-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInfluence of formulation and process parameters on the properties of Cu2+/alginate particles prepared by external ionic gelation evaluated by principal component analysis
Vliv formulačních a procesních parametrů na vlastnosti Cu2+/alginátových částic připravených vnější iontovou gelací hodnocený analýzou hlavních komponent Miroslava Pavelková
V současné době je metoda vnější iontové gelace v přípravě alginátových částic s úspěchem používána nejen na poli farmacie a medicíny, ale zejména v oblasti biotechnologie. Proto byla příprava alginátových částic a jejich následné hodnocení pomocí analýzy hlavních komponent stěžejním cílem našeho experimentu. Kvůli optimalizaci této metody jsme se zaměřili na hodnocení vlivu různých formulačních (koncentrace polymeru, koncentrace tvrdícího roztoku) a procesních parametrů (velikost vnějšího průměru injekční jehly) na vlastnosti vzniklých částic (výtěžek, sféricita, ekvivalentní průměr a bobtnavost při pH 6). Metodou analýzy hlavních komponent byl zásadní vliv na výsledné vlastnosti alginátových částic potvrzen pouze u koncentrace natrium-alginátu. Tyto výsledky potvrdily spolehlivý a bezpečný potenciál vnější iontové gelace v přípravě částicové lékové formy na bázi alginátu.
Klíčová slova:
hydrogelové částice – vnější iontová gelace – natrium-alginát – měďnaté ionty – hodnocení částicové lékové formy – analýza hlavních komponent
Authors: Miroslava Pavelková; Jakub Vysloužil; Kateřina Kubová; Sylvie Pavloková; Eliška Mašková; David Vetchý
Published in the journal: Čes. slov. Farm., 2019; 68, 69-77
Category: Původní práce
Summary
Currently, the method of external ionic gelation for the preparation of alginate particles is successfully used not only in the field of pharmacy and medicine, but also especially in the field of biotechnology. Therefore, the preparation of alginate particles and their subsequent evaluation using principal component analysis was the key task of our experiment. To optimize this method, we focused on the evaluation of the effect of formulation (the polymer concentration, the hardening solution concentration) and process parameters (the outer diameter of the injection needle) on the properties of the resulting beads (yield, sphericity factor, equivalent diameter and swelling capacity at pH 6). Using multivariate data analysis, the major influence on the resulting properties of the prepared particles was confirmed only in sodium alginate concentration. Obtained results verified the reliable and safe potential of the external ionic gelation for preparation alginate-based particulate dosage forms.
Keywords:
hydrogel particles – external ionic gelation – sodium alginate – copper ions – evaluation of the particulate dosage form – Principal component analysis
Introduction
External ionic gelation method is a widely employed technique for the formation of hydrogel beads. It belongs among promising tools in the development of novel biocompatible sustained and targeted controlled drug delivery systems, mainly due to its indisputable advantages, such as nontoxic process without using organic solvents, convenient and controllable procedure1). This technique is based on the cross-linking tendency of polyelectrolytes in the presence of polyvalent ions to form hydrogels2–4), when physical (electrostatic) forces between the polyelectrolyte and the present polyvalent ions are formed5).
During external ionic gelation, the polymer solution is extruded as drops into a hardening bath containing polyvalent ions, which immediately diffuse inward into the interstitial spaces between the alginate polymer chains to initiate cross-linking at the periphery of the polymer drop. A semisolid membrane encasing the drop is formed6). Subsequent leaving of the drops in the hardening solution for a certain time period allows further diffusion of ions across the membrane via a concentration gradient. After saturation of available binding sites, resulting beads are formed as three dimensional, water-insoluble gel network7, 8), with a higher concentration of ions at the periphery compared to the centre of the gel9).
The type and molecular weight of the polymer, surface tension and viscosity of the polymer solution, the type and concentration of the hardening solution (as formulation parameters) as well as temperature, hardening time, stirring intensity, the diameter of the drip device (as process parameters), all affect the resulting particle structure and their properties10, 11).
Alginates as naturally occurring anionic polyelectrolytes belong among the most commonly used polymers in the formation of hydrogel beads by external ionic gelation, especially due to their biocompatibility, biodegradability, low toxicity, vast availability, relatively low cost, and mild gelation12, 13). Commercially available alginates are typically extracted from brown seaweed (Phaeophyceae), including species Laminaria, Ascophyllum and Macrocystis. Bacterial alginates can be produced from Azotobacter and Pseudomonas species but these extraction methods are not economically viable for commercial applications14, 15).
Chemically, alginates are a family of linear unbranched polysaccharides which contain varying amounts of 1,4′-linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues. The residues may vary widely in composition and sequence and are arranged in a pattern of blocks along the chain as homogenous (poly-G, poly-M) or heterogeneous (MG) block-like patterns16, 17). The M/G ratio is a critical factor affecting the physical properties of the alginate and its resulting hydrogels14). The affinity of different block structures of the alginate to polyvalent ions increases in the order of Mg2+ << Mn2+ < Zn2+ < Ca2+ < Sr2+ < Ba2+ < Cu2+ < Pb2+ ions18–21). In general, it can be said that divalent cations preferentially bind toward the G-block rather than the M-block7, 22). However, in a more detailed study by Mørch et al. it has been found that Sr2+ ions are preferentially bound to the G-units, Ba2+ ions are bound to G - and M-blocks and Ca2+ are bound to G - and MG-blocks19).
The binding properties of the Cu2+ ions used in our study have been extensively investigated by researchers Lu et al. They have determined that no preference between M/G units exists in the formation of Cu2+ complexes with the alginate23). Moreover, the copper-alginate affinity is so strong that the outermost part of the beads is strongly complexed and the created outercoat partially prevents further diffusion of the Cu2+ ions toward the beads centre. Copper beads so develop a dense outer layer and they seem to lack Cu2+ ions in the centre24, 25).
The gel-forming property of sodium alginate, as well as its apparent biocompatibility14, 26), leads to an increased use in the medical, pharmaceutical and biotechnology industries. The alginate is employed for applications such as wound dressings27), gel matrix to encapsulate and to control the release of drugs including protein-based substances or as matrices for tissue engineering12, 14, 26, 28, 29).
The method of external ion gelation, especially when using polyvalent ions with antimicrobial and/or antiviral activity, provides a unique possibility to prepare a medical form with a biological activity without the need of drug encapsulation30). For instance, antimicrobial effects of copper have been known and used by humans since ancient times31). Therefore, its entrapment in the alginate network can provide a simple and effective way of treatment of vaginal or eye infections30).
The main objective of the presented study was to observe and evaluate the effect of several process and formulation parameters on the technological properties of Cu2+/alginate particles prepared by the ion external gelation method. An influence of needle diameter, polymer concentration and hardening solution concentration on the main important characteristics of prepared particles such as the yield, sphericity, equivalent diameter, swelling capacity were measured and investigated by principal component analysis.
Experimental part
Materials
Sodium alginate – SA (Sigma Aldrich, St. Louis, USA) served as the polymer for the particle preparation, copper (II) chloride (Sigma Aldrich, St. Louis, USA) was used as the gelation agent. Sodium hydroxide and potassium dihydrogen phosphate (both Dr. Kulich Pharma, Hradec Králové, Czech Republic) were used for the formation of pH 6.0 phosphate buffer. All materials were of Ph. Eur. quality.
Microparticle preparation
Beads were prepared by the external ionic gelation method. For each sample 100 g of sodium alginate solution (3% or 4%) was filled in a syringe with an appropriate needle (diameter 0.5, 0.7 or 0.9 mm) and they were added dropwise, using a linear dispenser pump NE-1000 (New Era Pump System, Farmingdale, NY, USA), to a hardening solution of copper (II) chloride (0.5M or 1M concentration) in Petri dishes, which were placed on a rotating pad to prevent deformation of the beads by constantly dripping to the same place. Here the crosslinking and hardening process took place. The injection needle tip distance from the surface of the curing solution was approximately 10 cm. Hardening time was 60 minutes. Thereafter, formed beads were washed with purified water, collected by filtration and then dried in a cabinet drier (HORO-048B, Dr. Hofmann GmbH, Ostfildern, Germany) for 12 hours at 25 °C. Samples were named accordingly to the parameter values used for their preparation (see Table 1).
Tab. 1. Designation of bead samples and variables used during their preparation 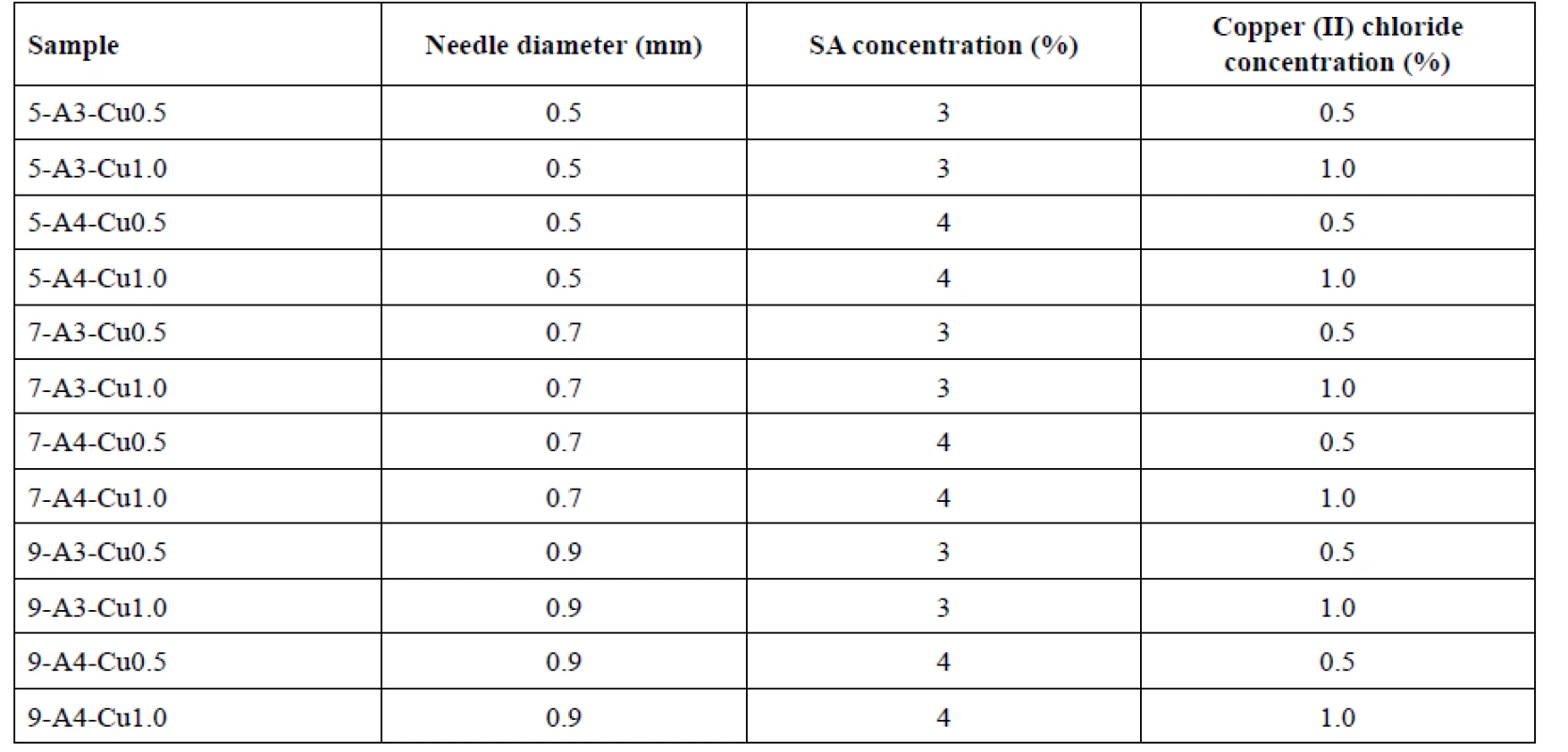
Microparticle characteristics
Yield
Effectiveness of the process was evaluated by yield. Dried samples were weighed on an analytical scales KERN 440-47N (KERN & SohnGmbH, Balingen, Germany). The yield was expressed as the weight of dry beads in grams obtained by dropping of 100 g of polymer solution (g/100 g).
Optical microscopy
To determine the morphology of the prepared beads, a stereoscopic microscope Nikon SMZ 1500 (Nikon, Tokyo, Japan) with a photo camera TV Lens 0.55xDS (Nikon, Tokyo, Japan) was employed. Beads were observed by a 0.75 objective. For each sample 200 random particles were measured using the threshold method in software NIS-Elements 4.00.06 (Nikon, Tokyo, Japan). The evaluated morphological properties included the sphericity factor (SF)32):
where A is a particle area in mm2 and p is perimeter in mm. Another determined property was the equivalent diameter (ED), which gives the diameter of a circle which has the same area as the observed object32):
ED and SF were calculated from the measured values and expressed as the arithmetic mean and standard deviation.
Swelling capacity
Swelling capacity (SSW) belongs among physical factors which may influence mucoadhesivity and drug release from dosage forms34). Every sample was tested for swelling capacity in pH 6.0 phosphate buffer. 100 mg of particles were placed into small pre-weighed metal baskets and then immersed into the buffer. In pre-determined intervals (30 minutes, 1, 2, 3, 4, 5 and 6 hours), baskets were picked up, properly dried from the outside and weighed on analytical scales. Each sample was measured in triplicate. Swelling capacity was calculated from the following equation3):
where Wt is the weight of beads in grams at the respective time interval, Wo is the weight of beads in grams before the first immersion35).
Data analysis
The primary goal of the data analysis was a study of the dependencies between formulation/process parameters and Cu2+/alginate particles properties. In order to determine statistical significance of these effects, the data were evaluated by means of analysis of variance (ANOVA) and for a better dependency visualization, the data were subsequently subjected to the principal component analysis (PCA). The p-values obtained by ANOVA are given in the parentheses for the discussed characteristics in the section Results and discussion, but only in the cases where statistical significance was confirmed (p < 0.05). The resulting PCA model was built based on the variables: yield, sphericity, equivalent diameter and swelling capacity at 6 h. The swelling capacities at all-time points were highly correlated, so redundant variables were excluded from the calculations. Data analysis was performed by means of the R software, version 3.4.4.36).
Results and discussion
Influence of needle diameter
Effect on yield
Influence of the needle diameter on the sample yields is displayed in Table 2. The sample yields prepared using 0.5 mm, 0.7 mm and 0.9 mm injection needles were in the range from 3.443 to 4.814 g/100 g, 3.184 to 4.386 g/100 g and 3.398 to 4.609 g/100 g, respectively. No dependence between the yield and the outside diameter of the used needle was found.
Tab. 2. Yield, sphericity factor and equivalent diameter results 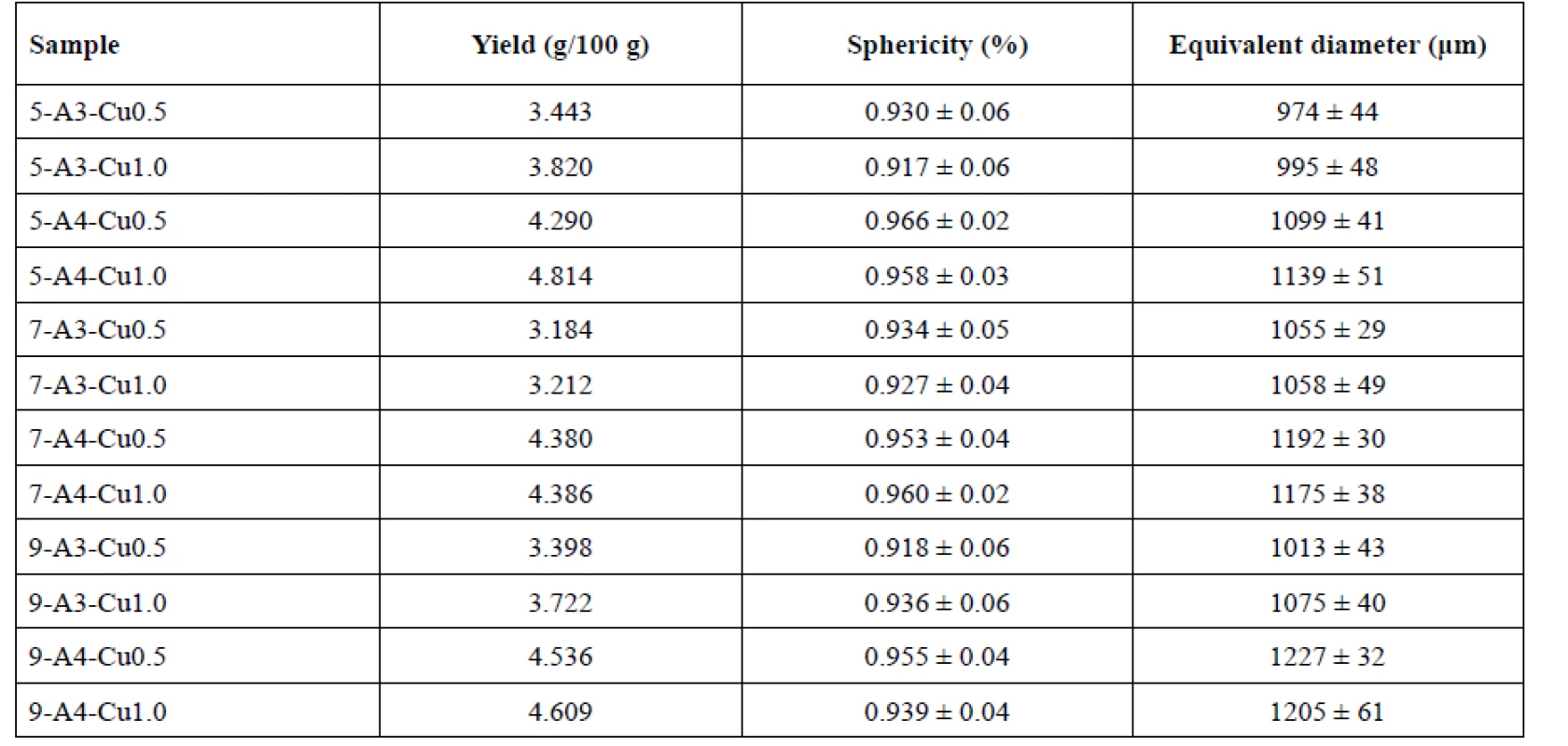
Effect on sphericity
Many studies have confirmed an impact of the needle size on the beads sphericity, specifically, that smaller and more spherical particles are formed using a smaller diameter syringe11, 37, 38). However, the aforementioned dependence has not been verified by our investigation, but the appropriate sphericity of all samples with minimal differences has been shown. The highest sphericity (0.966 ± 0.02) was shown in sample 5-A4-Cu0.5. The order of the rest of the samples was as follows: 7-A4-Cu1.0, 5-A4-Cu1.0, 9-A4-Cu0.5 and 7-A4-Cu0.5 with the sphericity values of 0.960 ± 0.02, 0.958 ± 0.03, 0.955 ± 0.04 and 0.953 ± 0.04, respectively (see Table 2).
Effect on equivalent diameter
The beads prepared using a 0.5 mm diameter needle showed the smallest equivalent diameter, namely samples 5-A3-Cu0.5 and 5-A3-Cu1.0 (974 ± 44 μm, 995 ± 48 μm). Conversely, the largest particles were prepared using a 0.9 mm needle, namely samples 9-A4-Cu0.5, 9-A4-Cu1.0 (1227 ± 32 μm, 1205 ± 61 μm). These results showed a slight increase in particle size, depending on the needle diameter (p < 0.001). However, A3-Cu0.5 sample was an exception. This sample prepared with the use of a 0.9 mm needle (1013 ± 43 μm) provided smaller beads than with the use of 0.7 mm needle (1055 ± 29 μm)
(Table 2) which could be caused by some random influences, such as human factor failure. The theories11, 37, 39, 40) about the dependence of the size syringe tip diameter on the drop size formation and subsequently on the size of the resulting beads have not been thus fully confirmed.Effect on swelling capacity
The beads dripped through a 0.5 mm injection syringe had the swelling capacity in the range of 76–130% after 30 minutes and 107–159% after 1 hour. The swelling of the samples obtained with a 0.7 mm injection needle was in the range of 38–151% (after 30 minutes) and 59 to 165% (after 1 hour). Swelling capacity of the samples prepared by using a 0.9 mm outer diameter injection needle ranged from 89 to 125% after 30 minutes, and 104 to 138% after 1 hour. Within next 5 hours, the swelling capacity increased, but significantly less, only about 20–30% (see Figs. 1, 2). No correlation between the outer diameter of the injection needle and the swelling of individual samples has been found.
Fig. 1. The swelling profiles of 3% Cu2+/alginate particles in phosphate buffer pH = 6.0 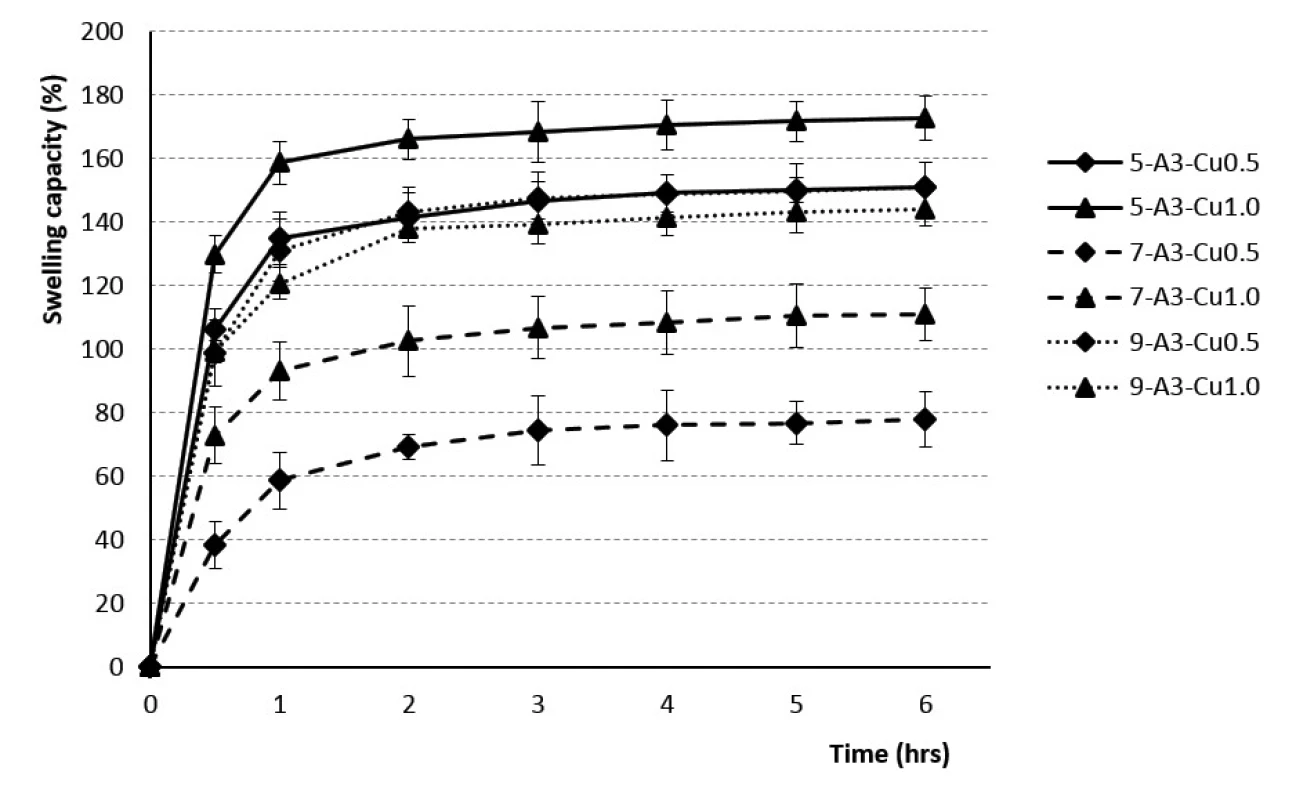
Fig. 2. The swelling profiles of 4% Cu2+/alginate particles in phosphate buffer pH = 6.0 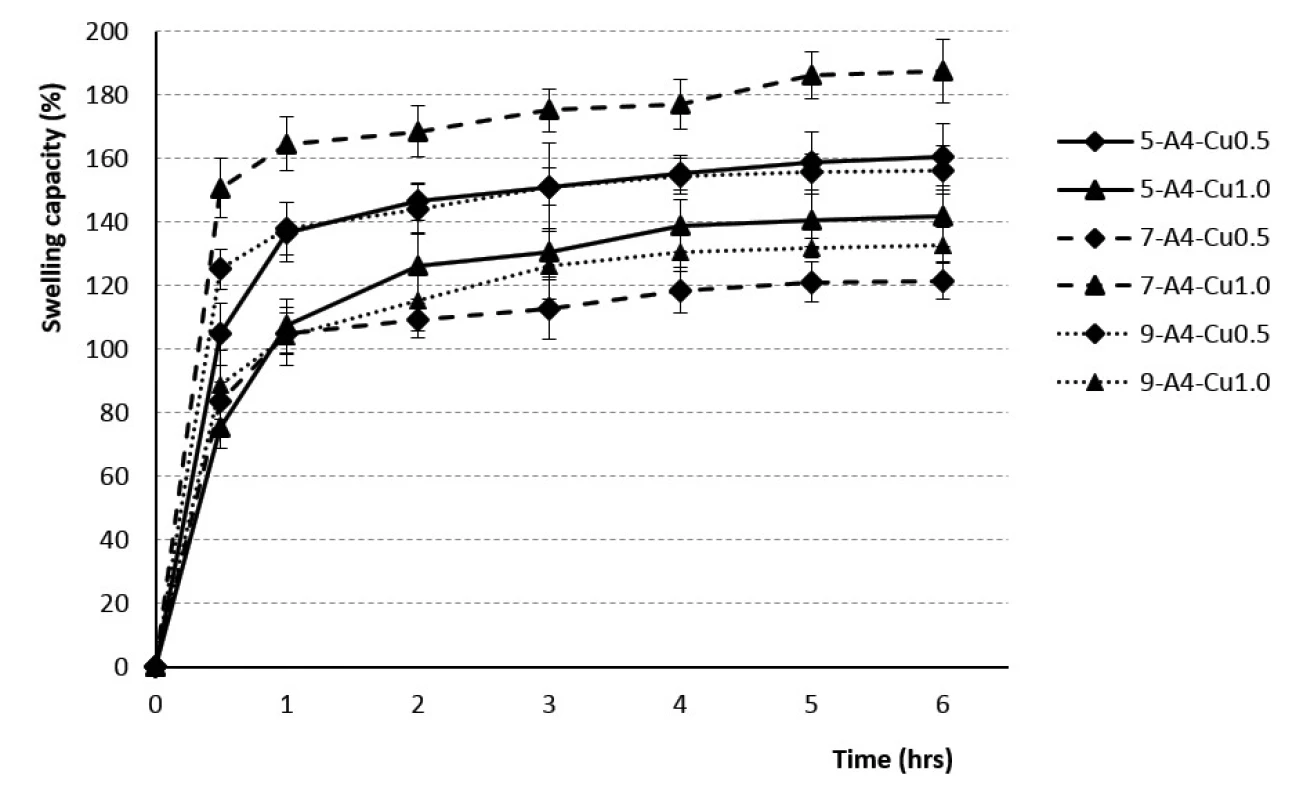
Influence of sodium alginate concentration
Effect on yield
The yields of beads prepared using 3% and 4% SA solutions were in the range from 3.184–3.820 g/100 g and 4.290–4.814 g/100 g. When comparing the corresponding samples in Table 2 (samples prepared using the same hardening solution concentration and the same size of needle diameter), it is obvious that the yields of individual samples statistically significantly depended on the SA concentration (p < 0.001). Specifically, the higher yields were achieved using more concentrated solution of alginates, which is consistent with the experimental study of Aswathy et al.41). The explanation could be seen in a higher number of binding sites for crosslinking by polyvalent ions in the higher-concentrated SA solution resulting in an increase in the process yield.
Effect on sphericity
The SA concentration exhibited a major influence on the sphericity of all samples (p < 0.001). All 4% samples showed higher sphericity values than the 3% samples (see Table 2). Our investigation has confirmed the theories of many researchers42, 43) about an increase in bead sphericity in dependence on rising polymer solution concentrations. At a low SA concentration, the particles are formed of a loose networks structure which may collapse during drying. On the other hand, a higher SA concentration creates a denser matrix structure which prevents collapse of the beads42, 44) and the resulting beads are more spherical. In addition, when the alginate liquid drop hits and enters the hardening bath, there are competing forces between the viscous surface tension forces and impact-drag forces to maintain the drop shape. However, the viscosity of the low-concentration SA solutions is not sufficient to cope with impact, resulting in bead deformation45).
Effect on equivalent diameter
Equivalent diameter of beads was in the range of 974 ± 44 μm (5-A3-Cu0.5) to 1075 ± 40 μm (9-A3-Cu1.0) for 3% SA samples and between 1099 ± 41 μm (5-A4--Cu0.5) and 1227 ± 32 μm (9-A4-Cu0.5) for 4% samples (see Table 2). Overall, in samples prepared with the same needle outer diameter, our results showed that all samples prepared from the 4% SA solution were larger in size compared to the 3% solution (p < 0.001). This could be attributed to an increase in relative viscosity at higher concentration of SA and formation of large droplets during dripping of polymer solution to the hardening bath42, 43, 46–48).
Effect on swelling capacity
The rate of swelling capacity was not dependent on the polymer concentration used. The swelling capacity was found to be the highest during the first hour, with the minimum value for the sample 7-A3-Cu0.5 (60%) and the maximum one for the sample 7-A4-Cu1.0 (168%). Consequently, during the next five hours, the swelling capacity changed less significantly, the batch 7-A3-Cu0.5 swelled up to 79% and the batch 7-A4-Cu1.0 up to 192% (see Figs. 1, 2). Our research did not confirm the findings reported by the majority of investigators about the dependence of the swelling extent on the SA concentration, which demonstrated that the beads prepared using the 4% SA polymeric solution exhibited a slower swelling rate than the more porous beads prepared with low-concentration 3% SA solution43, 47, 49, 50). Our results, indicating statistical insignificancy, were probably due to the unequal drying of the extracted baskets from the phosphate buffer during the test performance.
Influence of hardening solution concentration
Effect on yield
In samples prepared from the 3% SA solution and 0.5M hardening solutions, the yields took values from 3.184 to 3.433 g/100 g and in samples cross-linked with 1M hardening solution the values ranged from 3.212 to
3.820 g/100 g. Likewise, in the 4% samples cross-linked by 1M solution, the yield was slightly higher (see Table 2). Based on our experiment, it can be said that the yield of the samples increased with increasing concentrations of the hardening solution, but with no statistical insignificance. An explanation could be the following one: using a larger amount of Cu2+ ions provides a possibility of creating more electrostatic bonds within the crosslinking of the alginate polymer chains. Ultimately, there is a smaller loss of alginate within external ionic gelation process.Effect on sphericity
In our experiment, the beads sphericity dependence on the concentration of the hardening solution has not been demonstrated. The sphericity factor randomly grew and fell with increasing concentration of the hardening solution (see Table 2). Our results do not correspond with many other studies, which has confirmed the tendency to creation of the more spherical particles with their uniform size depending on the rising concentration of hardening solution42).
Effect on equivalent diameter
Based on previous researches46, 51), we expected that an increase in cross-linking ion concentration would significantly affect the mean diameter of the beads. However, our results were as follows: for the beads prepared from the 3% SA solution (using diameter needles of 0.5, 0.7 and 0.9 mm) and for the beads from 4% SA (using only a 0.5 mm needle), the equivalent diameter was smaller when the 0.5M hardening solution was used. Conversely, the beads prepared from 4% SA (using a 0.7 and 0.9 mm needle), the equivalent diameter was lower when we used the 1M hardening solution (see Table 2). An impact of hardening solution concentration on the beads equivalent diameter was confirmed (p < 0.001).
Effect on swelling capacity
The dependence of the swelling rate on the concentration of the hardening solution has not been demonstrated. The results within the corresponding samples were discrepant. Samples from 3% SA prepared using 0.5 mm and 0.7 mm diameter needles showed a higher swelling capacity when the beads were cross-linked by 1M solution CuCl2. 3% SA samples prepared with a 0.9 mm needle showed higher swelling when the 0.5M hardening solution was used for crosslinking. The samples from 4% SA exhibited similar behavior (see Figs. 1, 2). Despite the research which showed that the swelling capacity decreased with an increase in divalent ions concentration, dependence of swelling capacity on the crosslinking ion concentration has not confirmed in our study47).
Multivariate data analysis
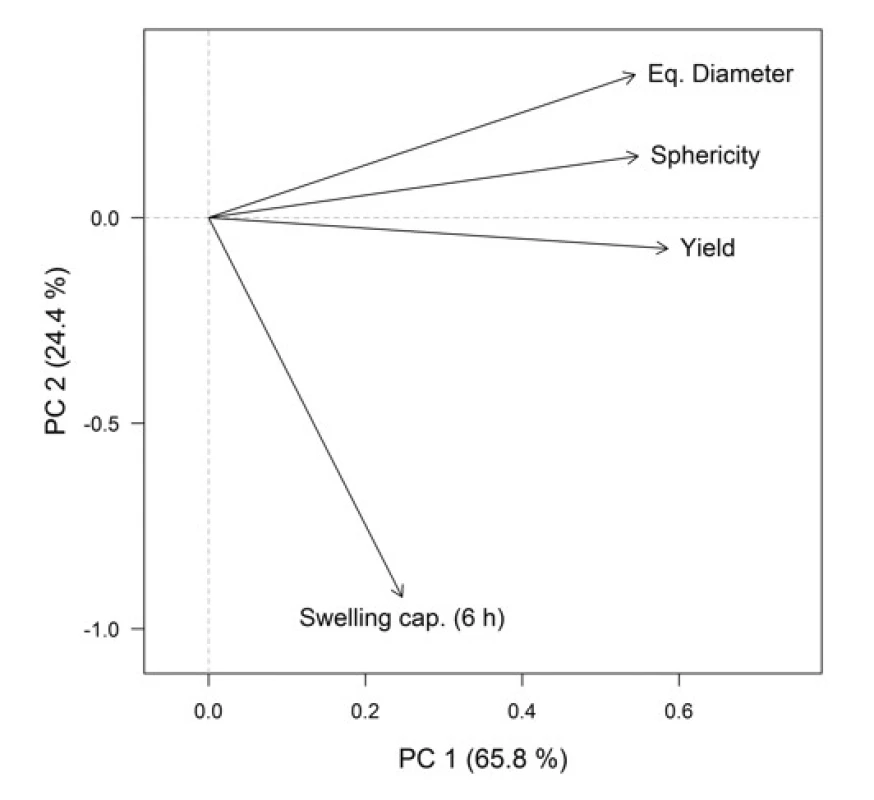
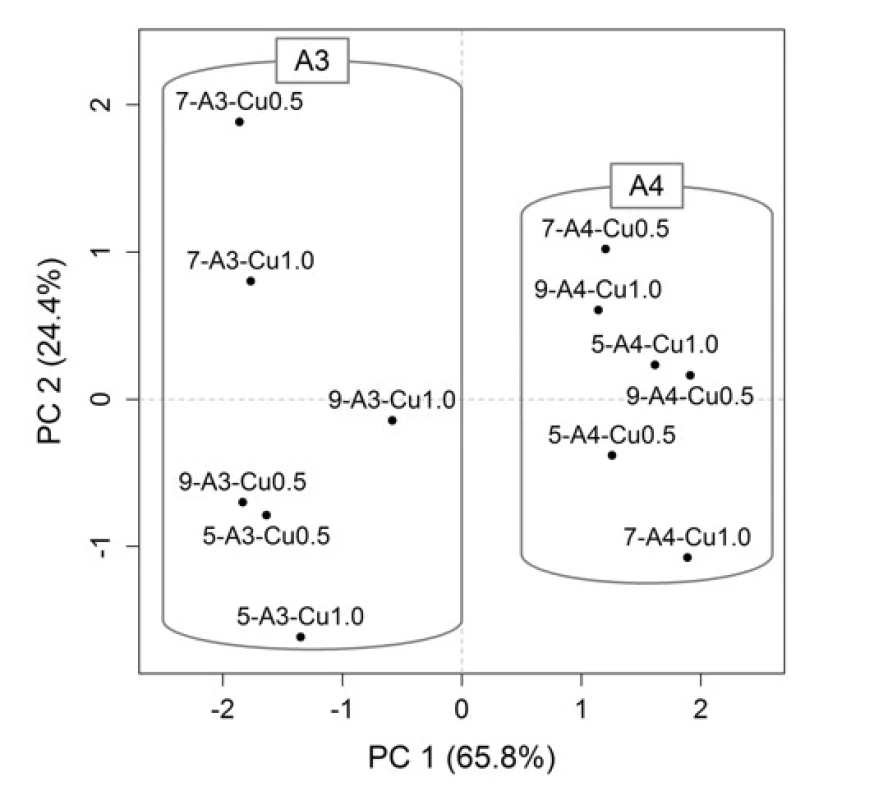
For a better representation of the relationships between the variables (formulation / process parameters) themselves and between the variables and objects (Cu2+/alginate particles properties), the PCA method was employed. Graphical representation is provided by the PCA loadings plot (Fig. 3), which displays the correlation structure of variables, and the PCA scores plot (Fig. 4), which displays the layout of objects. In the loadings plot, original variables are depicted as arrows in the ordination space of the first two principal components (PCs), their lengths are directly proportional to the explained variability and the amount of correlation can be interpreted based on the angle between any two arrows. In the scores plot, the samples with similar characteristics are closely spaced, while different samples are far apart from each other.
In the resulting model, the first two PCs explained 90.2% of variability, which is a sufficient amount to provide a reliable data interpretation (52). The relatively high positive correlation of equivalent diameter, sphericity and yields is obvious in the PCA loadings plot. These variables also correlated with the PC1. Swelling capacity at the time point 6 h was more associated with the PC2. In the PCA scores plot, the samples distribution along the PC1 is evident, clustering into two groups is based on SA concentration, which is also highlighted in the graph. When comparing the PCA graphical outputs (Figs. 3 and 4), it can be summarized that samples with a higher SA concentration exhibited a higher equivalent diameter, as well as higher sphericity and yield. Within both groups (A3 and A4), the samples differed mainly on the basis of swelling capacity, but without apparent statistical relationships with other variables. So the effects of varying needle diameter in conjunction with hardening solution concentration on the resulting particles properties cannot be unambiguously discussed.
Conclusion
The external ionic gelation resulted in the formation of all Cu2+/alginate particle samples with satisfactory properties. Their morphological parameters (ED, SF) as well as the enormous ability to swell in the environment of pH 6 were convenient, without a significant influence of most of our tested parameters. Based on multivariate data analysis results, it can be concluded that SA concentration was the only factor which fundamentally influenced the resulting properties of the prepared Cu2+/alginate particle. Therefore, our results have confirmed external ionic gelation as a safe, reliable and robust method for Cu2+/alginate beads. Despite a relatively wide variability of the input parameters, it resulted in the creation of beads with well-defined characteristics with a potential use for a development of a convenient particulate dosage form suitable either for drug encapsulation or for its own use due to the antimicrobial effect of copper.
Acknowledgement
This work was supported by the Ministry of Education, Youth and Sports project “FIT” (Pharmacology, Immunotherapy, nanoToxicology) CZ.02.1.01/0.0/0.0/15 003/0000495 and by the Ministry of Agriculture of the Czech Republic, institutional support MZE-R00518.
Conflicts of interest: none.
Department of Pharmaceutics, Faculty of Pharmacy
University of Veterinary and Pharmaceutical Sciences Brno
Palackého 1, 612 42 Brno, Czech Republic
e-mail: jakub.vyslouzil@gmail.com
*Department of Pharmacology and Immunotherapy, Veterinary Research Institute
Zdroje
1. Agnihotri S. A., Mallikarjuna N. N., Aminabhavi T. M. Recent advances on chitosan-based micro - and nanoparticles in drug delivery. J. Control. Release 2004; 100, 5–28.
2. Patil J. S., Kamalapur M. V., Marapur S. C., Kadam D. V. Ionotropic gelation and polyelectrolyte complexation: The novel techniques to design hydrogel particulate sustained, modulated drug delivery system: A review. Dig. J. Nanomater. Bios. 2010; 5, 241–248.
3. Patil P., Chavanke D., Wagh M. A. A review on ionotropic gelation method: Novel approach for controlled gastroretentive gelispheres. Int. J. Pharm. Pharm. Sci. 2012; 4, 27–32.
4. Cerciello A., Auriemma G., Del Gaudio P., Sansone F., Aquino R. P., Russo P. A novel core-shell chronotherapeutic system for the oral administration of ketoprofen. J. Drug Deliv. Sci. Tec. 2016; 32, 126–131.
5. Ahmadi F., Oveisi Z., Samani S. M., Amoozgar Z. Chitosan based hydrogels: characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015; 10, 1–16.
6. Zhang H., Tumarkin E., Peerani R., Nie Z., Sullan R. M. A., Walker G. C., Kumacheva E. Microfluidic production of biopolymer microcapsules with controlled morphology. J. Am. Chem. Soc. 2006; 128, 12205–12210.
7. Smidsrød O., Skjåk-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990; 8, 71–78.
8. Alonso B. C., Rayment P., Ciampi E., Ablett S., Marciani L., Spiller R. C., Norton I. T., Gowland P. A. NMR relaxometry and rheology of ionic and acid alginate gels. Carbohyd. Polym. 2010; 82, 663–669.
9. Skjåk-Braek G., Grasdalen H., Smidsrød O. Inhomogeneous polysaccharide ionic gels. Carbohyd. Polym. 1989; 10, 31–54.
10. Vysloužil J., Dvořáčková K., Kejdušová M. Příprava léčivých mikročástic metodou odpařování rozpouštědla. Chem. Listy 2013; 107, 16–23.
11. Lee B.-B., Ravindra P., Chan E.-S. Size and shape of calcium alginate beads produced by extrusion dripping. Chem. Eng. Technol. 2013; 36, 1627–1642.
12. Gombotz W. R., Wee S. F. Protein release from alginate matrices. Adv. Drug Deliver. Rev. 1998; 31, 267–285.
13. Marković D., Zarubica A., Stojković N., Vasić M., Cakić M., Nikolić G. Alginates and similar exopolysaccharides in biomedical application and pharmacy: Controled delivery of drugs. Advanced technologies 2016; 5, 39–52.
14. Lee K. Y., Mooney D. J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012; 37, 106–126.
15. Urtuvia V., Maturana N., Acevedo F., Peña C., Díaz-Barrera A. Bacterial alginate production: an overview of its biosynthesis and potential industrial production. World J. Microb. Biot. 2017; 33, 198.
16. Haug A., Larsen B., Smidsrød O. Studies on the sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 1967; 21, 691–704.
17. Haug A., Larsen B. Quantitative determination of the uronic acid composition of alginates. Acta Chem. Scand. 1962; 16, 1908–1918.
18. Agulhon P., Markova V., Robitzer M., Quignard F., Mineva T. Structure of alginate gels: Interaction of diuronate units with divalent cations from density functional calculations. Biomacromolecules 2012; 13, 1899−1907.
19. Mørch Y. A., Donati I., Strand B. L., Skjåk-Braek G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006; 7, 1471−1480.
20. Haug A., Smidsrød O. Selectively of some anionic polymers for divalent metal ions. Acta Chem. Scand. 1970; 24, 843–854.
21. Idota Y., Kogure Y., Kato T., Yano K., Arakawa H., Miyajima C., Kasahara F., Ogihara T. Relationship between physical parameters of various metal ions and binding affinity for alginate. Biol. Pharm. Bull. 2016; 39, 1893–1896.
22. Braccini I., Pérez. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules. 2001; 2, 1089–1096.
23. Lu L., Liu X., Qian L., Tong Z. Sol-gel transition in aqueous alginate solutions induced by cupric cations observed with viscoelasticity. Polym. J. 2003; 35, 804–809.
24. Velings N. M., Mestdagh M. M. Physico-chemical properties of alginate gel beads. Polym. Gels Netw. 1995; 3, 311–330.
25. Rodrigues J. R., Lagoa R. Copper ions binding in Cu-alginate gelation. J. Carbohyd. Chem. 2006; 25, 219–232.
26. Jain D., Bar-Shalom D. Alginate drug delivery systems: application in context of pharmaceutical and biomedical research. Drug Dev. Ind. Pharm. 2014; 40, online 1–9.
27. Thomas S. Alginate dressings in surgery and wound management – part 1. J. Wound Care. 2000; 9, 56–60.
28. Draget K. I., Taylor C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocolloid 2011; 25, 251–256.
29. Augst, A. D., Kong H. J., Mooney D. J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006; 6, 623–633.
30. Pavelková M., Kubová K., Vysloužil J., Kejdušová M., Vetchý D., Celer V., Molinková D., Lobová D., Pechová A., Vysloužil J., Kulich P. Biological effects of drug-free alginate beads cross-linked by copper ions prepared using external ionotropic gelation. AAPS PharmSciTech. 2017; 18, 1343–1354.
31. Grass G., Rensing C., Solioz M. Metallic copper as an antimicrobial surface. Appl. Environ. Microb. 2011; 77, 1541–1547.
32. Rabišková M., Häring A., Minczingerová K., Havlásek M., Musilová P. Microcrystalline cellulose in oral dosage forms. Chem. Listy 2007; 101, 70–77.
33. Smýkalová I., Horáček J., Hýbl M., Bjelková M., Pavelek M., Krulikovská T., Hampel D. Seed type identification by image analysis – correlation of nutrients with size, shape and colour characteristics of seeds. Chem. Listy 2011; 105, 138–145.
34. Dodou D., Breedveld P., Wieringa P. A. Mucoadhesives in the gastrointestinal tract: revisiting the literature for novel applications. Eur. J. Pharm. Biopharm. 2005; 60, 1–16.
35. Kubánková R., Vysloužil J., Kejdušová M., Vetchý D., Dvořáčková K. Impact of formulation and process parameters on the properties of chitosan-based microspheres prepared by external ionic gelation. Ces. slov, Farm. 2014; 63, 127–135.
36. The R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna,
Austria. 1993–2003. https://www.R-project.org/37. Chan E.-S., Lee B.-B., Ravindra P., Poncelet D. Prediction models for shape and size of ca-alginate macrobeads produced through extrusion – dripping method. J. Colloid Interf. Sci. 2009; 338, 63–72.
38. Rousseau I., Le Cerf D., Picton L., Argillier J. F., Muller G. Entrapment and release of sodium polystyrene sulfonate (SPS) from calcium alginate gel beads. Eur. Polym. J. 2004; 40, 2709–2715.
39. Kašpar O., Jakubec M., Štěpánek F. Characterization of spray dried chitosan-TPP microparticles formed by two - and tree fluid nozzles. Powder Technol. 2013; 204, 31–40.
40. Popa E. G., Gomes M. E., Reis R. L. Cell delivery systems using alginate - carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules 2011; 12, 3952–3961.
41. Aswathy K. S., Abraham A. M., Jomy L., Mehaladevi R., Rosemol K. J. Formulation and evaluation of Etodolac alginate beads prepared by ionotropic gelation for sustained release. Int. J. Sci. Innov. Res. 2014; 3, 527–531.
42. Manjanna K. M., Shivakumar B., Pramod kumar T. M. Diclofenac sodium microbeads for oral sustained drug delivery. Int. J. Pharm. Tech. Res. 2009; 1, 317–327.
43. Joshi S., Patel P., Lin S., Madan P. L. Development of cross-linked alginate spheres by ionotropic gelation technique for controlled release of naproxen orally. Asian J. Biomed. Pharm. Sci. 2012; 7, 134–142.
44. Rajesh K. S., Khanrah A., Biswanath S. Release of ketoprofen from alginate microparticles containing film forming polymers. J. Sci. Ind. Res. 2003; 62, 985–989.
45. Chan E.-S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohyd. Polym. 2011; 84, 1267–1275.
46. Østberg T., Vesterhus L., Graffner C. Calcium alginate matrices for oral multiple unit administration: II. Effect of process and formulation factors on matrix properties. Int. J. of Pharmaceut. 1993; 97, 183–193.
47. Sathali A. A. H., Varun J. Formulation, development and in vitro evaluation of candesartan cilexetil mucoadhesive microbeads. Int. J. Curr. Pharm. Res. 2012; 4, 109–118.
48. Khazaeli P., Pardakhty A., Hassanzadeh F. Formulation of ibuprofen beads by ionotropic gelation. Iran. J. Pharm. Res. 2008; 7, 163–170.
49. Blandino A., Macías M., Cantero D. Formation of calcium alginate gel capsules: Influence of sodium alginate and CaCl2 concentration on gelation kinetics. J. Biosci. Bioeng. 1999; 88, 686–689.
50. Bajpai S. K., Sharma S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004; 59, 129–140.
51. Striamornsak P., Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: II. Effect of formulation and processing variales on drug release. J Microencapsul. 1999; 16, 303–313.
52. Reimann C., Filzmoser P., Garret R. G., Dutter R. Statistical data analysis explained: applied environmental statistics with R. Ltd. Chichester, John Wiley & Sons; 2008.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2019 Číslo 2- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- Bevacizumab treatment in metastatic colorectal carcinoma – an economic perspective
- Prenylated phenols with cytotoxic and antiproliferative activity isolated from Morus alba
- Influence of formulation and process parameters on the properties of Cu2+/alginate particles prepared by external ionic gelation evaluated by principal component analysis
- OTC market – comparing Czech Republic and Greece
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- OTC market – comparing Czech Republic and Greece
- Bevacizumab treatment in metastatic colorectal carcinoma – an economic perspective
- Prenylated phenols with cytotoxic and antiproliferative activity isolated from Morus alba
- Influence of formulation and process parameters on the properties of Cu2+/alginate particles prepared by external ionic gelation evaluated by principal component analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání