-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaUpsetting the Dogma: Germline Selection in Human Males
article has not abstract
Published in the journal: . PLoS Genet 8(2): e32767. doi:10.1371/journal.pgen.1002535
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002535Summary
article has not abstract
As early as 1912, Wilhelm Weinberg, the visionary human geneticist, noted that infants with achondroplasia (short-limbed dwarfism) tended to be born late in their sibship [1]. From this he made the astonishing intellectual leap to the conclusion that this might signal a mutational origin for these infants' condition. This was an amazing insight considering the limited knowledge of mutation at that time. By the year 2000, the big picture seemed clear [2]: it was known that there are many more pre-meiotic cell divisions in the ancestry of a sperm than of an egg, and this seemed like a sufficient explanation for the much higher male than female mutation rate.
Yet there were exceptions. Some chromosome changes, including small duplications and deletions, seemed to have different rules of inheritance. And there were a few conditions, notably those associated with the genes FGFR2, FGFR3, and RET, that were more extreme: the new mutations were almost exclusively in males. Furthermore, there was a large increase in mutations with paternal age. It appeared as if these three loci, and very likely others, were marching to a different drummer [2].
The first major breakthrough came from the work of Andrew Wilkie, Anne Goriely, and their colleagues [3]. They studied FGFR2, which mutates to cause Apert syndrome. Using an enzyme that cuts the normal but not the mutant DNA at the relevant site, they identified FGFR2 mutations in sperm from normal males. The overall mutation rate, as inferred from sperm studies, agreed with the incidence data for Apert syndrome. But the distribution of mutants was quite different, somewhat resembling a Delbruck–Luria jackpot. Delbruck and Luria studied mutations that occur in a growing culture of bacterial cells: if a mutation occurs in a multiplying colony, the mutation is multiplied, leading to a cluster of mutations, or jackpot (the size of the jackpot depends on the number of cell divisions since the mutation occurred).
Definitive proof came with a study of the location of the mutants on one or the other of the two members of a chromosome pair, identified by marker genes. Rather than a binomial distribution, these showed a large excess of identical alleles. The authors inferred that there must be some sort of pre-meiotic selection favoring mutations. This was a remarkable result, considering the rarity of such a process in various species and the prevailing dogma that no such thing occurs in mammals. Such selection immediately supplied an explanation of the high “mutation rate” and the paternal age effect.
An attractive idea for the nature of the selection is that among the asymmetrical spermatogonial divisions, producing one daughter cell like the parent and one that develops into a sperm cell, occasional symmetrical divisions (two daughter cells like the parent) occur (Figure 1). These, of course, confer a large selective advantage by producing twice as many cell descendants. Arnheim and his colleagues attacked the problem head-on, studying the mutation underlying Apert syndrome [4] and, in this issue of PLoS Genetics, the mutation involved in multiple endocrine neoplasia type 2B (MEN2B) [5]. In the current study, they divided several normal testes into 192 segments each. The striking result was that an individual segment usually had no or only a few mutations among normal sperm, but that an occasional segment had a very large number. Thus the mutations occur in clusters, precisely as a selection hypothesis would predict. The number of clusters increases with age. By fitting adjustable parameters to the data, Arnheim and his associates found that a one percent probability of a symmetrical division best fits the data. This adds very strong support to the idea that “selection” is nothing more than symmetrical division producing two daughter cells instead of one. This explains not only the high mutation rate, but the strong paternal age effect. Other less appealing mechanisms are not ruled out, however. At first the result for MEN2B seemed erratic for the very old men, but a correction for age-related cell death was sufficient to remove the discrepancy.
Fig. 1. Symmetrical (A) and asymmetrical (B) divisions. 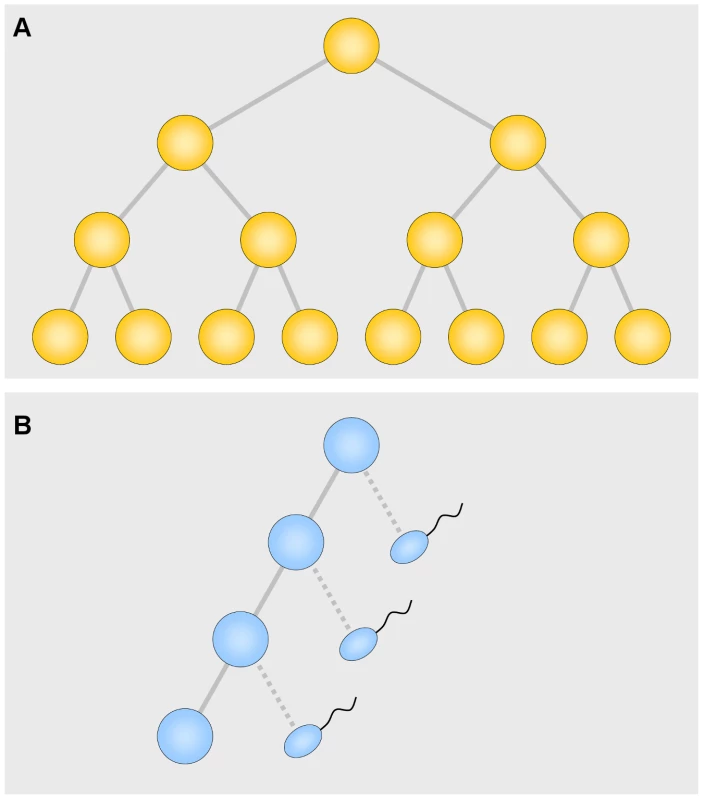
In the asymmetrical divisions each cell produces one daughter like itself and one that, after 6 divisions, develops into a sperm cell. Since there are many more asymmetrical divisions, especially in older men, most of the mutations occur during the period of asymmetrical divisions. This beautiful result immediately leads to several questions. How many more loci are there that use this device? There are a number of examples in biology of easy transition between symmetrical and asymmetrical division. Why are examples, especially in higher vertebrates, so rare? The symmetrical type may cause a harmful effect. If the zygotic property of a gametically favored trait is harmful, the harmful effect may well prevail. As Haldane once said: “Clearly a higher plant species is at the mercy of its pollen grains” [6]. Even a small difference in pollen tube growth, or in our example, a small difference in number of symmetrical divisions, may cause the trait to prevail, to the detriment of the species. If the process were frequent it could be devastating. So nature must have invented mechanisms to reduce the frequency of such a change.
The MEN2B system offers a promising way to approach this and other equally interesting problems because so much is known in the mouse: for example RET, the gene mutated in MEN2B, is necessary for spermatogonial self-renewal. The materials and technique are ready. We can look forward to much deeper biochemical and cytological knowledge of spermatogenesis and the ways in which it can be modified.
Asymmetrical division is one of nature's cleverest devices. In a human male it yields a constant daily supply of sperm. Yet mutation to symmetry occurs in a number of biological systems. The process of asymmetrical division is in constant danger of sabotage by mutants waiting to beat it.
Zdroje
1. WeinbergW 1912 Zur vererbung des zwergwuchses. Arch Rassen-u Gesell Biolog 9 710 718
2. CrowJF 2000 The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet 1 40 47
3. GorielyAMcVeanGATRöjmyrMIngemarssonBWilkieAOM 2003 Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science 301 643 646
4. ChoiS-KYoonS-RCalabresePArnheimN 2008 A germ-line-selective advantage rather than an increased mutation rate can explain some unexpectedly common human disease mutations. Proc Nat Acad Sci USA 105 10143 10148
5. ChoiS-KYoonS-RCalabresePArnheimN 2012 Positive selection for new disease mutations in the human germline: evidence from the heritable cancer syndrome multiple endocrine neoplasia type 2B. PLoS Genet 8 e1002420 doi:10.1371/journal.pgen.1002420
6. HaldaneJBS 1931 The causes of evolution New York Harper and Brothers 123
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 2
-
Všechny články tohoto čísla
- Upsetting the Dogma: Germline Selection in Human Males
- A Strong Deletion Bias in Nonallelic Gene Conversion
- Positive Selection for New Disease Mutations in the Human Germline: Evidence from the Heritable Cancer Syndrome Multiple Endocrine Neoplasia Type 2B
- Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
- Mixed Effects Modeling of Proliferation Rates in Cell-Based Models: Consequence for Pharmacogenomics and Cancer
- Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome
- Genome-Wide Association Study Identifies Chromosome 10q24.32 Variants Associated with Arsenic Metabolism and Toxicity Phenotypes in Bangladesh
- Structural Basis of Transcriptional Gene Silencing Mediated by MOM1
- Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour
- Genome-Wide Association Study Identifies Novel Loci Associated with Circulating Phospho- and Sphingolipid Concentrations
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- The Origin and Nature of Tightly Clustered Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution
- Ultrafast Evolution and Loss of CRISPRs Following a Host Shift in a Novel Wildlife Pathogen,
- Phosphorylation of Chromosome Core Components May Serve as Axis Marks for the Status of Chromosomal Events during Mammalian Meiosis
- Psoriasis Patients Are Enriched for Genetic Variants That Protect against HIV-1 Disease
- A Pathogenic Mechanism in Huntington's Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity
- The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
- Down-Regulating Sphingolipid Synthesis Increases Yeast Lifespan
- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Loss of Tgif Function Causes Holoprosencephaly by Disrupting the Shh Signaling Pathway
- Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability
- Discovery of a Modified Tetrapolar Sexual Cycle in and the Evolution of in the Species Complex
- The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
- A Regulatory Network for Coordinated Flower Maturation
- Coexpression Network Analysis in Abdominal and Gluteal Adipose Tissue Reveals Regulatory Genetic Loci for Metabolic Syndrome and Related Phenotypes
- Diced Triplets Expose Neurons to RISC
- The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
- The Empirical Power of Rare Variant Association Methods: Results from Sanger Sequencing in 1,998 Individuals
- Systematic Detection of Epistatic Interactions Based on Allele Pair Frequencies
- Familial Identification: Population Structure and Relationship Distinguishability
- Raf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
- Loss of Function of the Cik1/Kar3 Motor Complex Results in Chromosomes with Syntelic Attachment That Are Sensed by the Tension Checkpoint
- Computational Prediction and Molecular Characterization of an Oomycete Effector and the Cognate Resistance Gene
- The Dynamics and Prognostic Potential of DNA Methylation Changes at Stem Cell Gene Loci in Women's Cancer
- GTPase Activity and Neuronal Toxicity of Parkinson's Disease–Associated LRRK2 Is Regulated by ArfGAP1
- Evaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
- Neurophysiological Defects and Neuronal Gene Deregulation in Mutants
- Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
- Negative Supercoiling Creates Single-Stranded Patches of DNA That Are Substrates for AID–Mediated Mutagenesis
- Rewiring of PDZ Domain-Ligand Interaction Network Contributed to Eukaryotic Evolution
- The Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance
- Repression of a Potassium Channel by Nuclear Hormone Receptor and TGF-β Signaling Modulates Insulin Signaling in
- The Retrohoming of Linear Group II Intron RNAs in Occurs by Both DNA Ligase 4–Dependent and –Independent Mechanisms
- Cell Lineage Analysis of the Mammalian Female Germline
- Association of a Functional Variant in the Wnt Co-Receptor with Early Onset Ileal Crohn's Disease
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



