-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStrategies and Practices in Off-Label Marketing of Pharmaceuticals: A Retrospective Analysis of Whistleblower Complaints
Background:
Despite regulatory restrictions, off-label marketing of pharmaceutical
products has been common in the US. However, the scope of off-label
marketing remains poorly characterized. We developed a typology for the
strategies and practices that constitute off-label marketing.Methods and Findings:
We obtained unsealed whistleblower complaints against pharmaceutical
companies filed in US federal fraud cases that contained allegations of
off-label marketing (January 1996–October 2010) and conducted
structured reviews of them. We coded and analyzed the strategic goals of
each off-label marketing scheme and the practices used to achieve those
goals, as reported by the whistleblowers. We identified 41 complaints
arising from 18 unique cases for our analytic sample (leading to
US$7.9 billion in recoveries). The off-label marketing schemesdescribed in the complaints had three non–mutually exclusive goals:
expansions to unapproved diseases (35/41, 85%), unapproved disease
subtypes (22/41, 54%), and unapproved drug doses (14/41, 34%).
Manufacturers were alleged to have pursued these goals using fournon–mutually exclusive types of marketing practices:
prescriber-related (41/41, 100%), business-related (37/41,
90%), payer-related (23/41, 56%), and consumer-related (18/41,
44%). Prescriber-related practices, the centerpiece of company
strategies, included self-serving presentations of the literature (31/41,
76%), free samples (8/41, 20%), direct financial incentives to
physicians (35/41, 85%), and teaching (22/41, 54%) and
research activities (8/41, 20%).Conclusions:
Off-label marketing practices appear to extend to many areas of the health
care system. Unfortunately, the most common alleged off-label marketing
practices also appear to be the most difficult to control through external
regulatory approaches.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(4): e32767. doi:10.1371/journal.pmed.1000431
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000431Summary
Background:
Despite regulatory restrictions, off-label marketing of pharmaceutical
products has been common in the US. However, the scope of off-label
marketing remains poorly characterized. We developed a typology for the
strategies and practices that constitute off-label marketing.Methods and Findings:
We obtained unsealed whistleblower complaints against pharmaceutical
companies filed in US federal fraud cases that contained allegations of
off-label marketing (January 1996–October 2010) and conducted
structured reviews of them. We coded and analyzed the strategic goals of
each off-label marketing scheme and the practices used to achieve those
goals, as reported by the whistleblowers. We identified 41 complaints
arising from 18 unique cases for our analytic sample (leading to
US$7.9 billion in recoveries). The off-label marketing schemesdescribed in the complaints had three non–mutually exclusive goals:
expansions to unapproved diseases (35/41, 85%), unapproved disease
subtypes (22/41, 54%), and unapproved drug doses (14/41, 34%).
Manufacturers were alleged to have pursued these goals using fournon–mutually exclusive types of marketing practices:
prescriber-related (41/41, 100%), business-related (37/41,
90%), payer-related (23/41, 56%), and consumer-related (18/41,
44%). Prescriber-related practices, the centerpiece of company
strategies, included self-serving presentations of the literature (31/41,
76%), free samples (8/41, 20%), direct financial incentives to
physicians (35/41, 85%), and teaching (22/41, 54%) and
research activities (8/41, 20%).Conclusions:
Off-label marketing practices appear to extend to many areas of the health
care system. Unfortunately, the most common alleged off-label marketing
practices also appear to be the most difficult to control through external
regulatory approaches.
Please see later in the article for the Editors' SummaryIntroduction
In the US, a setting dominated by aggressive advertising of prescription drugs to patients and physicians, off-label marketing has been a controversial subject area. Physicians are permitted to prescribe drugs “off label”—that is, for purposes and patient populations outside of those formally approved by the US Food and Drug Administration (FDA). However, the FDA prohibits pharmaceutical companies from engaging in direct promotion of those unapproved uses [1]. The rationale is that such marketing can lead to widespread uses of a drug that are not based on evidence of efficacy and safety, expose patients to uncertain benefits and the prospect of adverse effects, and undermine incentives for manufacturers to conduct clinical trials necessary to achieve FDA approval for new uses [2]–[5].
Despite regulatory restrictions on off-label marketing, the practice appears to have flourished [6],[7]. In 2009, Pfizer paid US$2.3 billion to settle allegations that it marketed its drugs illegally to physicians—the largest federal health care fraud settlement in US history [8]. In 2010, at least six other manufacturers settled charges pertaining to off-label marketing, and more were under investigation [9]–[15]. The widely publicized litigation over the anti-inflammatory drug rofecoxib (Vioxx) also exposed marketing practices, such as seeding trials and ghost-writing of medical journal articles, that could promote off-label uses [16],[17]. What is known about off-label marketing practices comes largely in this form—namely, episodic reporting of high-profile prosecutions in the popular media [18]–[20], or personal testimony or congressional investigations arising from these same cases [21],[22]. There has been no systematic collection and analysis of these cases, which makes it difficult to identify larger themes and draw conclusions about the favored tactics.
An accumulating number of these cases over the last decade makes such an analysis feasible. Moreover, the data available to conduct this type of analysis are remarkably rich because virtually all of the major cases have been instigated by “whistleblowers” whose complaints provide detailed, firsthand knowledge of the practices at issue [23]. Because off-label marketing activities are secretive and difficult to detect and examine through other means [24], reports from these insiders provide a uniquely illuminating perspective on the range and nature of practices pursued.
We analyzed whistleblower-initiated legal complaints filed in off-label marketing cases over the last 15 y to shed more light on this widely discussed but poorly understood challenge for health regulation. We aimed to create a typology for understanding these cases and a coherent thematic model for mapping pharmaceutical companies' fraudulent promotional behaviors and strategies. Improved understanding in this area has the potential to contribute to the development of strategies for better detection and enforcement.
Methods
Design Overview
The primary data for this study consisted of complaints filed by whistleblowers in “qui tam” cases brought under the US federal False Claims Act (FCA). In brief, the FCA prohibits the submission of false claims to the government for reimbursement. Private citizens who notice potential violations of the FCA can file a sealed complaint in federal court; those who do nearly always retain a personal attorney to represent them and help them write their complaint. The allegations in the complaint are then investigated by the US Department of Justice (DOJ)-Civil Division, which, depending on the strength of the evidence, may elect to intervene and take over the enforcement action, essentially inserting the government as the lead party in the case. At this point, the original whistleblower's complaint is usually unsealed. Multiple complaints may be filed against the same company, but the DOJ intervenes only on the first complaint brought to its attention or subsequent complaints that provide new information (other nonintervened complaints against the same company are usually dismissed and remain sealed). Because of this screening process and the clout of the DOJ, nearly all complaints in which the DOJ intervenes lead to a settlement or judgment against the defendant company. This study focused on cases against pharmaceutical manufacturers for off-label marketing of prescription drugs in which the DOJ intervened.
Setting and Participants
Officials in the DOJ-Civil Division provided us with a full list of pharmaceutical-related federal qui tam cases in which the DOJ intervened and that were settled between January 1996 and 2005. We updated the list to include all DOJ-intervened cases through October 2010 by conducting a search of DOJ press releases [25] and electronic media reports in Lexis-Nexis. We cross-checked the final list with data compiled by Taxpayers Against Fraud, a nongovernmental organization that tracks federal fraud actions. We then obtained the unsealed complaints in these cases from the DOJ, on-line searches of archives of US federal court filings [26], and direct approaches to lawyers involved in the litigation.
Complaints are written documents that generally consist of a summary of the allegations, a description of the whistleblower(s) and defendants, and a detailed account of the allegations and the evidence supporting them. They may be amended during the course of the investigation. We used the most recent versions of the whistleblower-filed complaints available and accessible at the extraction date (6 November 2010). We searched the summaries of the allegations to determine which made allegations about unlawful off-label marketing by the defendant company; 41 complaints in 18 cases did. These complaints formed our analytical sample. Copies of the complaints can be found at http://www.drugepi.org/education/primarydocs.php.
Qualitative Analysis
We designed a structured instrument for abstracting information from the complaints. An initial typology was generated using a standard coding methodology [27],[28]. Two investigators (ASK and DMS) acting independently conducted a preliminary review of 20% of the complaints. After comparing and discussing results of these reviews, we identified two major descriptive domains for further analysis: the strategic goal of the off-label marketing scheme and the specific practices manufacturers used to achieve that goal. We also identified categories and subcategories within each of those domains. One of us (ASK) then read each complaint and coded the details provided into the prespecified categories and subcategories in each domain.
It is important to note that the range of off-label marketing strategies and behaviors we identified and report below are drawn from across the sample of cases as a whole; no manufacturer was accused of all of them.
Results
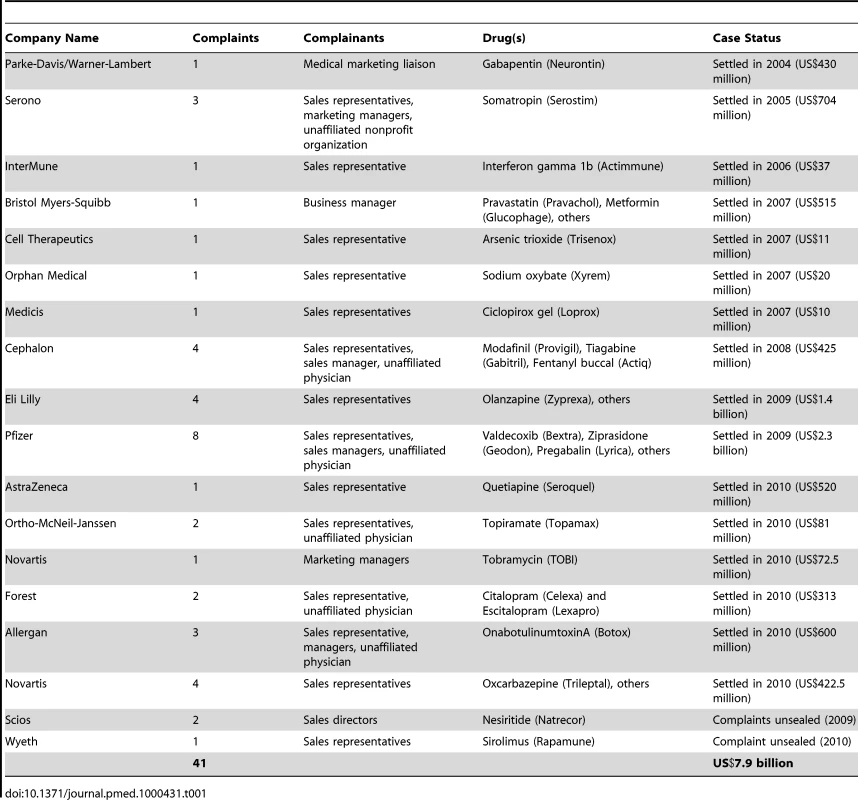
A total of 41 complaints arose from 55 whistleblowers (Table 1). At the time of the alleged fraud, the whistleblowers worked as pharmaceutical sales representatives (39/55, 71%), sales or accounting managers (11/55, 20%), and unaffiliated physicians (5/55, 9%). The cases were brought against 18 manufacturers, including both large companies with diverse drug portfolios (e.g., Pfizer, Eli Lilly) and smaller companies selling a relatively narrow range of products (e.g., Orphan Medical, Medicis). At the time of analysis, settlements had occurred in 16 of the 18 cases and totaled US$7.9 billion in damages.
Off-Label Marketing Strategies
According to the complaints, manufacturers aimed to increase use of their products through off-label marketing schemes in three non–mutually exclusive ways. They sought to expand uses to different disease entities, to variations on the approved indication, and to alternatives to the approved dosing schedule (Table 2).
Expansion to unapproved disease entities
The most prevalent strategy involved expanding use on the basis of diagnosis—that is, seeking off-label uses for disease entities distinct from those approved by the FDA (35/41, 85%). For example, gabapentin (Neurontin), approved as adjunctive treatment for certain types of epilepsy, was also allegedly promoted as therapy for patients with psychiatric disease such as bipolar disorder or depression [29]. Another case involved Pfizer's alleged promotion of sildenafil (Viagra) to treat low libido and to “restore and increase orgasmic sensations” in women [30].
In some cases, a reported rationale for pursuing this type of expansion was that limiting sales to the FDA-approved indication could not sustain needed levels of revenue. One whistleblower from a small, single drug-focused company stated that she was told “that management wanted to sell the company, and that in order to make it a more attractive acquisition target, it was necessary to show increased sales revenue” [31].
In many examples of this marketing strategy, the drug was promoted for treatment of similar symptoms across disease classes (17/35, 49%). For example, modafinil (Provigil), initially approved for narcolepsy-related sleepiness, was allegedly promoted for many types of sleepiness in non-narcoleptic patients [32]. Another example related to the anti-inflammatory drug valdecoxib (Bextra), which was approved for a limited number of pain-related indications and then allegedly promoted by Pfizer for pain relief more broadly [33].
Expansion to unapproved indications
The second most common strategy for off-label promotion was to expand the product's use to different variations of the same condition (22/41, 54%). In some cases, the off-label disease was closely related to the approved one—for example, when a product was specifically approved for a severe manifestation of a condition but then promoted for milder forms. In the case of nesiritide (Natrecor), the drug was approved for “acutely decompensated heart failure” and was allegedly promoted in patients with chronic stable heart failure as a preventative measure [34]. Although both groups of patients had heart failure, they were quite different manifestations of the disease.
One prominent subcategory of this type of off-label promotion focused on patient subgroups different from those contemplated in the FDA approval (10/22, 45%). For example, ciclopirox gel (Loprox) was approved for fungal dermatoses in patients over age 10, but allegedly promoted by its manufacturer to manage diaper-related fungal dermatitis in babies [35]. In some of the antidepressant drugs in our sample, the product was approved for adult use, but allegedly promoted to pediatricians and family practice physicians specifically for young patients who demonstrated signs of depression [30],[36]. In the case of citalopram (Celexa), studies that had shown dangers with using the drug in pediatric populations were allegedly withheld from physicians as part of the marketing campaign [36].
Expansion to unapproved dosing strategies
The final, and least common, variety of off-label expansion was off-label prescribing based on different dosing regimens than that approved by the FDA (14/41, 34%). Typically, manufacturers promoted higher doses to enhance revenues by encouraging sale of more units of the product. For example, the manufacturer of oxcarbazepine (Trileptal) allegedly promoted use of the antiepileptic drug “as monotherapy for seizures using extremely high dosages” [37]. By contrast, the manufacturer of sirolimus (Rapamune), which was approved for transplant patients in combination with cyclosporine and corticosteroids, allegedly trained its staff to encourage its use in combination with “any drug or combination of drugs that a physician could be convinced to prescribe” to enhance its market possibilities [38].
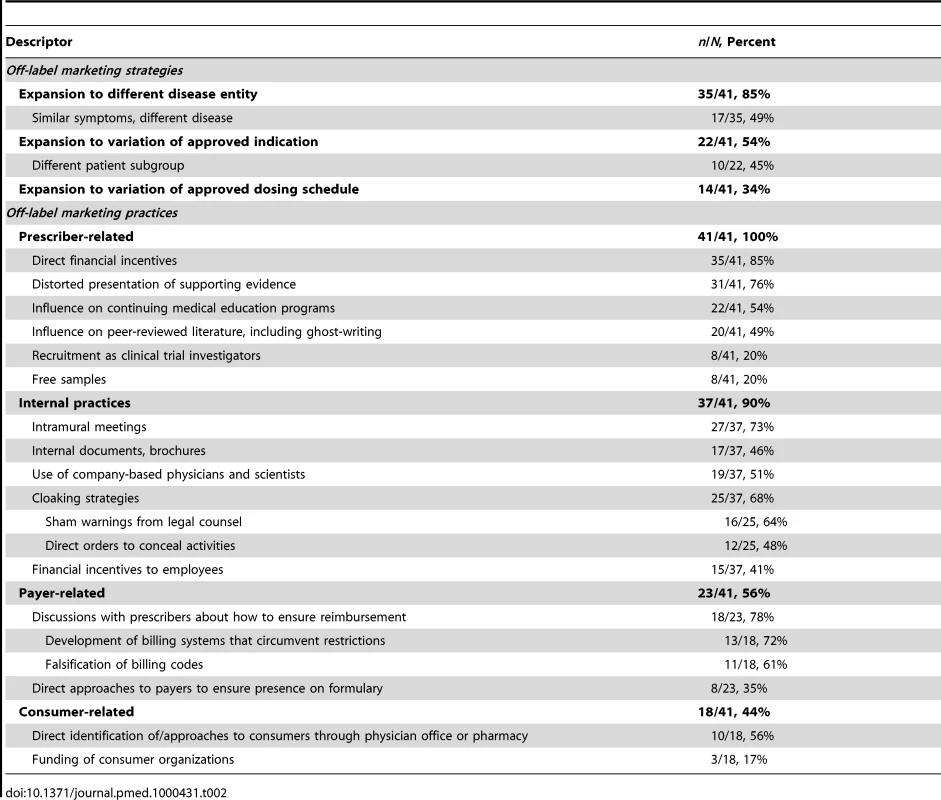
Off-Label Marketing Practices
The marketing practices manufacturers allegedly employed to achieve these strategic goals for off-label use fell into four non–mutually exclusive categories: internal practices, payer-related practices, prescriber-related practices, and consumer-related practices. We defined internal practices as incentives and other aspects of the employment environment at the defendant manufacturer that encouraged employees to promote off-label uses. Payer-related practices were strategies aimed at encouraging insurers to pay for off-label prescriptions. Prescriber-related and consumer-related practices involved direct promotion of off-label drug use to prescription writers and consumers, respectively.
Prescriber-related practices
All of the complaints we analyzed detailed off-label promotion to prescribers; this was generally the centerpiece of the whistleblowers' complaints. Though manufacturers are not supposed to discuss off-label uses unless a physician inquires, many were accused of either flouting that rule or designing their representatives' presentations in such a way as to guarantee that discussion would inevitably lead to off-label uses.
According to the complaints, off-label use was frequently encouraged through self-serving presentations of the scientific literature through which physicians were given false or unbalanced study data supporting the unapproved use (31/41, 76%). A common example was selective presentation of favorable studies, where dangers from the off-label uses allegedly being promoted were not mentioned [39]. Other examples included presenting one drug as being superior to another when no head-to-head studies had been conducted [40] and characterizing reports of individual cases or poorly designed studies as definitive evidence supporting an off-label use [41].
A number of whistleblowers alleged that free samples had been provided (8/41, 20%) as a way to promote off-label use. The whistleblowers in this group reported that these samples were intended to encourage physicians to use a product on the basis of convenience, even though it might not be approved for a certain use. In addition, many described how free samples were intended to introduce unapproved patient populations to the manufacturer's product with the intention of stimulating their continued use.
Complaints alleged that manufacturers also encouraged off-label use through direct financial incentives to physicians. Lavish gifts or honoraria were mentioned in most complaints (35/41, 85%), with many whistleblowers reporting strategies to target these gifts to physicians who were high off-label prescribers (18/41, 44%). In some cases, physicians might be invited to serve in focus groups or as consultants to the manufacturer, although it was alleged that the association was intended not to obtain expert advice, but to provide money to prescribers to positively reinforce off-label use (15/41, 37%).
Finally, off-label use was encouraged among prescribers through teaching and research activities. In over half the cases, Continuing Medical Education (CME) seminars were organized with speakers known to promote off-label uses (22/41, 54%). In a few cases, whistleblowers reported that CME activities were organized by shell corporations to impart an appearance of scientific neutrality [34]. Nearly half of whistleblowers also alleged that manufacturers sought to promote off-label drug use through journal publications (20/41, 49%). These practices included falsely reporting outcomes from patients in manufacturer-sponsored studies [42] and publishing “ghostwritten” articles supporting an unapproved use written by the manufacturer under the name of a respected scientist [43]. Finally, a minority of whistleblowers alleged that manufacturers recruited physicians to conduct clinical trials for them with the intent of encouraging off-label use (“seeding trials”), rather than for any useful scientific or information-gathering reasons (8/41, 20%).
Internal practices
Thirty-seven of the whistleblower (90%) complaints detailed particular internal manufacturer practices intended to bolster the off-label marketing (two of the four complaints where these were not mentioned were filed solely by whistleblowers positioned outside the companies). All of the practices described were reported to be company-wide, rather than the work of an individual manager or group of managers. In 73% (27/37) of these cases, the off-label marketing strategy was implemented through intramural meetings and seminars in which marketing practices were discussed; in 46% (17/37) of them, it was also implemented through development of brochures and other materials for dissemination; in 51% (19/37), employees other than the sales representatives, such as internal physicians and scientists, were involved.
Many of the complaints describing internal practices (25/37, 68%) pointed to specific efforts by drug manufacturers to conceal off-label marketing activities. Some described warnings from legal teams to avoid off-label marketing (16/25, 64%). These were generally understood by employees as providing “plausible deniability” to the company [33], and were widely undermined through strategies such as verbal orders diverging from what was declared in their company policies [31]. For example, one whistleblower reported that his company purposefully designed “do not detail” labels on materials related to off-label uses that could easily be removed by a sales representative [30]. A third of complaints included reports of direct orders to conceal, such as “cleaning” internal reports and memoranda of all mentions of off-label marketing (12/25, 48%).
The complaints frequently described use of financial incentives for employees to engage in off-label marketing. Forty-one percent (15/37) of the reports of internal strategies described incentives or other aspects of employees' compensation plans that were directly tied to effectively implementing an off-label prescription strategy. In one case involving a drug approved by the FDA for a rare indication, a whistleblower reported that the company imposed sales quotas on representatives that could only be met through expanding use beyond the limited approved indication [31]. Other examples included an internal sales “contest” for employees who could demonstrate greatest compliance with marketing programs encouraging off-label use [44] and direct payments to employees to encourage them not to report off-label marketing practices [35].
Payer-related practices
Payer-related promotional practices were reported in just over half of the complaints (23/41, 56%) and fell into two categories: discussions with prescribers about ways to ensure insurance reimbursement for their off-label prescriptions (18/23, 78%) and direct discussions with payers themselves (8/23, 35%) (three complaints described both). The reports of discussions with prescribers in complaints described efforts to educate them about how to manage the billing system to ensure that off-label prescriptions were reimbursed, including advice on ways to bypass insurers' restrictions on prescriptions of the product (13/18, 72%). For example, one whistleblower reported being taught to overcome a requirement that patients receive a trial of a competitor's drug first by instructing physicians to issue two different prescriptions at the same time: one for the competitor's drug that the patient could ignore, the other for the company's drug [45]. The other strategy commonly reported was to encourage providers to falsify billing codes (11/18, 61%).
Seven complaints reported that manufacturers interacted with payers to encourage off-label drug use by ensuring drugs were on a formulary for off-label uses (four reports) or developing organizational protocols that included the off-label use (four reports; one reported both). One whistleblower described a bolder tactic for ensuring formulary coverage for off-label use of a product: directing “their sales representatives to garnish physician and patient letters of support to encourage reimbursement” by Medicare intermediaries [46].
Consumer-related practices
Nearly half the complaints described off-label marketing practices focused directly on consumers (18/41, 44%). The most common example involved identifying consumers who could be off-label users (10/18, 56%)—for instance, by conducting chart reviews in physicians' offices. The next step was bringing those patients eligible for an off-label use to the physician's attention, thereby fusing a consumer-focused practice with a prescriber-focused one. Other practices intended to directly encourage off-label use among consumers allegedly included promotion of consumer demand for off-label uses through payments to nonprofit, consumer-focused disease management organizations in exchange for their support of the off-label use [43]. Another complaint described on-line resources presented by a “noncommercial public interest organization” that were intended to promote off-label use of the product, but which were developed by a marketing firm linked to the defendant company [47]. In a third case, the whistleblowers alleged that the company provided indigent patients with “gift certificates, phone cards, and bus tokens” as inducements to seek out prescriptions of a drug for an off-label purpose [48].
Discussion
Through a comprehensive review of whistleblower complaints, to our knowledge the first of its kind, we found descriptions of a range of marketing practices related to off-label promotion of prescription drugs. All of the strategies and behaviors we outlined were alleged by whistleblowers with special knowledge of company practices, although none of the complaints was subject to full trial and evaluation by a judge or jury. The study provides a basic empirical snapshot of the extent to which each of these strategies and practices have been employed, at least among cases exposed in qui tam litigation.
Our findings show that off-label marketing practices have a broad reach. Similar behaviors and strategies were linked to manufacturers of varying sizes across drugs in virtually all therapeutic classes; they extended to many aspects of the health care system; they affected a multitude of players (prescribers, pharmacies, disease advocacy groups, CME organizations, consumers); and were pursued through virtually every facet of physician-industry relationships (paid consultancies, preceptorships, and collaboration in clinical trials and research publications). The alleged tactics in our analytic sample ranged from subtly encouraging physicians to ask for information about off-label uses to providing strong financial rewards for encouraging off-label uses; they also included targeting multiple links in the prescription production chain, from company scientists and sales representatives to prescribers.
Some of the practices we identified have been highlighted in anecdotal reports and are relatively well known. Others have received little or no attention, such as pharmaceutical marketing representatives working directly with physicians and their office managers to circumvent reimbursement restrictions set by government payers and other insurers. Nearly a quarter of the whistleblowers alleged that pharmaceutical sales representatives were given access to patients' confidential medical records at physicians' offices for the purposes of trolling for prospective targets for illegal direct-to-consumer promotion of off-label uses. Despite the remarkable prevalence of this practice among the complaints we analyzed, media coverage has tended to center on other, more institutionally focused aspects of fraud.
New regulatory strategies, both public and private, aimed in part at preventing off-label marketing, have proliferated in recent years. Medical journals have changed their authorship standards to foil ghostwriting [49]; following the example of several states, the federal health care reform legislation requires disclosure of pharmaceutical industry payments to physicians [50]; the leading pharmaceutical manufacturers' association, PhRMA, has adopted a Code of Ethics that prohibits certain types of gifts [51]; and a handful of academic medical centers have restricted or prohibited visits by pharmaceutical sales representatives [52]. Our findings support the need for these measures to combat gifts to physicians, which we identified as the single most prevalent modality of off-label promotion reported by whistleblowers.
However, our results also suggest that additional steps will likely be necessary to curb off-label marketing. For example, interventions seeking to insulate physician education from industry influence have largely been limited to programs in which the manufacturer controls the content, but the reports in this study suggest that even so-called “unrestricted” educational grants from industry may be deployed to effect off-label marketing. A better policy solution would be fully independent programs of continuing medical education, an approach that has received limited support in a few states and has been proposed (but not enacted) in US Congress. Another potential solution is a central repository, independent from any physician or health care organization, where manufacturers can donate money that is then distributed for educational purposes.
Some experts have suggested that fraudulent off-label marketing might be prevented through more substantial fines for manufacturers under investigation or other penalties for company managers [53]. Criminal prosecutions of executives are rare [54], but the DOJ has signaled increasing interest in using this approach [53]. While seeking to fortify deterrence through such tactics might address some behaviors, our findings suggest that some common off-label marketing practices may be difficult to control through external regulatory approaches because of their deep-seated nature. Whistleblowers in most of the cases we reviewed reported that private conversations between sales representatives and prescribers were a leading strategy for off-label promotion. The opportunity to prompt and answer physicians' questions about off-label uses, address their individual concerns, and provide a digest of empirical evidence that can be slanted as needed likely makes these conversations a particularly effective form of marketing. The fact that so many of the communications are oral and take place in private offices makes them very difficult for regulators to monitor and sanction. It is impossible to conceive of how anyone other than a company insider or a physician could bring many of these marketing practices to light (indeed, this underlines the distinctive strength of our data source). The move by a few prominent academic medical centers to ban sales representatives from the premises is a bold and powerful one, but it has not, as yet, been followed by many hospitals or physician practices.
Changes in the PhRMA Code are a positive sign that the industry is responsive to public concerns about inappropriate marketing practices. In some news reports, manufacturers have described new corporate cultures that avowedly reject the illegal tactics described in the whistleblower complaints [55]. However, in many of the cases we studied, manufacturers were reported to demonstrate awareness of existing regulations and engage in strategic behaviors to work around them (e.g., by giving employees lectures about the regulatory environment that were understood to be a smokescreen) or to mask their violations of the law (e.g., by encouraging employees to not enter off-label marketing calls in their logs).
Our approach has limitations. First, although the DOJ conducted thorough investigations of each complaint in the study sample, the settled cases concluded without a full trial, which would have included formal fact-finding by a judge or jury. Thus, some allegations may be false and, for nearly all complaints, internal company documents that might have corroborated the complainants' specific reports remained confidential. Second, our analyses were conducted mainly at the complaint level, but nine of the 18 cases involved more than one complaint (the DOJ permits multiple complaints when each brings new information to bear on the case); the clustering of complaints in some cases may have inflated the reported prevalence of certain behaviors. Third, most whistleblowers were US-based sales representatives with a particular field of vision in relation to their companies' off-label marketing practices. It is possible that other behaviors and strategies exist that the whistleblower did not observe and the government investigations did bring to light. Our reliance on the text of the complaints means that we would have missed these. Finally, the complaints were composed to support claims of fraud under certain specific legislation, including the False Claims Act.
Conclusion
Off-label marketing has been ubiquitous in the health care system and features some behaviors and strategies that may be resistant to external regulatory approaches. Our findings suggest that no regulatory strategy will be complete and effective without physicians themselves serving as a bulwark against off-label promotion. Aside from sales representatives and other company insiders, who play important roles as whistleblowers, physicians are alone in having a full view of many of the most insidious forms of illegal marketing outlined in the complaints we reviewed. As physicians' understanding of these practices and the consequences of inappropriate off-label promotion for public health evolves, so may their enthusiasm for shutting them down.
Zdroje
1. Kefauver-Harris Drug Amendments
1962
Public Law 87-781 (codified as amended at 21 U.S.C. § 352
(n))
2. Fugh-Berman
A
Melnick
D
2008
Off-label promotion, on-target sales.
PLoS Med
5
e210
doi:10.1371/journal.pmed.0050210
3. Psaty
BM
Ray
W
2008
FDA guidance on off-label promotion and the state of the
literature from sponsors.
J Amer Med Assn
299
1949
1951
4. Henney
JE
2006
Safeguarding patient welfare: who's in
charge?
Ann Intern Med
145
305
307
5. Kesselheim
AS
Avorn
J
2008
Pharmaceutical promotion to physicians and First Amendment
rights.
New Engl J Med
358
1727
1732
6. Kesselheim
AS
Studdert
DM
2008
Whistleblower-initiated enforcement actions against health care
fraud and abuse in the United States, 1996 to 2005.
Ann Int Med
149
342
349
7. Almashat
S
Preston
C
Waterman
T
Wolfe
S
2010
December
16
Rapidly increasing criminal and civil monetary penalties against
the pharmaceutical industry: 1991 to 2010.
Available: http://www.citizen.org/hrg1924. Accessed 10 February
2011
8. Harris
G
2009
September
2
Pfizer pays $2.3 billion to settle marketing
case.
NY Times B4
9. Department of Justice
2010
April
29
Two Johnson & Johnson subsidiaries to pay over $81
million to resolve allegations of off-label promotion of
Topamax.
Available: http://www.justice.gov/opa/pr/2010/April/10-civ-500.html.
Accessed 10 February 2011
10. Department of Justice
2010
April
27
Pharmaceutical giant AstraZeneca to pay $520 million for
off-label drug marketing.
Available: http://www.justice.gov/opa/pr/2010/April/10-civ-487.html.
Accessed 10 February 2011
11. Department of Justice
2010
May
4
Novartis vaccines & diagnostics to pay more than $72
million to resolve False Claims Act allegations concerning
TOBI.
Available: http://www.justice.gov/opa/pr/2010/May/10-civ-522.html.
Accessed 10 February 2011
12. Department of Justice
2010
September
15
Drug maker Forest pleads guilty; to pay more than $313
million to resolve criminal charges and False Claims Act
allegations.
Available: http://www.justice.gov/opa/pr/2010/September/10-civ-1028.html.
Accessed 10 February 2011
13. Department of Justice
2010
September
30
Novartis Pharmaceuticals Corp. to pay more than $420
million to resolve off-label promotion and kickback allegations. 30 Sept
2010.
Available: http://www.justice.gov/opa/pr/2010/September/10-civ-1102.html.
Accessed 10 February 2011
14. Department of Justice
2010
September
1
Allergan agrees to plead guilty and pay $600 million to
resolve allegations of off-label promotion of Botox®.
Available: http://www.justice.gov/opa/pr/2010/September/10-civ-988.html.
Accessed 10 February 2011
15. Sandburg
B
2009
Health care fraud investigations bedevil Pharma industry: if
you're not under investigation, it's only because you've
recently settled.
Pink Sheet
71
21
24
16. Hill
KP
Ross
JS
Egilman
DS
Krumholz
HM
2008
The ADVANTAGE seeding trial: a review of internal
documents.
Ann Intern Med
149
251
258
17. Ross
JS
Hill
KP
Egilman
DS
Krumholz
HM
2008
Guest authorship and ghostwriting in publications related to
rofecoxib: a case study of industry documents from rofecoxib
litigation.
J Amer Med Assn
299
1800
1812
18. Spielmans
GI
2009
The promotion of olanzapine in primary care: an examination of
internal industry documents.
Soc Sci Med
69
14
20
19. Steinman
MA
Harper
GM
Chren
MM
Landefeld
CS
Bero
LA
2007
Characteristics and impact of drug detailing for
gabapentin.
PLoS Med
4
e134
doi:10.1371/journal.pmed.0040134
20. Steinman
MA
Bero
LA
Chren
MM
Landefeld
CS
2006
Narrative review: the promotion of gabapentin: an analysis of
internal industry documents.
Ann Intern Med
145
284
293
21. Fugh-Berman
A
Ahari
S
2007
Following the script: how drug reps make friends and influence
doctors.
PLoS Med
4
e150
doi:10.1371/journal.pmed.0040150
22. Waxman
HA
2005
May
5
Memorandum re: marketing of Vioxx to physicians.
Available: http://oversight.house.gov/documents/20050505114932-41272.pdf.
Accessed 10 February 2011
23. Kesselheim
AS
Studdert
DM
Mello
MM
2010
Experiences of whistle-blowers in major fraud litigation against
pharmaceutical companies.
New Engl J Med
362
1832
1839
24. Mello
MM
Studdert
DM
Brennan
TA
2009
Shifting terrain in the regulation of off-label promotion of
pharmaceuticals.
New Engl J Med
360
1557
1566
25. United States Department of Justice
2011
Office of Public Affairs Press Releases.
Available: http://www.usdoj.gov/03press/03_1_1.html. Accessed 10
February 2011
26. United States Judiciary
2011
Public Access to Court Electronic Records.
Available: http://pacer.psc.uscourts.gov/. Accessed 10 February
2011
27. Glaser
BG
Strauss
AL
1967
The discovery of grounded theory: strategies for qualitative
research
New York
Aldine De Gruyter Press
274
28. Constas
MA
1992
Qualitative data analysis as a public event: the documentation of
category development procedures.
American Educational Research Journal
29
253
266
29. US ex rel. Franklin v. Parke-Davis, Division of Warner-Lambert
Company
1996 August 13
Civil Action 1 : 96-CV-11651-PBS.
Available: http://www.drugepi.org/downloads/downloads/WarnerLambert_Complaint1.pdf.
Accessed 10 February 2011
30. US ex rel. Collins, et al. v. Pfizer, Inc
2007 August 13
Civil Action 1 : 04-CV-11780-DPW.
Available: http://www.drugepi.org/downloads/downloads/Pfizer_Complaint8.pdf.
Accessed 10 February 2011
31. US, et al. ex rel. Lauterbach v. Orphan Medical Inc., Jazz
Pharmaceuticals Inc., and Dr. Peter Gleason
2006 February 17
Civil Action 05-CV-0387-SJF-KAM.
Available: http://www.drugepi.org/downloads/downloads/OrphanMedical_Complaint1.pdf.
Accessed 10 February 2011
32. US, et al. ex rel. Boise v. Cephalon, Inc
2008 January 3
Civil Action 2 : 04-CV-04401-TON.
Available: http://www.drugepi.org/downloads/downloads/Cephalon_Complaint4.pdf.
Accessed 10 February 2011
33. US ex rel. Kopchinski v. Pfizer, Inc. and Pharmacia Corp
2005 October 24
Civil Action 1 : 05-CV-12115-DPW.
Available: http://www.drugepi.org/downloads/downloads/Pfizer_Complaint7.pdf.
Accessed 10 February 2011
34. US, et al. ex rel. Stromv. Scios, Inc. and Johnson & Johnson,
Inc
2005 July 22
Civil Action 3 : 05-CV-03004-CRB.
Available: http://www.drugepi.org/downloads/downloads/Scios_Complaint2.pdf.
Accessed 10 February 2011
35. US, et al. ex rel. Mulqueen, et al. v. Medicis Pharmaceutical
Corp
2007 April 5
Civil Action 2 : 04-CV-02389-KHV-GLR.
Available: http://www.drugepi.org/downloads/downloads/Medicis_Complaint1.pdf.
Accessed 10 February 2011
36. US, et al. ex rel. Gobble v. Forest Laboratories Inc., Forest
Pharmaceuticals, Inc
2010 January 8
Civil Action 03-10395-NMG.
Available: http://www.drugepi.org/downloads/downloads/Forest_Complaint1.pdf.
Accessed 10 February 2011
37. US, et al. ex rel. Copeland v. Novartis Pharmaceuticals Corp
2010 August 26
Civil Action 2 : 06-CV-01630-LDD.
Available: http://www.drugepi.org/downloads/downloads/NovartisCase2_Complaint1.pdf.
Accessed 10 February 2011
38. US, et al. ex rel. Sandler and Paris v. Wyeth Pharmaceuticals, Inc. and
Pfizer Inc
2010 May 24
Civil Action 2 : 05-CV-06609-JP.
Available: http://www.drugepi.org/downloads/downloads/Wyeth_Complaint1.pdf.
Accessed 10 February 2011
39. US ex rel. Marchese v. Cell Therapeutics, Inc., et al
2007 August 24
Civil Action 2 : 06-CV-00168-MJP.
Available: http://www.drugepi.org/downloads/downloads/CellTherapeutics_Complaint1.pdf.
Accessed 10 February 2011
40. US, et al. ex rel. Kruszewski v. Pfizer, Inc
2009 August 21
Civil Action 2 : 07-CV-04106-JCJ.
Available: http://www.drugepi.org/downloads/downloads/Pfizer_Complaint5.pdf.
Accessed 10 February 2011
41. US, et al. ex rel. Wetta v. AstraZeneca Corporation
2008 August 1
Civil Action 2 : 04-CV-03479-BMS.
Available: http://www.drugepi.org/downloads/downloads/AstraZeneca_Complaint1.pdf.
Accessed 10 February 2011
42. US ex rel. Gallagher v. Intermune, Inc
2004 July 9
Civil Action 04:CV-4323.
Available: http://www.drugepi.org/downloads/downloads/Intermune_Complaint1.pdf.
Accessed 10 February 2011
43. US ex rel. Westlock v. Pfizer, Inc., et al
2008 August 1
Civil Action 1 : 08-CV-11318-DPW.
Available: http://www.drugepi.org/downloads/downloads/Pfizer_Complaint2.pdf.
Accessed 10 February 2011
44. US ex rel. Liter v. Pfizer, Inc
2007 November 21
Civil Action 2 : 06-CV-00176-WOB.
Available: http://www.drugepi.org/downloads/downloads/Pfizer_Complaint1.pdf.
Accessed 10 February 2011
45. US ex rel. Farber and Schildhauer v. Pfizer, Inc
2007 June 12
Civil Action 1 : 07-CV-10304-DPW.
Available: http://www.drugepi.org/downloads/downloads/Pfizer_Complaint6.pdf.
Accessed 10 February 2011
46. US ex rel. Smith v. Scios, Inc., et al
2005 October 7
Civil Action 3 : 05-CV-04055-CRB.
http://www.drugepi.org/downloads/downloads/Scios_Complaint1.pdf.
Accessed 10 February 2011
47. US ex rel. Lang and Rushin v. Allergan, Inc
2007 June 5
Civil Action 1 : 07-CV-01288-WSD.
Available: http://www.drugepi.org/downloads/downloads/Allergan_Complaint2.pdf.
Accessed 10 February 2011
48. US, et al. ex rel. Garcia and Driscoll v. Serono, Inc
2003 October 6
Civil Action 03-CV-11892-GAO.
Available: http://www.drugepi.org/downloads/downloads/Serono_Complaint1.pdf.
Accessed 10 February 2011
49. Drazen
JM
de Leeuw
PW
Laine
C
Mulrow
C
DeAngelis
CD
2010
Toward more uniform conflict disclosures—the updated ICMJE
conflict of interest reporting form.
New Engl J Med
363
188
189
50. Physician Payments Sunshine Act
2009
H.R. 3590 § 6002
51. PhRMA
2008
Code on interactions with health care
professionals.
Available: http://www.phrma.org/files/attachments/PhRMA%20Marketing%20Code%202008.pdf.
Accessed 10 February 2011
52. Pollack
A
2006 September 12
Stanford to ban drug makers' gifts to doctors, even
pens.
NY Times C2
53. Edney
A
2010 October 15
Drugmaker CEOs may be targets for U.S. FDA in off-label cases,
lawyer says.
Bloomberg News. Available: http://www.bloomberg.com/news/2010-10-14/drugmaker-executives-may-become-targets-of-fda-for-off-label-promotions.html.
Accessed 10 February 2011
54. Wilson
D
2010 November 9
Ex-Glaxo executive is charged in drug fraud.
NY Times B2
55. Kindler
J
2010 March 17
Business must change to earn back the public's
trust.
Reuters. Available: http://blogs.reuters.com/great-debate/2010/03/17/business-must-change-to-earn-back-the-publics-trust/.
Accessed 10 February 2011
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
- Léčba bolesti u seniorů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Flexofytol® – přírodní revoluce v boji proti osteoartróze kloubů
-
Všechny články tohoto čísla
- Quality of Private and Public Ambulatory Health Care in Low and Middle Income Countries: Systematic Review of Comparative Studies
- A Multifaceted Intervention to Implement Guidelines and Improve Admission Paediatric Care in Kenyan District Hospitals: A Cluster Randomised Trial
- The Quality of Medical Care in Low-Income Countries: From Providers to Markets
- Neglect of Medical Evidence of Torture in Guantánamo Bay: A Case Series
- Improving Effective Surgical Delivery in Humanitarian Disasters: Lessons from Haiti
- Decline in Diarrhea Mortality and Admissions after Routine Childhood Rotavirus Immunization in Brazil: A Time-Series Analysis
- Effect of Pneumococcal Conjugate Vaccination on Serotype-Specific Carriage and Invasive Disease in England: A Cross-Sectional Study
- Effect of a Nutrition Supplement and Physical Activity Program on Pneumonia and Walking Capacity in Chilean Older People: A Factorial Cluster Randomized Trial
- Strategies and Practices in Off-Label Marketing of Pharmaceuticals: A Retrospective Analysis of Whistleblower Complaints
- A Call for Action: The Application of the International Health Regulations to the Global Threat of Antimicrobial Resistance
- Medical Complicity in Torture at Guantánamo Bay: Evidence Is the First Step Toward Justice
- A Public Health Emergency of International Concern? Response to a Proposal to Apply the International Health Regulations to Antimicrobial Resistance
- Global Health Philanthropy and Institutional Relationships: How Should Conflicts of Interest Be Addressed?
- Claims about the Misuse of Insecticide-Treated Mosquito Nets: Are These Evidence-Based?
- The African Women's Protocol: Bringing Attention to Reproductive Rights and the MDGs
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Global Health Philanthropy and Institutional Relationships: How Should Conflicts of Interest Be Addressed?
- A Call for Action: The Application of the International Health Regulations to the Global Threat of Antimicrobial Resistance
- Claims about the Misuse of Insecticide-Treated Mosquito Nets: Are These Evidence-Based?
- Neglect of Medical Evidence of Torture in Guantánamo Bay: A Case Series
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání