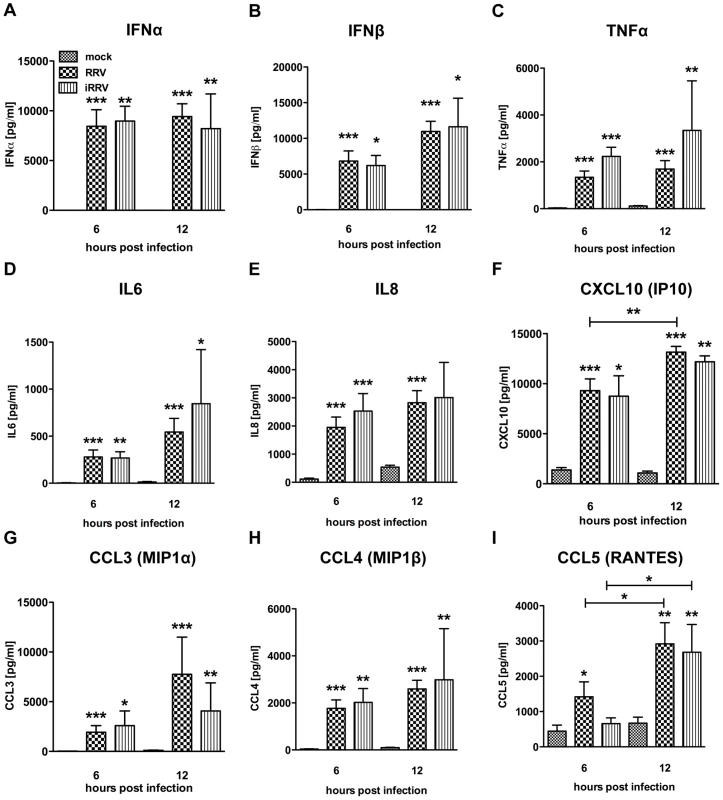
Detection of secreted IFNα (A), IFNβ (B), TNFα (C), IL6 (D), IL8 (E), CXCL10 (IP10) (F), CCL3 (MIP1α) (G), CCL4 (MIP1β) (H), and CCL5 (RANTES) (I) in culture supernatants (n = 3–18) from pDCs (1 million per mL) exposed to mock, live or inactivated RRV (moi 5) stimulus at various times post infection by Luminex or ELISA. TNFβ (LTA): ≤100 pg/mL detected post viral stimulus. sCD40L: no observed increase versus mock stimulus. Not detected: IFNγ, IL10, IL12p40, IL12p70, IL1β, IL1RA, IL2 and IL4. *: p≤0.0265; **: p≤0.0091; ***: p≤0.0008; Mann-Whitney. IFNα production is impaired in pDCs undergoing RRV replication
In contrast to what was observed with pDC activation and maturation, IFNα production was significantly reduced in NSP2+ pDCs (Figure 5). NSP2- bystander pDCs produced IFNα at a significantly increased frequency (p<0.0001; Wilcoxon signed rank test) compared to pDCs dim or bright for NSP2 (Figure 5A and 5B). The percent of IFNα+ pDCs was significantly increased in the NSP2dim population compared to that in the NSP2bright (p = 0.0006; Wilcoxon signed rank test), supporting the hypothesis that increased levels of RRV infection are inversely correlated with IFNα production. More significant was the sustained production of IFNα in uninfected (NSP2-) pDCs following RRV stimulus. A similar correlation between NSP2 expression and IFNβ production was also observed (Figure 5C). These data indicate that infection in a minor subset of pDCs results in the local suppression of the IFNα response in these specific cells.
Fig. 5. The effect of viral replication on IFNα induction and IRF7 phosphorylation. 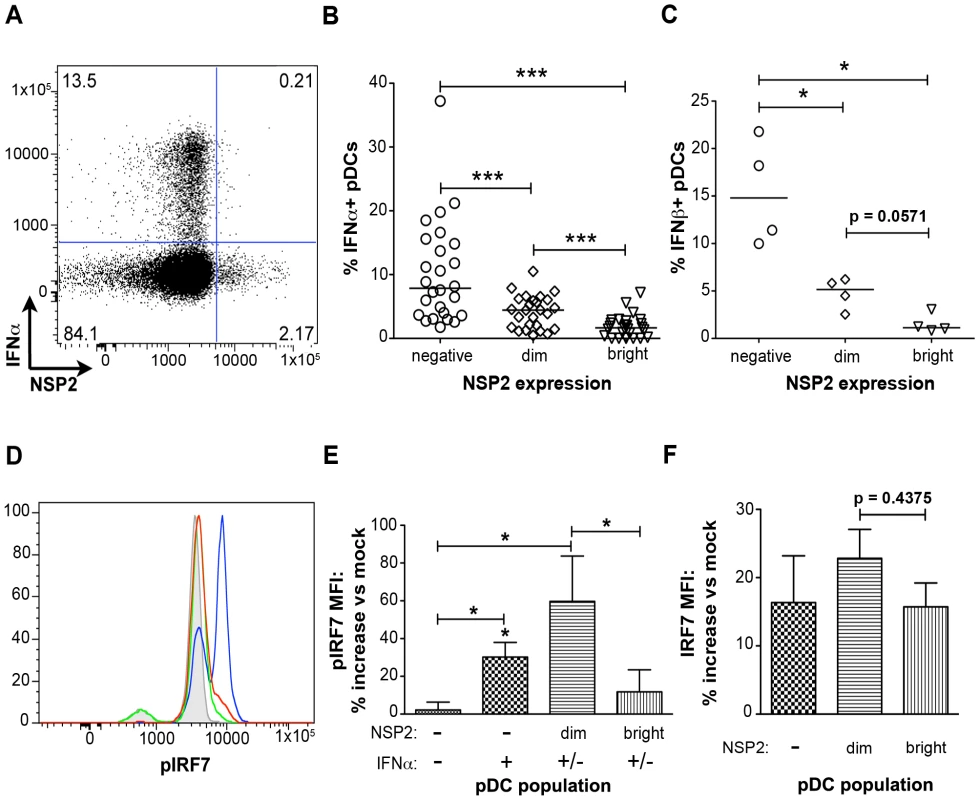
(A) Representative FACS plot of IFNα vs. NSP2 expression in pDCs stimulated with RRV 6hpi (moi 10). (B, C) pDCs from multiple donors were exposed to RRV and the percent of IFNα+ (B) (n = 26) or IFNβ+ (C) (n = 4) pDCs negative, dim or bright for NSP2 were determined. *:p = 0.0286, ***: p≤0.0006; Wilcoxon signed rank test. Donors with fewer than 0.5% of pDCs staining positive for NSP2 were excluded from this analysis. (D) Representative histograms of IRF7 phosphorylation in NSP2bright (red), NSP2dim (blue), and NSP2- (green) pDCs 6 hours post RRV or mock (gray) stimulus. (E, F) Percent increase in pIRF7 (E) or total IRF7 (F) MFI by indicated pDC populations relative to mock treated pDCs from multiple donors (n = 6), 6 hours post RRV inoculation. *: p = 0.0313; Wilcoxon signed rank test. IRF7 is phosphorylated in pDCs supporting low levels of viral replication
To further investigate the impairment of the IFNα response in pDCs undergoing rotavirus replication, phosphorylation of IRF7, the key transcription factor for the IFNα response in pDCs [50], was assessed by phosflow (Figure 5D and 5E). In accordance with the decreased production of IFNα by NSP2bright cells compared to that of NSP2dim pDCs, as well as previous findings of IRF7 degradation in rotavirus-infected cells [45], [46], phosphorylated IRF7 (pIRF7) was substantially decreased in pDCs bright for NSP2 (p = 0.03 versus NSP2dim pDCs; Wilcoxon signed rank test) (Figure 5D and 5E), and is similar to that of IFNα-NSP2- pDCs (p = 0.56; Wilcoxon signed rank test) (Figure 5E). To determine whether this decrease in pIRF7 was due to decreased levels of total IRF7 in pDCs, the MFI of total IRF7 was assessed (Figure 5F). While the total IRF7 MFI was slightly depressed in NSP2bright compared to NSP2dim pDCs, this decrease was not significant (p = 0.44; Wilcoxon signed rank test) and thus does not account for the observed differences in IRF7 phosphorylation. The increased levels of pIRF7 in NSP2dim pDCs may represent continual, direct stimulus of the IRF7 pathway in virus-positive pDCs, as well as a mechanism by which the IFNα response is directly initiated. Alternately, pIRF7 may be sequestered in a nontraditional cellular compartment prior to degradation, leading to increased protein accumulation. Interestingly, levels of IRF7 phosphorylation in IFNα+NSP2- pDCs were intermediate to that of NSP2dim (p = 0.31; Wilcoxon signed rank test) and NSP2bright or NSP2-IFNα- pDCs (p = 0.0313; Wilcoxon signed rank test) (Figure 5E). The slight depression of pIRF7 in the IFNα+ population compared to that observed in NSP2dim pDCs may be attributable to the enhanced IFNα production in this subpopulation. As pIRF7 is degraded following the initiation of IFNα transcription, cells in which IFNα has been transcribed would be expected to have lower levels of pIRF7 than those negative for IFNα. Notably, inhibition of IFNα secretion, achieved by simultaneous addition of brefeldin A with viral inoculation, decreased the percent of IFNα+ pDCs by an average of 63.5% (n = 6, data not shown). This supports the notion that recognition of secreted IFNα is an essential component of the IFNα response following rotavirus exposure, as has been reported with Newcastle disease virus [14]. Importantly, this is the first demonstration of impaired IRF phosphorylation in primary human cells infected with rotavirus.
Trypsin cleavage of VP4 is dispensable for pDC infection and IFNα induction
Trypsin-mediated cleavage of VP4 is canonically required for productive entry of rotavirus into permissive cells [33], [34], [35], [36]. However, it has previously been reported that murine bone marrow-derived DCs activate following exposure to uncleaved bovine rotavirus [40], indicating that VP4 cleavage may be dispensable for the induction of the DC response. To investigate the role of trypsin-mediated cleavage of VP4 in both rotavirus infection of, and IFNα production by, human pDCs, we exposed pDCs to RRV grown in the absence of trypsin (non-trypsinized; NT-RRV). Although subsequent exposure to trypsin (thus cleaving VP4) increased infectivity of the uncleaved NT-RRV preparation 63-fold by plaque titration and intracellular NSP2 staining by 20-fold in MA104s (data not shown), examination of NSP2 staining showed that similar proportions of pDCs were infected with either the cleaved or uncleaved NT-RRV preparations (p = 0.625; Wilcoxon signed rank test) (Figure 6A-C). Likewise, uncleaved NT-RRV induced IFNα production by a similar (p = 0.375; Wilcoxon signed rank test) percentage of pDCs, compared to cleaved NT-RRV (Figure 6A, 6B and 6D). Thus, cleavage of VP4 by trypsin is dispensable for both pDC infection and IFNα induction by RRV.
Fig. 6. The effect of trypsin activation of rotavirus on pDC infection and IFNα induction. 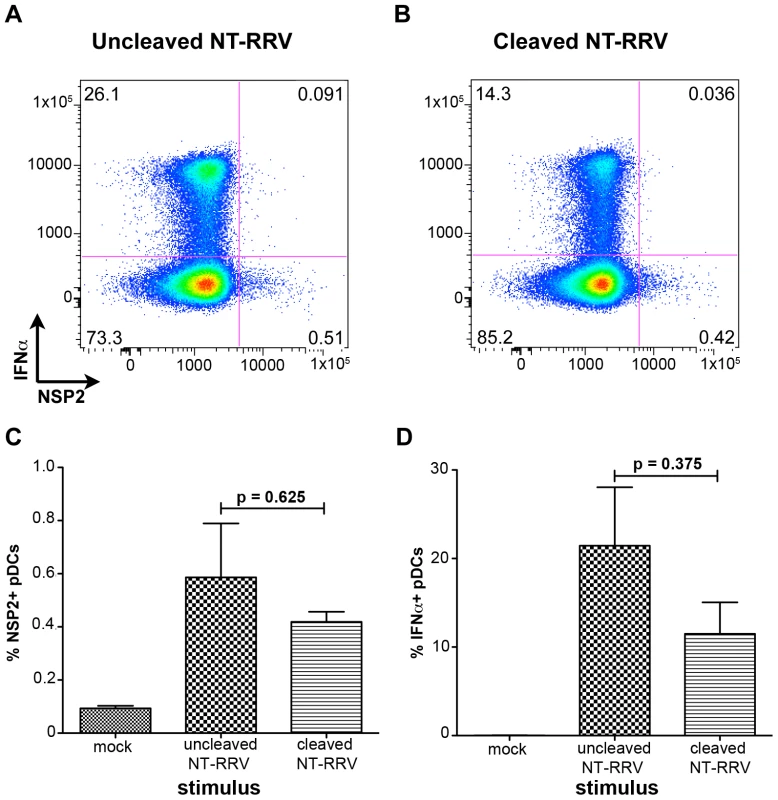
(A, B) Representative FACS plots of IFNα and NSP2 expression by pDCs exposed to equal quantities of non-trypsinized RRV (NT-RRV) with uncleaved (A, 3.77×105 focus forming units/ml; moi = 0.079) or cleaved (B, 2.38×107 focus forming units/ml; moi = 5) VP4. (C, D) Summary of percent NSP2+ and IFNα+ pDCs from multiple donors (n = 3) following exposure to cleaved or uncleaved RRV. p≥0.375; Wilcoxon signed rank test. IFNα induction requires VP4 and/or VP7, and dsRNA
Although the ability of pDCs to mount an IFNα response to viral infection is well described, the only documented viral TLR ligands for pDCs are ssRNA and DNA [2], [3], [7], [8], [9]. As rotavirus is a dsRNA virus and since psoralen-treated, transcriptionally-inactive rotavirus efficiently induced IFNα (Figures 3 and 4A), we investigated the viral requirements for IFNα induction. pDCs stimulated with equal quantities (see Methods describing particle quantification) of purified TLPs, DLPs, empty particles, or genome-free recombinant virus-like particles (VLPs) were stained for intracellular IFNα. As expected, density gradient purified RRV TLPs stimulated similar levels (p = 0.695; Wilcoxon signed rank test; n = 10) of IFNα production compared to pDCs treated with unpurified live or inactivated RRV expressing equivalent amounts of VP4, as measured by hemagglutination titer (Figure 7). pDCs exposed to 2/6 genome-containing RRV DLPs expressed neither IFNα nor NSP2, indicating a likely dependence on VP4 or VP7 for viral entry, as previously reported [32]. Alternately, one or both of these viral proteins could be serving as the pathogen-associated molecular pattern (PAMP) responsible for inducing the pDC IFNα response. To address this, pDCs were stimulated with 2/4/6/7 VLPs or 2/6/7 green fluorescent protein (GFP)-VLPs. Both 2/6/7 GFP-VLPs and 2/4/6/7 VLPs were capable of entering pDCs, but failed to induce IFNα (Figure 7 and data not shown). 2/6 VLPs were comparatively defective in the ability to enter pDCs (data not shown), thus supporting the hypothesis that DLPs fail to induce IFNα due to inefficient viral entry. The ability of infectious SA11, the parental virus from which the 2/4/6/7 VLPs were derived, to induce IFNα production (Figure 7) suggests that the lack of IFNα following inoculation with 2/4/6/7 VLPs was due to the absence of a viral genome, and not to strain variation in surface proteins. Consistent with this hypothesis, IFNα was detected in very few pDCs exposed to empty TLPs, as compared to pDCs exposed to equal quantities of genome-containing TLPs. Instead, IFNα production by pDCs exposed to empty particles was consistent with that observed in pDCs inoculated with an equal infectious dose of TLPs, suggesting that residual contaminating viral genome was responsible for IFNα production by the empty TLPs. Together, these data indicate that viral entry, mediated by VP4 and/or VP7, and the viral dsRNA genome are likely required for rotavirus-induced IFNα production by pDCs.
Fig. 7. Viral determinants of IFNα induction. 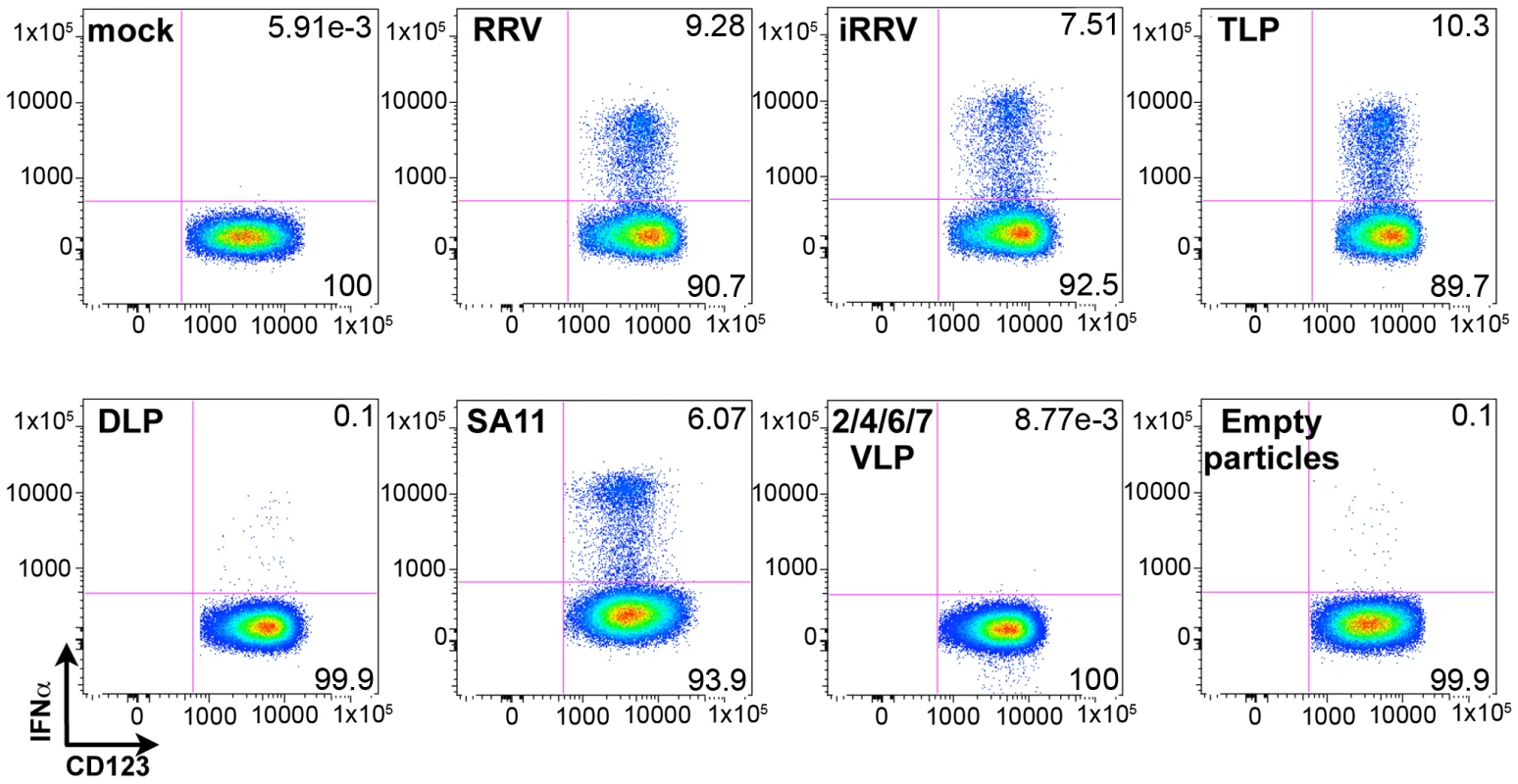
Representative FACS plots of IFNα production by pDCs at 12 hours post exposure to rotavirus-derived stimuli. RRV: live virus; iRRV: inactivated virus; TLP: purified triple-layered (2/4/6/7) genome-containing virus particles; DLP: purified double-layered (2/6) genome-containing virus particles; SA11: live virus strain from which VLPs are derived; 2/4/6/7 VLPs: recombinant genome-free virus-like particles; empty particles: purified genome-free TLPs. Endosomal acidification is required for initiation of the pDC IFNα response to rotavirus
To begin to characterize the mechanism by which pDC recognition and subsequent induction of the IFNα response occurs following rotavirus exposure, pDCs were treated with concanamycin A, a potent inhibitor of endosomal acidification, and thus TLR7/9 function [9], [51], [52], prior to inoculation with either live or inactivated RRV at an moi of 10 (Figure 8A–8C). Subsequent examination of intracellular IFNα production revealed significant inhibition in concanamycin-treated pDCs compared to untreated cells (mean ± SEM, RRV: 83.3%±4.6; iRRV: 83.2%±4.5; p = 0.01; Mann-Whitney). To determine whether the observed lack of IFNα production was due to a defect in viral entry, concanamycin-treated pDCs were examined for NSP2 expression. Interestingly, NSP2 expression was significantly increased in concanamycin-treated pDCs compared to untreated cells (Figure 8D and 8E) (mean fold increase: 5.9; p = 0.03; Wilcoxon signed rank test; n = 6). These data indicate that concanamycin-induced inhibition of endosomal acidification does not inhibit viral infection of pDCs and hence, presumably, does not restrict viral entry. These data support the conclusion that concanamycin A treatment prevents the pDC IFNα response at the level of ligand recognition, implicating TLR7 or TLR9. Additionally, the data suggest that the observed low levels of viral replication in pDCs (Figure 1D) are due, at least in part, to suppression by the pDC IFN response, as NSP2 expression significantly increases following IFNα inhibition (Figure 8C and 8D). Notably, these experiments demonstrate that endosomal acidification is required for the pDC IFNα response to rotavirus, and provide indirect evidence for the involvement of an endosomal receptor, such as TLR7 or TLR9, in the induction of this response.
Fig. 8. The effects of concanamycin A on IFNα induction and NSP2 expression. 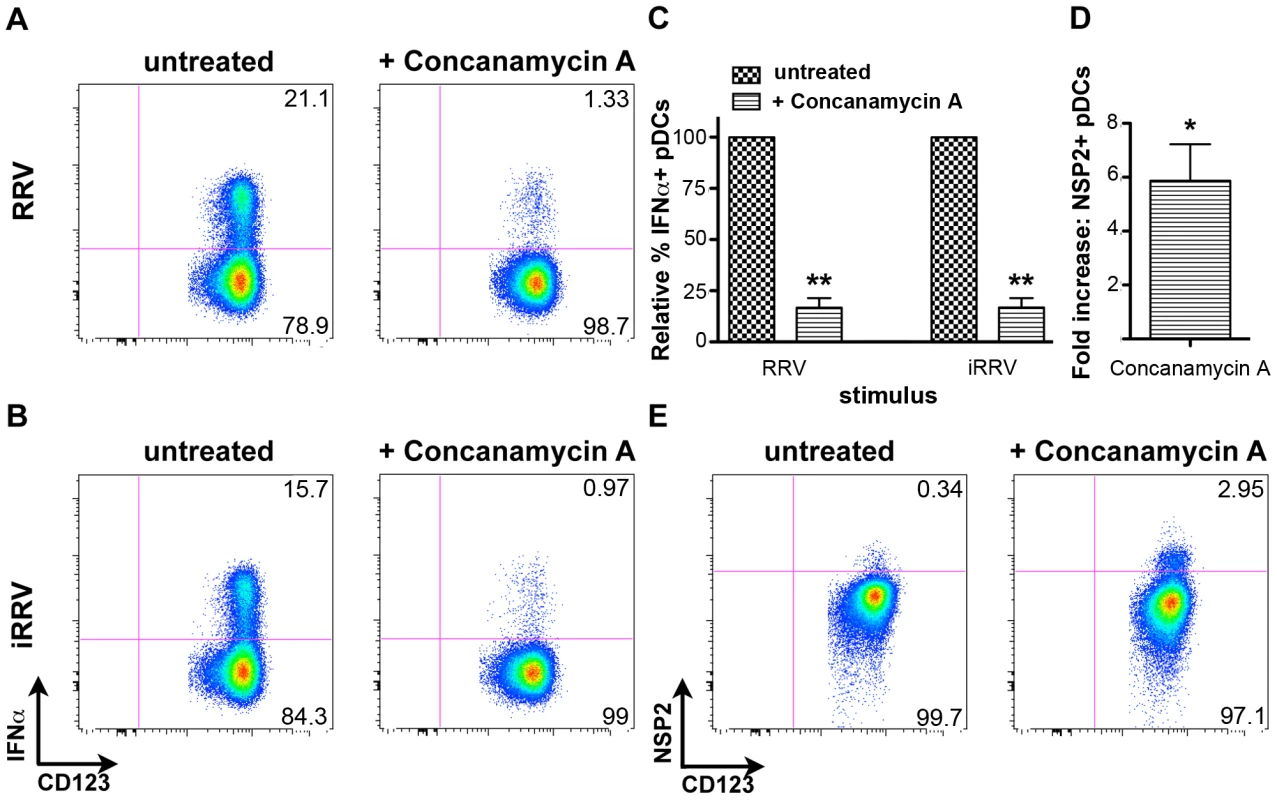
Intracellular IFNα was assessed in untreated or concanamycin A-treated pDCs following exposure to RRV (A) or iRRV (B). (C) Relative frequency of IFNα+ pDCs in untreated or concanamycin A-treated pDCs from multiple donors (n = 6) following exposure to live or inactivated rotavirus. **: p = 0.0097; Mann-Whitney. (D) Fold increase in NSP2 expression by pDCs from multiple donors (n = 6) treated with concanamycin A prior to infection, compared to expression by untreated pDCs. *: p = 0.0313; Wilcoxon signed rank test. (E) Representative FACS plots depicting NSP2 staining in untreated and concanamycin A-treated pDCs following RRV exposure. Discussion
pDCs play a vital role in the generation of innate and adaptive immunity following viral infection, primarily through the production of large quantities of IFNα in response to stimulus of TLR7 or TLR9 PRRs by ssRNA or DNA PAMPs, respectively. Traditional receptors for dsRNA, such as RIG-I, MDA5 and TLR3, are largely thought to not play a role in the initiation of the IFN response in human pDCs [2], [3], [4], [5], [6], [7], [8], [9]. Although Newcastle disease virus normally elicits an IFNα response in murine pDCs through triggering TLR7, RIG-I-like helicases have been recently implicated in IFNα induction by replicating virus in murine pDCs lacking the IFN receptor [14]. Hence, the mechanisms governing efficient IFNα induction in these cells by transcriptionally inactivated, replication-incompetent dsRNA rotavirus are not yet understood. As rotaviruses represent a large burden of human disease, it will be important to better understand the immune mechanisms underlying their recognition and subsequent generation of antiviral immunity.
In the present study, we demonstrate that primary human pDCs are largely resistant to rotavirus replication, as intracellular staining for NSP2 was observed in only a small percentage (≤5%) of cells following RRV exposure, even at a non-physiologically relevant moi of 100. While productive infection was observed in a subset of donors, this was significantly less than that observed in cells highly permissive for rotavirus infection [53]. As rotavirus is generally a lytic virus [31], [54], [55], the observed lack of significant death in pDC preparations exposed to live RRV, compared to those receiving mock stimulus or inactivated virus, further indicates that few pDCs are permissive to viral replication. These findings are in agreement with previous reports of low frequencies of pDC infection in virus models such as herpes simplex virus 2 [56], Dengue [57], human cytomegalovirus [17], influenza [58], HCV [24] and HIV [59]. The inability of the primary human peripheral pDC population to be uniformly infected by rotaviruses may be attributable to multiple factors, including age of the individual pDC, stage of the cell cycle, or cell-surface marker expression. Additionally, functionally-distinct pDC subsets have been recently identified on the basis of CD2 expression [60]; studies are currently underway to elucidate the role of these subsets in the pDC response to rotavirus.
Consistent with the general resistance to rotavirus infection, pDCs retained several important functional abilities following RRV exposure, as evidenced by their activation, maturation and cytokine production. Evidence of this functionality was observed primarily in the major NSP2- bystander population, although activation and maturation were significantly enhanced in the few NSP2+ pDCs, as well. The induction of this phenotype by inactivated RRV further indicates that the pDCs are responding to the presence of input virus or to secreted factors, and not to viral replication. The upregulation of DC costimulatory and maturation markers suggests antigen presentation to T cells is preserved, in line with previous reports that pDCs are necessary for stimulation of IFNγ-secreting memory T cells [42]. The antigen presentation hypothesis is supported by increased expression of CD86 and CD83 on NSP2+ pDCs compared to bystander pDCs, indicating that the presence of virus enhances this phenotype. Conversely, IFNα/β production is impaired in NSP2+ pDCs. Combined with decreased IRF7 phosphorylation in NSP2bright pDCs, this suggests the direct inhibition of the type I IFN response by rotavirus replication, as has been previously observed in fibroblasts and epithelial cells [45], [46], [47], [48].
This study is the first to demonstrate that rotavirus, a segmented dsRNA virus, directly induces IFNα and IFNβ production by primary human pDCs. While it has previously been reported that exposure to rotavirus induces IFNα secretion by murine FLT3 ligand-driven pDCs [39] and total human PBMCs [42], and that this response required pDCs [42], it was unclear until now whether pDCs produced IFNα directly in response to virus, or if pDCs amplified IFNα production in response to type I IFN or some other signal from a non-pDC. In a limited number of experiments, Mesa et al [42] observed not only pDCs singly positive for IFNα or rotavirus, but also cells double-positive for rotavirus and IFNα at a greater frequency than that reported here. It is important to note that Mesa et al used NSP4 as a marker for rotavirus infection. Extracellular NSP4 has been identified on uninfected cells [61]. Conversely, NSP2, employed in this report, to date has only been detected intracellularly. As such, the percentage of cells identified as positive for rotavirus on the basis of NSP4 staining could be inflated versus those expressing NSP2, as NSP4 staining may include pDCs not truly supporting viral replication; in turn, the percent of NSP4+IFNα+ pDCs would be artificially increased. This, combined with differences in sample size, may explain the discrepancies in the incidence of NSP4+IFNα+ pDCs (n = 3) observed by Mesa et al and the frequency of NSP2+IFNα+ pDCs (n = 27) reported here.
pDCs possess a seemingly unique phenotype in the context of rotavirus infection, as the ability of NSP1 to degrade multiple IRFs appears to subvert the type I IFN response in many other cells, including epithelial and fibroblastic cells [45], [46], [62]. Presumably, the constitutive expression of IRF7 in pDCs [63] allows for rapid induction of IFNα following rotavirus exposure, thus effectively suppressing viral replication—and the production of NSPs—in the majority of pDCs through the establishment of a largely paracrine-induced antiviral state. This conclusion is supported by the observed lack of significant increase in the NSP2+ or IFNα+ pDC populations when the moi is increased to 25 or 100 (data not shown). Importantly, the decrease in IFNα and pIRF7 in pDCs expressing high levels of NSP2 illustrates that the local suppression of the interferon response by actively replicating virus [45], [46], [47], [48] is conserved in human pDCs at an individual cell level. However, inhibition in this minor population is unlikely to substantially modulate the amount of cytokine produced by the total pDC population. The present study provides the first formal demonstration of type I IFN inhibition in primary human cells in which rotavirus replicates.
The uniqueness of pDCs in the context of rotavirus infection is further demonstrated by the ability of trypsinized and non-trypsinized rotavirus to induce IFNα and NSP2 expression at similar frequencies. The long-standing understanding of the requirement for VP4 proteolytic cleavage by luminal trypsin to activate viral entry and infection in the gut led to the assumption that efficient rotavirus infection could not occur systemically due to absence of appropriate extracellular proteases. Rotavirus infection was thought to be largely constrained to the intestine because this was the only anatomical location with substantial amounts of active extracellular trypsin available. The apparent dispensability of this proteolytic requirement for pDC infection may represent an alternate mechanism of rotavirus entry. This “non-trypsin dependent” mechanism of infectious entry might also account for the low levels of systemic organ infection and spread that normally occurs during rotavirus infection, and the elevated levels seen during rotavirus infection of highly immunocompromised people or animals, where the systemic availability of trypsin to proteolytically activate rotavirus is unlikely. Additionally, this provides a mechanism for the establishment of an antiviral state systemically, where circulating virus would not be expected to be able to infect cells in the traditional, trypsin cleavage-dependent, manner.
We have demonstrated that both live and inactivated rhesus rotavirus efficiently stimulate secretion of type I IFN, in addition to many other cytokines, suggesting that transcription of viral nucleic acid is not required for either viral recognition or subsequent cytokine production by primary human pDCs. Conversely, viral replication is required for activation of the IFN response in fibroblasts [62]. Indeed, further analysis of the viral requirements for IFNα induction suggests that pDCs recognize and respond to input rotavirus dsRNA or some degradation product of the rotavirus genome. This is supported by the inability of genome-free TLPs to effect IFNα secretion, indicating that the lack of IFNα induction following VLP-stimulus is due to the absence of a nucleic acid ligand and not to the lack of a minor structural protein, such as VP1 or VP3. To our knowledge, this is the first direct demonstration of the requirement for native viral dsRNA in the initiation of an IFNα response from primary human pDCs.
Importantly, this finding may represent an alternative mechanism of IFNα induction in primary human pDCs. The ability of a pDC that takes up rotavirus to mount a vigorous type I IFN response independently of rotavirus mRNA or protein expression provides a potential mechanism by which the host could effectively circumvent the anti-IFN effects of NSP1-mediated IRF degradation [62]. At least three possible mechanisms exist for this phenotype: first, input or self nucleic acid released from necrotic or apoptotic cells may serve as PAMPs in neighboring cells, as has been previously described [64]. As RRV exposure, either to replication-competent or inactivated virus, does not induce substantial pDC death (Figure 1), and as the cytokine response occurs rapidly following infection, this scenario seems unlikely. Secondly, input viral dsRNA may be exposed in the cytosol, the traditional cellular location for rotavirus replication, through the process of viral replication and interact with a cytosolic receptor such as RIG-I or protein kinase R (PKR). Rotavirus and reovirus, both members of the Reoviridae family, have been demonstrated to induce IFNβ production in epithelial and human embryonic kidney 293T cells, respectively, via RIG-I, but not PKR or TLR3 [65], [66]. The mechanism by which input viral genomic RNA recognition might occur is unclear, however, as viral dsRNA is thought to remain encapsidated in the DLP and not be free in the cytoplasm during viral replication. Finally, rotavirus particles may be taken up by and degraded within the endosome, where dsRNA or its degradation products, including ssRNA fragments or a single strand of the dsRNA [12], would be able to interact with TLR3, TLR7 or TLR9. As a biological role for known dsRNA receptors, such as RIG-I, PKR or TLR3, has not been demonstrated in human pDCs [2], [3], [4], [5], [6], it is unlikely that these receptors are responsible for the observed IFNα induction in this system, although RIG-I like helicases have been recently implicated in the generation of a type I IFN response in pDCs from IFN receptor knockout mice [14]. Signaling by a cytosolic receptor should be abrogated if inhibition of endosomal acidification in turn prevented rotavirus entry into the cytosol. However, the persistence and even increase of NSP2 expression following treatment with concanamycin A indicates that pDC entry by rotavirus is conserved under conditions that alter endosomal pH and decrease the pDC IFN response (Figure 8). Thus, the substantial reduction of the IFNα response following concanamycin A treatment supports the notion that an endosomal receptor, such as TLR7 or TLR9, is involved in the initiation of the pDC response to rotavirus. Hence we predict that dsRNA derived from input virus is exposed following viral degradation, and not replication, as inactivated RRV, but neither genome-free TLPs nor VLPs, is sufficient for pDC activation/maturation and IFNα production. While it is possible that recognition of some combination of VP4, VP7 and dsRNA are required for IFNα induction, we instead believe that viral entry—mediated by VP7 and/or VP4—and subsequent recognition of genomic dsRNA or its degradation products are the critical components of this pathway.
The induction of both IFNα and TNFα by rotavirus may create a synergistic effect in the suppression of viral replication, as has been observed following RIG-I stimulus by myxoma virus [67]. Although the precise kinetics of induction are unclear, both TNFα and IFNα are also observed in pDC supernatants following influenza infection [24], [68], [69]. Interestingly, production of IFNα is delayed until 14–20 h following infection with a laboratory strain of H1N1 influenza, at which point ∼4 ng/mL per million pDCs is detectable; this production was dependent on viral replication [69]. Conversely, we report that pDCs exposed to either live or inactivated rotavirus secrete approximately 10 ng/mL of IFNα by 6hpi. In agreement with previous findings [14], the recognition of secreted IFNα is an important component of the amplification of the pDC IFNα response to both live and inactivated rotavirus. As such, the recognition of input viral genome and subsequent induction of an initial IFNα response prior to possible IRF7 inhibition allows for the establishment of an antiviral state by the global pDC population. While inactivated herpes simplex virus also induces IFNα, replication of vesicular stomatitis virus, Sendai virus and human respiratory syncytial virus has been demonstrated to be required for IFNα induction in pDCs [70], [71]. Together, this demonstration of rapid, potent, replication-independent induction of cytokine by a dsRNA virus, largely dependent on endosomal acidification, points to a novel mechanism leading to production of IFNα by human pDCs.
Peripheral pDCs likely represent a significant source of the systemic IFNα observed following in vivo rotavirus infection [43], [72], [73], [74]; the dispensability of proteolytic activation of VP4 for pDC infection and IFNα stimulation by rotavirus provides a mechanism for the establishment of the antiviral state extraintestinally by a small amount of virus. Increased levels of serum IFNα have been reported in human pediatric patients infected with rotavirus [43], [44]; levels peaked within two days of symptom onset and disease resolved more quickly in patients with the highest levels of IFNα [43]. Likewise, type I IFN responses were observed in newborn calves infected with bovine rotavirus: animals receiving both high and low doses of viral inoculums generated multiple waves of IFN production, with higher doses correlating with both more rapid IFN responses and a lack of clinical signs [74]. As the ability of type I IFN to activate multiple arms of the innate and adaptive immune response is well documented (reviewed in [75], [76], [77]), and recent data have shown that type I IFN plays a critical role in restricting systemic rotavirus infection [78], we predict that pDC-derived type I IFN plays a significant role in confining rotavirus replication to the intestine while initiating the systemic immune response in the periphery.
In summary, these studies show that primary human pDCs are largely resistant to rotavirus replication, but that they remain responsive and functional following viral exposure, as indicated by cellular activation, maturation and production of multiple cytokines, including TNFα and large amounts of IFNα. The pDC response is demonstrated to require both the viral dsRNA genome and acidification of the pDC endosome, implying the likely initiation of the type I IFN response by TLR7 or TLR9. To our knowledge, these data are the first to show that inactivated rotavirus can efficiently induce a type I IFN response, which differs from what has been seen in fibroblasts and epithelial cells exposed to rotavirus [62]. The restricted ability of rotavirus to replicate in pDCs, combined with the ability of the pDC to effect a type I IFN response in the absence of viral replication, likely serves the purposes of enabling the host to mount an innate antiviral response despite the efficient degradation of the IRFs by rotavirus NSP1 in many host cells. Combined with the novel dispensability of trypsin cleavage of VP4 for the initiation of the pDC response, this creates a mechanism for the establishment of an antiviral state in the systemic extraintestinal environment and likely contributes to the substantial restriction of systemic replication compared to that observed in the intestine [38]. These experiments also provide the first indication that rotavirus infection is capable of locally suppressing IFNα production in primary human cells. Importantly, these findings demonstrate that rotavirus infection of primary human pDCs provides a valuable experimental system for the study of cellular processes, receptors and signaling pathways underlying pDC-mediated antiviral immunity while revealing a potentially novel dsRNA-mediated pathway of IFNα induction.
Materials and Methods
Virus preparation and inactivation
Simian (RRV and SA11) tissue-culture adapted rotaviruses were grown in fetal monkey kidney (MA104) cells in the presence of trypsin, except where noted, as previously described [79], [80]. Where indicated, RRV empty and genome-containing TLPs were purified from MA104 cell lysates by cesium chloride density gradient centrifugation following genetron extraction and pelleting through a sucrose cushion, as previously described [81], [82]. DLPs were created by treating TLPs with 20 nM EDTA, thus removing the outer viral proteins VP4 and VP7, as previously described [32]. Purified viral preparations were dialyzed to remove residual cesium chloride prior to pDC stimulus. A portion of the RRV lysate preparation was inactivated by treatment with 40 µg/ml psoralen, followed by exposure to ultraviolet light for 40 minutes, as described [83]. The inactivation of infectivity and transcriptional activity of the RRV in the psoralen preparations was confirmed by cell culture assay and the elimination of endogenous RNA polymerase activity by in vitro assay. Except where noted, viral preparations were trypsin activated (5 µg/ml) at 37°C for 20–30 minutes prior to pDC infection. Preparations were endotoxin-free, as determined by Limulus amebocyte lysate test (Charles River, Wilmington, MA).
Virus-like particles
Rotavirus-like particles (VLPs) expressing VP2 and VP6 (2/6 VLPs), or VP2, VP6 and VP7 (2/6/7 VLPs), were generated by coinfecting Spodoptera frugiperda 9 cells with two or three recombinant baculoviruses, respectively, at a moi ≥5 plaque forming units per cell, as previously described [84]. Briefly, the baculoviruses expressed RF (bovine rotavirus) VP6, RRV VP7, or a fusion protein consisting of GFP fused to the N terminus of RF VP2 deleted in the first 92 amino acids. Infected cultures were collected 5–7 days post infection and purified by cesium chloride density gradient centrifugation. VLPs composed of RF VP2, SA11 Cl3 VP6, SA11 Cl3 VP7 and SA11 4F VP4 (2/4/6/7 VLPs) were similarly prepared, as previously described [85], [86], [87]. The protein concentration of the purified VLP preparations was estimated by the Bradford method.
Virus and VLP quantification
All viral preparations were titrated by plaque assay on MA104 cells and expressed as plaque forming units per ml, as described [80]. Hemagglutination assays were performed to determine VP4 content of purified genome-containing and empty TLPs, unpurified RRV and 2/4/6/7 VLPs, as described [88], and used to ensure that equal quantities of TLPs were added to pDC preparations. Additionally, DLPs were prepared by treating purified genome-containing TLPs with 20 nM of EDTA, thus removing the outer capsid from the virion and resulting in equal particle counts between the two preparations.
Isolation of primary human pDCs
PBMCs were isolated from leukoreduction chambers obtained from the Stanford Blood Center (Palo Alto, CA) by centrifugation over ficoll-hypaque (GE Healthcare, Uppsala, Sweden). Plasmacytoid DCs were negatively selected using the pDC Isolation Kit (Miltenyi Biotec, Auburn, CA), according to manufacturer's instructions. To increase the purity of the pDC preparations, consecutive purifications were performed using an AutoMACS (Miltenyi Biotec, Auburn, CA). An average of 2 million pDCs, defined as viable, lineage-HLA-DR+CD11c-CD123+ cells, were isolated per donor; preparations were routinely >85% pure.
pDC culture, infection, stimulation and blocking studies
Approximately 2×105 pDCs were exposed to rotavirus particles or 4 µg/mL CpG ODN 2395 (Coley Pharmaceuticals, Ottawa, Canada), as indicated, for 1 hour in serum-free RPMI-1640 (CellGro, Manassas, VA) supplemented with penicillin/streptomycin and L-glutamine (Gibco, Carlsbad CA). pDCs were washed and suspended at a concentration of 1 million pDCs/mL in RPMI-1640 with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), penicillin/streptomycin, L-glutamine and 10 ng/mL IL3 (Peprotech, Rocky Hill, NJ) until harvest. Where indicated, rotavirus infection was blocked using neutralizing monoclonal antibodies to VP5 (mAb 2G4) or VP7 (mAb 159), or as a non-neutralizing control, a VP6 monoclonal antibody (mAb 1E11). In studies determining whether pDC infection was productive, highly-purified pDCs (mean purity: 91.51%) were exposed to RRV at an moi of 10 for one hour; neutralizing mAb 159 was then added for 30 minutes. pDCs were washed 3 times, and were frozen immediately or after overnight culture. The resulting pDC pellets and supernatants were freeze/thawed three times, and virus titer determined by FFU assay, as previously described [89].
Inhibition of endosomal acidification
Purified pDCs were incubated with 20 nM concanamycin A (Sigma-Aldrich, St Louis, MO) at 37 degrees for one hour prior to infection with live or inactivated RRV (moi 10).
Flow cytometry (FACS)
Supernatants were harvested by centrifugation of cultured pDCs, which were then washed once with PBS (CellGro, Manassas, VA). The LIVE/DEAD Aqua Dead Cell Stain Kit (Invitrogen, Carlsbad, CA) was utilized to assess cellular viability via amine exclusion. Surface staining was performed using antibodies against human CD3, CD14, CD16, HLA-DR, CD83, CD86, CD123 (BD Biosciences, San Jose, CA), CD11c, CD19, CD20 (eBioscience, San Diego, CA), and BDCA2 (Miltenyi Biotec, Auburn, CA); pDCs were defined as being lineage-HLA-DR+CD11c-CD123+. Cellular fixation and permeabilization were performed using Cytofix/Cytoperm (BD Biosciences, San Jose, CA), per the manufacturer's instructions. Following blocking of Fc receptors (Miltenyi Biotec, Auburn, CA), intracellular staining was performed with antibodies to IFNα, total IRF7, phosphorylated IRF7 (pS477/pS479) (BD Biosciences, San Jose, CA), IFNβ (Antigenix America, Huntington Station, NY) or NSP2 (mAb 191). The presence of NSP2 staining of exposed pDCs was considered to be indicative of rotaviral replication in those cells. Direct conjugation of mAb 191 to APC was performed by Chromaprobe Inc (Maryland Heights, MO). Data were acquired using a LSRII and DIVA software (BD Biosciences, San Jose, CA); analysis was performed using FlowJo (Treestar Inc, Ashland, OR).
Detection of secreted cytokines and chemokines
Supernatants of cultures >85% (mean ± SEM: 90.84%±0.7499) pure for pDCs were analyzed by Luminex using MILLIPLEX MAP (Millipore, Billerica, MA) per the manufacturer's instructions for the presence of IFNα, IFNγ, TNFα, TNFβ, IL1β, IL1RA, IL2, IL4, IL6, IL8, IL10, IL12p40, IL12p70, CXCL10 (IP10), CCL3 (MIP1α), CCL4 (MIP1β), CCL5 (RANTES) and sCD40L. Secreted IFNβ was detected by ELISA, per the manufacturer's instructions (PBL Interferon Source, Piscataway, NJ).
Statistical analysis
Mann-Whitney and Wilcoxon signed rank tests were performed using GraphPad Prism (GraphPad Software Inc, La Jolla, CA). A p-value of ≤0.05 was considered significant.
Supporting Information
Zdroje
1. LiuYJ
2005 IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 23 275 306
2. KadowakiN
HoS
AntonenkoS
MalefytRW
KasteleinRA
2001 Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 194 863 869
3. GillietM
CaoW
LiuYJ
2008 Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol 8 594 606
4. JarrossayD
NapolitaniG
ColonnaM
SallustoF
LanzavecchiaA
2001 Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol 31 3388 3393
5. SunQ
SunL
LiuHH
ChenX
SethRB
2006 The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24 633 642
6. KatoH
SatoS
YoneyamaM
YamamotoM
UematsuS
2005 Cell type-specific involvement of RIG-I in antiviral response. Immunity 23 19 28
7. KrugA
FrenchAR
BarchetW
FischerJA
DzionekA
2004 TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21 107 119
8. LundJ
SatoA
AkiraS
MedzhitovR
IwasakiA
2003 Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med 198 513 520
9. LundJM
AlexopoulouL
SatoA
KarowM
AdamsNC
2004 Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A 101 5598 5603
10. LoreK
BettsMR
BrenchleyJM
KuruppuJ
KhojastehS
2003 Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus - and HIV-1-specific T cell responses. J Immunol 171 4320 4328
11. LosekeS
Grage-GriebenowE
HeineH
WagnerA
AkiraS
2006 In vitro-generated viral double-stranded RNA in contrast to polyinosinic:polycytidylic acid induces interferon-alpha in human plasmacytoid dendritic cells. Scand J Immunol 63 264 274
12. HornungV
Guenthner-BillerM
BourquinC
AblasserA
SchleeM
2005 Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med 11 263 270
13. SugiyamaT
HoshinoK
SaitoM
YanoT
SasakiI
2008 Immunoadjuvant effects of polyadenylic:polyuridylic acids through TLR3 and TLR7. Int Immunol 20 1 9
14. KumagaiY
KumarH
KoyamaS
KawaiT
TakeuchiO
2009 Cutting Edge: TLR-Dependent Viral Recognition Along with Type I IFN Positive Feedback Signaling Masks the Requirement of Viral Replication for IFN-{alpha} Production in Plasmacytoid Dendritic Cells. J Immunol 182 3960 3964
15. GerosaF
GobbiA
ZorziP
BurgS
BriereF
2005 The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol 174 727 734
16. KempTJ
ElzeyBD
GriffithTS
2003 Plasmacytoid dendritic cell-derived IFN-alpha induces TNF-related apoptosis-inducing ligand/Apo-2L-mediated antitumor activity by human monocytes following CpG oligodeoxynucleotide stimulation. J Immunol 171 212 218
17. VaraniS
CederarvM
FeldS
TammikC
FrascaroliG
2007 Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J Immunol 179 7767 7776
18. PurthaWE
ChachuKA
VirginHWt
DiamondMS
2008 Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. J Virol 82 10964 10974
19. FreihorstJ
OgraPL
2001 Mucosal immunity and viral infections. Ann Med 33 172 177
20. LundJM
LinehanMM
IijimaN
IwasakiA
2006 Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol 177 7510 7514
21. ShenH
IwasakiA
2006 A crucial role for plasmacytoid dendritic cells in antiviral protection by CpG ODN-based vaginal microbicide. J Clin Invest 116 2237 2243
22. LichtnerM
RossiR
RizzaMC
MengoniF
SauzulloI
2008 Plasmacytoid dendritic cells count in antiretroviral-treated patients is predictive of HIV load control independent of CD4+ T-cell count. Curr HIV Res 6 19 27
23. TiltonJC
ManionMM
LuskinMR
JohnsonAJ
PatamawenuAA
2008 Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virol 82 3997 4006
24. DecalfJ
FernandesS
LongmanR
AhloulayM
AudatF
2007 Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med 204 2423 2437
25. LaiWK
CurbishleySM
GoddardS
AlabrabaE
ShawJ
2007 Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol 47 338 347
26. KapikianAZ
2001 A rotavirus vaccine for prevention of severe diarrhoea of infants and young children: development, utilization and withdrawal. Novartis Found Symp 238 153 171; discussion 171-159
27. ParasharUD
BreseeJS
GlassRI
2003 The global burden of diarrhoeal disease in children. Bull World Health Organ 81 236
28. ParasharUD
HummelmanEG
BreseeJS
MillerMA
GlassRI
2003 Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 9 565 572
29. ParasharUD
GibsonCJ
BresseJS
GlassRI
2006 Rotavirus and severe childhood diarrhea. Emerg Infect Dis 12 304 306
30. GlassRI
KilgorePE
HolmanRC
JinS
SmithJC
1996 The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis 174 Suppl 1 S5 11
31. EstesMK
2001 Rotaviruses and their replication.
KnipeDM
HowleyP
Fields Virology Philadelphia Lippincott Williams and Wilkins
32. DelormeC
BrussowH
SidotiJ
RocheN
KarlssonKA
2001 Glycosphingolipid binding specificities of rotavirus: identification of a sialic acid-binding epitope. J Virol 75 2276 2287
33. AriasCF
RomeroP
AlvarezV
LopezS
1996 Trypsin activation pathway of rotavirus infectivity. J Virol 70 5832 5839
34. ClarkSM
RothJR
ClarkML
BarnettBB
SpendloveRS
1981 Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J Virol 39 816 822
35. CrawfordSE
MukherjeeSK
EstesMK
LawtonJA
ShawAL
2001 Trypsin cleavage stabilizes the rotavirus VP4 spike. J Virol 75 6052 6061
36. EstesMK
GrahamDY
MasonBB
1981 Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol 39 879 888
37. DharakulT
Riepenhoff-TaltyM
AlbiniB
OgraPL
1988 Distribution of rotavirus antigen in intestinal lymphoid tissues: potential role in development of the mucosal immune response to rotavirus. Clin Exp Immunol 74 14 19
38. FenauxM
CuadrasMA
FengN
JaimesM
GreenbergHB
2006 Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice. J Virol 80 5219 5232
39. DouagiI
McInerneyGM
HidmarkAS
MiriallisV
JohansenK
2007 Role of interferon regulatory factor 3 in type I interferon responses in rotavirus-infected dendritic cells and fibroblasts. J Virol 81 2758 2768
40. IstrateC
DouagiI
CharpilienneA
McInerneyGM
HidmarkA
2007 Bone marrow dendritic cells internalize live RF-81 bovine rotavirus and rotavirus-like particles (RF 2/6-GFP-VLP and RF 8*2/6/7-VLP) but are only activated by live bovine rotavirus. Scand J Immunol 65 494 502
41. NarvaezCF
AngelJ
FrancoMA
2005 Interaction of rotavirus with human myeloid dendritic cells. J Virol 79 14526 14535
42. MesaMC
RodriguezLS
FrancoMA
AngelJ
2007 Interaction of rotavirus with human peripheral blood mononuclear cells: plasmacytoid dendritic cells play a role in stimulating memory rotavirus specific T cells in vitro. Virology 366 174 184
43. De BoissieuD
LebonP
BadoualJ
BompardY
DupontC
1993 Rotavirus induces alpha-interferon release in children with gastroenteritis. J Pediatr Gastroenterol Nutr 16 29 32
44. MangiarottiP
MoulinF
PalmerP
RavillyS
RaymondJ
1999 Interferon-alpha in viral and bacterial gastroenteritis: a comparison with C-reactive protein and interleukin-6. Acta Paediatr 88 592 594
45. BarroM
PattonJT
2005 Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc Natl Acad Sci U S A 102 4114 4119
46. BarroM
PattonJT
2007 Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7. J Virol 81 4473 4481
47. SenA
FengN
EttayebiK
HardyME
GreenbergHB
2009 IRF3 inhibition by rotavirus NSP1 is host cell and virus strain dependent but independent of NSP1 proteasomal degradation. J Virol 83 10322 10335
48. GraffJW
EttayebiK
HardyME
2009 Rotavirus NSP1 inhibits NFkappaB activation by inducing proteasome-dependent degradation of beta-TrCP: a novel mechanism of IFN antagonism. PLoS Pathog 5 e1000280
49. RuggeriFM
GreenbergHB
1991 Antibodies to the trypsin cleavage peptide VP8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J Virol 65 2211 2219
50. HondaK
YanaiH
NegishiH
AsagiriM
SatoM
2005 IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434 772 777
51. TownsleyAC
WeisbergAS
WagenaarTR
MossB
2006 Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J Virol 80 8899 8908
52. YiAK
TuetkenR
RedfordT
WaldschmidtM
KirschJ
1998 CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol 160 4755 4761
53. NarvaezCF
FrancoMA
AngelJ
MortonJM
GreenbergHB
2010 Rotavirus differentially infects and polyclonally stimulates human B cells depending on their differentiation state and tissue of origin. J Virol 0 JVI.02550-09
54. JayaramH
EstesMK
PrasadBV
2004 Emerging themes in rotavirus cell entry, genome organization, transcription and replication. Virus Res 101 67 81
55. PattonJT
Vasquez-Del CarpioR
SpencerE
2004 Replication and transcription of the rotavirus genome. Curr Pharm Des 10 3769 3777
56. DonaghyH
BosnjakL
HarmanAN
MarsdenV
TyringSK
2009 Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol 83 1952 1961
57. SunP
FernandezS
MarovichMA
PalmerDR
CelluzziCM
2009 Functional characterization of ex vivo blood myeloid and plasmacytoid dendritic cells after infection with dengue virus. Virology 383 207 215
58. ThitithanyanontA
EngeringA
EkchariyawatP
Wiboon-utS
LimsalakpetchA
2007 High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-alpha and TLR ligands. J Immunol 179 5220 5227
59. SchmidtB
ScottI
WhitmoreRG
FosterH
FujimuraS
2004 Low-level HIV infection of plasmacytoid dendritic cells: onset of cytopathic effects and cell death after PDC maturation. Virology 329 280 288
60. MatsuiT
ConnollyJE
MichnevitzM
ChaussabelD
YuCI
2009 CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol 182 6815 6823
61. SeoNS
ZengCQ
HyserJM
UtamaB
CrawfordSE
2008 Inaugural article: integrins alpha1beta1 and alpha2beta1 are receptors for the rotavirus enterotoxin. Proc Natl Acad Sci U S A 105 8811 8818
62. FengN
SenA
NguyenH
VoP
HoshinoY
2009 Variation in rotavirus NSP1 antagonism of the IFN response results in differential infectivity in mouse embryonic fibroblasts. J Virol
63. IzaguirreA
BarnesBJ
AmruteS
YeowWS
MegjugoracN
2003 Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol 74 1125 1138
64. GangulyD
ChamilosG
LandeR
GregorioJ
MellerS
2009 Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med 206 1983 1994
65. HirataY
BroquetAH
MenchenL
KagnoffMF
2007 Activation of innate immune defense mechanisms by signaling through RIG-I/IPS-1 in intestinal epithelial cells. J Immunol 179 5425 5432
66. HolmGH
ZurneyJ
TumilasciV
LeveilleS
DanthiP
2007 Retinoic acid-inducible gene-I and interferon-beta promoter stimulator-1 augment proapoptotic responses following mammalian reovirus infection via interferon regulatory factor-3. J Biol Chem 282 21953 21961
67. WangF
GaoX
BarrettJW
ShaoQ
BarteeE
2008 RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathog 4 e1000099
68. Di DomizioJ
BlumA
Gallagher-GambarelliM
MolensJP
ChaperotL
2009 TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood 114 1794 1802
69. SandbulteMR
BoonAC
WebbyRJ
RiberdyJM
2008 Analysis of cytokine secretion from human plasmacytoid dendritic cells infected with H5N1 or low-pathogenicity influenza viruses. Virology 381 22 28
70. LeeHK
LundJM
RamanathanB
MizushimaN
IwasakiA
2007 Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 315 1398 1401
71. HornungV
SchlenderJ
Guenthner-BillerM
RothenfusserS
EndresS
2004 Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol 173 5935 5943
72. La BonnardiereC
CohenJ
ContrepoisM
1981 Interferon activity in rotavirus infected newborn calves. Ann Rech Vet 12 85 91
73. La BonnardiereC
LaudeH
1983 Interferon induction in rotavirus and coronavirus infections: a review of recent results. Ann Rech Vet 14 507 511
74. Vanden BroeckeC
SchwersA
DagenaisL
GoossensA
MaenhoudtM
1984 Interferon response in colostrum-deprived newborn calves infected with bovine rotavirus: its possible role in the control of the pathogenicity. Ann Rech Vet 15 29 34
75. Fitzgerald-BocarslyP
DaiJ
SinghS
2008 Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev 19 3 19
76. Fitzgerald-BocarslyP
FengD
2007 The role of type I interferon production by dendritic cells in host defense. Biochimie 89 843 855
77. ZhangZ
WangFS
2005 Plasmacytoid dendritic cells act as the most competent cell type in linking antiviral innate and adaptive immune responses. Cell Mol Immunol 2 411 417
78. FengN
KimB
FenauxM
NguyenH
VoP
2008 Role of interferon in homologous and heterologous rotavirus infection in the intestines and extraintestinal organs of suckling mice. J Virol 82 7578 7590
79. CuadrasMA
FeigelstockDA
AnS
GreenbergHB
2002 Gene expression pattern in Caco-2 cells following rotavirus infection. J Virol 76 4467 4482
80. HoshinoY
WyattRG
GreenbergHB
FloresJ
KapikianAZ
1984 Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis 149 694 702
81. BridgerJC
WoodeGN
1976 Characterization of two particle types of calf rotavirus. J Gen Virol 31 245 250
82. CohenJ
1977 Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol 36 395 402
83. GroeneWS
ShawRD
1992 Psoralen preparation of antigenically intact noninfectious rotavirus particles. J Virol Methods 38 93 102
84. CharpilienneA
NejmeddineM
BeroisM
ParezN
NeumannE
2001 Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J Biol Chem 276 29361 29367
85. CrawfordSE
LabbeM
CohenJ
BurroughsMH
ZhouYJ
1994 Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J Virol 68 5945 5952
86. BluttSE
WarfieldKL
O'NealCM
EstesMK
ConnerME
2006 Host, viral, and vaccine factors that determine protective efficacy induced by rotavirus and virus-like particles (VLPs). Vaccine 24 1170 1179
87. ConnerME
ZarleyCD
HuB
ParsonsS
DrabinskiD
1996 Virus-like particles as a rotavirus subunit vaccine. J Infect Dis 174 Suppl 1 S88 92
88. KalicaAR
JamesJDJr
KapikianAZ
1978 Hemagglutination by simian rotavirus. J Clin Microbiol 7 314 315
89. IshidaS
FengN
TangB
GilbertJM
GreenbergHB
1996 Quantification of systemic and local immune responses to individual rotavirus proteins during rotavirus infection in mice. J Clin Microbiol 34 1694 1700
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin NeutralizationČlánek Serum-Dependent Selective Expression of EhTMKB1-9, a Member of B1 Family of Transmembrane KinasesČlánek Host Cell Invasion and Virulence in Sepsis Is Facilitated by the Multiple Repeats within FnBPA
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Cognitive Dysfunction Is Sustained after Rescue Therapy in Experimental Cerebral Malaria, and Is Reduced by Additive Antioxidant Therapy
- DNA Watermarking of Infectious Agents: Progress and Prospects
- Self-Protection against Gliotoxin—A Component of the Gliotoxin Biosynthetic Cluster, GliT, Completely Protects Against Exogenous Gliotoxin
- Modifies the Tsetse Salivary Composition, Altering the Fly Feeding Behavior That Favors Parasite Transmission
- Requirement of NOX2 and Reactive Oxygen Species for Efficient RIG-I-Mediated Antiviral Response through Regulation of MAVS Expression
- The Enteropathogenic Effector EspF Targets and Disrupts the Nucleolus by a Process Regulated by Mitochondrial Dysfunction
- Epithelial p38α Controls Immune Cell Recruitment in the Colonic Mucosa
- Coexpression of PD-1, 2B4, CD160 and KLRG1 on Exhausted HCV-Specific CD8+ T Cells Is Linked to Antigen Recognition and T Cell Differentiation
- Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin Neutralization
- EspA Acts as a Critical Mediator of ESX1-Dependent Virulence in by Affecting Bacterial Cell Wall Integrity
- An RNA Element at the 5′-End of the Poliovirus Genome Functions as a General Promoter for RNA Synthesis
- Tetherin Restricts Productive HIV-1 Cell-to-Cell Transmission
- Epstein-Barr Virus-Encoded LMP2A Induces an Epithelial–Mesenchymal Transition and Increases the Number of Side Population Stem-like Cancer Cells in Nasopharyngeal Carcinoma
- Protein Kinase A Dependent Phosphorylation of Apical Membrane Antigen 1 Plays an Important Role in Erythrocyte Invasion by the Malaria Parasite
- Requirement for Ergosterol in V-ATPase Function Underlies Antifungal Activity of Azole Drugs
- The SOCS-Box of HIV-1 Vif Interacts with ElonginBC by Induced-Folding to Recruit Its Cul5-Containing Ubiquitin Ligase Complex
- A Crucial Role for Infected-Cell/Antibody Immune Complexes in the Enhancement of Endogenous Antiviral Immunity by Short Passive Immunotherapy
- Modulation of the Arginase Pathway in the Context of Microbial Pathogenesis: A Metabolic Enzyme Moonlighting as an Immune Modulator
- Role of Abl Kinase and the Wave2 Signaling Complex in HIV-1 Entry at a Post-Hemifusion Step
- Serum-Dependent Selective Expression of EhTMKB1-9, a Member of B1 Family of Transmembrane Kinases
- Epigenetic Repression of by Latent Epstein-Barr Virus Requires the Interaction of EBNA3A and EBNA3C with CtBP
- Host Cell Invasion and Virulence in Sepsis Is Facilitated by the Multiple Repeats within FnBPA
- Role of PKR and Type I IFNs in Viral Control during Primary and Secondary Infection
- A Kinome RNAi Screen Identified AMPK as Promoting Poxvirus Entry through the Control of Actin Dynamics
- Cryptococcal Cell Morphology Affects Host Cell Interactions and Pathogenicity
- NleG Type 3 Effectors from Enterohaemorrhagic Are U-Box E3 Ubiquitin Ligases
- Human Cytomegalovirus UL29/28 Protein Interacts with Components of the NuRD Complex Which Promote Accumulation of Immediate-Early RNA
- Complement Receptor 1 Is a Sialic Acid-Independent Erythrocyte Receptor of
- A Viral microRNA Down-Regulates Multiple Cell Cycle Genes through mRNA 5′UTRs
- Immunotoxin Complementation of HAART to Deplete Persisting HIV-Infected Cell Reservoirs
- Protein Expression Redirects Vesicular Stomatitis Virus RNA Synthesis to Cytoplasmic Inclusions
- Entry and Fusion of Emerging Paramyxoviruses
- Paramyxovirus Entry and Targeted Vectors for Cancer Therapy
- Suppressing Glucose Transporter Gene Expression in Schistosomes Impairs Parasite Feeding and Decreases Survival in the Mammalian Host
- Formation of Complexes at Plasmodesmata for Potyvirus Intercellular Movement Is Mediated by the Viral Protein P3N-PIPO
- Fungal Cell Gigantism during Mammalian Infection
- Two Novel Point Mutations in Clinical Reduce Linezolid Susceptibility and Switch on the Stringent Response to Promote Persistent Infection
- Rotavirus Structural Proteins and dsRNA Are Required for the Human Primary Plasmacytoid Dendritic Cell IFNα Response
- The Epigenetic Landscape of Latent Kaposi Sarcoma-Associated Herpesvirus Genomes
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requirement of NOX2 and Reactive Oxygen Species for Efficient RIG-I-Mediated Antiviral Response through Regulation of MAVS Expression
- Formation of Complexes at Plasmodesmata for Potyvirus Intercellular Movement Is Mediated by the Viral Protein P3N-PIPO
- Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin Neutralization
- Two Novel Point Mutations in Clinical Reduce Linezolid Susceptibility and Switch on the Stringent Response to Promote Persistent Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



