-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaUnravelling the Genetics of Ischaemic Stroke
article has not abstract
Published in the journal: . PLoS Med 7(3): e32767. doi:10.1371/journal.pmed.1000225
Category: Research in Translation
doi: https://doi.org/10.1371/journal.pmed.1000225Summary
article has not abstract
Summary Points
-
Epidemiological studies suggest genetic predisposition is important in stroke risk.
-
Monogenic conditions are important for the individual patient but do not account for much population attributable risk. An increasing number of such conditions are being described, particularly for the small vessel disease stroke subtype.
-
The candidate genes approach has proved disappointing in identifying genes contributing to the risk of multifactorial or polygenic stroke. This is a situation shared with other complex diseases.
-
Recently the GWAS (genome-wide association study) approach has identified genetic loci for many other cardiovascular diseases such as coronary heart disease, diabetes, and hypertension, and is just being applied to stroke. Some novel genetic variants initially associated with other cardiovascular diseases have recently been identified as risk factors in stroke populations.
Are Genetic Factors Important in Stroke Risk?
Stroke is the third commonest cause of death and the major cause of adult neurological disability. It is a major health problem not only in the developed world, but is increasing in incidence in much of the developing world. Cerebrovascular disease is also an important cause of dementia and age-related cognitive decline, and the commonest cause of late-onset epilepsy. Conventional risk factors such as high blood pressure account for a significant proportion of stroke risk but much risk remains unexplained, and we do not understand why some individuals with risk factors such as hypertension develop stroke while others with similar risk factor profiles remain disease free. It has been suggested that genetic factors may be responsible for some of this unexplained risk, but what is the evidence for this?
Limited data from twin studies suggests stroke is more common in monozygotic compared with dizygotic twins, consistent with a role for genetic factors [1]. Considerably more data is available for family history studies, which show a family history of stroke is more common in individuals with ischaemic stroke [1]. The association is stronger for younger individuals, and those with the large artery disease and small vessel disease subtypes of stroke [2],[3]. This association may represent genetic predisposition, but could also be explained by shared environmental factors. More robust data come from studying intermediate phenotypes of stroke. These are markers of disease, usually detected on imaging, which represent intermediate stages of disease pathology leading to stroke. Both twin and family studies have shown that MRI white matter hyperintensities, which usually represent small vessel disease [4], are the most heritable cerebrovascular phenotype, with a heritability (proportion of variation explained by genetic factors) estimated to be between 55% and 71% [5]–[7]. Carotid artery intima-media thickness, measured by ultrasound and believed to represent early stages of atherosclerosis and therefore relate to large artery stroke [8],[9], has been estimated to have a heritability of 30% to 68% [10]–[13].
Why Should We Identify Genes for Stroke?
A small proportion of ischaemic stroke is monogenic [14]. A mutation in a specific gene results in disease, and most individuals with the abnormality are likely to develop stroke at some stage in their life. For these diseases identifying the underlying mutation allows diagnosis, prognostic information, specific treatments in some cases, and enables counselling of other family members. However, the vast majority of stroke appears to be “polygenic”; multiple genes, each likely to confer a small risk, interact with environmental risk factors to result in disease [15]. Identifying these underlying genetic risk factors may allow improved risk profiling, although as each genetic variant is likely to confer only a small risk, any useful risk prediction is likely to require a combination of multiple markers. Genetic testing for other polygenic diseases has already been developed, and indeed some gene tests for stroke are already marketed. However, their clinical relevance has been debated; most panels of genetic markers available explain only a small portion of total “genetic risk” [16]. Furthermore, their use has been questioned when clinicians already have difficulty treating known risk factors such as hypertension, which make a stronger contribution to risk, and concern has been expressed over the psychological consequences of testing. Perhaps more importantly, discovering genetic variants conferring increased stroke risk may allow new pathways involved in the pathogenesis of stroke to be identified and new treatments to be developed. This approach is just beginning to bear fruit in other “complex” diseases involving interactions between multiple genes and environment, such as macular degeneration and Crohn's disease [17],[18].
How Can We Identify Genes for Stroke?
Linkage techniques have been successfully used to identify a number of genes causing monogenic stroke. This relies on identifying associations between chromosomal markers and disease phenotype within families. Linkage is good at identifying genes conferring greatly increased risk, but has been less successful in common polygenic complex disease such as stroke. There was excitement when this approach identified the STRK1 gene in the Icelandic population as phosphodiesterase 4D [19], but this could not be replicated in other European populations [20].
Until recently the mainstay of investigating stroke genetics was the candidate gene method. Genetic variants (usually single nucleotide polymorphisms [SNPs]) are identified in a “candidate” gene that is hypothesised to be involved in stroke risk. The frequency of the SNP is compared in a group of stroke patients compared with controls. Many hundreds of such studies have been carried out in stroke with largely disappointing results [21]. This picture is common to many other complex diseases, and the reasons for lack of success have been explored in detail [21]. Important factors include small sample sizes, failure to phenotype cases accurately, and failure to replicate positive associations, combined with publication bias resulting in preferential publication of positive associations. A major problem with the candidate gene method is that associations can be identified only in genes already discovered and implicated in the disease. Completely novel genes involved in disease risk cannot be identified.
The Genome-Wide Association Study (GWAS) Approach
Recently the field of complex genetics has been revolutionised by the genome-wide association study (GWAS) method [22]. This allows up to 1 million SNPs spanning the whole genome to be genotyped in a single individual. Using a case control methodology, and rigorous statistical methods to account for the multiple comparisons made, associations between completely unexpected chromosomal loci and disease can be identified. With this approach, combined with much larger study sizes involving thousands of patients and rigorous replication of positive results, over 600 new genetic loci have been discovered predisposing to other complex disorders such as diabetes and myocardial infarction (Figure 1) [23]. An early success was in age-related macular degeneration, which, like stroke, is primarily a disease of the elderly in which cardiovascular risk factors increase risk [24]. Robust and replicable associations were identified with complement factor H, and this has allowed new treatment approaches to be developed. Novel genetic association have now been reported in many cardiovascular diseases including myocardial infarction, diabetes, obesity, hypertension, and hyperlipidaemia [23]. An important message from these studies is that most genetic variants discovered using this approach individually account for only a small increase in risk, with odds ratios usually between 1.1 and 1.3. This means large sample sizes are required to identify such variants.
Fig. 1. Published associations between chromosomal loci and many diseases which have been identified by genome-wide associations up to June 2009. 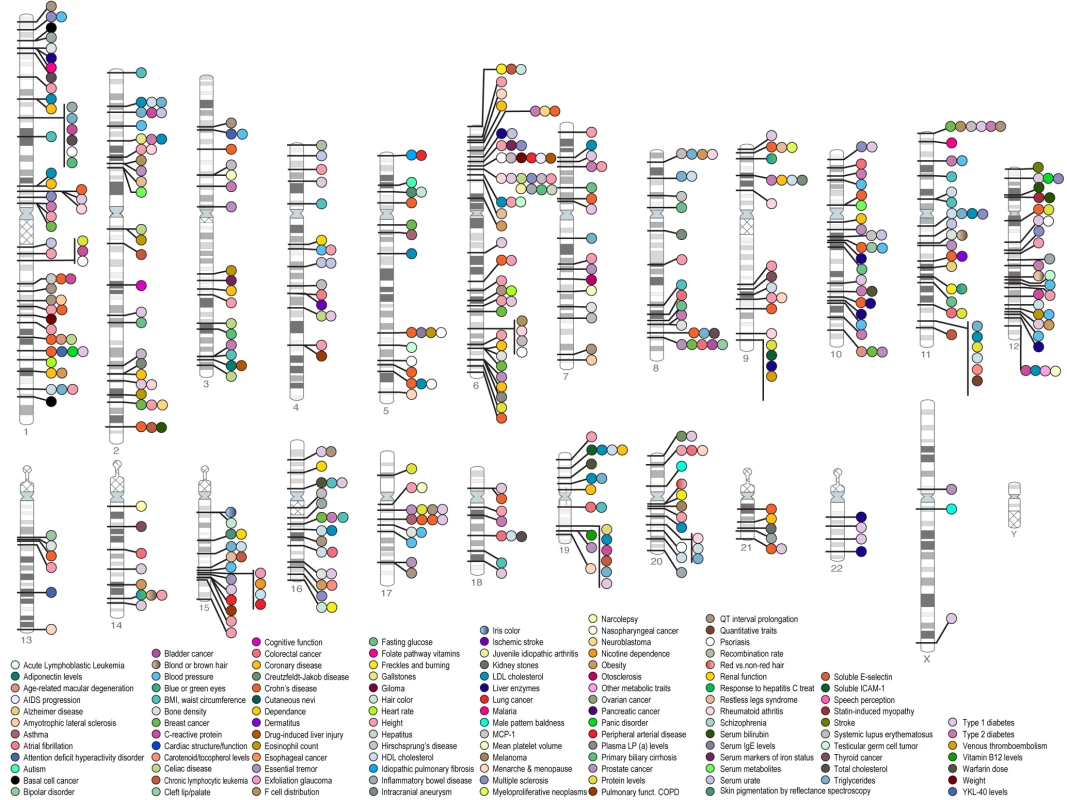
A significance level of p<5×10−8 has been used to determine whether associations are significant. As can be seen a large number of associations have been identified with many common diseases. Credit: Darryl Leja and Teri Manolio; available from http://www.genome.gov/gwastudies. Accessed 25 September 2009. The GWAS Approach in Stroke
Stroke genetics has lagged behind that of many other complex diseases, and the GWAS approach is only just beginning to be applied. An early study in 500 individuals found no definite associations, but we now appreciate this was underpowered [25]. Larger studies are currently taking place in the UK (as part of the Wellcome Case Control Consortium 2), the US, Australia, and other countries. As yet most data, and most current studies, are from white populations. Other complex diseases have taught us that we may need very large sample sizes to identify genetic variants. For example, in hypertension, initial studies in a few thousand cases were disappointing. More recently meta-analysis of over 80,000 cases identified eight novel genetic loci [26]. The importance of robust replication prior to publication was demonstrated by the recent high-profile publication of a novel Chromosome 12 locus associated with stroke identified on GWAS [27], which unfortunately could not be replicated in a sample of over 10,000 individuals, although differences in study designs—the original study was based on prospective cohorts and the replication was cross-sectional—could potentially account for some of the discrepancy [28]. The lack of success in stroke so far is likely to reflect the relatively small sample sizes studied to date, but other issues may also be important. For example, the GWAS approach relies on the existence of common variants, each of which confer risk. It is less good at detecting rare variants which could also confer increased risk.
Some novel genetic variants initially associated with other cardiovascular diseases have recently been identified as risk factors in stroke populations. A Chromosome 9 variant associated with myocardial infarction and coronary artery disease [29] was found to also be associated with stroke across multiple populations, but this association was due to an association with large artery stroke, and no association was found with other stroke subtypes [30]. Two other variants identified as risk factors for atrial fibrillation have also been associated with stroke; here the association was primarily with cardioembolic stroke [31],[32]. These findings emphasise that genetic risk factors may predispose to specific subtypes of stroke. Therefore, identifying such risk factors will depend on rigorous stroke phenotyping and large numbers of cases with each stroke subtype.
Monogenic Stroke Disorders
A large number of rare monogenic disorders can cause stroke (Table 1) Some of these result in stroke as part of a systemic disorder, while others produce a clinical phenotype limited to the central nervous system [14]. Progress has been particularly exciting in cerebral small vessel disease. This causes lacunar stroke, which accounts for about 20% of ischaemic stroke, and is the most common cause of vascular dementia. The most common monogenic form of small vessel disease is CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), which results from mutations in the NOTCH3 gene [33]. A striking feature of CADASIL is that, even within families, disease severity is highly variable. This variation is not accounted for by the site of the disease mutation. Confluent white matter hyperintensity called leukoaraiosis, and best seen on MRI, is a prominent feature in CADASIL. A recent study measuring leukoaraiosis volume demonstrated that the variability in leukoariosis volume between different CADASIL carriers has a strong genetic predisposition, with a heritability as high as 63% [34]. This raises the intriguing possibility that other genes modulate the damage caused by the NOTCH3 mutation to modify the disease phenotype. GWAS studies are currently being planned to try to identify these modifying genes. Whether such genes modify only leukoaraiosis severity in CADASIL, or whether they could also play an important role in modifying white matter damage in response to other more common risk factors such as hypertension, remains to be determined. Similar GWAS studies are being carried out using the intermediate phenotype of white matter hyperintensity lesion volume both in normal populations and in populations with stroke and stroke risk factors. It will be interesting to compare the results of the studies in different populations.
Tab. 1. Monogenic or single gene disorders causing stroke, classified according to the each one's stroke subtype. 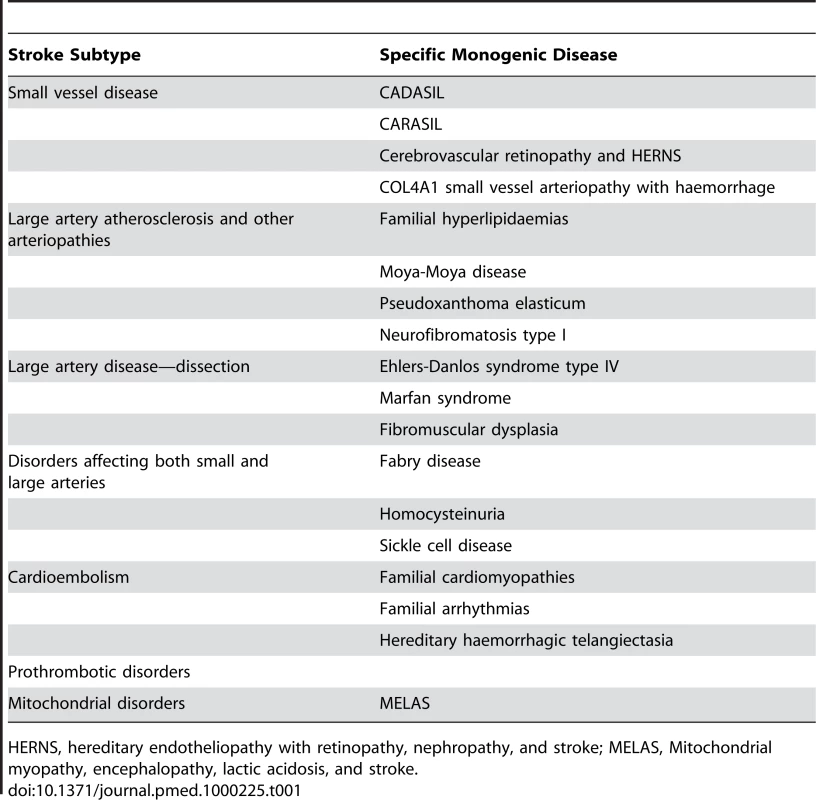
HERNS, hereditary endotheliopathy with retinopathy, nephropathy, and stroke; MELAS, Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke. Genes causing a number of rarer monogenic forms of small vessel disease have recently been identified. CARASIL (cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy), which causes lacunar stroke, leukoaraiosis, and early onset vascular dementia, has been shown to results from mutations in the HtrA serine protease 1 (HTRA1) gene, which is involved in TGF-β signalling [35]. Autosomal dominant retinal vasculopathy with cerebral leukodystrophy is a microvascular endotheliopathy presenting with visual loss, stroke, and dementia with onset in middle age. C-terminal frameshift mutations in TREX1, which encodes a DNA-specific 3′ to 5′ exonuclease ubiquitously expressed in mammalian cells were identified [36]. These truncated proteins retain exonuclease activity but lose normal perinuclear localization. COL4A1, a gene encoding the type IV collagen alpha1 chain, has been found to be involved in families with autosomal-dominant porencephaly and infantile hemiparesis [37]. Patients have been reported to present with adult-onset white matter ischaemic changes consistent with small vessel disease with microbleeds in the absence of infantile hemiparesis or intracerebral haemorrhage [38]. Fabry disease is an X-linked recessive lysosomal storage disease resulting from deficient alpha-galactosidase which causes organ failure in multiple beds including the brain. Stroke may result from a number of mechanisms including cerebral small vessel disease, and confluent white matter ischaemic changes are seen on MRI. It was reported be an important cause of young-onset cryptogenic ischemic stroke, accounting for 4.9% of male cases and 2.4% of female cases, although further studies are required to confirm this [39]. Therefore multiple different gene defects resulting in disruption of multiple different pathways can result in the a similar clinical phenotypes of cerebral small vessel disease.
Conclusions
In summary, genetic factors appear to be important in stroke risk, although we do not really know how important. Candidate gene studies have been largely disappointing but with GWAS technology we may well be at the dawn of a new era in stroke genetics. Future technological advances such as copy number variant determination and, whole genome sequencing are also likely to be important; the latter will allow rare variants associated with disease to be identified. Studies to date have emphasised the importance of careful phenotyping or stroke subtyping, and the experience of other complex diseases has taught us that we need large sample studies and rigorous replication of results.
Zdroje
1. FlossmannE
SchulzUGR
RothwellPM
2004 A systematic review of the methods and results of studies of the genetic epidemiology of ischaemic stroke. Stroke 35 212 227
2. Jerrard-DunneP
CloudG
HassanA
MarkusHS
2003 Evaluating the genetic component of ischemic stroke subtypes: a family history study. Stroke 34 1364 1369
3. PolychronopoulosP
GioldasisG
EllulJ
MetallinosIC
LekkaNP
2003 Family history of stroke in stroke types and subtypes. J Neurol Sci 195 117 122
4. FazekasF
KleinertR
OffenbacherH
SchmidtR
KleinertG
1993 Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43 1683 1689
5. CarmelliD
DeCarliC
SwanGE
JackLM
ReedT
1988 Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke 29 1177 1181
6. AtwoodLD
WolfPA
Heard-CostaNL
MassaroJM
BeiserA
2004 Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke 35 1609 1613
7. TurnerST
FornageM
JackCRJr
MosleyTH
KardiaSL
2005 Genomic susceptibility loci for brain atrophy in hypertensive sibships from the GENOA study. Hypertension 45 793 8
8. HumphriesSE
MorganL
2004 Genetic risk factors for stroke and carotid atherosclerosis: insights into pathophysiology from candidate gene approaches. Lancet Neurol 3 227 235
9. LorenzMW
MarkusHS
BotsML
RosvallM
SitzerM
2007 Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115 459 467
10. DuggiralaR
Gonzalez VillalpandoC
O'LearyDH
SternMP
1996 Genetic basis of variation in carotid artery wall thickness. Stroke 27 833 837
11. JarttiL
RonnemaaT
KaprioJ
JarvisaloMJ
ToikkaJO
2002 Population-based twin study of the effects of migration from Finland to Sweden on endothelial function and intima-media thickness. Arterioscler Thromb Vasc Biol 22 832 837
12. MoskauS
GollaA
GrotheC
BoesM
PohlC
2005 Heritability of carotid artery atherosclerotic lesions: an ultrasound study in 154 families. Stroke 36 5 8
13. Hank JuoSuh-Hang
LinHsiu-Fen
RundekTanja
SabalaEdisonA.
Boden-AlbalaBernadette
2004 Genetic and Environmental Contributions to Carotid Intima-Media Thickness and Obesity Phenotypes in the Northern Manhattan Family Study. Stroke 35 2243 2247
14. HassanA
MarkusHS
2000 Genetics and ischaemic stroke. Brain 123 1784 1812
15. DichgansM
2007 Genetics of ischaemic stroke. Lancet Neurol 6 149 161
16. KraftP
HunterDJ
2009 Genetic risk prediction–are we there yet? N Engl J Med 360 1701 1703
17. RohrerB
LongQ
CoughlinB
WilsonRB
HuangY
2009 A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest Ophthalmol Vis Sci 50 3056 3064
18. KlionskyDJ
2009 Crohn's disease, autophagy, and the Paneth cell. N Engl J Med 360 1785 1786
19. GretarsdottirS
ThorleifssonG
ReynisdottirST
ManolescuA
JonsdottirS
2003 The gene encoding phosphodiesterase 4D confers risk of ischaemic stroke. Nat Genet 353 131 138
20. BevanS
DichgansM
GschwendtnerA
KuhlenbäumerG
RingelsteinEB
2008 Variation in the PDE4D Gene and Ischemic Stroke Risk. A Systematic Review and Meta-analysis on 5200 Cases and 6600 Controls. Stroke 39 1966 1971
21. DichgansM
MarkusHS
2005 Genetic association studies in stroke: methodological issues and proposed standard criteria. Stroke 36 2027 2031
22. HardyJ
SingletonA
2009 Genomewide association studies and human disease. N Engl J Med 360 1759 1768
23. HindorffLA
JunkinsHA
MehtaJP
ManolioTA.
A catalog of published genome-wide association studies. Available: http://www.genome.gov/gwastudies. Accessed 25 September 2009
24. EdwardsAO
RitterRIII
AbelKJ
ManningA
Panhuysen
2005 Complement factor H polymorphism and age-related macular degeneration. Science 308 421 424
25. MatarínM
BrownWM
ScholzS
Simón-SánchezJ
FungHC
2007 A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol 6 414 420
26. Newton-ChehC
JohnsonT
GatevaV
TobinMD
BochudM
2000 Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009 May 10. Epub ahead of print. doi:10.1038/ng.361
27. IkramMA
SeshadriS
BisJC
FornageM
DeStefanoAL
2009 Genomewide association studies of stroke. N Engl J Med 23 1718 1728
28. International Stroke Genetics Consortium and Wellcome Trust Case-Control Consortium 2 2010 Failure to validate association between variants on 12p13 and ischemic stroke. N Engl J Med In press
29. SchunkertH
GötzA
BraundP
McGinnisR
TregouetDA
2008 Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation 117 1675 1684
30. GschwendtnerA
BevanS
ColeJW
PlourdeA
MatarinM
2009 International Stroke Genetics Consortium.Sequence variants on chromosome 9p21.3 confer risk for atherosclerotic stroke. Ann Neurol 65 531 539
31. GretarsdottirS
ThorleifssonG
ManolescuA
StyrkarsdottirU
HelgadottirA
2008 Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Ann Neurol 64 402 409
32. GudbjartssonDF
HolmH
GretarsdottirS
ThorleifssonG
WaltersGB
2009 A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet 41 876 878
33. JoutelA
CorpechotC
DucrosA
VahediK
ChabriatH
1996 Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383 707 710
34. OpherkO
PetersN
HoltmannspotterM
GschwendtnerA
Muller-MyhsokB
DichgansM
2006 Heritability of MRI Lesion Volume in CADASIL. Evidence for Genetic Modifiers. Stroke 37 2684 2689
35. HaraK
ShigaA
FukutakeT
NozakiH
MiyashitaA
2009 Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med 360 1729 1739
36. RichardsA
van den MaagdenbergAM
JenJC
KavanaghD
BertramP
2007 C-terminal truncations in human 3′-5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat Genet 39 1068 1070
37. GouldDB
PhalanFC
van MilSE
SundbergJP
VahediK
2006 Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med 354 1489 96
38. VahediK
KubisN
BoukobzaM
ArnoultM
MassinP
2007 COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke 38 1461 1464
39. RolfsA
BöttcherT
ZschiescheM
MorrisP
WinchesterB
2005 Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet 366 1794 6. Erratum in: Lancet. 2006;368 : 2210
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 3- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Nutraceutikum Armolipid Plus podle klinických důkazů zlepšuje lipidový profil − metaanalýza
- Snižuje terapie betablokátory kardiovaskulární benefit aerobního cvičení u pacientů s arteriální hypertenzí?
-
Všechny články tohoto čísla
- CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials
- BMI and Risk of Serious Upper Body Injury Following Motor Vehicle Crashes: Concordance of Real-World and Computer-Simulated Observations
- Preventing Road Deaths—Time for Data
- Essential Surgery at the District Hospital: A Retrospective Descriptive Analysis in Three African Countries
- Drivers of Inequality in Millennium Development Goal Progress: A Statistical Analysis
- Where Will the Next Generation of Stroke Treatments Come From?
- Unravelling the Genetics of Ischaemic Stroke
- Chronic Obstructive Pulmonary Disease: Effects beyond the Lungs
- The Promise of Prevention: The Effects of Four Preventable Risk Factors on National Life Expectancy and Life Expectancy Disparities by Race and County in the United States
- Can Animal Models of Disease Reliably Inform Human Studies?
- New Approaches to Preventing, Diagnosing, and Treating Neonatal Sepsis
- Protecting Vulnerable Road Users from Injury
- Providing Alcohol-Related Screening and Brief Interventions to Adolescents through Health Care Systems: Obstacles and Solutions
- Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- Accelerating Policy Decisions to Adopt Type b Vaccine: A Global, Multivariable Analysis
- Human Resource and Funding Constraints for Essential Surgery in District Hospitals in Africa: A Retrospective Cross-Sectional Survey
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- BMI and Risk of Serious Upper Body Injury Following Motor Vehicle Crashes: Concordance of Real-World and Computer-Simulated Observations
- Unravelling the Genetics of Ischaemic Stroke
- Providing Alcohol-Related Screening and Brief Interventions to Adolescents through Health Care Systems: Obstacles and Solutions
- Human Resource and Funding Constraints for Essential Surgery in District Hospitals in Africa: A Retrospective Cross-Sectional Survey
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



