-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaAre Human Intestinal Eukaryotes Beneficial or Commensals?
article has not abstract
Published in the journal: . PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1005039
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005039Summary
article has not abstract
Multiple Biomes (Virome, Microbiome, and Eukaryome) Govern Human Health
Since the advent of microbiology, it has been well known that each human body hosts a multitude of microbes. The magnitude of our microbial system is best reflected by the widely discussed ratio of one human cell to ten microbes. Indeed, humans and other mammals live in a consortium composed of vast arrays of viruses (these are typically called the virome), archaea and bacteria (i.e., the microbiome), along with fungi and other uni - and multicellular eukaryotes (protists and helminths, respectively) historically thought of as “parasites.” It was the advent of next generation sequencing (NGS) that first allowed deeper insight not only into the composition of this “microbial zoo” but also its dynamics in relation to age, diet, health, sex, and geographic location of the host. Attention has focused primarily on the bacterial microbiome, which constitutes the most abundant and diverse segment of the human intestinal ecosystem. However, we argue that eukaryotes play important, but largely unrecognized roles and that there is much to gain by turning our attention to eukaryotic members of the gut ecosystem.
The Eukaryome Is Primarily Commensal
Diverse eukaryotes inhabit the human gut, including protists, fungi, and helminths [1,2], and we suggest the designation “eukaryome” for this collection, which appears more practical than the previously suggested “eukaryotome” [3]. Historically, any protist or helminth species found in humans was considered a parasite and assumed to have a pathogenic effect on the host organism [2]. Pathogenicity is certainly the rule for some intestinal protists, such as Cryptosporidium spp. and Entamoeba histolytica, as well as for the helminths Ascaris lumbricoides and Strongyloides stercoralis, especially in heavy infections or in malnourished or immunocompromised hosts [4]. However, critical evaluation of the literature indicates that for a range of human-associated protists and helminths, unambiguous data about their pathogenicity are hard to find (for a review, see [5]).
Emerging evidence suggests that many common eukaryotic residents of the human gut are commensal or beneficial rather than parasitic, at least in some contexts. For example, the gut protist Blastocystis (Fig 1A) frequently associated in the literature with gastrointestinal disease, is very common, present in 10% to 100% of surveyed individuals (for a review, see [6]). Interestingly, several recent studies have found Blastocystis frequently in healthy individuals [7], sometimes at higher prevalence than in those with gastrointestinal disease [8]. Moreover, available data suggest that another protist, Dientamoeba fragilis (Fig 1E), has a similarly variable ecological role in the human intestinal ecosystem, occasionally associated with disease [9] but also highly prevalent in healthy individuals [7,8].
Fig. 1. 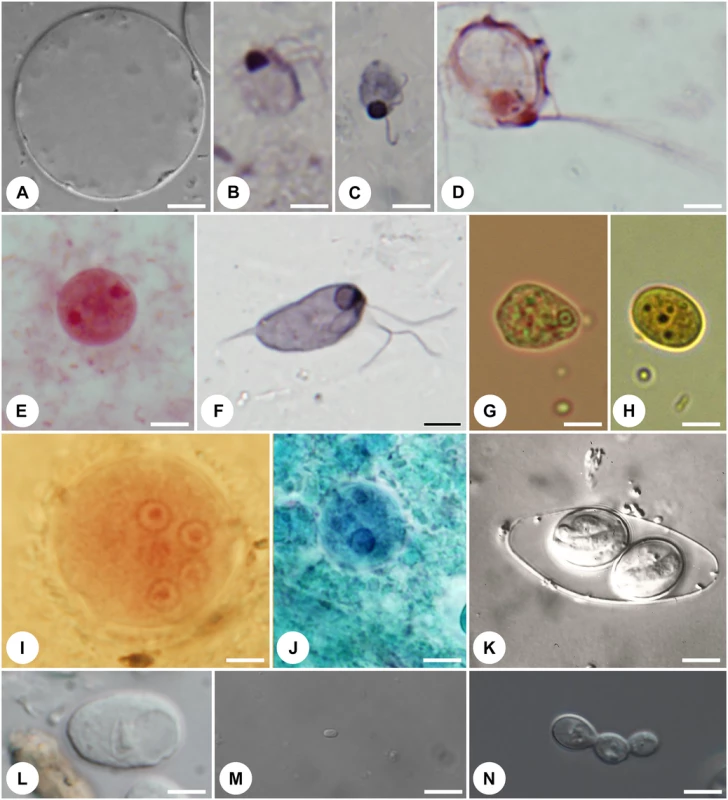
Representative protist symbionts that are part of the human gut eukaryome: (A) Stramenopile <i>Blastocystis hominis</i>; (B) diplomonadid <i>Enteromonas hominis</i>; (C) retortamonadid <i>Retortamonas intestinalis</i>; (D) trichomonadid <i>Pentratrichomonas hominis</i>; (E) tritrichomonadid <i>Dientamoeba fragilis</i>; (F) retortamonadid <i>Chilomastix mesnili</i>; (G) amoebozoans <i>Entamoeba hartmani</i>; (H) <i>Endolimax nana</i>; (I) <i>Entamoeba coli</i>; (J) <i>Entamoeba dispar</i>; (K) coccidian <i>Isospora belli</i>; (L) amoebozoan <i>Iodamoeba buetschli</i>; (M) microsporidian <i>Encephalitozoon cuniculi</i>; (N) ascomycete <i>Candida albicans</i>. Magnification is to the same scale (scale bar = 5 μm). Living cells (A,J–M), protargol-stained cells (B–D,F), trichrome-stained cell (E), Giemsa-stained cell (G), iodine-stained cell (H), and Gomori trichrome-stained cell (I). Pictures were kindly provided by K. Jirků-Pomajbíková (A,I,L), Ivan Čepička (B,C,E,F) (Charles University, Prague), Jaroslav Kulda (D) (Charles University, Prague), Marianne Lebbad (G,H) (Public Health Agency, Solna, Sweden), Martin Kostka (I) (University of South Bohemia, České Budějovice), Jiří Vávra (K) (Charles University, Prague), Martin Kváč and Bohouš Sak (M) (Institute of Parasitology, České Budějovice), and Miroslav Kolařík (N) (Institute of Microbiology, Prague). What differentiates pathogenic eukaryotes (true parasites) from commensals and mutualists? Many questions remain, but organismal factors such as duration of infection and/or colonization and localization within the gut are likely important. Organisms that cause acute disease and provoke an immune response that results in rapid clearance and long-term immunity (e.g., most Microsporidia (Fig 1M), Cryptosporidium spp., and Giardia intestinalis) are true parasites. At the other end of the spectrum, we find the beneficial eukaryotes that establish life-long associations with their host, including the cellulose-digesting ciliates and flagellates in herbivores [1]. In between, there are many eukaryotic organisms that colonize the intestine stably over long time periods and appear to be well tolerated by the immune system, including Entamoeba coli, Blastocystis, and Dientamoeba. Localization within the gut is also important. True parasites, such as Entamoeba histolytica, invade host tissue and migrate out of the gut, causing lesions, abscesses, and inflammation within the gut and throughout the body. In contrast, a commensal relationship is more likely for those organisms confined to the lumen of the gut, as exemplified by Escherichia coli, Blastocystis, Dientamoeba, Enteromonas hominis (Fig 1B), and Retortamonas intestinalis (Fig 1C). Moreover, the nature of the host–eukaryote relationship will change depending on the context of the host and the gut ecosystem, so that the same organism may be parasitic in some cases and commensal in others. The nutritional status and health of the host are clearly important in this equation. For example, symbionts will have a different effect on healthy humans with permanent access to food rich in vitamins and trace elements (as is common for citizens of industrialized countries) compared with malnourished or immunocompromised individuals.
Is the eukaryome beneficial overall? We do not know, and clear-cut cases of beneficial eukaryotes in the human gut are few. Yet, new findings in diverse fields suggest that we may ignore possible beneficial roles of the eukaryome at our peril. Potential benefits of the eukaryome may stem from increasing diversity in general or from the ability of eukaryotes to modulate the host immune system—each of which can induce changes in host physiology and the overall gut ecosystem.
Diversity of the Eukaryome and the Gut Ecosystem
Diversity of the eukaryome as a whole may be beneficial. High diversity across all components of the gut ecosystem, including the eukaryome, is associated with healthy individuals and lower incidence of autoimmune and inflammatory disease [10–12]. Eukaryotes in the gut may signal, or even stimulate, higher diversity overall. Bacterial diversity is higher in the presence of Entamoeba (Fig 1G, 1I, and 1J) and some nematodes in the gut [13,14], but others did not see that effect [15,16]. However, eukaryome diversity is substantially lower in industrialized populations compared to populations with traditional lifestyles [7], just as it has been shown for bacteria [12,17]. This is a result of lifestyle changes (e.g., increased hygiene), as well as targeted removal. As mentioned above, most eukaryotes found in our body have been historically defined as parasites and assumed to have a negative impact. As such, doctors are (almost invariably) trained to remove them, even from asymptomatic patients [18,19]. Efficient drugs that disrupt their life cycles stand behind the virtual elimination of most protists and helminths from the populations of industrialized countries [20], considered a success of their medical systems. However, the true picture is possibly much more complicated, especially as eliminating eukaryotes is associated with increased incidence of immune-mediated and inflammatory diseases [10,11,20,21]. Indeed, the fast increase of the autoimmune and inflammatory diseases including Crohn’s disease, ulcerative colitis, various allergies, rheumatic arthritis, and others, are firmly associated with changes in the gut microbiome [11,12,22]. Amidst these changes, we propose that the overall rareness or even absence of the gut eukaryotes contributes significantly to this negative development.
Immune System Modulation
Specific eukaryotes in the gut ecosystem can also have beneficial effects stemming from their ability to modulate the host immune system, which is well established for helminths. A positive role for helminths in the eukaryome is suggested by the negative correlation between their presence and incidence of immune-mediated disease and by studies documenting a rise in disease symptoms after clearance of parasites [10,20]. Indeed, direct introduction of helminths (helminth therapy) as a prophylactic or therapeutic agent has often been successful at preventing or treating autoimmune and inflammatory disease [10,11,20,21], with important counter examples. While currently unexplored, possible immuno-modulatory roles for Blastocystis, Dientamoeba, and other common intestinal protists that have co-evolved with humans are worth investigating thoroughly. One mechanism of action is the stimulation of mucus production, via the cytokine IL-22, which alleviates symptoms of colitis, improving gut health [23]. These sorts of indirect effects may be more common than currently appreciated because we have not looked for them. Perhaps Blastocystis is more common in healthy people because it helps maintain a healthy mucus layer in the intestine, either directly or through interactions with beneficial bacteria or the immune system.
Rethinking the Term “Parasite”
In light of our evolving understanding of eukaryotes associated with humans and other hosts, we suggest referring to them as symbionts rather than parasites to capture the diversity of their ecological roles. The term “symbiont” encompasses all host-associated organisms across the spectrum of possible relationships (beneficial, detrimental, or neutral). This name change is part of the general shift away from viewing the eukaryome as inherently harmful, echoing the shift in perspective that already occurred for bacteria as we learned more about the vital and beneficial role of the bacterial microbiome in the human gut [24]. Many intestinal eukaryotes are commensal [2,5] or beneficial, for example, the ciliates and flagellates that break down cellulose (for a review, see [1]).
Eukaryome and Its Relationships with Microbiome
Eukaryotic microbes co-evolved with mammals over millions of years and are a normal component of the microbiome from an evolutionary point of view [7,11]. Many are stable, long-term colonists rather than transient invaders [25]. The eukaryome can have strong effects on the composition and dynamics of the microbiome [14], likely with cascading consequences for our health. Although less numerous than bacteria, gut-dwelling eukaryotes are much bigger and they may have a disproportionate influence, similar to large animals in other ecosystems. For example, sharks on tropical reefs and wolves in Yellowstone have a profound effect on the entire ecosystem, and removal of these keystone species has wide consequences. It is worth testing whether targeted removal of eukaryotes—potential keystone components of the gut microbiome—in industrialized countries has contributed disproportionately to the diversity loss observed in the bacterial microbiome [12] and other negative health consequences discussed above. In summary, there are many exciting prospects for investigating potential benefits of the human eukaryome, all while keeping in mind the well-documented detrimental impact of some eukaryotic symbionts, particularly when present in large numbers and in mammalian hosts experiencing food limitation [26].
Future Prospects
Calling intestinal eukaryotic microbes symbionts—which encompasses mutualists, commensals, and parasites—rather than parasites conveys the diverse interactions they have with hosts above and beyond pathogenicity.
There is a need to change therapeutic strategies to target elimination only of demonstrably pathogenic species—the true parasites—from host organisms, while avoiding removal of the harmless or commensal species.
Now is the right time to determine the diversity of eukaryotic microbes in the healthy human population using NGS and to disentangle their relationships with bacterial communities.
Characterizing eukaryotic microbes will enable us to associate the occurrence or absence of various eukaryotic microbes with human diseases.
With the aim to bring some of the “lost” intestinal eukaryotes back, controlled colonization by commensal eukaryotes in human volunteers should be performed to confirm the predicted positive impact on diversification of human gut microbiome.
If successful, these trials should be followed by controlled infections of patients with, e.g., functional and organic gastrointestinal diseases with a goal to inform novel approaches in the treatment of such diseases.
Zdroje
1. Parfrey LW, Walters WA, Knight R (2011). Microbial eukaryotes in the human microbiome: Ecology, evolution, and future directions. Front Cell Infect Microbiol 2 : 1–6.
2. Bogitsh BJ, Carter CE, Oeltmann TN (2012). Human Parasitology, 4the edition. Elsevier Academic Press, San Diego, USA; 430 pp.
3. Andersen LO, Nielsen HV, Stensvold CR (2013). Waiting for the human intestinal eukaryotome. ISME J 7 : 1253–1255. doi: 10.1038/ismej.2013.21 23407309
4. Lewthwaite P, Gill GV, Hart CA, Beeching NJ (2005). Gastrointestinal parasites in the immunocopromised persons. Curr Opin Infect Dis 18 : 427–35. 16148530
5. Lukeš J, Kuchta R, Scholz T, Pomajbíková K (2014) (Self-) infections with parasites: re-interpretation for the present. Trends Parasitol 30 : 377–385. doi: 10.1016/j.pt.2014.06.005 25033775
6. Scanlan PD, Stensvold CR, Rajilic-Stojanovic M, Heilig HGHJ, De Vos WM, O’Toole PW, Cotter PD (2014) The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microb Lett 90 : 326–330.
7. Parfrey LW, Walters WA, Lauber CL, Clemente JC, Berg-Lyons D, Teiling C, Kodira C, Mohiuddin M, Brunelle J, Driscoll M, Fierer N, Gilbert JA, Knight R (2014). Communities of microbial eukaryotes in the mammalian gut within the context of environmental eukaryotic diversity. Front Microbiol 5 : 298. doi: 10.3389/fmicb.2014.00298 24995004
8. Rossen NG, Bart A, Verhaar N, van Nood E, Kootte R, de Groot PF, D'Haens GR, Ponsioen CY, van Gool T (2015). Low prevalence of Blastocystis sp. In active ulcerative colitis patients. Eur J Clin Microbiol Infect Dis 34 : 1039–1044. doi: 10.1007/s10096-015-2312-2 25680316
9. Barratt JLN, Harkness J, Marriott D, Ellis JT, Stark D (2011). A review of Dientamoeba fragilis carriage in humans: Several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes 2 : 3–12. doi: 10.4161/gmic.2.1.14755 21637013
10. Velasquez-Manoff M (2012). An epidemic of absence. New York, Scribner.
11. Rook GAW, Raison CL, Lowry CA (2014). Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol 177 : 1–12. doi: 10.1111/cei.12269 24401109
12. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012). Diversity, stability and resilience of the human gut microbiota. Nature 489 : 220–230. doi: 10.1038/nature11550 22972295
13. Pliekatt JL, Deenonpoe R, Mulvenna JP, Krause L, Sripa B, Bethony JM, Brindley P (2013) Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB J 27 : 4572–4584. doi: 10.1096/fj.13-232751 23925654
14. Morton ER, Lynch J, Froment A, Lafosse S, Heyer E, Przeworski M, Blekhman R, Segurel L (2015) Variation in rural African gut microbiomes is strongly shaped by parasitism and diet. http://biorxiv.org/content/early/2015/03/24/016949. Accessed 17 July 2015.
15. Cooper P, Walker AW, Reyes J, Chico M, Salter SJ, Vaca M, Parkhill J (2013) Patent human infections with the whipworm, Trichuris trichiura, are not associated with alterations in the faecal microbiota. PLoS One 8: e76573. doi: 10.1371/journal.pone.0076573 24124574
16. Cantacessi C, Giacomin P, Croese J, Zakrzewski M, Sotillo J, McCann L, Nolan MJ, Mitreva M, Krause L, Loukas A (2014). Impact of experimental hookworm infection on the human gut microbiota. J Infect Dis 210 : 1431–1434. doi: 10.1093/infdis/jiu256 24795483
17. Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, Turroni S, Biagi E, Peano C, Severgnini M, Fiori J, Gotti R, De Bellis G, Luiselli D, Brigidi P, Mabulla A, Marlowe F, Henry AG, Crittenden AN (2014). Gut microbiome of the Hadza hunter-gatherers. Nat Commun 5 : 3654. doi: 10.1038/ncomms4654 24736369
18. Committee (1998). Dientamoeba fragilis: A harmless commensal or a mild pathogen? Paediatr Child Health 3 : 81–82. 20401204
19. Coyle CM, Varughese J, Weiss LM, Tanowitz HB (2012) Blastocystis: to treat or not to treat. Clin Infect Dis 54 : 105–110. doi: 10.1093/cid/cir810 22075794
20. Wiria AE, Djuardi Y, Supali T, Sartono E, Yazdanbakhsh M (2012). Helminth infection in populations undergoing epidemiological transition: A friend or foe? Semin Immunopathol 34 : 889–901. doi: 10.1007/s00281-012-0358-0 23129304
21. Elliott DE, Weinstock JV (2012). Where are we on worms? Curr Opin Gastroenterol 28 : 551–556. doi: 10.1097/MOG.0b013e3283572f73 23079675
22. Clemente JC, Ursell LK, Parfrey LW, Knight R (2012). The impact of the gut microbiota on human health: An integrative view. Cell 148 : 1258–1270. doi: 10.1016/j.cell.2012.01.035 22424233
23. Leung JM, Davenport M, Wolff MJ, Wiens KE, Abidi WM, Poles MA, Cho I, Ullman T, Mayer L, Loke P (2014). Il-22-producing cd4+ cells are depleted in actively inflamed colitis tissue. Mucosal Immunol 7, 124–133. doi: 10.1038/mi.2013.31 23695510
24. Sommer F., Backhed F. (2013) The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 11 : 227–38. doi: 10.1038/nrmicro2974 23435359
25. Scanlan PD, Stensvold CR (2013). Blastocystis: getting to grips with guileful guest. Trends Parasitol 29 : 523–529. doi: 10.1016/j.pt.2013.08.006 24080063
26. Graham AL, Hayward AD, Watt KA, Pilkington JG, Pemberton JM, Nussey DH (2010) Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science 330 : 662–665. doi: 10.1126/science.1194878 21030656
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type GenesČlánek Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion IsolatesČlánek -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated GlycolipidsČlánek Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Long and Winding Road (Apologies to the Beatles)
- The Ebola Virus: From Basic Research to a Global Health Crisis
- Riding the R Train into the Cell
- The Two-Phase Emergence of Non Pandemic HIV-1 Group O in Cameroon
- Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies
- E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3
- The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type Genes
- Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-κB Protein p65/RelA by Meningococcal IgA Protease
- Active Transport of Phosphorylated Carbohydrates Promotes Intestinal Colonization and Transmission of a Bacterial Pathogen
- HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates
- Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion
- Inhibiting the Recruitment of PLCγ1 to Kaposi’s Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells
- Goblet Cell Derived RELM-β Recruits CD4 T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation
- HLA Class-II Associated HIV Polymorphisms Predict Escape from CD4+ T Cell Responses
- An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses
- Extracellular Adenosine Protects against Lung Infection by Regulating Pulmonary Neutrophil Recruitment
- : Adaptations to the Dixenous Life Cycle Analyzed by Genome Sequencing, Transcriptome Profiling and Co-infection with
- Which Way In? The RalF Arf-GEF Orchestrates Host Cell Invasion
- Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
- A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane
- Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect
- -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids
- The Activation of Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b
- A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in
- Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation
- The Rsb Phosphoregulatory Network Controls Availability of the Primary Sigma Factor in and Influences the Kinetics of Growth and Development
- Inhibits Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis
- Illuminating Targets of Bacterial Secretion
- Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment
- Interdisciplinarity and Infectious Diseases: An Ebola Case Study
- Fungi That Infect Insects: Altering Host Behavior and Beyond
- Plasticity and Redundancy in Proteins Important for Invasion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- A Novel Virus Causes Scale Drop Disease in
- STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control
- Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
- Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
- AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
- Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice
- Transgenic Rabbits Expressing Ovine PrP Are Susceptible to Scrapie
- Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of . Virulence Pathways
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors
- Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins the Activation of the MnK/eIF4E Pathway
- Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission
- Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
- sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic
- Evolutionary and Functional Analysis of Old World Primate TRIM5 Reveals the Ancient Emergence of Primate Lentiviruses and Convergent Evolution Targeting a Conserved Capsid Interface
- Hepcidin and Host Defense against Infectious Diseases
- Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis
- Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIV Rectal Challenge
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Illuminating Targets of Bacterial Secretion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



