-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaA Splice Region Variant in Lowers Non-high Density Lipoprotein Cholesterol and Protects against Coronary Artery Disease
Cholesterol levels in the bloodstream, in particular elevated low-density lipoprotein cholesterol (LDL-C), are strong risk factors for cardiovascular disease, and LDL-C reduction reduces mortality in people at risk. One of the major determinants of plasma LDL-C levels is the low density lipoprotein receptor (LDLR) that acts as a scavenger for cholesterol rich lipoprotein particles. Mutations that disrupt the function of the LDLR or lead to reduction in the number of LDLR usually result in elevated LDL-C in blood. In the current study, we identified, through whole-genome sequencing and imputation into a large fraction of the Icelandic population, four LDLR gene variants that affect non-HDL-C levels (that includes cholesterol in LDL and other pro-atherogenic lipoproteins) and risk of coronary artery disease (CAD). Two variants are known and two are novel. One of them, a splice region variant in intron 14 (rs72658867-A), affects normal splicing and is predicted to generate a truncated LDLR, lacking domains essential for receptor function. Despite this, rs72658867-A lowers non-HDL-C substantially and protects against CAD in the general population, demonstrating that variants that disrupt the LDLR can result in lower cholesterol levels.
Published in the journal: . PLoS Genet 11(9): e32767. doi:10.1371/journal.pgen.1005379
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005379Summary
Cholesterol levels in the bloodstream, in particular elevated low-density lipoprotein cholesterol (LDL-C), are strong risk factors for cardiovascular disease, and LDL-C reduction reduces mortality in people at risk. One of the major determinants of plasma LDL-C levels is the low density lipoprotein receptor (LDLR) that acts as a scavenger for cholesterol rich lipoprotein particles. Mutations that disrupt the function of the LDLR or lead to reduction in the number of LDLR usually result in elevated LDL-C in blood. In the current study, we identified, through whole-genome sequencing and imputation into a large fraction of the Icelandic population, four LDLR gene variants that affect non-HDL-C levels (that includes cholesterol in LDL and other pro-atherogenic lipoproteins) and risk of coronary artery disease (CAD). Two variants are known and two are novel. One of them, a splice region variant in intron 14 (rs72658867-A), affects normal splicing and is predicted to generate a truncated LDLR, lacking domains essential for receptor function. Despite this, rs72658867-A lowers non-HDL-C substantially and protects against CAD in the general population, demonstrating that variants that disrupt the LDLR can result in lower cholesterol levels.
Introduction
The low-density lipoprotein receptor (LDLR) is a cell-surface receptor responsible for binding and uptake of circulating cholesterol-containing lipoprotein particles. This uptake is the primary pathway for removal of cholesterol from the circulation [1]. It is well established that high levels of low-density lipoprotein-cholesterol (LDL-C) are a key risk factor for coronary artery disease (CAD) and is a primary target for therapeutic intervention [2]. Recent studies show that non-high density lipoprotein cholesterol (non-HDL-C) is a better predictor for cardiovascular risk than LDL-C as it encompasses all cholesterol containing pro-atherogenic lipoproteins such as very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), chylomicron remnants (CR) as well as LDL-C [3]. LDL receptors primarily clear LDL-C from blood but they also bind VLDL-C and remnant particles [4].
The LDL receptor and its role in LDL-C regulation was discovered 40 years ago when Goldstein and Brown set out to unravel the cause of familial hypercholesterolemia (FH) [5], a severe autosomal dominant disorder characterized by high levels of LDL-C in blood and premature cardiovascular disease [6]. The most common sequence variants causing FH are loss-of-function LDLR mutations that disrupt the receptor function leading to reduced hepatic LDL-C clearance and elevated plasma LDL-C. So far over 1,200 rare LDLR mutations have been reported in FH families [7,8]. Common variants at the LDLR locus with modest effects on LDL-C levels and risk of coronary artery disease (CAD) in the general population have been identified through genome-wide association studies (GWAS) [9–11]. More recently GWAS studies based on whole-exome sequencing have confirmed the association between very rare LDLR missense and loss-of-function variants (MAF <1%) with LDL-C levels and risk of myocardial infarction (MI) [12,13]. The design of these studies, however, had little capacity to detect rare and low frequency non-coding variants at the LDLR locus that affect cholesterol levels and the risk of CAD and MI. High-coverage whole-genome sequencing (WGS) based GWAS in contrast have the potential to identify such variants if present.
Here we applied high-coverage WGS to a large fraction of the Icelandic population to search for LDLR sequence variants affecting serum levels of non-HDL-C in the general population. We found four highly significant variants each representing independent signals at the LDLR locus that associate with levels of non-HDL-C and risk of CAD. Two of these associations are novel and represented by non-coding variants of low frequency that lower non-HDL-C levels and protect against CAD. One of them affects splicing of the LDLR that is predicted to truncate the receptor.
Results
Identification of two low frequency non-coding variants that associate with non-HDL-C
In our study we elected to use the measurement non-HDL-C instead of LDL-C as it encompasses all potential atherogenic cholesterol containing lipoproteins including LDL-C. We examined the association of 7,351 sequence variants in a 1 Mb region centered on LDLR (chr19 : 10,559,187–11,559,187 (NCBI build36/hg18)) with non-HDL-C levels in 119,146 Icelanders. These sequence variants (SNPs and INDELs) were identified by WGS of 2,636 Icelanders and imputed, assisted by long-range phased haplotypes, into 104,220 Icelanders genotyped with Illumina SNP arrays [14]. In addition, we used genealogical information to calculate genotype probabilities for 294,212 first and second degree relatives of array genotyped individuals[15].
After performing stepwise conditional analysis we identified four highly significant variants each representing an independent signal at the LDLR locus (Fig 1 and Table 1 and S1 Table). Two of the variants are non-coding and low frequency and are novel with respect to association with non-HDL-C, rs72658867-A, a splice region variant at position +5 in intron 14 of LDLR (NM_000527.4:c.2140+5G>A, minor allelic frequency (MAF) = 2.2%), and rs17248748-T, a variant in the first intron of LDLR (NM_000527.4:c.68-4859C>T, MAF = 3.4%), that lower non-HDL-C by 0.44 mmol/l (Padj = 2.0 × 10−70) and 0.13 mmol/l (Padj = 5.0 × 10−11), respectively. The splice region variant rs72658867-A has been described in FH families, however, it has been disputed whether it is pathogenic [16–19]. The third signal is captured by a common variant rs17248720-T (NM_000527.4:c.-2038C>T, MAF = 8.8%) located at the 5’ end of LDLR that lowers non-HDL-C by 0.24 mmol/l (Padj = 1.8× 10−80) and has been reported to lower LDL-C levels with similar effect as shown here and confer protection against CAD [9,20]. The fourth signal at the LDLR locus, is represented by a rare variant rs200238879-C (MAF = 0.06%), reported to be an Icelandic founder FH mutation [21]. This variant is located in the donor splice site of intron 4 (NM_000527.4:c.694+2T>C) and increases non-HDL-C serum levels by 1.33 mmol/l (Padj = 2.2 × 10−22). The four variants associate with LDL-C with similar effect sizes as with non-HDL-C, the P-values are however slightly higher due to smaller sample size for LDL-C (S2 Table). None of the variants associate with high-density cholesterol (HDL-C) or triglycerides except rs72658867-A, that associates weakly with increased HDL-C levels (Padj = 0.0035) (Table 1).
Fig. 1. Overview of non-HDL-C associations in the region around LDLR. 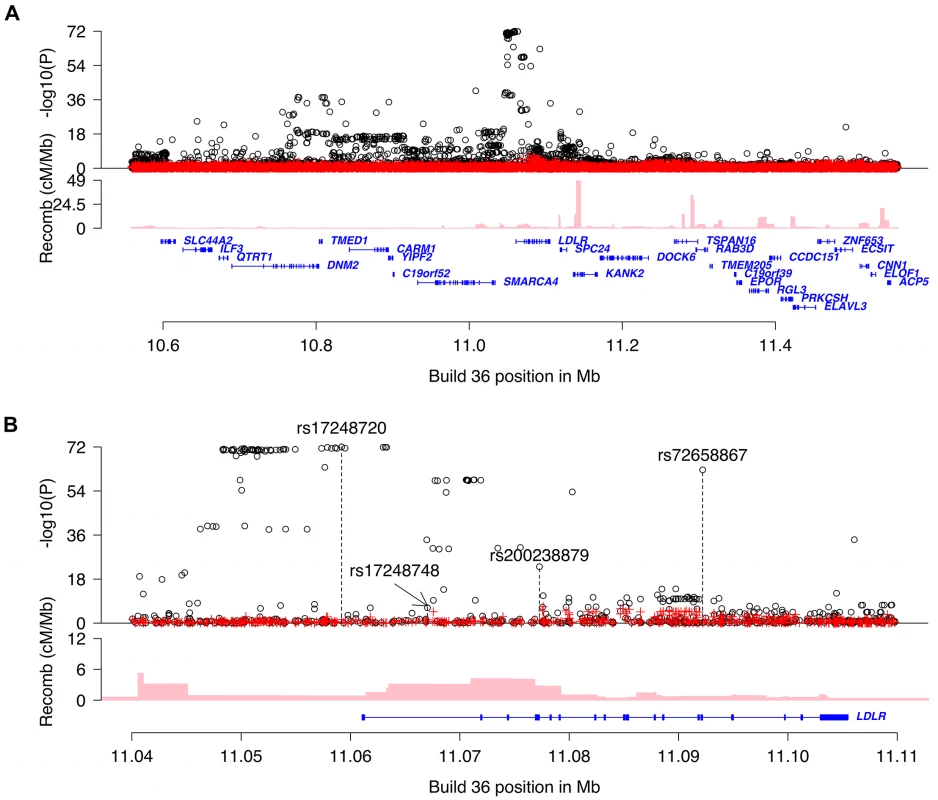
Plot A is a 0.8Mb overview centered on LDLR and plot B is a 70kb overview around the LDLR gene. Black circles show-log10 P as a function of build 36 coordinates for associations with non-HDL-C and red crosses correspond to non-HDL-C associations after adjusting for the four variants rs17248720, rs72658867, rs200238879 and rs17248748 that are indicated by vertical broken lines in plot b. Genes are shown in blue and recombination rates are reported in cM/Mb. Tab. 1. Association of LDLR sequence variants with non-HDL-C, TG and HDL-C in Iceland. 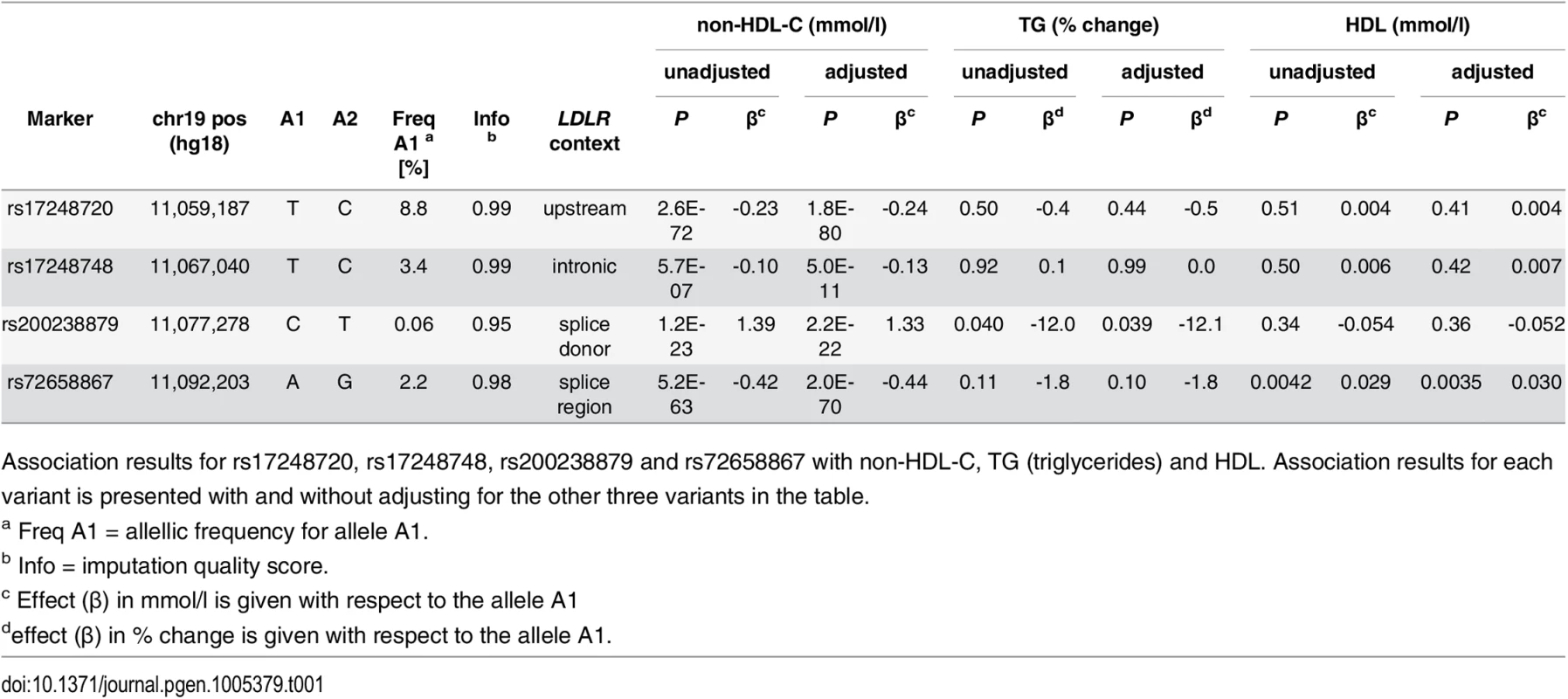
Association results for rs17248720, rs17248748, rs200238879 and rs72658867 with non-HDL-C, TG (triglycerides) and HDL. Association results for each variant is presented with and without adjusting for the other three variants in the table. No combination of non-HDL-C lowering alleles (minor alleles) of rs72658867-A (splice region variant), rs17248748-T (intronic) and rs17248720-T (common) occur on the same haplotype in our data (S1 Fig). The splice donor variant rs200238879-C is very rare and is weakly correlated with the other three variants (S3 Table). The non-HDL-C lowering effects of the two low frequency variants and the common variant are additive (S2 Fig and S4 Table). Homozygous carriers of each of these variants have lower non-HDL-C levels than heterozygotes, with the lowest values observed for the homozygous carriers of the splice region variant (rs72658867-A).
Follow up of rs72658867-A and rs17248748-T association with non-HDL-C in other populations
We attempted to follow up the association of the two novel variants with non-HDL-C by direct genotyping in samples from Denmark, the Netherlands and Iran. In all three populations we replicate the association of both variants with lower non-HDL-C with similar effect sizes as in Iceland (effect of rs72658867-A on non-HDL-C is -0.41 mmol/l, P = 1.2 × 10−11 and for rs17248748-T is -0.14 mmol/l, P = 0.0082) (Table 2 and S5 Table). Joined with the Icelandic discovery data the combined effect on non-HDL-C for rs72658867-A is -0.44 mmol/l, P = 1.1 × 10−80 and for rs17248748-T is -0.13 mmol/l, P = 1.3 × 10−12 (Table 2 and S5 Table).
Tab. 2. Association of LDLR splice region variant rs72658867-A and intronic variant rs17248748-T with non-HDL-C in Denmark, Netherlands and Iran. 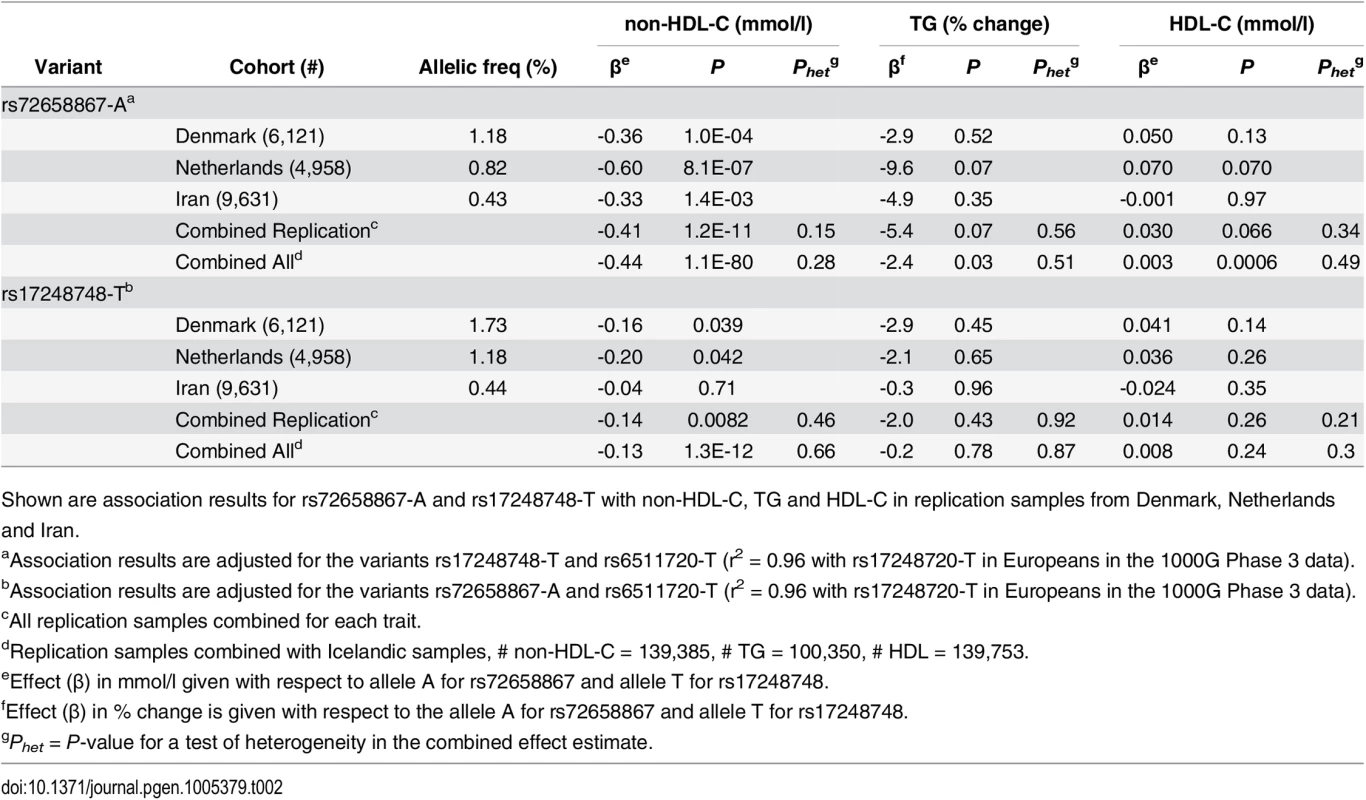
Shown are association results for rs72658867-A and rs17248748-T with non-HDL-C, TG and HDL-C in replication samples from Denmark, Netherlands and Iran. Association of LDLR variants with CAD
We tested the four LDLR variants for association with CAD in a sample of 33,090 cases and 236,254 controls from Iceland. All variants associate with CAD in a direction consistent with the known correlation between non-HDL-C and CAD (Table 3). The three non-HDL-C lowering variants, rs72658867-A (splice region variant), rs17248748-T (intronic) and rs17248720-T (common), all associate with a reduced risk of CAD and both rs72658867-A and rs17248720-T delay the age at diagnosis of CAD (Table 3). The rare splice donor variant that raises non-HDL-C, rs200238879-C, increases CAD risk and lowers the age at diagnosis by almost nine years (Table 3). Further, consistent with the effect on CAD, rs72658867-A and rs17248720-T both associate with increased lifespan (0.59 and 0.61 years per allele, respectively) and the rare splice donor mutation rs200238879-C associates with decreased lifespan (-6.46 years per allele) (S6 Table).
Tab. 3. Association of LDLR sequence variants with CAD and age at diagnosis of CAD in Iceland. 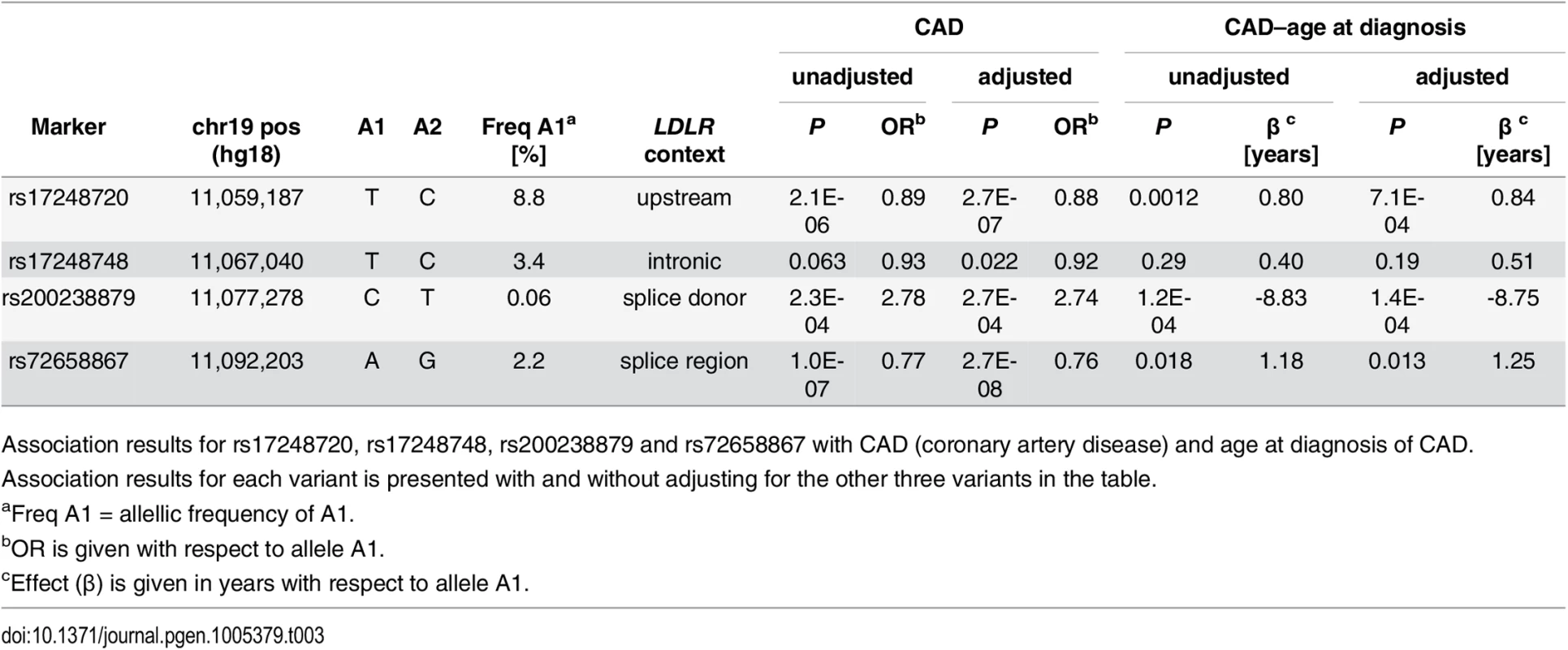
Association results for rs17248720, rs17248748, rs200238879 and rs72658867 with CAD (coronary artery disease) and age at diagnosis of CAD. Association results for each variant is presented with and without adjusting for the other three variants in the table. The low frequency variants rs72658867-A and rs17248748-T are likely causative
In our WGS data the coverage at the LDLR locus was high (~20X) apart from a small region (50bp) in intron 1 of low coverage that was analysed separately in an independent set of individuals (n = 738) whole-genome sequenced with the Illumina PCR-free sequencing method (S3 Fig). Our dataset is thus likely to represent all sequence variants (SNPs and INDELs) at the LDLR locus that are present in the Icelandic population at a frequency over 0.1%, allowing for fine mapping and identification of the causative variants of the four LDLR signals [14]. For that purpose we explored all variants in the Icelandic dataset that correlated (r2>0.8) with the four index variants in a 2 Mb window centered on each variant. The rare splice donor variant rs200238879-C is the most likely causative variant for that signal as it correlates with no other coding mutation and the mutation is known to cause abnormal splicing of the LDLR [21]. For the common upstream variant rs17248720 we found 56 correlates (46 variants with r2>0.99) none of which are in the LDLR coding region (S7 Table). Three of the most strongly correlated SNPs are located in sequence motifs with strong promoter or enhancer activities in the liver cell line (HepG2) (HaploReg v3, see URLs), suggesting that any one of them could be causative.
The novel intronic variant (rs17248748-T) has no strong correlates. It is located in a sequence motif within intron 1 with strong enhancer activity in the HepG2 liver cell line and binds regulatory proteins, including c/EBPbeta known to regulate transcription of LDLR (HaploReg v3, see URLs), supporting a causative role of the variant [22,23]. For the splice region variant rs72658867-A we found only one correlate, rs180760728-C, an intronic variant in the LDLR gene (MAF = 1.98%, r2 = 0.89). Conditional analysis revealed that rs180760728-C does not account for the non-HDL-C association of rs72658867-A (Padj = 0.32 for rs180760728 adjusting for rs72658867; Padj = 8.7×10−11 for rs72658867-A adjusting for rs180760728-C). Furthermore, in the 1000 Genomes European ancestry Phase 3 dataset (see URLs) rs180760728-C is more weakly correlated with rs72658867-A (r2 = 0.49) and rs180760728 is the only marker with r2>0.3 with rs72658867-A, indicating that rs72658867-A is likely the causative variant for this signal. The replication of the non-HDL-C associations of rs72658867-A and rs17248748-T with similar effect sizes in the three distinct populations further supports a causative role for these variants.
The splice region variant rs72658867-A associates with higher LDLR mRNA expression in white blood cells
To further characterize the two novel non-HDL-C signals at the LDLR locus we analysed the effect of rs72658867 and rs17248748 on LDLR mRNA expression in a microarray mRNA expression dataset for white blood cells (1,001 individuals) and adipose tissue (667 individuals). The non-HDL-C lowering allele of the splice region variant rs72658867 associates with increased LDLR mRNA expression in blood (~22% increase, P = 1.2 × 10−11) (S4 Fig) and no other variant in a ~1Mb region centered on LDLR correlated more strongly with LDLR expression than rs72658867 (S8 Table). These findings were replicated in an independent RNA-sequencing dataset from blood (252 individuals) where similar increase was detected in carriers (P = 0.0075) (Fig 2A). Using that dataset we also performed allele specific analysis of heterozygous carriers and non-carriers and show that the chromosomes carrying rs72658867-A have greater expression than the chromosomes carrying the reference allele (S9 Table and S5 Fig). In heterozygous carriers of rs72658867-A about 60% of the transcripts are derived from the mutated chromosome compared to a baseline proportion of 0.52 in non-carriers. In the adipose tissue microarray dataset the correlation with LDLR expression is in the same direction but much weaker (P = 0.020, S4 Fig). No significant correlation with LDLR expression in blood or adipose tissue was found for the intronic rs17248748 variant (P = 0.40 and P = 0.10, respectively).
Fig. 2. RNA sequencing data from blood demonstrates increased expression and abnormal splicing characterized by intron 14 retention in carriers of the splice region variant rs72658867-A. 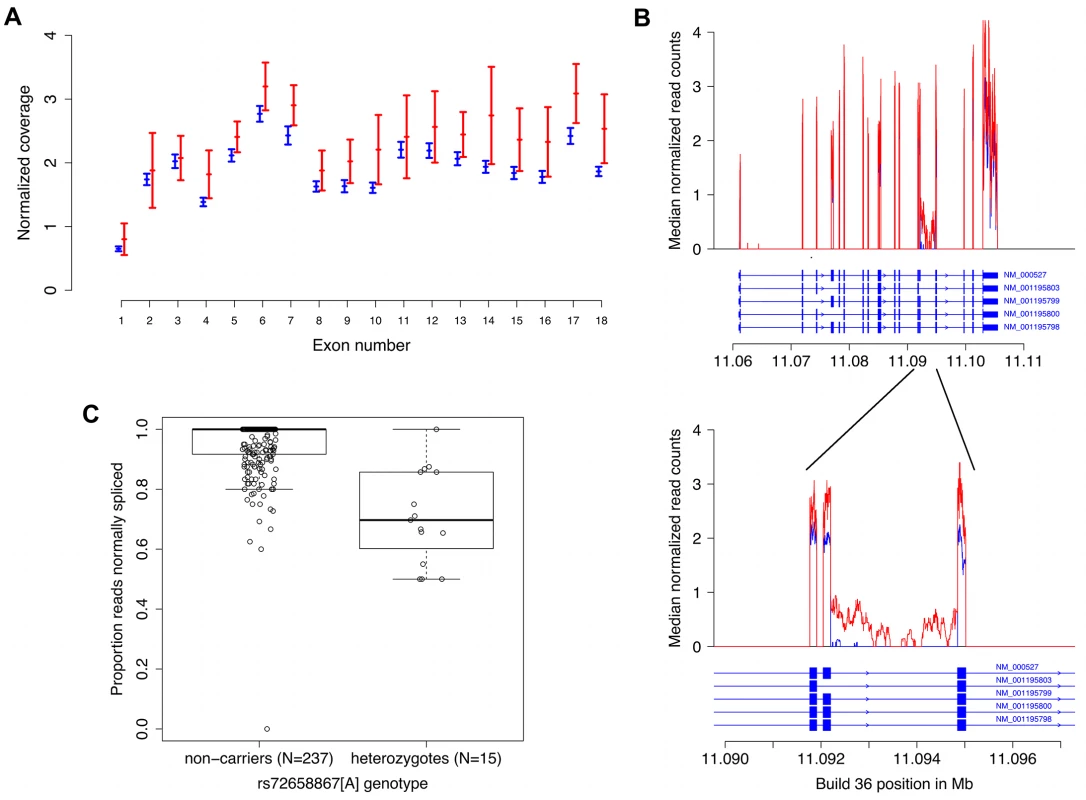
A. Normalized average LDLR exon coverage for non-carriers (N = 238, in blue) and heterozygotes (N = 15, in red) of rs72658867-A demonstrates increased expression of LDLR transcripts in heterozygotes by ~22%, P = 0.0075. The X-axis is the exon number corresponding to RefSeq transcript NM_000527 for LDLR. The Y-axis shows the median normalized coverage (normalized for each individual to the total number of aligned reads). The error bars are based on the median absolute deviation within each group and is calculated separately for each exon. B. Using the same samples as in a) preferential intron 14 retention is observed in heterozygous carriers of rs72658867-A (shown in red). The X-axis is the genomic position in Mb (hg18/Build36). The Y-axis is the median count of normalized reads as in a). The structure of all LDLR RefSeq transcript variants is shown. The upper panel shows the full length gene whereas the lower panel shows the exons 13, 14 and 15 and the intron retention in intron 14. C. Quantitation of the proportion of transcripts with intron 14 retention in heterozygotes. The Y-axis corresponds to the proportion of RNA sequencing reads that are spliced from exon 14 to exon 15 (correctly spliced) out of the total number of reads that cover the last base of exon 14 (individuals that do not have coverage at this position are omitted). Median proportion: 1.00 (non-carriers); 0.70 (heterozygotes). Mean proportion: 0.95 (non-carriers); 0.71 (heterozygotes). Mann-Whitney test for location shift gives P = 6.0×10−9. The splice region variant rs72658867-A disrupts LDLR splicing and is predicted to truncate the receptor
The splice region variant rs72658867 is located at position 5 of intron 14, a position that is conserved and could potentially affect splicing. To investigate this, we analysed the mRNA sequence data from blood and observed abnormal splicing in rs72658867-A carriers, characterized by retention of intron 14 (i.e. transcription through intron 14 in the LDLR transcripts) (Fig 2B). When looking at the proportion of RNA sequencing reads that are spliced from exon 14 to exon 15 (correctly spliced) out of the total number of reads that cover the last base of exon 14 we observed a mean proportion of 0.95 in non-carriers (n = 238) compared to 0.71 in heterozygous carriers (n = 15) (Mann-Whitney test for location shift P = 6.0×10−9). This indicates that approximately 30% of the transcripts in heterozygous carriers are abnormally spliced (Fig 2C). Analysis of blood RNA sequence data from homozygous carriers of rs72658867 - A (n = 3) demonstrated that about half of the LDLR transcripts are characterized by intron retention (S10 Table). Together these data from the hetero - and homozygous carriers indicate that from chromosomes carrying rs72658867-A, about half of the transcripts are normally spliced and half of them are abnormal. This also indicates that even though the total amount of LDLR transcripts is increased by 22% in heterozygotes (Figs 2A and S4), the estimated amount of normally spliced LDLR transcripts will not exceed 90% of normal levels. This was confirmed by RT-PCR analysis of LDLR mRNA from blood that showed similar levels of wild type LDLR mRNA in heterozygous rs72658867-A/G carriers (n = 20) and rs72658867-G/G non-carriers (n = 343), (Mann-Whitney test: P = 0.87) (S6 Fig). The non-HDL-C lowering effect of rs72658867-A is thus not mediated by a net increase in the wild type LDLR transcripts.
The retention of intron 14 alters the LDLR reading frame after amino acid position 713 (end of exon 14, NP000518:p.Thr713fsTer33) such that 33 amino acids are added until a premature stop codon is reached. It is unlikely that the introduction of the premature stop codon renders the transcript susceptible to nonsense-mediated decay as a high fraction of the LDLR transcripts in heterozygotes and homozygotes are characterized by retention of intron 14 (Fig 2C and S10 Table). The abnormally spliced mRNA is predicted to produce a truncated LDLR lacking the O-linked glycan region and the transmembrane and cytoplasmic domains (S7 Fig). The transmembrane domain anchors the LDLR in the lipid bilayer and endocytosis and intracellular transport of the LDLR are regulated via its cytoplasmic domain [24] but the role of the O-linked glycan region is unclear[25].
Discussion
We have identified, by high coverage whole-genome sequencing and subsequent imputation into a large fraction of the Icelandic population, four independent sequence variants at the LDLR locus that associate with levels of non-HDL-C and risk of CAD in the general population. Two of them are of low frequency and novel with respect to non-HDL-C association: a splice region variant (rs72658867-A, c.2140+5G>A) and an intronic variant (rs17248748-T). Both variants associate with lowering of non-HDL-C and protection against CAD. We show that the splice region variant causes retention of intron 14 altering the LDLR reading frame after amino acid position 713 such that 33 amino acids are added until a premature stop codon is reached. This splicing defect affects about half of the transcripts generated from the chromosome carrying the variant. The same variant also increases mRNA expression of LDLR that includes both normally and abnormally spliced transcripts and this increase seems to be driven by the chromosome carrying rs72658867-A. This study highlights the importance of including non-coding variants, in all segments of the frequency spectrum (common, low frequency and rare), in GWAS.
Although the abnormal transcript is predicted to translate into a truncated LDLR lacking domains essential for receptor function, the splice region variant associates with a strong non-HDL-C lowering effect and protection against CAD in the general population. These data contrast LDLR truncating mutations that lead to an increase in non-HDL-C because of reduced function of the LDLR [24–26]. The evidence for the non-HDL-C lowering effect of rs72658867-A is further strengthened by replication of the effect in three additional populations. Since the splice region mutation also increases expression of the LDLR mRNA, the non-HDL-C lowering could be mediated by an increase above normal levels in wild type LDLR transcripts. We, however, demonstrate that the wild type mRNA levels are comparable in heterozygous rs72658867-A carriers and non-carriers. Furthermore, others have shown that in a lymphoblastoid cell line generated from a heterozygous carrier of rs72658867-A, the membrane bound LDLR levels and internalization of LDL are similar to that of cell lines that do not carry LDLR mutations [27]. The non-HDL-C lowering effect of rs72658867-A is thus not mediated by a net increase in the wild type LDLR transcripts. LDLR mutations have been described that cause truncation of the receptor at similar location as rs72658867-A is predicted to do [24–26], however, these mutations are different from rs72658867-A in that they appear to lead to reduction in wild type transcripts. In contrast to rs72658867-A, these mutations have been linked to FH and an increase in non-HDL-C. Perhaps the combination of a truncated receptor and normal wild type levels of the LDLR mediate the non-HDL-C lowering effect of rs72658867-A.
The observed effect of rs72658867-A on LDLR splicing can be attributed to its spatial relation to the site of splicing (c.2140+5G>A). Allele specific analysis of mRNA sequence data indicates that the increase in LDLR transcripts in rs72658867-A carriers is derived from the chromosome carrying rs72658867-A. The LDLR splicing defect and increased LDLR mRNA expression are thus likely both mediated by the splice region variant rs72658867-A itself since in Iceland no other variant than rs72658867-A can fully explain the association with non-HDL-C and in the 1000G European ancestry data (Phase 3 dataset, see URL) no variant is correlated with r2>0.5 with rs72658867-A. It is however, unlikely that the effect of rs72658867-A on LDLR expression is mediated by the splicing defect itself. Based on ENCODE data, rs72658867 overlaps a RNA polymerase II binding site in number of different cell lines which may possibly reflect an enhancer site that could mediate altered LDLR expression.
In conclusion we have identified two non-coding low frequency variants in the LDLR gene that associate with lower non-HDL-C and protection against CAD. One of them, the splice region variant rs72658867-A, affects splicing and introduces a premature stop codon that is expected to produce a truncated LDLR lacking the O-linked glycan region and the transmembrane and cytoplasmic domains, domains that are both essential for function of the receptor. The same mutation increases transcription of the LDLR, albeit the normal wild type transcript levels do not exceed levels detected in non-carriers. These data contrast the effects of other reported LDLR truncating mutations that increase LDL-C levels and the risk of CAD. Further functional studies are warranted to gain better understanding of the biology of the splice region variant rs72658867-A.
Materials and Methods
Ethics statement
The study was approved by The National Bioethics Committee in Iceland (Approval no. 07–085, with amendments), and the Data Protection Authority in Iceland (Approval no. 2007060474ÞS/—, with amendments). All donors of biological samples gave informed written consent.
Study populations
Iceland
We obtained blood lipid measurements (non-high-density lipoprotein (non-HDL-C), low-density lipoprotein (LDL-C) and high-density lipoprotein (HDL-C) cholesterol as well as triglycerides (TG)) from three of the largest laboratories in Iceland: (i) Landspítali—The National University Hospital of Iceland (LUH), Reykjavík, Iceland (measurements performed between the years 1993 and 2012, hospitalized and ambulatory patients); (ii) The Laboratory in Mjódd (RAM), Reykjavík, Iceland (measurements performed between 2004 and 2012, ambulatory patients); and (iii) Akureyri Hospital, The Regional Hospital in North Iceland, Akureyri, Iceland (performed between 2004 and 2010, hospitalized and ambulatory patients). Measurements performed at the Icelandic Heart Association at the time of recruitment for deCODE´s studies between the years 1999 and 2004 were also included. The participants had a median of 2 (1–86) and geometric average of 2.5 non-HDL-C measurements. The mean non-HDL-C value was 4.00 mmol/l (SD = 1.18). Lipid levels were adjusted for sex, year of birth and age at measurement, lipid lowering medication and measurement site, using the average of multiple measurements for an individual, and then normalized to a standard normal distribution using quantile normalization. To obtain effect estimates in mmol/L the estimates from the regression analysis were multiplied by the estimated standard deviation of lipid level in the population. Given their approximately log-normal distribution, triglyceride levels were log-transformed before adjustment and the corresponding effect estimates are presented as percentage change instead of units of mmol/L. A total of 119,146 individuals with non-HDL-C were included in the study, where 69,277 were chip-typed and directly imputed; the remaining 49,869 were first and second degree relatives of chip-typed individuals and had their genotypes inferred based on genealogy[15]. Non-HDL-C is obtained by subtracting HDL-C from total cholesterol and gives a measure of the amount of cholesterol carried within all atherogenic lipoprotein particles (VLDL, IDL and LDL).
Coronary artery disease (CAD) was defined as: (a) individuals in the MONICA registry who suffered myocardial infarction (MI) before the age of 75 in Iceland between 1981 and 2002 and satisfied the MONICA criteria[28], (b) subjects with CAD discharge diagnoses (ICD 9 codes 410.*, 411.*, 412.*, 414.* or ICD 10 codes I20.0, I21.*, I22.*. I23.*, I24.*, I25.*) from LUH, (c) subjects diagnosed with significant angiographic CAD (see below) identified from a nationwide clinical registry of coronary angiography and percutaneous coronary interventions (PCI) at LUH between the years 1987 and 2012, (d) subjects undergoing coronary artery bypass grafting (CABG) procedures at LUH between the years 2002 and 2011 or (e) cause of death or contributing cause of death listed as MI or CAD (ICD 9 or 10 codes) on death registries between the years 1996 and 2012. Coronary angiograms in the nationwide registry were evaluated by an interventional cardiologist. Patients were considered to have significant angiographic CAD if one or more of the three major epicardial coronary vessels or the left main coronary artery was found to have at least 50% diameter stenosis by visual estimation. A total of 33,090 CAD cases were used in the study. Of those 14,640 were chip-typed and imputed and the remaining 18,450 were first and second degree relatives of chip-typed individuals and had their genotypes inferred based on genealogy. The 236,254 Icelandic controls were study participants from various deCODE genetics programs without known CAD. The lifespan variable was based on 62,558 individuals that were born after 1890 and lived to be at least 50 years old. Personal identities of the patients and biological samples were encrypted by a third party system provided by the Icelandic Data Protection Authority.
Denmark
The Danish samples are part of the randomised population-based intervention study (Inter99) which has been described in details elsewhere[29]. Danish study participants gave informed consent for use of their biological samples for genetic studies. The current research protocol was approved by The Danish National Ethical Committee on Health Research and is in accordance with the ethical scientific principles of the Helsinki Declaration II.
The Netherlands
Subjects from the Netherlands were recruited within the ‘Nijmegen Biomedical Study’. The details of this study were reported previously [30]. Briefly, this is a population-based survey conducted by the Department for Health Evidence and the Department of Clinical Chemistry of the Radboud. Age - and sex-stratified randomly selected adult inhabitants of Nijmegen were invited to participate and to donate a blood sample for DNA isolation and biochemical studies. The Nijmegen Biomedical Study was approved by the Institutional Review Board of the Radboud University medical center.
Iran
The Iranian samples are part of the Tehran Lipid and Glucose Study (TLGS) cohort. The TLGS design has been described in detail previously [31]. The study has been ongoing for 20 years and 15,005 residents of District 13 of Tehran have been enrolled. For the purpose of this study we included only individuals ≥18 years of age. The study was approved by the National Research Council of the Islamic Republic of Iran.
Lipid values for the Danish, Dutch and Iranian samples were adjusted and standardized as in Iceland.
Genotyping
Illumina SNP chip-genotyping
Icelandic chip-typed samples were assayed using the Illumina HumanHap300, HumanCNV370, HumanHap610, HumanHap1M, HumanHap660, Omni-1, Omni 2.5 or Omni Express bead chips at deCODE genetics. SNPs were excluded if they had (i) yield <95%, (ii) MAF <1% in the population or (iii) significant deviation from Hardy–Weinberg equilibrium in the controls (P <0.001), (iv) if they produced an excessive inheritance error rate (over 0.001) or (v) if there was substantial difference in allele frequency between chip types (from just a single chip if that resolved all differences, but from all chips otherwise). All samples with a call rate below 97% were excluded from the analysis.
Single track assay SNP genotyping
Single SNP genotyping was carried out applying the Centaurus (Nanogen) platform[32].
Whole-genome sequencing
Whole-genome sequencing was performed for 2,636 individuals selected for various conditions. All individuals were sequenced at a minimum depth of 10x (average 22x). Sample preparation, DNA sequencing and alignment were recently described in detail [15]. Briefly, paired-end libraries for sequencing were prepared according to the manufacturer’s instructions (Illumina, TruSeqTM), sequencing by synthesis was performed on Illumina GAIIx and/or HiSeq 2000 instruments and reads were aligned to NCBI Build 36 of the human reference sequence using Burrows-Wheeler Aligner (BWA) 0.5.9[33].
Long-range phasing and genotype imputation
Long-range phasing of all chip-genotyped individuals was performed with methods previously described[34,35]. For the HumanHap series of chips, 304,937 SNPs were used for long-range phasing, whereas for the Omni series of chips 564,196 SNPs were included. The final set of SNPs used for long-range phasing was composed of 707,525 SNPs. A detailed description of imputation methods used for the Icelandic population was recently published[15]. In brief, SNPs and INDELs identified through sequencing were imputed into 104,220 chip-genotyped and long-range phased Icelanders. Approximately 28.3 million SNPs and small INDEL variants were imputed based on this set of individuals. The imputation quality score for the four highly significant LDLR sequence variants, rs17248720-T, rs17248748-T, rs200238879-C and rs72658867-A was 0.99, 0.99, 0.95 and 0.98, respectively (Table 1).
Association analysis
A generalized form of linear regression that accounts for relatedness between individuals was used to test for the association of quantitative traits with sequence variants[36]. Conditional analysis was performed by including the sequence variant being conditioned on as a covariate in the model under the null and the alternative in the generalized linear regression. Stepwise forward selection was used starting with sequence variants as a covariate in the model then adjusting for the most significant sequence variant and repeating that process until no variant remained significant in the region. Logistic regression was used to test for association between sequence variants and disease (CAD), treating disease status as the response and expected genotype counts from imputation or allele counts from direct genotyping as covariates. Other available individual characteristics that correlate with disease status were also included in the model as nuisance variables. These characteristics were: sex, county of birth, current age or age at death (first - and second-order terms included), blood sample availability for the individual and an indicator function for the overlap of the lifetime of the individual with the timespan of phenotype collection. Testing was performed using the likelihood ratio statistic.
Correction for relatedness of Icelandic subjects and population stratification
Individuals in both the Icelandic case and control groups are related, causing the χ2 test statistic to have a mean >1 and median >0.675. We estimated the inflation factor λg based on a subset of about 300,000 common variants and the P-values were adjusted by dividing the corresponding χ2 values by this factor to adjust for both relatedness and potential population stratification[37]. Genomic control correction factors: non-HDL-C: 1.36, triglycerides: 1.40, HDL-C: 1.575, CAD: 1.71, CAD age of onset: 1.41, lifespan: 1.49.
Genotype imputation information
The informativeness of genotype imputation was estimated by the ratio of the variance of imputed expected allele counts and the variance of the actual allele counts:
where θ∈{0,1} is the allele count. Var[E(θ|chipdata)] was estimated by the observed variance of the imputed expected counts and Var(θ) was estimated by p(1 − p), where p is the allele frequency. Sequence variants with imputation information below 0.8 were excluded from the analysis.Gene and variant annotation
Sequence variants were annotated with information from Ensembl release 70 using Variant Effect Predictor (VEP) version 2.8[38]. Variants annotated as having high impact include loss-of-function variants, i.e. stop-gained variants, frameshift indels and essential splice variants, and moderate impact variants include missense, inframe indels, and splice region variants.
Gene Expression Microarrays
Samples of RNA from human peripheral blood were hybridized to Agilent Technologies Human 25K microarrays as described previously[39]. We quantified expression changes between two samples as the mean logarithm (log10) expression ratio (MLR) compared to a reference pool RNA sample. In comparing expression levels between groups of individuals with different genotypes, we denoted the expression level for each genotype as 10 (average MLR), where the MLR is averaged over individuals with the particular genotype. We determined s.e.m. and significance by regressing the MLR values against the number of risk alleles carried. We took into account the effects of age, gender and differential cell type count in blood as explanatory variables in the regression. P-values were adjusted for familial relatedness of the individuals by simulation.
RNA-analysis
Preparation of Poly-A cDNA sequencing libraries
The quality and quantity of isolated total RNA samples was assessed using the Total RNA 6000 Nano chip for the Agilent 2100 Bioanalyzer. cDNA libraries derived from Poly-A mRNA were generated using Illumina´s TruSeq RNA Sample Prep Kit. Briefly, Poly-A mRNA was isolated from total RNA samples (1–4 μg input) using hybridizaton to Poly-T beads. The Poly-A mRNA was fragmented at 94°C, and first-strand cDNA was prepared using random hexamers and the SuperScript II reverse transcriptase (Invitrogen). Following second-strand cDNA synthesis, end repair, addition of a single A base, adaptor ligation, AMPure bead purification, and PCR amplification, the resulting cDNA was measured on a Bioanalyzer using the DNA 1000 Lab Chip.
Sequencing
Samples were clustered on to flowcells using Illumina´s cBot and the TruSeq PE cluster kits v2, respectively. Paired-end sequencing (2x76 cycles) was performed with either GAIIx instruments using the TruSeq SBS kits v5 from Illumina or HiSeq 2000 instruments using TruSeq v3 flowcells/SBS kits. In some instances the readlength was 2x50 or 2x101 cycles. Approximately 125–175 million forward reads (250–350 M total reads) were sequenced per sample.
Read alignment
RNA sequencing reads were aligned to Homo sapiens Build 36 with TopHat[40] version 1.4.1 with a supplied set of known transcripts in GTF format (RefSeq hg18; Homo sapiens, NCBI, build36.3). TopHat was configured such that it attempts first to align reads to the provided transcriptome then, for reads that do not map fully to the transcriptome, it attempts to map them onto the genome. Read mapping statistics used for read count normalization were calculated using the CollectRnaSeqMetrics tool in Picard version 1.79 (see URLs).
RNA-seq data analysis
For each sample, the normalized read count was determined for each bp location as # reads covering the base / the total # of aligned reads in the individual in billions. Then the median normalized read count for each genotype group was determined for each bp location and plotted graphically. In order to quantify the levels of expression in different regions, the median of normalized read counts over the genomic segment in question was determined for each individual. We then did a logarithmic transformation of the normalized read counts and standardized them so that the response variable had a mean 0 and standard deviation of 1.
Allele specific expression analysis of LDLR for rs72658867 heterozygous carriers
To analyze allele specific expression for heterozygous carriers of rs72658867-A we looked at base counts in the RNA-Seq data at positions of synonymous variants in heterozygous state (see S8 Table for list of the allele specific synonymous variants tested). All the synonymous variants we considered had D’≥0.99 with the splice region variant rs72658867-A. Using the phasing information for the imputed genotypes of the samples, we calculated the proportion of read bases at the positions of the synonymous variants, where the proportion was #ALT bases/#total bases if the alternative allele (ALT) of the synonymous variant was positively correlated with rs72658867-A and #REF bases/#total bases if the reference allele (REF) of the synonymous variant was negatively correlated with rs72658867-A. The median proportions over all synonymous variants for rs72658867 GG and GA carriers were 0.52 and 0.61, respectively (P = 0.0016, Mann-Whitney test, making a simplifying assumption of independence of proportions); the median proportion for rs72658867 GG being greater than 0.50 reflects a reference bias in the mapping of RNA-Seq reads.
Quantitative RT-PCR
Quantitative RT-PCR of the LDLR cDNA, specific for the correct removal of intron 14, was performed with an assay from the Roch Universal probe library. This was run on an ABI 7900HT Real-time PCR system according to standard protocol (forward primer: ccactcgcccaagtttacc, reverse primer: gcagcctcagcctctgtg, probe #17).
URLs
Picard: http://broadinstitute.github.io/picard/command-line-overview.html#CollectRnaSeqMetrics
HaploReg v3 (accessed May 2015):
http://www.broadinstitute.org/mammals/haploreg/haploreg_v3.php
1000genomes Phase 3 (May 2013): ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/release/20130502/
Supporting Information
Zdroje
1. Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29 : 431–8. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2740366&tool=pmcentrez&rendertype=abstract doi: 10.1161/ATVBAHA.108.179564 19299327
2. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285 : 2486–97. http://www.ncbi.nlm.nih.gov/pubmed/11368702 11368702
3. Rana JS, Boekholdt SM, Kastelein JJP, Shah PK. The role of non-HDL cholesterol in risk stratification for coronary artery disease. Curr Atheroscler Rep. 2012;14 : 130–4. http://www.ncbi.nlm.nih.gov/pubmed/22203405 doi: 10.1007/s11883-011-0224-x 22203405
4. Lagor WR, Millar JS. Overview of the LDL receptor: relevance to cholesterol metabolism and future approaches for the treatment of coronary heart disease. J Receptor Ligand Channel Res. 2010;3 : 1–14.
5. Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974;249 : 5153–5162. 4368448
6. Varghese MJ. Familial hypercholesterolemia: A review. Ann Pediatr Cardiol. 2014;7 : 107–17. doi: 10.4103/0974-2069.132478 24987256
7. Singh S, Bittner V. Familial hypercholesterolemia-epidemiology, diagnosis, and screening. Curr Atheroscler Rep. 2015;17 : 482. http://www.ncbi.nlm.nih.gov/pubmed/25612857 doi: 10.1007/s11883-014-0482-5 25612857
8. Usifo E, Leigh SEA, Whittall RA, Lench N, Taylor A, Yeats C, et al. Low-Density Lipoprotein Receptor Gene Familial Hypercholesterolemia Variant Database: Update and Pathological Assessment. Ann Hum Genet. 2012;76 : 387–401. doi: 10.1111/j.1469-1809.2012.00724.x 22881376
9. Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40 : 161–169. doi: 10.1038/ng.76 18193043
10. Aulchenko YS, Ripatti S, Lindqvist I, Boomsma D, Heid IM, Pramstaller PP, et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41 : 47–55. doi: 10.1038/ng.269 19060911
11. Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43 : 333–8. doi: 10.1038/ng.784 21378990
12. Lange LA, Hu Y, Zhang H, Xue C, Schmidt EM, Tang Z-Z, et al. Whole-exome sequencing identifies rare and low-frequency coding variants associated with LDL cholesterol. Am J Hum Genet. 2014;94 : 233–45. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3928660&tool=pmcentrez&rendertype=abstract doi: 10.1016/j.ajhg.2014.01.010 24507775
13. Do R, Stitziel NO, Won H-H, Jørgensen AB, Duga S, Angelica Merlini P, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2014; http://www.ncbi.nlm.nih.gov/pubmed/25487149
14. Gudbjartsson DF et al. Large-scale whole-genome sequencing of the Icelandic population. Nat Genet.: In press.
15. Styrkarsdottir U, Thorleifsson G, Sulem P, Gudbjartsson DF, Sigurdsson A, Jonasdottir A, et al. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature. 2013;497 : 517–20. http://www.ncbi.nlm.nih.gov/pubmed/23644456 doi: 10.1038/nature12124 23644456
16. Bourbon M, Duarte MA, Alves AC, Medeiros AM, Marques L, Soutar AK. Genetic diagnosis of familial hypercholesterolaemia: the importance of functional analysis of potential splice-site mutations. J Med Genet. 2009;46 : 352–7. http://www.ncbi.nlm.nih.gov/pubmed/19411563 doi: 10.1136/jmg.2007.057000 19411563
17. Whittall RA, Matheus S, Cranston T, Miller GJ, Humphries SE. The intron 14 2140+5G>A variant in the low density lipoprotein receptor gene has no effect on plasma cholesterol levels. J Med Genet. 2002;39: e57. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1735227&tool=pmcentrez&rendertype=abstract 12205127
18. Jensen HK. The molecular genetic basis and diagnosis of familial hypercholesterolemia in Denmark. Dan Med Bull. 2002;49 : 318–45. 12553167
19. Huijgen R, Kindt I, Fouchier SW, Defesche JC, Hutten BA, Kastelein JJP, et al. Functionality of sequence variants in the genes coding for the low-density lipoprotein receptor and apolipoprotein B in individuals with inherited hypercholesterolemia. Hum Mutat. 2010;31 : 752–60. doi: 10.1002/humu.21258 20506408
20. Linsel-Nitschke P, Götz A, Erdmann J, Braenne I, Braund P, Hengstenberg C, et al. Lifelong reduction of LDL-cholesterol related to a common varriant in the LDL-receptor gene decreases the risk of coronary artery disease—A Mendelian randomisation study. PLoS One. 2008;3.
21. Gudnason V, Sigurdsson G, Nissen H, Humphries SE. Common founder mutation in the LDL receptor gene causing familial hypercholesterolaemia in the Icelandic population. Hum Mutat. 1997;10 : 36–44. 9222758
22. Zhang F, Lin M, Abidi P, Thiel G, Liu J. Specific interaction of Egr1 and c/EBPbeta leads to the transcriptional activation of the human low density lipoprotein receptor gene. J Biol Chem. 2003;278 : 44246–54. http://www.ncbi.nlm.nih.gov/pubmed/12947119 12947119
23. Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40: D930–4. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3245002&tool=pmcentrez&rendertype=abstract doi: 10.1093/nar/gkr917 22064851
24. Lehrman MA, Russell DW, Goldstein JL, Brown MS. Alu-Alu recombination deletes splice acceptor sites and produces secreted low density lipoprotein receptor in a subject with familial hypercholesterolemia. J Biol Chem. 1987;262 : 3354–61. http://www.ncbi.nlm.nih.gov/pubmed/3818645 3818645
25. Takada D, Emi M, Ezura Y, Nobe Y, Kawamura K, Iino Y, et al. Interaction between the LDL-receptor gene bearing a novel mutation and a variant in the apolipoprotein A-II promoter: molecular study in a 1135-member familial hypercholesterolemia kindred. J Hum Genet. 2002;47 : 656–64. 12522687
26. Lehrman MA, Schneider WJ, Südhof TC, Brown MS, Goldstein JL, Russell DW. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985;227 : 140–6. http://www.ncbi.nlm.nih.gov/pubmed/3155573 3155573
27. Holla ØL, Nakken S, Mattingsdal M, Ranheim T, Berge KE, Defesche JC, et al. Effects of intronic mutations in the LDLR gene on pre-mRNA splicing: Comparison of wet-lab and bioinformatics analyses. Mol Genet Metab. 2009;96 : 245–52. http://www.ncbi.nlm.nih.gov/pubmed/19208450 doi: 10.1016/j.ymgme.2008.12.014 19208450
28. Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation. 1979;59 : 607–9. http://www.ncbi.nlm.nih.gov/pubmed/761341 761341
29. Jørgensen T, Borch-Johnsen K, Thomsen TF, Ibsen H, Glümer C, Pisinger C. A randomized non-pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. Eur J Cardiovasc Prev Rehabil. 2003;10 : 377–86. 14663300
30. Wetzels JFM, Kiemeney LALM, Swinkels DW, Willems HL, den Heijer M. Age - and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007;72 : 632–7. http://www.ncbi.nlm.nih.gov/pubmed/17568781 17568781
31. Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials. 2009;10 : 5. doi: 10.1186/1745-6215-10-5 19166627
32. Kutyavin I V, Milesi D, Belousov Y, Podyminogin M, Vorobiev A, Gorn V, et al. A novel endonuclease IV post-PCR genotyping system. Nucleic Acids Res. 2006;34: e128. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1636472&tool=pmcentrez&rendertype=abstract 17012270
33. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25 : 1754–60. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2705234&tool=pmcentrez&rendertype=abstract doi: 10.1093/bioinformatics/btp324 19451168
34. Kong A, Steinthorsdottir V, Masson G, Thorleifsson G, Sulem P, Besenbacher S, et al. Parental origin of sequence variants associated with complex diseases. Nature. 2009;462 : 868–874. doi: 10.1038/nature08625 20016592
35. Kong A, Masson G, Frigge ML, Gylfason A, Zusmanovich P, Thorleifsson G, et al. Detection of sharing by descent, long-range phasing and haplotype imputation. Nat Genet. 2008;40 : 1068–1075. doi: 10.1038/ng.216 19165921
36. Steinthorsdottir V, Thorleifsson G, Sulem P, Helgason H, Grarup N, Sigurdsson A, et al. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat Genet. 2014;46 : 294–8. doi: 10.1038/ng.2882 24464100
37. Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55 : 997–1004. 11315092
38. McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;26 : 2069–70. doi: 10.1093/bioinformatics/btq330 20562413
39. Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J, et al. Genetics of gene expression and its effect on disease. Nature. 2008;452 : 423–8. http://www.ncbi.nlm.nih.gov/pubmed/18344981 doi: 10.1038/nature06758 18344981
40. Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7 : 562–78. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3334321&tool=pmcentrez&rendertype=abstract doi: 10.1038/nprot.2012.016 22383036
Štítky
Genetika Reprodukční medicína
Článek The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis inČlánek Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 9
-
Všechny články tohoto čísla
- Retraction: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Signaling from Within: Endocytic Trafficking of the Robo Receptor Is Required for Midline Axon Repulsion
- A Splice Region Variant in Lowers Non-high Density Lipoprotein Cholesterol and Protects against Coronary Artery Disease
- The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis in
- A NIMA-Related Kinase Suppresses the Flagellar Instability Associated with the Loss of Multiple Axonemal Structures
- Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
- Expression of Concern: Protein Under-Wrapping Causes Dosage Sensitivity and Decreases Gene Duplicability
- Mutagenesis by AID: Being in the Right Place at the Right Time
- Identification of as a Genetic Modifier That Regulates the Global Orientation of Mammalian Hair Follicles
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- Evaluating the Performance of Fine-Mapping Strategies at Common Variant GWAS Loci
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs
- Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception
- Cognitive Function Related to the Gene Acquired from an LTR Retrotransposon in Eutherians
- Critical Function of γH2A in S-Phase
- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
- Integration of Genome-Wide SNP Data and Gene-Expression Profiles Reveals Six Novel Loci and Regulatory Mechanisms for Amino Acids and Acylcarnitines in Whole Blood
- A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism
- Cell Cycle Regulates Nuclear Stability of AID and Determines the Cellular Response to AID
- A Genome-Wide Association Analysis Reveals Epistatic Cancellation of Additive Genetic Variance for Root Length in
- Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
- RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization
- Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes
- Dynamic Contacts of U2, RES, Cwc25, Prp8 and Prp45 Proteins with the Pre-mRNA Branch-Site and 3' Splice Site during Catalytic Activation and Step 1 Catalysis in Yeast Spliceosomes
- ARID1A Is Essential for Endometrial Function during Early Pregnancy
- Predicting Carriers of Ongoing Selective Sweeps without Knowledge of the Favored Allele
- An Interaction between RRP6 and SU(VAR)3-9 Targets RRP6 to Heterochromatin and Contributes to Heterochromatin Maintenance in
- Photoreceptor Specificity in the Light-Induced and COP1-Mediated Rapid Degradation of the Repressor of Photomorphogenesis SPA2 in Arabidopsis
- Autophosphorylation of the Bacterial Tyrosine-Kinase CpsD Connects Capsule Synthesis with the Cell Cycle in
- Multimer Formation Explains Allelic Suppression of PRDM9 Recombination Hotspots
- Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling
- A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary
- Cell-Autonomous Gβ Signaling Defines Neuron-Specific Steady State Serotonin Synthesis in
- Discovering Genetic Interactions in Large-Scale Association Studies by Stage-wise Likelihood Ratio Tests
- The RCC1 Family Protein TCF1 Regulates Freezing Tolerance and Cold Acclimation through Modulating Lignin Biosynthesis
- The AMPK, Snf1, Negatively Regulates the Hog1 MAPK Pathway in ER Stress Response
- The Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway
- Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens
- Recurrent Domestication by Lepidoptera of Genes from Their Parasites Mediated by Bracoviruses
- Three Different Pathways Prevent Chromosome Segregation in the Presence of DNA Damage or Replication Stress in Budding Yeast
- Identification of Four Mouse Diabetes Candidate Genes Altering β-Cell Proliferation
- The Intolerance of Regulatory Sequence to Genetic Variation Predicts Gene Dosage Sensitivity
- Synergistic and Dose-Controlled Regulation of Cellulase Gene Expression in
- Genome Sequence and Transcriptome Analyses of : Metabolic Tools for Enhanced Algal Fitness in the Prominent Order Prymnesiales (Haptophyceae)
- Ty3 Retrotransposon Hijacks Mating Yeast RNA Processing Bodies to Infect New Genomes
- FUS Interacts with HSP60 to Promote Mitochondrial Damage
- Point Mutations in Centromeric Histone Induce Post-zygotic Incompatibility and Uniparental Inheritance
- Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality
- Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins
- A Large-Scale Functional Analysis of Putative Target Genes of Mating-Type Loci Provides Insight into the Regulation of Sexual Development of the Cereal Pathogen
- A Genetic Selection for Mutants Reveals an Interaction between DNA Polymerase IV and the Replicative Polymerase That Is Required for Translesion Synthesis
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




