-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaGenetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
Weight-loss interventions generally improve lipid profiles and reduce cardiovascular disease risk, but effects are variable and may depend on genetic factors. We performed a genetic association analysis of data from 2,993 participants in the Diabetes Prevention Program to test the hypotheses that a genetic risk score (GRS) based on deleterious alleles at 32 lipid-associated single-nucleotide polymorphisms modifies the effects of lifestyle and/or metformin interventions on lipid levels and nuclear magnetic resonance (NMR) lipoprotein subfraction size and number. Twenty-three loci previously associated with fasting LDL-C, HDL-C, or triglycerides replicated (P = 0.04–1×10−17). Except for total HDL particles (r = −0.03, P = 0.26), all components of the lipid profile correlated with the GRS (partial |r| = 0.07–0.17, P = 5×10−5–1×10−19). The GRS was associated with higher baseline-adjusted 1-year LDL cholesterol levels (β = +0.87, SEE±0.22 mg/dl/allele, P = 8×10−5, Pinteraction = 0.02) in the lifestyle intervention group, but not in the placebo (β = +0.20, SEE±0.22 mg/dl/allele, P = 0.35) or metformin (β = −0.03, SEE±0.22 mg/dl/allele, P = 0.90; Pinteraction = 0.64) groups. Similarly, a higher GRS predicted a greater number of baseline-adjusted small LDL particles at 1 year in the lifestyle intervention arm (β = +0.30, SEE±0.012 ln nmol/L/allele, P = 0.01, Pinteraction = 0.01) but not in the placebo (β = −0.002, SEE±0.008 ln nmol/L/allele, P = 0.74) or metformin (β = +0.013, SEE±0.008 nmol/L/allele, P = 0.12; Pinteraction = 0.24) groups. Our findings suggest that a high genetic burden confers an adverse lipid profile and predicts attenuated response in LDL-C levels and small LDL particle number to dietary and physical activity interventions aimed at weight loss.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002895
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002895Summary
Weight-loss interventions generally improve lipid profiles and reduce cardiovascular disease risk, but effects are variable and may depend on genetic factors. We performed a genetic association analysis of data from 2,993 participants in the Diabetes Prevention Program to test the hypotheses that a genetic risk score (GRS) based on deleterious alleles at 32 lipid-associated single-nucleotide polymorphisms modifies the effects of lifestyle and/or metformin interventions on lipid levels and nuclear magnetic resonance (NMR) lipoprotein subfraction size and number. Twenty-three loci previously associated with fasting LDL-C, HDL-C, or triglycerides replicated (P = 0.04–1×10−17). Except for total HDL particles (r = −0.03, P = 0.26), all components of the lipid profile correlated with the GRS (partial |r| = 0.07–0.17, P = 5×10−5–1×10−19). The GRS was associated with higher baseline-adjusted 1-year LDL cholesterol levels (β = +0.87, SEE±0.22 mg/dl/allele, P = 8×10−5, Pinteraction = 0.02) in the lifestyle intervention group, but not in the placebo (β = +0.20, SEE±0.22 mg/dl/allele, P = 0.35) or metformin (β = −0.03, SEE±0.22 mg/dl/allele, P = 0.90; Pinteraction = 0.64) groups. Similarly, a higher GRS predicted a greater number of baseline-adjusted small LDL particles at 1 year in the lifestyle intervention arm (β = +0.30, SEE±0.012 ln nmol/L/allele, P = 0.01, Pinteraction = 0.01) but not in the placebo (β = −0.002, SEE±0.008 ln nmol/L/allele, P = 0.74) or metformin (β = +0.013, SEE±0.008 nmol/L/allele, P = 0.12; Pinteraction = 0.24) groups. Our findings suggest that a high genetic burden confers an adverse lipid profile and predicts attenuated response in LDL-C levels and small LDL particle number to dietary and physical activity interventions aimed at weight loss.
Introduction
Dyslipidemia is a strong risk factor for atherosclerotic heart disease [1]–[3], has a well-defined genetic basis [4], and is modifiable through therapeutic lifestyle changes and weight-loss interventions [5], [6]. Individuals at risk for diabetes are also at high risk of cardiovascular disease [7], and individualized lifestyle intervention programs, like the one incorporated into the Diabetes Prevention Program (DPP), have a salutary effect on dyslipidemia and cardiovascular disease risk in this population. However, the cost of widespread implementation of such interventions has been highlighted as a major limitation [8] and not all benefit equally from such interventions. Identifying persons most likely to benefit from intensive lifestyle modification could provide justification for targeting this subpopulation first, making the clinical translation of findings from studies such as the DPP more feasible.
Selection of persons whose dyslipidemia is likely to respond well to lifestyle interventions or pharmacotherapy could help target resources and optimize prevention strategies. To do so requires knowledge of the underlying risk factors for the trait and knowledge of how personal characteristics interact with exercise, diet, and weight loss. Although the heritability of polygenic dyslipidemia [9]–[11] and its sequelae [12] have been elucidated, little is known of how lifestyle interventions modify the effects of these loci, singly or in combination, on lipid profiles. Thus, learning how a person's genetic background modulates his or her response to therapeutic lifestyle changes and weight-loss interventions might help optimize the targeting of interventions designed to mitigate cardiovascular and metabolic disease risk.
The purpose of this study was to examine whether loci reliably associated with polygenic dyslipidemia modified the response to cardio-protective interventions in the DPP, a randomized clinical trial of intensive lifestyle modification, metformin treatment, or placebo with standard care. We hypothesized i) that the baseline lipid profiles of DPP participants would be associated with gene variants known to associate with polygenic dyslipidemia and ii) that improvement in lipidemia following treatment would depend on these same genetic variants. We also used NMR spectroscopy to characterize the associations of these previously reported loci with lipoprotein subfractions.
Results
Table 1 shows participant characteristics stratified by DPP treatment arm. The effects of the DPP interventions on 1 yr changes in weight [14], insulin secretion [13], beta-cell function [13], and lipid traits [29] are reported in detail elsewhere.
Tab. 1. Baseline Characteristics of the Study Population by Treatment Group [Quantitative Traits Are Shown as Median (Interquartile Range)]. ![Baseline Characteristics of the Study Population by Treatment Group [Quantitative Traits Are Shown as Median (Interquartile Range)].](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/8e0129764303914d0e98746173edb9df.png)
Individual SNP Replication
Thirty-two SNPs previously associated with triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C) and/or high-density lipoprotein-cholesterol (HDL-C) levels were considered [10]. Thirty-one of these were successfully genotyped in the DPP, and two SNPs in CETP, serving as HapMap proxies (r2≥0.90) for rs173539, including rs247616, were subsequently successfully genotyped, with rs247616 retained as the replacement for rs173539. Twenty-three of these 32 non-redundant SNPs replicated with their respective traits in a directionally consistent manner (P≤0.05), including 8/11 for TG, 9/14 for HDL-C and 8/11 for LDL-C. Two of the SNPs, rs12678919 and rs964184, replicated for both HDL-C and TG (Table S1).
Association of Individual SNPs with All Four Lipid Traits and Ten Lipoprotein Traits
Additionally, we evaluated the associations of the 32 lipid loci with baseline lipids and nuclear magnetic resonance (NMR)-derived lipoprotein traits (Large HDL particles, Small HDL particles, Total HDL particles, HDL size, LDL size, Total LDL particles, Small LDL particles, Total VLDL particles, Large VLDL particles, VLDL size). Of all analyses of baseline traits, roughly one third of the tests were nominally significant associations, and 35 associations were significant after correcting for all 448 hypothesis tests; these involved 12 SNPs and 13 traits (Table S2). Interestingly, SNP rs10401969 did not replicate for LDL-C (C vs. T: β±SEM = −0.1±1.6 mg/dl, additive P = 0.94), but was associated with decreased large VLDL (mean 5.43, 4.26, 4.07 nmol/L for TT, TC, CC genotypes respectively, additive P = 4×10−5) and smaller VLDL size (53.18, 50.94, 49.55 nm, P = 2×10−6). SNP rs7679 did not quite reach nominal significance for decreased HDL-C (C vs. T: β ±SEM = −0.016±0.009 ln mg/dl, additive P = 0.07), but was very strongly associated with increased small HDL particle number (17.93, 20.14 and 21.89 µmol/L for TT, CT and CC genotypes respectively; additive P = 4×10−18) and consequently total HDL particle number (34.07, 35.10 and 36.78 µmol/L, P = 2×10−5).
Association of Genetic Risk Score (GRS) with Baseline Lipid and Lipoprotein Traits
A lipid GRS was calculated for each individual by first replacing missing genotypes with ethnicity-specific imputed means and then adding up the number of risk alleles possessed for each of the 32 independent SNPs. Of the 32 SNPs evaluated, 11 were originally associated in the meta-analysis with LDL cholesterol, 10 with HDL cholesterol only, seven with triglycerides only, and four with both HDL cholesterol and triglycerides. A risk allele was defined as one associated with increased TG or LDL-C or decreased HDL in the original meta-analysis [10]. After adjustment for age, sex, ethnicity, and BMI, the GRS was significantly associated with all baseline traits evaluated except total HDL particles (P = 0.26, Table 2). The following are P-values for the effects of the GRS, as a quantitative covariate, and geometric means for the upper and lower ethnicity-specific GRS quartiles for each trait. A higher GRS was associated with elevated baseline levels of: total cholesterol (P = ×410−11, 206 vs. 195 mg/dl), LDL-C (P = ×910−8;, 129 vs. 121 mg/dl arithmetic means), TG (P = ×410−19, 160 vs. 127 mg/dl), total VLDL particles (P = ×610−14, 67 vs. 53 nmol/L), large VLDL particles (P = ×110−14, 6.57 vs. 4.21 nmol/L), total LDL particles (P = ×210−10, 1412 vs. 1262 nmol/L), small LDL particles (P = ×210−11, 743 vs. 543 nmol/L), small HDL particles (P = µ0.0005, 19.17 vs. 18.10 mol/L), and VLDL particle size (P = ×110−5, 53.86 vs. 51.84 nm). A higher GRS was also associated with lower baseline levels of: HDL-C (P = ×110−15, 43 vs. 47 mg/dl), LDL particle size (P = ×110−19, 0.256 vs. 0.269 nm), large HDL particles (P = ×210−8µ, 2.98 vs. 3.68 mol/L), and HDL particle size (P = 0.0003, 8.82 vs. 8.90 nm). All of these results are consistent with a greater number of risk alleles increasing the atherogenicity of the lipoprotein profile.
Tab. 2. Association of 32-SNP GRS with Baseline Lipid and Lipoprotein Traits (n2,843). 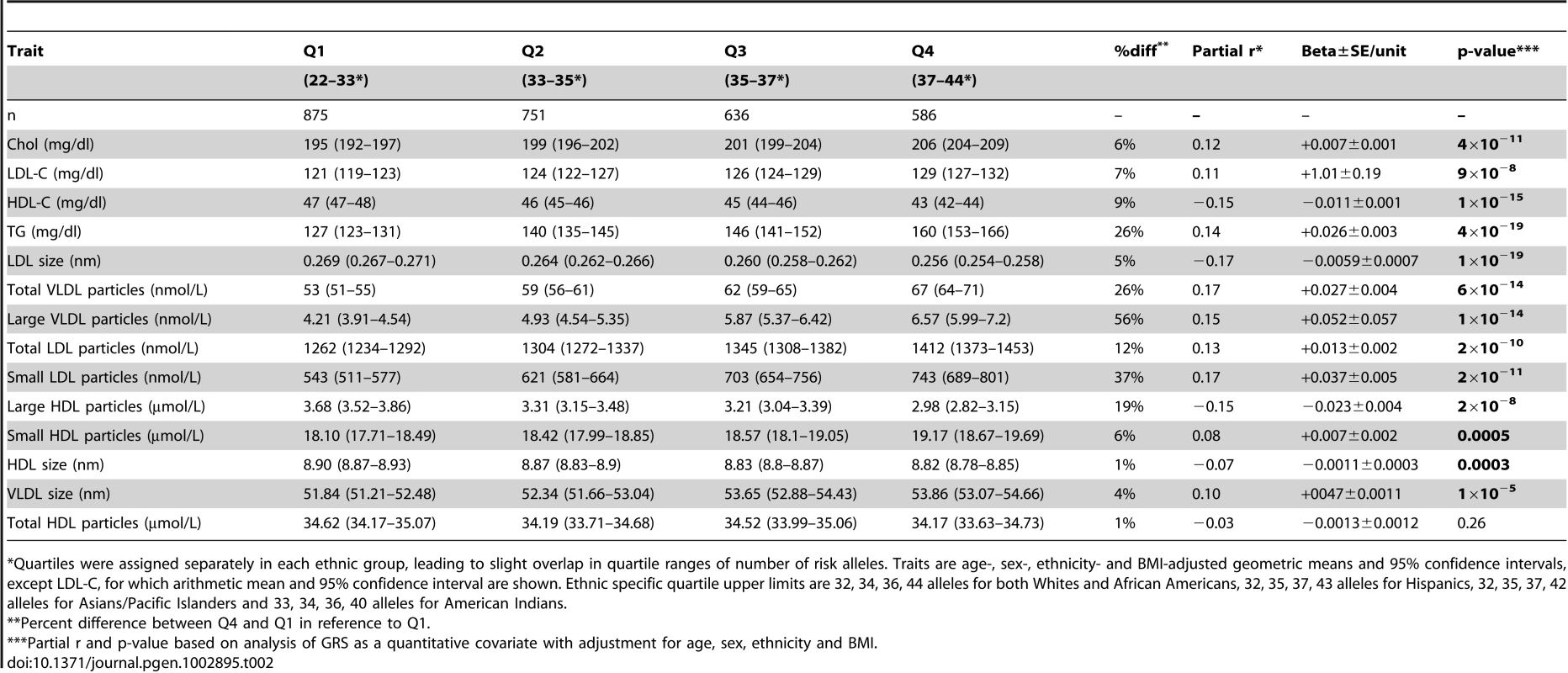
%%Quartiles were assigned separately in each ethnic group, leading to slight overlap in quartile ranges of number of risk alleles. Traits are age-, sex-, ethnicity- and BMI-adjusted geometric means and 95 confidence intervals, except LDL-C, for which arithmetic mean and 95 confidence interval are shown. Ethnic specific quartile upper limits are 32, 34, 36, 44 alleles for both Whites and African Americans, 32, 35, 37, 43 alleles for Hispanics, 32, 35, 37, 42 alleles for Asians/Pacific Islanders and 33, 34, 36, 40 alleles for American Indians. GRS×Intervention Interactions of Baseline-Adjusted One-Year Traits
Two traits showed evidence of GRS×lifestyle interaction: LDL-C (P = 0.02) and small LDL particles (P = 0.01, Table 3; Figure 1; Figure S1a–S1f). For these two traits, there was a residual detrimental impact of GRS in the lifestyle (i.e., the GRS was associated with higher levels at one year even after adjusting for baseline levels) but not the metformin or placebo group, suggesting that the lifestyle intervention was less effective at lipid-lowering in those with a higher genetic burden. A unit (allele) GRS increase was associated with higher residual LDL-C levels in the lifestyle group (β+±0.087, SEE0.022 mg/dl, P = ×810−5) but not in the metformin (β−±0.03, SEE0.22 mg/dl, P = 0.90) or placebo (β+±0.20, SEE0.22 mg/dl, P = 0.35) groups (Figure 1). Similarly, the GRS was associated with higher residual ln-small LDL particles in the lifestyle group (β+±0.030, SEE0.0.012 ln nmol/L, P = 0.01), but not in the metformin (β−±0.013, SEE0.008 ln nm/L, P = 0.12) or placebo (β−±0.002, SEE0.008, P = 0.74) groups (Figure 1×). There were no metforminGRS interactions significant at the P = ×0.05 level. In addition to the three traits discussed, several traits showed residual detrimental effects of the GRS in one or more strata (total cholesterol, TG, LDL size, total VLDL particles, large HDL particles, and HDL size) without any statistical evidence of treatmentGRS interaction (Table 3).
Fig. 1. LDL-C levels. 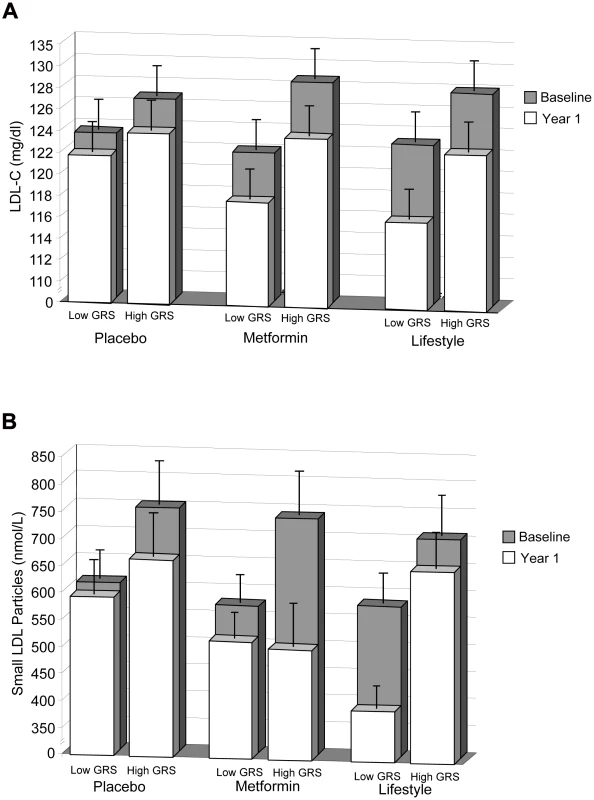
LDL-C levels at baseline and 1 year (A) and small LDL particle levels at baseline and 1 year (B) stratified by treatment group and lipid GRS. Each column shows ethnicity-adjusted arithmetic (for LDL-C) or geometric (for small LDL particles) means (with upper 95 confidence), stratified above and below (less than or equal to) the ethnic-specific median GRS value. Ethnic-specific median GRS values are 34 alleles for Caucasian, African American and American Indian ethnicities and 35 for Hispanic and Asian/Pacific Islander ethnicities. Tab. 3. Association of 32-SNP GRS with Baseline-Adjusted One-Year Lipid and Lipoprotein Traits (n2,686). 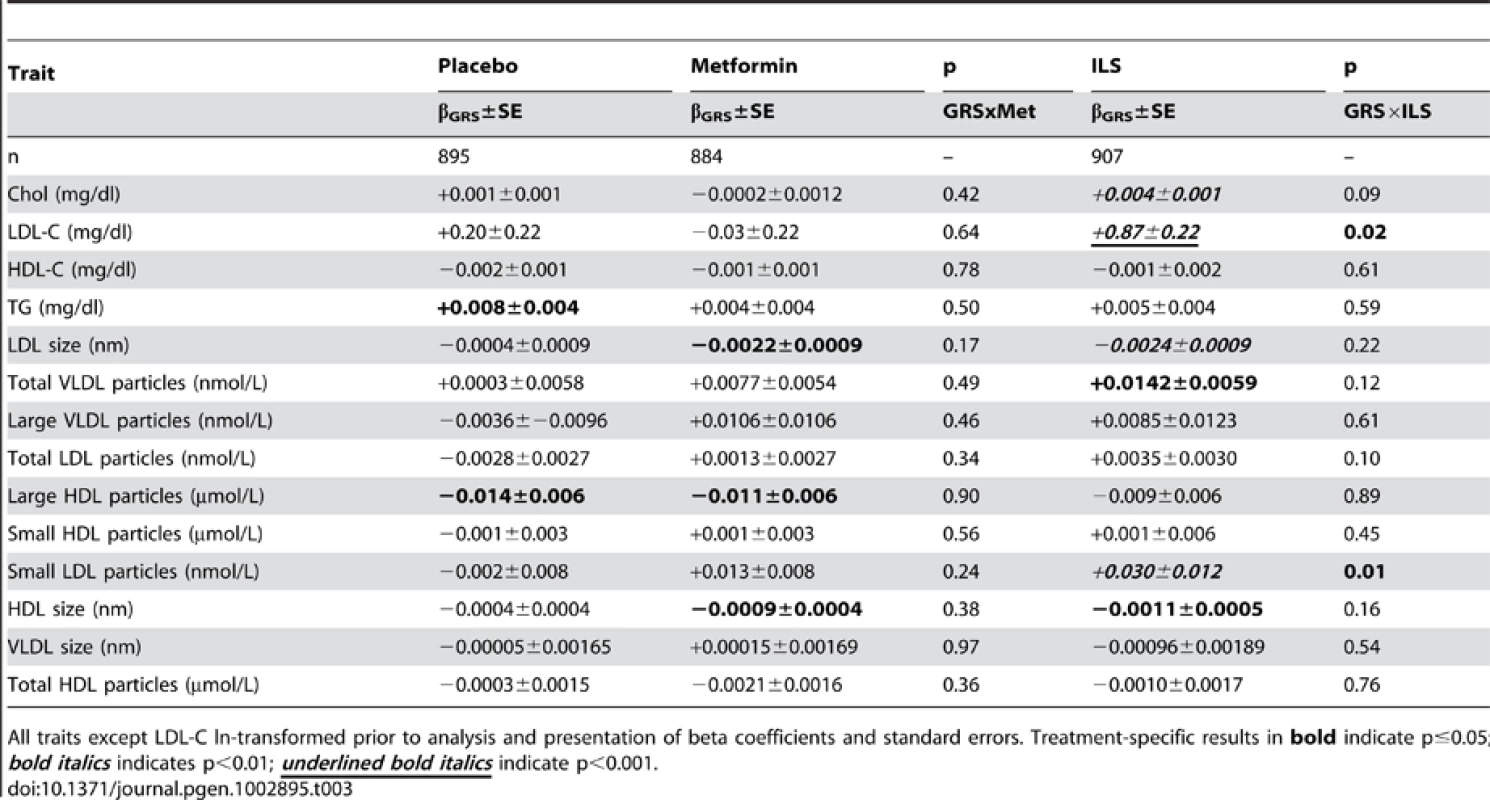
All traits except LDL-C ln-transformed prior to analysis and presentation of beta coefficients and standard errors. Treatment-specific results in bold≤; indicate p0.05 bold italics<; indicates p0.01 underlined bold italics< indicate p0.001. Discussion
In the present study, the majority of previously associated SNPs replicated for baseline lipid traits, and there was a statistically significant relationship between the GRS and the vast majority of standard lipid traits and NMR lipoprotein subfractions. Importantly, in several cases the evidence for association with NMR subfractions was much stronger than for the original standard lipid trait, which may be owing to the relative proximity of the subfractions to the genetic loci. For example, SNP rs7679, originally associated with total HDLC levels in previous GWASs, in the DPP was not significantly associated with HDL-C (P = 0.07) but was strongly associated with small HDL particle levels (P = ×410−18). This SNP is near PLTP, encoding phospholipid transfer protein, a molecule directly influencing HDL particle size [14]. Such findings extend our understanding of lipid biology and suggest that, compared to standard lipid levels, measurements of lipoprotein subfractions may provide a more effective way of capturing genetically influenced risk. This is particularly important, as recent studies have shown that HDL-C is a heterogeneous trait, which in the context of clinical use may benefit from sub-stratification by genotype [15].
We also observed that within the lifestyle group but not the placebo or metformin groups, the GRS was associated with higher LDL-C and small LDL particle levels after one year of intervention. These findings suggest that the genetic burden on these traits cannot be completely overcome by lifestyle modification. However, even those with the greatest genetic burden benefit to a limited extent from lifestyle intervention in terms of LDL-C reduction (Figure 1A), although the effect on LDL particle size reduction is almost completely ablated (Figure 1B'). Even a true residual effect of lifestyle on LDL-C in people with the highest GRS does not negate the clinical relevance of our findings in terms of potential to facilitate tailored treatment decisions. Seeing less of an effect of lifestyle in a particular patient subgroup indicates that these persons may benefit from more frequent surveillance, more intense lifestyle interventions, or aggressive pharmaceutical interventions to supplement lifestyle interventions. Conversely, knowing that lifestyle intervention is likely to be adequate in persons with the lowest genetic burden may maximize the patients diet adherence and potentially reduce the costs and side effects associated with prescribing lipid-lowering medications unnecessarily. The availability of information on genetic background may also facilitate patient-provider dialogue, owing to improved diagnostic accuracy. This is similar to the strategy used to control cholesterol levels in patients with a monogenic disorder such as familial hypercholesterolemia due to a severe loss of function mutation in LDLR, where lifestyle intervention combined with pharmacotherapy is needed to bring LDL-C levels within an acceptable range (Third Report of the NCEP-ATP III on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults).
×It is also important to bear in mind that the interaction effects may be underestimated in our paper. This is because the majority of SNPs included in the GRS are likely to be imperfect proxies for unobserved functional variants, resulting in some degree of genotype misclassification. Moreover, all 32 SNPs were included within the GRS, even though not all SNPs convey statistically significant effects in the DPP and do not individually modify the effects of the interventions. A parsimonious GRS including only those SNPs that are statistically significant in the DPP would likely be overfitted to our data, resulting in biased conclusions about the strength and magnitude of genetreatment interactions.
Dyslipidemia is a long-established risk factor for CVD [1]–[3]. Thus, the primary and secondary prevention of atherosclerotic CVD often involves intervening on lipid levels [16]. Lifestyle interventions [17] and metformin treatment [18]; that result in weight loss have the potential to improve lipid profiles nevertheless, as long recognized [19], changes in lipid profiles following interventions vary greatly from one person to the next. Some of the variability in response to interventions may be because genotypes modulate the effects of preventive interventions on lipid homeostasis and CVD risk [20].
Of the many known dyslipidemia-predisposing loci discovered so far [10]×, only a handful have been the focus of studies testing hypotheses of genetreatment interactions [21]–[28], and most of these studies are small (N<150), non-randomized trials of dietary intervention. Although some of these studies have focused on genomic regions that are confirmed to harbor dyslipidemia-predisposing loci, such as APOB, CETP, LIPC and LPL [21], [22], [24]–[28], no exhaustive studies testing whether GWAS-discovered loci [10], [29], [30] modify response to treatments have been previously reported.
The GRS used in this study attenuated the impact of the DPP lifestyle intervention on LDL levels and small LDL particle number. This suggests that a genetic predisposition to high LDL levels and more small LDL particles is difficult to overcome through lifestyle intervention alone. These data also unmask the effects of an underlying genetic defect of LDL levels and small LDL particles found in individuals with a high genetic burden, which becomes visible when adiposity and blood TG content are reduced through lifestyle intervention. This information may justify the combination of lifestyle and lipid lowering drug treatment from the outset in these individuals, rather than the usual approach of stepping from lifestyle to drug therapy when the former fails.
;The DPP lifestyle intervention prioritized weight loss, daily fat gram intake and physical activity goals over intake of saturated fat, cholesterol, viscous fiber and plant stanols/sterols this may have influenced the nature of the changes in the lipid profile. When compared to the metformin and placebo groups, the lifestyle intervention group reported improved physical activity levels and reductions in calorie intake, resulting in significantly greater weight losses [31], each of which has major influences on TG levels. The lifestyle intervention group reported significantly greater reductions in percent calories from total fat and saturated fat than the metformin and placebo groups [31]. However, they did not, on average, achieve the National Cholesterol Education Program target for saturated fat intake and did not focus on the other therapeutic lifestyle changes, such as the additional dietary changes mentioned above, that often have the largest effects on LDL concentrations. The ethnic diversity of the DPP cohort facilitates the generalizability of results, but may also lead to confounding by population stratification in genetic analyses. However, Sensitivity analyses in the European White sub-cohort of the DPP yielded comparable effect estimates to the results obtained in the entire DPP genetics cohort (results for baseline traits shown in Table S3), supporting the conclusion that confounding by population stratification is unlikely to explain our findings.
×Interestingly, no significant interaction was observed between the GRS and other biochemical components of the lipid profile in the present study. It is important to bear in mind, however, that despite being the largest clinical trial of its kind, the DPP is only moderately powered to detect genetreatment interactions [32];× it is likely, therefore, that genetreatment interactions that are small in magnitude will have been overlooked here. Moreover, during the course of writing this paper, many smaller impact lipid loci have been discovered [9], [11]. Thus, it is possible that with a larger sample size and the inclusion of some or all of these additional loci, we may have discovered interaction effects on other lipid traits.
In summary, we have shown that common genetic loci that influence polygenic dyslipidemia also modify the effects of clinical interventions designed to mitigate cardiovascular and metabolic risk. This report is the first comprehensive effort to examine validated lipid loci within the context of a large randomized clinical trial. The findings of this study may facilitate the implementation of complex trait genetics into the clinical setting.
Methods
Participants
The DPP was a multi-center randomized controlled trial that examined the effects of metformin or intensive lifestyle modification on the incidence of type 2 diabetes [33], [34]∼∼%%∼ – = . Briefly, overweight persons with elevated but non-diabetic fasting and post-challenge glucose levels were randomized to receive placebo, metformin (850 mg twice daily) or a program of intensive lifestyle modification. The lifestyle intervention was designed to achieve 150 min/wk of physical activity and 7 weight loss via focus on daily fat gram goals. Fat gram goals were based on initial weight and 25 of calories from fat using a calorie level estimate to produce a weight loss of 0.51 kg/wk. The principal endpoint was the development of diabetes by confirmed semi-annual fasting plasma glucose or annual oral glucose tolerance testing (OGTT). Other phenotypes, such as changes in weight, waist circumference, lipids, insulin and glucose, were also ascertained. Written, informed consent was obtained from each participant, and each of the 27 DPP centers obtained institutional review board approval prior to initiation of the study protocol. A total of 2,993 participants in the placebo, lifestyle and metformin groups had DNA available and provided consent for genetic analysis. Individuals taking lipid lowering medications at baseline (n145) were excluded from all analyses.
Measurements
≥[]All participants fasted for 12 hrs the night before blood was drawn from an antecubital vein. Standard blood lipid measurements (triglyceride TG, total cholesterol, HDL-C, calculated LDL-C) were performed at the DPP central biochemistry laboratory. TG and total cholesterol levels were measured using enzymatic methods standardized to the Centers for Disease Control and Prevention reference methods [35]. HDL fractions for cholesterol analysis were obtained by the treatment of whole plasma with dextran sulfate Mg+2 [36]. LDL cholesterol was calculated by the Friedewald equation [37]>β. In participants with TGs4.5 mmol/l, the lipoprotein fractions were separated using preparative ultracentrifugation of plasma by quantification [38]. Lipoprotein subclass particle concentrations and average VLDL, LDL, and HDL particle diameters were measured by NMR spectroscopy at LipoScience, Inc (Raleigh, NC) with modification of existing methods [39].
Genotyping
Thirty-two SNPs previously associated with lipid concentrations in GWAS meta-analyses [10] were selected. DNA was extracted from peripheral blood leukocytes using standard methods. Genotyping was performed by allele-specific primer extension of multiplex amplified products and detection using matrix-assisted laser desorption ionization time-of-flight mass spectrometry on a Sequenom iPLEX platform [40]%%. The mean genotyping success rate was 96.7. The minimum call rate was 94.0. All SNPs were in Hardy-Weinberg equilibrium within each self-reported ethnic group.
Statistical Analysis
The SAS software v9.2 (SAS, Carey, NC) was used for analyses. Baseline total cholesterol, HDL-C, TG and all lipoprotein sub-fraction levels were natural log transformed for non-normality, and LDL-C was evaluated directly. For replicating the previously reported associations of SNPs with baseline traits and evaluating the association of the individual SNPs with NMR lipoprotein particle sizes and numbers, measurements were compared across genotypic groups by ANCOVA (general model, 2 df F test for three possible genotypes), and evidence for an additive effect of genotype was also evaluated using the measured genotype approach, in which each genotype was assigned a value of 0, 1 or 2 according to the number of minor alleles. Analyses of baseline traits were adjusted for age, sex, self-reported ethnicity (to minimize confounding due to potential differences in both allele frequency and lipid traits across ethnicities) and BMI. For the individual SNP analyses, the Bonferroni-corrected P-value for significance was set at P<× = ; = 0.0001 to account for multiple comparisons (32 SNPs14 traits448 tests 0.05/4480.0001).
A genetic risk score (GRS) was calculated from the 32 SNPs using the direction of association from the initial association seen in the published meta-analysis [10]; for each SNP, an allele was designated as a risk allele if it was associated with higher TG or LDL-C and/or lower HDL-C. In order to be able to incorporate all individuals in the analysis, including those missing genotypes at one or more loci, a simple imputation procedure within each self-reported ethnic group was implemented (in order to account for allele frequency differences across ethnicities) prior to score calculation. First, after coding the genotype as the number of minor alleles (0, 1 or 2), an ethnicity-specific mean genotype was calculated and rounded to the nearest whole number. Missing genotypes were replaced by the appropriate rounded mean genotype [41]%××. We calculated a GRS for each individual by adding up the number of risk alleles for each of the 32 tested SNPs, where a risk allele was defined as one associated with increased TG or LDL-C or decreased HDL. The GRS was then included as a quantitative independent variable in a multiple regression model for each baseline lipid/lipoprotein trait to test for association, adjusted for age, sex, self-reported ethnicity, and BMI. GRS quartiles were constructed separately within each self-reported ethnicity prior to calculating quartile-specific arithmetic means or geometric means and 95 confidence intervals. To test for interaction of the risk score with treatment, a multiple regression model was constructed with the 1 year value as the outcome variable and including GRS, lifestyle and metformin treatment and GRSlifestyle and GRSmetformin terms, along with adjustments for the corresponding baseline trait, baseline age, sex and self-reported ethnicity.
Sample Size and Power
A priori power calculations are an important study-planning tool, providing relevant information on likely effect sizes and variances is accessible. It is possible to obtain a broad understanding of the power constraints of our study by extrapolating results from other experimental settings (as described in detail in [42]), but specific a priori× power calculations could not be performed for the current study because reliable effect estimates and variances for tests of genetreatment interactions for the index genotypes and phenotypes were unavailable in the published literature at the time this study was planned. Post-hoc' power calculations were not performed, as these are well known to cause bias when interpreting a studys results [43]–[46]. However, confidence intervals are included in the figures, which give insight into the precision of the GRS effect estimates and hence the power to detect those estimates in the DPP cohort.
Supporting Information
Zdroje
1. DownsJR, ClearfieldM, WeisS, WhitneyE, ShapiroDR, et al. (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279 : 1615–1622.
2. SacksFM, PfefferMA, MoyeLA, RouleauJL, RutherfordJD, et al. (1996) The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 335 : 1001–1009.
3. ShepherdJ, CobbeSM, FordI, IslesCG, LorimerAR, et al. (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 333 : 1301–1307.
4. NamboodiriKK, KaplanEB, HeuchI, ElstonRC, GreenPP, et al. (1985) The Collaborative Lipid Research Clinics Family Study: biological and cultural determinants of familial resemblance for plasma lipids and lipoproteins. Genet Epidemiol 2 : 227–254.
5. Yu-PothS, ZhaoG, EthertonT, NaglakM, JonnalagaddaS, et al. (1999) 'Effects of the National Cholesterol Education Programs Step I and Step II dietary intervention programs on cardiovascular disease risk factors: a meta-analysis. Am J Clin Nutr 69 : 632–646.
6. KrausWE, HoumardJA, DuschaBD, KnetzgerKJ, WhartonMB, et al. (2002) Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 347 : 1483–1492.
7. CederbergH, SaukkonenT, LaaksoM, JokelainenJ, HarkonenP, et al. (2010) Postchallenge Glucose, HbA1c, and Fasting Glucose as Predictors of Type 2 Diabetes and Cardiovascular Disease: A 10-year Prospective Cohort Study. Diabetes Care
8. BenjaminSM, ValdezR, VinicorF (2002) Diabetes prevention. N Engl J Med 346 : 1829–;–1830 author reply 18291830.
9. ChasmanDI, PareG, MoraS, HopewellJC, PelosoG, et al. (2009) Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet 5: e1000730 doi:10.1371/journal.pgen.1000730.
10. KathiresanS, WillerCJ, PelosoGM, DemissieS, MusunuruK, et al. (2009) Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 41 : 56–65.
11. TeslovichTM, MusunuruK, SmithAV, EdmondsonAC, StylianouIM, et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 : 707–713.
12. KathiresanS, MelanderO, AnevskiD, GuiducciC, BurttNP, et al. (2008) Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med 358 : 1240–1249.
13. KitabchiAE, TemprosaM, KnowlerWC, KahnSE, FowlerSE, et al. (2005) Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 54 : 2404–2414.
14. YazdanyarA, YeangC, JiangXC (2011) Role of phospholipid transfer protein in high-density lipoprotein - mediated reverse cholesterol transport. Curr Atheroscler Rep 13 : 242–248.
15. VoightBF, PelosoGM, Orho-MelanderM, Frikke-SchmidtR, BarbalicM, et al. (2012) Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet
16. De BackerG, AmbrosioniE, Borch-JohnsenK, BrotonsC, CifkovaR, et al. (2004) European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of eight societies and by invited experts). Atherosclerosis 173 : 381–391.
17. RatnerR, GoldbergR, HaffnerS, MarcovinaS, OrchardT, et al. (2005) Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care 28 : 888–894.
18. DespresJP (2003) Potential contribution of metformin to the management of cardiovascular disease risk in patients with abdominal obesity, the metabolic syndrome and type 2 diabetes. Diabetes Metab 29 : 6S53–61.
19. KeysA (1965) Effects of Different Dietary Fats on Plasma-Lipid Levels. Lancet 1 : 318–319.
20. CorellaD, OrdovasJM (2009) Nutrigenomics in cardiovascular medicine. Circ Cardiovasc Genet 2 : 637–651.
21. BernsteinMS, CostanzaMC, JamesRW, MorrisMA, CambienF, et al. (2003) ×No physical activityCETP 1b.-629 interaction effects on lipid profile. Med Sci Sports Exerc 35 : 1124–1129.
22. KilpelainenTO, LakkaTA, LaaksonenDE, MagerU, SalopuroT, et al. (2008) Interaction of single nucleotide polymorphisms in ADRB2, ADRB3, TNF, IL6, IGF1R, LIPC, LEPR, and GHRL with physical activity on the risk of type 2 diabetes mellitus and changes in characteristics of the metabolic syndrome: The Finnish Diabetes Prevention Study. Metabolism 57 : 428–436.
23. NettletonJA, SteffenLM, SchulzeMB, JennyNS, BarrRG, et al. (2007) Associations between markers of subclinical atherosclerosis and dietary patterns derived by principal components analysis and reduced rank regression in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 85 : 1615–1625.
24. SpielmannN, LeonAS, RaoDC, RiceT, SkinnerJS, et al. (2007) CETP genotypes and HDL-cholesterol phenotypes in the HERITAGE Family Study. Physiol Genomics 31 : 25–31.
25. Teran-GarciaM, RankinenT, KozaRA, RaoDC, BouchardC (2005) Endurance training-induced changes in insulin sensitivity and gene expression. Am J Physiol Endocrinol Metab 288: E1168–1178.
26. TodorovaB, KubaszekA, PihlajamakiJ, LindstromJ, ErikssonJ, et al. (2004) The G-250A promoter polymorphism of the hepatic lipase gene predicts the conversion from impaired glucose tolerance to type 2 diabetes mellitus: the Finnish Diabetes Prevention Study. J Clin Endocrinol Metab 89 : 2019–2023.
27. TuckerAJ, MackayKA, RobinsonLE, GrahamTE, BakovicM, et al. (2010) The effect of whole grain wheat sourdough bread consumption on serum lipids in healthy normoglycemic/normoinsulinemic and hyperglycemic/hyperinsulinemic adults depends on presence of the APOE E3/E3 genotype: a randomized controlled trial. Nutr Metab (Lond) 7 : 37.
28. ZhangC, Lopez-RidauraR, RimmEB, RifaiN, HunterDJ, et al. (2005) −→Interactions between the 514CT polymorphism of the hepatic lipase gene and lifestyle factors in relation to HDL concentrations among US diabetic men. Am J Clin Nutr 81 : 1429–1435.
29. WillerCJ, SannaS, JacksonAU, ScuteriA, BonnycastleLL, et al. (2008) Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 40 : 161–169.
30. SandhuMS, WaterworthDM, DebenhamSL, WheelerE, PapadakisK, et al. (2008) LDL-cholesterol concentrations: a genome-wide association study. Lancet 371 : 483–491.
31. Mayer-DavisEJ, SparksKC, HirstK, CostacouT, LovejoyJC, et al. (2004) Dietary intake in the diabetes prevention program cohort: baseline and 1-year post randomization. Ann Epidemiol 14 : 763–772.
32. JablonskiKA, McAteerJB, de BakkerPI, FranksPW, PollinTI, et al. (2010) Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes 59 : 2672–2681.
33. The Diabetes Prevention Program Research Group (1999) Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 22 : 623–634.
34. KnowlerWC, Barrett-ConnorE, FowlerSE, HammanRF, LachinJM, et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346 : 393–403.
35. WarnickGR (1986) Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol 129 : 101–123.
36. WarnickGR, BendersonJ, AlbersJJ (1982) +Dextran sulfate-Mg2 precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem 28 : 1379–1388.
37. FriedewaldWT, LevyRI, FredricksonDS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18 : 499–502.
38. Hainline AJ, Karon J, Lippel K (1983) Manual of Laboratory Operations. 2nd ed. . Washington, DC, US.: Lipid Research Clinics Program, Lipid and Lipoprotein Analysis, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health
39. OtvosJD (2002) Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab 48 : 171–180.
40. TangK, FuDJ, JulienD, BraunA, CantorCR, et al. (1999) Chip-based genotyping by mass spectrometry. Proc Natl Acad Sci U S A 96 : 10016–10020.
41. Fontaine-BissonB, RenstromF, RolandssonO, PayneF, HallmansG, et al. (2010) Evaluating the discriminative power of multi-trait genetic risk scores for type 2 diabetes in a northern Swedish population. Diabetologia
42. MooreAF, JablonskiKA, McAteerJB, SaxenaR, PollinTI, et al. (2008) Extension of type 2 diabetes genome-wide association scan results in the diabetes prevention program. Diabetes 57 : 2503–2510.
43. GoodmanSN, BerlinJA (1994) The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med 121 : 200–206.
44. SmithAH, BatesMN (1992) Confidence limit analyses should replace power calculations in the interpretation of epidemiologic studies. Epidemiology 3 : 449–452.
45. DetskyAS, SackettDL (1985) “”?When was a negative clinical trial big enough How many patients you needed depends on what you found. Arch Intern Med 145 : 709–712.
46. GreenlandS (1988) On sample-size and power calculations for studies using confidence intervals. Am J Epidemiol 128 : 231–237.
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



