-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Gamma Interferon Independent Mechanism of CD4 T Cell Mediated
Control of Infection
CD4 T cell deficiency or defective IFNγ signaling render humans and mice
highly susceptible to Mycobacterium tuberculosis (Mtb)
infection. The prevailing model is that Th1 CD4 T cells produce IFNγ to
activate bactericidal effector mechanisms of infected macrophages. Here we test
this model by directly interrogating the effector functions of Th1 CD4 T cells
required to control Mtb in vivo. While Th1 CD4 T cells specific for the Mtb
antigen ESAT-6 restrict in vivo Mtb growth, this inhibition is independent of
IFNγ or TNF and does not require the perforin or FAS effector pathways.
Adoptive transfer of Th17 CD4 T cells specific for ESAT-6 partially inhibited
Mtb growth while Th2 CD4 T cells were largely ineffective. These results imply a
previously unrecognized IFNγ/TNF independent pathway that efficiently
controls Mtb and suggest that optimization of this alternative effector function
may provide new therapeutic avenues to combat Mtb through vaccination.
Published in the journal: . PLoS Pathog 7(5): e32767. doi:10.1371/journal.ppat.1002052
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002052Summary
CD4 T cell deficiency or defective IFNγ signaling render humans and mice
highly susceptible to Mycobacterium tuberculosis (Mtb)
infection. The prevailing model is that Th1 CD4 T cells produce IFNγ to
activate bactericidal effector mechanisms of infected macrophages. Here we test
this model by directly interrogating the effector functions of Th1 CD4 T cells
required to control Mtb in vivo. While Th1 CD4 T cells specific for the Mtb
antigen ESAT-6 restrict in vivo Mtb growth, this inhibition is independent of
IFNγ or TNF and does not require the perforin or FAS effector pathways.
Adoptive transfer of Th17 CD4 T cells specific for ESAT-6 partially inhibited
Mtb growth while Th2 CD4 T cells were largely ineffective. These results imply a
previously unrecognized IFNγ/TNF independent pathway that efficiently
controls Mtb and suggest that optimization of this alternative effector function
may provide new therapeutic avenues to combat Mtb through vaccination.Introduction
IFNγ is essential for defense against Mtb infection, as revealed by experimental studies using knockout mice and the unusually severe mycobacterial infections in patients with defects in the IFNγ or IL-12 signaling pathways [1], [2], [3], [4]. The role of CD4 T cells in defense against Mtb infection has been inferred from the increased reactivation of latent Mtb infections in CD4 T cell deficient patients following HIV infection and severe tuberculosis observed in CD4 T cell-deficient mice [3], [5]. These clinical and experimental findings have led to a widely accepted model positing that the critical immunologic mechanism of anti-mycobacterial immunity involves CD4 T cells that secrete IFNγ to activate bactericidal functions of Mtb-infected macrophages. Substantial evidence indicates that IFNγ can activate murine macrophages to limit Mtb growth [6]–[7] but the relative importance of this bactericidal mechanism and the cellular sources of IFNγ are unknown. Evidence for an CD4 T cell dependent, IFNγ independent mechanism of killing has been suggested by the finding that the frequency of Mtb-specific, IFNγ-producing cells following immunization does not correlate with protection against infection and that depletion of CD4 cells exacerbates Mtb infection in mice despite the ongoing expression of IFNγ [8], [9], [10], [11], [12].
In this report, we have assessed the requirement of IFNγ in protection by Mtb specific CD4 T cells. Using a model of adoptive transfer of Mtb specific effector CD4 T cells, we provide evidence for the surprising conclusion that IFNγ is not a required mediator of CD4 T cell defense to Mtb. In support of this finding is our discovery that key mediators of IFNγ - dependant immunity, inducible nitric oxide synthase and phagocyte oxidase, were not required for the early protective events mediated by adoptively transferred Th-1 skewed CD4 T cells. Although Th1-skewed cells were superior to Th2 or Th17-skewed cells in defense to Mtb, surprisingly, protection by Th1 –skewed cells was independent of the master regulator of Th1 differentiation, T-bet. Our results are contrary to a dominant role for IFNγ production by effector CD4 in Mtb protection, but strongly support a requirement for Th1 mediated immunity to Mtb.
Results/Discussion
IFN-gamma and iNOS independent control of M. tuberculosis infection
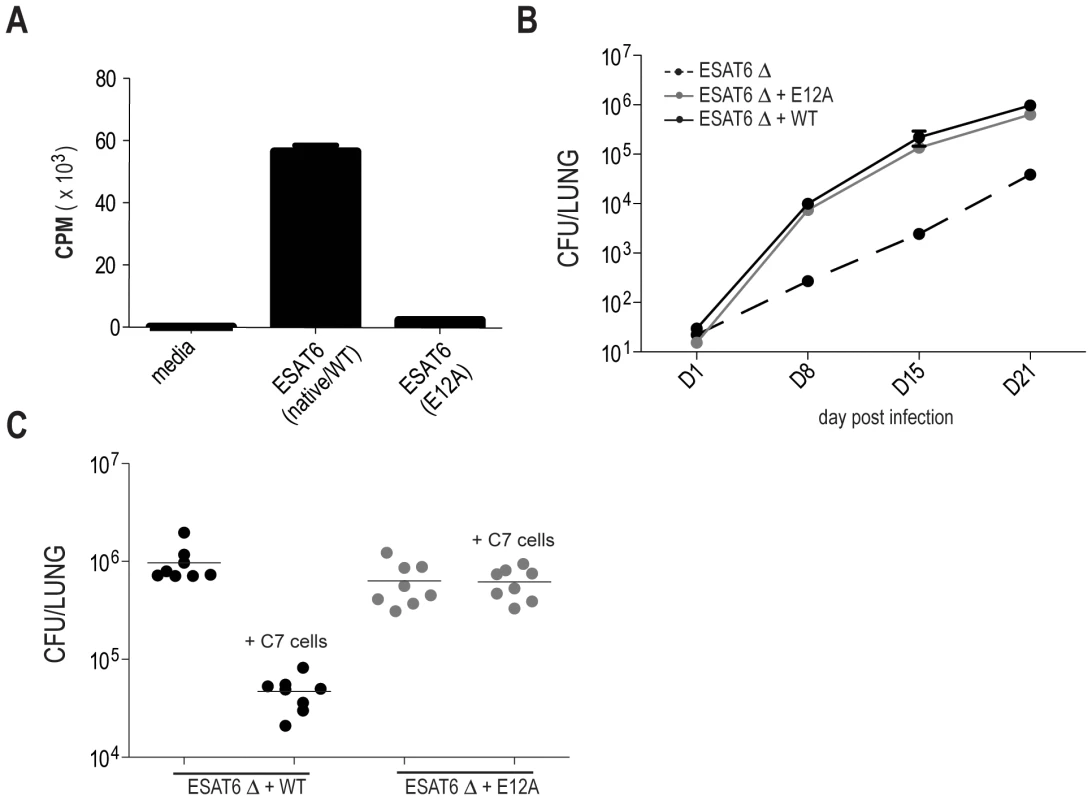
To investigate the contribution of IFNγ production by Mtb-specific CD4 T cells during infection, we compared the ability of WT and IFNγ deficient, Mtb-specific CD4 T cells derived from the C7 TCR transgenic mouse [13] , to protect mice from infection. Naive C7 CD4 T cells, specific for the immunodominant Mtb antigen ESAT-6 in the context of the IAb MHC class II molecule, were activated in vitro under Th1-skewing conditions [13] and transferred into WT mice. To be certain that protection mediated by C7 CD4 T cells is antigen specific, we generated a strain of M. tuberculosis in which a key TCR contact residue in the ESAT-6 epitope (E12) was mutated to alanine to abolish C7 recognition (Figure 1A). M. tuberculosis ESAT6-E12A was fully virulent, but was not affected by Th1-differentiated C7 cells, whereas wild type M. tuberculosis titers were reduced by approximately 50 fold (Figure 1B and C).
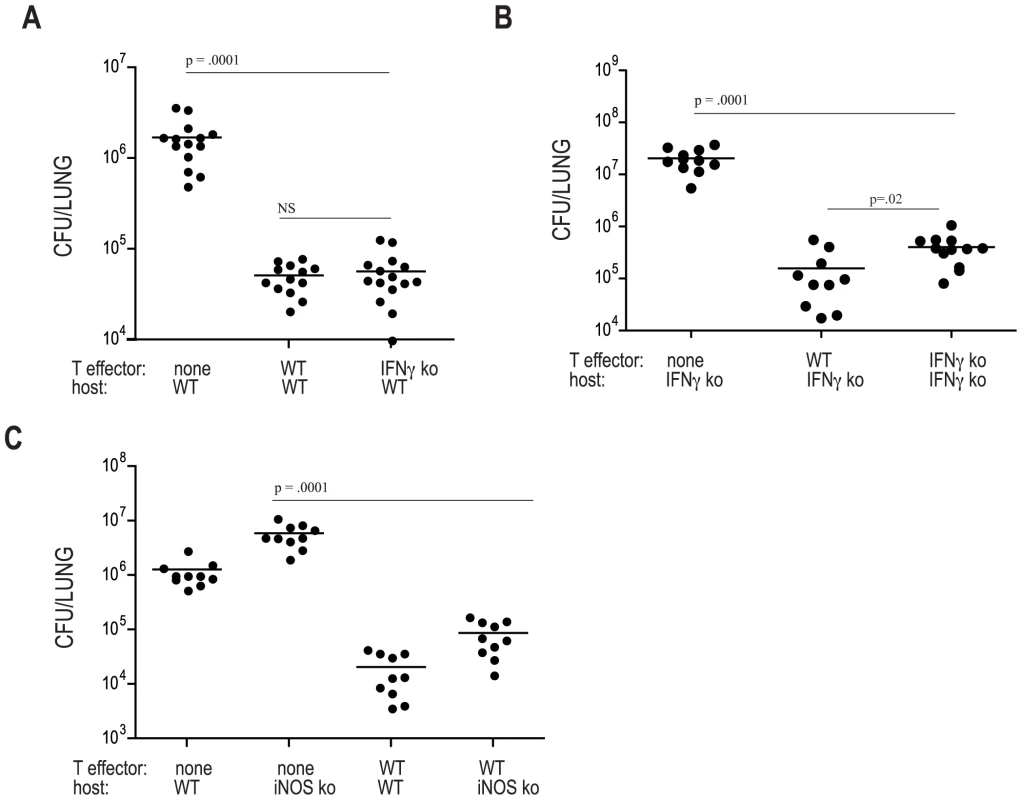
Next, we transferred ten million C7.WT or C7.IFNγ deficient Th1 effector cells into WT mice that were subsequently aerosol-infected with Mtb and, twenty-one days later, the number of bacteria in the lungs was determined. Surprisingly, both C7.WT and C7.IFNγ deficient cells provided similar levels of protection compared to animals that did not receive cells (Figure 2A), indicating that IFNγ production by adoptively transferred effector CD4 T cells is not required for protection when these cells are present at the time of infection. To examine whether IFNγ-independent restriction of Mtb growth is a specific property of C7 transgenic T cell populations or a general property of Mtb specific T cells, we generated bone marrow chimeric mice in which wild type recipient C57BL/6 mice received a 50∶50 mixture of bone marrow from Rag2-deficient and IFNγ-deficient donors. In these chimeric mice, all T cells are IFNγ-deficient, while Rag2-independent innate immune cells, such as NK cells, macrophages, monocytes and DCs are capable of producing IFNγ. Bone marrow chimeric mice with IFNγ-deficient T cells were similarly resistant to Mtb infection as mice receiving wild type T cells, supporting our conclusion that T cells can mediate protection without producing IFNg and that our results with transgenic T cells extend to native T cell populations (Figure S1). We also examined whether naïve C7 cells could limit M. tuberculosis growth and whether this effect is independent of IFN-γ. 10,000 naïve C7 cells significantly reduced M. tuberculosis bacterial load in the lung at 22 days (Figure S1B). IFNγ deficient T cells also significantly reduced bacterial loads and there was no significant difference in the ability of wild type and IFNγ deficient naïve cells to control M. tuberculosis growth (Figure S1B).
Because IFNγ is essential for effective immune control of Mtb, we speculated that IFNγ deficient C7 cells might recruit IFNγ-expressing host-derived cells (e.g. Natural Killer cells or endogenous CD4 or CD8 T cells) to sites of mycobacterial infection. In this way, host-derived IFNγ might activate the expression of mycobactericidal factors. To address this hypothesis, we tested that ability of adoptively transferred T cells to provide protection in mice lacking IFNγ. Remarkably, both WT and IFNγ-deficient C7 effector cells protected hosts lacking IFNγ, although in this setting IFNγ-deficient T cells were slightly but significantly less effective than WT C7 cells at limiting in vivo growth of Mtb. Nevertheless, compared to IFNγ deficient mice that did not receive T cells, animals that received C7 IFNγ deficient effectors had ∼30 fold reduction in bacterial numbers in the lungs at day 21 following infection (Figure 2B). This result demonstrates that CD4 T cells have a highly effective effector pathway to control Mtb that is completely independent of IFNγ.
During murine infection with Mtb, IFNγ signaling induces NOS2 (inducible nitric oxide synthase), leading to the generation of nitric oxide (NO) which can kill mycobacteria [14]. To determine whether adoptively transferred C7 T cells mediate protection by inducing NOS2, we transferred C7 T cells into NOS2 deficient mice. WT C7 effectors were effective at protecting both NOS2 and PHOX deficient mice from infection, resulting in ∼70 fold reduction in bacterial numbers in NOS2 or PHOX deficient C7-recipients compared to deficient mice that did not receive cells (Figure 2C) and Figure S2. NOS2 induction is a major IFNγ-dependent effector mechanism controlling defense against Mtb in mice, yet our results show that C7 T cells that produce IFNγ are similarly protective in WT and NOS2-deficient hosts. Taken together, our results demonstrate the existence of an IFNγ/NOS2-independent mechanism of CD4 T cell mediated killing of Mtb that is operative at the early time points examined in this study.
Optimal control of M. tuberculosis growth can be independent of IFNγ and TNF production by effector T cells
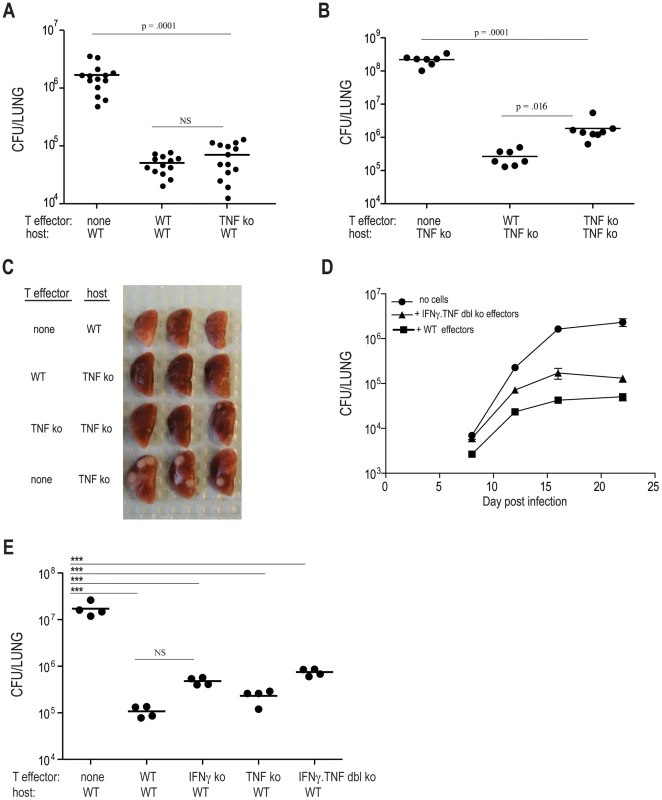
Tumor necrosis factor (TNF) is another critical regulator of host defense that is secreted by Th1 CD4 T cells. The precise contribution of TNF to defense against Mtb infection is difficult to define since it has been implicated in lymphocyte recruitment, cell survival, and mycobacterial killing [3], [15], [16]. We next determined whether TNF deficient C7 cells could protect WT and TNF deficient mice from Mtb infection. The protection provided to recipient mice either by WT or TNF deficient C7 effector cells was comparable, demonstrating that TNF production by effector CD4 T cells is not required for protection against Mtb infection (Figure 3A). Similarly, TNF-deficient naïve C7 T cells provided the same level of protection as adoptively transferred wild-type C7 T cells (Figure S1B). Experiments using TNF deficient hosts showed that TNF deficient effectors reduced the number of bacteria by ∼100 fold, compared to TNF deficient hosts that did not receive C7 effectors (Figure 3B). Although adoptively transferred TNF-deficient C7 effectors were slightly less effective than WT C7 effectors at providing protection in TNF deficient recipients, TNF-deficient C7 effector T cells still provided a high degree of protection in TNF deficient recipients, an effect that could also be observed macroscopically in infected lungs, with TNF deficient hosts having large lesions that are diminished in size in TNF deficient recipients of either WT or TNF deficient C7 effectors (Figure 3C).
It is possible that the protection provided by IFNγ or TNF deficient C7 T cells might result from partial functional redundancy of these two cytokines. To test this idea, we generated C7 cells lacking both IFNγ and TNF (C7.IFNγ.TNF dbl deficient cells), and transferred these cells, following in vitro Th1 differentiation, into WT hosts and then infected with Mtb. Bacterial numbers were determined 8, 12, 16, and 22 days following aerosol infection and compared to control animals (recipients of WT effectors, or, animals that did not receive cells). The data in Figure 3Dγ demonstrate that when C7 T cells are unable to produce both TNF and IFN, their capacity to provide protection is modestly restricted. In comparison to mice that did not receive T cells and mice that received wild type C7 Th1 cells, double deficient T cells continued to provide ∼60% of maximal protection. TNF and IFNγ double deficient naïve C7 T cells also conferred protection against Mtb (Figure S1B). These results indicate that CD4 T cells, independent of both TNF and IFNγ, provide roughly 10 to 15 fold inhibition of Mtb growth.
We considered the possibility that the gamma/TNF independent pathway of antimycobacterial immunity demonstrated above might depend on cell dose. In the above experiments, the high cell dose (10 million) might allow a minor pathway of antimycobacterial immunity to substitute for the gamma/TNF pathway. To address this question, we repeated our experiments with one million transferred cells, which in our prior experience still provided substantial killing of M. tuberculosis [13]. One million transferred WT C7 cells reduced the bacterial load in the lungs of infected mice by 160 fold compared with animals that received no cells (Figure 3E). One million IFNγ deficient T cells retained substantial antimycobacterial effect, reducing bacterial loads by 36 fold compared to control animals (Figure 3E). Similarly, TNF deficient cells reduced bacterial loads by 74 fold. IFNγ/TNF deficient cells were somewhat impaired in their antimycobacterial effect, but still reduced bacterial loads by 23 fold. Taken together, these experiments indicate both IFNγ/TNF dependent and independent pathways of T cell mediated protection. The majority of protection conferred by the C7 cells is, however, IFNγ independent, even at lower T cell doses.
Cytolysis via perforin and FAS are not required to control of M. tuberculosis infection
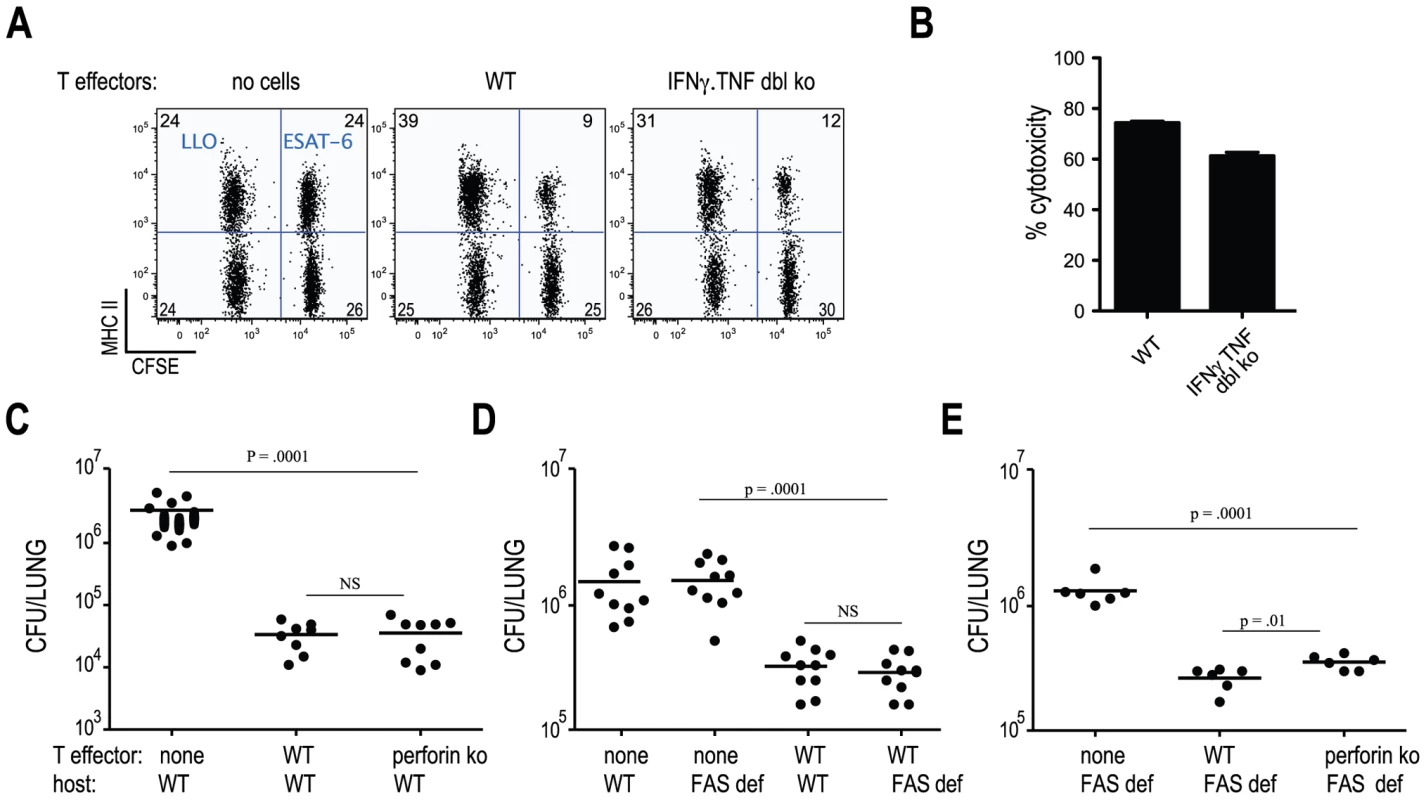
MHC-class II restricted cytolytic activity has been observed following Mtb infection and has been suggested to contribute to protective immunity [17], [18]. Since Mtb-specific cells deficient in both IFNγ and TNF protected mice from infection, we investigated whether ESAT-6-specific CD4 T cell cytolytic activity contributed to in vivo protection. To determine whether C7 effector cells killed target cells presenting the ESAT-6 epitope in vivo, we transferred C7 WT or C7.IFNγ.TNF double deficient CD4 T cells into mice and 3 days later injected these mice with CFSE-labeled, ESAT-6-coated target cells. We detected approximately 60–80% specific lysis of ESAT-6-coated target cells in recipients of either C7.WT or C7.IFNγ.TNF double deficient effectors (Figure 4A and B). This high degree of cytolysis is similar to what was observed for endogenous populations of Mtb-specific CD4 T cells [17]. To determine the contribution of perforin in CD4 T cell-mediated protection against Mtb infection, we compared bacterial numbers in infected mice that did not receive cells to recipients of C7.WT or C7.perforin deficient, Th1-differentiated effector T cells. Figure 4C - demonstrates that perforin deficient T cells were able to restrict Mtb growth to the same extent as wild type T cells, indicating that perforinmediated cytolytic activity does not contribute to the T cell-mediated control of mycobacterial infection we observe.
Mice lacking functional FAS or FASL are more susceptible to Mtb infection than WT animals [19]. To determine if FAS mediated signaling contributes to C7 T cell-mediated protection of mice from Mtb infection, we modified our adoptive transfer protocol since C7.WT cells transferred before infection into FAS deficient hosts were undetectable 21 days following infection (data not shown). Instead of transferring C7 cells prior to infection, C7 effector cells were transferred 7 days following infection and bacterial numbers were measured 7 days later. The shorter experiment led to a similar recovery of C7 cells in WT and FAS deficient hosts (data not shown). Figure 4D( shows that deficiency in FAS signaling did not alter the ability of C7 effectors to protect against Mtb infection, since comparable bacterial numbers were cultured from WT and FAS deficient recipient mice. Elimination of both perforin and FAS had only a minor, albeit statistically significant effect on protection mediated by C7 cells Figure 4E). In addition, Perforin deficient C7 cells were also as capable as C7.WT cells at protecting iNOS deficient mice from infection (Figure S3). Taken together, these results demonstrate that early control of mycobacterial infection does not require CD4 T cells to kill Mtb-infected target cells by FAS or perforin-mediated cytolytic activity.
Optimal protection to M. tuberculosis requires a Th-1 lineage population, yet is independent of T-bet
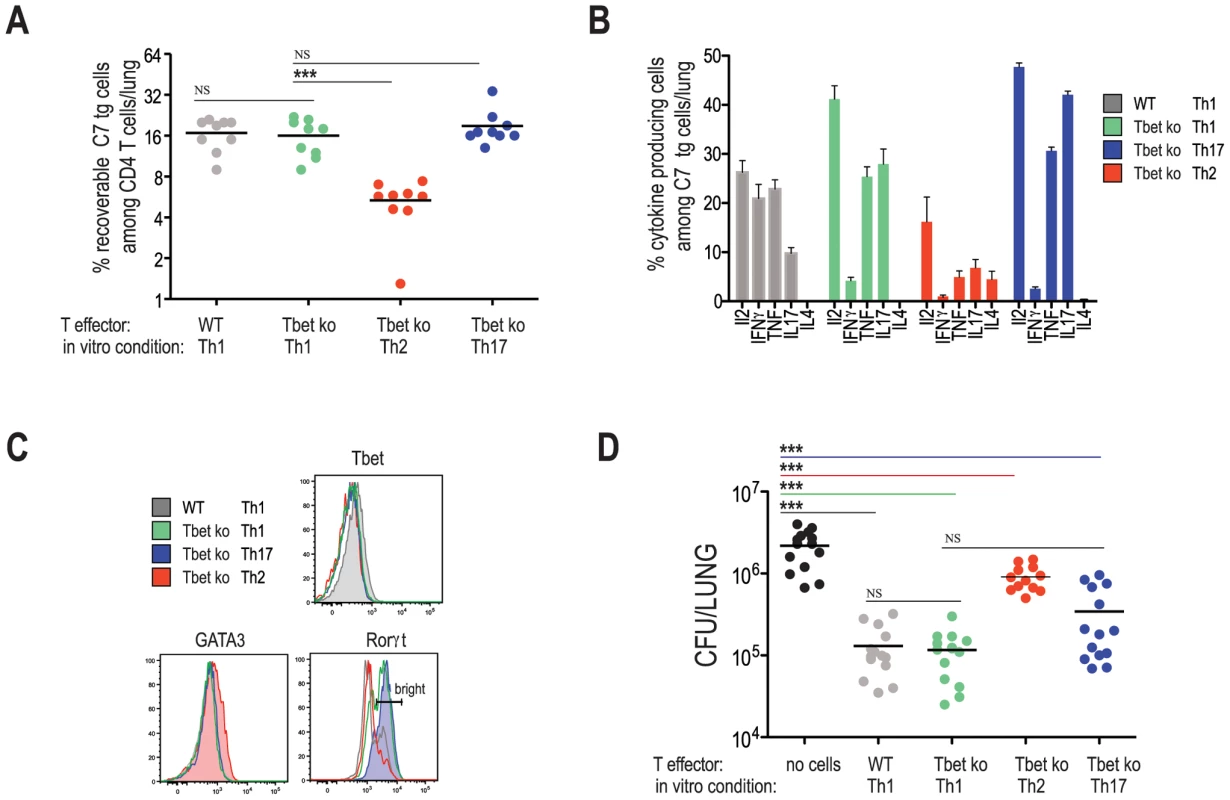
Because no single Th1-associated effector pathway was essential for T cell mediated protection against Mtb infection (i.e., IFNγ, TNF, perforin, and FAS), we asked whether protection is a general property of helper T cells regardless of their effector phenotype. To address this question, naïve C7 cells were differentiated into Th2 and Th17 cells and transferred into WT hosts prior to Mtb infection. C7 TCR tg mice were crossed to the T-bet deficient background to prevent in vivo conversion of Th2 and Th17 populations into cells with a Th1 profile (AMG and EGP unpublished data, and [20]). Twenty-one days following infection, lungs were harvested from infected animals and the frequencies and phenotypes of transferred populations were determined (Figure 5). While frequencies of Th1-skewed cells (on either a WT or T-bet deficient background), and Th17-skewed cells were similar, compared to these populations, the frequencies of Th2-skewed cells were reduced. Of note, adoptively transferred T cells, including Th2-skewed cells, inhibited the priming of host-derived endogenous populations of ESAT-6 specific cells, indicating that adoptively transferred cells were participating in the immune response (Figure S4). Since the degree of inhibition is directly correlated with the number of transferred effector cells (data not shown and [21]), we concluded from this result that recipient mice, at least at the time of endogenous T cell priming, harbored similar frequencies of adoptively transferred cells.
The cytokine and transcription factor profiles of adoptively transferred T cells correspond to the expected phenotypes based on the in vitro culturing conditions [22]. As expected, while WT Th1 cell populations contained IFNγ producing cells, all T-bet deficient cells examined (i.e., Th1, Th2, and Th17 skewed cells) did not produce detectable levels of IFNγ (Figure 5B). Th2-differentiated T-bet deficient C7 T cells expressed IL4 and GATA3, while Th17-skewed C7 cells expressed IL17 and RORγt (Figure 5B and C). Th1-skewed T-bet deficient C7 T cells also expressed IL17 and RORγt, albeit in lower proportions. Figure 5D demonstrates that Th2-skewed cells marginally protected mice from infection, demonstrating that protection is not a general feature of all effector CD4 T cells. Th1-skewed, T-bet-deficient C7 T cells protected mice as well as Th1-skewed, WT C7 T cells, corroborating our finding that IFNγ production by Mtb-specific CD4 T cells is unnecessary for early protection against Mtb infection. T-bet deficient C7 T cells differentiated under Th17 conditions provided protection, however [23], we consistently observed better protection by Th1-differentaited, T-bet deficient C7 T cells, despite seemingly similar cytokine and transcription factor profiles between these two populations (Figure 5B and C). Taken together, our results suggest that protection against Mtb infection is optimal when effector T cells are differentiated under Th1 conditions, however in a T-bet independent fashion.
IFNγ and TNF are central to host defense against Mtb infection in both mice and humans. However, because these cytokines have pleiotropic roles in T cell differentiation, cell trafficking, and macrophage activation, and are produced by a wide variety of immune cells, the exact mechanism(s) by which they confer protection has not been clearly defined. One predominant model is that Th1 effector T cells, which are known to produce both IFNγ and TNF, are the important in vivo sources of these cytokines and thereby activate macrophage mycobactericidal effector functions. However, there have been occasional reports in which protection against Mtb infection did not correlate with production of IFNγ by CD4 effector cells [5], [8], [9], [10], suggesting either that other cellular sources of these cytokines are important, or that IFNγ independent mechanisms of protection may exist. The data presented here provide strong support for a mycobactericidal effector function of CD4 T cells that is independent of both IFNγ and TNF. These results may indicate that the central role of these cytokines is to prime CD4 differentiation to the Th1 phenotype, after which other effector functions kill Mtb. When cells with a Th1 phenotype are supplied, IFNγ becomes dispensable. Our findings do not dispute the evidence that IFNγ plays an essential role in immunity to Mtb, rather they show that antigen experienced effector cells have mechanisms to control infection that do not rely on IFNγ mediated signals [24], [25]. When these cells are supplied at the time of infection, T cells can be highly effective at limiting Mtb growth. Our findings suggest that vaccination strategies that seek to maximize IFNγ producing CD4 T cells may miss an important effector mechanism of Th1 CD4 T cells that we demonstrate is highly effective at controlling Mtb growth in vivo. Further exploitation of this new pathway therefore holds promise for the design of vaccines to control Mtb infection.
Materials and Methods
Mice
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the MSKCC Institutional Animal Care and Use Committee. No non-human primates were used in this research. C57BL/6J mice, iNOS ko (#002609), and FAS deficient mice (#000482) were purchased from Jackson Laboratory. C7.IFNγ ko, C7.TNF ko, C7.IFNγ.TNF dl ko, C7.perforin ko, C7.T-bet ko, IFNγ ko, TNF ko, and gp91 phox ko mice were maintained at the animal facility in the memorial Sloan-Kettering Research Animal Resource Center. The genotypes of the animals were confirmed by PCR analysis and phenotypic confirmation of the transgenic T cells was performed by intracellular staining (C7.IFNγ ko, C7.TNF ko, C7.IFNγ.TNF dbl ko, C7.T-bet ko). The Memorial Sloan-Kettering Institutional Animal Care and Use committee approved all animal procedures.
Generation of effector T cells
4×106 purified C7 TCR tg CD4+T cells were cultured with 12×106 irradiated T cell–depleted splenocytes and 5 µg/ml of ESAT-6 1–20 peptide. At days 2 and 3 of culture, the cells were split 1∶2, and 50 U/ml IL-2 was added (R&D Systems). For Th1 cultures, 10 ng/ml IL-12, and 5 µg/ml of neutralizing anti–IL-4 antibody (R&D Systems) were added at day 0 of culture. For Th17 cultures, 10 µg/ml of anti-IFNγ and anti-IL-4 and 5 ng/ml of hTGF-β, 20 ng/ml of IL6 and 20 ng/ml of IL-23 were added at day 0 of culture. For Th2 cultures, 10 µg/ml of anti-IFNγ. and 20 ng/ml of IL4 were added to cultures.
Aerosol infections with M. tuberculosis and generation of M. tuberculosis Erdman strains
Mice were infected at 8–10-wks of age with M. tuberculosis Erdman and plated as described [13] M. tuberculosis Erdman strain, Δesat6::hyg (SSM6), has a deletion in the gene encoding ESAT-6 [26]. This strain was complemented with PMH406, which integrates at the chromosomal attB site, which uses the mop promoter to drive expression of CFP-10 and ESAT-6 [27]. Either wild type ESAT-6 or mutated ESAT-6 in which amino acid number 12 was changed from glutamic acid to alanine (E12A) were used to generate Δesat6::hyg attB::pMH406 or Δesat6::hyg attB::pMH406-ESAT6(E12A) .
Statistical analysis
For comparison of means between two groups we performed the unpaired Student's t test in GraphPad Prism software. For experiments that involved more than three groups (e.g Figures 3E, 5A, 5D), we compared the groups using a one way ANOVA with a Bonferroni's multiple comparison test.
Supporting Information
Zdroje
1. CooperAMDaltonDKStewartTAGriffinJPRussellDG
1993
Disseminated tuberculosis in interferon gamma gene-disrupted
mice.
J Exp Med
178
2243
2247
2. FlynnJLChanJTrieboldKJDaltonDKStewartTA
1993
An essential role for interferon gamma in resistance to
Mycobacterium tuberculosis infection.
J Exp Med
178
2249
2254
3. van de VosseEHoeveMAOttenhoffTH
2004
Human genetics of intracellular infectious diseases: molecular
and cellular immunity against mycobacteria and salmonellae.
Lancet Infect Dis
4
739
749
4. Filipe-SantosOBustamanteJChapgierAVogtGde BeaucoudreyL
2006
Inborn errors of IL-12/23 - and IFN-gamma-mediated immunity:
molecular, cellular, and clinical features.
Semin Immunol
18
347
361
5. CarusoAMSerbinaNKleinETrieboldKBloomBR
1999
Mice deficient in CD4 T cells have only transiently diminished
levels of IFN-gamma, yet succumb to tuberculosis.
J Immunol
162
5407
5416
6. EhrtSSchnappingerDBekiranovSDrenkowJShiS
2001
Reprogramming of the macrophage transcriptome in response to
interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric
oxide synthase-2 and phagocyte oxidase.
J Exp Med
194
1123
1140
7. SatoKAkakiTTomiokaH
1998
Differential potentiation of anti-mycobacterial activity and
reactive nitrogen intermediate-producing ability of murine peritoneal
macrophages activated by interferon-gamma (IFN-gamma) and tumour necrosis
factor-alpha (TNF-alpha).
Clin Exp Immunol
112
63
68
8. MajlessiLSimsovaMJarvisZBrodinPRojasMJ
2006
An increase in antimycobacterial Th1-cell responses by
prime-boost protocols of immunization does not enhance protection against
tuberculosis.
Infect Immun
74
2128
2137
9. MittruckerHWSteinhoffUKohlerAKrauseMLazarD
2007
Poor correlation between BCG vaccination-induced T cell responses
and protection against tuberculosis.
Proc Natl Acad Sci U S A
104
12434
12439
10. LambertPHHawkridgeTHanekomWA
2009
New vaccines against tuberculosis.
Clin Chest Med
30
811
826
11. ScangaCAMohanVPYuKJosephHTanakaK
2000
Depletion of CD4(+) T cells causes reactivation of murine
persistent tuberculosis despite continued expression of interferon gamma and
nitric oxide synthase 2.
J Exp Med
192
347
358
12. KaginaBMAbelBScribaTJHughesEJKeyserA
2010
Specific T Cell Frequency and Cytokine Expression Profile do not
Correlate with Protection against Tuberculosis, Following BCG Vaccination of
Newborns.
Am J Respir Crit Care Med
182
1073
9
13. GallegosAMPamerEGGlickmanMS
2008
Delayed protection by ESAT-6-specific effector CD4+T cells
after airborne M. tuberculosis infection.
J Exp Med
205
2359
2368
14. ShilohMUNathanCF
2000
Reactive nitrogen intermediates and the pathogenesis of
Salmonella and mycobacteria.
Curr Opin Microbiol
3
35
42
15. LocksleyRMKilleenNLenardoMJ
2001
The TNF and TNF receptor superfamilies: integrating mammalian
biology.
Cell
104
487
501
16. FlynnJLGoldsteinMMChanJTrieboldKJPfefferK
1995
Tumor necrosis factor-alpha is required in the protective immune
response against Mycobacterium tuberculosis in mice.
Immunity
2
561
572
17. WoodworthJSWuYBeharSM
2008
Mycobacterium tuberculosis-specific CD8+T cells require
perforin to kill target cells and provide protection in
vivo.
J Immunol
181
8595
8603
18. LewinsohnDMBementTTXuJLynchDHGrabsteinKH
1998
Human purified protein derivative-specific CD4+T cells use
both CD95-dependent and CD95-independent cytolytic
mechanisms.
J Immunol
160
2374
2379
19. TurnerJD'SouzaCDPearlJEMariettaPNoelM
2001
CD8 - and CD95/95L-dependent mechanisms of resistance in mice with
chronic pulmonary tuberculosis.
Am J Respir Cell Mol Biol
24
203
209
20. LohningMHegazyANPinschewerDDBusseDLangKS
2008
Long-lived virus-reactive memory T cells generated from purified
cytokine-secreting T helper type 1 and type 2 effectors.
J Exp Med
205
53
61
21. BadovinacVPHaringJSHartyJT
2007
Initial T cell receptor transgenic cell precursor frequency
dictates critical aspects of the CD8(+) T cell response to
infection.
Immunity
26
827
841
22. ReinerSL
2007
Development in motion: helper T cells at work.
Cell
129
33
36
23. KhaderSABellGKPearlJEFountainJJRangel-MorenoJ
2007
IL-23 and IL-17 in the establishment of protective pulmonary
CD4+T cell responses after vaccination and during Mycobacterium
tuberculosis challenge.
Nat Immunol
8
369
377
24. HartyJTBevanMJ
1995
Specific immunity to Listeria monocytogenes in the absence of IFN
gamma.
Immunity
3
109
117
25. CowleySCElkinsKL
2003
CD4+T cells mediate IFN-gamma-independent control of
Mycobacterium tuberculosis infection both in vitro and in
vivo.
J Immunol
171
4689
4699
26. StanleySARaghavanSHwangWWCoxJS
2003
Acute infection and macrophage subversion by Mycobacterium
tuberculosis require a specialized secretion system.
Proc Natl Acad Sci U S A
100
13001
13006
27. GuinnKMHickeyMJMathurSKZakelKLGrotzkeJE
2004
Individual RD1-region genes are required for export of
ESAT-6/CFP-10 and for virulence of Mycobacterium
tuberculosis.
Mol Microbiol
51
359
370
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Distribution of the Phenotypic Effects of Random Homologous Recombination between Two Virus SpeciesČlánek SIV Nef Proteins Recruit the AP-2 Complex to Antagonize Tetherin and Facilitate Virion ReleaseČlánek Dual Function of the NK Cell Receptor 2B4 (CD244) in the Regulation of HCV-Specific CD8+ T CellsČlánek A Large and Intact Viral Particle Penetrates the Endoplasmic Reticulum Membrane to Reach the CytosolČlánek Interleukin-13 Promotes Susceptibility to Chlamydial Infection of the Respiratory and Genital Tracts
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Lymphoadenopathy during Lyme Borreliosis Is Caused by Spirochete Migration-Induced Specific B Cell Activation
- Infections Are Virulent and Inhibit the Human Malaria Parasite in
- A Gamma Interferon Independent Mechanism of CD4 T Cell Mediated Control of Infection
- MDA5 and TLR3 Initiate Pro-Inflammatory Signaling Pathways Leading to Rhinovirus-Induced Airways Inflammation and Hyperresponsiveness
- The OXI1 Kinase Pathway Mediates -Induced Growth Promotion in Arabidopsis
- An E2F1-Mediated DNA Damage Response Contributes to the Replication of Human Cytomegalovirus
- Quantitative Subcellular Proteome and Secretome Profiling of Influenza A Virus-Infected Human Primary Macrophages
- Distribution of the Phenotypic Effects of Random Homologous Recombination between Two Virus Species
- Inhibition of Both HIV-1 Reverse Transcription and Gene Expression by a Cyclic Peptide that Binds the Tat-Transactivating Response Element (TAR) RNA
- A Viral Satellite RNA Induces Yellow Symptoms on Tobacco by Targeting a Gene Involved in Chlorophyll Biosynthesis using the RNA Silencing Machinery
- Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Investigating the Host Binding Signature on the PfEMP1 Protein Family
- Human Neutrophil Clearance of Bacterial Pathogens Triggers Anti-Microbial γδ T Cell Responses in Early Infection
- Septation of Infectious Hyphae Is Critical for Appressoria Formation and Virulence in the Smut Fungus
- A Family of Helminth Molecules that Modulate Innate Cell Responses via Molecular Mimicry of Host Antimicrobial Peptides
- Phospholipids Trigger Capsular Enlargement during Interactions with Amoebae and Macrophages
- CTL Escape Mediated by Proteasomal Destruction of an HIV-1 Cryptic Epitope
- Evolution of Th2 Immunity: A Rapid Repair Response to Tissue Destructive Pathogens
- Extensive Genome-Wide Variability of Human Cytomegalovirus in Congenitally Infected Infants
- The Antiviral Efficacy of HIV-Specific CD8 T-Cells to a Conserved Epitope Is Heavily Dependent on the Infecting HIV-1 Isolate
- Epstein-Barr Virus Infection of Polarized Epithelial Cells via the Basolateral Surface by Memory B Cell-Mediated Transfer Infection
- Reactive Oxygen Species Hydrogen Peroxide Mediates Kaposi's Sarcoma-Associated Herpesvirus Reactivation from Latency
- Crystal Structure and Functional Analysis of the SARS-Coronavirus RNA Cap 2′-O-Methyltransferase nsp10/nsp16 Complex
- The Dot/Icm System Delivers a Unique Repertoire of Type IV Effectors into Host Cells and Is Required for Intracellular Replication
- AAV Exploits Subcellular Stress Associated with Inflammation, Endoplasmic Reticulum Expansion, and Misfolded Proteins in Models of Cystic Fibrosis
- Suboptimal Activation of Antigen-Specific CD4 Effector Cells Enables Persistence of In Vivo
- SIV Nef Proteins Recruit the AP-2 Complex to Antagonize Tetherin and Facilitate Virion Release
- Dual Function of the NK Cell Receptor 2B4 (CD244) in the Regulation of HCV-Specific CD8+ T Cells
- Transition of Sporozoites into Liver Stage-Like Forms Is Regulated by the RNA Binding Protein Pumilio
- A Large and Intact Viral Particle Penetrates the Endoplasmic Reticulum Membrane to Reach the Cytosol
- Transcriptome Analysis of in Human Whole Blood and Mutagenesis Studies Identify Virulence Factors Involved in Blood Survival
- Interleukin-13 Promotes Susceptibility to Chlamydial Infection of the Respiratory and Genital Tracts
- Structural Insights into Viral Determinants of Nematode Mediated Transmission
- Protective Efficacy of Serially Up-Ranked Subdominant CD8 T Cell Epitopes against Virus Challenges
- Viral CTL Escape Mutants Are Generated in Lymph Nodes and Subsequently Become Fixed in Plasma and Rectal Mucosa during Acute SIV Infection of Macaques
- Taking Some of the Mystery out of Host∶Virus Interactions
- Viral Small Interfering RNAs Target Host Genes to Mediate Disease Symptoms in Plants
- : An Emerging Cause of Sexually Transmitted Disease in Women
- Mitochondrial Ubiquitin Ligase MARCH5 Promotes TLR7 Signaling by Attenuating TANK Action
- The Hexamer Structure of the Rift Valley Fever Virus Nucleoprotein Suggests a Mechanism for its Assembly into Ribonucleoprotein Complexes
- Acquisition of Human-Type Receptor Binding Specificity by New H5N1 Influenza Virus Sublineages during Their Emergence in Birds in Egypt
- Stromal Down-Regulation of Macrophage CD4/CCR5 Expression and NF-κB Activation Mediates HIV-1 Non-Permissiveness in Intestinal Macrophages
- A Component of the Xanthomonadaceae Type IV Secretion System Combines a VirB7 Motif with a N0 Domain Found in Outer Membrane Transport Proteins
- Perturbs Iron Trafficking in the Epithelium to Grow on the Cell Surface
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Crystal Structure and Functional Analysis of the SARS-Coronavirus RNA Cap 2′-O-Methyltransferase nsp10/nsp16 Complex
- Lymphoadenopathy during Lyme Borreliosis Is Caused by Spirochete Migration-Induced Specific B Cell Activation
- The OXI1 Kinase Pathway Mediates -Induced Growth Promotion in Arabidopsis
- : An Emerging Cause of Sexually Transmitted Disease in Women
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání