-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaDevelopment and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma
Using Italian and Taiwanese cohorts, Alessandro Vitale and colleagues develop and validate a staging system and prognostic model for hepatocellular carcinoma.
Published in the journal: . PLoS Med 13(4): e32767. doi:10.1371/journal.pmed.1002006
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002006Summary
Using Italian and Taiwanese cohorts, Alessandro Vitale and colleagues develop and validate a staging system and prognostic model for hepatocellular carcinoma.
Introduction
Liver cancer is the sixth most common cancer and the second most common cause of cancer death worldwide, leading to nearly 746,000 deaths in 2012 [1]. Hepatocellular carcinoma (HCC) is largely a problem in low - and middle-income countries and regions, where 83% (50% in China alone) of the estimated 782,000 new HCC cases occurred in 2012 [1]. In addition, in recent decades, HCC incidence and HCC-related deaths have been rising, not only in low - and middle-income countries but also in high-income countries [2]. The highest HCC risk, measured by age-standardized rates and classified as “very high risk,” has been recorded in eastern (31.9) and in southeastern Asia (22.2). The HCC risk in Italy (11.0) was classified as “moderately high risk,” and it was one of the highest in high-income countries [1].
Over the last 20 years, a variety of prognostic systems have been proposed in an attempt to address the interrelationship of prognostic factors among HCC patients and the complexity of HCC treatment [3–17]. In the literature, several HCC prognostic systems have been described but no single system for HCC has been universally adopted. Some systems—such as Barcelona Clinic Liver Cancer (BCLC) staging, United Network for Organ Sharing (UNOS) tumor node metastases (TNM) staging, Hong Kong Liver Cancer (HKLC) staging, and American Joint Committee on Cancer (AJCC) TNM staging—are “staging classification” schemas, typically based on systematic reviews of the literature and/or expert opinions [6–9]. These systems stratify the HCC population in stages exclusively or mainly defined by tumor characteristics. The aim of these schemas is usually to link stages to guidelines for the management of patients with HCC and the design of clinical trials. However, these systems often lack prognostic power [10] or strong statistical support such as external validation [8].
Other systems—such as the Model to Estimate Survival in Ambulatory HCC Patients (MESIAH), Cancer of the Liver Italian Program (CLIP), and Japanese Integrated Staging (JIS) scores—are “conventional” prognostic scores that incorporate variables that were significant in multivariable Cox survival analyses [11–17]. The strength of these scores consists in objective and reproducible variables and rigorous statistical methodology. Unfortunately, even if these prognostic scores correlate well with outcome, often they are not suitably generalizable to populations different from the one that generated the score, and they don’t define tumor stages easily usable in clinical practice [11–17].
Based on these considerations, we sought to develop and validate a new prognostic system for HCC patients including both a tumor staging and a prognostic score. The Italian Liver Cancer (ITA.LI.CA) dataset, including more than 5,000 Italian HCC patients prospectively enrolled from 1 January 1987 to 31 March 2012, was used for the training and internal validation sets. A large HCC population from Taiwan served as the external validation cohort, to test the prognostic value of this new system in an Asian population.
Methods
Study Groups
Prospectively collected data of 5,290 consecutive patients with HCC, each managed in one of the 19 institutions participating in the ITA.LI.CA study group and enrolled between 1 January 1987 and 31 March 2012 were analyzed.
The institutional review boards of the participating institutions approved the study. According to the Italian and Taiwanese laws, no patient approval is needed for retrospective studies. Informed consent was obtained as usual for medical, surgical, and radiological treatments, but not specifically for patient data to be used in this retrospective study. Patients gave written consent for every procedure performed in the hospitals, including use of data for medical purposes.
Details about patient data collected for this study are described in S1 Text. For each patient the following composite variables were calculated and recorded: Child—Pugh score (CPS), BCLC stage, UNOS modified TNM stage, HKLC stage, CLIP score, JIS stage, MESIAH score, and modified BCLC stage [6–8,12,15,17–20]. After exclusion of 107 cases without complete follow-up data or lost to follow-up, a total of 5,183 patients were included in the analysis and randomly allocated into the training or test (internal validation) set in an approximately 2 : 1 ratio (3,628 : 1,555). In addition, in order to test the general application of our scheme to HCC patients in another center, an external validation was performed in a cohort of 2,651 patients from Taipei (Taiwan) with HCC diagnosed between 1 January 2002 and 31 December 2012. In the Taiwanese cohort, there were no patients lost to follow-up or with incomplete follow-up data.
Descriptive Statistics
Baseline characteristics were examined based on frequency distribution; continuous data are presented as median (interquartile range) unless indicated otherwise. Univariate comparisons were assessed using Student’s t test, Wilcoxon rank-sum test, or chi-squared test as appropriate. Missing data relative to study covariates always involved less than 10% of patients. Thus, missing values were imputed using the maximum likelihood estimation method [21].
Overall survival was defined from the date of first diagnosis of HCC to the date of death, last follow-up evaluation, or data censoring (31 December 2012). Kaplan—Meier survival curves were used to estimate median overall survival and the 1-, 3-, and 5-y overall survival rates. The survival curves were also stratified according to ITA.LI.CA prognostic system quartiles, and the log-rank test was used to compare differences in survival.
Specific statistical analyses [22] were conducted to develop and validate the ITA.LI.CA prognostic system. Here we describe only the rationale behind this process and the main statistical analyses, while more statistical details are described in S2 and S3 Texts.
Development of the ITA.LI.CA Prognostic System
We first defined the ITA.LI.CA tumor staging as a composite variable based on four main stages: 0 (very early), A (early), B (intermediate), and C (advanced) (Table 1). These stages were similar to the BCLC stages but were designed to have some important differences from the BCLC system so as to avoid several confusing aspects of that system. First, ITA.LI.CA stages are based only on tumor characteristics (i.e., Eastern Cooperative Oncology Group (ECOG) performance status test (PST) and CPS did not contribute to stage definition). Second, single tumor >5 cm was considered B stage, regardless of the treatment received. Third, based on published data and clinical knowledge [7,8,23], B stage patients were further stratified into three sub-stages: B1, single HCC >5 cm or 2–3 nodules measuring 3–5 cm; B2, 2–3 nodules measuring >5 cm or >3 nodules measuring ≤5 cm; B3, >3 nodules measuring >5 cm or presence of intrahepatic vascular invasion. Stage C included only HCC patients with extrahepatic vascular invasion or metastases [23].
Tab. 1. The ITA.LI.CA tumor staging system. 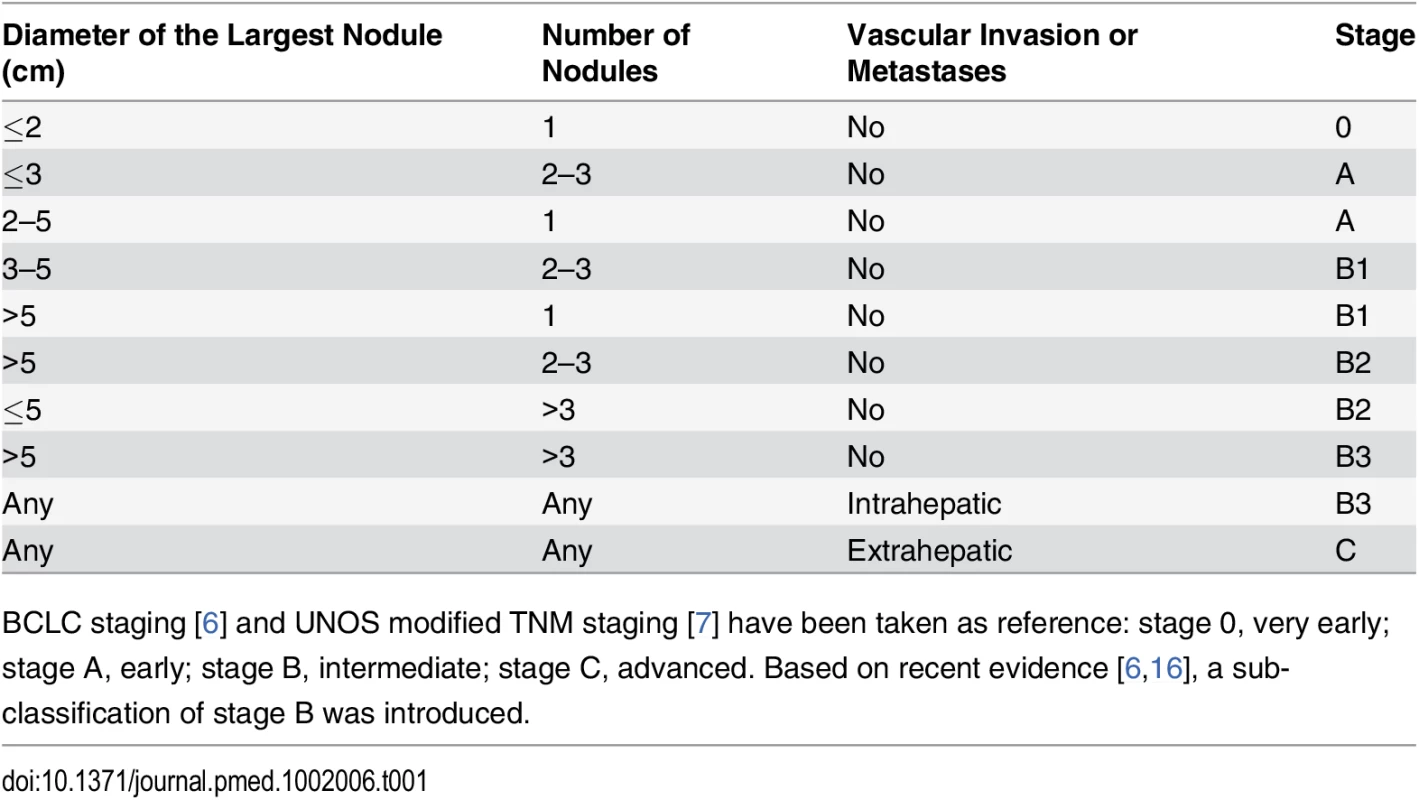
BCLC staging [6] and UNOS modified TNM staging [7] have been taken as reference: stage 0, very early; stage A, early; stage B, intermediate; stage C, advanced. Based on recent evidence [6,16], a sub-classification of stage B was introduced. Similar to in BCLC and HKLC staging [6,8], established variables that have determinative roles in prognostic assessment of HCC patients—ITA.LI.CA tumor staging, CPS, ECOG PST, and alpha-fetoprotein (AFP)—were selected a priori in building the prognostic system. AFP was included in the ITA.LI.CA system because of its known prognostic relevance [12] and new evidence suggesting its utility as an exclusion criterion for liver transplantation [24,25]. AFP was added as a categorical variable (AFP > or ≤ 1,000 μg/l); 1,000 μg/l was used as the cutoff because this value represented the AFP threshold yielding the best prognostic discrimination in the multivariate survival model (better also than AFP used as a continuous variable).
To develop the ITA.LI.CA integrated system for individual prognostic prediction, we selected overall survival as the outcome of interest. We therefore modeled overall survival based on the above four prognostic factors (i.e., ITA.LI.CA tumor staging, CPS, ECOG PST, and AFP) using a multivariable survival model to account for their relative effects.
Exploratory Analyses
As explained in S2 Text and S1 and S2 Figs, a multivariable parametric model was chosen to construct the prognostic score in the training set.
Performance Assessment of the ITA.LI.CA Prognostic System
The performance of the ITA.LI.CA prognostic system was assessed based on a rigorous validation methodology (through internal validation and external validation in a large population from Taiwan). To compare the prognostic performance of the ITA.LI.CA prognostic score with that of other systems, we calculated the Akaike information criterion (AIC), the concordance index (C index), and the test for trend chi-square [22,26]. The lower the AIC value, the higher the discriminatory ability of the staging system. The higher the C index and the test for trend chi-square, the higher the discriminatory ability and monotonicity of gradients of the staging system. To measure whether the performance of the ITA.LI.CA score was significantly better than that of other systems we used the likelihood ratio test.
Due to the long enrollment period (from 1987 to 2012) of the ITA.LI.CA population, a subgroup analysis for time period was also performed, to evaluate and overcome potential time-related biases.
Results
Characteristics of the Study Groups
The characteristics of the study groups are presented in Table 2. There were no differences in the baseline characteristics of the training set and internal validation set. The median age of the three groups (training, internal validation, and external validation) was 68, 67, and 65 y, respectively; in all groups, there was a preponderance of male gender (75%, 76%, and 78%, respectively). As expected, the majority of patients in the Taiwan validation group were hepatitis B carriers (55%), whereas in the Italian cohort, the majority of patients were hepatitis C carriers (61%). Liver function was better preserved in the external validation group (median Model for End-Stage Liver Disease [MELD] score = 8) than in the Italian cohort (median MELD score = 11). While the number of tumors was similar among the three groups, patients from Taiwan were more likely to have larger tumors and vascular invasion. Taiwanese patients also tended to have a high ECOG PST, compared with the two Italian sets. As expected, hepatic resection was the most common therapeutic approach in the Taiwanese cohort [20].
Tab. 2. Patient characteristics of the three groups. 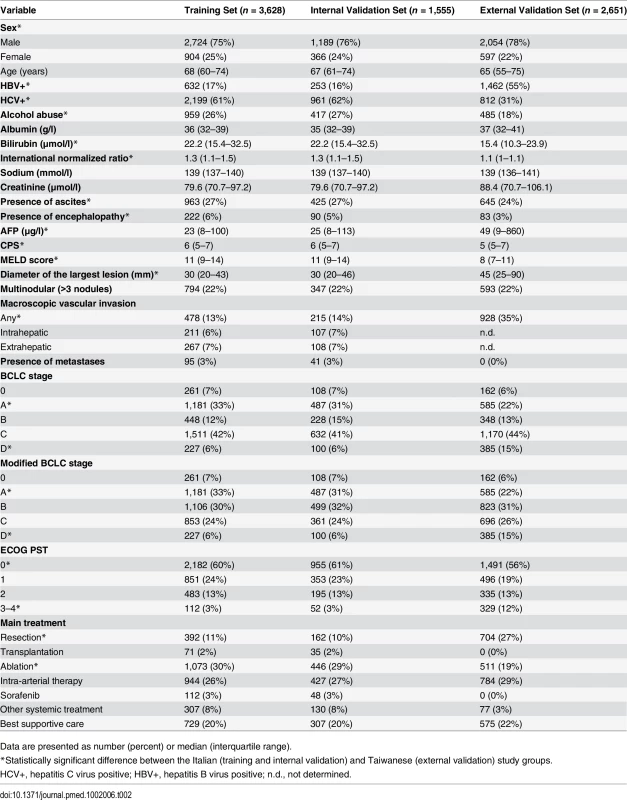
Data are presented as number (percent) or median (interquartile range). The ITA.LI.CA Prognostic System
Median duration of follow-up was 58 mo for the Italian patients (interquartile range, 26–106 mo) and 39 mo for the Taiwanese patients (interquartile range, 12–61 mo). Overall survival was significantly lower (log-rank test, p < 0.001) in the Italian patients than in the Taiwanese patients (S3 Fig). For the Italian patients, the median overall survival was 32 mo (interquartile range, 14–64 mo), and the 1-, 3-, and 5-y overall survival rates were 80%, 48%, and 29%, respectively. For Taiwanese patients, the median overall survival was 57 mo (interquartile range, 15–104 mo), and the 1-, 3-, and 5-y overall survival rates were 78%, 61%, and 49%, respectively.
In building the model, four main prognostic factors were selected: ITA.LI.CA tumor staging, ECOG PST, CPS, and AFP (Table 3). These variables had the following impact in determining the final score. For ITA.LI.CA tumor staging, one point was assigned for each increase in stage (from 0 to 5 for 0, A, B1, B2, B3, and C). For CPS to define liver function, zero points were assigned for a CPS of 5, one point for a CPS of 6 or 7, two points for a CPS of 8 or 9, and three points for a CPS > 10. For ECOG PST, zero points were assigned for a PST of 0, one point for a PST of 1–2, and three points for a PST of 3–4. CPS and PST points together formed the ITA.LI.CA functional score. For AFP level, two points were assigned for AFP > 1,000 μg/l.
Tab. 3. Development of the ITA.LI.CA prognostic system. 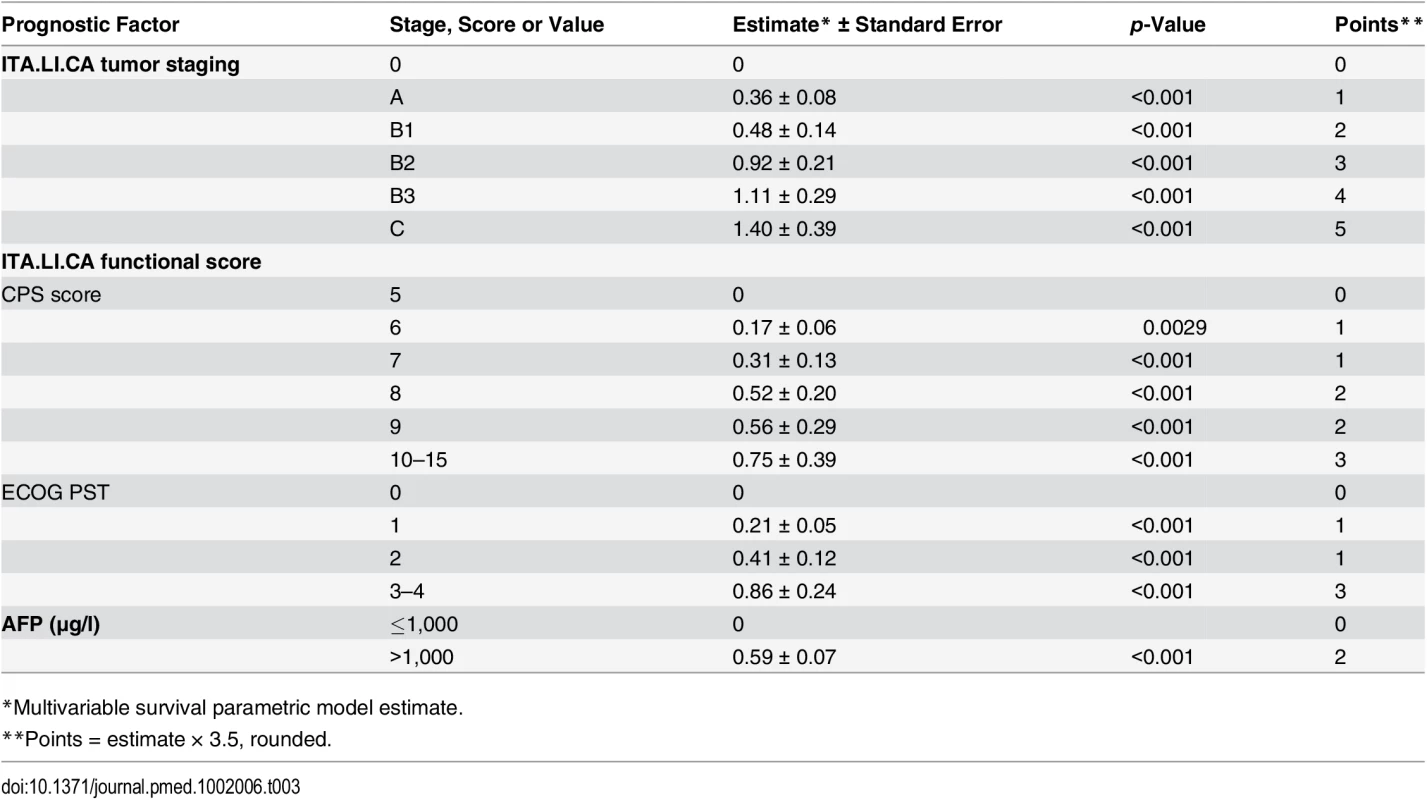
*Multivariable survival parametric model estimate. The lowest score (ITA.LI.CA score = 0) of the model corresponded to the best prognosis, and the highest score (ITA.LI.CA score = 13) was associated with the worst prognosis (S2 Table; p < 0.001 at log-rank test). To test the prognostic calibration of the ITA.LI.CA score, patients were divided into quartiles at the 25th, 50th, and 75th percentiles of the risk score. Quartile 1 coincided with ITA.LI.CA score ≤ 1, quartile 2 with score 2–3, quartile 3 with score 4–5, and quartile 4 with score > 5.
Observed median survival in the training, internal, and external validation cohort (Figs 1–3) was 57, 61, and >129 mo, respectively, in quartile 1; 43, 38, and 75 mo, respectively, in quartile 2; 23, 23, and 56 mo, respectively, in quartile 3; and 9, 8, and 10 mo, respectively, in quartile 4. The calibration was optimal in the ITA.LI.CA training and validation sets (Figs 1 and 2). In the external validation set, calibration was better for quartiles 1 and 2 than for quartiles 3 and 4 (Fig 3).
Fig. 1. Expected versus observed survival in the training cohort. 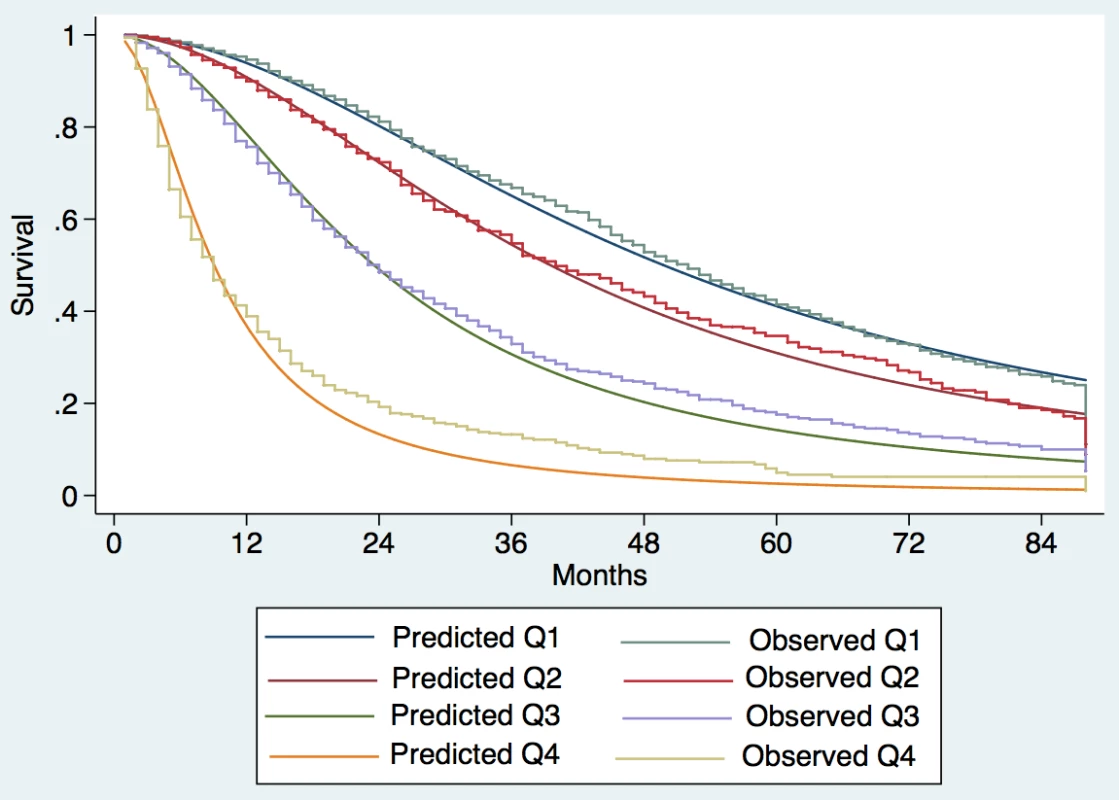
Patients were divided into quartiles at the 25th, 50th, and 75th percentiles of the risk score. Quartile 1 coincided with ITA.LI.CA score ≤ 1, quartile 2 with score 2–3, quartile 3 with score 4–5, quartile 4 with score >5. Fig. 2. Expected versus observed survival in the internal validation cohort. 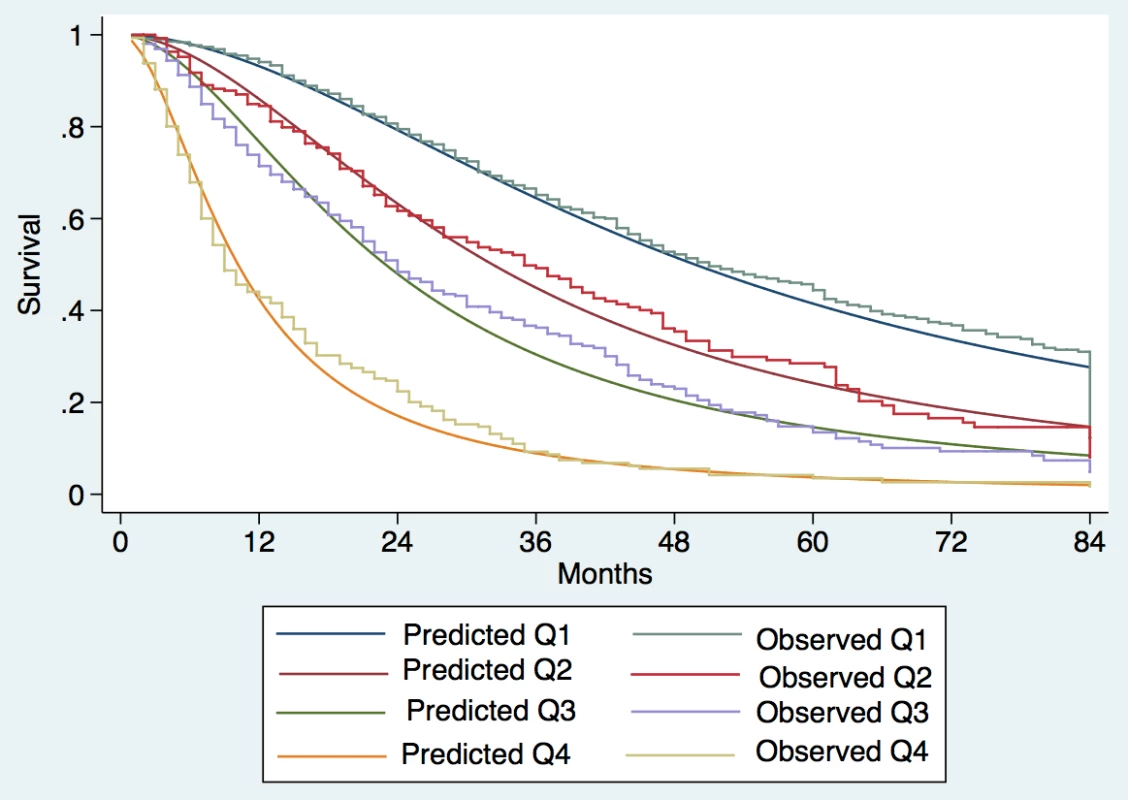
Patients were divided into quartiles at the 25th, 50th, and 75th percentiles of the risk score. Quartile 1 coincided with ITA.LI.CA score ≤ 1, quartile 2 with score 2–3, quartile 3 with score 4–5, quartile 4 with score > 5. Fig. 3. Expected versus observed survival in the external validation cohort. 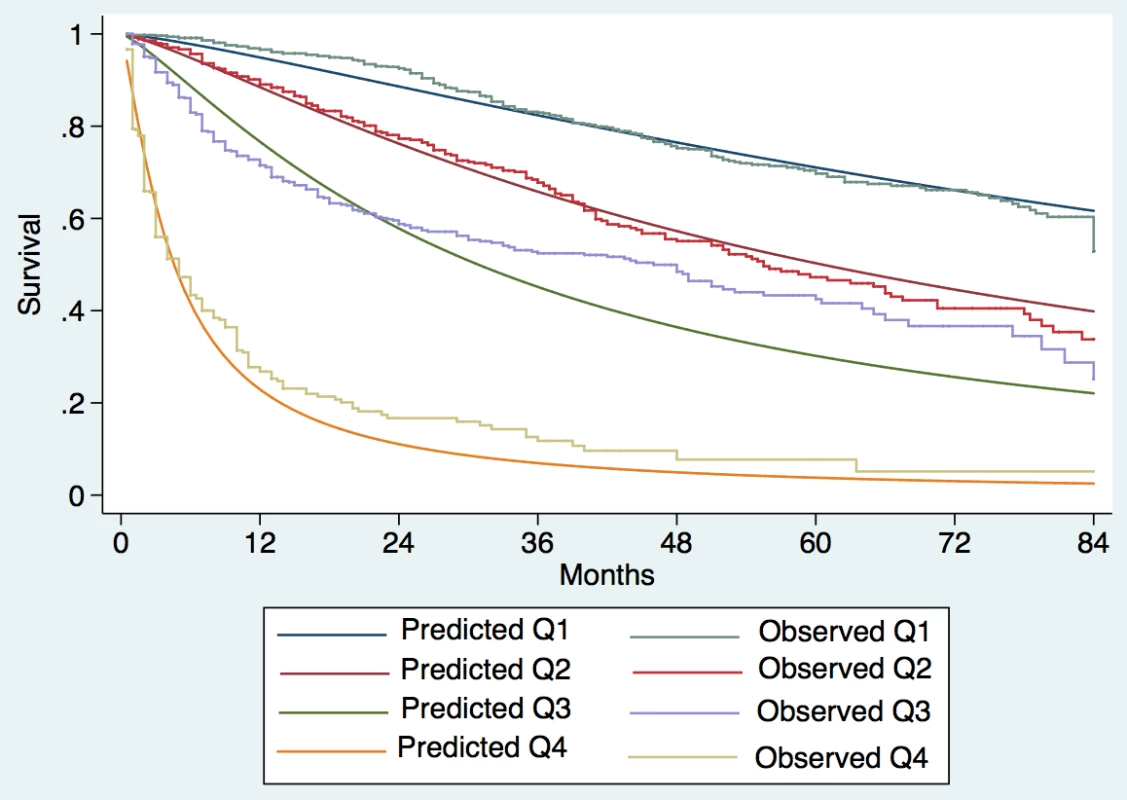
Patients were divided into quartiles at the 25th, 50th, and 75th percentiles of the risk score. Quartile 1 coincided with ITA.LI.CA score ≤ 1, quartile 2 with score 2–3, quartile 3 with score 4–5, quartile 4 with score > 5. The ITA.LI.CA score showed the best discriminatory ability and monotonicity of gradients among the most common HCC staging systems (Table 4) in all three study cohorts (training, internal validation, and external validation). In particular, the C index of the ITA.LI.CA score in the internal and external validation cohorts was 0.71 and 0.78, respectively. The ITA.LI.CA score’s prognostic ability was superior to that of BCLC stage (respective C indexes of 0.64 and 0.73), CLIP score (0.68 and 0.75), JIS stage (0.67 and 0.70), MESIAH score (0.69 and 0.77), and HKLC stage (0.68 and 0.75). By using the likelihood ratio test to compare different survival models, the prognostic performance of the ITA.LI.CA system always resulted in significantly better discrimination ability (p < 0.001) than the other systems in all three study groups. The superiority of the ITA.LI.CA score was also confirmed after stratification of the analysis for time period (Table 5).
Tab. 4. Discrimination ability of the integrated ITA.LI.CA prognostic system and comparison with other staging systems in the training, internal validation, and external validation cohorts. 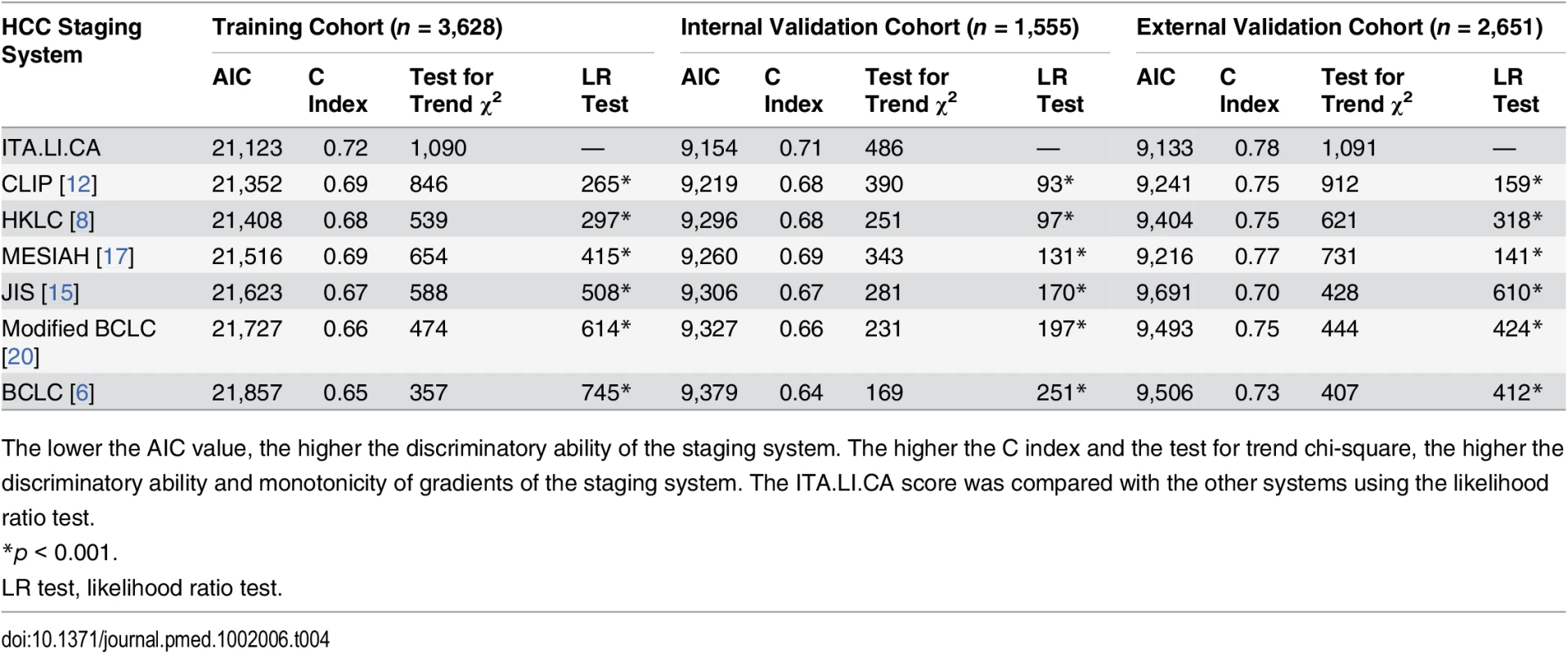
The lower the AIC value, the higher the discriminatory ability of the staging system. The higher the C index and the test for trend chi-square, the higher the discriminatory ability and monotonicity of gradients of the staging system. The ITA.LI.CA score was compared with the other systems using the likelihood ratio test. Tab. 5. Discrimination ability of the integrated ITA.LI.CA prognostic system and comparison with other staging systems in the training and internal validation cohorts (n = 5,183) stratified based on study period. 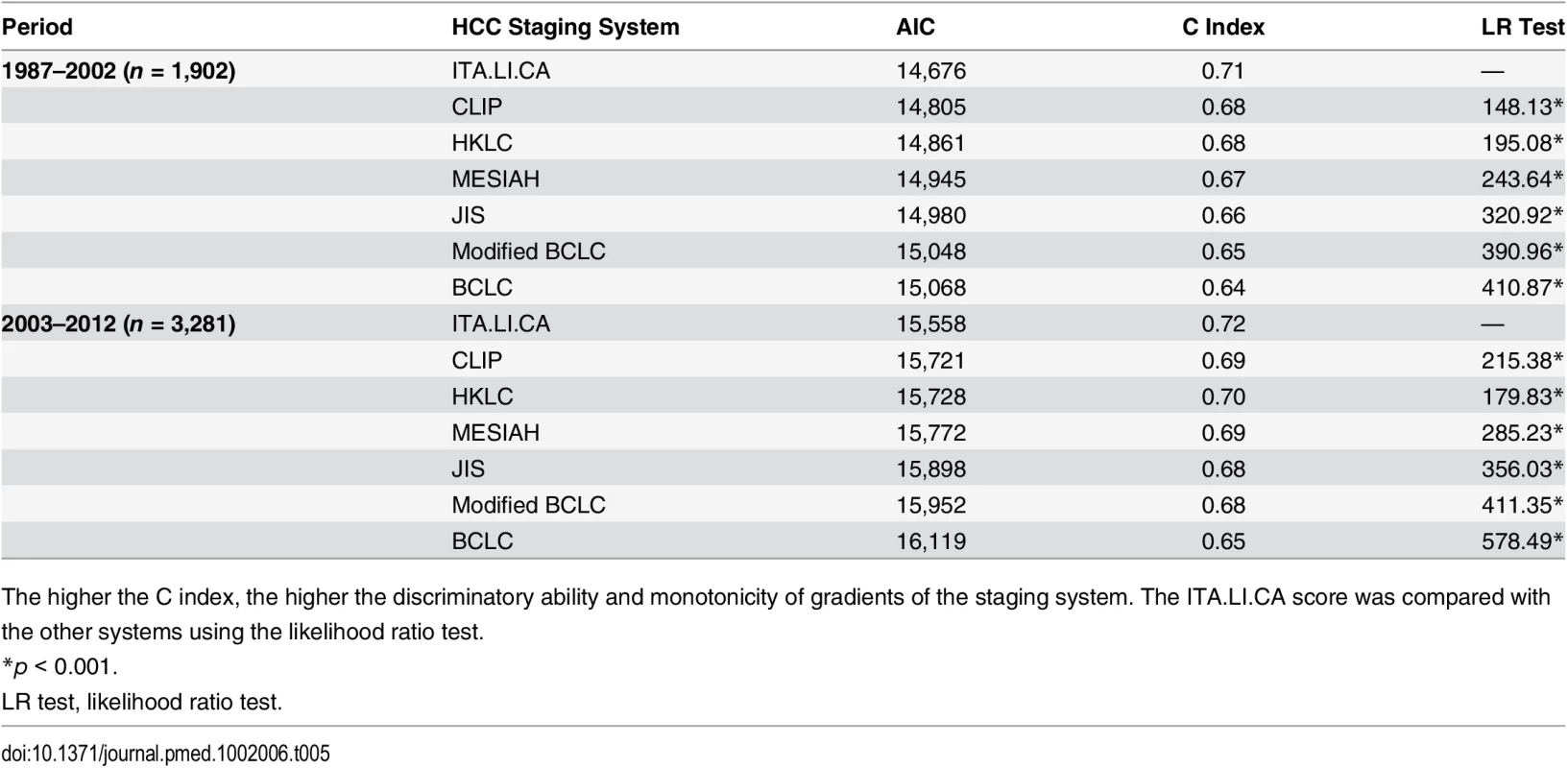
The higher the C index, the higher the discriminatory ability and monotonicity of gradients of the staging system. The ITA.LI.CA score was compared with the other systems using the likelihood ratio test. Discussion
The main purpose of this study is the proposal of a new ITA.LI.CA prognostic system for HCC including both tumor staging, with the potential to be used in the clinical management of HCC patients, and an integrated prognostic score, to predict individual survival. This new prognostic system has a strong prognostic ability in at least one European and one Asian population.
The ITA.LI.CA tumor staging was defined by specific stages (0, A, B1, B2, B3, and C) representing a synthesis of recent data from the literature on HCC prognosis (Table 1) [7,8,23,27]. The use of ITA.LI.CA tumor staging and CPS to develop the prognostic score was also supported by their comparison with other tumor staging and liver function assessment systems described in the literature (S1 Table). In particular, the ITA.LI.CA tumor staging showed a higher discrimination ability than UNOS TNM staging [7] and HKLC staging [8], and CPS had a higher prognostic power than MELD score [28] and the new albumin—bilirubin grade [29].
The definition of some main prognostic subgroups of HCC patients (i.e., tumor stages) may be useful to drive common therapeutic strategies or to design clinical trials [3–5]. However, tumor staging may be inaccurate in determining individual prognosis [10]. For this reason, we integrated the ITA.LI.CA tumor staging with CPS, ECOG PST, and AFP in a multivariable survival model to construct the ITA.LI.CA prognostic system. We investigated the discrimination ability of the ITA.LI.CA score relative to other systems. Of note, our system showed the highest discrimination ability in all study cohorts (Table 4). BCLC staging has been considered by American and European guidelines to be the best system to predict survival in HCC patients [4,5]. However, several studies have shown that this staging system has several limitations, mainly related to the large heterogeneity of BCLC stage B and C patients [23], the controversial prognostic role of ECOG PST [20] (which can be influenced by liver function, cancer symptoms, or both), and the lack of statistical weighting of different factors such as tumor status, liver function variables, and ECOG PST [8,10]. In the current study, BCLC staging had the lowest prognostic power among the different prognostic systems, while the ITA.LI.CA score had the highest prognostic power (Table 4).
Therapeutic choice is probably the most important prognostic factor for HCC patients [3]. However, none of the proposed HCC prognostic systems [6–17] include this variable for two main reasons. From a clinical point of view, treatment choice is extremely difficult to predict, especially in HCC patients, for whom many therapies are available and many variables influence treatment decision. From a statistical point of view, there are many interactions between therapy and other prognostic variables (i.e., tumor size and CPS influence simultaneously treatment decision and survival). A potential solution to this problem is to not include treatment as a prognostic variable, but rather consider treatment decision as an outcome variable, proposing a treatment scheme linked to the staging system (i.e., BCLC or HKLC algorithm) [3–6,8]. Treatment algorithms, such as the BCLC and HKLC ones, however, are by nature rigid tools, giving only one treatment option for each stage or sub-stage, and intrinsically not open to treatment alternatives and evolution [23]. This difficulty explains why, in everyday clinical practice, HCC guidelines are usually disregarded [30–35].
Based on these considerations, we deliberately did not propose a treatment algorithm in the present study. Although this study was designed with a prognostic intent, the variables included in the ITA.LI.CA prognostic system have an established impact on treatment decision, as recently underlined by the position paper of the Italian Association for the Study of the Liver [28]: (a) size and number of nodules and vascular invasion (used to determine tumor stage 0, A, B1, B2, B3, C), (b) the liver function and patient-related variables CPS and ECOG PST (the ITA.LI.CA functional score), and (c) AFP level. In particular, the ITA.LI.CA tumor staging preserves the basic staging system of very early (0), early (A), intermediate (B1, B2, and B3), and advanced (C) stages, since staging according to this system is the main criterion for treatment decisions in current guidelines. CPS and ECOG PST (composing the ITA.LI.CA functional score) and AFP level are well known variables used to evaluate treatment feasibility and aggressiveness within each tumor stage [3–5,28].
For clinical use, the ITA.LI.CA prognostic system can be synthetized in a single formula, TSFA, where TS is the tumor stage, F is point value of the ITA.LI.CA functional score, and A is the AFP point value. For example, the formula A22 indicates a patient with a stage A HCC, with an ITA.LI.CA functional score of two points (CPS of 6–7 and PST of 1–2 or CPS of 8–9 and PST of 0), and with two points for AFP (>1,000 μg/l); the formula C00 indicates a stage C HCC, with a CPS of 5 and a PST of 0 and with AFP ≤ 1,000. Considering that one point is assigned to each tumor stage (Table 3, from 0 to 5 for stages from 0 to C), this simplified, user-friendly formula can synthetize all ITA.LI.CA system components and the prognostic score, but also provide an accurate clinical description of each HCC patient with the potential to be used for deciding patient treatment or designing clinical trials.
Unlike other HCC prognostic scores not linked to treatment recommendations [11–17], the ITA.LI.CA integrated prognostic system is compatible both with current guidelines [3–5,28] and with a more modern management of HCC patients. Recent evidence, for example, supports expanded use of surgery as first-line therapy regardless of tumor status, provided that it is technically and oncologically feasible [30–33], and the use of percutaneous ablation and intra-arterial therapies in conjunction for early and intermediate HCCs [34,36].
This study has several potential limitations, however. The main limitation of this study is its retrospective nature. A prospective trial would be the ideal setting to validate the ITA.LI.CA prognostic system. On the other hand, the high number of enrolled patients, the strong methodology (internal and external validation), and the heterogeneity in treatment allocation during the study period gave us the opportunity to compare different prognostic systems. Such a comparison would be really difficult in a prospective trial in both ethical and statistical (sample size) terms. Moreover, it has to be underlined that all main prognostic systems currently used for HCC patients [6–17] were developed using retrospective series based on cohorts significantly smaller than that used in the present study.
A second limitation concerns the intrinsically significant differences between the Taiwanese and Italian groups (Table 2), particularly in cancer etiology, laboratory data, tumor stage distribution, and, most importantly, treatment choices. In particular, we would underline the crucial issue of differences in etiology among European and Asian countries [33,36,37]. Another important difference between the Italian and Taiwan cohorts is the different enrollment periods. All these differences could potentially introduce selection biases. In this study, we performed specific subgroup analyses showing that the prognostic power of the ITA.LI.CA prognostic system was not influenced by enrollment period (Table 5).
The differences between the Taiwanese and Italian groups probably explain why the survival of Taiwanese patients was significantly greater than that of Italian patients with the same ITA.LI.CA score (Figs 1–3 and S2 Fig). On the other hand, the ITA.LI.CA system performed as the best prognostic score also in the Taiwanese patients (Tables 4 and 5). Thus, although the Italian and Taiwanese groups differed in their survival estimations, this study demonstrated a broad applicability of the proposed system both in European and Asian populations.
In conclusion, the ITA.LI.CA prognostic system demonstrated the best ability of the compared systems to predict the survival of Italian and Taiwanese patients. Moreover, it allows a simple but accurate clinical description of each HCC patient, with the potential to be used for deciding treatment or designing clinical trials.
Supporting Information
Zdroje
1. Zhao C, Nguyen MH. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J Clin Gastroenterol. 2016;50 : 120–133. 26583266
2. Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24 : 1–17. doi: 10.1016/j.soc.2014.09.001 25444466
3. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35 : 421–430. 11592607
4. European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56 : 908–943. doi: 10.1016/j.jhep.2011.12.001 22424438
5. Bruix J, Sherman M, Practice Guidelines Committee of the American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42 : 1208–1236. 16250051
6. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19 : 329–338. 10518312
7. United Network for Organ Sharing. Important policy notice. 2011 Dec 15 [cited 5 Mar 2015]. Available: http://optn.transplant.hrsa.gov/SharedContentDocuments/2011-11_Policy_Notice.pdf.
8. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146 : 1691–1700. doi: 10.1053/j.gastro.2014.02.032 24583061
9. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th edition. New York: Springer; 2010.
10. Kim BH, Park JW, Nam BH, Kwak HW, Kim WR. Validation of a model to estimate survival in ambulatory patients with hepatocellular carcinoma: a single-centre cohort study. Liver Int. 2014;34:e317–e323. doi: 10.1111/liv.12519 24606128
11. Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56 : 918–928. 2990661
12. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28 : 751–755. 9731568
13. Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31 : 133–141. 10424293
14. Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94 : 1760–1769. 11920539
15. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38 : 207–215. 12673442
16. Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54 : 419–425. 15710994
17. Yang JD, Kim WR, Park KW, Chaiteerakij R, Kim B, Sanderson SO, et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56 : 614–621. doi: 10.1002/hep.25680 22370914
18. Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1 : 1–85. 4950264
19. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60 : 646–649. 4541913
20. Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Lin HC, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology. 2013;57 : 112–119. doi: 10.1002/hep.25950 22806819
21. Baraldi AN, Enders CK. An introduction to modern missing data analyses. J Sch Psychol. 2010;48 : 5–37. doi: 10.1016/j.jsp.2009.10.001 20006986
22. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21 : 128–138. 20010215
23. Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32 : 348–359. doi: 10.1055/s-0032-1329906 23397536
24. Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143 : 986–994. doi: 10.1053/j.gastro.2012.05.052 22750200
25. Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20 : 945–951. doi: 10.1002/lt.23904 24797281
26. Stone M. Akaike’s criteria. In: Armitage P, Colton T, editors. Encyclopedia of biostatistics. Chichester: Wiley; 1998. pp. 123–124.
27. Bolondi L, Cillo U, Colombo M, Craxi A, Farinati F, Giannini EG, et al. Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis. 2013;45 : 712–723. 23769756
28. Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6 : 1416–1421. 16686765
29. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—the ALBI grade. J Clin Oncol. 2015;33 : 550–558. doi: 10.1200/JCO.2014.57.9151 25512453
30. Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62 : 440–451. doi: 10.1002/hep.27745 25678263
31. Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol. 2015;62 : 617–624. doi: 10.1016/j.jhep.2014.10.037 25450706
32. Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257 : 929–937. 23426336
33. Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260 : 329–340. 24096763
34. Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31 : 426–432. doi: 10.1200/JCO.2012.42.9936 23269991
35. Wang Y, Deng T, Zeng L, Chen W. Efficacy and safety of radiofrequency ablation and transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2015;46 : 58–71. doi: 10.1111/hepr.12568 26265000
36. McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19 : 223–238. 25921660
37. Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH, et al. Prognosis of hepatocellular carcinoma: assessment of eleven staging systems. J Hepatol. 2016;64 : 601–608. doi: 10.1016/j.jhep.2015.10.029 26551516
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 4- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Nutraceutikum Armolipid Plus podle klinických důkazů zlepšuje lipidový profil − metaanalýza
- Snižuje terapie betablokátory kardiovaskulární benefit aerobního cvičení u pacientů s arteriální hypertenzí?
-
Všechny články tohoto čísla
- Why Are Some Population Interventions for Diet and Obesity More Equitable and Effective Than Others? The Role of Individual Agency
- Risk of Bias in Systematic Reviews of Non-Randomized Studies of Adverse Cardiovascular Effects of Thiazolidinediones and Cyclooxygenase-2 Inhibitors: Application of a New Cochrane Risk of Bias Tool
- The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control
- The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials
- Disentangling the Association between Statins, Cholesterol, and Colorectal Cancer: A Nested Case-Control Study
- Gender Differences in Homicide of Neonates, Infants, and Children under 5 y in South Africa: Results from the Cross-Sectional 2009 National Child Homicide Study
- Mobile Phones As Surveillance Tools: Implementing and Evaluating a Large-Scale Intersectoral Surveillance System for Rabies in Tanzania
- Building Learning Health Systems to Accelerate Research and Improve Outcomes of Clinical Care in Low- and Middle-Income Countries
- The Future of the RTS,S/AS01 Malaria Vaccine: An Alternative Development Plan
- Birth “Out-of-Hours”: An Evaluation of Obstetric Practice and Outcome According to the Presence of Senior Obstetricians on the Labour Ward
- A Nested Case–Control Study of Metabolically Defined Body Size Phenotypes and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC)
- Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma
- Underweight, Markers of Cachexia, and Mortality in Acute Myocardial Infarction: A Prospective Cohort Study of Elderly Medicare Beneficiaries
- The Impact of Hotspot-Targeted Interventions on Malaria Transmission in Rachuonyo South District in the Western Kenyan Highlands: A Cluster-Randomized Controlled Trial
- Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial
- Is There Evidence of Poorer Birth Outcomes for Mothers and Babies When the Most Senior Obstetrician Is Not On Site?
- Clinical Implications of Cancer Genomics: A Call for Papers
- The ITA.LI.CA Staging System: A Novel Staging System for Hepatocellular Carcinoma
- Observational Evidence of For-Profit Delivery and Inferior Nursing Home Care: When Is There Enough Evidence for Policy Change?
- Child Homicide: A Global Public Health Concern
- The Chernobyl Disaster and Beyond: Implications of the Sendai Framework for Disaster Risk Reduction 2015–2030
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Observational Evidence of For-Profit Delivery and Inferior Nursing Home Care: When Is There Enough Evidence for Policy Change?
- Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial
- The Chernobyl Disaster and Beyond: Implications of the Sendai Framework for Disaster Risk Reduction 2015–2030
- Is There Evidence of Poorer Birth Outcomes for Mothers and Babies When the Most Senior Obstetrician Is Not On Site?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



