-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBiotic elicitation of the L. suspension culture
Biotická elicitace suspenzní kultury Trifolium pratense L.
Mezi významné látky sekundárního metabolismu patří flavonoidy a isoflavonoidy. Suspenzní kultura Trifolium pratense L. (Fabaceae) má však nízkou produkci těchto metabolitů, a proto byla snaha zvýšit jí elicitací. K endogenním signálním látkám rostlinných obraných reakcí patří kyselina jasmínová, která v případě exogenní aplikace působí také jako biotický elicitor. V práci byl sledován vliv čtyř koncentrací kyseliny jasmínové na produkci suspenzní kultury Trifolium pratense L. (varieta DO-8 a varieta DO-9). Kultura byla kultivovaná na mediu podle Gamborga s přídavkem 2 mg.l⁻¹ 2,4-dichlorfenoxyoctové kyseliny a 2 mg.l⁻¹ 6-benzylaminopurinu. Maximální zvýšení produkce flavonoidů oproti kontrole vyvolala u obou variet koncentrace 500 μmol (6hodinová aplikace o 140 % u variety DO-9 a 24hodinová aplikace o 65 % u variety DO-8). Produkci isoflavonoidů (genistinu, daidzeinu, genisteinu a formononetinu) stimulovala u obou variet nejvíce koncentrace 50 μmol (u variety DO-9 zvýšila 48hodinová aplikace obsah genistinu až o 845 %).
Klíčová slova:
suspenzní kultura Trifolium pratense L. – flavonoidy a isoflavonoidy – biotická elicitace – kyselina jasmínová
Authors: M. Kašparová; T. Siatka; J. Dušek
Authors place of work: Department of Pharmacognosy ; Charles University in Prague, Faculty of Pharmacy in Hradec Králové
Published in the journal: Čes. slov. Farm., 2008; 57, 107-110
Category: Původní práce
Summary
The important substances of secondary metabolism include flavonoids and isoflavonoids. The Trifolium pratense L. (Fabaceae) suspension culture’s yield of these metabolites is low, thus an attempt was made to increase the production by elicitation. The endogenous signal substances of the plants’ defensive responses include jasmonic acid that also functions as a biotic elicitor in the case of exogenous application. In the experiment the authors monitored the impact of 4 different concentrations of jasmonic acid on the Trifolium pratense L. (variety DO-8 and variety DO-9) suspension culture’s yield. The culture was cultivated in Gamborg medium to which 2 mg.l⁻¹ of 2,4‑dichlorophenoxyacetic acid and 2 mg.l⁻¹ of 6-benzylaminopurine were added. The maximum increase in the production of flavonoids was achieved, when compared with the control samples, with both varieties of the 500 μmol concentration (DO-9, 6-hour application by 140%; DO-8, 24‑hour application by 65%). The production of isoflavonoids (genistin, daidzein, genistein, and formononetine) was best stimulated in both varieties by the 50 μmol concentration (in the case of DO-9 variety, the 48-hour application increased the content of genistin by up to 845%).
Key words:
Trifolium pratense L. suspension culture – flavonoids and isoflavonoids – biotic elicitation – jasmonic acidThe elicitation method is one of the means to increase the production of secondary metabolites in explant cultures of plants. The elicitation utilizes substances possessing the signal effect properties–the elicitors. These function as stress factors, induce increased synthesis of phytoalexins and of other secondary metabolites that contribute to the defense potential of the plant or of the explant.
Jasmonic acid and its precursors and derivates are some of the endogenous signal substances of the plants’ defensive responses 1). Jasmonic acid and its methyl ester are natural hormonal regulators controlling the plant’s aging and inducing processes that take place after the plant is injured. In the exogenic application case, they may also function as elicitors 2).
Important secondary metabolites of intact plants and of explant cultures include flavonoids and isoflavonoids which posses a wide range of biological effects 3–12). In the observed suspension culture, Trifolium pratense L. (Fabaceae), the production rate of these metabolites is low, thus there was an attempt to increase their production with the jasmonic acid biotic elicitor.
EXPERIMENTAL PHASE
Instruments
A 200S analytical scales manufactured by Sartorius, Göttingen; a PS 20A autoclave by Chirana, Brno; an HS 31A hot-air sterilizer by Chirana, Brno; a laminar flow workbench by Fatran LF, Žilina; a roller by Vývojové Dílny AV ČR, Praha; a 2010 shaker by Unimax, Heidolph; a CE 1010 spectrophotometer by Cecil Instruments, Cambridge; a liquid chromatograph (a PU-2089 pump, a MD-2015 detector, an AS 2055 automatic sample injector) by Jasco, Tokyo.
Trifolium pratense L. suspension culture
The suspension cultures (variety DO-8, DO-9) were derived from callus cultures. First by mechanical loosening on the shaker followed by the cultivation in Gamborg nutrient media 13) with the addition of 2 mg.1-1 of 2,4--dichlorophenoxyacetic acid and 2 mg.1-1 of 6 benzylaminopurine, at the temperature of 25 °C, 16-hr light/8-hr dark period. The subcultivation interval lasted 14 days 14).
Elicitation
To elicitate, we used jasmonic acid dilutions in 96% ethanol in the concentrations of 5 μmol, 50 μmol, 500 μmol, and 5000 μmol. During the elicitation process, we added 1.0 ml of the elicitor to a two-year-old suspension culture on the 21st day of cultivation 14). The control cultures received 1.0 ml of distilled water. The elicitor application durations were 6, 24, 48, and 168 hours. The cells were separated from the media by vacuuming, rinsed in distilled water, and dried at the laboratory room temperature.
Determining the flavonoids and isoflavonoids
The elicited and the inspection samples underwent photometric determination of flavonoids in accordance with the Czech Pharmacopoeia 2005 15) and the determination of isoflavonoids using the HPLC method 16). The HPLC determination process conditions were as follows: a RP-18 Lichrospher column (250 × 4 mm, particles size 5 μm) with a precolumn made of the same material; elution: linear gradient of the mobile phase A (methanol) in phase B (water containing 0.15% of phosphoric acid) 30–80% from 0 to 9 minutes was followed by the isocratic elution mobile phase in the composition of 80% of phase A in phase B from 9 to 15 minutes; the elution speed was 1.1 ml/min; the detection was carried out at the 260 nm wave length. The obtained results were statistically evaluated in the t-test for at least 3 members of the team and at the p = 0.05 level of statistical significance.
RESULTS AND DISCUSSION
Increasing the production of therapeutically important secondary metabolites in in vitro cultures is one of the closely watched spheres in pharmaceutical biotechnology. Tangibly speaking, the elicitation of explant cultures is a very promising method.
The Trifolium pratense L. suspension culture (variety DO-8, DO-9) was observed for the effect of jasmonic acid’s biotic elicitor on the production of flavonoids and isoflavonoids.
The findings of previous experiments imply 14) that the elicitation of the Trifolium pratense L. suspension culture is best on day 21 of subcultivation; our culture’s elicitation was therefore carried out on that day of cultivation.
Four concentrations of jasmonic acid were tested (5 μmol, 50 μmol, 500 μmol, and 5000 μmol); these concentrations had been chosen from within the range that is usually applied with this type of elicitor 17–19). The time periods of the elicitor’s effect that were tested (6, 24, 48 and 168 hours) had been based on the results of the already performed tests 20) and on different pieces of published information that deal with an increase in the production of the secondary metabolites after the addition of an elicitor in the range of several hours to several days 17, 18, 21, 22). Inspection cultures were collected after 6 and 168 hours since their production does not change notably in such short time intervals.
The results of the biotic elicitation of the Trifolium pratense L. suspension culture (variety DO-8) with jasmonic acid (Table 1) clearly indicate that flavonoid production was influenced in a positive way. The maximum content (0.112%) was recorded after a 24-hour exposure to a 500 μmol concentration and, after comparing this result with the control culture result, we can state that a statistically important increase in the production by 65% was achieved. Statistically important results were also achieved with the remaining concentrations of the elicitor after a 24-hour exposure when compared with the control samples.
Tab. 1. Production of flavonoids in suspension culture of Trifolium pratense L. (variety DO-8, DO-9) elicited with jasmonic acid 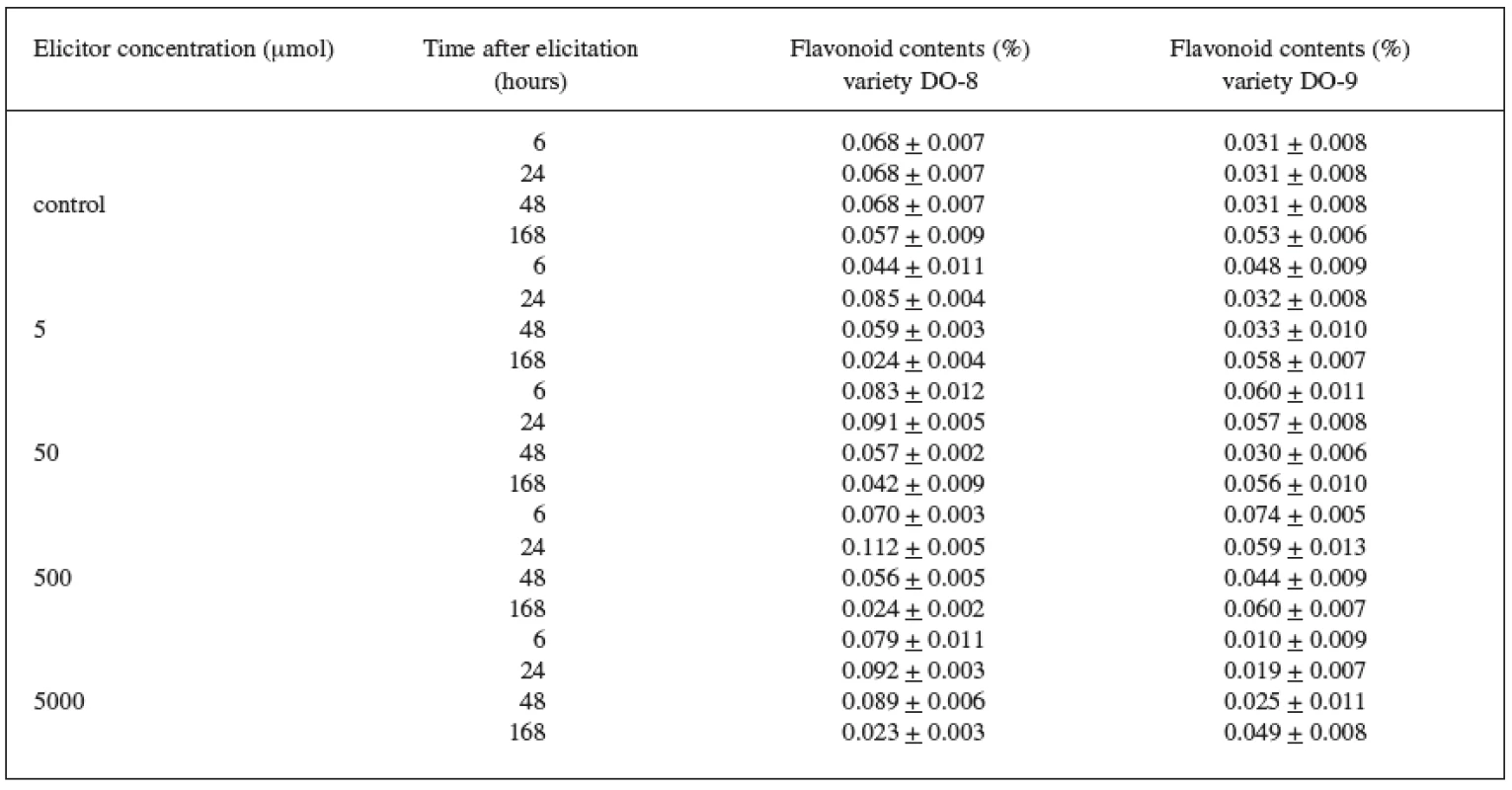
The Trifolium pratense L. suspension culture (variety DO-9) showed a better elicitation effect with a 500 μmol concentration, too (Table 1). The maximum content of flavonoids (0.074%) and a statistically important increase in the production by 140%, compared with the control samples, were recorded after a 6-hour application.
In the Trifolium pratense L. suspension culture (variety DO-8, DO-9) elicited with jasmonic acid, we also checked the production of isoflavonoids. In the control culture we recorded the isoflavonoids genistin, daidzein, genistein, and formononetin. In the case of the DO-8 variety (Table 2), we can value namely the 6-hour application of the 50 μmol concentration which increased, compared with the control culture, the production of all isoflavonoids under observation – genistin by 150%, daidzein by 123%, genistein by 285%, and formononetin by 30%. The increase in the production of these isoflavonoids was also induced by the 500 μmol concentration.
Tab. 2. Production of isoflavonoids in suspension culture of Trifolium pratense L. (variety DO-8) elicited with jasmonic acid 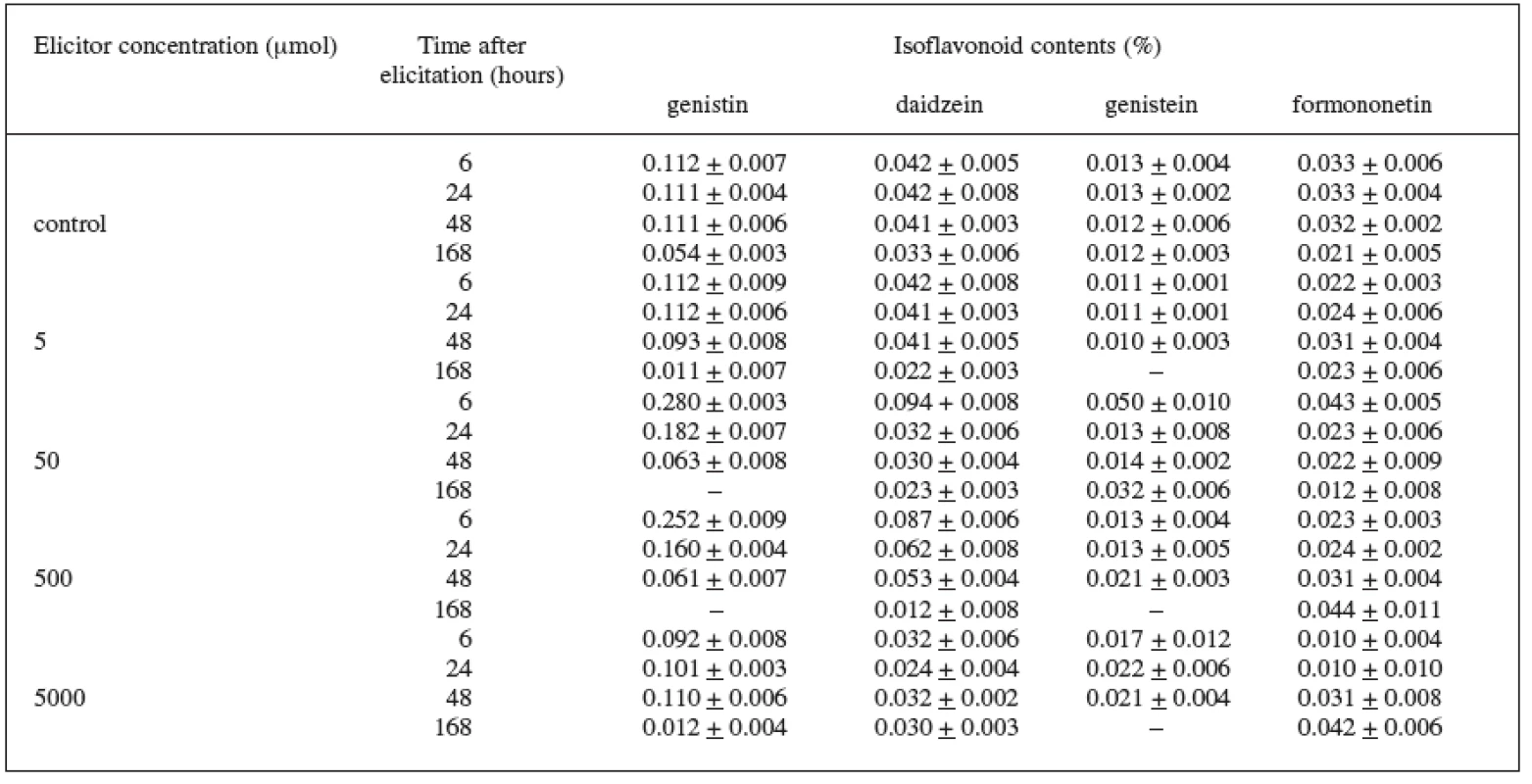
In the case of the variety DO-9 (Table 3), the best elicitation effect of jasmonic acid was also recorded at the 50 μmol concentration. The maximum increase in the content of all isoflavonoids under observation, when compared with the control culture, was brought about during the 48-hour application of the 50 μmol concentration when the increase was as follows: genistin by 845%, daidzein by 300%, genistein by 30%, and formononetin by 250%. The production of genistin was also increased by the other concentrations of the elicitor and the increase values were again highest after the 48 hour application.
Tab. 3. Production of isoflavonoids in suspension culture of Trifolium pratense L. (variety DO-9) elicited with jasmonic acid 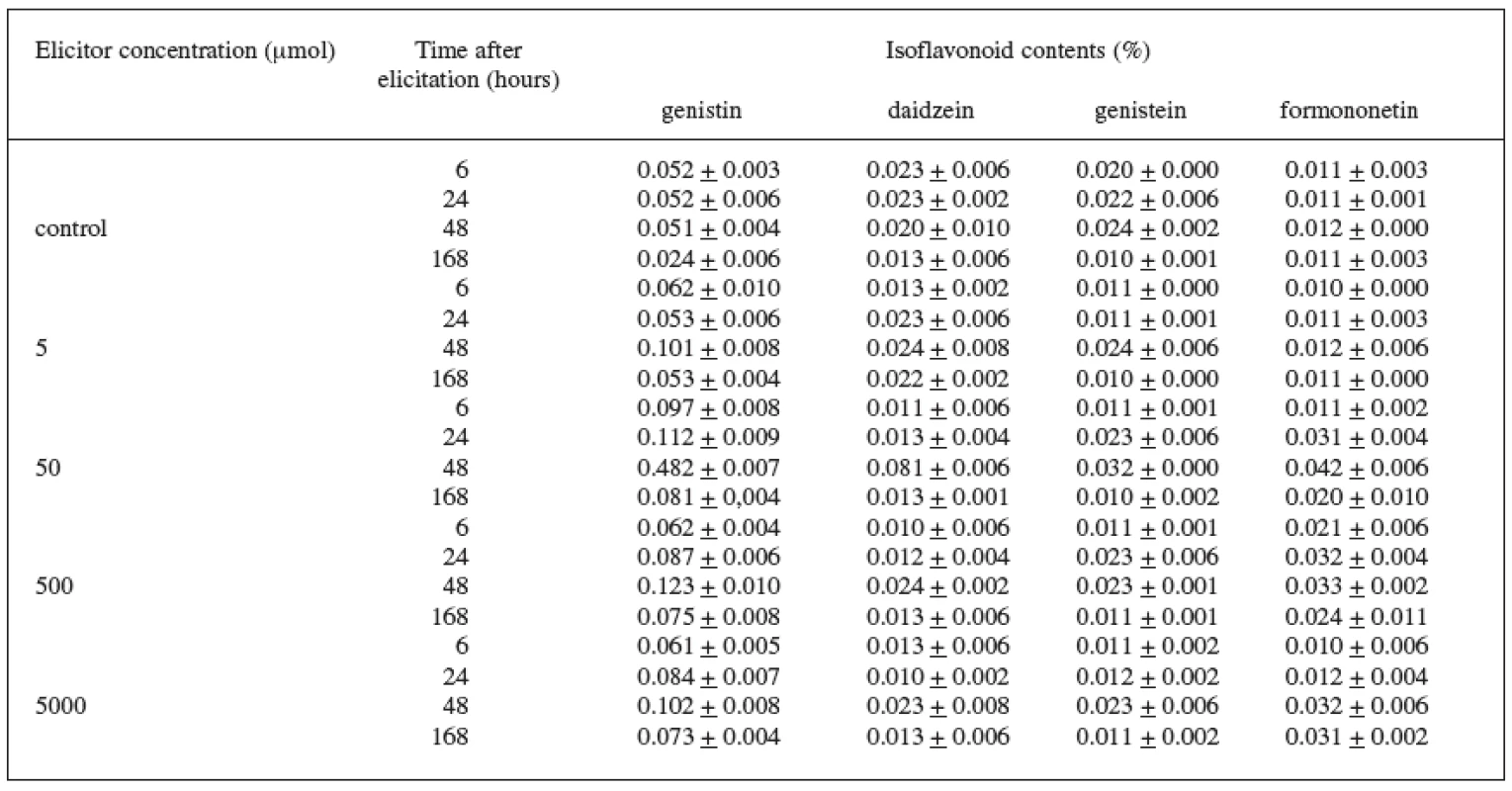
The 48-hour application of the 50 μmol concentration also stimulated best the production of anthracene derivatives in the Rheum palmatum L. suspension culture 20). Other studies confirm the fact that jasmonic acid is one of the important signal substances of the plants’ defensive responses that result in an increased production of secondary metabolites 23–26).
This work was sponsored under the Research Project No. MSM 0021620822.
Received 17 March 2008
Accepted 21 April 2008
Corresponding author:
PharmDr. Marie Kašparová, Ph.D.
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmacognosy
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: kasparova@faf.cuni.cz
Zdroje
1. Nürnberger, T., Scheel, D.: Trends Plant Sci., 2001; 6, 372.
2. Gundlach, H. et al.: Proc. Natl. Acad. Sci. USA, 1992; 89, 2389.
3. Luczkiewicz, M., Glód, D.: Plant Sci., 2003; 165, 1101.
4. Thiem, B.: Plant Sci., 2003; 165, 1123.
5. Lozovaya, V. V. et al.: Plant Physiol. Biochem., 2004; 42, 671.
6. Federici, E. et al.: Phytochemistry, 2003; 64, 717.
7. Souza, K. C. B., Bassani, V. L., Schyloval, E. E. S.: Phytomedicine, 2007; 14, 102.
8. Verhoeven, H.: J. Menopause, 2002; 2, 14.
9. Zhang, M. et al.: Nutr. Cancer, 2004; 49, 125.
10. Aaby, K., Hvattum, E., Skrede, G.: J. Agric. Food Chem., 2004; 28, 4595.
11. Michels, G. et al.: Toxicol. Lett., 2004; 151, 105.
12. Kim, J. D. et al.: J. Nutr. Biochem., 2006; 17, 165.
13. Gamborg, O. L., Miller, R. A., Ojima, K.: Exp. Cell Res., 1968; 50, 151.
14. Kašparová, M. et al.: Čes. slov. Farm., 2006; 55, 44.
15. Czech Pharmacopoeia 2005, Praha, Grada, 2005, s. 1368.
16. De Rijke, E. et al.: Anal. Chim. Acta, 2002; 468, 3.
17. Tebayashi, S. et al.: Phytochemistry, 2000; 54, 387.
18. Wei, W. et al.: Appl. Microbiol. Biotechnol., 2006; 70, 298.
19. Chong, T. et al.: Process Biochem., 2005; 40, 3397.
20. Kašparová, M., Siatka, T., Dušek, J.: Čes. slov. Farm., 2003; 52, 148.
21. Tůmová, L., Zápalková, R.: Čes. slov. Farm., 2002; 51, 96.
22. Pomahačová, B. et al.: Folia Pharm. Univ. Carol., 2006; 34, 29.
23. Satdive, R. K., Futzele, D. P., Eapen, S.: J. Biotechnol., 2007; 128, 281.
24. Filella, I., Penuelas, J., Llusia, J.: New Phytologist., 2006; 169, 135.
25. Gadzovska, S. et al.: Plant Cell Tiss. Org. Cult., 2007; 89, 1.
26. Kuzovkina, I. U. et al.: Rus. J. Plant Physiol., 2005; 52, 77.
Štítky
Farmacie Farmakologie
Článek NOVÉ KNIHY
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2008 Číslo 3- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Biotic elicitation of the L. suspension culture
- Effects of Mg-ATP on the flavonoid production in the Scutellaria baicalensis Georgii suspension cultures
- Synthesis of 2-{3-[4-(4-fluorophenyl)-1-piperazinyl]-2-hydroxy-propoxy}-phenylcarbamic acid alkylesters and in vitro evaluation of their ß-antiadrenergic and vasodilatative activities
- Studies on local anesthetics Part 183: Micellization and thermodynamic parameters of heptacainium chloride in the solution of potassium bromide
- Medicinal preparations in Mattioli’s Herbarium of 1596
- On the content of the national part of the Czech Pharmacopoeia from the aspect of the formulation of medicinal preparations in pharmacies
- X. sjezd české farmaceutické společnosti ČLS JEP
- XXX. pracovní dny sekce radiofarmacie české společnosti nukleární medicíny čls jep
- Slovenská farmaceutická spoločnosť 55 ročná
- Význačný farmaceut RNDr. PhMr. Lumír Jindra se dožil 80 let
- Docent Ing. Ivan Lacko je už sedemdesiatnikom
- Oslavy životního jubilea nestorů české farmacie
- NOVÉ KNIHY
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Medicinal preparations in Mattioli’s Herbarium of 1596
- On the content of the national part of the Czech Pharmacopoeia from the aspect of the formulation of medicinal preparations in pharmacies
- Studies on local anesthetics Part 183: Micellization and thermodynamic parameters of heptacainium chloride in the solution of potassium bromide
- XXX. pracovní dny sekce radiofarmacie české společnosti nukleární medicíny čls jep
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



