-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPhenolic compounds of the genus Iris plants (Iridaceae)
Fenolické sloučeniny rostlin rodu Iris (Iridaceae)
Článek shrnuje výsledky testování fenolických sloučenin (flavonoidy, isoflavonoidy, xanthony, fenolkarboxylové kyseliny, taniny, kumariny atd.) obsažených v oddencích čtyř druhů kosatců (Iris sibirica L., Iris pseudacorus L., Iris imbricatа Lindl., Iris hungarica Waldst. et Kit.). S využitím papírové a tenkovrstvé chromatografie bylo identifikováno patnáct fenolických sloučenin: kyselina gallová, kumarová, skořicová, chlorogenová, neochlorogenová, ferulová, kávová; kaempferol, quercetin, hispidulin, daidzein, genistein, formononetin, mangiferin a isomangiferin. Byly stanoveny kvantitativní obsahy flavonoidů (1,2–3,7 %), hydroxyskořicových kyselin (0,6–6,5 %), γ-pyronů (0,01–0,8 %), taninů (6–14 %), isoflavonoidů (1–2 %) a polyfenolických sloučenin (až 3 %) v oddencích druhů kosatců. Vybrané rostliny patří k přírodní flóře a jsou často pěstovány. Nicméně tato fytochemická analýza pro hlavní skupiny biologicky aktivních látek ukazuje na perspektivní využití druhů Iris v lékařství.
Klíčová slova:
druhy Iris • Iridaceae • fenolické sloučeniny • chromatografie • kvalitativní analýza • kvantitativní obsah
Authors: Olga O. Mykchailenko; Vladimir M. Kovalyov
Published in the journal: Čes. slov. Farm., 2016; 65, 70-77
Category: Přehledy a odborná sdělení
Summary
This article presents the results of testing of phenolic compounds (flavonoids, isoflavonoids, xanthones, phenolcarboxylic acids, tannins, coumarins, etc.) in the rhizomes of four Iris species (Iris sibirica L., Iris pseudacorus L., Iris imbricatа Lindl., Iris hungarica Waldst. et Kit.). With the use of paper and thin-layer chromatography, fifteen phenolic compounds were identified: gallic, coumaric, cinnamic, chlorogenic, neochlorogenic, ferulic, caffeic acids; kaempferol, quercetin, hispidulin, daidzein, genistein, formononetin, mangiferin and isomangiferin. Quantitative contents of flavonoids (1.2–3.7%), hydroxycinnamic acids (0.6–6.5%), γ-pyrones (0.01–0.8%), tannins (6–14%), isoflavonoids (1–2%), polyphenolic compounds (up to 3%) in the rhizomes of the Iris species were determined. Chosen plants belong to natural flora and have been often cultivated. However, this phytochemical analysis for the main groups of the biologically active substances shows a perspective use of the Iris species in medicine.
Key words:
Iris species • Iridaceae • phenolic compounds • chromatography • qualitative analysis • quantitative contentsIntroduction
Phenolic compounds are the aromatic compounds that have one or more bound hydroxyl groups as a substituent in the aromatic ring; this is one of the most widely-distributed classes of natural compounds exhibiting biological activity. Phenolic compounds are formed almost in every plant cell and participate in the basic life processes of plants: respiration, photosynthesis, the formation of cell walls, protection of the plant from diseases, viruses, the action of abiotic and biotic stresses1). Phenolic and polyphenolic compounds provide antioxidant, anti-inflammatory, capillary-restorative, anti-cancerous, hepatoprotective effect2). The most widely-distributed in nature are caffeic acid and its derivatives (chlorogenic acid and its isomers) that provide anti-inflammatory and cholagogue effect. Ferulic acid is a part of the plant cells. Chlorogenic, ferulic, caffeic, coumaric acids together provide gipoazotemic effect, increase renal function, stimulate antitoxic function of the liver3, 4). Coumarins are used as anticoagulants, providing bactericidal, fungicidal, anti-cancerous activity5, 6). Isoflavonoids have antioxidant, antibacterial, anti-inflammatory and estrogenic effects4, 7, 8).
The objects of the study were Irises (Iridaceae)9–11). Irises accumulate various secondary metabolites: flavonoids12), isoflavonoids and their glycosides, benzoquinones, triterpenoids, stilbens13–15); they provide anti-inflammatory, antioxidant, antituberculosis, diuretic and other effects2, 16, 17). The genus Iris plants grows on the territory of Ukraine18) and contiguous countries19) in large quantities. The richest diversity of wild Irises species is not only the basis for the introduction of valuable species into the culture, but also a source of biologically active substances (BAS). The aim of the study is to perform qualitative and quantitative analysis of the phenolic compounds in the rhizomes of the four Iris species.
Experimental part
Plant material
In the preliminary investigation, twenty-one samples of materials (leaves, rhizomes) were analysed, including eleven Iris species and four Iris varieties. Species and varieties were harvested from the collections of the following botanical gardens: V. N. Karazin Kharkiv National University (Kharkiv, Ukraine; 2012, 2014), M. M. Gryshko National Botanical Garden of the National Academy of Sciences of Ukraine (Kiev, Ukraine; 2012, 2014, 2015), National University of Pharmacy (Kharkiv, Ukraine, 2007–2009), Azerbaijan Medical University (Baku, Azerbaijan, 2005) and in the surrounding area of Kharkiv.
The raw material was dried to air-dry state, cut into pieces, packed in cloth bags and stored in a cool, dry place. For chemical analysis an average sample of raw material was reduced to the particle size of 2–3 mm20–23).
Qualitative analysis
Qualitative analysis of the phenolic compounds of the studied species of irises was performed using generally accepted methods and techniques of phytochemical analysis. For preliminary analysis, the rhizomes of irises were exposed to exhaustive extraction of the raw materials with water, 20% and 70% ethanol (in the ratio of the raw material to the extractant in the proportion of 1 : 5) in a boiling water bath with the reverse refrigerator. The obtained extractions were concentrated by evaporation of the solvent in a water bath (to decrease volume by half). Further it was used for qualitative reactions and chromatographic analysis1, 4–6, 8).
Coumarins were determined according to the results of the reactions: lactone reaction (yellow colouring) and formation of azo dye (red colouring).
Glycosides and aglycons of flavonoids were determined in hydro-alcoholic extracts in the reactions of identification: cyanidine reaction by Bryant (yellow-red colouring of the aqueous phase and yellow-hot colouring of the octal phase), the reaction with 3% solution of iron (III) chloride (dark green colour of flavonols, flavones); the reaction with an alkaline solution (bright yellow colour); the reaction with 5% solution of aluminium chloride (yellow-green colouring); the boric-acid reaction (yellow colouring on detection of 3 - and 5-hydroxyflavones and 5-hydroxyflavanones); the reaction with ammonia (flavones, flavonols, flavanones and flavanonols dissolve with formation of yellow colour, which, when heated, changes to orange or brown colour).
To determine tannins, the reactions of the sediment were carried out with 1% gelatin solution, 1% solution of quinine hydrochloride, 10% solution of basic acetate of lead. The group of tannins was detected by the reaction with a solution of iron ammonium alum.
Chromatographic tests were carried out on the paper «Filtrak» (FN-1, FN-4), for thin-layer chromatography using silica gel 60 F254 TLC plates (by “Mersk”, Germany). The process of chromatography was carried out by the methods of ascending, descending unidimensional, two-dimensional chromatography. The solvents relation is indicated by figures and taken in volumetric units.
The systems of solvents were used for chromatography:
- n-butanol – acetic acid – water (4 : 1 : 2 by volume) is for testing of the phenolic compounds, flavonoids, phenolcarboxylic acids, xanthones
- 15% acetic acid is for determining phenolcarboxylic acids, xanthones
- 2% acetic acid is for determining phenolcarboxylic acids
- chloroform – acetic acid – water (13 : 6 : 1 by volume) is for determining flavonoids, isoflavonoids
- benzole – ethyl acetate – acetic acid – water (50 : 50 : 1 : 1 by volume) is similar №No 4
- chloroform (formamide 25%) is for determining coumarins
- petroleum ether (formamide 25%) is for determining coumarins
- benzole (formamide 25%) is for determining coumarins
- benzole – ethyl acetate (2 : 1 by volume) – is for determining coumarins; TLC
- ethyl acetate – formic acid – glacial acetic acid – water (100 : 11 : 11 : 26 by volume) – is for determining flavonoids, xanthones; TLC
- chloroform – methanol (8 : 2 by volume) is for determining flavonoids, isoflavonoids, phenolcarboxylic acids; TLC
On the chromatograms the substances were detected by the characteristic fluorescence in UV-light at the wavelength of 365 nm and 254 nm before and after processing the chromatograms with ammonium vapours, 2% alcoholic solution of aluminum chloride; 10% sodium/potassium hydroxide solution; 5% alcoholic solution of diazotized sulfanilic acid (diazo reagent) (for determining coumarins, hydroxycinnamic acids) that allows to obtain bands with the brighter fluorescence in UV-light. For the identification of the substances, the value of Rf was determined and compared with the standard samples (flavonoids aglycones and isoflavonoids, xanthones, phenolcarboxylic acids).
For further analysis of the flavonoids aglycones and isoflavonoids, acid hydrolysis of the sum of the phenolic compounds was conducted with 5% sulfuric acid for 5 hours in a water bath. After cooling, the solution was transferred into a separatory funnel and worked with ethyl acetate (6 times and 10 ml by volume). The ethyl acetate extractions were combined, then transferred to the separatory funnel and processed with water to eliminate residua of acid (by the universal indicator). Received ethyl acetate extractions were evaporated and 2 ml of 70% alcohol were added. Analysis of the substances was carried out by PC and TLC systems: 1, 4, 5, 10, 11.
Extraction of raw material
Air-dried rhizomes of Iris pseudacorus (1.0 kg) were extracted with EtOH (50%) in a percolator for 24 h. The extraction was repeated twice under the same conditions. The aqueous EtOH extracts were combined, filtered, evaporated in a rotary evaporator to 0.7 L of aqueous residue, and left for 1 d. The supernatant liquid was separated. The resulting extract was worked up successively with CHCl3, EtOAc, and n-BuOH. The resulting extracts were evaporated in vacuo. The qualitative composition of chloroform, EtOAc and butanol fractions controlled by paper chromatography in a solvent system number 1, 2, 3. In aqueous residue, substances 1–4 were identified by preparative chromatography. EtOAc and butanol extracts were combined and subjected to CC on silica gel and eluted with gradient: CHCl3 and ethanol-mixtures with increasing ethanol concentration (0–100%) to afford 152 fractions. After analysis, the presence of compounds 5–10 was found.
Experimental
When determining the structures of received substances, physical and physicochemical methods of analysis (UV-, IR-, 1H-NMR spectroscopy, mass spectrometry, chromatography in thin layer of sorbent (Silufol UV-254 plates; silica gel 60 F254 TLC plates), column chromatography on silica gel of Silica Gel 100–200 (75–150 mrm) (USА)) were used. Silica gel was pre-purified from ions of metals with 0.5% solution of chloric acid, then it was dried at room temperature and then it was activated at a temperature of 130 – 140 ºC for 2 hours in a drying cabinet. Melting point (MP) was determined on the Kofler block (Franz Kustner nqch K:G:Dresden; N.K.70/3314k). The recrystallization of selected substances was carried out in 96% ethanol with an addition of 2–3 drops of water. The substances were dried under vacuum (10–2 mm Hg) over P2O5 at 110 až 115 °C for 5 hours.
UV-absorption spectra and optical density were recorded with a spectrophotometer module of Carl Zeіss (Germany) in cells with layer thickness of 10 mm. IR - -spectra were recorded on the instrument Tensor 27, UR-20 (GDR) in the tablets of potassium bromide at the ratio of substance and filler of 1 : 200 – 1 : 400. 1H-NMR-spectra were recorded with the device Varіan Mercury-VX-200 (200 MHz) (USA), the solvent DMSO-d6 (the internal standard TMS). Mass-spectra were measured on the instrument Varian 1200 L (USA) (the temperature of the ionization chamber is 150–300 °C, the ionizing voltage is 70 eV, 40–600 m/z).
Compounds
Caffeic acids 1. C9H8O4, mp 194–195 °C. UV λmax (C2H5OH) nm: 325, 300, 235. Rf 0.32, system 3; Rf 0.92, system 11. IR (KBr), νν, cm−1: 3400, 3235, 2975 (OH), 1647, 1630 (C = O), 1607, 1540 (Ar), 880, 860 (substituted benzene).
Ferulic acids 2. C10H10O4, mp 168–170 °C. UV λmax (C2H5OH) nm: 320, 290sh. Rf 0.43, system 3; Rf 0.18, system 11.
Chlorogenic acids 3. C16H18O9, mp 203–205 °C. UV λmax (C2H5OH) nm: 325, 300, 245. Rf 0.66, system 3; IR (KBr), ν, cm−1: 2970, 2900, 2780 (OH), 1740–1710 (ester group), 1660, 1625 (CС = ОO), 1605–1550 (Ar), 840 (substituted benzene).
Neochlorogenic acids 4. C16H18O9, amorphous solid; UV λmax (C2H5OH) nm: 325, 300, 243. Rf 0.70, system 3.
Kaempferol 5. C15H10O6, mp 275–277 °C. UV λmax (C2H5OH) nm: 265, 369. Rf 0.55, system 4; Rf 0.63, system 5. IR (KBr), νν, cm−1: 3340 (-OHОН), 1660 (CС = OО), 1615, 1570, 1510 (СCС = СC), 820 (n-substitution in the ring “B”). 1H NMR (200 MHz, DMSO-d6, TMS): δ 6.11 (1HН, s, 6-HН), 6,24 (1HН, s, 8-H), 7.90 (2HН, d, HН-2’, 6’), 6.80 (2H, d, H-3′’, 5’′).
Hispidulin 6. C16H12O6, mp 287–290 °C. UV λmax (C2H5OH) nm: 336, 275. Rf 0.86, system 5; Rf 0.39, system 10. IR (KBr), νν, cm−1: 3300, 3100 (-ОНOH), 1655 (CС = OО), 1610, 1570, 1490 (CС = СC), 2950–2850 (-OCH3). 1H NMR (200 MHz, DMSO-d6, TMS): δ 6.6 (1HН, s, 3-HН), 13.1 (1НH, s, 5-H), 6.7 (1HН, s, 8-H), 7.9 (2HН, d, HН-2’, 6’), 6.9 (2H, d, H-3’′, 5’′).
Genistein 7. C15H10O5, mp 290–292 °C. UV λmax (C2H5OH) nm: 260, 325. Rf 0.88, system 5; Rf 0.41, system 11. IR (KBr), ν ν, cm−1: 3726, 3410, 3104 (-ОНOH), 1651 (СC = ОO), 1615, 1569, 1424, 1503 (CС = СC), 885 (n-substitution in the ring “B”). 1H NMR (200 MHz, DMSO-d6, TMS): δ 8.42 (s, 2-НH), 6.72 (d, 6-HН), 6.47 (d, 8-H), 7.40 (d, HН-2’, 6’), 6.83 (d, H-3’, 5’′), 9.64 (s, OH-4’), 12.94 (s, OH-5).
Daidzein 8. C15H10O4, mp 307–308 °C. UV λmax (C2H5OH) nm: 249 sh, 303. Rf 0.77, system 4. IR (KBr), νν, cm−1: 3666, 3171 (-OHОН), 1631 (CС = СO), 1595, 1518, 1188, 1460 (CС = CС), 887, 820 (n-substitution in the ring “B”).1H NMR (200 MHz, DMSO-d6, TMS): δ 8.31 (s, 2-НH), 8.05 (d, 5-H), 7.14 (dd, 6-HН), 7.23 (d, 8-H), 7.41 (d, НH-2’, 6’), 6.82 (m, H-3’′, 5’′).
Mangiferin 9. C19H18O11, mp 267–269 °C. UV λmax (C2H5OH) nm: 364, 315, 257, 240. Rf 0.57, system 1. Rf 0.49, system 10. IR (KBr), ν, cm−1: 1650 (CС = OО), 3100–3700 (ОН-OH), 1591, 1565, 1494 (CС = CС). 1H NMR (200 MHz, DMSO-d6, TMS): δ 6.35 (1HН, s, 4-НH), 6.83 (1HН, s, 5-H), 7.38 (1HН, s, 8-HН), 13.77, 10.65, 9.85 (1H, s, OH).
Isomangiferin 10. C19H18O11, mp 248–250 °C. UV λmax (C2H5OH) nm: 366, 314, 257, 240. Rf 0.55, system 1. Rf 0.44, system 10. IR (KBr), ν, cm−1: 1650 (CС = OО), 3100–3700 (OHОН), 1591, 1565, 1494 (CС = CС); 1H NMR (200 MHz, DMSO-d6, TMS): δ 6.88 (1HН, s, 5-HН), 7.45 (1HН, s, 8-H), 6.25 (1HН, s, 2-HН), 13.78, 10.60, 9.85 (1H, s, OH).
Quantitative analysis
For the quantitative determination of the contents in the raw materials of flavonoids, phenolic compounds, hydroxycinnamic acids, isoflavonoids the spectrophotometric methods were used; for tannins, the titrimetric method was used in accordance with the recommendations of the SP XI, SFU. For the quantitative determination of flavonoids, the method based on the reaction of complex formation of flavonoids with the solution of 3% aluminium chloride was used24), in terms of the standard pharmacopoeia sample (PSS) rutin (PA 42--2508-87; «Herb of Hypericum») at the wavelength of 410 nm23).
The content of the sum of the hydroxycinnamic acids was determined on conversion to the PSS chlorogenic acid at the wavelength of 327 nm, as the basis took the technique of “Herb of Erigeron canadensis” (TPA 42-U - -6/37-323-96)23).
The content of the sum of the phenolic compounds was determined at the wavelength of 270 nm on conversion to the PSS gallic acid according to the method of standardization of the Piphlamin drug on the basis of the herb of pea field25). For the analysis of tannins, the Neubauer-Löwenthal titrimetric method was used, which based on the oxidation of polyphenols with potassium permanganate20).
To establish the content of isoflavonoids, the method of quantitative determination of isoflavonoids in the roots of restharrow of the PA “Roots of restharrow” was taken as the basis25), but as the extractant 50% alcohol was used. The optical density of the extract was determined at the wavelength of 271 nm; the conversion of the content of isoflavonoids was conducted to onosid (PA-42U “Herb of haricot”)8), as the maximum rhizomes of irises solution absorption is on this wave length.
Quantitative determination of the sum of γ-pyrones in terms of mangiferin at the wavelength of 369 nm conducted with the previously published methods26).
UV-absorption spectra and optical density were recorded with a spectrophotometer module of SP-46, Carl Zeіss (Germany) Specord M-80, Thermo Scientific Evolution 60S UV – Visible Spectrophotometer (USA), in the wavelength range of 200–500 nm.
Statistical analysis of the results was conducted in accordance with the requirements of the State Pharmacopeia of Ukraine, the 1st issue, supplement 1, p. 5.3 using Microsoft Office Excel 7.0 application.
Results and discussion
Primary screening
The presence of flavonoids, coumarins, hydroxycinnamic acids and tannins was established with the qualitative reactions and chromatographic analysis in the aqueous and hydro-alcoholic extracts of 21 samples of the leaves and rhizomes of irises (Table 1).
Tab. 1. Qualitative analysis of BAS in the leaves and rhizomes of Irises 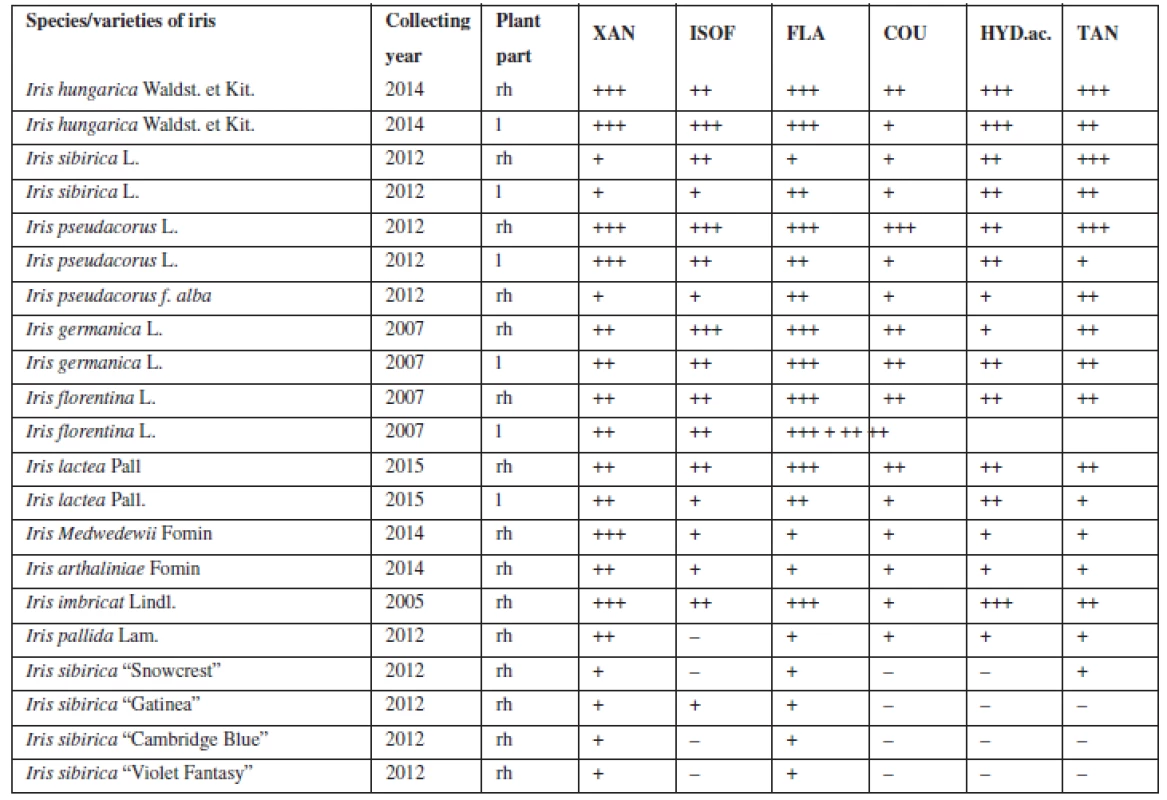
rh – rhizomes, l – leaves, XAN – xanthones, ISOF – isoflavonoids, FLA – flavonoids, COU – coumarins, HYD.ac. – hydroxycinnamic acids, TAN – tannins evaluation of reactions: – any reaction, + weak reaction, ++ strong reaction, but with some deficiencies in coloration, +++ best reaction For the next study, four Iris species (Iris hungarica Waldst. et Kit., Iris sibirica L., Iris imbricata Lindl., Iris pseudacorus L.) (Fig. 1) that showed good results were chosen, they have a sufficient resource base and belong to different sections. According to the classification of Rodionenko (1961)27), I. hungarica, I. imbricata belong to the group of bearded irises (Barbatae are the species with flowers, bearing on the outer perianth lobes beard of multicellular hairsprings) from section Iris, the series Elatae Lawrence. I. pseudacorus (series Laevigatae (Diels) Lawrence), I. sibirica (series Sibiricae (Diels) Lawrence) belong to the Limniris (Tausch) Spach em. Rodion section, to the group of beardless irises (Imberbis are the species with flowers, deprived of their beards).
Fig. 1. General view of the living plants and studied raw material of the Irises species 
The identification of phenolic compounds was carried out by paper chromatography to confirm the results of the qualitative reactions.
Identification of Iris phenolic compounds
Phenolic compounds were determined in the extracts of the rhizomes of irises by two-dimensional PC which is the most efficient for separation of phytogenic phenolic compounds.
The results of the chromatographic alcoholic extracts of irises analysis showed that when identified in UV-light and according to the results of the qualitative reactions with the alcoholic solutions of sodium hydroxide, aluminum chloride and diazoreagent on the chromatograms of I. pseudacorus, I. imbricatа, I. sibiricа, I. hungarica not less 8–21 substances of phenolic nature were found.
Some bands had yellow and light-yellow colour in UV-light that is specific to flavones and their 7-glycosides, aglycones. Bands with yellow-brown or dark-brown colour were characterized as flavones, flavone-3-glycosides, and flavanones. Bands with bright-orange fluorescence are specific to the derivatives of dibenzo-γ-pyrones. Absorption bands with blue, violet, violet-blue fluorescence are specific to coumarins, phenolcarboxylic acids, isoflavonoids, flavone-5-glycosides, stilbens. Bands with red fluorescence are probably chalcones, aurones.
Chromatograms differed in the number of spots, their chromatographic behaviour, and colour of spots before and after they were exposed to UV-light, Rf values.
After processing chromatograms with ammonia vapours and 2% alcoholic solution of aluminum chloride spots of aglycones got bright-yellow fluorescence, dark-brown spots got yellow-green colour that is specific of flavones glycosides. Acid hydrolysis was carried out to establish their nature.
Flavonoids (C6 – C3 – C6). Extracts were chromatographed in the systems 1, 4, 5, 10, 11. By the characteristic fluorescence in UV-light, Rf value and colour of spots on chromatograms after they were exposed to ammonia vapours and solution of aluminum chloride in comparison with authentic samples and literature data flavonoids aglycones and isoflavonoids were identified as quercetin, kaempferol, hispidulin, daidzein, formononetin, genistein (Table 2).
Tab. 2. Chromatographic characterization of phenolcarboxylic acids identified in the rhizomes of Irises 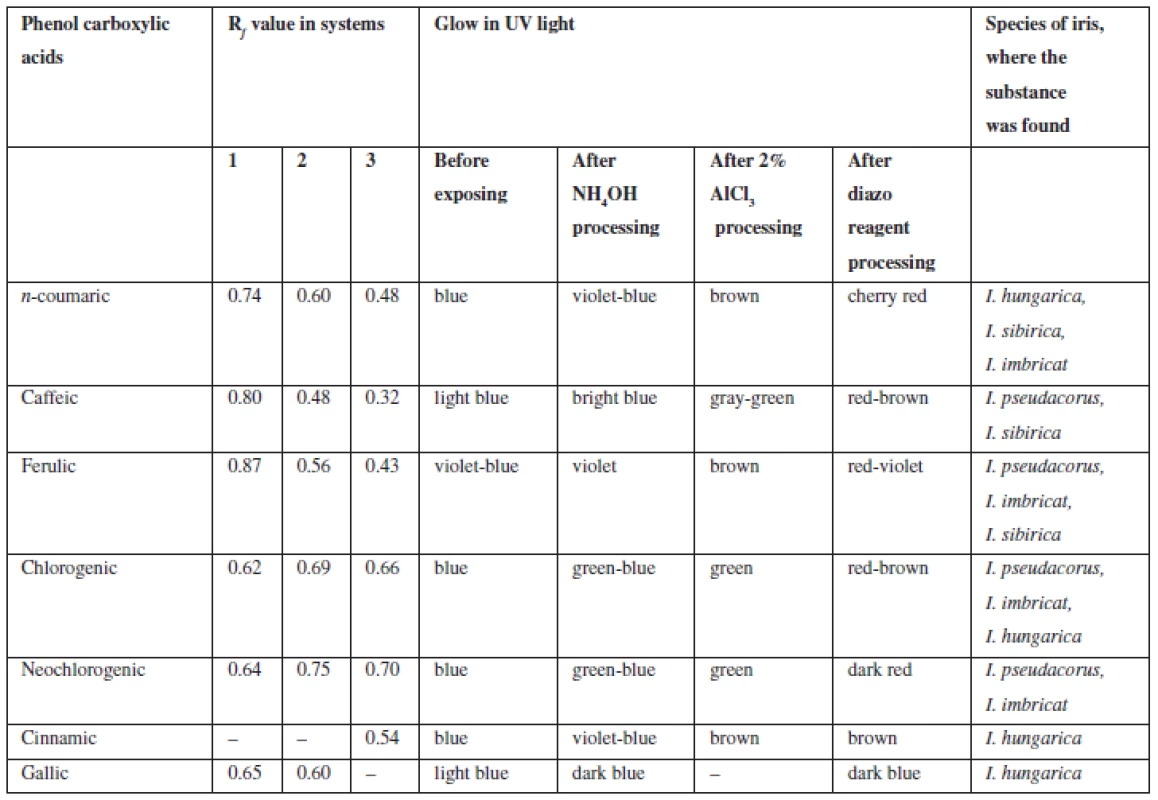
On the grounds of spots colour of isoflavonoids in UV-light coincided with some phenolic acids, further chromatographic analysis was carried out with authentic samples of substances, their colour and Rf values of the spots were compared.
Phenolcarboxylic acids (С6 – С1, С6 – С3). Extracts from rhizomes of irises were chromatographed in the systems 1, 2, 3, 11. In UV-light at the wavelength of 365 nm after processing chromatograms with ammonia vapours spots became visible, which fluorescence intensified from blue to blue-green, after processing with diazoreagent solution the spots became reddish-brown, which is specific of hydroxycinnamic acids. When processing chromatograms with solution of aluminum chloride colour of spots intensified or changed.
By chromatographic behaviour (colour of spots in UV-light, colour after processing with chromogenic reagents, Rf values when compared with previously known substances) caffeic, cinnamic, n-coumaric, ferulic, chlorogenic, neochlorogenic, gallic acids were identified (Table 3).
Tab. 3. Chromatographic characterization of phenolic substances identified in the rhizomes of Irises 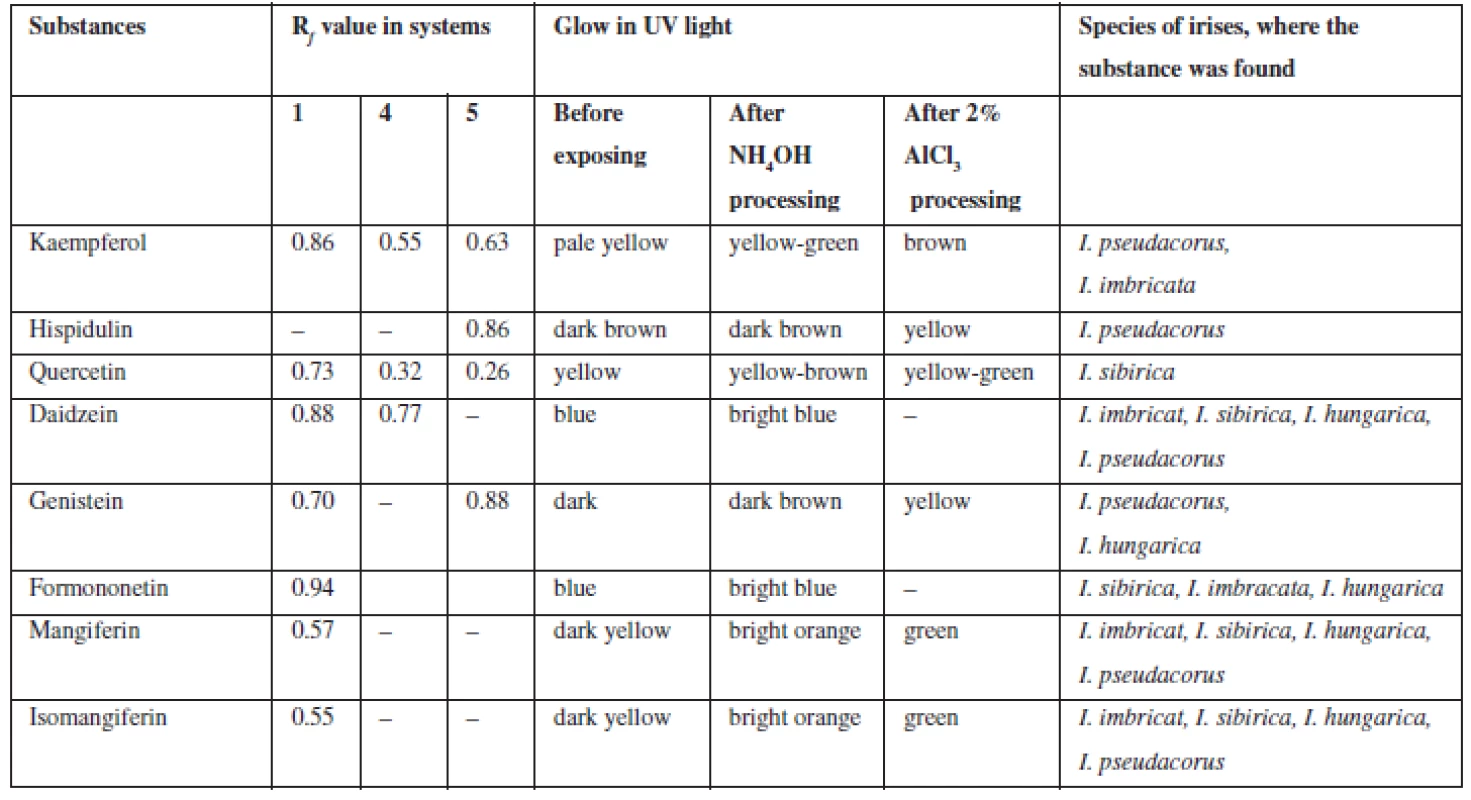
Coumarins (C6 – C3). Extracts were chromatographed in the systems 6, 7, 8, 9. After processing chromatograms with 10% solution of potassium hydroxide and diazoreagent spots with purple, bluish-green, grey-green, green, yellow fluorescence were identified which indicated the presence of derivatives benzo-α-pyrones5). Then coumarins (coumarin, umbelliferone, aesculetin, scopoletin, isoscopoletin, herniarin, daphnoretin) were separated from rhizomes of Iris pseudacorus and the results were presented in the paper28).
Xanthones (C6 – C1 – C6). In UV-light on chromatograms the substances were identified by distinctive dark-yellow light emission that is specific for benzo-γ-pyrones8, 26). These bands were exposed to ammonia vapours and got bright-orange, after processing chromogenic reagent these bands got green which confirms they are xanthones. In all four species of irises, mangiferin (Rf 0.52; system 2; Rf 0.49; system 10) and isomangiferin (Rf 0.38; system 2) were identified.
Tannins ((C6 – C3 – C6)n). Extracts from rhizomes of irises were dark-purple that is specific for the group of hydrolysable tannins.
Isolation and determination of phenolic compounds
To separate and identify the individual phenolic compounds, division was used of their sum on a column with silica gel or preparative chromatographic separation on paper in several systems of solvents with subsequent separated crystallization of the separated compounds. Extracts of the raw materials of irises were received by maceration with 50% alcohol, evaporated under vacuum to 700 ml. The received residual was sequentially processed in a separating funnel with chloroform, ethyl acetate and n-butanol. The received fractions were evaporated and applied on silica gel, as the eluents used chloroform, alcohol-chloroform mixture in various combinations and ethyl alcohol. The process of elution was monitored by paper and thin-layer chromatography in systems 1, 2, 3, 10 and 11. Identical fractions were combined, solvent was distilled, and substances were recrystallized from ethanol. The following substances were separated: caffeic, ferulic, chlorogenic, neochlorogenic acids; kaempferol, hispidulin; genistein, daidzein; mangiferin and isomangiferin.
Quantitative analysis
The results of quantitative determination of biologically active substances in rhizomes of irises are presented in Table 4.
Tab. 4. The quantitative content of the main groups of BAS in the rhizomes of Irises 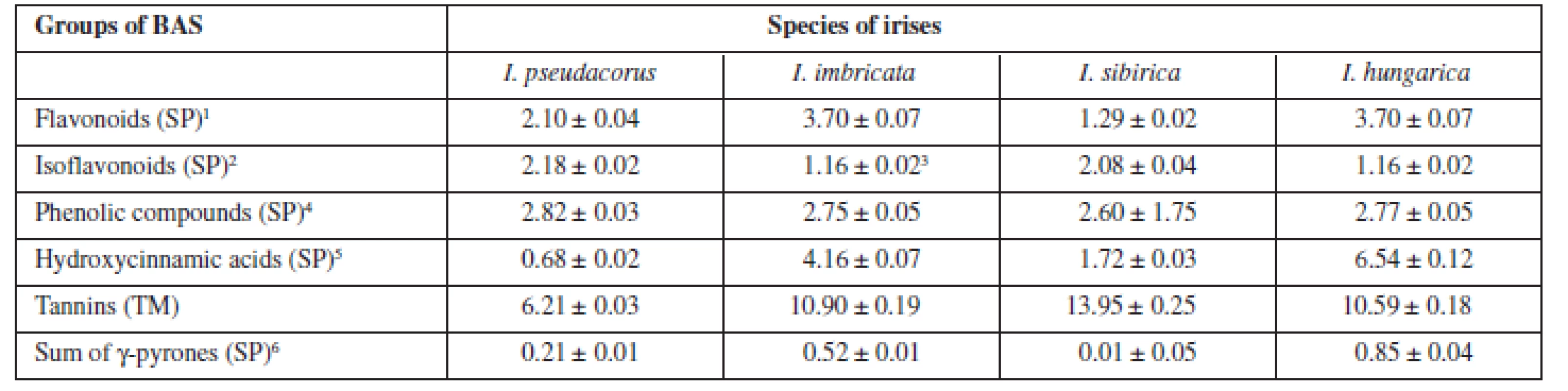
SP – spectrophotometrical method, TM – titrimetric method 1is in terms of rutin 2is in terms of onosid 3is in terms of ononin 4is in terms of gallic acid 5is in terms of chlorogenic acid 6is in terms of mangiferin The highest content of flavonoids and hydroxycinnamic acids was pointed out for Iris hungarica and Iris imbricatа more 3.5% and 4%, respectively. While isoflavonoids were contained more in beardless irises (more than 2%), all irises contain the sum of γ-pyrones to 1%. The high content of tannins in the raw material (from 6 to 14 %) explains the historical use of rhizomes of irises for the tanning of leather13).
Conclusion
In the rhizomes of I. pseudacorus, I. imbricatа, I. sibiricа, I. hungarica from 7 to 21 substances of phenolic nature were revealed by paper and thin-layer chromatography, among them there were phenolcarboxylic acids, coumarins, flavonoids, isoflavonoids. In the rhizomes 15 substances were identified: gallic, coumaric, cinnamic, chlorogenic, neochlorogenic, ferulic, caffeic acids; kaempferol, quercetin, hispidulin, daidzein, genistein, formononetin, mangiferin and isomangiferin. Later on, these substances were separated from the rhizomes of irises. The quantitative content of phenolic compounds, flavonoids, isoflavonoids, hydroxycinnamic acids, γ-pyrones, and tannins was determined. Detailed phytochemical analysis of irises was carried out for the first time. With the exception of kaempferol and hispidulin in the rhizomes of Iris pseudacorus, all substances have been identified in the investigated species of irises for the first time.
Conflict of interest: The authors have declared that no financial relationships with any organizations that might have an interest in the submitted work; nor any other relationships or activities that could appear to have influenced the submitted work.
Acknowledgement
The authors are grateful to acting as Head of Department of the Ornamental plants, Senior Researcher of the National Botanical Garden n.a. M.M. Gryshko of NAS of Ukraine (Kyiv), Cand. Biol. Sci. Yu.V. Buydin, as well as Head of Department of the Floriculture of Botanical garden of the Kharkiv National University named after V.N. Karazin T.G. Orlova for help in the preparation and determination of systematical classification of plants.
Conflicts of interest: none.
ass. prof. Olga O. Mykchailenko, PhD (∗)
National University of Pharmacy, Department of Botany
str. Bluhera (Valentinivska) 4,
61168 Kharkiv,
Ukraine
e-mail: z_ola07@mail.ru
prof. V. M. Kovalyov, D.Sc.
National University of Pharmacy, Department of Pharmacognosy,
Kharkiv,
Ukraine
Received 26 Februar 2016
Accepted 9 March 2016
Zdroje
1. Kochetkov N. K. Chemistry of biologically active natural compounds. Moscow: Chemistry 1970.
2. Khare C. P. Indian medicinal plants. Berlin, Heidelberg: Springer-Verlag 2007; 336–337.
3. Chekman J. S., Zavalko I. V. Flavonoids: pharmacological aspects. Phytotherapy J. 2008; 1, 3–11.
4. Korulkin D. Y., Abilov D. A., Muzychkina R. A., Tolstikov G. A. Natural flavonoids. Novosibirsk: Geo 2007.
5. Kuznetsova G. A. Natural coumarins and furokumariny. Leningrad: Nauka 1967.
6. Kochetova M. V., Semenistaya E. N., Larionov O. G., Revina A. A. Determination of biologically active phenols and polyphenols in various objects by chromatographic techniques. Russian Chemical Reviews 2007; 76(1), 79–90.
7. Zatylnikova O. A., Osolodchenko T. P., Kovalev V. N. Antimicrobial activity of extracts of Iris pseudacorus L. Scientific J. Annals of Mechnikov’s Institute 2010; 4, 43–47.
8. Kovalev V. N. (ed.) Practicum on Pharmacognosy: Textbook for students. Kharkiv: Golden Pages 2003.
9. Goldblatt P., Manning J. C. The Iris Family: Natural History and Classification, Timber Press: Portland 2008.
10. Dykes W. R. The Genus Iris. New York: Dover Publications 1974.
11. Austin C. Irises: a gardeners encyclopedia; Portland: Timber Press 2005.
12. Williams Ch. A., Harborne J. B., Colasante M. Flavonoid and xanthone patterns in bearded Iris species and the pathway of chemical evolution in the genus. Biochemical Systematics and Ecology. 1997; 25(4), 309–325.
13. Plant Resources of Russia and neighboring countries: flowering plants, their chemical composition, the use of: family Butomaceae-Typhacea. St. Petersburg: Nauka 1994; 77–82.
14. Kassak P. Secondary metabolites of the choosen genus Iris spesies. J. acta universitatis agriculturae et silviculturae mendelianae brunensis. 2012; 32(8), 269–280.
15. Kukula-Koch W., Sieniawska E., Widelski J., Urjin O. Major secondary metabolites of Iris spp. Phytochemistry reviews. 2013; 12(4), 1–32.
16. Rahman A. U., Nasim S., Baig I., Jalil S., Orhan I., Sener B., Choudhary M. I. Anti-inflammatory isoflavonoids from the rhizomes of Iris germanicа. J. of Ethnopharmacology. 2003; 86, 177–180.
17. Wollenweber E., Stevens J. F., Klimo K., Knauft J., Frank N., Gerhäuser C. Cancer Chemopreventive in vitro activities of isoflavones from Iris germanica. Planta Med. 2003; 69, 15–20.
18. Mosyakin S. L., Fedoronchuk M. M. Vascular plants of Ukraine: a nomenclatural checklist. Kiev 1999; 31–33.
19. Czerepanov S. K. Vascular plants of Russia and adjacent states (the former USSR). Cambridge 2007; 280–282.
20. State pharmacopoeia of the USSR. 11th ed. Vol. 1. General methods of analysis. Herbal drugs/USSR Ministry of Health. Moscow: Medicine 1987. 21. State pharmacopoeia of the USSR. 11th ed. Vol. 2. General methods of analysis. Herbal drugs/USSR Ministry of Health. Moscow: Medicine 1990.
22. State Pharmacopoeia of Ukraine. 1st ed. Appendix 1. Kharkiv: State Enterprise: Scientific and Expert Pharmacopoeial Centre 2004.
23. State Pharmacopoeia of Ukraine. 1st ed. Appendix 2. Kharkiv: State Enterprise: Scientific and Expert Pharmacopoeial Centre 2008.
24. Belikov V. V., Schreiber M. S. Methods of analysis of flavonoid compounds. Pharmacy 1970; 19(1), 68–72.
25. Kovalyova A. I., Georgiyevskiy G. V., Kovalyov V. M., Komisarenko A. I., Tymchenko N. I. Development of new piflamin medicine standardization methods. Farmakom 2002; 2, 92–97.
26. Aslanukov A. K., Ayrapetova A. U., Serebryanaya F. C. Identification and quantification of the amount of xanthones in terms mangiferin in the grass kopeck Caucasus (Hedysarum caucasicum Bieb.). Development, research and marketing of new pharmaceutical products. 2009; 64, 11–13.
27. Rodionenko G. I. An outline of a new and evolutionary botanical classification of Irises. The Iris Year Book. The British Iris Society 1962; 103–119.
28. Kovalev V. N., Mykhailenko O. A., Kovalev S. V. Coumarins from Yellow iris. Khimiya Rastitelnogo Surya 2013; 3, 201–205.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2016 Číslo 2- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Potential immunomodulatory and antiinflammatory mechanisms of probiotics
- Mice lacking individual molecular forms of cholinesterases
- Oral adverse drug reactions in various medications
- Phenolic compounds of the genus Iris plants (Iridaceae)
- The evaluation of the prevalence of constipation at the Centre of clinical gerontology
- Výbor Slovenskej farmaceutickej spoločnosti na roky 2016–2020
-
Zemřel doc. RNDr. PhMr. Václav Rusek, CSc.
(25. února 1928 – 30. ledna 2016) - Bartunek A. Dejiny slovenského lekárnictva I, 10. storočie až 1918.
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Oral adverse drug reactions in various medications
- Potential immunomodulatory and antiinflammatory mechanisms of probiotics
- The evaluation of the prevalence of constipation at the Centre of clinical gerontology
- Phenolic compounds of the genus Iris plants (Iridaceae)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



