-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaFour Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
There is increasing evidence that the microcirculation plays an important role in the pathogenesis of cardiovascular diseases. Changes in retinal vascular caliber reflect early microvascular disease and predict incident cardiovascular events. We performed a genome-wide association study to identify genetic variants associated with retinal vascular caliber. We analyzed data from four population-based discovery cohorts with 15,358 unrelated Caucasian individuals, who are members of the Cohort for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium, and replicated findings in four independent Caucasian cohorts (n = 6,652). All participants had retinal photography and retinal arteriolar and venular caliber measured from computer software. In the discovery cohorts, 179 single nucleotide polymorphisms (SNP) spread across five loci were significantly associated (p<5.0×10−8) with retinal venular caliber, but none showed association with arteriolar caliber. Collectively, these five loci explain 1.0%–3.2% of the variation in retinal venular caliber. Four out of these five loci were confirmed in independent replication samples. In the combined analyses, the top SNPs at each locus were: rs2287921 (19q13; p = 1.61×10−25, within the RASIP1 locus), rs225717 (6q24; p = 1.25×10−16, adjacent to the VTA1 and NMBR loci), rs10774625 (12q24; p = 2.15×10−13, in the region of ATXN2,SH2B3 and PTPN11 loci), and rs17421627 (5q14; p = 7.32×10−16, adjacent to the MEF2C locus). In two independent samples, locus 12q24 was also associated with coronary heart disease and hypertension. Our population-based genome-wide association study demonstrates four novel loci associated with retinal venular caliber, an endophenotype of the microcirculation associated with clinical cardiovascular disease. These data provide further insights into the contribution and biological mechanisms of microcirculatory changes that underlie cardiovascular disease.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001184
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001184Summary
There is increasing evidence that the microcirculation plays an important role in the pathogenesis of cardiovascular diseases. Changes in retinal vascular caliber reflect early microvascular disease and predict incident cardiovascular events. We performed a genome-wide association study to identify genetic variants associated with retinal vascular caliber. We analyzed data from four population-based discovery cohorts with 15,358 unrelated Caucasian individuals, who are members of the Cohort for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium, and replicated findings in four independent Caucasian cohorts (n = 6,652). All participants had retinal photography and retinal arteriolar and venular caliber measured from computer software. In the discovery cohorts, 179 single nucleotide polymorphisms (SNP) spread across five loci were significantly associated (p<5.0×10−8) with retinal venular caliber, but none showed association with arteriolar caliber. Collectively, these five loci explain 1.0%–3.2% of the variation in retinal venular caliber. Four out of these five loci were confirmed in independent replication samples. In the combined analyses, the top SNPs at each locus were: rs2287921 (19q13; p = 1.61×10−25, within the RASIP1 locus), rs225717 (6q24; p = 1.25×10−16, adjacent to the VTA1 and NMBR loci), rs10774625 (12q24; p = 2.15×10−13, in the region of ATXN2,SH2B3 and PTPN11 loci), and rs17421627 (5q14; p = 7.32×10−16, adjacent to the MEF2C locus). In two independent samples, locus 12q24 was also associated with coronary heart disease and hypertension. Our population-based genome-wide association study demonstrates four novel loci associated with retinal venular caliber, an endophenotype of the microcirculation associated with clinical cardiovascular disease. These data provide further insights into the contribution and biological mechanisms of microcirculatory changes that underlie cardiovascular disease.
Introduction
Although both macrovascular and microvascular pathology are associated with cardiovascular disease, including coronary artery disease and stroke [1], [2], most studies on the genetic determinants of cardiovascular disease have primarily focused on macrovascular disease traits, and genetic analyses of microvascular disease phenotypes are rare [2], [3]. This paucity of data is due to difficulties in non-invasively assessing the microcirculation. However, retinal arterioles and venules, which range between 50 to 300 µm in diameter, can be directly imaged, and provide an ideal opportunity to study the microcirculation in vivo [4].
Quantitative measurement of retinal blood vessel caliber from photographs allows a non-invasive direct assessment of the human microcirculation [4]. Studies using this technique have shown that changes in retinal vascular caliber (e.g., narrower arteriolar and wider venular caliber) are associated with a range of cardiovascular diseases and their risk factors [5], [6], including hypertension [7], diabetes mellitus [8], [9], stroke [10], coronary heart disease [11], and cerebral small vessel disease [12], [13]. Retinal vascular caliber is also an early marker of major eye diseases such as diabetic retinopathy and age-related macular degeneration [14]–[16].
Recent studies suggest that genetic factors may play a role in influencing retinal vascular caliber [17]–[20], so understanding specific genetic factors underlying retinal vascular caliber could therefore demonstrate novel insights into the mechanisms that contribute to the microvascular pathways of cardiovascular and eye diseases. To identify the underlying genetic determinants of retinal arteriolar and venular caliber, we meta-analyzed results of genome-wide association studies (GWAS) of 15,358 white participants from four large, prospective population-based cohorts included in the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium [21]: the Age Gene/Environment Susceptibility – Reykjavik Study (AGES) [22], the Atherosclerosis Risk in Communities Study (ARIC) [23], the Cardiovascular Health Study (CHS) [24] and the Rotterdam Study [25]. We replicated our findings in four independent cohorts of Caucasian ethnicity [the Australian Twins Study [26], the UK Twins Study [27], the Beaver Dam Eye Study (BDES) [11], and the Blue Mountains Eye Study (BMES)] [11]. Finally, in order to examine the association between the replicated hits and cardiovascular diseases, we used data on coronary artery disease from the Wellcome Trust Case Control Consortium (WTCCC) [3], on stroke and myocardial infarction from the Heart and Vascular Health (HVH) Study [28], [29], on hypertension from the Global Blood Pressure Genetics (Global BPgen) Consortium [30], and on diabetes mellitus from the Diabetes Genetics Replication and Meta-analysis + (DIAGRAM+) Consortium [31].
Results
Study samples
The total study sample for the discovery analyses was 15,358 and for the replication analyses 6,652. Characteristics of both the discovery and replication samples are presented in Table 1.
Tab. 1. Baseline characteristics of both the discovery and replication cohorts. 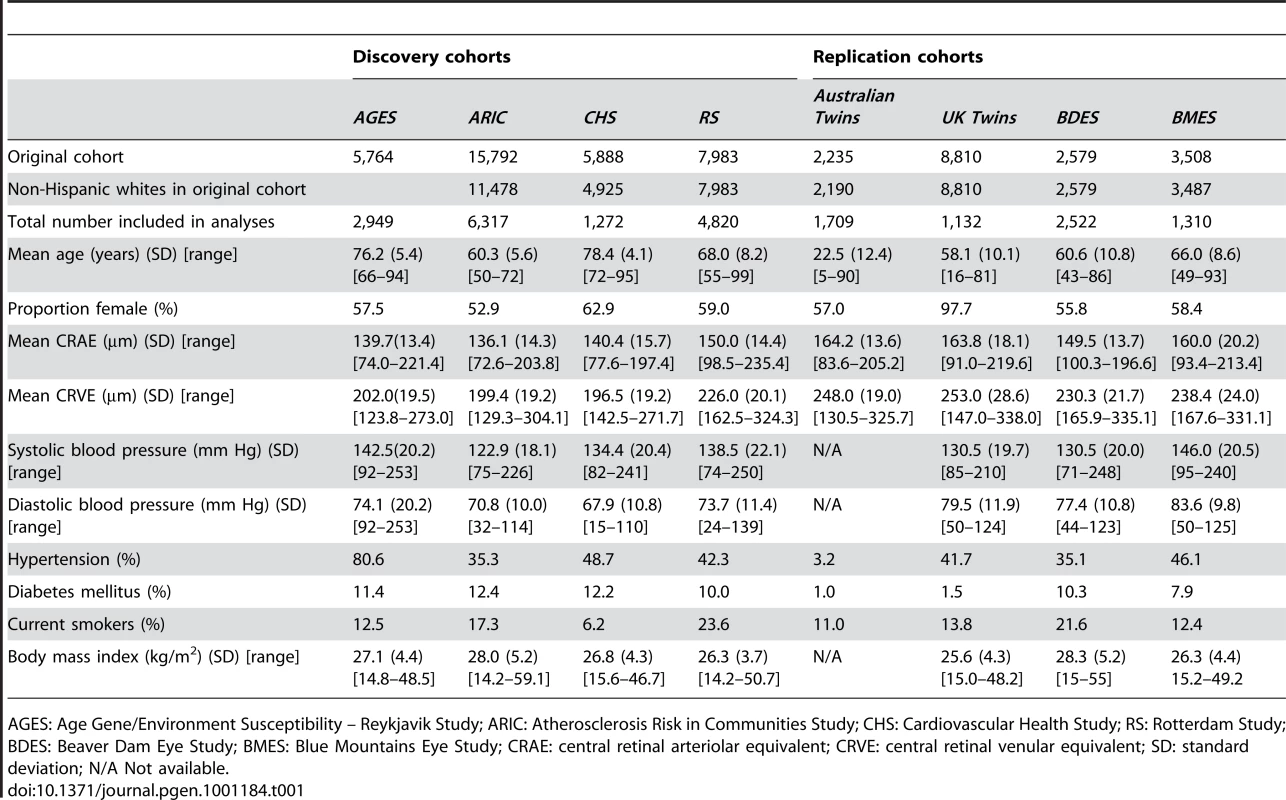
AGES: Age Gene/Environment Susceptibility – Reykjavik Study; ARIC: Atherosclerosis Risk in Communities Study; CHS: Cardiovascular Health Study; RS: Rotterdam Study; BDES: Beaver Dam Eye Study; BMES: Blue Mountains Eye Study; CRAE: central retinal arteriolar equivalent; CRVE: central retinal venular equivalent; SD: standard deviation; N/A Not available. Meta-analysis of CHARGE cohort results
A total of 179 single nucleotide polymorphisms (SNPs) at five loci surpassed our preset threshold (p<5.0×10−8) for genome-wide significance for retinal venular caliber. Collectively, these five independent loci explain 1.0–3.2% of the variation in retinal venular caliber within our discovery cohorts. The QQ-plots (Figure S1A) show departure from the line of identity at approximately p<1.0×10−3. Figure 1A displays the minus log-transformed p-values for the individual SNPs against their genomic position. Table 2 summarizes both the meta-analyzed results and results from each discovery cohort individually for the most significant SNPs at each locus that were associated with retinal venular caliber.
Fig. 1. P-values (minus log-transformed) are shown in a signal intensity (Manhattan) plot relative to their genomic position. 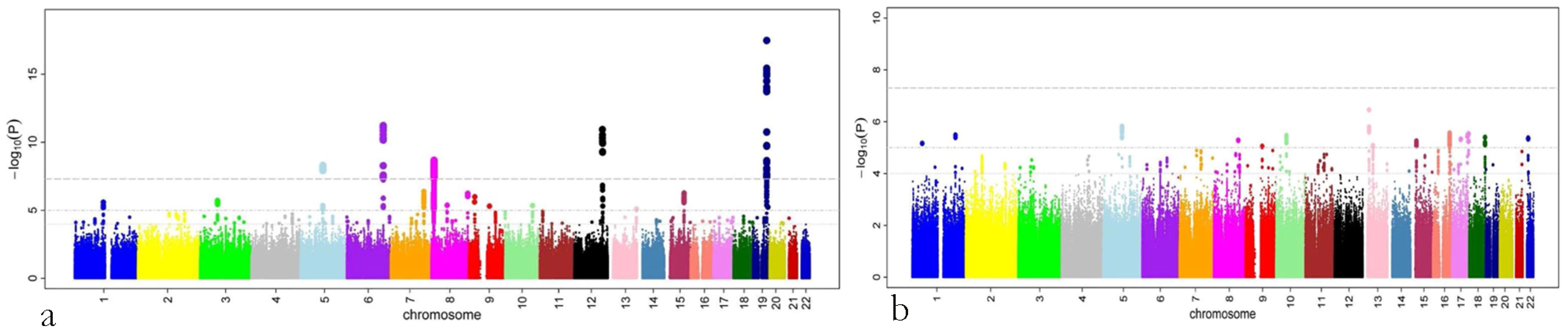
For (a) retinal venular caliber and (b) retinal arteriolar caliber. Tab. 2. Results for the five loci associated (p<5.0×10−8) with retinal venular caliber in the discovery cohorts both combined and individually. 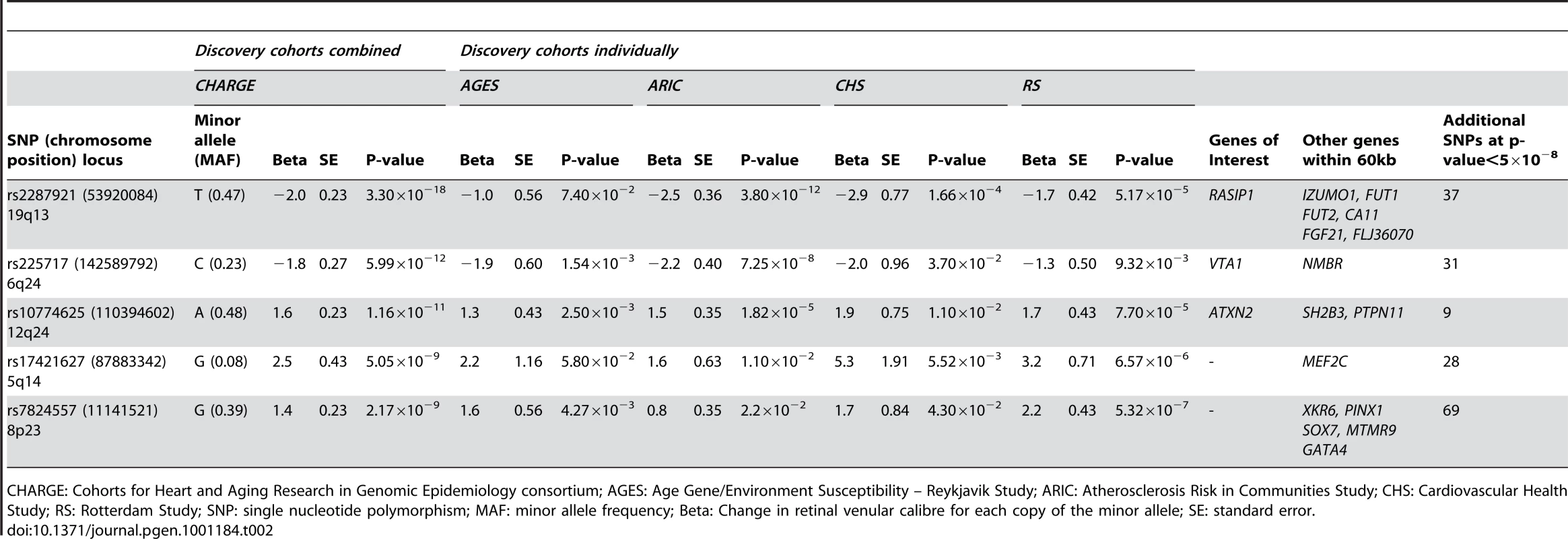
CHARGE: Cohorts for Heart and Aging Research in Genomic Epidemiology consortium; AGES: Age Gene/Environment Susceptibility – Reykjavik Study; ARIC: Atherosclerosis Risk in Communities Study; CHS: Cardiovascular Health Study; RS: Rotterdam Study; SNP: single nucleotide polymorphism; MAF: minor allele frequency; Beta: Change in retinal venular calibre for each copy of the minor allele; SE: standard error. No genome-wide significant locus was identified for retinal arteriolar caliber and only one SNP was associated with retinal arteriolar caliber at a significance threshold between 5.0×10−8 and 1.0×10−6. The QQ-plot (Figure S1B) showed a departure from the line of identity at approximately p<1.0×10−4. Figure 1B displays the minus log-transformed p-values for the individual SNPs against their genomic position. The most significant signal was on chromosome 13q12 (rs2281827, per minor allele (T) copy 1.0 µm (SE: 0.21) increase in arteriolar caliber; minor allele frequency (MAF): 0.23; p = 3.53×10−7). This signal on chromosome 13q12 was located in FLT1, also known as vascular endothelial growth factor receptor.
Replication in independent cohorts
Table 3 shows the results within each replication cohort for the five loci that were genome-wide significant in the discovery phase. Minor allele frequencies in the replication cohorts were very similar to that in the discovery cohorts. Four out of the five loci showed consistent effects in the combined analyses of the replication cohorts at a Bonferroni-corrected significance threshold of p<0.01 (0.05/5, as five loci were tested in the replication phase), the exception was rs7824557 (8p23). The combined analyses of the discovery and replication cohorts yielded an overall p-value of 1.61×10−25 for rs2287921 (19q13). The corresponding values for the other loci were p = 1.25×10−16 for rs225717 (6q24), p = 2.15×10−13 for rs10774625 (12q24) and p = 7.32×10−16 for rs17421627 (5q14). Finally, for rs7824557 (8p23) the overall p-value did not reach genome-wide significance (p = 3.80×10−7).
Tab. 3. Results for the five loci associated with retinal venular caliber for the discovery, replication, and combined cohorts. 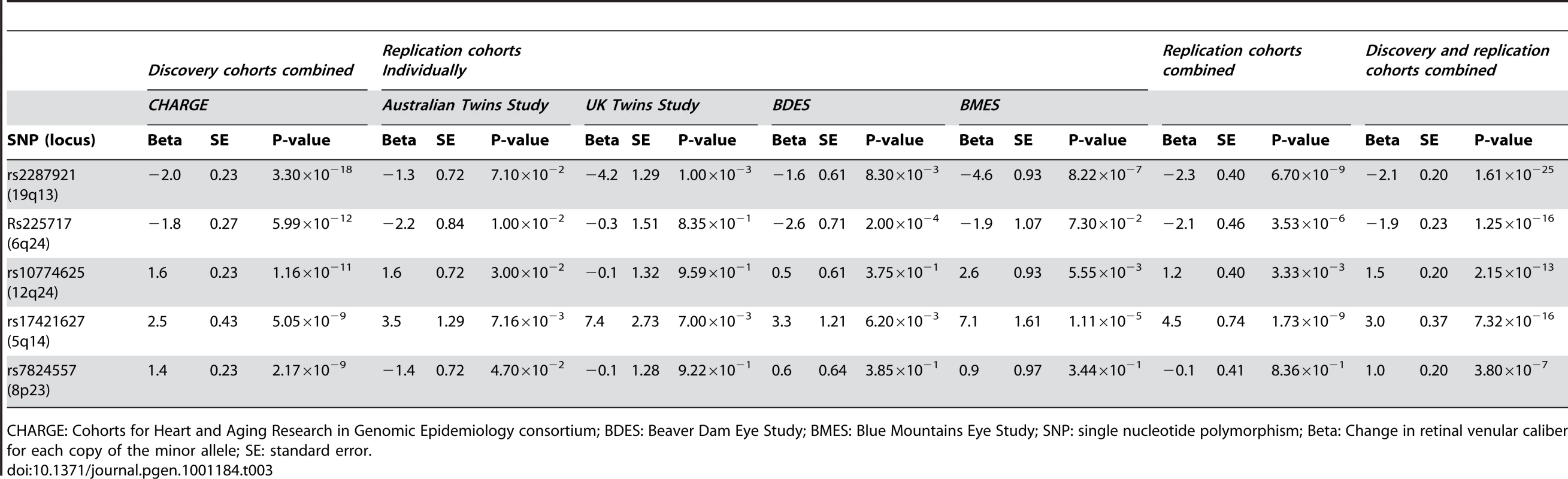
CHARGE: Cohorts for Heart and Aging Research in Genomic Epidemiology consortium; BDES: Beaver Dam Eye Study; BMES: Blue Mountains Eye Study; SNP: single nucleotide polymorphism; Beta: Change in retinal venular caliber for each copy of the minor allele; SE: standard error. The regional association plots for these four loci are presented in Figure 2A–2D. After additional adjustments for hypertension and diabetes mellitus, the associations between the four replicated loci and retinal venular caliber remained the same (Table S1).
Fig. 2. Regional association plots for the four novel loci. 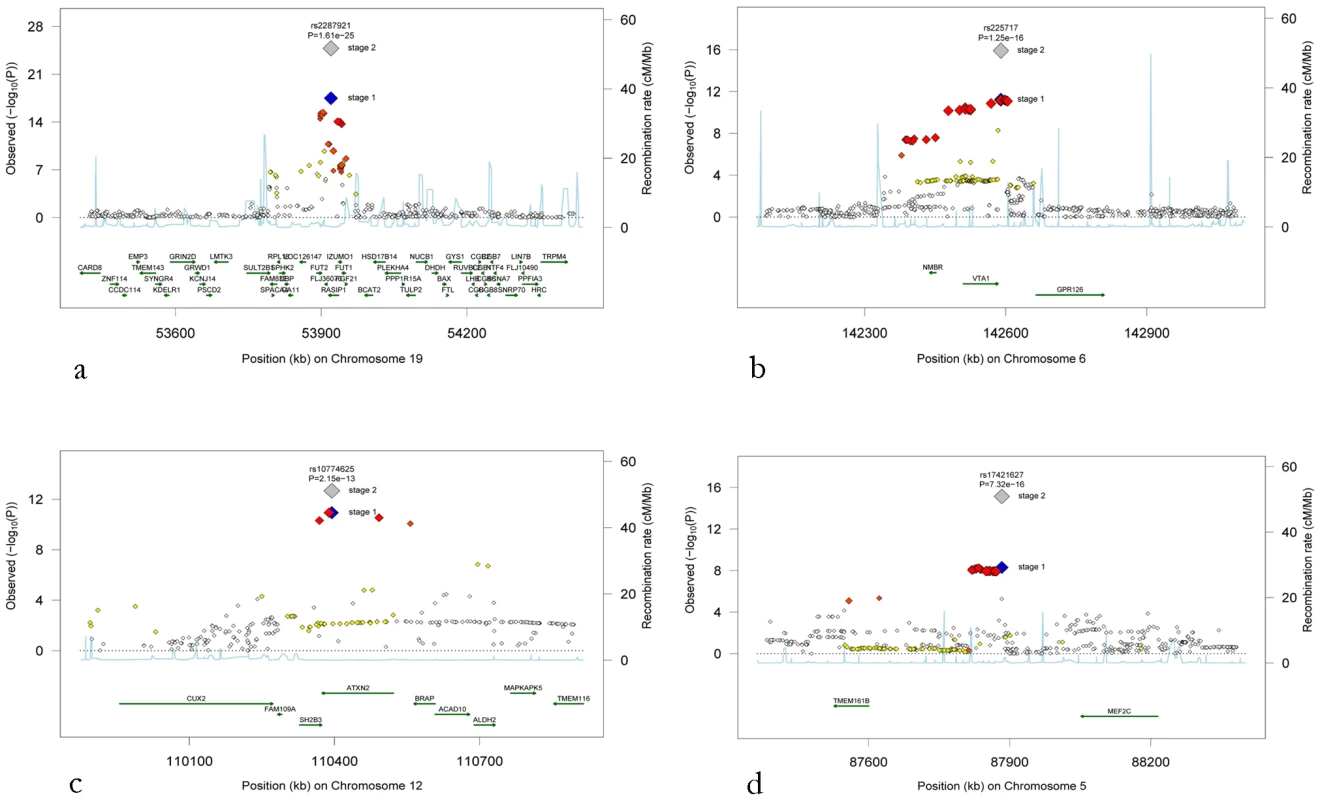
(a) Chromosome 19q13, (b) chromosome 6q24, (c) chromosome 12q24, and (d) chromosome 5q14. The blue diamonds show stage 1 p-values (discovery phase) for the top SNP at each locus, whereas the grey diamonds show the p-values following stage 2 meta-analysis including the replication cohorts for that top SNP. P-values from stage 1 for additional SNPs at each locus are colour-coded according to their linkage disequilibrium with the top SNP as follows: r2<0.2 white, 0.2<r2<0.5 yellow, 0.5<r2<orange-red, r2>0.8 red. Associations with cardiovascular diseases
Table 4 presents the results with clinical cardiovascular diseases for the four loci that were successfully replicated in the replication cohorts. These association results provided evidence for 12q24 as a risk locus for coronary artery disease and hypertension. The allelic odds ratios of rs10774625 were 1.13 (95% confidence interval (CI): 1.03–1.24; p = 0.008) for coronary artery disease and 1.06 (95% CI: 1.01–1.12; p = 0.019) for hypertension. As we found the most convincing evidence for rs10774625 to be associated with coronary artery disease, we examined the association with coronary artery disease for all 10 SNPs on locus 12q24 that were genome-wide significant in the current analysis with retinal venular caliber. Figure 3 shows a plot in which the p-values for these 10 SNPs from the current analysis are combined with those for coronary artery disease from WTCCC. We found that all 10 SNPs were significantly associated with coronary artery disease at a nominal p-value of 0.05 suggesting a strong overlap between the association signals of retinal venular caliber and coronary artery disease.
Fig. 3. A combined regional association plot showing p-values from CHARGE for the 10 SNPs on 12q24 for retinal venular caliber and from WTCCC for coronary artery disease. 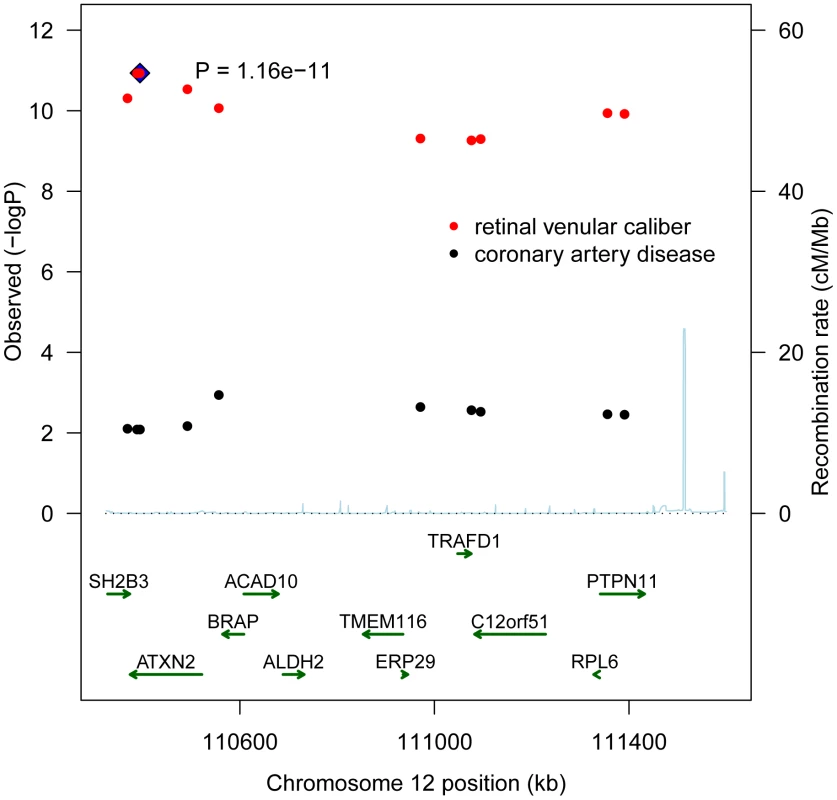
Tab. 4. The association between the four novel loci and cardiovascular diseases. 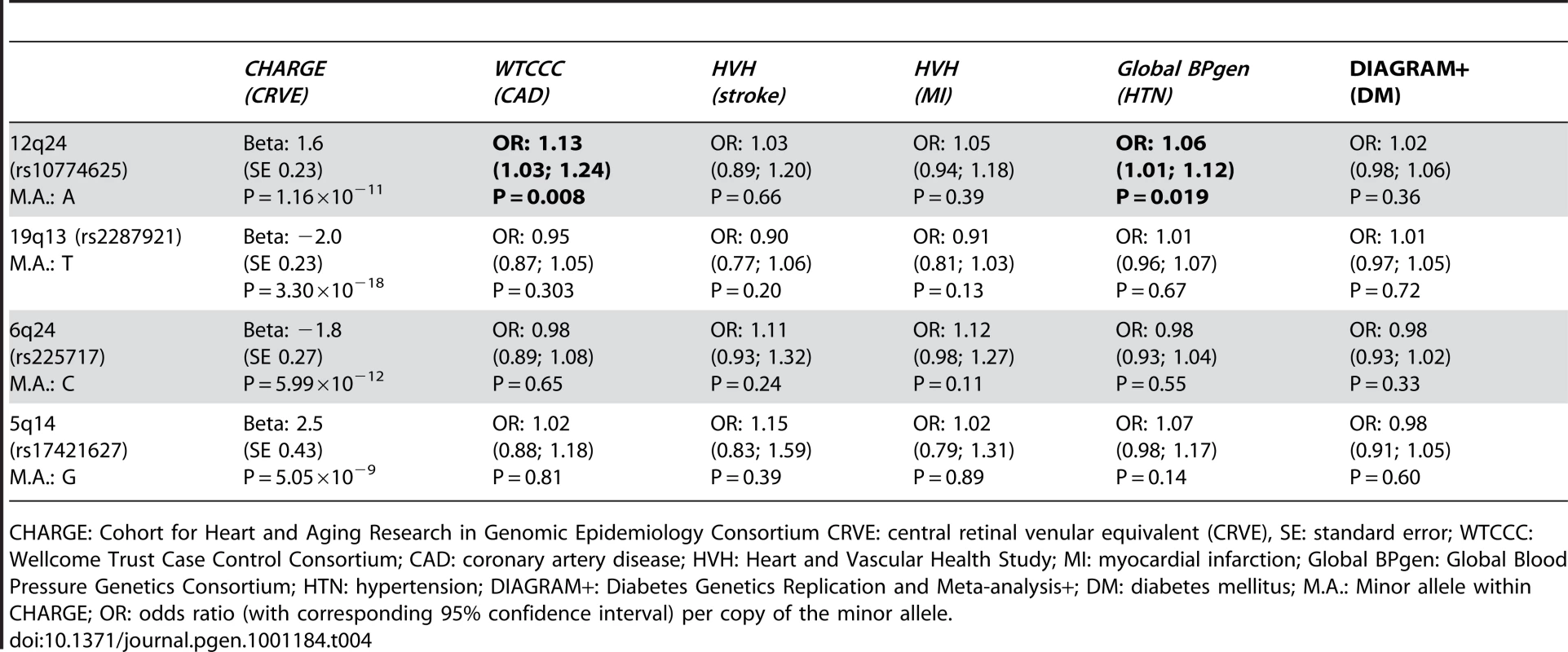
CHARGE: Cohort for Heart and Aging Research in Genomic Epidemiology Consortium CRVE: central retinal venular equivalent (CRVE), SE: standard error; WTCCC: Wellcome Trust Case Control Consortium; CAD: coronary artery disease; HVH: Heart and Vascular Health Study; MI: myocardial infarction; Global BPgen: Global Blood Pressure Genetics Consortium; HTN: hypertension; DIAGRAM+: Diabetes Genetics Replication and Meta-analysis+; DM: diabetes mellitus; M.A.: Minor allele within CHARGE; OR: odds ratio (with corresponding 95% confidence interval) per copy of the minor allele. Discussion
In this meta-analysis of GWAS data from four populations on retinal microcirculation and subsequent replication in four independent cohorts, we identified four novel loci on chromosomes 19q13, 6q24, 12q24 and 5q14 that were consistently associated with retinal venular caliber in persons of Caucasian descent at genome-wide significance of <5.0×10−8. The most significant SNPs at each of the four loci were associated with an approximate 2.0µm change in retinal venular caliber for each copy of the minor allele. Locus 12q24 was also associated with coronary heart disease and hypertension. We did not find any loci that reached genome-wide significance for retinal arteriolar caliber, and only one SNP reached highly suggestive levels.
Our study is the first large study to evaluate common genetic variants of the microcirculation, increasingly thought to play a substantial role in the pathogenesis and development of clinical cardiovascular diseases, including coronary heart disease and stroke. The retinal vasculature provides a non-invasive direct view of the human microcirculation. Retinal venular caliber has been shown to predict a range of subclinical [5] and clinical cardiovascular disease [6]. In a recent meta-analysis, wider retinal venules were associated with a hazard ratio of 1.16 (95% CI: 1.06–1.26) for coronary artery disease in women while controlling for other known cardiovascular risk factors [11]. Furthermore, wider venular caliber predicted risk of stroke and is associated with progression of cerebral white matter lesions [10], [12]. Both systemic and environmental factors likely induce variation in retinal venular caliber along with individual genetic differences [5], [6], [17]–[20]. Wider retinal venular caliber has been hypothesized to reflect the effects of hypoxia [32], and an increased nitric oxide production and release of cytokines resulting from activated endothelial cells [33]. This is supported by clinical and epidemiological studies showing larger venular caliber to be associated with systemic biomarkers of inflammation, including C-reactive protein and interleukin-6, and with impaired fasting glucose metabolism, dyslipidemia, obesity and cigarette smoking [5], [34].
The most significant SNP associated with retinal venular caliber was in the RASIP1 gene (rs2287921, p = 1.61×10−25) on chromosome 19q13. RASIP1 belongs to the family of RAS molecules, which have recently been implicated in animal models to be involved in vascular development, endothelial cell migration, capillary tube assembly, blood vessel homeostasis and vascular permeability [35]. Specifically, RASIP1 is expressed in the endothelium of the developing blood vessels and is essential for proper endothelial cell angiogenic assembly and migration [35].
On chromosome 6q24, the top SNPs were located in or adjacent to VTA1 and NMBR genes. VTA1 encodes a protein involved in trafficking of the multivesicular body, an endosomal compartment involved in sorting membrane proteins for degradation in lysosomes [36]. Neuromedin B (NMB) is a peptide that acts by binding to a specific receptor protein (NMBR) and is involved in a number of physiological processes including immune defense, thyroid, adrenocortical function and cognition. NMB is also aberrantly expressed by a variety of cancers and is involved in tumor cell proliferation [37].
The signals for association on chromosome 12q24 were spread across a large 1 Mb LD block, including genes such as SH2B3, ATXN2 and PTPN11. The most significant SNP was located in ATXN2. Defects in the ATXN2 are the cause of spinocerebellar ataxia type 2 (SCA 2), which belongs to the autosomal cerebellar ataxias characterized by cerebellar ataxia, optic atrophy, ophthalmoplegia and dementia. SCA 2 is caused by extension of a CAG repeat in the coding region of this gene. Another gene in this region is SH2B3, which is expressed by vascular endothelial cells and regulates growth factor and cytokine receptor-mediated pathways implicated in lymphoid, myeloid and platelet homeostasis [38]. Our study showed that the most significant SNP in the SH2B3 region was rs3184504 (p = 4.88×10−11). Interestingly, this variant is associated with type 1 diabetes mellitus, a disease in which the risk of developing complications was found to be associated with wider retinal venular caliber [38]. Recent GWAS studies have shown that several SNPs at the locus 12q24 (e.g. rs11065987 in ATXN2 and rs11066301 in PTPN11) are associated with platelet count, hemoglobin concentration, hematocrit, and blood pressure [39]–[41]. Furthermore, replication in independent case-control series including 9,479 cases and 10,527 controls have shown odds ratios of 1.14 (95% CI: 1.10–1.20; p = 2.52×10−9) and 1.15 (95% CI: 1.10–1.20; p = 7.05×10−11) per minor allele copy for the association of these two SNPs with coronary artery disease [39]. The corresponding allelic odds ratios for myocardial infarction were 1.17 (95% CI: 1.11–1.22; p = 3.43×10−10) and 1.18 (95% CI: 1.12–1.23; p = 2.42×10−12) [39]. In our discovery cohort, apart from rs10774625 we found nine additional SNPs in the region that were genome-wide significant, including both rs11065987 and rs11066301 (1.5 increase in venular caliber per minor allele for both) that have also been shown to be associated with coronary heart disease and myocardial infarction. Finally, in the present study the association results from WTCCC and Global BPgen confirmed locus 12q24 to be a risk locus for both coronary artery disease and hypertension. Specifically, we found a strong overlap between the association signals of retinal venular caliber and coronay artery disease.
The most significant SNPs at the 5q14 locus were located in an intergenic region. The closest gene in this region is MEF2C, which is located about 200 kb downstream. Myocyte enhancer factor 2 (MEF2) family proteins are key transcription factors, consisting of four members MEF2A, MEF2B, MEF2C and MEF2D, controlling gene expression in myocytes, lymphocytes, and neurons. MEF2 also plays an important role in cardiogenesis, epithelial cell survival and maintenance of blood vessel integrity. Knockout of MEF2C gene in mice is embryologically lethal due to failure in cardiac development [42].
We did not find any loci that reached genome-wide significance for retinal arteriolar caliber. It is possible that genetic factors play a smaller role in arteriolar caliber, which is strongly associated with increasing age and blood pressure [5]–[8]. It is also possible that multiple genetic loci determine retinal arteriolar caliber and each locus exerts only a very weak association that is not detectable using our current study sample size. Thus, in order to examine genetic associations with retinal arteriolar caliber more fully, we are currently in the process of building collaborations with several other studies to increase the sample size of the discovery cohort.
While we have identified four loci associated with retinal venular caliber, the identified SNPs may not represent the causal variants but could be in high linkage disequilibrium (LD) with the causal variants, which remain to be discovered. Further fine mapping of this genomic region will be required to facilitate expression and translational studies. Our study suggests that the effect of common genetic variants on retinal vascular caliber is small, and explain only a small proportion of the heritability of these traits [43]. It remains possible that low frequency variants might be important, but GWAS provides poor coverage of rare variants. With the study populations of predominantly Caucasian descent and stringent checks for latent population substructure, the associations are unlikely to be due to population stratification.
To conclude, our population-based GWAS demonstrate four novel loci on chromosomes 19q13 (within the RASIP1 locus), 6q24 (adjacent to the VTA1 and NMBR loci), 12q24 (in the region of the SH2B3, ATXN2 and PTPN11 loci) and 5q14 (adjacent to the MEF2C locus) associated with retinal venular caliber, an endophenotype of the microcirculation associated with clinical cardiovascular disease. Furthermore, locus 12q24 was also associated with coronary heart disease and hypertension. While further studies are needed to determine the causal genetic variants that underlie the heritability of this endophenotype, our findings will help focus research on novel genes and pathways involving the microvasculature and its role in the pathogenesis and development of cardiovascular disease.
Materials and Methods
Ethics statement
Each cohort secured approval from their respective institutional review boards, and all participants provided written informed consent in accordance with the Declaration of Helsinki.
Consortium organization
The CHARGE consortium included large prospective community-based cohort studies that have genome-wide marker data and extensive data on multiple phenotypes [21]. All participating studies approved guidelines for collaboration, and a working group arrived at a consensus on phenotype harmonization, covariate selection and analytic plans for within-study analyses and meta-analyses of results.
Setting
Details of cohort selection, risk factor assessment and retinal vascular caliber measurements in the four studies have been described in Text S1, section 1 [11], [22]–[25]. The AGES is a prospective study with subject recruitment from 2002–2006 of 5,764 surviving members, aged 66 years and older, of the established Reykjavik Study, a cohort of 19,381 participants assembled in 1967 to study cardiovascular disease and its risk factors among those born between 1907 and 1935 [22]. The ARIC study enrolled 15,792 men and women (including 11,478 non-Hispanic whites) from four U.S. communities to investigate the etiology and sequelae of atherosclerosis and cardiovascular risk factors [23]. Participants were between age 45 and 64 years at their baseline examination in 1987–1989. The CHS enrolled 5,888 adults 65 years and older from four field centers to study coronary artery disease and stroke. The baseline examination took place either in 1989–90 or 1992–93 [24]. The Rotterdam Study enrolled 7,983 inhabitants from a district of Rotterdam aged 55 years and older to study cardiovascular, neurological, ophthalmic and endocrine diseases. The baseline examination was in 1990–93 [25].
Study population
The AGES and Rotterdam cohorts consisted predominantly of Caucasian whites. Only non-Hispanic white participants were included from the ARIC and CHS. Retinal photographs were obtained from participants at the third examination in ARIC and the tenth in CHS. Participants were excluded if their photographs could not be graded (due to cataract, corneal opacities or poor focus) or if genotyping data were unavailable (Table 1).
Retinal vascular caliber measurements
Retinal vascular caliber was measured using standardized protocols and software that were developed initially at the University of Wisconsin and used in the ARIC study and the CHS, and following slight modifications, also in the Rotterdam and AGES studies (Text S1, section 2) [4], [5], [9], [11], . In brief, participants underwent retinal photography and optic disc-centered images were used to measure vascular caliber. Pharmacological mydriasis was used in the AGES and Rotterdam studies. For ARIC, CHS and Rotterdam the photographs of one eye were digitized using a high-resolution scanner and for the AGES study, photographs of both eyes were captured digitally. All digital retinal images were analyzed with a semi-automated retinal vessel measurement system and the calibers of all retinal arterioles and venules were measured in an area between half and one disc-diameter from the optic disc margin. The Parr-Hubbard-Knudtson formulae were used to compute summary measures for retinal arteriolar and venular calibers in micrometers (µm) and referred to as the “central retinal arteriolar and venular equivalents” [44]. Quality control (QC) measures for intergrader and intragrader intraclass correlation coefficients for retinal vascular calibers for each of the cohorts ranged from 0.76–0.99 in AGES, 0.69–0.89 in ARIC, 0.67–0.91 in CHS to 0.67–0.95 in the Rotterdam Study [4], .
Genotyping
The consortium was formed after the individual studies had finalized their GWAS platform selection. The four studies included used different platforms: the Affymetrix GeneChip SNP Array 6.0 for the ARIC study, Illumina HumanCNV370-Duo for the AGES study and the CHS and the Illumina Infinium HumanHap550-chip v3.0 for the Rotterdam Study. All studies used their genotype data to impute to the 2.2 million non-monomorphic, autosomal, SNPs identified in HapMap (CEU population). Extensive QC analyses were performed in each cohort (Text S1, sections 3 and 4) [21].
Statistical analyses within discovery cohorts
Based on an a priori analysis plan, each study fitted an additive genetic model with a 1-degree of freedom trend test relating the retinal arteriolar or venular caliber to genotype dosage (0–2 copies of the minor allele) for each SNP, adjusting for age and sex. For the CHS and ARIC studies, the analyses were additionally adjusted for study site. We used linear regression models to calculate regression coefficients (beta) and their standard errors (SE) using the ProbABEL program (http://mga.bionet.nsc.ru/~yurii/ABEL/) in AGES, ARIC and Rotterdam study and the R software in CHS (http://www.r-project.org). Genomic control correction was applied in each study prior to the meta-analysis. To implement genomic control, the λgc value was used to correct the SE as follows: SE_corrected = SE×√λgc. All four cohorts showed low dispersion with inflation factors in the range of 1.030–1.071.
Meta-analysis
We conducted a meta-analysis of the beta estimates obtained from the linear regression models from the four cohorts using an inverse-variance weighting using the R software (MetABEL) (Text S1, section 5) [45]. Strand information was available from all cohorts, facilitating the meta-analysis. After QC, filtering, and imputation within each study, we restricted our meta-analysis to the 2,194,468 autosomal SNPs that were common to all cohorts. We decided a priori on a genome-wide significance threshold of p<5.0×10−8 which corresponds to a p-value of 0.05 with Bonferroni correction for one million independent tests. For 2.2 million tests, it corresponds to an expectation of only 0.11 false positives, regardless of test-dependence [46]. Use of this threshold is also supported by LD patterns observed in deep sequencing work within European populations [47].
Replication analyses
The genome-wide significant SNPs for each locus from the discovery phase were examined in four replication cohorts. The four replication sample sets included 1,709 participants from the Australian Twins Study, 1,132 from the UK Twins Study, 2,501 from the BDES and 1,310 from the BMES. Retinal vascular caliber measurements used the same methodology and formulas as in the CHARGE cohorts. Details of this and the procedures for genotyping are described in the Text S1, sections 1 and 2. In brief, in the Australian Twins Study, genotyping was performed on the Illumina Human Hap610W Quad array. In the UK Twins Study, 56% of the participants were genotyped using the Illumina 317k HumanHap duo array, whereas the remaining 44% using the Illumina HumanHap610Quad array. In the BDES, SNPs were genotyped using TaqMan SNP genotyping assays (Applied Biosystems, CA). Finally, in the BMES genotyping was performed using the Illumina 610K array.
Analyses with cardiovascular diseases
In order to examine the association between SNPs that were successfully replicated in the current study and cardiovascular diseases, we obtained association statistics for each of these SNPs from several GWA studies. We obtained these data from the WTCCC on 2000 cases with coronary artery disease and 3000 controls [3], from HVH Study on 501 cases with stroke [28], 1,172 cases with myocardial infarction and 1,314 controls [29], from Global BPgen on 8,871 cases with hypertension and 9,027 controls [30], and from DIAGRAM+ on 8,130 cases with diabetes mellitus and 38,987 controls [31]. Details of each these studies have been described in the Text S1, section 6.
Supporting Information
Zdroje
1. CaminiPG
CreaF
2007 Coronary microvascular dysfunction. N Engl J Med 356 830 840
2. WatkinsH
FarrallM
2006 Genetic susceptibility to coronary artery disease: from promise to progress. Nat Rev Genet 7 163 173
3. Wellcome Trust Case Control Consortium 2007 Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 661 678
4. HubbardLD
BrothersRJ
KingWN
CleggLX
KleinR
1999 Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 106 2269 2280
5. IkramMK
De JongFJ
VingerlingJR
WittemanJC
HofmanA
2004 Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 45 2129 2134
6. WongTY
MitchellP
2004 Hypertensive retinopathy. N Engl J Med 351 2310 2317
7. WongTY
KleinR
SharrettAR
DuncanBB
CouperDJ
2004 Atherosclerosis Risk in Communities Study. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med 140 248 255
8. WongTY
KleinR
SharrettAR
SchmidtMI
PankowJS
2002 Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA 287 2528 2533
9. QiuC
CotchMF
SigurdssonS
GarciaM
KleinR
2008 Retinal and cerebral microvascular signs and diabetes: the age, gene/environment susceptibility-Reykjavik study. Diabetes 57 1645 1650
10. McGeeshanK
LiewG
MacaskillP
IrwigL
KleinR
2009 Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol 170 1323 1332
11. McGeechanK
LiewG
MacaskillP
IrwigL
KleinR
2009 Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med 151 404 413
12. IkramMK
De JongFJ
Van DijkEJ
PrinsND
HofmanA
2006 Retinal vessel diameters and cerebral small vessel disease: The Rotterdam Scan Study. Brain 129 182 188
13. LongstrethWJr
LarsenEK
KleinR
WongTY
SharrettAR
2007 Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: the Cardiovascular Health Study. Am J Epidemiol 165 78 84
14. JeganathanVS
KawasakiR
WangJJ
AungT
MitchellP
2008 Retinal vascular caliber and age-related macular degeneration: the Singapore Malay Eye Study. Am J Ophthalmol 146 954 959
15. NguyenTT
WangJJ
SharrettAR
IslamFM
KleinR
2008 Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 31 544 549
16. KleinR
KleinBE
MossSE
WongTY
2007 Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology 114 1884 1892
17. XingC
KleinBE
KleinR
JunG
LeeKE
2006 Genome-wide linkage study of retinal vessel diameters in the Beaver Dam Eye Study. Hypertension 47 797 802
18. WangJJ
WongTY
2006 Genetic determinants of retinal vascular caliber: additional insights into hypertension pathogenesis. Hypertension 47 644 645
19. LiewG
ShankarA
WangJJ
KleinR
BrayMS
2007 Apolipoprotein E gene polymorphisms and retinal vascular signs: the atherosclerosis risk in communities (ARIC) study. Arch Ophthalmol 125 813 818
20. De JongFJ
IkramMK
DesprietDD
UitterlindenAG
HofmanA
2007 Complement factor h polymorphism, inflammatory mediators, and retinal vessel diameters: the rotterdam study. Invest Ophthalmol Vis Sci 48 3014 3018
21. PsatyBM
O'DonnellCJ
GudnasonV
LunettaKL
FolsomAR
2009 Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from five cohorts. Circ Cardiovasc Genet 2 73 80
22. HarrisTB
LaunerLJ
EiriksdottirG
KjartanssonO
JonssonPV
2007 Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 165 1076 1087
23. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives 1989 The ARIC investigators. Am J Epidemiol 129 687 702
24. FriedLP
BorhaniNO
EnrightP
FurbergCD
GardinJM
1991 The Cardiovascular Health study: design and rationale. Ann Epidemiol 1 263 276
25. HofmanA
BretelerMM
van DuijnCM
JanssenHL
KrestinGP
2009 The Rotterdam Study: 2010 objectives and design update. Eur J Epidemiol 24 553 572
26. MackeyDA
MackinnonJR
BrownSA
KearnsLS
RuddleJB
2009 Twins Eye Study in Tasmania (TEST): Rationale and Methodology to Recruit and Examine Twins. Twin Res Hum Genet 12 441 454
27. HammondCJ
SniederH
SpectorTD
GilbertCE
2000 Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med 342 1786 1790
28. PsatyBM
HeckbertSR
KoepsellTD
SiscovickDS
RaghunathanTE
1995 The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA 274 620 625
29. KlungelOH
HeckbertSR
LongstrethWTJr
FurbergCD
KaplanRC
2001 Antihypertensive drug therapies and the risk of ischemic stroke. Arch Intern Med 161 37 43
30. Newton-ChehC
JohnsonT
GatevaV
TobinMD
BochudM
2009 Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 41 666 676
31. VoightBF
ScottLJ
SteinthorsdottirV
MorrisAP
DinaC
2010 Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 42 579 589
32. TamaiK
MatsubaraA
TomidaK
MatsudaY
MoritaH
2002 Lipid hydroperoxide stimulates leukocyte-endothelium interaction in the retinal microcirculation. Exp Eye Res 75 69 75
33. ChesterAH
BorlandJA
ButteryLD
MitchellJA
CunninghamDA
1998 Induction of nitric oxide synthase in human vascular smooth muscle: interactions between proinflammatory cytokines. Cardiovasc Res 38 814 821
34. WongTY
IslamFM
KleinR
KleinBE
CotchMF
2006 Retinal vascular caliber, cardiovascular risk factors and inflammation: the Multi-Ethnic Study of Atherosclerosis. Invest Ophthalmol Vis Sci 47 2341 2350
35. XuK
ChongDC
RankinSA
ZornAM
CleaverO
2009 RASIP1 is required for endothelial cell motility, angiogenesis and vessel formation. Dev Biol 329 269 279
36. XiaoJ
XiaH
ZhouJ
AzmiIF
DaviesBA
2008 Structural Basis of Vta1 Function in the Multivesicular Body Sorting Pathway. Dev Cell 14 37 49
37. MatusiakD
GloverS
NathanielR
MatkowskyjK
YangJ
2005 Neuromedin B and its receptor are mitogens in both normal and malignant epithelial cells lining the colon. Am J Physiol Gastrointest Liver Physiol 288 718 728
38. SmythDJ
PlagnolV
WalkerNM
CooperJD
DownesK
2008 Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med 359 2767 2777
39. SoranzoN
SpectorTD
ManginoM
KühnelB
RendonA
2009 A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat Genet 41 1182 1190
40. GaneshSK
ZakaiNA
Van RooijFJ
SoranzoN
SmithAV
2009 Multiple loci influence erythrocyte phenotypes in the CHARGE consortium. Nat Genet 41 1191 1198
41. LevyD
EhretGB
RiceK
VerwoertGC
LaunerLJ
2009 Genome-wide association study of blood pressure and hypertension. Nat Genet in press
42. MaK
ChanJK
ZhuG
WuZ
2005 Myocyte enhancer factor 2 acetylation by p300 enhances its DNA binding activity, transcriptional activity, and myogenic differentiation. Mol Cell Biol 25 3575 3582
43. ManolioTA
CollinsFS
CoxNJ
GoldsteinDB
HindorffLA
2009 Finding the missing heritability of complex diseases. Nature 461 747 753
44. KnudtsonMD
LeeKE
HubbardLD
WongTY
KleinR
2003 Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 27 143 149
45. SchlesselmanJJ
1997 Risk of endometrial cancer in relation to use of combined oral contra-ceptives: a practitioner's guide to meta-analysis. Human Reprod 12 1851 1863
46. GordonA
GlazkoG
QiuX
YakovlevA
2007 Control of the mean number of false discoveries, Bonferroni and stability of multiple testing. Ann Appl Statistics 1 179 190
47. HoggartCJ
ClarkTG
De IorioM
WhittakerJC
BaldingDJ
2008 Genome-wide significance for dense SNP and resequencing data. Genet Epidemiol 32 179 185
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



