-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaRrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
During the synthesis of small ribosomal subunits in eukaryotes, the pre-40S particles formed in the nucleolus are rapidly transported to the cytoplasm. The mechanisms involved in the nuclear export of these particles and its coordination with other steps of the 40S synthesis pathway are mostly unknown. In this work we studied the function of Rrp12, the only major non-ribosomal factor of nuclear pre-40S particles that does not remain stably associated to them during maturation in the cytoplasm. We demonstrate that Rrp12 is required for the exit of pre-40S particles to the cytoplasm. Remarkably, we also found that Rrp12, together with the Crm1 exportin, participates in processes that occur in early pre-ribosomes in the nucleolus, including the processing of the pre-rRNA and the elimination of processing byproducts. Thus, Rrp12 and Crm1 participate in maturation steps that take place upstream of nuclear export. Our results indicate that, in the 40S subunit synthesis pathway, the completion of early pre-40S particle assembly, the initiation of byproduct degradation and the priming for nuclear export occur in an integrated manner in nucleolar pre-ribosomes.
Published in the journal: . PLoS Genet 10(12): e32767. doi:10.1371/journal.pgen.1004836
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004836Summary
During the synthesis of small ribosomal subunits in eukaryotes, the pre-40S particles formed in the nucleolus are rapidly transported to the cytoplasm. The mechanisms involved in the nuclear export of these particles and its coordination with other steps of the 40S synthesis pathway are mostly unknown. In this work we studied the function of Rrp12, the only major non-ribosomal factor of nuclear pre-40S particles that does not remain stably associated to them during maturation in the cytoplasm. We demonstrate that Rrp12 is required for the exit of pre-40S particles to the cytoplasm. Remarkably, we also found that Rrp12, together with the Crm1 exportin, participates in processes that occur in early pre-ribosomes in the nucleolus, including the processing of the pre-rRNA and the elimination of processing byproducts. Thus, Rrp12 and Crm1 participate in maturation steps that take place upstream of nuclear export. Our results indicate that, in the 40S subunit synthesis pathway, the completion of early pre-40S particle assembly, the initiation of byproduct degradation and the priming for nuclear export occur in an integrated manner in nucleolar pre-ribosomes.
Introduction
The formation of ribosomes in eukaryotic cells requires the production and subsequent assembly of four rRNAs and ≈80 ribosomal proteins into small (40S) and large (60S) ribosome subunits. In the yeast S. cerevisiae, three out of those four rRNAs (18S, 5.8S and 25S) are transcribed together in the nucleolus in the context of a common polycistronic 35S pre-rRNA (see scheme in Fig. 1A) [1], [2]. This primary rRNA precursor is bound by ribosomal proteins, as well as by the U3 small nucleolar ribonucleoprotein (snoRNP) and ≈70 non-ribosomal factors, to form the large 90S pre-ribosomal particle [3]. This process involves the recruitment of smaller multiprotein subunits that associate to the nascent transcript in a stepwise manner [4], [5], [6]. The 35S pre-RNA then undergoes serial cleavages at the A0, A1 and A2 sites to generate the 20S and 27SA2 pre-rRNAs (Fig. 1A) [1], [2]. These three cleavages can also occur co-transcriptionally within the so-called small subunit (SSU) processome, a complex very similar in composition to the 90S pre-ribosome [7], [8]. The disassembly of the 90S pre-ribosome leads to the formation of pre-40S and pre-60S particles containing the 20S and the 27SA2 pre-rRNAs, respectively [2], [9]. This process is accompanied by the release of the non-ribosomal components originally present in the 90S pre-ribosome and the rapid degradation of processing byproducts [2].
Fig. 1. Defects in Rrp12 function block the synthesis of 40S subunits but not of 60S subunits. 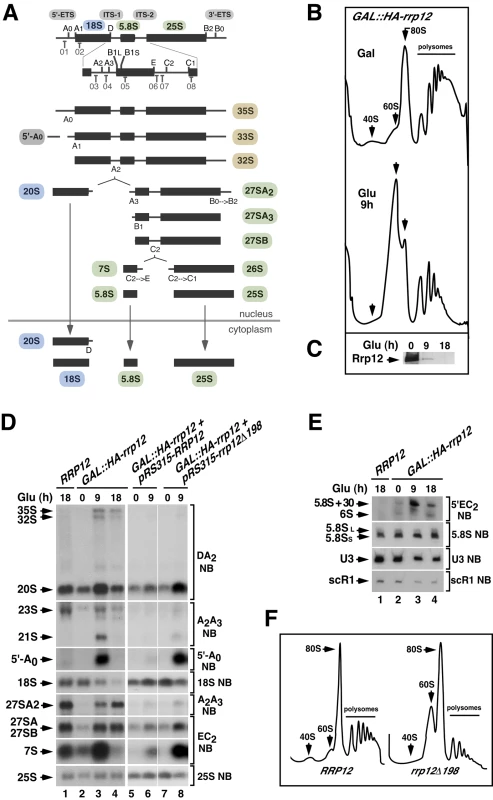
(A) Structure of the 35S pre-rRNA and major intermediates of the rRNA processing pathway. The names of the initial pre-rRNA species are highlighted in brown. Those for the 18S pre-rRNA precursor, 5.8S/25S precursors, and 5′-A0 processing byproduct are highlighted in blue, green and grey, respectively. For simplicity, an alternative pathway to form 27SBL pre-rRNA is not shown. Binding sites for oligonucleotide probes (01 to 08) used in Northern blot experiments are indicated in the upper diagram. Those included probe 03 for the DA2 region, probe 04 for the A2–A3 region, probe 01 for the 5′-A0 region, probe 02 for the 18S region, probe 07 for the E-C2 region, probe 08 for the 25S region, probe 06 for the 5′EC2 region and probe 05 for the 5.8S region. (B) Sucrose-gradient sedimentation analysis of ribosomal fractions (40S, 60S, 80S and polysomes) of cell lysates from GAL::HA-rrp12 cells that have been grown in galactose (Gal)-containing media or shifted to a glucose (Glu)-containing media for 9 hours. Depletion of Rrp12 protein was analyzed by Western blot (C). (D and E) Northern blot (NB) analysis of total RNAs extracted from RRP12, GAL::HA-rrp12, and GAL::HA-rrp12 cells containing plasmids encoding either wild type Rrp12 or the hypomorphic Rrp12 (deletion Δ1-198) mutant. Cells were grown at 30°C in galactose-containing media and shifted to glucose-containing media for the indicated times. The specific region of the 35S pre-rRNA recognized by the Northern blot probe is indicated on the right. This will be similarly indicated in the rest of analyses presented in this work. The thin white lines between lanes 6 and 7 indicate the presence of in-between lanes in the same blot that have been removed. The experiment shown in E also includes, as a loading control, the RNA of the signal recognition particle scR1. (F) Sucrose-gradient sedimentation analysis of ribosomal fractions (40S, 60S, 80S and polysomes) of cell lysates from GAL::HA-rrp12 cells containing plasmids encoding either wild type Rrp12 or the hypomorphic Rrp12 (Δ1-198) mutant. Cells were grown continuously in glucose-containing media. The early pre-60S particles contain >40 associated factors, and undergo multiple maturation steps that are accompanied by major changes in composition until exiting the nucleus [2], [10], [11], [12]. In contrast, the early pre-40S particles are thought to have a relatively low compositional complexity and are rapidly exported, consistent with the fact that the 20S pre-rRNA is not further processed inside the nucleus (Figure 1A) [2], [10], [11], [13]. Final maturation of 40S subunits, which includes a proofreading step through association to 60S subunits and the cleavage of the 20S pre-rRNA at site D, takes place in the cytoplasm [14], [15], [16], [17]. Due to the rapid kinetics of transit thorough the nucleoplasm, it is assumed that the major events of pre-40S particle assembly take place concurrently with the 35S pre-RNA cleavage steps in the nucleolus. Despite this, the pre-40S particles released from 90S pre-ribosomes have to undergo some transformations before leaving the nucleus. These include the recruitment of factors that will participate in cytoplasmic maturation processes as well as transport proteins involved in particle transit through nuclear pores [18], [19]. Pre-40S particles are also known to undergo a kinase-dependent conformational rearrangement that might be required for nuclear export [20].
Despite the significant progress in the understanding of the compositional changes that take place between 90S and pre-40S pre-ribosomes, there are still many questions about the nucleolar assembly and nuclear maturation of 40S subunits that remain unanswered. For example, it is still unclear how the early pre-40S particles are assembled within the 90S pre-ribosome and how similar they are, at the structural level, to the pre-40S particles that reach the cytoplasm. It is also unknown how and when pre-40S particles become competent for export, and how the export process itself takes place. The Ran GTPase and the Crm1 exportin are both essential for pre-40S particles to exit the nucleus [19], [21], but the factors or mechanisms that mediate their interaction with those particles are not known. Tackling these questions has been difficult so far due to the large number of components involved, the transient nature of nucleoplasmic transit and nuclear exit, and the lack of success in dissecting these activities in separable or mechanistically simple steps.
Rrp12 is a karyopherin-like protein previously described as essential for the export of pre-40S and pre-60S particles out of the nucleus [22]. In addition, Rrp12 has been found to facilitate ribosome-unrelated nuclear import processes [23]. In relation with the role of Rrp12 in pre-ribosome export, it is presently unclear whether such function is due to an implication in the assembly of pre-ribosomal complexes, their maturation in the nucleus, the actual transport event, or compound roles in some of the above processes. To address those issues, we studied in detail the consequences of a partial or total loss of function of Rrp12 in S. cerevisiae. Our results show that Rrp12 is required for nuclear export of pre-40S particles. However, and unlike previously-published observations, we found that Rrp12 is not essential for 60S subunit production. During the course of our experiments, we additionally uncovered that this protein, together with the Crm1 exportin, is important for the last processing events of the 35S pre-rRNA within the 90S pre-ribosome, and for the rapid elimination of the 5′-A0 byproduct. The characterization of these new roles indicates that the completion of assembly and the nuclear export of the pre-40S particle are intertwined processes.
Results
Rrp12 is primarily required for the synthesis of 40S ribosomal subunits
A previous report described that Rrp12 was required for export of both pre-40S and 60S ribosomal subunits from the nucleus to the cytoplasm [22]. However, we observed using a yeast strain with the RRP12 gene under a galactose-inducible promoter (GAL::HA-rrp12) that this protein was specifically involved in the biosynthesis of 40S subunits. Evidence in favor of such conclusion included: (i) Polysome profile analyses showing that the loss of Rrp12 was associated with reductions in the content of free 40S subunits and polysomes, but not of free 60S subunits (Figure 1B and Figure 1C). In fact, the relative abundance of the large subunits was clearly increased in the absence of Rrp12 (Figure 1B and Figure 1C). (ii) Northern blot analyses demonstrating a decrease in the steady-state amount of the 18S rRNA (present in 40S subunits) but not in those of the 5.8S and 25S rRNAs (present in 60S subunits) in Rrp12-depleted cells (Figure 1D, left panels; and Figure 1E). Such alterations were found to be associated with an increase in the abundance of the 20S pre-rRNA, the immediate upstream precursor for the 18S rRNA (Figure 1D; see scheme in Figure 1A), indicating that the cleavage at site D is inhibited. Consistent with previously published data [22], we also observed some accumulation of the 35S and 32S pre-rRNAs, a reduction in the content of the 27SA2 pre-rRNA, and the generation of the aberrant 21S pre-rRNA (a species produced from direct cleavage of the 32S pre-rRNA at site A3) (Figure 1D; see scheme in Figure 1A). These results indicate that, in addition to the major defect in the cleavage at site D, the loss of Rrp12 causes partial defects in the early cleavages at sites A0 and A1 and, to a larger extent, at site A2. We also detected a delay in the processing events of 5.8S rRNA precursors manifested by the presence of both the 7S pre-rRNA and aberrant 3′-extended forms of the 5.8S rRNA (5.8S+30) in Rrp12-depleted cells (Figure 1D and Figure 1E). Curiously, we found that the absence of Rrp12 led to an increase in the abundance of the 5′-A0 fragment (Figure 1D), a byproduct produced when the rRNA precursor is cleaved at site A0 (Figure 1A). Similar defects, although milder in intensity, were observed in a constitutive manner when pre-rRNA analyses were performed in a yeast strain (rrp12-Δ198) expressing a hypomorphic version of Rrp12 (Figure 1D, right panels; Figure 1F). Taken together, these data indicate that Rrp12 is absolutely required for the generation of the 18S rRNA from 20S pre-rRNA and, in addition, important for both the rapid elimination of the 5′-A0 fragment and the normal processing of both 32S and 5.8S pre-rRNA precursors. Despite this latter function, Rrp12 does not seem to have any major influence on the overall production of 60S ribosomal subunits.
Rrp12 is present in both 90S and pre-40S particles
Our group and others have previously shown that Rrp12 copurifies with components of 90S and pre-40S particles [3], [18], [22], [23]. However, there is no detailed information about its relative content in different subsets of pre-40S complexes. Using coimmunoprecipitation experiments, we observed that endogenous Rrp12 interacted with green fluorescent protein (GFP)-tagged versions of factors present in nucleolar 90S (Pwp2, Enp1, Dim1, Pno1; Figure 2A and Figure 2B, lanes 1 to 4 and lanes 19 to 22) and nucleoplasmic pre-40S (Enp1, Dim1, Pno1, Tsr1; Figure 2A and Figure 2B, lanes 3 to 6 and lanes 19 to 22) particles. These interactions took place within the context of ribonucleproteic complexes, because they were eliminated by RNase treatment (Figure 2B, lanes 1 to 6 and lanes 19 to 22). By contrast, we did not detect any association of Rrp12 in these experiments with either Nob1 or Rio2, two proteins mostly present in cytoplasmic pre-40S particles (Figure 2A and Figure 2B, lanes 7,8 and lanes 23,24). Rrp12 did show an interaction with Ltv1, a protein that, like Nob1 and Rio2, is mainly detected in cytoplasmic pre-40S complexes (Figure 2A and Figure 2B, lanes 25,26). This interaction is the only one that cannot be disrupted by RNase treatment (Figure 2B, lanes 25,26), indicating that it survives pre-40S particle disassembly or, alternatively, that takes place outside those particles. In agreement with the results presented in Figure 1, we could not detect interactions of Rrp12 with proteins present in early (Ssf1, Nop7; Figure 2A and Figure 2B, lanes 9 to 12), intermediate nuclear (Rix1; Figure 2A and Figure 2B, lanes 13,14), late nuclear (Arx1; Figure 2A and Figure 2B, lanes 15,16) or cytoplasmic (Kre35; Figure 2A and Figure 2B, lanes 17,18) pre-60S complexes. These results suggest that Rrp12 is predominantly associated to both nucleolar and nuclear pre-40S pre-ribosomes while it is weakly associated, or not bound at all, to the cytoplasmic ones. Further analyses of Rrp12-MYC immunoprecipitates by Northern blot confirmed the predominant presence of this protein in the 40S synthesis pathway and, in addition, evidenced that its interactions with nucleolar and nucleoplasmic particles exhibit differential features. Thus, we observed that the association of Rrp12 to pre-40S particles had to be rather strong, as inferred by the stable coimmunoprecipiation of the 20S pre-rRNA with Rrp12-MYC (Figure 2C). Indeed, the amount of this pre-RNA in those complexes is even higher than that seen in the case of immunoprecipitations performed with Tsr1, a factor that stably associates with both nucleolar - and cytoplasmic-located pre-40S particles (Figure 2A and Figure 2C). By contrast, we could not detect any significant amount of 35S and 32S pre-RNAs in the Rrp12-MYC immunoprecipitates, suggesting that the association with the 90S particle is either labile or restricted to a minor pool of Rrp12-containing complexes (Figure 2C). As control, we found that these two pre-RNAs do coimmunoprecipitate with Pwp2 (Figure 2C), an integral component of the 90S pre-ribosome (Figure 2A). Consistent with the lack of Rrp12 in the purifications of pre-60S complexes (see above Figure 2B), we could not observe any interaction of Rrp12-MYC with the 27S or 7S pre-rRNAs. As expected, these two pre-rRNAs do coimmunoprecipitate with the early pre-60S particle component Nop7-MYC (Figure 2D, see scheme in Figure 2A). These results indicate that Rrp12 does not stably associate with pre-60S particles.
Fig. 2. Rrp12 is present in both 90S pre-ribosomes and pre-40S particles. 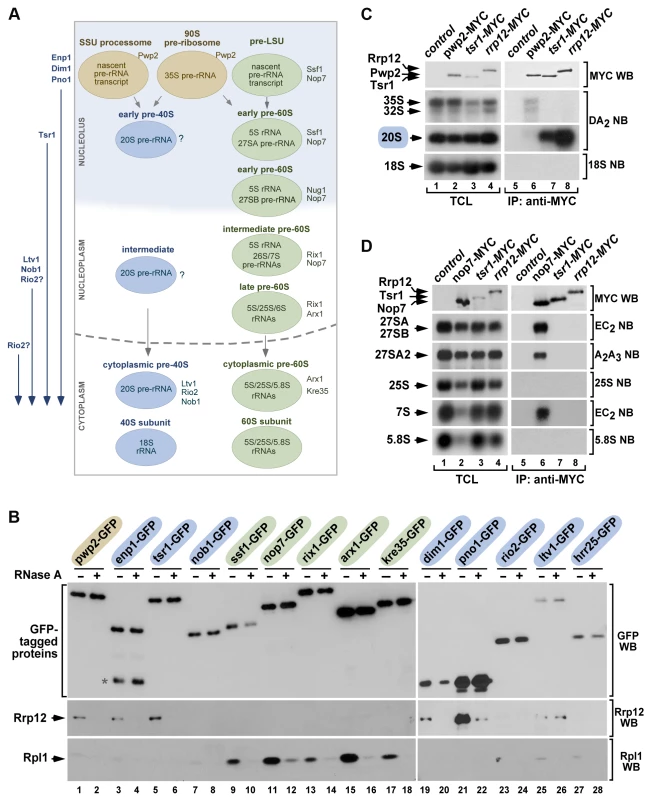
(A) Scheme of the maturation of pre-ribosomes. The names of specific factors frequently used for purifying each pre-ribosome are indicated on the right. In rapidly growing cells, ∼60% of primary transcripts are cleaved at A0–A1–A2 co-transcriptionally within the small subunit (SSU) processome and, after this, the precursor of the large subunit (pre-LSU) is assembled onto the nascent pre-rRNA. When not cleaved co-transcriptionally, the full-length 35S pre-rRNA is assembled into the 90S pre-ribosome, a particle very similar to the SSU-processome. The order of incorporation of the seven major maturation factors present in cytoplasmic pre-40S particles is shown on the left. Enp1, Dim1 and Pno1 are recruited to 90S/SSU particles. Tsr1 is recruited to early pre-40S particles in the nucleolus. Ltv1 and Nob1 join pre-40S particles in the nucleus. The step of incorporation of Rio2 remains ill defined. (B) Western blot analysis showing coimmunoprecipitation of Rrp12 (second panels from top) and of the control protein Rpl1 (bottom panels) with the indicated 90S pre-ribosome and nuclear pre-40S factors (top) in the presence (+) or absence (−) of RNase A in cell lysates. Factors present in 90S, pre-40S and pre-60S particles are shaded in brown, blue and green, respectively. The amount of GFP-Trap purified bait is shown in the first panels from top. The asterisk indicates a protein species in the Enp1-GFP purification lane that probably corresponds to a partial degradation product. (C and D) Northern blot analysis showing coimmunoprecipitation of pre-rRNA species (second to bottom panels on the right) with the indicated MYC-tagged proteins in normal cells. As control, parallel Northern blots were performed on total RNAs prepared from the same total cell lysate samples used for the immunoprecipitations (second to bottom panels on the left). Western blot experiments were performed to analyze the amount of MYC-tagged protein present in the total cell lysates (top panel on the left) and immunoprecipitations (top panel on the right). TCL, total cell lysates. IP, immunoprecipitation. Rrp12 is not required for pre-40S particle assembly
We next focused on the cause of the block in the maturation of 20S pre-rRNA to 18S rRNA found in Rrp12-depleted cells. Given the restricted presence of Rrp12 to nucleolar 90S and nucleoplasmic pre-40S complexes, this phenotype could be due to defects in the assembly of the pre-40S particle inside the nucleus. However, this does not seem to be the case because the depletion of Rrp12 does not affect the stability of both early and late nuclear pre-40S components (Enp1, Dim1, Tsr1, Rio2, Nob1; Figures 3A to 3C; top panels). Likewise, it does not block the interaction of those proteins with the 20S pre-rRNA (Figures 3A to 3C; bottom panels). However, the depletion of Rrp12, although not affecting the steady state levels of Ltv1 in cell lysates prepared by TCA precipitation (Figure 3D, compare lanes 7 and 9), does cause a destabilization of that protein under the conditions used for the pre-rRNA coimmunoprecipitation analyses (Figure 3C; top panel, compare lanes 4 and 6). Such behavior may reflect a functional relationship of Rrp12 and Ltv1 in vivo, because we observed using sucrose gradient fractionation experiments that the loss of Rrp12 leads to a substantial decrease in the amount of Ltv1 that is stably incorporated onto ∼40S complexes (Figure S1A). This effect is specific, because the depletion of Rrp12 does not affect the incorporation of both Enp1 and Rio2 onto those complexes (Figure S1B and Figure S1C).
Fig. 3. Rrp12 is not involved in pre-40S particle assembly. 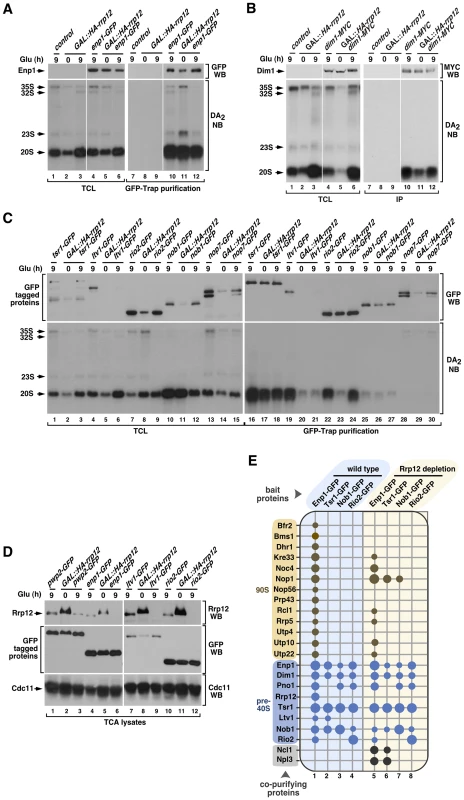
(A to C) Bottom panels, Northern blot analysis showing coimmunoprecipitation of the 20S pre-rRNA with Enp1-GFP (A), Dim1-MYC (B), Tsr1-GFP (C), Ltv1-GFP (C), Rio2-GFP (C), Nob1-GFP (C) and Nop7-GFP (C) before (0) and upon depletion of Rrp12 for 9 hours. Top panels, Western blot analysis showing the amount of immunoprecipitated proteins in these experiments. Mobility of pre-RNA species is indicated on the left of each bottom panel. Antibodies used in the immunoblots and Northern blot probes are shown on the right of the top and bottom panels, respectively. The thin white lines between lanes 3 and 4, and 9 and 10, shown in A and B, indicate the presence of in-between lanes in the same blot that have been removed. (D) Western blot analyses of trichloroacetic acid (TCA) precipitated cell lysates showing the amount of Rrp12 (top panels) and the indicated GFP-tagged proteins (middle panels) under the indicated growth conditions. The amount of Cdc11 was used as loading control (bottom panel). (E) Pre-ribosomal factors (listed on the left) copurifying with the indicated GFP-tagged proteins (top) in the presence (columns 1 to 4) or absence (columns 5 to 8) of Rrp12. Copurification of a factor with the bait is indicated with a dot. For Rrp12 depletion, GAL::HA-rrp12 cells were shifted from galactose-containing media to glucose-containing media for 12 hours. The pre-ribosomal particles that contain the prey proteins are indicated on the left. Size of dots represents the relative amount of coimmunoprecipitated protein in each case (see Materials and Methods). Mass spectrometry experiments further confirmed that the absence of Rrp12 does not have a major effect in the composition of pre-40S complexes. Indeed, we found that both the pattern and strength of the associations exhibited by four pre-40S factors (Enp1, Tsr1, Nob1 and Rio2) with the rest of major pre-40S particle components are quite similar to those observed in wild-type cells (Figure 3E, compare columns 1 to 4 with columns 5 to 8). The only exception observed is the loss of the interaction of both Enp1 and Tsr1 with Ltv1 (Figure 3E, compare columns 1 and 2 with columns 5 and 6), a defect probably derived from the impaired recruitment of Ltv1 to the pre-40S particle seen in above experiments. Interestingly, we observed that the loss of Rrp12 promotes the formation of new interactions of both Enp1 and Tsr1 with the tRNA methyltransferase Ncl1 and the abundant hnRNP protein Npl3 (Figure 3E, compare columns 1 and 2 with columns 5 and 6). Likewise, Tsr1 and Nob1 interact with the 90S particle component Nop1 (Figure 3E, compare columns 2 and 3 with columns 6 and 7). These results indicate that Rrp12 is not required for the formation of the core structure of the pre-40S particle, although it may contribute to the release of specific nucleolar factors such as Nop1. In addition, they show that Rrp12 appears to be dispensable for the recruitment of some factors with hitherto unknown roles in the synthesis of 40S subunits (i.e., Ncl1, Npl3). Also consistent with a correct particle assembly in the absence of Rrp12, we found using Western blot analyses that Prp43 and Mex67 [24], [25], [26], [27], two factors that are not usually detected in this type of proteomics analysis due to their weak interaction with pre-40S particles, remain particle-associated in the absence of Rrp12 (Figure S2). Interestingly, the absence of Rrp12 does promote a reduction in the association of Enp1 with some, but not all, of its usual partners within the 90S particle (Figure 3E, compare columns 1 and 5). These data indicate that the lack of Rrp12 may affect either the composition or maturation dynamics of 90S pre-ribosomes.
Rrp12 is required for nuclear export of pre-40S particles
The above findings indicated that the lack of Rrp12 blocks the 40S synthesis pathway at a step downstream the assembly of pre-40S particles. To investigate if this block occurred in the nucleolus, nucleoplasm or cytoplasm, we analyzed the subcellular localization of GFP-tagged versions of pre-40S particle (Enp1, Dim1, Pno1, Tsr1, Ltv1, Nob1, Rio2) and mature 40S subunit (Rps2) components in control and Rrp12-depleted cells. Consistent with previous reports [18], [28], [29], [30], [31], we found that these proteins exhibit nucleolar (Enp1, Figure 4A), nucleolar and nucleoplasmic (Dim1, Tsr1; Figure 4B and Figure 4C, respectively), and nucleoplasmic plus cytoplasmic (Pno1, Ltv1, Nob1, Rio2 and Rps2; Figures 4D to G, and Figure S3, respectively) localizations in both wild type cells and control GAL::HA-rrp12 cells. However, in Rrp12-depleted GAL::HA-rrp12 cells, we detected that most of those proteins undergo a major relocalization towards the nucleoplasm (Figures 4A to G; and Figure S3). The only exception was again Ltv1, since its subcellular distribution is fully Rrp12-independent (Figure 4E). The nuclear accumulation of Rio2, but not of the cytosolic Pgk1 control protein, in the absence of Rrp12 was demonstrated using independent subcellular fractionation experiments (Figure 4H). This effect is specific for the 40S subunit synthesis pathway, because the loss of Rrp12 does not alter the normal subcellular distribution of Rpl25 and Rpl11 (Figure S3), two 60S subunit components. These results show that pre-40S particles are blocked in the nucleoplasm when Rrp12 is absent. Collectively, our data indicate that Rrp12 does not participate in the major assembly events of pre-40S particles in the nucleus, and that it is essential for some event that immediately precedes or is concomitant to nuclear export.
Fig. 4. Rrp12 is required for the export of pre-40S particles out of the nucleus. 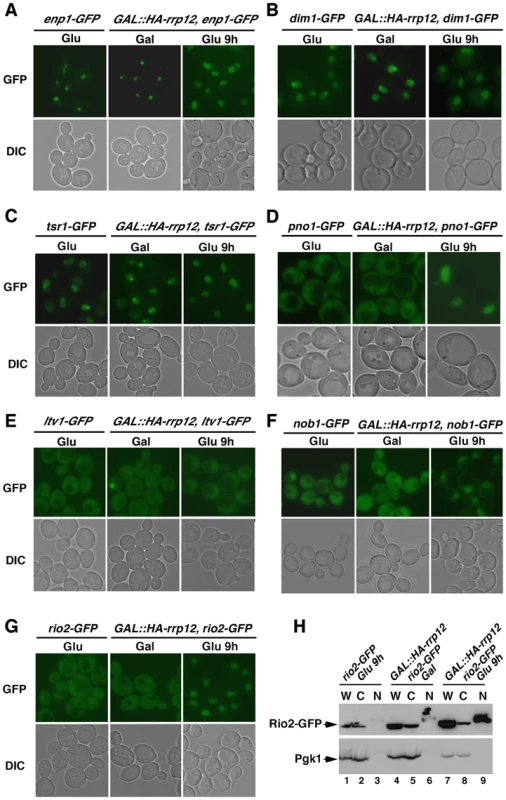
(A to G) Top panels, epifluorescence microscopy analysis of the subcellular distribution of GFP-tagged Enp1 (A), Dim1 (B), Tsr1 (C), Pno1 (D), Ltv1 (E), Nob1 (F) and Rio2 (G) in the indicated yeast strains and culture conditions (top). Bottom panels, differential interference contrast (DIC) images of the above preparations. (H) Western blot analysis showing the distribution of Rio2-GFP (top panel) and Pgk1 (bottom panel) in whole cell lysates (W), cytosolic (C) and nuclear (F) fractions obtained from either control rio2-GFP cells (lanes 1 to 3) or GAL::HA-rrp12/rio2-GFP cells growing in galactose-containing medium (lanes 4 to 6) or upon a shift to glucose-containing medium for 9 hours (lanes 7 to 9). Rrp12 influences an intermediate maturation step within a 90S transitional particle
In addition to the block in pre-40S particle export, the depletion of Rrp12 causes defects in the cleavage of the pre-rRNA at site A2 and in the elimination of the 5′-A0 fragment. The accumulation of this byproduct appears to be a rather specific feature, because it is not observed upon depletion of other factors, like Pno1, that do not affect the A0 cleavage but are essential for the A2–A3 cleavages (Figure S4A). We also found that the 5′-A0 fragment associates to Rrp12 in wild type cells (Figure S5), suggesting that Rrp12 might influence directly the elimination of this fragment. As a first approximation to obtain clues about the role of Rrp12 in this process, we decided to study the sedimentation behavior of the 5′-A0 fragment on sucrose gradients in the presence and absence of Rrp12. These experiments corroborated the increase in the abundance of the 5′-A0 fragment already seen by Northern blot analyses in Rrp12-depleted cells (see above, Figure 1D) and, in addition, revealed that this fragment was present in complexes that sediment broadly between the 60S and 90S regions of the gradient (Figure 5A; right panels, gradient fractions 12 to 15). A significant proportion of these entities cosedimented with the 32S pre-rRNA and U3 snoRNA (Figure 5A; right panels, gradient fractions 14,15), suggesting that they form part of a 90S transitional particle that has initiated, but not completed, the processing of the 35S pre-rRNA. This interpretation is consistent with the delay in the A2 cleavage evidenced by the formation of aberrant 21S pre-rRNA (see above, Figure 1D), and the increased coimmunoprecipitation of the 5′-A0 fragment with the 90S pre-ribosome-specific Pwp2 protein in Rrp12-depleted cells (Figure 5B, compare lanes 10 and 12). The interaction of Pwp2 with the 5′-A0 fragment appears to take place in the context of a 90S pre-ribosome-like particle, as inferred from the presence of Pwp2 in 80–90S complexes in Rrp12-depleted cells (Figure 5C). In agreement with an abnormal accumulation of a 90S transitional particle, we observed by microscopy experiments that Pwp2 shifted from an exclusively nucleolar localization to a more disperse distribution between the nucleolus and the nucleoplasm upon depletion of Rrp12 (Figure 5D). These results indicate that the loss of Rrp12 delays some event during pre-40S particle assembly in the nucleolus, leading to both the accumulation and delocalization of 90S transitional particles in the nucleoplasm.
Fig. 5. Loss of Rrp12 causes accumulation of 5′-A0-containing 90S pre-ribosomes. 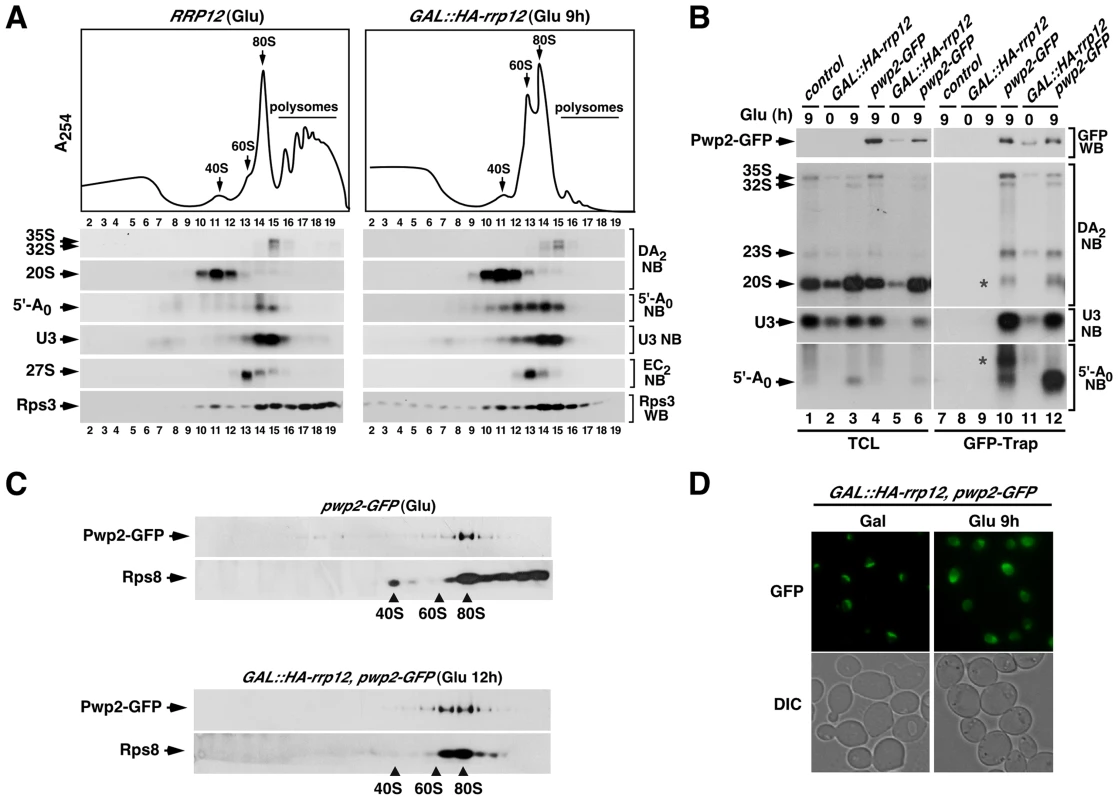
(A) Top panel, sucrose-gradient sedimentation analysis of ribosomal fractions (40S, 60S, 80S and polysomes) of cell lysates from the control wild type strain grown in glucose-containing media, and the GAL::HA-rrp12 strain grown in galactose-containing media and shifted to glucose-containing media for 9 hours. Bottom panels, Northern (second to sixth panels from top) and Western (bottom panel) blot analyses of indicated components of pre-ribosomal particles in fractions obtained in the gradients. Numbers of fractions are shown at the bottom. Blotting probes and antibodies are indicated on the right. (B) Northern blot analysis showing copurification (second to fourth panels on the right) of the indicated pre-RNA species, U3 snoRNA and 5′-A0 fragment with Pwp2-GFP in the indicated yeast strains and culture conditions (top). As control, parallel Northern blots were performed on total RNAs prepared from the same total cell lysate samples used for the immunoprecipitations (second to third panels on the left). Western blot experiments were performed to analyze the amount of Pwp2-GFP present in the total cell lysates (top panel on the left) and GFP-Trap purified complexes (top panel on the right). Asterisks indicate pre-rRNA species that do not correspond to any major processing intermediate, which probably are 35S partial degradation products. (C) Sucrose gradient analysis showing the sedimentation behavior of Pwp2-GFP and Rps8 in the presence (top two panels) and absence (bottom two panels) of Rrp12. The positions of the gradient where 40S, 60S and 80S complexes sedimented are indicated by arrows. (D) Top panels, epifluorescence microscopy analysis of the subcellular distribution of Pwp2-GFP before (top left panel) and upon depletion (top right panel) of Rrp12. Bottom panels, DIC images of above preparations. The Rrp12-dependent maturation step precedes the A2 cleavage and the exosome-mediated degradation of the 5′-A0 fragment
We next characterized by mass spectrometry the complexes formed by Pwp2 in the absence of Rrp12 to investigate possible differences in the composition of 90S pre-ribosomes. Although highly similar to those formed in control cells, we observed the presence of new Pwp2 partners in the absence of Rrp12 (Figure 6A). Those included 90S pre-ribosome components involved in the cleavage of the 35S precursor at the A0–A1–A2 (Utp20, Rcl1) and A1–A2 (Dim1, Pno1) sites [29], [32], [33], [34], [35], [36]. Interestingly, we observed using RNA coimmunoprecipitation experiments that two of the above partners, Dim1 and Pno1, preferentially bind the 32S rather than the earliest 35S pre-rRNA (Figure 6B). This suggests that they become stably assembled onto the 90S pre-ribosome upon cleavage of the 35S precursor at the A0 and A1 sites (see above, Figure 1A). We also found among the new partners the nuclease Rrp44 (also known as Dis3), an exosome subunit shown to be involved in the direct physical interaction with the 5′-A0 fragment [37]. This finding was quite interesting for us, because previous results have shown that this interaction seems to be crucial for poising the 5′-A0 fragment for productive degradation [37], [38]. Thus, we surmised that the Rrp44-Pwp2 interaction detected in Rrp12-depleted cells could indicate that the exosome is normally recruited to 90S pre-ribosomes and that, in the absence of Rrp12, there is an enrichment or stabilization of some of those exosome-containing 90S pre-ribosomes. In agreement with this idea, we found using sucrose gradient sedimentation analyses that Rrp44 is indeed present in 80–90S complexes both in control and Rrp12-depleted cells (Fig. S1D). These data raised the possibility that the defect in the elimination of 5′-A0 fragment found in Rrp12-depleted cells could be due to an impairment of exosome function. Consistent with this idea, we found that the elimination of the exosome cofactor Mtr4 (also known as Dob1) elicited the expected accumulation of the 5′-A0 fragment (Figure 6C and Figure 6D) [39] and, most importantly, that such accumulation occurs in the context of 80–90S complexes, similarly to what is observed in Rrp12-depleted cells (Figure 6D; see above, Figure 5). Interestingly, Rrp12-depleted cells do not exhibit the sustained high levels of the 5′-A0 fragment seen in Mtr4-depleted cells (Figure 1D and Figure 6C), indicating that the exosome activity is affected but not fully compromised upon the loss of Rrp12. Consistent with this, we have seen that the loss of this protein does not trigger other terminal defects typically observed in exosome-deficient cells, such as the abnormal accumulation of the 35S pre-rRNA, the total block of 7S pre-rRNA maturation, and the balanced decrease in the contents of both ribosomal subunits (Figure 6C and Figure 6D) [39], [40]. Taken together, our data indicate that the loss of Rrp12 causes a 90S pre-ribosome maturation defect that precedes the A2 cleavage and the exosome-dependent 5′-A0 fragment degradation steps. As a result, it promotes either a delay or partial inhibition, but not a block, in the A2 cleavage of the pre-rRNA and the elimination of the 5′-A0 fragment.
Fig. 6. Rrp12 is required at a 90S particle-mediated maturation step that precedes exosome action. 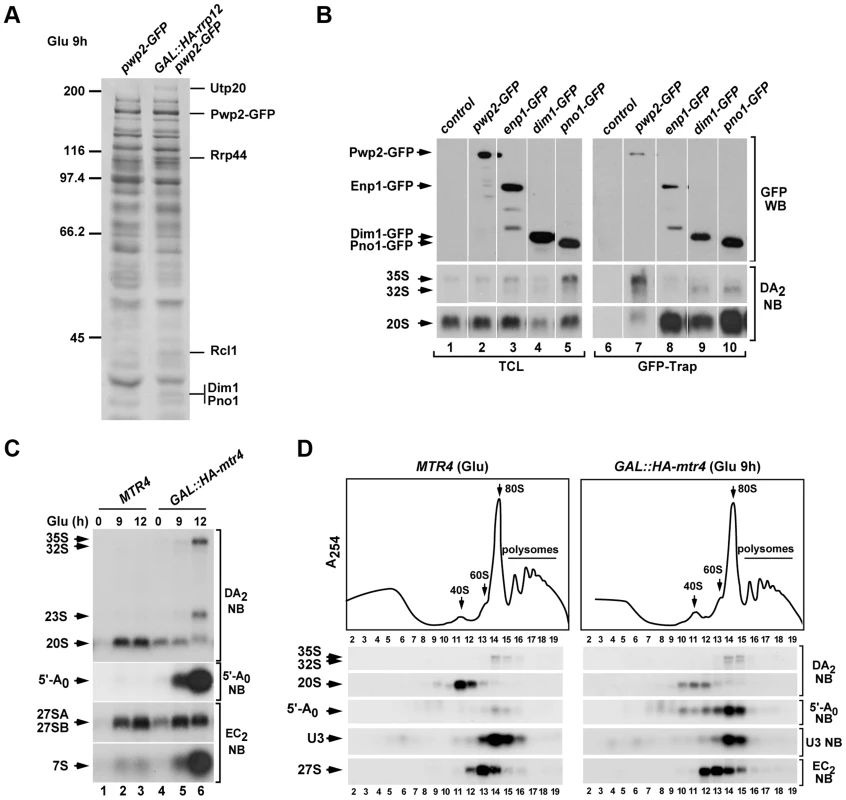
(A) Protein complexes formed by Pwp2-GFP in control and Rrp12-depleted cells. Bands and proteins identified by mass spectrometry are indicated on the right. Molecular weight markers (in kDa) are indicated on the left. (B) Northern blot analysis showing copurification (lanes 6 to 10) of the indicated pre-RNA species with GFP-tagged Pwp2, Enp1, Dim1 and Pno1 in normal cells. As control, parallel Northern blots were performed on total RNAs prepared from the same samples used for the immunoprecipitations (second and third panels on the left). Western blot experiments were performed to analyze the amount of the GFP-tagged protein present in the corresponding total cell lysates (top panel on the left) and GFP-Trap purifications (top panel on the right). (C) Northern blot analysis of total RNAs extracted from MTR4, and GAL::HA-mtr4 cells to show the relative contents of pre-rRNA species and 5′-A0 fragment. Cells were grown at 30°C in galactose-containing media and shifted to glucose-containing media for the indicated times. Northern blot probes are indicated on the right. (D) Top panel, sucrose-gradient sedimentation analysis of ribosomal fractions (40S, 60S, 80S and polysomes) of cell lysates from control (MTR4) and Mtr4-depleted (GAL::HA-mtr4 (Glu 9 h)) strains. Bottom panels, Northern blot analysis of indicated components of pre-ribosomal particles in gradient fractions obtained in the above experiment. Numbers of fractions are shown at the bottom. Blotting probes and antibodies are indicated on the right. The Crm1 exportin is also involved in the Rrp12-dependent 90S pre-ribosome maturation step
Given the implication of Rrp12 in the export of pre-40S particles (see above, Figure 3 and Figure 4), we decided to investigate whether the pre-40S export step was associated to the Rrp12-dependent 90S pre-ribosome maturation step. If that were the case, we expected that the elimination of any other protein involved in pre-40S export would induce the same defects seen in Rrp12-depleted cells. To test this idea, we chose a yeast strain that constitutively expressed a mutant version of the Crm1 (Crm1T539C) exportin. This mutant protein, unlike its wild type counterpart, can be specifically inhibited by leptomycin B [41]. Using this strategy, we found that the inhibition of Crm1 recapitulates all the defects observed in Rrp12-depleted cells, including increased abundance of the 35S, 32S and 21S pre-RNA species (Figure 7A), abnormal levels of the 5′-A0 fragment (Figure 7A and Figure 7B), accumulation of this fragment in 80–90S complexes (Figure 7B) and, as expected [19], an increase in the content of the 20S pre-rRNA due to the halt in pre-40S particle nuclear export (Figure 7A). These results indicate that the 40S subunit export machinery facilitates a late 90S pre-ribosome maturation event that promotes the rapid cleavage of the pre-rRNA at site A2 and the efficient degradation of the 5′-A0 fragment. This function is quite specific for export regulators, because the elimination of factors specifically involved in the cytoplasmic maturation of pre-40S complexes (Rio2 and Ltv1) does not trigger any of the above defects [16], [28], [42] (Figure S4B).
Fig. 7. Crm1 participates in the Rrp12-mediated 90S maturation step. 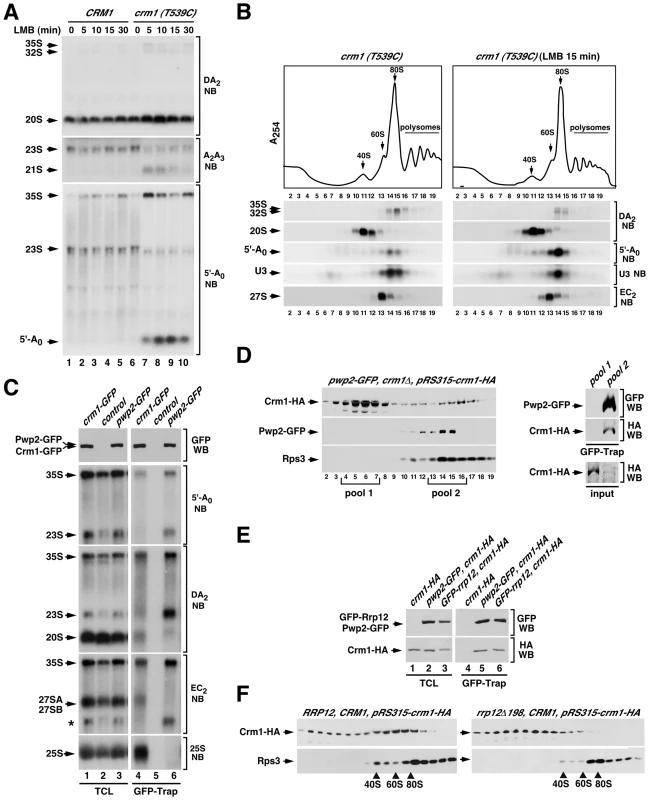
(A) Northern blot analysis showing the amount of the indicated pre-RNA intermediaries and 5′-A0 byproduct (left) in control CRM1 and mutant crm1 (T539C) strains treated with leptomycin B (LMB) for the indicated periods of time (top). (B) Top panel, sucrose-gradient sedimentation analysis of ribosomal complexes (40S, 60S, 80S and polysomes) of cell lysates from crm1 (T539C) cells that were either nontreated (left panels) or treated (right panels) with leptomycin B for 15 min. Bottom panels, Northern blot analysis of the indicated pre-rRNA species in the gradient fractions. Numbers of fractions are shown at the bottom. Blotting probes are indicated on the right. (C) Northern blot analysis showing copurification (second to fifth panels on the right) of the indicated pre-RNA species with GFP-tagged Crm1 (lane 4) and GFP-tagged Pwp2 (lane 6) in normal cells. Control samples were wild-type cells expressing endogenous untagged Crm1 and Rrp12. Parallel Northern blots were performed on total RNAs prepared from the same total cell lysate samples used for the purifications (second to fifth panels on the left). Western blot experiments were performed to analyze the amount of Crm1-GFP and Pwp2-GFP present in the total cell lysates (top panel on the left) and GFP-Trap purified complexes (top panel on the right). The asterisk in the EC2 blot indicates the signal of the 23S pre-rRNA from previous hybridization with the DA2 probe. (D) Sucrose gradient analysis of Crm1-HA, Pwp2-GFP and Rps3 in pwp2-GFP/crm1Δ cells containing a pRS315-crm1-HA plasmid. Gradient fractions were analyzed by Western blot with anti-HA, anti-GFP and anti-Rps3 (left three panels). The right panels show copurification of Crm1-HA with GFP-Trap purified complexes from pooled fractions of the 80–90S gradient region (pool 2). A GFP-Trap purification on pooled fractions of the 10–20S gradient region (pool 1) was used as a control. Parallel Western blots analyzed the amount of Crm1-HA in each one of the pool samples used for the GFP-Trap purifications (input) (right bottom panel). (E) Western blot analysis showing copurification of Crm1-HA with GFP-tagged Pwp2 (lane 5) and with GFP-tagged Rrp12 (lane 6) in pwp2-GFP cells containing a pRS315-crm1-HA plasmid (lane 5), and in GFP-rrp12 cells containing a pRS315-crm1-HA plasmid (lane 6), respectively. Parallel Western blots were performed to analyze the amounts of the coimmunoprecipitated proteins in total cell lysates (lanes 1 to 3). (F) Sucrose gradient analysis showing the sedimentation behavior of Crm1-HA and Rps3 in RRP12/CRM1 (left panels) and rrp12-Δ198/CRM1 (right panels) cells containing a pRS315-crm1-HA plasmid. The positions of the gradient where 40S, 60S and 80S complexes sedimented are indicated by arrows. The above results led us to investigate whether Crm1, like Rrp12, was present in 90S pre-ribosomes. We first assessed the potential interaction of Crm1 with two 90S pre-ribosome components, the 35S pre-RNA and Pwp2, using coimmunoprecipitation analyses similar to those that detect Rrp12 in 90S and pre-40S particles (see above Figure 2). This approach however did not reveal associations of Crm1 with any pre-ribosomal component, not even with pre-rRNAs or proteins present in the pre-40S and pre-60S complexes transported by this exportin. We therefore decided to change the experimental conditions of our coimmunoprecipitation assays. In particular, we changed the Triton X-100-containing lysis buffer by a NP-40-containing buffer that was similar to buffers used by others to detect interactors of Crm1 in vivo [43], [44], [45]. Notably, when we purified Crm1-GFP using the NP-40 buffer, we could readily observe that it interacts with the 35S pre-rRNA, the 20S pre-rRNA, 27S pre-rRNAs and the 25S rRNA (Figure 7C). The associations with these RNAs were specific because in the same Northern blots Pwp2-GFP coprecipitated the 35S and 23S pre-rRNAs, but not the 20S, 27S and 25S RNAs. These results indicate that Crm1 binds to pre-40S and pre-60S particles, as expected from its role in export, and also that it is already recruited to early 90S particles. Consistent with this, we found using sucrose gradient sedimentation analysis that Crm1 is indeed present in large 80–90S complexes that co-sediment with Pwp2 (Figure 7D). Furthermore, when 90S pre-ribosomes were purified from sucrose gradients using Pwp2 as bait it was confirmed that they do contain Crm1 (Figure 7D, right set of panels). Western blot analysis of Rrp12-containing complexes from total cell lysates evidenced that Crm1 interacts with Rrp12 (Figure 7E), a result consistent with the common presence of the two proteins in both 90S and pre-40S pre-ribosomes.
In our final set of experiments, we investigated whether the recruitment of Crm1 to 90S pre-ribosomes was Rrp12-dependent. For this purpose we analyzed the sedimentation behavior in sucrose gradients of a HA-tagged version of Crm1 that was coexpressed with the endogenous Crm1 either in wild type or in rrp12Δ198 cells. We found that in wild type cells the Crm1-HA protein is recruited to large assemblies, including 80–90S-like complexes (Figure 7F, left two panels). This sedimentation in large complexes is drastically reduced in rrp12Δ198 cells (Figure 7F, right two panels), suggesting that the incorporation of Crm1 onto large 80–90S pre-ribosomal particles is Rrp12-dependent. Altogether, our data indicate that Rrp12 and Crm1 act on 90S pre-ribosomes in a concerted manner.
Discussion
The results presented here identify Rrp12 as a factor required for a number of intertwined steps of the 40S ribosomal subunit synthesis pathway (Figure 8). We have observed that Rrp12, together with Crm1, is first recruited to the pathway to facilitate the processing of the 35S pre-rRNA and the elimination of the 5′-A0 fragment in the context of a late 90S transitional particle (Figure 8). A lack of Rrp12 or Crm1 at this step delays but does not halt the assembly and release of early pre-40S particles. Interestingly, this early function of Rrp12 occurs immediately upstream and temporally close to the export of the pre-40S particles, a process that absolutely requires Rrp12 and Crm1. In addition to revealing a hitherto unknown role for export-related factors in a specific maturation step in the nucleolus, our results shed light onto the dynamics of 90S pre-ribosome factors upon cleavage of the 35S pre-rRNA at site A2. Indeed, some authors previously suggested that, after the A2 cleavage, the non-ribosomal components of the 90S particle are released en bloc in association with the 5′-A0 fragment [3], [18]. However, the formation of such disassembly complexes, and when and how was the exosome recruited, remained unclear. We find no evidence for the formation of a post-disassembly complex containing the 5′-A0 fragment upon which the exosome acts (Figure 6D). Rather, our results indicate that the exosome is present in transitional 90S pre-ribosomes to degrade the 5′-A0 fragment, either in the last step of pre-40S particle assembly or at the very time of pre-40S particle release (Figure 8). The implication of Crm1 in steps of ribosome synthesis, other than nuclear export, is also a new finding in yeast. In human cells, Crm1 has been implicated in the targeting of snoRNP complexes to the nucleolus [43], [46]. Whether Rrp12 and Crm1 utilize the same domains for the export-related and maturation-related functions, and whether the two proteins need to interact directly to exert their functions, remains to be determined. We have found that Rrp12 and Crm1 purified from bacteria do not stably interact in vitro (unpublished data). However, we cannot exclude the possibility that such interaction could require the participation of other proteins. Indeed, it has been shown before that the interaction of Crm1 with other molecules involves the participation of additional factors, including the Ran GTPase in its GTP-bound state. Ran can in fact be involved in these interactions, as suggested by the identification of allele-specific Ran mutants that elicit defects in the degradation of the 5′-A0 fragment [47]. Based on the present results, we hypothesize that such defects could be associated to the Rrp12 - and Crm1-dependent mechanism reported here. An involvement of Ran on the association of Crm1 with pre-ribosomes could also explain the difficulties for detecting Crm1 in purified 90S and pre-40S pre-ribosomes, because these complexes are normally prepared under conditions that favor the conversion of Ran-GTP to Ran-GDP. Here we describe that, using a buffer that contains 0.2% NP-40, it is possible to detect the specific association of Crm1 to both pre-rRNAs and pre-ribosomal components by coimmunoprecipitation analysis. The reason for the efficiency of this buffer is unclear, but it must somehow favor the maintenance of some Ran-GTP levels and/or affect other currently unknown features that improve the stability or solubilization of Crm1-containing complexes.
Fig. 8. Model for the integration of different processes in the nucleolus during synthesis of 40S subunits. 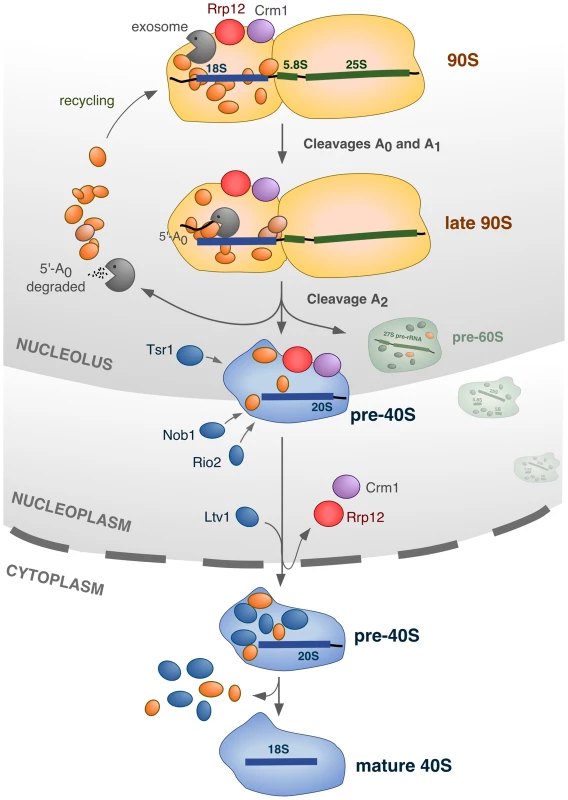
The 90S pre-ribosome contains ∼70 factors (represented in orange) that are specifically required for the cleavage of the primary pre-rRNA at sites A0, A1 and A2, and for the assembly of ribosomal proteins (not represented). In addition, the 90S pre-ribosome engages two other sets of proteins that participate in activities that will be initiated at the time of, or immediately after, the A2 cleavage: the exosome complex, and Rrp12/Crm1. The exosome degrades the 5′-A0 fragment, allowing the liberation and recycling of bound 90S proteins. Rrp12 and Crm1 act as export factors for the released pre-40S particle. The cleavage of the pre-rRNA at site A2 is intertwined with the initiation of 5′-A0 degradation and the priming of the emergent pre-40S particle for nuclear export. During the rapid transit of the pre-40S particle from the nucleolus to the cytoplasm, a few maturation factors (Tsr1, Rio2, Nob1) that will be required in the cytoplasm are incorporated in a manner independent of nuclear export. Another maturation factor, Ltv1, requires Rrp12 for its stable incorporation onto pre-40S particles, but whether or not it is dependent on the export process itself remains to be ascertained. Further details about this model, and the evidence supporting it, is given in the text. In our model we propose that Rrp12 is an export factor rather than a nuclear maturation factor (Figure 8). Consistent with this, we have observed that the elimination of Rrp12 leads to the accumulation of pre-40S particles that, in addition to being dissociated from the 90S pre-ribosome machinery, are fully-assembled. This is evidenced by the recruitment to those particles of factors that are predominantly cytoplasmic in normal cells (Rio2, Nob1), and that therefore must join the pathway just before nuclear exit. One important inference of our results is that the major assembly events involved in the formation of pre-40S particles are separable and fully independent from the subsequent export step. A direct participation of Rrp12 in the export process is also supported by the previously-described interactions of this protein with some nucleoporins and with Ran [22]. Unexpectedly, we could not find any significant role for Rrp12 in the export of pre-60S subunits, as it had been previously published [22]. In addition to the phenotypic analysis of Rrp12-depleted cells, the prominent role of Rrp12 in the 40S rather than the 60S subunit pathway is supported by the RNA-protein interaction data showing the specific binding of Rrp12 to the 20S but not the 27S and 7S pre-rRNAs. The reason for these different results is not readily apparent. We have found that the loss of Rrp12 elicits the 40S subunit-specific phenotype both in the W303 and in BY4743 strains, indicating no influence of the genetic background. Still, it is worth noting that the depletion of Rrp12 causes delays in the processing of 5.8S rRNA precursors in the nucleus by a hitherto unknown mechanism. According to our results, such delays do not impact the overall production of 60S subunits, but it could be possible that, under some experimental conditions or in strains with genetic modifications that subtly affect ribosome biogenesis, the defect in 5.8S rRNA production became exacerbated and caused nuclear accumulation of pre-60S particles. It is also plausible that Rrp12 could interact either weakly or very transiently with some pre-60S particle subpools, as it would be expected if its influence on the processing of 5.8S precursors were direct. This possibility would be in agreement with the previously-reported detection of Rrp12 bound to 27SB pre-rRNAs using primer-extension analyses [22]. Despite the possibility of these alternative scenarios, we believe that our data clearly indicate that Rrp12 is not essential for 60S subunit synthesis. Consistent with this idea, it is also worth noting that mammalian Rrp12 has been shown to be required exclusively for 40S subunit synthesis [48], [49].
One distinctive feature of the intermediate particle formed in the absence of Rrp12 is the lack of Ltv1, a factor not essential for nuclear export. Previous studies indicate that this protein is recruited in the nucleus [31], [50], but some evidence suggests that its interaction with the nuclear pre-ribosomes that are about to be exported might be weak [20]. Thus, a possible explanation for the absence of Ltv1 in the pre-40S particles of Rrp12-depleted cells is that those particles are ready to be exported and have Ltv1 loosely associated. Alternatively, it is possible that Rrp12 could be actively required for the docking of Ltv1 to those particles during the export process. We currently favor the latter possibility, since we have observed that the interaction of these two proteins can occur in a pre-rRNA-independent manner. Based on the present data, we believe that Rrp12 probably promotes the recruitment of Ltv1 onto the pre-40S particle immediately prior to the step of transport (Figure 8). Upon this docking step, Rrp12 is carried along with the particle through the nuclear pores to be finally released when the particles reach the cytosol. Consistent with this hypothesis, our co-purification experiments and other proteomic analyses have shown that Rrp12 is not a major component of cytoplasmic pre-40S particles. Alternatively, it is also possible that Rrp12 could remain associated to cytoplasmic pre-40S particles and only becomes released upon completion of a specific maturation event that takes place right after the nuclear export step. This model would explain previous results indicating that Rrp12 can associate with di-methylated 20S pre-rRNA, a modified form of the 20S pre-rRNA that is generated in the cytoplasm [22]. Further work will be required to dissect the fate and specific roles of Rrp12 in these late maturation stages.
The reason for using the pre-40S export machinery to facilitate late 90S pre-ribosome-mediated processes is unknown. We propose that such mechanism could ensure a timely coordination of the recycling kinetics of 90S pre-ribosome components with pre-40S particle release and rapid export (Figure 8). An inter-relation between these three processes is indicated by our data, which shows that the impairment of nuclear export causes defects in the function, disassembly and subcellular localization of the 90S pre-ribosome. Future work will be needed to explain the precise mechanisms by which the export factors influence the activities of the exosome and A2 cleavage complexes within the 90S pre-ribosome.
Materials and Methods
Yeast strains, genetic methods and plasmids
The Saccharomyces cerevisiae strains and plasmids used in this study are listed in Tables S1 and S2, respectively. The conditional strain for RRP12 under the control of the GAL1 promoter (YPM7) was generated by one-step insertion of a KAN-MX6-GAL1 cassette upstream of the ATG of the RRP12 gene [51]. This strain (referred to in the text as GAL::HA-rrp12), and the other GAL1-driven strains used in this study, JDY144, WDG72, YGM168, YO470 and YGM174 (referred to in the text as GAL::HA-spb4, GAL::rsa4, GAL::HA-pno1, GAL::rio2-ProtA and GAL::HA-mtr4, respectively) were cultured at 30°C in media containing galactose (YPGal, 0.4% yeast extract, 0.8% peptone, 0.1 mM adenine, 2% galactose) or glucose (YPD, 0.4% yeast extract, 0.8% peptone, 0.1 mM adenine, 2% glucose). For protein depletion, the incubation times in YPD varied from 9 to 18 h, as indicated in figure labelings. The ltv1Δ strain was cultured at 25°C, the temperature at which the 40S subunit biogenesis defects of this strain are more exacerbated. For the experiments of inactivation of Crm1 we employed a strain with the CRM1 gene depleted that carried a plasmid for the expression of the crm1-T539C-HA allele (strain MNY8, plasmid pDC-crm1-T539C). As a control for those experiments, we employed the corresponding strain carrying a plasmid for the expression of crm1-HA (strain MNY7, plasmid pDC-CRM1). MNY7 and MNY8 cells were treated with 100 ng/ml of leptomycin B (LMB) for 5–15 min. All strains with MYC, hemagglutinin (HA) or green fluorescent protein (GFP) carboxy-terminal tagged alleles, except the crm1-HA and GFP-rrp12 ones, were generated by in-frame one-step integration of PCR cassettes in the corresponding locus of wild type cells. In these strains, the epitope-tagged versions are the only source of the proteins in the cell, and their expression is driven from the endogenous gene promoters. All epitope-tagged alleles were fully functional, as measured by normal growth rates and normal contents of rRNAs, pre-rRNAs and ribosomal subunits. The sedimentation analysis of Crm1-HA shown in Figure 7D was performed on the YGM193 strain (referred to in the figure as pwp2-GFP, crm1Δ, pRS315-crm1-HA). The coimmunoprecipitation experiment in Figure 7E was performed with the YMD6 strain carrying the pDC-CRM1 plasmid (referred to in the figure as pwp2-GFP, crm1-HA) and with the YPM7 strain carrying the pGM58 and pDC-CRM1 plasmids (referred to in the figure as GFP-rrp12, crm1-HA). The sedimentation analysis of Crm1-HA shown in Figure 7F was performed on the following strains maintained in glucose-containing media: YPM7 carrying the pBN18 and pDC-CRM1 plasmids (referred in the figure as RRP12, CRM1, pRS315-crm1-HA), and YPM7 carrying the pBN19 and pDC-CRM1 plasmids (referred in the figure as rrp12Δ198, CRM1, pRS315-crm1-HA). Preparation of media, yeast transformation and genetic manipulations were performed according to established procedures.
RNA preparation and northern blot analysis
RNAs from total cellular lysates, gradient fractions and coimmunoprecipitations were prepared by the hot-phenol method [52]. Oligonucleotide labeling, RNA separation, Northern blotting and hybridization were performed as described previously [53]. The sequences of the oligonucleotides used as probes are shown in Table S3.
Protein purification and analysis
Preparation of total celular lysates for immunoblot, Western blot analysis, purification of GFP-tagged proteins and mass spectrometry analysis were performed as described previously [23], except for the Pwp2-GFP/Crm1-HA and GFP-Rrp12/Crm1-HA coimmunoprecipitation analysis in Figure 7E. In this case, instead of lysing cells in IP buffer (20 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 150 mM potassium acetate, 1 mM dithithreitol, 0.2% Triton X-100, supplemented with Complete [Roche]), cells were lysed in IP-NP40 buffer (15 mM Na2HPO4, 10 mM NaH2PO4, pH 7.2, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 0.1 mM NaVO4, 0.5% NP-40 Alternative [Calbiochem], supplemented with Complete). Before purification of Pwp2-GFP and Rrp12-GFP with GFP-TRAP (Chromotek), the pre-cleared lysates were diluted to 0.2% NP-40. The anti-Rrp12 antibody used for Western blot in Figure 2B is a rabbit polyclonal antibody raised against a peptide mapping at the C-terminus of yeast Rrp12 (this study). Other antibodies used in Western blot analysis were: anti-MYC (Roche), anti-GFP (Clontech), anti-HA (Covance), anti-Nop1 (Pierce), anti-Mex67 (kind gift of C. Dargemont, Institut Jacques Monod), anti-Rps3 (kind gift of M. Seedorf, University of Heidelberg), anti-Rps8 (kind gift of G. Dieci, University of Parma), anti-Rpl1 (kind gift of F. Lacroute, Centre de Génétique Moléculaire, Gif-sur-Yvette), anti-Pgk1 (Abcam), and anti-Cdc11 (Santa Cruz). For the represention of the results of the proteomic analysis shown in Figure 3E, the four different dot sizes are indicative of the amount of the copurifying protein relative to the amount of bait: >80%, 60–80%, 40–60%, and <40%.
Polysome preparation and sucrose gradient analysis
Cell cultures (200 ml) were grown to an optical density at 600 (OD600) between 0.8 and 0.1 and, before harvesting, cycloheximide was added to a final concentration of 0.1 mg/ml. After an incubation on ice for 5 min, cells were collected and lysed in 700 µl of HK buffer (20 mM HEPES, pH 7.5, 10 mM KCl, 2.5 mM MgCl2, 1 mM EGTA, 1 mM dithiothreitol (DTT) and 0.1 mg/ml cycloheximide) using a Fastprep apparatus. Cell lysates were pre-cleared by high-speed centrifugation, and extracts equivalent to 5–20 absorption units at 260 nm (A260) were loaded on 7–50% sucrose gradients (10 ml), which had been prepared in HK buffer without cycloheximide. Ultracentrifugation, subsequent fraction collection and polysome profile recording were performed as previously described [53]. For Western blot analysis, 40 µl samples of each fraction were mixed directly with 10 µl of SDS-PAGE loading buffer (SPLB) and loaded onto SDS polyacrylamide gels. For Northern blot analysis, total RNA was prepared by the hot-phenol procedure from 100 µl samples of each fraction and separated on 1.2% agarose-formadehyde gels. For the analysis of purified complexes shown in Figure 7D, two sets (pools 1 and 2) of four combined fractions were concentrated 6-fold by spinning on Microcon-10 (Millipore) filters. The recovery of proteins after the concentration step was ∼10 fold more efficient for pool 1 than for pool 2, probably due to the higher sucrose concentration in pool 2. Before performing the GFP-Trap purification, each concentrated pool was taken to 1 ml with NP-40 buffer (0,2% final concentration).
Protein-RNA coimmunoprecipitation experiments
Cell cultures were grown to OD600 between 0.8 and 1.0, and polysome extracts were prepared as described above. Extract equivalents to 15 A260 units were taken to 250 µl with HK buffer and mixed with 0.5 ml of IP buffer containing Complete and 600 U/ml of RNasin (Promega). In the Crm1-RNA coimmunoprecipitations shown in Figure 7C, instead of using IP buffer it was used IP-NP40 (0.2% final concentration) buffer. For evaluation of protein content in total cell lysates, a 30 µl aliquot of the pre-cleared lysate was mixed with 30 µl of SPLB and kept frozen until analysis by Western blot. The rest of the extract was incubated with 2 µg of anti-MYC 9E10 (Roche) antibody or with 25 µl of GFP-TRAP beads at 4°C for 2 h. When using anti-MYC antibody, immunoprecipitates were immobilized with GammaBind sepharose beads (GE Healthcare). Immunoprecipitates were washed four times at 4°C with IP or IP-NP40 buffer. For protein analyses, one fifth of the immunoprecipitated material was resuspended in SPLB and analyzed, in paralel with the samples of total protein, by Western blot. For RNA analyses, the rest of the immunoprecipitated material was resuspended in 400 µl of 50 mM sodium acetate, 10 mM EDTA (pH 5.2), and processed for RNA extraction by the hot phenol method. After ethanol precipitation, the whole amount of recovered RNA was resuspended in formaldehyde loading buffer, separated on 1.2% agarose-formadehyde gels and analyzed by Northern blot. In parallel, in the same Northern blot experiments, it was evaluated the pre-rRNA content in cell lysates before immunoprecipitation, using 5 µg of total RNA prepared by the hot phenol method directly from extract equivalents to 10 A260 units of the corresponding polysome preparations.
Fluorescence microscopy
Cells were visualized using a Zeiss Axioplan 2 microscope equiped with a 63× objective, a Hammamutsu ORCA-ER digital camera and Openlab (Improvision) cell imaging analysis software. The Rpl25-EGFP and Rps2-GFP reporter assays to monitor pre-40 and pre-60S nuclear accumulation were performed as previously described [54].
Subcellular fractionation
Cells were grown to OD600 between 0.8 and 0.1, harvested and spheroplasts prepared by incubation in S buffer (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1.2 M sorbitol, 1 mM dithiothreitol, 5 mg/ml Zymolyase T-100 (Seikagaku) at 30°C for 15 min. After two washes with the same buffer, the spheroplasts were lysed using a manual homogenizer in Ficoll buffer (10 mM Tris-HCl, pH 7.5, 20 mM KCl, 5 mM MgCl2, 3 mM dithiothreitol, 1 mM EDTA, 1 mM PMSF, 180 mg/ml Ficoll-400, supplemented with Complete). Pre-cleared lysates were ultracentrifuged in a TLA 100.3 rotor at 23.000 rpm for 15 min, and the supernatant cytosolic fraction collected. The nuclei pellet was resuspended in 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 30 mM MgCl2, 0.25% NP-40 supplemented with Complete. Aliquots of the precleared whole lysate (W), cytosolic fraction (C) and nuclei (N) were mixed with SPLB and loaded onto a SDS polyacrilamide gel for Western blot analysis.
Supporting Information
Zdroje
1. VenemaJ, TollerveyD (1999) Ribosome synthesis in Saccharomyces cerevisiae. Annu Rev Genet 33 : 261–311.
2. WoolfordJLJr, BasergaSJ (2013) Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 195 : 643–681.
3. GrandiP, RybinV, BasslerJ, PetfalskiE, StraussD, et al. (2002) 90S pre-ribosomes include the 35S pre-rRNA, the U3 snoRNP, and 40S subunit processing factors but predominantly lack 60S synthesis factors. Mol Cell 10 : 105–115.
4. Perez-FernandezJ, RomanA, De Las RivasJ, BusteloXR, DosilM (2007) The 90S preribosome is a multimodular structure that is assembled through a hierarchical mechanism. Mol Cell Biol 27 : 5414–5429.
5. GallagherJE, DunbarDA, GrannemanS, MitchellBM, OsheimY, et al. (2004) RNA polymerase I transcription and pre-rRNA processing are linked by specific SSU processome components. Genes Dev 18 : 2506–2517.
6. Perez-FernandezJ, Martin-MarcosP, DosilM (2011) Elucidation of the assembly events required for the recruitment of Utp20, Imp4 and Bms1 onto nascent pre-ribosomes. Nucleic Acids Res 39 : 17.
7. DragonF, GallagherJE, Compagnone-PostPA, MitchellBM, PorwancherKA, et al. (2002) A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417 : 967–970.
8. PhippsKR, CharetteJM, BasergaSJ (2011) The SSU Processome in Ribosome Biogenesis - Progress and Prospects. WIREs RNA 2 : 1–21.
9. ThomsonE, Ferreira-CercaS, HurtE (2013) Eukaryotic ribosome biogenesis at a glance. J Cell Sci 126 : 4815–4821.
10. KresslerD, HurtE, BasslerJ (2010) Driving ribosome assembly. Biochim Biophys Acta 1803 : 673–683.
11. HenrasAK, SoudetJ, GerusM, LebaronS, Caizergues-FerrerM, et al. (2008) The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol Life Sci 65 : 2334–2359.
12. TschochnerH, HurtE (2003) Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends Cell Biol 13 : 255–263.
13. KarbsteinK (2011) Inside the 40S ribosome assembly machinery. Curr Opin Chem Biol 15 : 657–663.
14. LebaronS, SchneiderC, van NuesRW, SwiatkowskaA, WalshD, et al. (2012) Proofreading of pre-40S ribosome maturation by a translation initiation factor and 60S subunits. Nat Struct Mol Biol 19 : 744–753.
15. StrunkBS, NovakMN, YoungCL, KarbsteinK (2012) A translation-like cycle is a quality control checkpoint for maturing 40S ribosome subunits. Cell 150 : 111–121.
16. PertschyB, SchneiderC, GnadigM, SchaferT, TollerveyD, et al. (2009) RNA helicase Prp43 and its co-factor Pfa1 promote 20 to 18 S rRNA processing catalyzed by the endonuclease Nob1. J Biol Chem 284 : 35079–35091.
17. KarbsteinK (2013) Quality control mechanisms during ribosome maturation. Trends Cell Biol 23 : 242–250.
18. SchaferT, StraussD, PetfalskiE, TollerveyD, HurtE (2003) The path from nucleolar 90S to cytoplasmic 40S pre-ribosomes. Embo J 22 : 1370–1380.
19. MoyTI, SilverPA (2002) Requirements for the nuclear export of the small ribosomal subunit. J Cell Sci 115 : 2985–2995.
20. SchaferT, MacoB, PetfalskiE, TollerveyD, BottcherB, et al. (2006) Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit. Nature 441 : 651–655.
21. MoyTI, SilverPA (1999) Nuclear export of the small ribosomal subunit requires the ran-GTPase cycle and certain nucleoporins. Genes Dev 13 : 2118–2133.
22. OeffingerM, DlakicM, TollerveyD (2004) A pre-ribosome-associated HEAT-repeat protein is required for export of both ribosomal subunits. Genes Dev 18 : 196–209.
23. DosilM (2011) Ribosome synthesis-unrelated functions of the preribosomal factor rrp12 in cell cycle progression and the DNA damage response. Mol Cell Biol 31 : 2422–2438.
24. CombsDJ, NagelRJ, AresMJr, StevensSW (2006) Prp43p is a DEAH-box spliceosome disassembly factor essential for ribosome biogenesis. Mol Cell Biol 26 : 523–534.
25. LeedsNB, SmallEC, HileySL, HughesTR, StaleyJP (2006) The splicing factor Prp43p, a DEAH box ATPase, functions in ribosome biogenesis. Mol Cell Biol 26 : 513–522.
26. LebaronS, FromentC, Fromont-RacineM, RainJC, MonsarratB, et al. (2005) The splicing ATPase prp43p is a component of multiple preribosomal particles. Mol Cell Biol 25 : 9269–9282.
27. FazaMB, ChangY, OcchipintiL, KemmlerS, PanseVG (2012) Role of Mex67-Mtr2 in the nuclear export of 40S pre-ribosomes. PLoS Genet 8: e1002915.
28. VanrobaysE, GelugneJP, GleizesPE, Caizergues-FerrerM (2003) Late cytoplasmic maturation of the small ribosomal subunit requires RIO proteins in Saccharomyces cerevisiae. Mol Cell Biol 23 : 2083–2095.
29. VanrobaysE, GelugneJP, Caizergues-FerrerM, LafontaineDL (2004) Dim2p, a KH-domain protein required for small ribosomal subunit synthesis. Rna 10 : 645–656.
30. ChenW, BucariaJ, BandDA, SuttonA, SternglanzR (2003) Enp1, a yeast protein associated with U3 and U14 snoRNAs, is required for pre-rRNA processing and 40S subunit synthesis. Nucleic Acids Res 31 : 690–699.
31. SeiserRM, SundbergAE, WollamBJ, Zobel-ThroppP, BaldwinK, et al. (2006) Ltv1 is required for efficient nuclear export of the ribosomal small subunit in Saccharomyces cerevisiae. Genetics 174 : 679–691.
32. LafontaineD, VandenhauteJ, TollerveyD (1995) The 18S rRNA dimethylase Dim1p is required for pre-ribosomal RNA processing in yeast. Genes Dev 9 : 2470–2481.
33. DezC, DlakicM, TollerveyD (2007) Roles of the HEAT repeat proteins Utp10 and Utp20 in 40S ribosome maturation. Rna 13 : 1516–1527.
34. BillyE, WegierskiT, NasrF, FilipowiczW (2000) Rcl1p, the yeast protein similar to the RNA 3′-phosphate cyclase, associates with U3 snoRNP and is required for 18S rRNA biogenesis. Embo J 19 : 2115–2126.
35. WoollsHA, LamannaAC, KarbsteinK (2011) Roles of Dim2 in ribosome assembly. J Biol Chem 286 : 2578–2586.
36. SenapinS, Clark-WalkerGD, ChenXJ, SeraphinB, DaugeronMC (2003) RRP20, a component of the 90S preribosome, is required for pre-18S rRNA processing in Saccharomyces cerevisiae. Nucleic Acids Res 31 : 2524–2533.
37. SchneiderC, KudlaG, WlotzkaW, TuckA, TollerveyD (2012) Transcriptome-wide analysis of exosome targets. Mol Cell 48 : 422–433.
38. SchneiderC, LeungE, BrownJ, TollerveyD (2009) The N-terminal PIN domain of the exosome subunit Rrp44 harbors endonuclease activity and tethers Rrp44 to the yeast core exosome. Nucleic Acids Res 37 : 1127–1140.
39. de la CruzJ, KresslerD, TollerveyD, LinderP (1998) Dob1p (Mtr4p) is a putative ATP-dependent RNA helicase required for the 3′ end formation of 5.8S rRNA in Saccharomyces cerevisiae. Embo J 17 : 1128–1140.
40. AllmangC, MitchellP, PetfalskiE, TollerveyD (2000) Degradation of ribosomal RNA precursors by the exosome. Nucleic Acids Res 28 : 1684–1691.
41. NevilleM, RosbashM (1999) The NES-Crm1p export pathway is not a major mRNA export route in Saccharomyces cerevisiae. Embo J 18 : 3746–3756.
42. LebaronS, PapinC, CapeyrouR, ChenYL, FromentC, et al. (2009) The ATPase and helicase activities of Prp43p are stimulated by the G-patch protein Pfa1p during yeast ribosome biogenesis. Embo J 28 : 3808–3819.
43. BoulonS, VerheggenC, JadyBE, GirardC, PesciaC, et al. (2004) PHAX and CRM1 are required sequentially to transport U3 snoRNA to nucleoli. Mol Cell 16 : 777–787.
44. StrubBR, EswaraMB, PierceJB, MangrooD (2007) Utp8p is a nucleolar tRNA-binding protein that forms a complex with components of the nuclear tRNA export machinery in Saccharomyces cerevisiae. Mol Biol Cell 18 : 3845–3859.
45. WangW, BudhuA, ForguesM, WangXW (2005) Temporal and spatial control of nucleophosmin by the Ran-Crm1 complex in centrosome duplication. Nat Cell Biol 7 : 823–830.
46. Pradet-BaladeB, GirardC, BoulonS, PaulC, AzzagK, et al. (2011) CRM1 controls the composition of nucleoplasmic pre-snoRNA complexes to licence them for nucleolar transport. Embo J 30 : 2205–2218.
47. SuzukiN, NoguchiE, NakashimaN, OkiM, OhbaT, et al. (2001) The Saccharomyces cerevisiae small GTPase, Gsp1p/Ran, is involved in 3′ processing of 7S-to-5.8S rRNA and in degradation of the excised 5′-A0 fragment of 35S pre-rRNA, both of which are carried out by the exosome. Genetics 158 : 613–625.
48. TafforeauL, ZorbasC, LanghendriesJL, MullineuxST, StamatopoulouV, et al. (2013) The complexity of human ribosome biogenesis revealed by systematic nucleolar screening of Pre-rRNA processing factors. Mol Cell 51 : 539–551.
49. WildT, HorvathP, WylerE, WidmannB, BadertscherL, et al. (2010) A protein inventory of human ribosome biogenesis reveals an essential function of exportin 5 in 60S subunit export. PLoS Biol 8: e1000522.
50. Leger-SilvestreI, MilkereitP, Ferreira-CercaS, SaveanuC, RousselleJC, et al. (2004) The ribosomal protein Rps15p is required for nuclear exit of the 40S subunit precursors in yeast. Embo J 23 : 2336–2347.
51. LongtineMS, McKenzieA3rd, DemariniDJ, ShahNG, WachA, et al. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14 : 953–961.
52. Ausubel FM, Brent R., Kingston R E., Moore D D., Seidman J G., et al.. (1994) Current Protocols in Molecular Biology. New York: John Wiley & Sons, Inc.
53. DosilM, BusteloXR (2004) Functional characterization of Pwp2, a WD family protein essential for the assembly of the 90 S pre-ribosomal particle. J Biol Chem 279 : 37385–37397.
54. GadalO, StraussD, KesslJ, TrumpowerB, TollerveyD, et al. (2001) Nuclear export of 60s ribosomal subunits depends on Xpo1p and requires a nuclear export sequence-containing factor, Nmd3p, that associates with the large subunit protein Rpl10p. Mol Cell Biol 21 : 3405–3415.
Štítky
Genetika Reprodukční medicína
Článek Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart DiseaseČlánek Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the NicheČlánek Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial TetheringČlánek Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature SeasonalityČlánek Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell DivisionsČlánek Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity inČlánek Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration inČlánek ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression inČlánek Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex EnvironmentsČlánek The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 12
-
Všechny články tohoto čísla
- Stratification by Smoking Status Reveals an Association of Genotype with Body Mass Index in Never Smokers
- Genome Wide Meta-analysis Highlights the Role of Genetic Variation in in the Regulation of Circulating Serum Chemerin
- Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication
- Distinct Genealogies for Plasmids and Chromosome
- Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease
- Non-coding RNAs Prevent the Binding of the MSL-complex to Heterochromatic Regions
- Plasmid Flux in ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences
- Epigenome-Guided Analysis of the Transcriptome of Plaque Macrophages during Atherosclerosis Regression Reveals Activation of the Wnt Signaling Pathway
- The Inventiveness of Nature: An Interview with Werner Arber
- Mediation Analysis Demonstrates That -eQTLs Are Often Explained by -Mediation: A Genome-Wide Analysis among 1,800 South Asians
- Generation of Antigenic Diversity in by Structured Rearrangement of Genes During Mitosis
- A Massively Parallel Pipeline to Clone DNA Variants and Examine Molecular Phenotypes of Human Disease Mutations
- Genetic Analysis of the Cardiac Methylome at Single Nucleotide Resolution in a Model of Human Cardiovascular Disease
- Genetic Analysis of Circadian Responses to Low Frequency Electromagnetic Fields in
- The Dissection of Meiotic Chromosome Movement in Mice Using an Electroporation Technique
- Altered Chromatin Occupancy of Master Regulators Underlies Evolutionary Divergence in the Transcriptional Landscape of Erythroid Differentiation
- Syd/JIP3 and JNK Signaling Are Required for Myonuclear Positioning and Muscle Function
- Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the Niche
- Mutation of Leads to Blurred Tonotopic Organization of Central Auditory Circuits in Mice
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- RAN-Binding Protein 9 is Involved in Alternative Splicing and is Critical for Male Germ Cell Development and Male Fertility
- Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import
- Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering
- Recurrent Loss of Specific Introns during Angiosperm Evolution
- Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature Seasonality
- SEEDSTICK is a Master Regulator of Development and Metabolism in the Arabidopsis Seed Coat
- Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell Divisions
- Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity in
- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression in
- Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex Environments
- Global Analysis of Photosynthesis Transcriptional Regulatory Networks
- Mucolipin Co-deficiency Causes Accelerated Endolysosomal Vacuolation of Enterocytes and Failure-to-Thrive from Birth to Weaning
- Controlling Pre-leukemic Thymocyte Self-Renewal
- How Malaria Parasites Avoid Running Out of Ammo
- Echoes of the Past: Hereditarianism and
- Deep Reads: Strands in the History of Molecular Genetics
- Keep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
- Analysis of a Plant Complex Resistance Gene Locus Underlying Immune-Related Hybrid Incompatibility and Its Occurrence in Nature
- Epistatic Adaptive Evolution of Human Color Vision
- Increased and Imbalanced dNTP Pools Symmetrically Promote Both Leading and Lagging Strand Replication Infidelity
- Genetic Basis of Haloperidol Resistance in Is Complex and Dose Dependent
- Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development
- Interaction between Conjugative and Retrotransposable Elements in Horizontal Gene Transfer
- The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
- is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
- PRL1, an RNA-Binding Protein, Positively Regulates the Accumulation of miRNAs and siRNAs in Arabidopsis
- Genetic Control of Contagious Asexuality in the Pea Aphid
- Early Mesozoic Coexistence of Amniotes and Hepadnaviridae
- Local and Systemic Regulation of Plant Root System Architecture and Symbiotic Nodulation by a Receptor-Like Kinase
- Gene Pathways That Delay Reproductive Senescence
- The Evolution of Fungal Metabolic Pathways
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- Formation of Linear Amplicons with Inverted Duplications in Requires the MRE11 Nuclease
- Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
- Rrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
- The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
- Topoisomerase I Plays a Critical Role in Suppressing Genome Instability at a Highly Transcribed G-Quadruplex-Forming Sequence
- A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation
- Transcriptional Frameshifting Rescues Type VI Secretion by the Production of Two Length Variants from the Prematurely Interrupted Gene
- Association Mapping across Numerous Traits Reveals Patterns of Functional Variation in Maize
- Genome-Wide Analysis of -Regulated and Phased Small RNAs Underscores the Importance of the ta-siRNA Pathway to Maize Development
- Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
- The Tau Tubulin Kinases TTBK1/2 Promote Accumulation of Pathological TDP-43
- Germline Signals Deploy NHR-49 to Modulate Fatty-Acid β-Oxidation and Desaturation in Somatic Tissues of
- Microevolution of in Macrophages Restores Filamentation in a Nonfilamentous Mutant
- Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells Is Required for Outflow Tract Lengthening during Cardiac Development
- Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
- A ABC Transporter Regulates Lifespan
- RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
- , and Reprogram Thymocytes into Self-Renewing Cells
- The miR9863 Family Regulates Distinct Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling
- Detection of Pleiotropy through a Phenome-Wide Association Study (PheWAS) of Epidemiologic Data as Part of the Environmental Architecture for Genes Linked to Environment (EAGLE) Study
- Extensive Copy-Number Variation of Young Genes across Stickleback Populations
- The and Genetic Modules Interact to Regulate Ciliogenesis and Ciliary Microtubule Patterning in
- Analysis of the Genome, Transcriptome and Secretome Provides Insight into Its Pioneer Colonization Strategies of Wood
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- Echoes of the Past: Hereditarianism and
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



