-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTowards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
Type VI secretion systems (T6SS) are trans-envelope machines dedicated to the secretion of virulence factors into eukaryotic or prokaryotic cells, therefore required for pathogenesis and/or for competition towards neighboring bacteria. The T6SS apparatus resembles the injection device of bacteriophage T4, and is anchored to the cell envelope through a membrane complex. This membrane complex is composed of the TssL, TssM and TagL inner membrane anchored proteins and of the TssJ outer membrane lipoprotein. Here, we report the crystal structure of the enteroaggregative Escherichia coli Sci1 TssJ lipoprotein, a two four-stranded β-sheets protein that exhibits a transthyretin fold with an additional α-helical domain and a protruding loop. We showed that TssJ contacts TssM through this loop since a loop depleted mutant failed to interact with TssM in vitro or in vivo. Biophysical analysis of TssM and TssJ-TssM interaction suggest a structural model of the membrane-anchored outer shell of T6SS. Collectively, our results provide an improved understanding of T6SS assembly and encourage structure-aided drug design of novel antimicrobials targeting T6SS.
Published in the journal: . PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002386
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002386Summary
Type VI secretion systems (T6SS) are trans-envelope machines dedicated to the secretion of virulence factors into eukaryotic or prokaryotic cells, therefore required for pathogenesis and/or for competition towards neighboring bacteria. The T6SS apparatus resembles the injection device of bacteriophage T4, and is anchored to the cell envelope through a membrane complex. This membrane complex is composed of the TssL, TssM and TagL inner membrane anchored proteins and of the TssJ outer membrane lipoprotein. Here, we report the crystal structure of the enteroaggregative Escherichia coli Sci1 TssJ lipoprotein, a two four-stranded β-sheets protein that exhibits a transthyretin fold with an additional α-helical domain and a protruding loop. We showed that TssJ contacts TssM through this loop since a loop depleted mutant failed to interact with TssM in vitro or in vivo. Biophysical analysis of TssM and TssJ-TssM interaction suggest a structural model of the membrane-anchored outer shell of T6SS. Collectively, our results provide an improved understanding of T6SS assembly and encourage structure-aided drug design of novel antimicrobials targeting T6SS.
Introduction
Pathogenic bacteria have evolved numerous and original strategies to invade host tissues, colonize new niches or to kill predators. Bacteria are able to adhere to various surfaces and to actively release protein toxins. The delivery of effectors in the milieu, into host cells or bacteria involves dedicated machineries called secretion systems. Among the six secretion systems identified in Gram negative bacteria, the recently identified Type VI secretion system (T6SS) is composed of 13 core components which form a trans-envelope apparatus [1]. The T6SS are highly versatile in terms of functions [1]–[4]. T6SS have been found to be required for resisting predation or for pathogenesis in several bacteria: in Vibrio cholerae, the T6SS is required to escape amoeba predation, or for killing host cells by modification of the host cell cytoskeleton and subsequent impairing phagocytic activity [5]–[6]. Beside the role of several T6SS in pathogenesis towards animal or plant models, it was recently reported that T6SS are involved in stress sensing, in regulating bacteria-bacteria interactions or in targeting other bacterial cells, and may therefore help in competition towards a specific niche [2], [7], [8]. When not required for pathogenesis, T6SS yet provide a critical advantage to neighbouring bacteria, allowing an improved colonization efficiency.
A hallmark of T6SS is that two proteins are found in culture supernatants of bacteria producing T6SS: Hcp and VgrG [1]. The crystal structures of Hcp and VgrG have been reported: Hcp forms hexameric rings leaving a pore of ∼40 Å [9] whereas three VgrG assemble to form a syringe-like structure [10]–[12]. Phylogenetic and structural data have shown that these two proteins share remarkable homologies with bacteriophage components. The Hcp structure is superimposable to the major tail protein gpV of bacteriophage λ (bacteriophage T4 gp19 protein; [9], [13]) whereas VgrG has a fold highly similar to the gp27-gp5 complex, the cell puncturing device of bacteriophage T4 [10], [11], [14]. Several other subunits of Type VI secretion systems also share a common evolutionary history with other bacteriophage baseplate or sheath components [1], [15]. These include TssE, a homologue of the baseplate gp25 protein, and TssB and TssC, which have been shown to form tubular structures resembling the bacteriophage tail sheath (the nomenclature used in this manuscript follows the general Tss nomenclature [16]). Interestingly, the Vibrio cholerae TssB/TssC (VipA/VipB) tubular structures are disassembled by TssH, an AAA+ traffic ATPase of the Clp family [17]. The current model suggests that these proteins may assemble an extracellular tubular structure composed of the Hcp protein carrying the VgrG protein at the tip [18]. This upside-down bacteriophage structure will thus deliver the VgrG protein in the milieu or into host cells [12], [19]. Several VgrG proteins carry an additional C-terminal domain which acts as an effector module with functions interfering with the host cytoskeleton or the host physiology [12].
Beside bacteriophage-derived components, a number of membrane-associated proteins were shown to be critical for T6SS. Among these components, TssL and TssM have close homologues in Type IVb secretion systems [1], [15], [20]. Two other T6SS genes, tssJ and tssH encode an outer membrane (OM) lipoprotein and an AAA+ ATPase, two components regularly found in bacterial secretion systems or in trans-envelope structures allowing the assembly of cell surface appendages [1], [21]. An immunoprecipitable complex composed of four proteins, TssJ, TssL, TssM and TagL has been evidenced in enteroaggregative Escherichia coli (EAEC) [22], [23]. TssM is an inner membrane (IM) protein with three transmembrane segments. Homologues of TssM in Agrobacterium tumefaciens and Edwardsiella tarda have been shown to interact with homologues of the TssL inner membrane protein and of the outer membrane lipoprotein TssJ [20], [24]. TssL interacts with TagL, an inner membrane protein carrying a peptidoglycan-binding motif of the OmpA/Pal/MotB family that anchors the T6SS to the cell wall [22], [23]. This membrane complex therefore links both membranes and the peptidoglycan layer. Although in vivo data have been accumulated on the topology of the membrane complex subunits and their interactions, little is known on the structural organization of these proteins. To gain structural information on the assembly of this complex, we initiated the purification of the different subunits. We report here the crystal structure of the TssJ protein of the enteroaggregative Escherichia coli Sci1 T6SS which likely constitute a prototype for all TssJ-like proteins. We also present biochemical data on the TssM protein and on the TssJ-TssM interaction. We provide in vitro and in vivo evidence for the function of a specific loop of TssJ in mediating contact with the TssM subunit, which therefore provide fundamental insight into T6SS biogenesis and topology.
Results
Crystal structure of the EAEC TssJ protein
A fragment of the tssJ gene of the sci1 cluster from enteroaggregative Escherichia coli consisting of amino-acid residues 2-155 of the processed TssJ lipoprotein (residues 25-178 of the full-length protein) was cloned in the Gateway vector pETG20A, with an N-terminal fusion hexahistidine tagged thioredoxin for purification [25]. This construct consists of a polypeptide chain starting at the glycine residue following the cysteine anchoring TssJ to an acyl chain. The numbering used in this report follows the sequence of the mature lipoprotein, between residues Cys1 (here mutated in Gly) and Lys155. The TssJ protein was purified by affinity chromatography and gel filtration, and the native TssJ protein was obtained upon fusion and tag cleavage by the TEV protease.
TssJ was analyzed by MALS/QELS/UV/RI (on-line multi-angle laser light scattering/quasi-elastic light scattering/absorbance/refractive index detectors) experiments [26]. The protein was shown to be a monomer at a concentration of 4 mg/mL (230 µM) at pH 7.5 in the presence of 100 mM NaCl. Mass and hydrodynamic radius calculation performed with the ASTRA software (Wyatt Technology) using a dn/dc value of 0.185 mL/g indicated a mass of 17260±800 Da, close to the theoretical mass of 16,899 Da (Figure S1, Tables S1 & S2).
TssJ crystallized readily with 2.2 M ammonium sulfate as a precipitant at pH 6.0 in sitting nano-drops [27]. We collected a native dataset and a dataset from a crystal soaked in CsI/NaI at beamline Proxima 1 (Soleil synchrotron, Saint-Aubin, France). The structure was solved from 2.0 Å resolution SIRAS (single isomorphous replacement with anomalous scattering) maps calculated using CsI as phasing agents, and the resolution limit was extended to 1.35 Å with the native data set (Figure S2, Table S3). The polypeptide chain could be traced from residue Ile23 to Pro151. The segment 1-22 anchoring the protein to the membrane via Cys1 and its phospholipid thioester as well as the last four residues were not ordered in the crystal.
A unique TssJ molecule is contained in the asymmetric unit, and the PISA server [28] did not identify any sufficient interactions between TssJ molecules related by crystallographic symmetry. We can therefore conclude that TssJ is a monomer in solution.
TssJ has the topology of a β-sandwich formed by two four-stranded β-sheets (Figure 1). Sheet one is composed or β-strands 4(-1), 1, 7 and 8 (1), and is packed against sheet 2 which contains β-strands 3(-1), 2, 5(-1) and 6. The other face of β-sheet 2 one is covered in part by three short helices (h1-3) occurring between β-strands 2 and 3. These helices exhibit B-factors larger than average, in particular the segment 56-71, between helices 2 and 3 which has very weak electron density. Of particular interest are the loops located between strands 1 and 2, and between strands 5 and 6. Another long loop incorporates the η1 helix between strands 6 and 7.
Fig. 1. Structure of the enteroaggregative E. coli T6SS TssJ subunit. 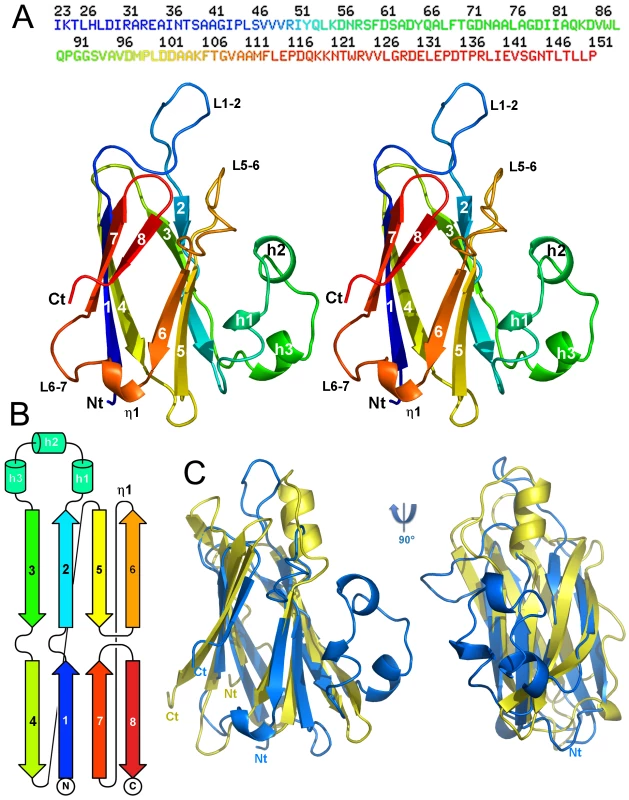
(A) Stereoview of TssJ in ribbon representation and rainbow coloring, from blue (N-term) to red (C-term); the sequence is represented above. Figure made with Pymol [61]. (B) Topology cartoon of TssJ (same coloring as in (A)). (C) Structural comparison of TssJ and its nearest homologue, transthyretin (1sn5), after superimposition. The topology is identical for both proteins and their β-sandwiches superimpose within 3.2 Å. Note the presence of an extra helical domain in TssJ, and an extra helix (top) in transthyretin. Searching the protein database for structurally related proteins with Dali [29] returned significant hits with transthyretin (1sn5 [30]; Z = 6.4; rmsd [root mean-square deviation] = 3.2 Å) on 87 residues among 116 involved in comparison (Figure 1C) and with 5-hydroxy-isourate hydrolase (3iwu [31]; Z = 6.3; rmsd = 3.3 Å). Both proteins originate from vertebrates, a mammalian blood transport protein and an enzyme from zebra fish, respectively [30], [31]. Dali also returned the recently determined structure of the ExsB lipoprotein from the P. aeruginosa Type III secretion system (T3SS) [32], although with lower scores (2yjl; Z = 5.2; rmsd = 4.0 Å; Figure S3). Noteworthy, TssJ helical domain (residues 57-71) is absent in transthyretin, 5-hydroxy-isourate hydrolase and ExsB, but a single helix is located on the same face, between strands 4 and 5 of transthyretin (Figure 1C). The extended loop (residues 38-45) between stands 1 and 2, forming a protruding extension, is also absent in the three proteins (Figure 1C, Figure S3). In the crystal, this loop points out of the core of the protein and is stabilized by contacts with a symmetry related molecule, although this contact is not biologically relevant due to its limited interface.
Sequence alignments of TssJ from the enteroaggregative E. coli Sci1 have been performed with the 49 closest sequences in the non redundant (NR) database (Figure S4). The 33 first sequences do not present insertions nor deletions, while the 17 more divergent ones exhibit a 3-residue insertion in the loop between stands 1 and 2, making this loop even longer. On the 16 conserved residues, 13 are present in the X-ray structure (Figure S5). The two first residues (Cys1 and Gly2) are of functional importance in TssJ proteins: Cys1 is the N-terminal acylated residue whereas Gly2 is responsible for the Lol-dependent outer membrane targeting [21]. Three conserved prolines (at positions 90, 99 and 116) are involved in structural integrity of strands-joining loops. A group of aromatic residues, Tyr65, Phe113 and Trp123 form also a structurally important hydrophobic cluster stabilizing the α-helical domain against the β-sandwich core. The other residues are scattered along the polypeptide chain and do not reveal interpretable features.
Analysis using the CASTp software [33] did not identify significant surface cavities with a volume larger than 100 Å3. In contrast, when the TssJ structure is overlaid with transthyretin and ExsB (Figure 1C, Figure S3), two domains protrude from the core of the protein: the loop between strands 1 and 2 (residues 38-45) and the helical domain (residues 57-81), the latter one presenting very high B-factors, especially between residues 65 and 75 (Figure S6A). The calculation of contact electrostatic potential did not highlight any hydrophobic or positively or negatively charged surface patches, but indicated a scattered and balanced charges distribution over the whole surface (Figure S6B).
TssM ekto-domain and sub-domains production and characterization
Enteroaggregative E. coli TssM is a large - 1129 amino-acids - protein with an N-terminal cytoplasmic domain (1-387) bearing three trans-membrane helices and a periplasmic domain of 744 residues (termed hereafter ekto-domain; Aschtgen and Cascales, unpublished data). JPRED [34] secondary structure predictions reveal that the first ∼500 residues (386-930) of the ekto-domain are helical while the C-terminus (931-1129) is essentially a β-domain (Table S2). We expressed three constructs of TssM domains in fusion with Trx and His tags: the ekto-N-terminal domain (ekto-Nt, 386-930), the ekto-C-terminal domain (ekto-Ct, 931-1129) and the full-length ekto domain (386-1129). The ekto-Nt domain was well expressed and soluble up to 0.7 mg/mL without the need of detergent addition. The ekto-Ct domain was produced as inclusion bodies and could not be purified. The full-length TssM-ekto was expressed in large quantities and remained soluble after TEV cleavage. It could be concentrated up to 9.0 mg/ml without the need of detergent. The CD spectra of these domains indicated that TssM-ekto-Nt is predominantly formed of α-helices, while a contribution of β-strands appears in the full-length TssM (Table S2, Figure S7A).
In vivo and in vitro interaction of TssJ with TssM
Since the TssJ lipoprotein has been proposed to facing the periplasm [21], we tested whether TssJ interacted with TssM-ekto in an in vivo co-immunoprecipitation assay. TssM-ekto and the two deletion variants, TssM-ekto-Nt and TssM-ekto-Ct were cloned downstream a signal peptide allowing their targeting to the periplasm. All TssM constructs were fused to a FLAG epitope. The full-length, acylated TssJ protein was produced with a C-terminal hemagglutinine (HA) tag [21]. Both proteins were produced from compatible plasmids in E. coli K12 (i.e., devoid of T6SS gene cluster) to test for direct interaction. Cells expressing both TssM-ekto and TssJ proteins were treated with formaldehyde and subjected to membrane solubilization. Solubilized extracts were then used in an immunoprecipitation assay with the anti-FLAG antibody. TssJ co-precipitated with TssM, whereas no TssJ was found associated with the resin in absence of TssM-ekto (Figure 2A). These results demonstrate that TssJ interacts with the periplasmic domain of TssM in vivo. Similar experiments performed with the TssM-ekto sub-domains further showed that TssJ interacts with TssM-ekto-Ct but not with the TssM-ekto-Nt variant (Figure 2A).
Fig. 2. TssJ interacts with the C-terminal domain of TssM. 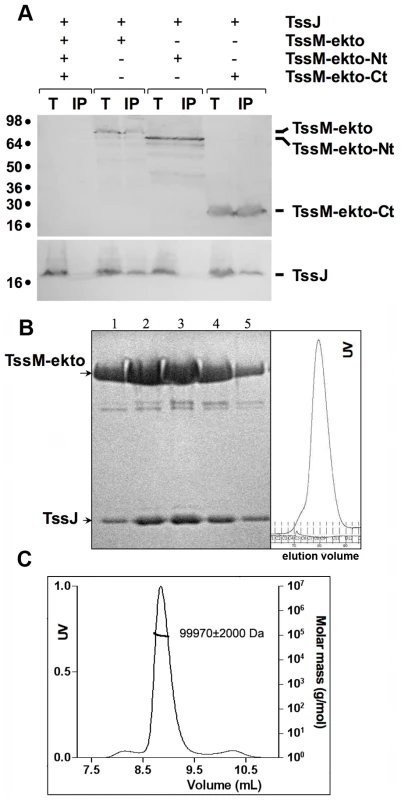
(A) Solubilized extracts of E. coli K12 W3110 strain producing (+) or not (-) HA-tagged TssJ and FLAG-tagged TssM-ekto or -Nt or –Ct derivatives were subjected to immunoprecipitation with anti-FLAG-coupled beads. The total solubilized material (T) and the immunoprecipitated material (IP) were loaded on a 12.5%-acrylamide SDS PAGE, and immunodetected with anti-HA (TssJ; lower panel) and anti-FLAG (TssM-ekto and sub-domains; upper panel) monoclonal antibodies. Immunodetected proteins are indicated on the right. Molecular weight markers are indicated on the left. (B) Gel filtration showing the direct interaction of TssM-ekto with TssJ. The SDS-PAGE analysis of the fractions is shown on the left panel. The chromatogram of the gel filtration is shown on the right panel. (C) MALS/QELS/UV/RI analysis of the TssM-ekto/TssJ complex. Purified TssJ and TssM-ekto produced in E. coli were tested in an in vitro interaction assay. Both proteins were mixed with a slight molar excess of TssJ and were subjected to gel-filtration. A peak was observed at an elution time slightly shorter compared to that of TssM-ekto alone, and analysis of this peak by SDS page revealed the presence of both partners (Figure 2B). Mass and hydrodynamic radius calculations confirmed the formation of the TssJ-TssM complex with a mass of 99,970±2,000 Da and a 1∶1 stoichiometry (Figure 2C, Table S2). Together, these results demonstrated that TssM-ekto and TssJ interact directly and form a complex of 1∶1 stoichiometry.
We analyzed the strength of the interaction by performing a surface plasmon resonance study (SPR) with a Biacore X100. TssJ was covalently coupled to a CM5 chip and TssM-ekto was injected in the microfluidic channel. The sensorgrams indicated that TssM-ekto was released in a short time without the necessity of chip regeneration (Figure 3A). The Kd could be calculated from the levels of the sensorgrams at equilibrium, since the values of Kon and Koff could not be obtained in such a context. The average of three experiments yielded a Kd value of 2.1±0.25 µM (Figure 3B). We then performed the symmetrical experiment using covalently linked TssM-ekto to the chip. Upon TssJ injection, a Kd value of 4.0±0.5 µM was measured (Figure 3B). In contrast, injection of TssM-ekto-Nt domain on the TssJ-coupled CM5 chip did not result in any signal on the sensorgram, revealing that TssJ does not interact with the TssM N-terminal, α-helical domain in vitro. The TssM-ekto-Ct domain was not tested in this assay as it was produced as inclusion bodies and could not be purified. Collectively, the data from the in vivo and in vitro experiments demonstrate the existence of a 1∶1 stoichiometry complex between the C-terminal periplasmic domain of TssM and the TssJ lipoprotein, with a Kd of 2-4 µM.
Fig. 3. Measure of the interaction between TssM-ekto and TssJ by Surface Plasmon Resonance. 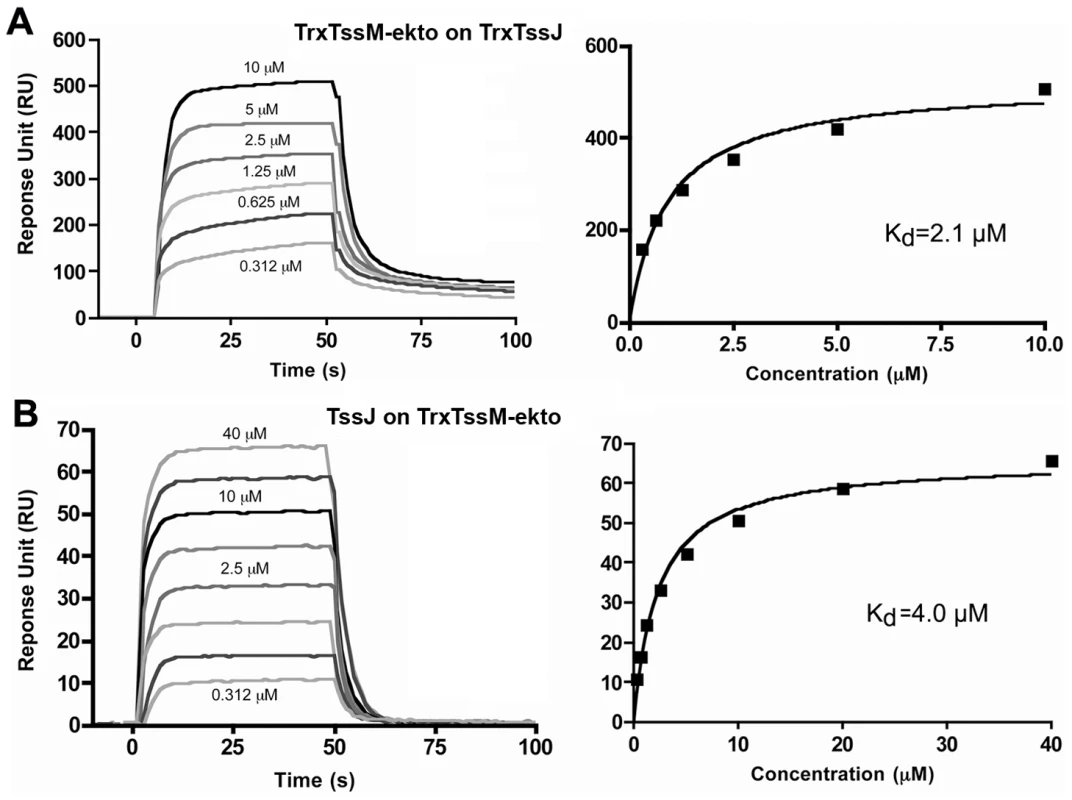
(A) Sensorgram and saturation curve of the titration of Trx-TssJ by Trx-TssM-ekto. The CM5 chip (BIAcore) was coated with TssJ N-terminal thioredoxine fusion with 600 response units (RU) and the Trx-TssM-ekto was injected in the microfluidic channel. (B) Sensorgram and saturation curve of the titration of Trx-TssM-ekto by TssJ. The CM5 chip was coated with TssM-ekto N-terminal thioredoxine fusion with 3000 response units, and TssJ was injected in the microfluidic channel. The KD values were obtained using the fitting tool of the BIAevaluation software (BIAcore). TssJ interaction mutants
Interactions between proteins require surface complementarities. The three dimensional structure of TssJ shows that it lacks any crevice able to host any putative TssM-ekto protruding domain. In contrast, TssJ exhibits extensions, compared to transthyretin and ExsB, which could interact with crevices on TssM. The helical domain is located on the face of the β-sandwich opposite to the N - and C-termini, while the extended loop is located at the apical side of the β-sandwich, opposite to the N-terminus, and hence to the outer membrane. These sites are however close enough to participate to a large interaction area with TssM. We therefore constructed TssJ mutants deleted of residues 39-42 from the L1-2 loop (TssJ-ΔL1-2) and of the helical domain (TssJ-Δαdom). The TssJ-ΔL1-2 mutant was expressed at similar level that the native protein, both in vivo and in vitro. The TssJ-ΔL1-2 mutant was found to be soluble, monomeric, and with a CD spectrum closely similar to that of the native protein (Figure S7B), indicating that the TssJ-ΔL1-2 mutant was properly folded. By contrast, TssJ mutants deleted or partly deleted of the helical domain were little produced in vivo, and not produced in vitro, precluding further studies with them. An hydrophobic patch at the interface between TssJ core and the helical domain (Phe113, Trp123) might have been exposed to solvent, probably inducing aggregation.
The TssJ-ΔL1-2 mutant was tested (i) for function in a complementation assay and (ii) in vivo and in vitro interaction with TssM-ekto. The presence of Hcp in the culture supernatant has been previously shown to reflect the correct assembly of the machine. Whole cells and supernatants of ΔtssJ cells expressing full-length wild-type or mutant TssJ were separated by centrifugation and the presence of Hcp in both fractions was assessed by western-blot. As shown in Figure 4A, the wild-type TssJ protein was functional while TssJ-ΔL1-2 did not complement the tssJ mutation for Hcp release. We then tested whether the deletion mutant of TssJ interacts with the periplasmic domain of TssM in an in vivo co-immunoprecipitation assay. Figure 4B shows that TssJ-ΔL1-2 did not co-immunoprecipitate with TssM-ekto.
Fig. 4. The L1-2 loop of TssJ is required for TssJ-TssM complex formation. 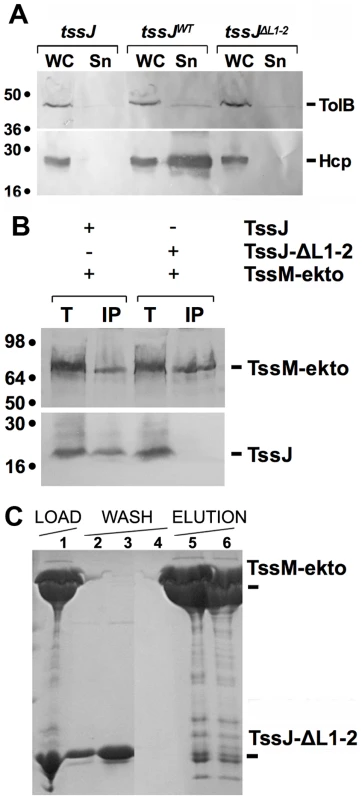
(A) In vivo Hcp release assay. HcpFLAG release was assessed by separating whole cells (WC) and supernatant (Sn) fractions from tssJ cells carrying the empty vector (tssJ), the vector encoding wild-type TssJ (tssJWT) or the vector encoding the TssJ-ΔL1-2 mutant (tssJΔL1-2). 2 ×108 cells and the TCA-precipitated material of the supernatant from 5×108 cells were loaded on a 12.5%-acrylamide SDS-PAGE and immunodetected using the anti-FLAG monoclonal antibody (lower panel) and the anti-TolB polyclonal antibodies (lysis control; upper panel). (B) Solubilized extracts of E. coli K12 W3110 strain producing (+) or not (-) FLAG-tagged TssM-ekto and HA-tagged TssJ or TssJ-ΔL1-2 mutant were subjected to immunoprecipitation with anti-FLAG-coupled beads. The total solubilized material (T) and the immunoprecipitated material (IP) were loaded on a 12.5%-acrylamide SDS PAGE, and immunodetected with anti-HA (TssJ and TssJ-ΔL1-2; lower panel) and anti-FLAG (TssM-ekto; upper panel) monoclonal antibodies. Immunodetected proteins are indicated on the right. Molecular weight markers are indicated on the left. (C) Affinity purification of TssJ-ΔL1-2 with TRX-His6-TssM-ekto. The Coomassie blue-stained SDS-PAGE shows the fractions of the purification steps (Load, fraction 1; Wash, fractions 2-4; Elution, fractions 5 and 6). The positions of the proteins of interest are indicated on the right. This conclusion was validated by in vitro analyses. First, analysis of a mixture of TssM-ekto and TssJ-ΔL1-2 by gel filtration revealed a unique peak eluting at the same volume as TssM-ekto alone. SDS-PAGE analysis of the different fractions demonstrated the presence of TssM only (Figure 4C). We then performed SPR experiments with TssM-ekto covalently linked to a CM5 chip. Whereas we detected a 2-4 µM interaction between TssM-ekto and TssJ, injection of the TssJ-ΔL1-2 mutant at a concentration up to 10 µM failed to produce any deviation of the sensorgram, indicating thus a lack of interaction between TssM and TssJ-ΔL1-2. Coupled to the observation that TssJ-ΔL1-2 was correctly folded, we concluded that the L1-2 loop of TssJ is a critical determinant for the TssJ-TssM interaction.
Discussion
Type VI secretion systems are important determinants of bacterial pathogenesis, either by the secretion of toxic molecules to host cells, or by competing with bacterial rivals towards the colonization of a specific niche [1], [2]. Several studies have demonstrated the implications of T6SS in the virulence of Vibrio cholerae towards amoeba or animal/human host cells, of Edwardsiella tarda towards fishes, or of Burkholderia sp. towards animal host cells [5], [6], [24], [35], [36]. Similarly, high levels of antibodies directed against the HSI-1 Hcp protein of Pseudomonas aeruginosa have been detected in the sputum of cystic fibrosis patients [9]. With the observation that the HSI-1 T6SS is dedicated to the competition against other bacteria, including pathogens [8], [37], the presence of Hcp antibodies suggests that an intense bacterial warfare occurs in specific niches of animal or human bodies. As an inroad to better understand how T6SS are built, we initiated a structural, biophysical and functional characterization of the T6SS core components. We reported here the crystal structure of the TssJ lipoprotein, a critical core-component of Type VI secretion systems, and its interaction with TssM.
TssJ structure and interaction with TssM
The TssJ-TssM complex is part of a larger complex involving the inner membrane TssL protein and the peptidoglycan-associated TagL protein [22]. This membrane sub-complex of T6SS therefore links both membranes and the peptidoglycan layer, forming a trans-envelope, periplasm spanning, structure. Few structures have been reported so far in T6SS: the Hcp and VgrG proteins which are structural/exported components that share homologies with bacteriophage subunits [9], [11]. This study thus reports the first structure of a T6SS membrane complex component and provides clues on specific interaction sites.
TssJ is a β-sandwich resembling transthyretin with two additional elements: a protruding loop and a small helical domain. Sequence alignment of the T6SS TssJ homologues showed that all TssJ proteins share these additional elements (Figure S4), suggesting that the EAEC TssJ structure likely constitutes a prototype for T6SS-associated TssJ proteins. Because these regions are not required for the canonical fold but are conserved among TssJ homologues, we thought they may represent potential site of functionality. Indeed, using in vivo assays, we found that deletion of the extended loop L1-2 abrogates Hcp secretion, while the helical domain is necessary for TssJ stability. In vivo and in vitro protein-protein interaction studies further showed that the L1-2 loop of TssJ is required for efficient interaction with the periplasmic domain of TssM. Although present in all TssJ homologues, this loop is not well conserved in terms of amino-acid composition and length. The sequence alignment presented Figure S4 reveals two TssJ sub-families based on the L1-2 length (5 - or 8-amino-acids). Because this loop is a critical determinant of the TssJ-TssM interaction, one may hypothesize that these variations may modulate specificity between TssJ and TssM homologues. Specificity between these two proteins can therefore be a determinant during T6SS assembly to ensure proper recognition between the cognate subunits when several T6SS gene clusters are encoded within the same genome. SPR experiments showed that the Kd between TssJ and TssM was ∼2-4 ìM, while a 1∶1 stoichiometry was determined by MALS/QELS/UV/RI. Sub-domain dissection of TssM further demonstrated that TssJ does not interact with the TssM-ekto-Nt domain, but rather with the TssM-ekto-Ct domain.
Organization of the T6SS outer shell
TssM-ekto-Ct domain localization in the sequence, opposite to the N-terminal trans-membrane helices imbedded in the IM, suggests that it should be close to the outer membrane. The hypothesis of the vicinity of TssM-ekto-Ct with the OM is further reinforced by the interaction of TssM-ekto-Ct with TssJ. However, formation of a T6SS outer shell based on TssM implies the establishment of lateral TssM-TssM interactions. The monomeric state of TssM-ekto in solution, as determined by MALS/QELS/UV/RI experiments, as well as the SPR data, are not in favor of this hypothesis. However, these interactions may still be established with the full length TssM molecules, since they could interact through their transmembrane helices. Our data, as well as the current knowledge on T6SS, made it possible to suggest a T6SS topology model (see Figure 5). As previously reported, TssL, TagL and TssM interact at the IM [22] whereas our data showed that the C-terminal domain of TssM interacts with TssJ at the OM (Figure 5). We propose that the TssL-TagL-TssM-TssJ complex may therefore form a trans-envelope spanning channel, as exemplified in other secretion systems [38]-[41].
Fig. 5. Schematic representation of the enteroaggregative E. coli T6SS. 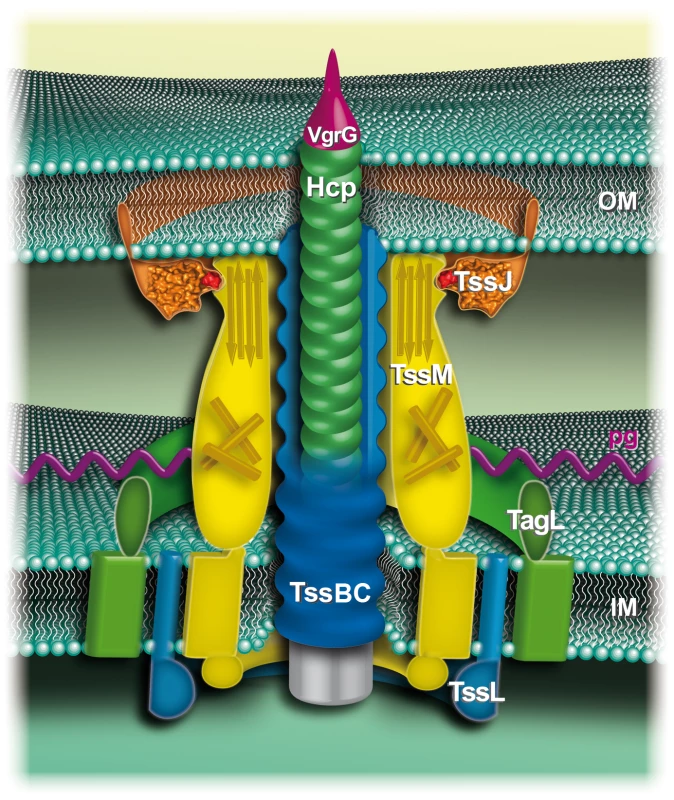
The outer (OM) and inner membranes (IM) are represented in light green. The T4 phage-like central puncturing device includes Hcp (green disks) and VgrG (purple). The “tail sheath” TssBC (VipAB) proteins are shown in blue, around the central Hcp/VgrG pilum. The TssBC proteins constituting a sheath encompassing the Hcp tube has not been evidenced but is speculated based on the similarities between the T6SS TssBC subunits and the bacteriophage T4 sheath [4], [17]. The three-transmembrane inner membrane TssM protein (yellow) interacts with the TssL IM protein (blue) [20]. TssL interacts with TagL (green), an IM protein that anchors the T6SS to the cell wall [22]. TssM C-terminal domain interacts with a loop of the outer membrane lipoprotein TssJ [this study]. In this model, the TssL-TagL-TssM-TssJ complex forms a trans-envelope spanning channel. A chaperone role of TssJ?
The TssJ core domain (residues 2-155) is anchored to the membrane through a thioether linkage between Cys1 and a diacylglycerol. Residues 2-22 have been found to be non-ordered in the crystal structure, providing to TssJ a wide radius of periplasm exploration for catching TssM. Interestingly, the TssM-ekto-Ct domain is predicted to be essentially formed by β-strands. One interesting hypothesis that remains to be tested is that the TssJ lipoprotein may contribute to the folding or the stability of the TssM-ekto-Ct domain. A chaperone-like role for lipoproteins has already been demonstrated in most bacterial secretion systems: in Type II secretion systems (T2SS), the GspS lipoprotein acts as a pilotine to allow the passage of the GspD secretin through the periplasm and help to its proper insertion in the outer membrane [42]. Similarly, in T3SS, cognate lipoproteins serve as insertion helper for outer membrane secretins [43], [44]. More generally, lipoproteins associated with cell envelope spanning structures are often involved in machine assembly or nucleation factor. In T4SS, the VirB7 lipoprotein is covalently linked to the VirB9 outer-membrane associated component through a disulfide bridge [45], is required for stability of VirB9 and of the VirB9-VirB10 outer membrane translocon [39], and has been suggested to be necessary for the early stages of T4SS biogenesis [46]–[48]. Interestingly, the TssJ fold is similar to that of the P. aeruginosa T3SS-associated ExsB outer membrane lipoprotein [32]. This observation suggests that these two structures may represent a family of lipoproteins associated with macromolecular systems sharing a common evolutionary history. It is noteworthy that ExsB does not exhibit the L1-2 loop and the helical domain found in TssJ (Figure S3) highlighting the specific role of these two elements in Type VI secretion.
Drugability of the TssM-TssJ interaction
Our data showed that the 4 amino-acids protruding loop L1-2 of TssJ is required for a functional TssJ-TssM interaction. This should be analyzed in light of the SPR experiments, reporting a Kd value in the µM range, suggesting that the interaction does not involve a large surface area of interaction, such as the 600-900 Å2 typical of Fab/protein interfaces that would assemble with Kd values down to the nM range [49]. This observation as well as the complete loss of TssJ-TssM interaction when the L1-2 loop is deleted let us to conclude that there is no other interaction area between TssM and TssJ. Interestingly, the size of the L1-2 loop is comparable with that of medium sized organic molecules that could therefore be good candidates to fit into the TssM complementary cavity and compete for TssJ binding. T6SS being important determinant of pathogenesis, biofilm formation or inter-bacterial competition, this observation paves the way for the identification of anti-microbial molecules targeting T6SS assembly [50].
Materials and Methods
Bacterial strains, medium, growth conditions and chemicals
Escherichia coli K12 DH5α was used for cloning procedures. The enteroaggregative E. coli strain 17-2 (kindly provided by Arlette Darfeuille-Michaud, University of Clermont-Ferrand, France) and its ΔtssJ derivative [ΔsciN; 21] were used for this study. EAEC strains were routinely grown in LB medium at 37°C with shaking. Plasmids were maintained by the addition of ampicillin (200 µg/ml) or kanamycin (100 µg/ml).
Constructions for in vivo studies
Constructions of plasmid pSciNHA (encoding the WT EAEC TssJ protein fused to a C-terminal HA epitope) and pHcpFLAG (encoding the Hcp protein fused to a C-terminal FLAG epitope) have been previously reported [21]. Deletion of the L1-2 loop has been introduced into pSciNHA by site-directed mutagenesis using mutagenic primers annealing upstream and downstream of the sequence to be deleted (5′-CCAGGGAGGCCATTAACACCGGTGGCGCCTCGGTTGTGGTGCGGATTTATC and 5′-GATAAATCCGCACCACAACCGAGGCGCCACCGGTGTTAATGGCCTCCCTGG). pSciSp, encoding the EAEC TssM-ekto domain (amino-acids 386 to 1129) fused to a signal peptide for periplasm targeting, has been constructed by a double PCR technique using pASK-IBA4 as template and oligonucleotides IBA-Sp5 (5′-CGCTACCGTAGCGCAGGCCGCTAGCGATTATAAAGACGACGATGACAAAAGTCTGGTTGCTGAAGTACAGGAACAGATTCGTCCG [sequence encoding the FLAG epitope tag underlined]) and IBA-Sp3 5′-GCCTTTTTCGAACTGCGGGTGGCTCCATCAGTCAGTCTCCTCCACGGTATCCCCGG). Plasmid encoding TssM-ekto-Nt (amino-acids 386 to 973) has been obtained by site-directed mutagenesis by introduction of a stop codon at codon Asn974 using pSciSp as template and oligonucleotides 5′ - GGATGTGGCGTTCACCACAGGTTAAGCGGGGCTGCATTTTGAGCTGC and 5′ - GCAGCTCAAAATGCAGCCCCGCTTAACCTGTGGTGAACGCCACATCC. Plasmid encoding TssM-ekto-Ct (amino-acids 972 to 1129) has been constructed by a double PCR technique using pASK-IBA4 as template and oligonucleotides IBA-Spβ5 (5′-CGCTACCGTAGCGCAGGCCGCTAGCGATTATAAAGACGACGATGACAAAGGTAACGCGGGGCTGCATTTTGAGCTGCG) and IBA-Sp3. Primers were obtained from custom oligonucleotides synthesized by Eurogentec. Polymerase Chain Reactions (PCR) were performed with a Biometra thermocycler, using the Pfu Turbo DNA polymerase (Stratagene; La Jolla, CA). All constructs have been verified by DNA sequencing (GATC).
In vivo Hcp release assay
Supernatant and cell fractions have been separated as previously described [21]. Briefly, 2×109 cells producing FLAG epitope-tagged Hcp were harvested and collected by centrifugation at 2,000 × g for 5 min. The supernatant fraction was then subjected to a second low-speed centrifugation and then at 16,000 × g for 15 min. The supernatant was then filtered on sterile polyester membranes with a pore size of 0.2 µm (membrex 25 PET, membraPure GmbH) before precipitation with trichloroacetic acid (TCA) 15%. Cells and precipitated supernatant were then resuspended in loading buffer and analyzed by SDS-PAGE and immunoblotting with the anti-FLAG M2 monoclonal antibody (Sigma-Aldrich). As control for cell lysis, Western blots were probed with antibodies raised against the periplasmic TolB protein.
In vivo co-immunoprecipitation assays
Co-immunoprecipitation experiments were performed essentially as previously described [51]. 2×109 exponentially growing cells were harvested, washed with 20 ml of 10 mM sodium phosphate buffer (NaPi, pH 6.8), and resuspended in NaPi buffer supplemented with para-formaldehyde 1%. After incubation at room temperature for 20 minutes, the cross-linking reaction was quenched by the addition of 0.3 M Tris-HCl pH 6.8, and the cells were washed twice in Tris-HCl 20 mM pH 6.8. The cell pellet was then subjected to solubilization for 30 min at 37°C in TES (Tris-HCl 10 mM, pH 7.5, EDTA 5 mM, SDS 1%) in presence of protease inhibitors (Complete, Roche), and diluted 15-fold in TNE (Tris-HCl 10 mM, pH 7.5, EDTA 5 mM, NaCl 150 mM) supplemented with 1% Triton X-100. After incubation 2 hours at room temperature with vigorous shaking, the extract was centrifuged 15 min at 18000 x g to remove unsolubilized material. Supernatants were then incubated overnight at 4°C with anti-HA antibody coupled to Agarose-Protein G beads (Roche). Beads were then washed twice with TNE supplemented with 1% Triton X-100, once in TNE supplemented with 0.1% Triton X-100 and Tween 0.1% and once in TNE supplemented with 0.1% Triton X-100. The immunoprecipitated material was heated in loading buffer prior to analyses by SDS-PAGE and immunoblotting.
Constructions for in vitro studies and purification procedures
tssJ and tssM-ekto of enteroaggregative Escherichia coli strain 17-2 were cloned into pETG-20A (a kind gift from Dr Arie Geerlof, EMBL, Hamburg) expression vector according to standard Gateway™ protocols. The final constructs encoded the target genes and an N-terminal fusion with hexahistidine tagged thioredoxine followed by a TEV protease cleavage site. Both plasmids were transformed in Escherichia coli T7 Iq pLysS (New England Biolabs) expression strain. Cells were grown at 37°C in Terrific Broth until the OD600 reached 0.9 and the tssJ or tssM expression was inducted with 0.5 mM isopropyl-β-thio-galactoside (IPTG) overnight at 25°C and 17°C, respectively. After cells harvesting, the lysis was done by adding 0.25 mg/ml lysozyme, followed by sonication. Soluble protein was separated from inclusion bodies and cell debris by 30 min of centrifugation at 20,000 g. We used an AKTA FPLC system to four steps of purification: a Ni2+ affinity chromatography (HisTrap 5 ml GE Healthcare) with a step gradient of 250 mM Imidazole, an overnight TEV His protease digestion at 4°C with a 1∶10 (w/w) protease: protein ratio, a second Ni2+ affinity chromatography; a preparative Superdex 75 (GE Healthcare) gel filtration run in 20 mM Tris pH 8, 100 mM NaCl. Gel filtration of TssM-ekto was performed in a preparative Superose 6 in 20 mM Tris pH 8.0, 200 mM NaCl, 5% glycerol.
In vitro co-purification assays
Co-purification of TssM-ekto and TssJ was performed in three steps on a AKTA FPLC system: a Ni2+ affinity chromatography in a step gradient of 250 mM Imidazole of the mixture Trx-(His6)-TssM-ekto and TssJ, an overnight TEV His protease digestion at 4°C with a 1∶10 (w/w) protease: protein ratio and a preparative Superose S6 (GE Healthcare) gel filtration run in 20 mM Tris pH 8.0, 200 mM NaCl, 5% glycerol.
Biophysical experiments
Size exclusion chromatography (SEC) was performed on an Alliance 2695 HPLC system (Waters) using a KW803 and KW804 columns (Shodex) run in 10 mM HEPES pH 7.5, 150 mM NaCl at 0.5 ml/min. MALS, UV spectrophotometry, QUELS and RI were achieved with MiniDawn Treos (Wyatt Technology), a Photo Diode Array 2996 (Waters), a DynaPro (Wyatt Technology) and an Optilab rEX (Wyatt Technology), respectively, as described [26]. Mass and hydrodynamic radius calculation was done with ASTRA software (Wyatt Technology) using a dn/dc value of 0.185 mL/g.
Circular dichroism spectra of TssJ, TssJ-ΔL1-2 mutant and Trx-TssM-ekto were recorded in 20 mM NaH2PO4 pH 7,2, 150 mM NaCl using a Jasco J-810 spectropolarimeter.
The Surface Plasmon Resonance measurements were performed in a 10 mM Tris pH 8, 150 mM NaCl, 0.005% detergent TWEEN using a BIACORE X100 (BIAcore). The chip CM5 (BIAcore) was coated with TssJ N-terminal thioredoxine fusion with 600 response units (RU). We also used the inverse set-up, coating the chip CM5 with TssM-ekto N-terminal thioredoxine fusion with 3000 response units. Binding assays with TssJ N-terminal thioredoxine fusion covalently linked to the chip CM5 were performed with TssM N-terminal thioredoxine fusion at 10, 5, 2.5, 1.25, 0.625, 0.312 µM. We also performed the binding assays with the chip CM5 coated with TssM N-terminal thioredoxine fusion at 40, 20, 10, 5, 2.5, 1.25, 0.625, 0.312 µM. The signal from the uncoated reference cell and the buffer response was subtracted from all measurements. The KD values were obtained using the fitting tool of the BIAevaluation software (BIAcore). A 1∶1 binding model was assumed in all cases.
Crystallization and structure determination
TssJ crystallization trials were carried out in sitting-drop vapor diffusion method at 20°C in 96-well Greiner crystallization plates using a nanodrop-dispensing robot (Cartesian Inc.) [27]. Crystals grew in a few days by mixing 300 nL protein at 8mg/mL with 100 nL 2.2 M AmSO4, 0.2 M Na+-thiocyanate pH 6.0. Crystals were cryo-protected with mother-liquor supplemented with 20% ethylene glycol and flash frozen in liquid nitrogen. Some crystals were soaked in the cryo-protecting solution that also contained 0.5 M NaI and 0.5 M CsI. Two data sets were collected: a native data set (λ = 0.98011) and I-SAD (λ = 1.37760) at Proxima I beamline (SOLEIL, Gif-sur-Yvette, France) using an ADSC Q315r detector. Data processing and scaling were done using XDS, XSCALE [52], and POINTELESS (Table S3.). The crystals of TssJ belong to space group P3121 with unit-cell parameters a = b = 78.07 Å, c = 46.95 Å. TssJ structure was solved by SIRAS with Sharp [53] and initial automatic building was performed with Buccaneer [54]. Manual model building was performed with COOT [55]. Refinement was carried out at 1.35 Å using Buster-TNT [56] and anisotropic refinement with REFMAC [57]. Structure analyzes was assisted by the PISA server [28] and electrostatic potential calculation was done with APBS [58].
Accession codes
The atomic coordinates and structure factors have been deposited at the Protein Data Bank with accession code 3RX9.
Supporting Information
Zdroje
1. CascalesE 2008 The Type VI secretion toolkit. EMBO Rep 9 735 741
2. SchwarzSHoodRDMougousJD 2010 What is Type VI secretion doing in all those bugs? Trends Microbiol 18 531 537
3. BernardCSBrunetYRGueguenECascalesE 2010 Nooks and crannies in Type VI secretion regulation. J Bacteriol 192 3850 60
4. RecordsAR 2011 The Type VI secretion system: A multipurpose delivery system with a phage like machinery. Mol Plant Microbe Interact 24 751 7
5. PukatzkiSMaATSturtevantDKrastinsBSarracinoD 2006 Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103 1528 33
6. MaATMekalanosJJ 2010 In vivo actin cross-linking induced by Vibrio cholerae Type VI secretion system is associated with intestinal inflammation. Proc Natl Acad Sci U S A 107 4365 70
7. SchwarzSWestTEBoyerFChiangWCCarlMA 2010 Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog 6 e1001068
8. RussellABHoodRDBuiNKLerouxMVollmerW 2011 Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475 343 7
9. MougousJDCuffMERaunserSShenAZhouM 2006 A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312 1526 1530
10. PukatzkiSMaATRevelATSturtevantDMekalanosJJ 2007 Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci U S A 104 15508 15513
11. LeimanPGBaslerMRamagopalUABonannoJBSauderJM 2009 Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci U S A 106 4154 4159
12. PukatzkiSMcAuleySBMiyataST 2009 The Type VI secretion system: translocation of effectors and effector-domains. Curr Opin Microbiol 12 11 17
13. PellLGKanelisVDonaldsonLWHowellPLDavidsonAR 2009 The phage lambda major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system. Proc Natl Acad Sci U S A 106 4160 4165
14. KanamaruSLeimanPGKostyuchenkoVAChipmanPRMesyanzhinovVV 2002 Structure of the cell-puncturing device of bacteriophage T4. Nature 415 553 557
15. BingleLEBaileyCMPallenMJ 2008 Type VI secretion: a beginner's guide. Curr Opin Microbiol 11 3 8
16. ShalomGShawJGThomasMS 2007 In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology 153 2689 99
17. BonemannGPietrosiukADiemandAZentgrafHMogkA 2009 Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J 28 315 325
18. KanamaruS 2009 Structural similarity of tailed phages and pathogenic bacterial secretion systems. Proc Natl Acad Sci U S A 106 4067 4068
19. MaATMcAuleySPukatzkiSMekalanosJJ 2009 Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe 5 234 43
20. MaLSLinJSLaiEM 2009 An IcmF family protein, ImpLM, is an integral inner membrane protein interacting with ImpKL, and its walker a motif is required for type VI secretion system-mediated Hcp secretion in Agrobacterium tumefaciens. J Bacteriol 191 4316 4329
21. AschtgenMSBernardCSde BentzmannSLloubesRCascalesE 2008 SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli. J Bacteriol 190 7523 7531
22. AschtgenMSGavioliMDessenALloubesRCascalesE 2010 The SciZ protein anchors the enteroaggregative Escherichia coli Type VI secretion system to the cell wall. Mol Microbiol 75 886 899
23. AschtgenMSThomasMSCascalesE 2010 Anchoring the type VI secretion system to the peptidoglycan: TssL, TagL, TagP... what else? Virulence 1 535 540
24. ZhengJLeungKY 2007 Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol 66 1192 1206
25. VincentelliRCanaanSOffantJCambillauCBignonC 2005 Automated expression and solubility screening of His-tagged proteins in 96-well format. Anal Biochem 346 77 84
26. SciaraGBlangySSiponenMMc GrathSvan SinderenD 2008 A topological model of the baseplate of lactococcal phage Tuc2009. J Biol Chem 283 2716 2723
27. SulzenbacherGGruezARoig-ZamboniVSpinelliSValenciaC 2002 A medium-throughput crystallization approach. Acta Crystallogr D Biol Crystallogr 58 2109 2115
28. KrissinelEHenrickK 2007 Inference of macromolecular assemblies from crystalline state. J Mol Biol 372 774 797
29. HolmLRosenstromP 2010 Dali server: conservation mapping in 3D. Nucleic Acids Res 38 W545 549
30. EneqvistTLundbergEKarlssonAHuangSSantosCR 2004 High resolution crystal structures of piscine transthyretin reveal different binding modes for triiodothyronine and thyroxine. J Biol Chem 279 26411 26416
31. ZanottiGCendronLRamazzinaIFolliCPercudaniR 2006 Structure of zebra fish HIUase: insights into evolution of an enzyme to a hormone transporter. J Mol Biol 363 1 9
32. IzoreTPerduCJobVAttreeIFaudryE 2011 Structural characterization and membrane localization of ExsB from the Type III secretion system (T3SS) of Pseudomonas aeruginosa. J Mol Biol 413 236 246
33. DundasJOuyangZTsengJBinkowskiATurpazY 2006 CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res 34 W116 118
34. ColeCBarberJDBartonGJ 2008 The Jpred 3 secondary structure prediction server. Nucleic Acids Res 36 W197 201
35. SchellMAUlrichRLRibotWJBrueggemannEEHinesHB 2007 Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol Microbiol 64 1466 85
36. BurtnickMNBrettPJHardingSVNgugiSARibotWJ 2011 The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect Immun 79 1512 25
37. HoodRDSinghPHsuFGüvenerTCarlMA 2010 A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7 25 37
38. FronzesRSchäferEWangLSaibilHROrlovaEV 2009 Structure of a type IV secretion system core complex. Science 323 266 268
39. ChandranVFronzesRDuquerroySCroninNNavazaJ 2009 Structure of the outer membrane complex of a type IV secretion system. Nature 462 1011 1015
40. SchraidtOMarlovitsTC 2011 Three-dimensional model of Salmonella's needle complex at subnanometer resolution. Science 331 1192 1195
41. ReichowSLKorotkovKVHolWGGonenT 2010 Structure of the cholera toxin secretion channel in its closed state. Nat Struct Mol Biol 17 1226 1232
42. HardieKRLorySPugsleyAP 1996 Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J 15 978 988
43. CragoAMKoronakisV 1998 Salmonella InvG forms a ring-like multimer that requires the InvH lipoprotein for outer membrane localization. Mol Microbiol 30 47 56
44. DaeflerSRusselM 1998 The Salmonella typhimurium InvH protein is an outer membrane lipoprotein required for the proper localization of InvG. Mol Microbiol 28 1367 1380
45. SpudichGMFernandezDZhouXRChristiePJ 1996 Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport apparatus. Proc Natl Acad Sci U S A 93 7512 7517
46. FernandezDSpudichGMZhouXRChristiePJ 1996 The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J Bacteriol 178 3168 3176
47. CascalesEChristiePJ 2004 Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304 1170 1173
48. ChristiePJAtmakuriKKrishnamoorthyVJakubowskiSCascalesE 2005 Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu Rev Microbiol 59 451 85
49. DaviesDRCohenGH 1996 Interactions of protein antigens with antibodies. Proc Natl Acad Sci U S A 93 7 12
50. ShahianTLeeGMLazicAArnoldLAVelusamyP 2009 Inhibition of a viral enzyme by a small-molecule dimer disruptor. Nat Chem Biol 5 640 646
51. CascalesELloubesRSturgisJN 2001 The TolQ-TolR proteins energize TolA and share homologies with the flagellar motor proteins MotA-MotB. Mol Microbiol 42 795 807
52. KabschW 2010 Xds. Acta Crystallogr D Biol Crystallogr 66 125 132
53. BricogneGVonrheinCFlensburgCSchiltzMPaciorekW 2003 Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr D Biol Crystallogr 59 2023 2030
54. CowtanK 2006 The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr D Biol Crystallogr 62 1002 1011
55. EmsleyPCowtanK 2004 Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60 2126 2132
56. BlancERoversiPVonrheinCFlensburgCLeaSM 2004 Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr D Biol Crystallogr 60 2210 2221
57. MurshudovGVaginAADodsonEJ 1997 Refinement of Macromolecular Structures by the Maximum-Likelihood Method. Acta Crystallogr D Biol Crystallogr D 53 240 255
58. BakerNASeptDJosephSHolstMJMcCammonJA 2001 Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A 98 10037 10041
59. CorpetF 1988 Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16 10881 90
60. GouetPCourcelleEStuartDIMetozF 1999 ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15 305 8
61. DeLanoW 2002 The PyMOL Molecular Graphics System. DeLano Scientific LLC (San Carlos, CA) Available: http://www.pymol.org. Accessed 11 October 2011
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



