-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaReassessment of HIV-1 Acute Phase Infectivity: Accounting for Heterogeneity and Study Design with Simulated Cohorts
Using simulated cohorts that account for previously unmeasured bias, Steve Bellan and colleagues provide new estimates of the duration and relative infectivity of the HIV-1 acute phase based on data from the retrospective cohort of serodiscordant couples in Rakai, Uganda.
Published in the journal: . PLoS Med 12(3): e32767. doi:10.1371/journal.pmed.1001801
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001801Summary
Using simulated cohorts that account for previously unmeasured bias, Steve Bellan and colleagues provide new estimates of the duration and relative infectivity of the HIV-1 acute phase based on data from the retrospective cohort of serodiscordant couples in Rakai, Uganda.
Introduction
Antiretroviral therapy (ART) reduces the infectiousness of HIV-infected individuals [1]. Both mathematical modeling and empirical research have suggested that scaling up antiretroviral treatment could substantially reduce the rate of new HIV infections [2,3]. However, there are numerous practical challenges for treatment as prevention (TasP) interventions, including broad implementation of HIV testing and treatment programs and ensuring adherence. In addition, HIV transmission immediately following infection may evade TasP if it occurs before infected persons are diagnosed, linked to care, and virally suppressed [4]. The success of TasP may therefore hinge on the fraction of HIV incidence attributable to transmission early after infection (AFearly).
In general, HIV transmission depends on both sexual contact patterns and biological factors that influence the probability of infection per coital act, both of which can change throughout the course of infection. HIV viral load trajectories rise rapidly during the first few weeks following infection (acute phase) and then, after a cell-mediated host immune response, decrease to a relatively stable “viral set point” for many years (chronic phase), before rising again and leading to AIDS (late phase) [5]. These viral dynamics and the well-established relationship between viral load and infectivity [6,7] suggest that biological infectiousness is greatest during the acute phase, when viral load peaks. The enhanced acute phase infectivity is often characterized using two quantities: the relative hazard of transmission during the acute versus chronic phase (RHacute) and the acute phase duration (dacute). To clarify, “acute” refers to a period of elevated biological infectivity following infection, and “early” refers to a post-infection period (often longer than the acute phase) with a duration set by policy considerations (e.g., the lag between infection and first treatment) [4].
Acute phase infectivity may be even higher than expected based on viral load alone. Virion infectivity may decrease after the acute phase, for example because of viral evolution away from highly infectious strains that survive transmission bottlenecks or because of the accumulation of antibody coatings that reduce infectivity [8,9]. A macaque SIV experiment found that 7.5 to 750 times fewer virions were required to establish successful intravenous infection when the injected virus was derived from recently versus chronically infected macaques, suggesting that acutely infected animals may have higher per virion infectiousness [9]. However, we do not know whether HIV virion infectivity in humans is elevated during the acute phase and, if so, how quickly it declines to chronic phase levels.
The epidemiological implications of acute phase infectivity depend on the sociological context. In a serially monogamous population with long-lasting partnerships, elevated infectivity during the acute phase will contribute negligibly to transmission since acutely infected individuals will likely only re-expose the partner that infected them (unless they happen to change partners in that short period). In contrast, if partnerships are less stable, acutely infectious individuals may often expose new susceptible partners, and the acute phase may thereby contribute substantially to transmission. This will occur, for example, when there is a high prevalence of concurrent partnerships [10], when there is a generally fast partner switching rate, or if individuals exhibit episodic risk behavior (risk “volatility”) [11]. Nonlinear interactions between acute phase infectivity and patterns of sexual contact may increase AFearly far more than the sum of their separate effects [10,12]. Thus, it is necessary to understand acute phase infectivity in the context of sexual contact patterns to assess their joint contributions to transmission dynamics.
Although a high AFearly presents a challenge for TasP, it does not necessarily doom it to failure, because of an important trade-off between the timing and extent of transmission [13]. The observed exponential rise in HIV prevalence at the start of an epidemic can be explained by, at one extreme, infected individuals rapidly infecting a relatively small number of people (low R0 but high AFearly) or, at the other extreme, infected individuals more slowly transmitting to many more people (high R0 but low AFearly). In general, the amount of intervention required to contain an epidemic decreases with R0 [14]. Thus, early transmission (high AFearly), which implies relatively low R0, makes early intervention more critical, but generally lowers the bar for success [4,13]. Consequently, some have proposed that the net effect of AFearly on the projected effectiveness of TasP interventions may be small [15], though this remains debated [16]. Even if our ability to control HIV transmission is not fundamentally limited by AFearly, understanding the timing and magnitude of early transmission is critical for the design of cost-effectiveness interventions that maximally interrupt transmission.
However, estimates of AFearly range widely [4,8], depending on assumptions about RHacute, dacute, and sexual network characteristics. Here, we review the evidence for elevated acute phase infectivity, identify possible biases in widely accepted estimates of RHacute and dacute, and reanalyze the available data to revise them accordingly.
Among 11 estimates of AFearly reviewed by Cohen et al. [8], all five studies focusing on sub-Saharan Africa used estimates of RHacute and dacute based on a retrospective cohort in Rakai, Uganda [17–22], which provides the only direct epidemiological measurement of acute phase infectivity and duration published to date. Of the remaining studies, none relied on a direct measure of acute phase infectivity. Instead, they considered the relationship between viral load trajectory and viral load infectivity [23], or relied on indirect estimates based on fitting a particular model to observed epidemic growth rates [24–29]. The lack of other data sources on newly infected HIV cases is not surprising. First, newly infected individuals are rarely tested within the short acute phase time window, and tests identifying recently infected individuals are not very reliable [8,30]. Second, newly infected individuals who are infected by their current sexual partner (who cannot be reinfected) provide no information on acute phase infectivity. Finally, ethics dictate that, when infected individuals with potentially susceptible partners are identified, interventions should be taken to prevent further transmission, so that identified individuals no longer transmit at rates reflective of the general population.
Given the influence of the Rakai study on the general understanding of the acute phase, we reanalyzed the results reported from this study to account for several previously overlooked sources of bias. Specifically, we fit a couples transmission model that accounted for the study design and for unmeasured transmission heterogeneity between couples. We then replicated previous analyses on simulated data to systematically explore the differences between our results, and those reported by the original study [17] and by the most widely cited reanalysis [18].
Methods
S1 Text provides a complete model description and all scripts needed to reproduce our analyses. All analyses were performed in R [31].
Excess Hazard-Months Attributable to Elevated Acute Infectivity
Previous studies focused on estimating RHacute, the relative hazard (i.e., infectivity) of the acute phase relative to the chronic phase. However, such estimates are not directly comparable across studies that assume (or estimate) different dacute values (Fig. 1A and 1B). To overcome this limitation, we introduce a new measure: the excess hazard-months attributable to the acute phase (EHMacute), which equals (RHacute − 1) × dacute. EHMacute is defined, intuitively, in units of chronic phase hazard-months. If infectivity is constant throughout disease progression, then an infectious individual who dies 10 y after infection produces 120 hazard-months. If the acute phase is 3 mo long and 26 times as infectious as the chronic phase, then the acute phase contributes an additional EHMacute = (26 − 1) ×3 mo = 75 hazard-months, for a total of 195 hazard-months. EHMacute quantifies the total impact of physiologically elevated acute infectivity, and is comparable across studies. The overall contribution of each disease phase to population-level transmission is also influenced by sexual behavior (e.g., partner switching and concurrency). However, by focusing on data from stable couples, we separate the contribution of EHMacute from that of partnership dynamics.
Fig. 1. Excess hazard-months due to the acute phase. 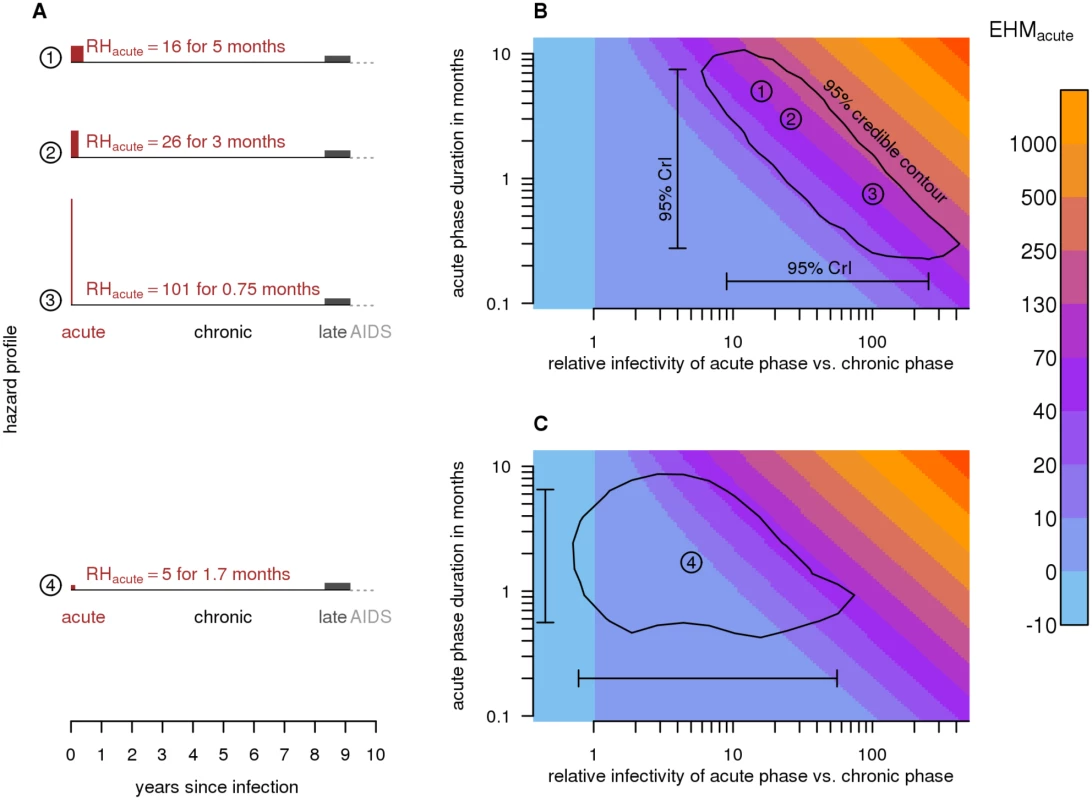
(A) Schematic diagram of relative infectiousness during HIV progression. In scenarios 1–3, the duration (dacute) and relative hazard of the acute phase (RHacute) differ; however, they all generate 75 excess hazard-months (EHMacute = [RHacute − 1] × dacute). The area of each acute phase rectangle (red; drawn to scale) represents the magnitude of EHMacute. Scenario 2 is the widely assumed acute phase infectivity that was estimated from the Rakai retrospective cohort using a variable hazard survival model [18]. Scenario 4 is our revised estimate obtained by fitting a couples transmission model to the same Rakai data (EHMacute = 8.4). Unlike previous estimates, it accounts for unmodeled heterogeneity and the Rakai study’s exclusion criteria. (B) RHacute versus dacute for scenarios 1–3, along with 95% credibility intervals (95% CrIs) and a 95% credibility contour around estimates from the variable hazard survival model (scenario 2). Colors indicate EHMacute. Because couples in the Rakai cohort were observed at 10-mo intervals, the duration of the acute phase is not easily identified—shorter, highly infectious and longer, mildly infectious acute phases are both consistent with the data. EHMacute, however, can be estimated with greater relative precision. (C) Our best estimate of acute phase characteristics (scenario 4) and associated 95% CrI and credibility contour. Estimating EHMacute from Viral Load
Published estimates of acute phase infectivity are believed to be higher than would be expected based on viral load alone [4]. However, viral load trajectories vary throughout the acute phase, increasing to a peak before declining to the chronic phase set point. If, as is commonly assumed, infectivity varies with viral load, then the instantaneous RHacute also changes throughout the acute phase, and thus EHMacute attributable to elevated acute phase viral load cannot be reliably inferred from snapshot estimates of RHacute at the viral load peak [13]. Thus, we estimated the expected EHMacute based on the viral load trajectory during the acute phase, rather than just the peak viral load. Combining empirical acute phase viral load trajectories [32] with a fitted log-linear model of infectivity as a function of viral load (with 95% CI [7]), we generated a relative hazard profile over an average disease progression, and summed the area under this profile to estimate EHMacute caused solely by elevated acute phase viral load (Fig. 2).
Fig. 2. Viral-load-based estimates of excess hazard-months due to the acute phase. 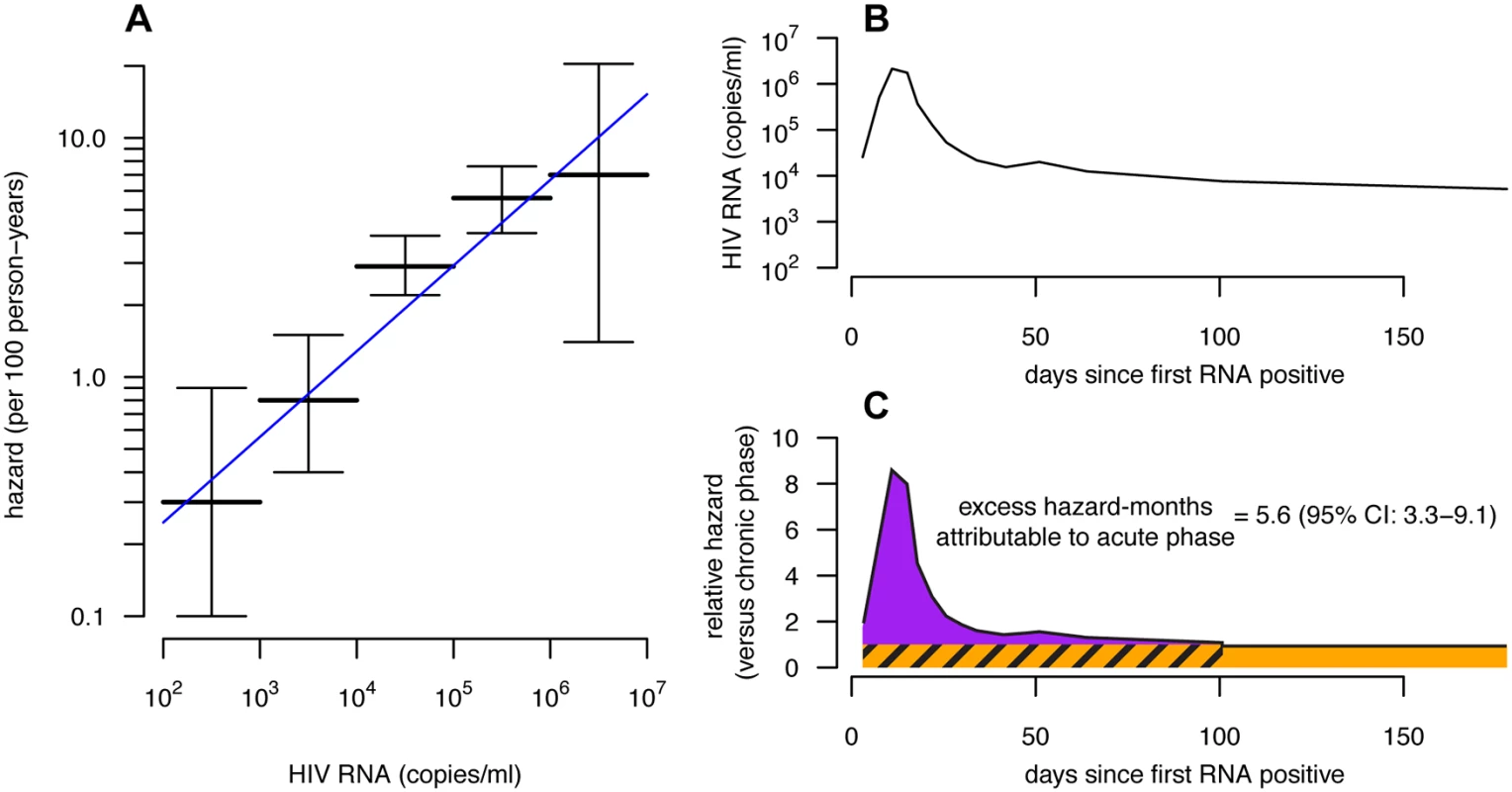
(A) The hazard of transmission by viral load category (horizontal bars with 95% confidence intervals [95% CIs]) from [7] with a fitted log-linear model (blue line). We compare these data to other studies in S7 Fig. (B) The average viral load trajectory of 19 recently infected individuals in East Africa from the ECHO cohort [32]. (C) Combining the fitted log-linear model (and 95% CIs on model coefficients) from (A) and the average viral load trajectory from (B), we estimated the relative hazard by disease phase (black line). The expected EHMacute is the excess hazard-months occurring in the acute phase (area of the purple region), which can be compared with the baseline chronic hazard of equal duration (hatched orange area). While we drew the acute phase cutoff at 100 d based on the stabilization of the viral load near this time, it can be seen that EHMacute, because it is defined as excess hazard-months, is relatively insensitive to the cutoff time once the relative hazard approaches that of the chronic phase level (orange). Couples Transmission Model
We adapted our previously published couples transmission model [33] for two purposes. First, we fit it to the Rakai retrospective cohort data to generate an independent estimate of EHMacute (Fig. 3). Second, we used the model to simulate cohort data and thereby investigate discrepancies between prior estimates of EHMacute and our own lower estimates.
Fig. 3. Model Diagram. 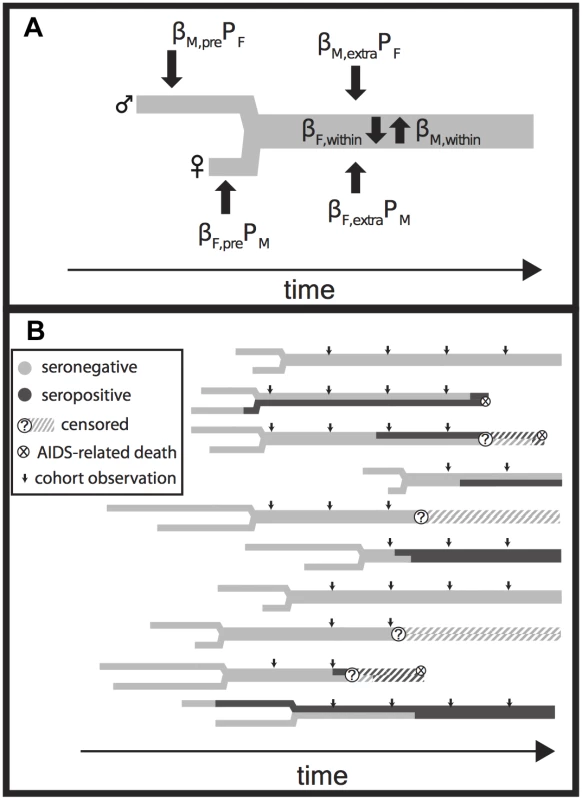
(A) The relationship history of an example couple. Male (M, upper) and female (F, lower) branches begin at each partner’s sexual debut and then join together into a single thick gray line when they form a couple. Male and female partners are at risk of transmission prior to couple formation at a rate equal to the product of a transmission coefficient (βM,pre and βF,pre) and the time-varying population prevalence in the opposite gender (PF and PM). Transmission after the couple has formed from extra-couple partners is similarly dependent on the population prevalence. Infected individuals infect their stable partner at a rate equal to the product of a chronic phase transmission rate (βM,within or βF,within) and the relative hazard of their current disease phase versus the chronic phase (not shown). Once infected, individuals are given Weibull distributed survival times [33,34] (not shown). (B) A simulated time series of infection, AIDS mortality, and censorship histories for ten couples. Small arrows indicate longitudinal observations of each couple, up to five times at 10-mo intervals if they have already formed at the start of observation, if they are not censored due to loss to follow-up or couple dissolution, and if both partners remain alive. These observations are then used to create a retrospective cohort (Fig. 4). Fig. 4. Rakai retrospective cohort study design. 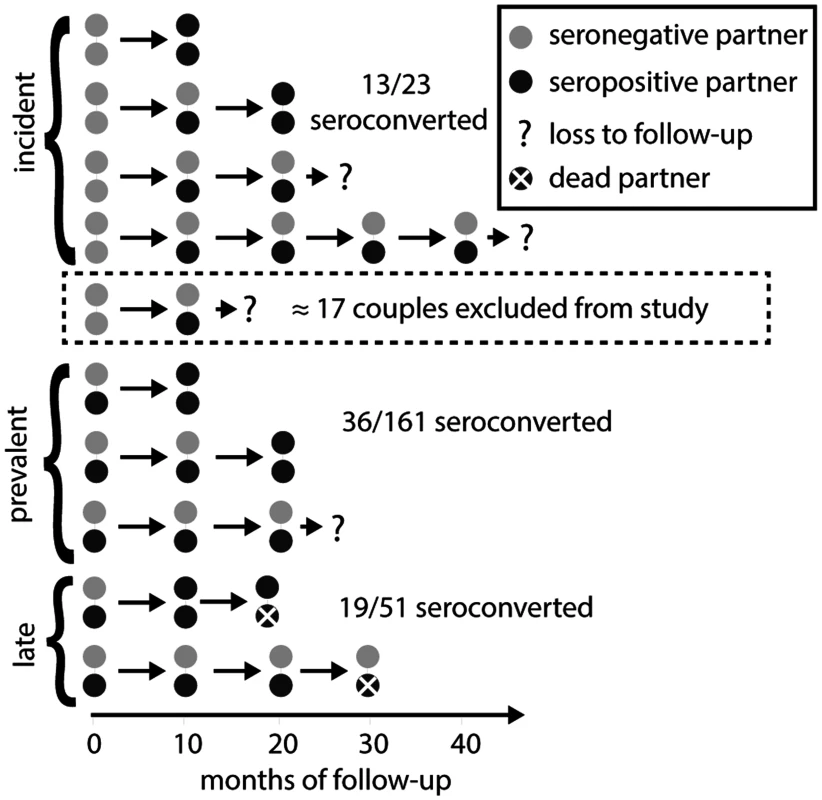
In both the original Rakai study and our simulated cohorts (Fig. 3), retrospectively identified serodiscordant couples (SDCs) were divided into those in which (1) the index partner’s infection occurred between study visits (incident SDCs), (2) the index partner’s infection occurred prior to study enrollment and neither partner died during follow-up (prevalent SDCs), and (3) the index partner’s infection occurred prior to study enrollment and the index partner died of AIDS during follow-up (late SDCs). Incident, prevalent, and late SDCs were assumed to reflect acute, chronic, and late phase infectivity exposure for the secondary partner (i.e., non-index partner). Couples recorded as serodiscordant only once and never seen again were excluded from the analysis under the assumption that these couples did not contribute any person-time at risk for transmission, while couples transitioning directly from concordant negative to concordant positive were included, whether or not they were subsequently observed. However, just as an immediate transition from concordant negative to concordant positive provides evidence for higher acute phase infectivity, a transition from concordant negative to serodiscordant provides evidence for lower acute phase infectivity. Thus, a sampling bias arises from this asymmetric exclusion of couples. In the model, partners can be infected prior to couple formation, by a stable partner, or by an extra-couple partner while in a stable couple. We allowed the transmission rates between stable partners to vary according to the disease phase of the infected partner—acute, chronic, late, or AIDS. We also incorporated heterogeneity in risk by drawing individual hazards of infection from log-normal distributions with median λ¯hazard and standard deviation σhazard. We set uninformative uniform priors on acute phase parameters, median transmission rates, and σhazard (S1 Text). For each parameter set, we simulated a population of couples (see below), recording the timing of key events in disease progression (i.e., date of infection, death, and corresponding infection phases) and each individual’s hazard.
We constructed a “cohort” from the output of each simulation above according to the Rakai Community Cohort Study design [17]. Specifically, each couple’s serostatus was “observed” at 10-mo intervals from January 1994 through mid-1999. We then censored observations to simulate loss to follow-up and couple dissolution. Censorship was modeled as a serostatus-dependent process: couples that were concordant negative, serodiscordant, or incident serodiscordant (i.e., changed from concordant negative to serodiscordant between successive cohort observations) at a given cohort observation had a 25%, 35%, and 47% probability, respectively, of being censored before the subsequent cohort observation, reflecting empirically observed rates [35,36].
Using the criteria of Wawer et al. [17], we selected a “retrospective cohort” from each of these simulated “cohorts” that included all couples that were observed serodiscordant and then observed in at least one more subsequent visit, along with all couples that were observed concordant negative and then concordant positive at the subsequent visit. Importantly, these criteria exclude couples that were observed concordant negative and then serodiscordant only once before being censored by loss to follow-up, couple dissolution, or the end of the study (Fig. 4), under the assumption that these couples did not contribute any person-time at risk. However, each couple that transitions from concordant negative to serodiscordant provides evidence for lower acute phase infectivity, while each couple that transitions from concordant negative to concordant positive provides evidence for higher acute phase infectivity. The exclusion of the former but not the latter couples creates sampling bias. By including this sampling process in our model, we explicitly accounted for this bias.
Retrospective cohort couples were then classified as “incident,” “prevalent,” or “late,” depending on information about the stage of the index partner (i.e., first partner infected). Specifically, “incident” couples were those in which the index partner became infected while the couple was under cohort observation; the secondary partners (i.e., non-index partner) in such couples were therefore exposed to an acutely infected index partner during the observation period. Couples that were not incident were classified as “prevalent” unless the index partner was recorded as having died during the study, in which case they were classified as “late.” Late couples thus constitute couples in which the secondary partner was exposed to an index partner in the AIDS phase (too sick to infect), and possibly in the late phase (increased infectivity) preceding it.
The Rakai study used molecular viral linkage assays to identify seroconverting secondary partners who had been infected by an extra-couple partner, and to exclude them from the cohort. To replicate this in our fitted model, we similarly excluded all couples where the second partner was infected via extra-couple transmission. We conducted a sensitivity analysis to this exclusion in the simulation analysis below because such couples do contribute person-time at risk up until the secondary partner’s infection.
Estimating EHMacute from the Rakai Retrospective Cohort
We fit the couples transmission model to the Rakai retrospective cohort data using approximate Bayesian computation with sequential Monte Carlo (ABC-SMC) [37] to estimate transmission rates, RHacute, dacute, and σhazard. We describe our approach in detail in S1 Text. Briefly, we used the model to simulate 4,875 couples (i.e., the Rakai couples cohort size [36]) for each of hundreds of thousands of parameter sets drawn from uninformative prior distributions (S1 Table). Parameter sets that generated retrospective cohorts sufficiently similar to the Rakai cohort, as measured by several summary statistics, were accepted, while others were rejected. Summary statistics included proportions of secondary partners seroconverting in incident and prevalent couples and the extent of individual heterogeneity as indicated by discrepancies between unadjusted and adjusted regression analyses (S2 Table). New parameter sets, sampled randomly around those accepted in the previous step, were simulated and then again filtered based on similarity to the Rakai data. This filtering procedure was repeated with increasingly strict criteria for similarity, until the distribution of parameters converged and the simulation summary statistics sufficiently matched the real data.
Simulating Previous Estimates of EHMacute
To identify biases underlying discrepancies between our estimates and prior estimates from the Rakai cohort data, we replicated the two most highly cited analyses of the Rakai retrospective cohort (Poisson regression [17] and variable hazard survival model [18]) on simulated data across a wide range of parameter sets (S3 Table). We examined differences between estimated parameters and the “true” values used in the simulation. To reduce the effect of sampling error, we simulated cohorts of 100,000 couples in this analysis.
Poisson Regression Model
We replicated the approach of Wawer et al. [17] and estimated RHacute from each simulated retrospective cohort using a Poisson regression of secondary partner seroconversion against index partner disease phase (acute, chronic, late), controlling for secondary partner person-time at risk (as an offset term). Person-time at risk was calculated by assuming that infections or deaths occurring in a 10-mo interval occurred at the 5-mo midpoint of the interval. Similarly, when both partners were infected in the same interval, this analysis assumed that secondary partner infection occurred at 7.5 mo. For incident couples, only the interval in which the index partner seroconverted was considered representative of acute phase exposure for the secondary partner. Consequently, later observation intervals were excluded from the regression. Given the person-time assumptions above, this interval represents the secondary partner’s exposure to the first 0–5 mo of the index partner’s infection. Thus, this approach implicitly assumes that the acute phase lasts 5 mo.
In [17], observed covariates that potentially affect transmission (coital rate, genital ulcer disease [GUD], viral load, and age) were included in the regression. Adjusting for observed covariates controls for some, but not all, of the heterogeneity between couples because other sources of variation remain unobserved. We similarly simulated “observed covariates” that were partially correlated with each individual’s actual hazard and adjusted for these covariates. This allowed us to assess estimation accuracy under the assumption that the study included observed covariates that account for some but not all of the variance in risk between individuals.
Variable Hazard Survival Model
Hollingsworth et al. [18] reanalyzed the Rakai retrospective cohort by fitting a variable hazard survival model to the data. Because their estimates of acute and late phase infectivity and duration are frequently considered the best available estimates [4,8,10,19,38,39], we also replicated their analysis on our simulated retrospective cohorts. This model explicitly specifies different hazards and durations for the acute, chronic, and late phases, and also specifies the existence of an AIDS phase prior to death during which no transmission occurs (Fig. 1A here and Fig. 1 in [18]). Rather than assuming a 5-mo acute phase as in [17], this approach explicitly estimates both phase infectivity and duration.
This model makes the following assumptions. Secondary partners in prevalent couples are exposed to a chronically infected index partner. Secondary partners in incident SDCs are exposed to an acutely infected index partner for a duration dacute beginning when their partner was infected, and are exposed to a chronically infected index partner thereafter. Similarly, secondary partners in late SDCs cannot be infected during the AIDS phase preceding their partner’s death, are exposed to the late phase preceding that, and are exposed to the chronic phase preceding that. Rather than assuming that infections occurred at the midpoint of an interval, this model more realistically considered the timing of the index partner’s infection as an unknown, hidden event that occurred with equal prior probability at each time during the interval of occurrence.
Hollingsworth et al. estimated phase durations and hazards and 95% CIs using a maximum likelihood approach, but did not estimate confidence intervals for derived parameters such as RHacute or EHMacute. We fitted the same model using a Bayesian Markov chain Monte Carlo algorithm, which better facilitated estimation of 95% CrIs (the Bayesian analogue of confidence intervals) for all parameters of interest. We validated our fitting procedure by fitting to data simulated by this same variable hazard survival model with known parameters, and assessing our ability to accurately recover these parameters.
Results
We found that analyses of cohort data with 10-mo survey intervals cannot distinguish between shorter, highly infectious and longer, less infectious acute phases because of the relatively long intervals between couple observations. While collinearity between dacute and RHacute prevents these parameters from being identifiable, EHMacute can be estimated with relatively greater precision (Fig. 1A and 1B). EHMacute values can be compared to the 120 hazard-months an individual would produce over 10 y of infection if infectivity was constant. Thus, an EHMacute of 12 would indicate that the acute phase contributes an additional 10% of the hazard that an individual would produce during 10 y of untreated chronic infection.
Based on viral load–infectivity relationships alone, we estimate that the hazard of transmission at peak viral load is approximately nine times greater than at the chronic phase set point. However, this peak is transient, and the viral load trajectory across the entire acute phase suggests an EHMacute of only 5.6 (95% CI: 3.3–9.1) (Fig. 2).
We fit a couples transmission model that explicitly accounts for couple dissolution, loss to follow-up and the cohort exclusion criteria of the Rakai retrospective cohort data (Figs. 3 and 4) to estimate acute phase infectivity and duration, mean transmission rates into and between couples, and inter-individual heterogeneity in these transmission rates. Our model fit the data well (S1 Fig.). From this analysis, we estimated EHMacute to be 8.4 (95% CrI: −0.27 to 63), RHacute = 5.3 (95% CrI: 0.79–57), and dacute = 1.7 mo (95% CrI: 0.55–6.8) (Figs. 1C and 5; Table 1). We estimated the median transmission rate between partners to be λ¯hazard = 12 (95% CrI: 4.6–30) per 100 person-years and the heterogeneity in transmission to be σhazard = 2.0 (95% CrI: 1.2–2.8). A σhazard of 2.0 corresponds to individuals at the 97.5% highest risk quantile, experiencing transmission rates 50-fold greater than the median.
Fig. 5. Revised acute phase estimates. 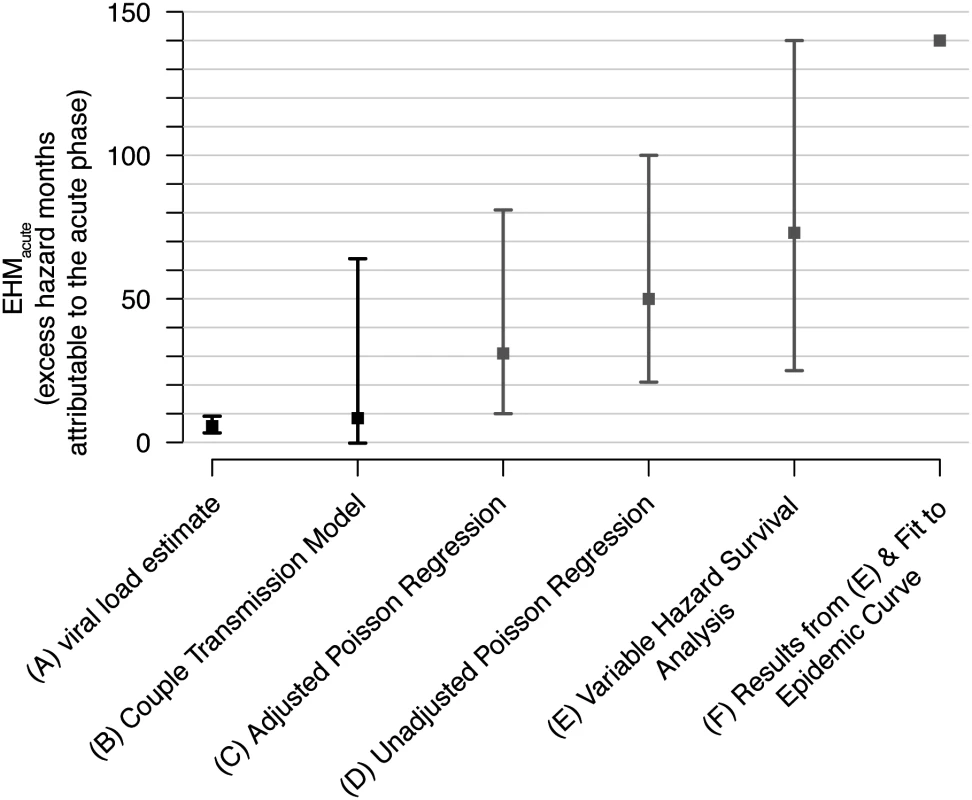
Our estimates (black) of the excess hazard-months attributable to the acute phase (EHMacute) based on (A) viral load trajectories (Fig. 2) and (B) our fit of a couples transmission model to the Rakai retrospective cohort. We compare these estimates with previous Rakai-based estimates that did not adjust for these biases (gray). These include (C) Wawer et al.’s adjusted and (D) unadjusted Poisson regressions [17], (E) Hollingsworth et al.’s variable hazard survival analysis [18], and (F) Powers et al.’s estimates that used a Bayesian framework to combine estimates from (E) and a mathematical modeling fit to an epidemic curve [19]. Tab. 1. Acute phase infectivity estimates from Rakai cohort data. 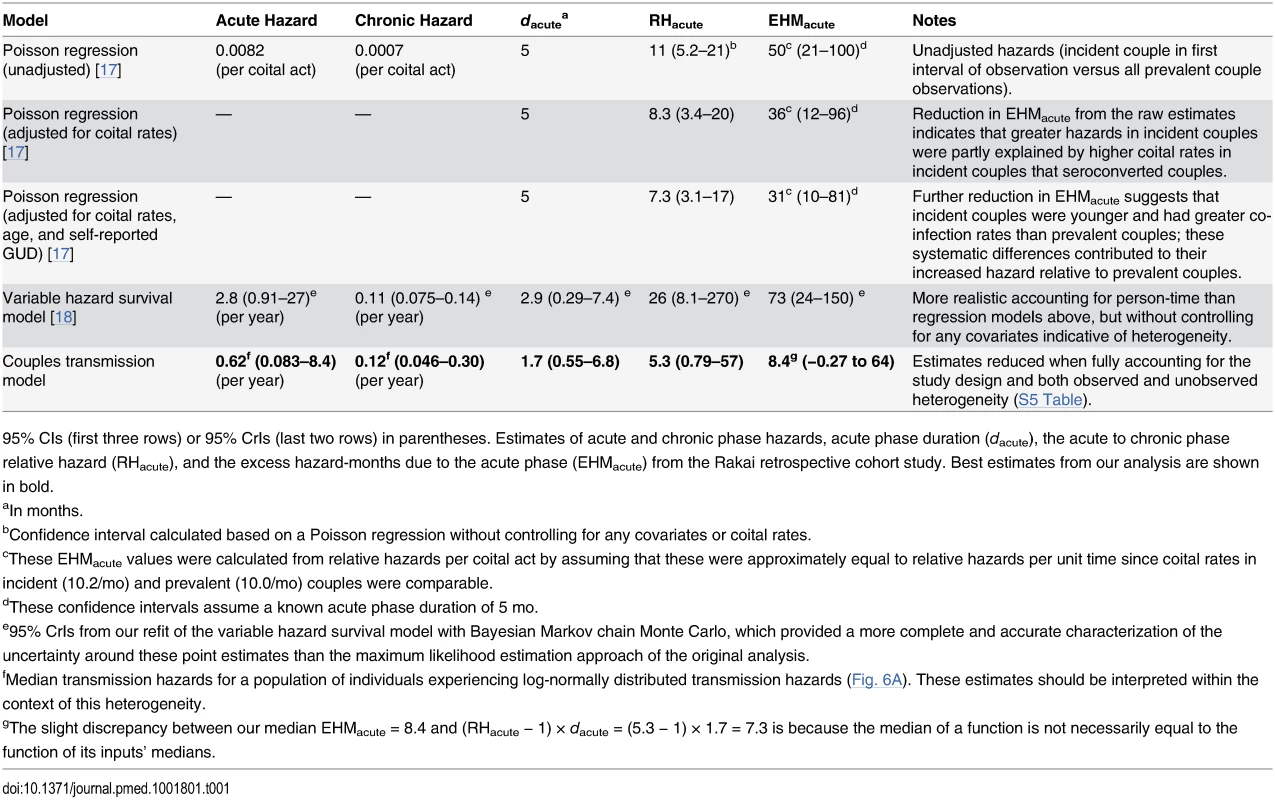
95% CIs (first three rows) or 95% CrIs (last two rows) in parentheses. Estimates of acute and chronic phase hazards, acute phase duration (dacute), the acute to chronic phase relative hazard (RHacute), and the excess hazard-months due to the acute phase (EHMacute) from the Rakai retrospective cohort study. Best estimates from our analysis are shown in bold. Fig. 6. Multiple sources of bias for acute phase estimates. 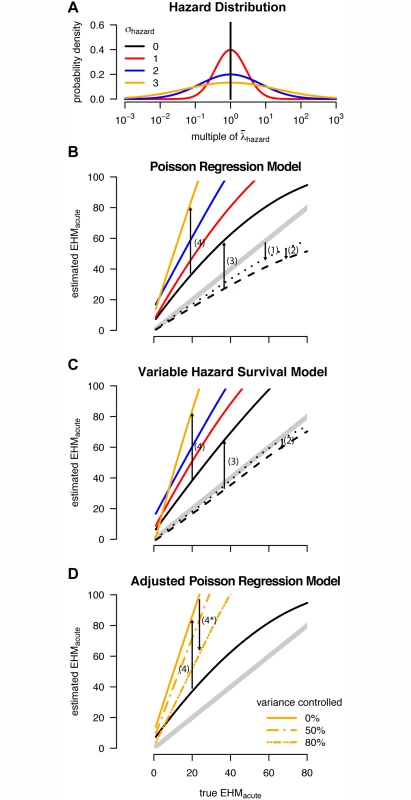
(A) The log-normal distributions used to model variability in individual hazard of infection (color-coding of σhazard used throughout the figure). (B and C) Estimated excess hazard-months attributable to the acute phase (EHMacute) versus the true (simulated) EHMacute when analyzing simulated cohort data with the (B) Wawer et al. [17] Poisson regression and (C) Hollingsworth et al. [18] variable hazard survival model. Thick gray diagonal lines represent unbiased estimates. Arrows 1–4 indicate how each bias affects estimates of EHMacute. Arrow locations along the x-axis are chosen for ease of display only; for any true EHMacute, each bias is quantified by the vertical separations between lines. Dotted lines show the “best case” scenario for these models: if the underlying population is truly homogenous, the analysis includes all seroincident couples, and late and chronic phase infectivity are equal. The small downward bias (1) in the Poisson regression arises from assumptions regarding person-time at risk. The dashed lines reveal additional downward bias (2) in both models stemming from misclassification of late couples as prevalent couples (assuming the excess hazard-months attributable to the late phase was 40). Solid lines show estimates from simulated cohorts when seroincident couples lost to follow-up are excluded (Fig. 4), causing bias (3). Finally, both analyses are further biased upward (4) when used to analyze heterogeneous populations. (D) The same trends for σhazard = 0 and 3 from (B), but also showing how bias (4) can be partly mitigated (4*) when variance between individuals is controlled for by adjusting for measured covariates corresponding to some (but not all) of the heterogeneity. (B–D) were created by fitting smoothers through individual simulations (S3 Fig.). We replicated previously published methods for estimating acute phase infectivity in simulated populations of 100,000 couples, yielding an average retrospective cohort size of 3,000 couples (i.e., after excluding couples with no infections or as dictated by the study’s exclusion criteria), which was sufficient to distinguish inherent biases from random fluctuations (S2 Fig.). We identified four sources of bias that influenced estimates of EHMacute produced by either the Poisson regression [17] or variable hazard survival model [18] (Fig. 6B–6D). The first bias stems from assumptions about the timing of seroconversion between cohort observations. The Poisson regression approach assumed that the first infection in a given 10-mo observation interval occurred at the 5-mo midpoint. When an incident infection and secondary infection occurred in the same interval, this approach assumed that the latter occurred at 7.5 mo (i.e., the midpoint between the first infection and the end of the interval). In theory, the interacting effects of these three assumptions can be complex (S3 Fig.), but our simulations show that, on the whole, these assumptions led to a small downward bias in estimates of EHMacute. By assuming instead that infection events occurred at unknown times with equal prior probability distributed throughout an interval, the variable hazard survival model removed this bias.
The second bias stems from misclassification of some late phase couples as chronic phase couples when they are lost to follow-up just prior to a partner’s death. The accidental inclusion of some late phase couples leads to overestimates of chronic phase infectivity, which, in turn, biases estimates of RHacute and EHMacute downward (Figs. 6B, 6C, and S4). Both the regression and survival models were affected similarly by this small downward bias.
The last two biases caused overestimates of EHMacute that far outweighed the first two downward biases (Fig. 6). Specifically, the exclusion of incident SDCs lost to follow-up (Fig. 4) nearly doubles estimates of EHMacute relative to the true value. This bias is best illustrated with unadjusted hazard calculations. Assuming that index and secondary infections occurred at the 5-mo and 7.5-mo points within each study interval, we can calculate the acute phase hazard from the original data in which ten of 23 incident couples had both partners seroconvert in the same interval (S4 Table):
However, based on empirical rates of loss to follow-up in Rakai, it is likely that approximately 17 incident SDCs were excluded because they were not seen again after their incident serodiscordant visit, either because they dissolved, were subsequently lost to follow-up or because their incident serodiscordant visit coincided with the end of the study period [35,36]. If we correctly include these couples in our calculation, then the estimated acute phase hazard is much lower: Our analysis finds that this effect approximately doubled previous estimates of acute phase transmission that relied on this retrospective cohort. We assumed 17 (43%) of incident SDCs were excluded from the original data for this illustrative example. However, in fitting our couples transmission model, we explicitly modeled this sampling procedure, and the proportion of incident SDCs excluded was variable between simulations fitted to the data and was driven by empirical estimates of the loss-to-follow-up and couple dissolution rates in Rakai. Our ABC-SMC fitted posterior median number of incident SDCs excluded was 17 (95% CrI: 8–35), corresponding to 43% (95% CrI: 27%–60%) of incident SDCs being excluded (S1 Text; S5 Fig.). Importantly, this uncertainty in the exact number excluded by the Rakai study is reflected by our new estimates of EHMacute.The fourth bias emerges from unmodeled heterogeneity in the risk of transmission within SDCs. Couples with higher risk are, by definition, more likely to transmit infection. Thus, couples that have remained persistently serodiscordant (prevalent and late couples) represent couples with a lower transmission risk, on average, than newly formed “naïve” SDCs (incident couples). Thus, a portion of the estimated EHMacute may actually reflect sampling-based differences between couples that enter the study serodiscordant versus seroconcordant negative, rather than biological differences between acute and chronic phase infectivity. The adjusted Poisson regression analysis partly corrects for this bias, by adjusting for covariates, which accounts for some of this heterogeneity (Fig. 6D), whereas the variable hazard survival analysis does not correct for any covariates. In the original regression analysis, adjustment for covariates reduced the estimated EHMacute from 50 to 31. Inclusion of additional risk covariates, had they been measured, would have reduced this estimate further. By fitting our heterogeneous couples transmission model above, we explicitly accounted for this bias while estimating the extent of heterogeneity.
These four biases account for virtually all of the difference between the Poisson regression and variable hazard survival estimates and the true simulated values being estimated (S5 Table); thus, other remaining sources of bias are necessarily minor. In particular, the exclusion of couples in which the secondary partner was infected in an extra-couple partnership was not a substantial source of bias (S6 Fig.), in part because such couples were excluded from both incident and prevalent categories, and these effects approximately balanced each other.
Discussion
In addition to reducing morbidity, ART also reduces the risk that HIV-infected individuals infect their sexual partners [1]. TasP has consequently become a primary focus of HIV control strategies [40]. Still, many have highlighted that TasP prevents transmission only from individuals who have been diagnosed and treated [19]. This has spawned an energetic debate concerning the proportion of transmission likely to occur too early after infection to be preventable by realistic TasP interventions [4]. In particular, the assumption that individuals are extremely infectious during the several months immediately following infection has led to arguments that a large proportion of all transmission occurs too early to be averted by TasP [4,19,39].
We have found that the evidence for elevated acute phase infectivity, a key component of early transmission, is not nearly as strong as commonly thought. Acute phase infectivity has been directly measured only once, from a retrospective couples cohort in Rakai [17]. We reanalyzed the reported results from this study, accounting for the sampling procedure and individual-level heterogeneity in the risk of HIV transmission, which were overlooked in all previous analyses. Our new estimate for the acute phase hazard is nine times less than the currently most frequently used estimate [8,18]. Thus, physiologically elevated infectiousness early in infection alone is unlikely to undermine TasP campaigns. Furthermore, intervention efforts targeted at identifying acutely infected individuals [41] may be less cost-effective at preventing forward transmission than previously thought.
In general, long intervals between observations may preclude precise estimation of shorter duration events. The 10-mo Rakai observation interval contributes substantial uncertainty to estimates of the duration (dacute) and relative hazard (RHacute) of the acute phase. A short, intense acute phase and a long, milder acute phase will exhibit similar transmission patterns at a 10-mo level of resolution. To circumvent this uncertainty, we introduced EHMacute, the excess chronic-phase-equivalent hazard-months attributable to elevated acute infectivity (EHMacute = [RHacute − 1] × dacute). Unlike RHacute, EHMacute can be estimated from cohort studies, even when the duration of the acute phase is unknown. The magnitude of EHMacute can be compared to the baseline of 120 chronic phase hazard-months untreated infected individuals would generate over their approximately 10 y of constant infectiousness if the acute and late phases had infectivity equal to that of the chronic phase. We estimated an EHMacute of 8.4 (95% CrI: −0.27 to 64), lower than the original estimate of 31 and far lower than the currently most frequently used estimate of 73 [17,18]. We find that the upward revision from the first regression-based estimate of EHMacute = 31 to the survival-analysis-based estimate of 73 was almost entirely attributable to the latter’s exclusion of covariates that the former used to captured some, but not all, of the heterogeneity in transmission risk. Furthermore, we showed that both previous estimates were biased upward by the study design itself and by additional unobserved heterogeneity in couple transmission rates.
The effect of controlling for heterogeneity can be seen in the original regression analysis [17]. Adjusting for coital rates reduced the estimated EHMacute from 50 to 36 (Table 1), and adjusting for age and GUD further reduced the estimate to 31. Correcting for other sources of inter-couple heterogeneity (e.g., host and viral genotypes affecting susceptibility and infectiousness, tendency to use condoms, other co-infections) would have likely reduced the estimate even further.
Our findings demonstrate the utility of simulation approaches for validating epidemiological study design and analysis [42,43]. Bias may arise in unexpected ways from interactions between epidemiological, observation, and sampling processes. All of these processes can be included in simulation models that can be fit directly to empirical data with modern statistical approaches. Such models can also be used to simulate data for analysis to compare the performance of alternative methods. Comparisons between estimates from simulated data, where the underlying true parameters are known, provides a powerful tool with which to discover biases and evaluate the robustness of estimators when not all assumptions are met, or in the presence of sampling bias. For example, our initial aim was to examine the effect of heterogeneity on estimates of acute phase infectivity. Unexpectedly, replication of previous approaches on our homogenous simulations also yielded biased estimates, leading us to discover three other sources of bias.
Our two independent estimates of EHMacute—one based on elevated acute phase viral load and the other based on the Rakai data—were similar, with each inside the confidence bounds of the other. Thus, contrary to the prevailing consensus [8,18,19], we cannot reject the null hypothesis that elevated acute phase infectivity in humans is caused solely by the transient elevation in viral load (and not elevated per virion infectivity). However, we emphasize that the variance in all estimates based on this small cohort reflects considerable uncertainty, which should be propagated in all analyses of acute phase infectivity, particularly those calculating AFearly. To that end, in addition to providing our estimates of EHMacute, RHacute, and dacute and their credibility intervals above, we have provided our fitted posterior distribution of acute phase infectivity and duration and individual heterogeneity in transmission to facilitate future modeling work (S1 Data). We emphasize that models relying on these estimates of acute phase infectivity and duration should also adequately account for their collinearity (i.e., the upper confidence bounds of both RHacute and dacute are not, as a pair, within their joint credibility contour; Fig. 1) and also consider individual heterogeneity.
The Acute Phase Debate
There is considerable disagreement regarding the impact of early transmission on the effectiveness of TasP [4,13,15,16]. Powers et al. estimated that the fraction of HIV incidence attributable to transmission from acutely infected individuals (AFacute) was 40% [19], while Williams et al. argued that AFacute was more likely to be 2%–4% [44]. The discrepancy arises from the former’s confidence and the latter’s skepticism in the variable hazard survival analysis’s estimates of acute phase infectivity and duration from Rakai (Fig. 1B; [18]), which we show are upward-biased by unmodeled heterogeneity and study design (Figs. 1C and 6).
Powers et al. fit an HIV transmission model to antenatal clinic prevalence trends in Lilongwe, Malawi, using a Bayesian procedure to update prior estimates of acute phase infectivity from Rakai [19]. Their analysis provided posterior estimates of EHMacute = 141, nearly double the estimate that formed the basis for their prior, and 16 times our best estimate. Their further inflation of EHMacute stems from their fit to the Lilongwe epidemic, which (like many HIV epidemic trajectories) exhibits a steep initial rise in prevalence followed by deceleration to a lower epidemic peak than would be expected based on the initial rise. However, the observed steep epidemic growth in Lilongwe is largely driven by one antenatal clinic observation in 1987 with substantial uncertainty. More importantly, this characteristic epidemic trajectory can be explained by mechanisms other than high acute phase infectivity. For example, heterogeneity and assortativity in risk behavior can drive rapid early growth as HIV spreads through high-risk subpopulations [15,45]. Declining risk behavior over the course of the epidemic can also explain relatively rapid early growth [15,46]. Therefore, high estimates of EHMacute derived by fitting to epidemic trajectories are unreliable.
Phylogenetic clustering of incident infections has also been used to infer the proportion of transmission attributable to early infection [47–49]. However, these studies make varying assumptions regarding the time window after infection considered “early,” which precludes direct comparison of the AFearly estimates. Furthermore, recent work has uncovered several questionable assumptions in phylogenetic and phylodynamic inference of transmission events [50–52] and has suggested that conclusions reached from these approaches should be interpreted cautiously. For example, phylogenetic tree topologies may not correspond to transmission networks, sampled individuals are not unbiased random samples of infected individuals, several viral genotypes may be transmitted during infection, and certain genotypes may be preferentially transmitted.
In addition, AFearly estimates may be strongly influenced by the intervention history in the focal population. Large AFearly values are often interpreted as an obstacle to future TasP success, but they could instead indicate ongoing TasP success. Successful TasP will decrease transmission following the initiation of treatment, thereby increasing the relative transmission rate of the pretreatment period (i.e., AFearly). As increasingly ambitious TasP strategies are implemented, AFearly should thus increase even while incidence decreases. For instance, a recent phylodynamic analysis of Detroit’s population of men who have sex with men concluded that half of all transmission occurs within the first year of infection [49], and that individuals are 20 times as infectious in the first year post-infection (corresponding to EHMacute = 228) [53]. However, this relative infectivity of the acute phase compares transmission from untreated, acutely infected individuals to that from treated, chronically infected individuals, and therefore overestimates relative acute phase infectivity. Future studies should interpret estimated AFearly in the context of ART coverage, noting that successful TasP interventions should increase AFearly.
Finally, we again note arguments that because larger estimates of AFearly imply smaller reproductive numbers, TasP effectiveness may be less sensitive to AFearly in the long term than commonly assumed [4,13,15]. An observed epidemic trajectory can be explained by, at one extreme, infected individuals transmitting to relatively few people relatively quickly (low R0 but high AFearly) or, at the other extreme, by infected individuals transmitting to relatively many people over a longer duration (high R0 but low AFearly). Infectious diseases with smaller R0 are more sensitive to interventions [14]. Thus, in the former scenario, TasP would be proportionally less effective because AFearly is high, but reducing transmission would be easier (because R0 is low). In the latter scenario, TasP would avert a greater proportion of transmission, but population-level transmission would be more difficult to reduce (because R0 is high) [4,13]. Thus, the net effect of AFearly on the projected effectiveness of TasP interventions may be small [15], though this is still under debate [16]. Our results help to mediate the controversy over the impact of AFearly on intervention effectiveness. If AFearly is smaller than previously assumed, then any potential interference with TasP is also smaller and efforts to target early transmission may be less cost-effective, compared to more broad-scale interventions.
Assumptions and Limitations
Biases arise when assumptions influence results but do not hold in the real world. The two prior studies that estimated HIV acute phase infectivity from the Rakai cohort data unknowingly suffered from four distinct sources of bias, each stemming from a specific problematic assumption. In our analysis, we used a detailed simulation model to explicitly correct these assumptions (S5 Table). While our model necessarily makes other assumptions, we have demonstrated that differences between our results and earlier analyses rest entirely on the four corrected assumptions. When making additional assumptions, we used the best available data, including age-at-seroconversion-dependent Weibull survival times [34]. Censorship rates due to loss to follow-up and couple dissolution [17,35,36] were informed by a recent study of the Rakai couples cohort [35] that largely overlapped with the original retrospective cohort study. While the exact number of incident SDCs excluded remains unknown, uncertainty in this quantity is reflected in our new estimates of EHMacute.
As with the preceding studies of acute phase infectivity [17,18], our goal was to accurately estimate the excess physiological infectivity due to the acute phase, which we measure by EHMacute. SDC cohort data that track susceptible individuals with both acutely infected and chronically infected partners is uniquely suited for this analysis. However, factors other than elevated physiological infectivity can also cause transmission in stable couples to occur more quickly from newly infected partners. In particular, various types of heterogeneity in infectiousness and susceptibility can lead to increased early transmission in couples cohorts and, if left unmodeled, can spuriously inflate estimates of physiologically elevated acute phase infectivity. While controlling for these confounding factors is critical, they should not all be dismissed as analytic nuisances. Some, but not all, forms of risk heterogeneity that bias couples cohort data can also transiently increase infectivity following infection in the broader population, and should be considered in addition to physiological infectivity when estimating AFacute and designing interventions.
In particular, we distinguish between persistent heterogeneity and time-varying heterogeneity. Persistent heterogeneity arises from systematic variation between individuals in susceptibility or infectiousness that remains relatively stable over the course of individuals’ sexually active lifetimes. This includes persistent biological states (e.g., circumcision, host or virus genotypes, chronic co-infections) or persistent behavioral differences between individuals (e.g., condom usage). Highly susceptible individuals (or partners of highly effective transmitters) will be infected relatively quickly after their first exposure. In these cases, early transmission is not a consequence of high acute phase infectivity but instead of persistently high risk. Outside of the stable couple context, this mechanism will not create the same bias toward early transmission, because relationship initiation and fast transmission can happen during either the acute or the (longer) chronic phase.
The effects of time-varying heterogeneity are more complex. In some cases, time-varying heterogeneity in infectiousness can contribute to AFearly at the population level as strongly as in stable couples cohorts. This will occur when increased infectiousness is correlated with recency of infection. For example, newly infected individuals often have other sexually transmitted infections (STIs) that elevate HIV infectiousness (either because the STI increased their risk of acquiring HIV or because they acquired the STI and HIV through the same risk behaviors), and consequently are more infectious early after HIV infection prior to STI treatment [54]. This could, for instance, account for the observation in the Rakai cohort that, because incident couples exhibited a higher prevalence of GUD compared to prevalent couples, adjusting for self-reported GUD reduced estimated EHMacute. Unlike persistent heterogeneity, this mechanism would increase infectivity during the early phase not only within stable partnerships but also for the broader population. Temporal variation in safe sex practices or coital rate could cause similar effects. Time-varying heterogeneity was not incorporated because data were lacking regarding its magnitude and volatility and because, as discussed above, we expected it to contribute to early transmission in couples cohorts in a manner similar to that of persistent heterogeneity. Nonetheless, we did simulate persistent heterogeneity to explore how unmodeled heterogeneity biases EHMacute. Finally, some forms of risk heterogeneity will affect AFearly at the population level but cannot be observed in stable couples cohorts, because they do not affect transmission to a stable partner. Examples include partner acquisition rates and tendency to maintain concurrent relationships (i.e., episodic risk behavior [11,12]).
While we separate physiologically elevated acute phase infectivity from various types of heterogeneity, we emphasize that estimates of AFearly must consider not only EHMacute and sexual network assumptions, but also sources of heterogeneity that could potentially amplify early transmission at the population level, including some that have been observed in the couples cohort that we have separated from EHMacute. Thus, while studies assuming larger values of EHMacute have generally produced larger estimates of AFearly (Fig. 7; S6 Table), we suggest caution when considering the intuitive conclusion that studies relying on upward-biased estimates of EHMacute have also overestimated AFearly. Because our analysis focused on estimating the relative infectivity and duration of the acute phase from SDCs, our model did not specify population-level sexual mixing patterns and thus was unable to produce an estimate of AFearly. We believe new estimates of AFearly are needed that carefully consider our updated EHMacute estimate, along with both persistent and time-varying heterogeneity, sexual network assumptions, and explicit consideration of differences in ART coverage between acutely and chronically infected individuals.
Fig. 7. Proportion of transmission due to acute infectivity. 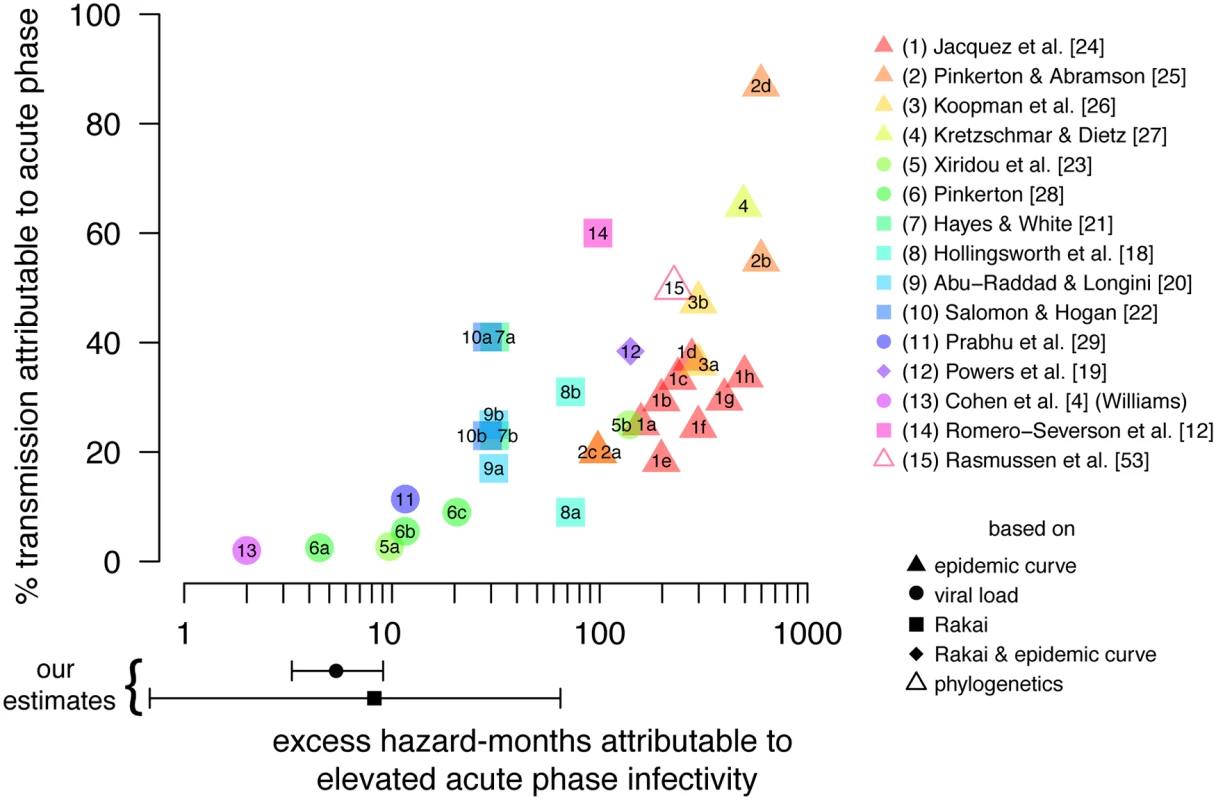
Published estimates of the proportion of incidence attributable to early transmission (AFearly) versus the assumed excess hazard-months attributable to physiologically elevated acute phase infectivity (EHMacute). Shapes indicate whether EHMacute was estimated from epidemic growth rates, viral load trajectories and viral load–infectivity relationships, the Rakai retrospective cohort, phylogenetics, or a combination thereof. Points reflecting studies that published more than one result are identified with letters; explanations of differences between estimates are available in S6 Table. Points and error bars below the x-axis indicate our estimated EHMacute from the Rakai retrospective cohort data and based on viral load trajectories; we do not specify a sexual network model and therefore do not estimate AFearly in this study. Conclusion
By analyzing a seminal HIV couples cohort study using stochastic models and approximate Bayesian computation, we have reestimated the relative infectivity of the acute phase and found that the most highly cited estimates are substantially biased upward by unmodeled heterogeneity and by the study exclusion criteria. Thus, the proportion of transmission occurring immediately after infection should be reevaluated, and may have more to do with risk heterogeneity and HIV intervention measures than with physiological differences between the acute and chronic stages of infection. These revised estimates should be considered when designing population-scale interventions and communicating individual-level risk in clinical or community settings. It is becoming increasingly clear that infected individuals should initiate ART as early as possible both to achieve the greatest reductions in transmission and for its direct clinical benefits [55]. Our findings cautiously suggest that the population-level benefits might be larger than predicted by earlier estimates.
Supporting Information
Zdroje
1. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365 : 493–505. doi: 10.1056/NEJMoa1105243 21767103
2. Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG (2009) Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373 : 48–57. doi: 10.1016/S0140-6736(08)61697-9 19038438
3. Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L (2013) High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 339 : 966–971. doi: 10.1126/science.1228160 23430656
4. Cohen MS, Dye C, Fraser C, Miller WC, Powers KA, et al. (2012) HIV treatment as prevention: debate and commentary—will early infection compromise treatment-as-prevention strategies? PLoS Med 9: e1001232. doi: 10.1371/journal.pmed.1001232 22802728
5. Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, et al. (2003) Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17 : 1871–1879. 12960819
6. Attia S, Egger M, Mueller M, Zwahlen M, Low N (2009) Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 23 : 1397–1404. doi: 10.1097/QAD.0b013e32832b7dca 19381076
7. Lingappa JR, Hughes JP, Wang RS, Baeten JM, Celum C, et al. (2010) Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS ONE 5: e12598. doi: 10.1371/journal.pone.0012598 20856886
8. Cohen MS, Shaw GM, McMichael AJ, Haynes BF (2011) Acute HIV-1 infection. N Engl J Med 364 : 1943–1954. doi: 10.1056/NEJMra1011874 21591946
9. Ma Z-M, Stone M, Piatak M, Schweighardt B, Haigwood NL, et al. (2009) High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol 83 : 3288–3297. doi: 10.1128/JVI.02423-08 19129448
10. Eaton JW, Hallett TB, Garnett GP (2011) Concurrent sexual partnerships and primary HIV infection: a critical interaction. AIDS Behav 15 : 687–692. doi: 10.1007/s10461-010-9787-8 20890654
11. Zhang X, Zhong L, Romero-Severson E, Alam SJ, Henry CJ, et al. (2012) Episodic HIV risk behavior can greatly amplify HIV prevalence and the fraction of transmissions from acute HIV infection. Stat Commun Infect Dis 4 : 1–21.
12. Romero-Severson EO, Alam SJ, Volz E, Koopman J (2013) Acute-stage transmission of HIV: effect of volatile contact rates. Epidemiology 24 : 516–521. doi: 10.1097/EDE.0b013e318294802e 23689754
13. Williams BG (2011) How important is the acute phase in HIV epidemiology? Cornell University Library. Available: http://arxiv.org/abs/1105.2767v1. Accessed 25 May 2014.
14. Grassly NC, Fraser C (2008) Mathematical models of infectious disease transmission. Nat Rev Microbiol 6 : 477–487. doi: 10.1038/nrmicro1845 18533288
15. Eaton JW, Hallett TB (2014) Why the proportion of transmission during early-stage HIV infection does not predict the long-term impact of treatment on HIV incidence. Proc Natl Acad Sci U S A 111 : 16202–16207. doi: 10.1073/pnas.1323007111 25313068
16. Powers KA, Kretzschmar ME, Miller WC, Cohen MS (2014) Impact of early-stage HIV transmission on treatment as prevention. Proc Natl Acad Sci U S A 111 : 15867–15868. doi: 10.1073/pnas.1418496111 25368195
17. Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li XB, et al. (2005) Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 191 : 1403–1409. doi: 10.1086/429411 15809897
18. Hollingsworth TD, Anderson RM, Fraser C (2008) HIV-1 transmission, by stage of infection. J Infect Dis 198 : 687–693. doi: 10.1086/590501 18662132
19. Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, et al. (2011) The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 378 : 256–268. doi: 10.1016/S0140-6736(11)60842-8 21684591
20. Abu-Raddad LJ, Longini IM Jr (2008) No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS 22 : 1055–1061. doi: 10.1097/QAD.0b013e3282f8af84 18520349
21. Hayes RJ, White RG (2006) Amplified HIV transmission during early-stage infection. J Infect Dis 193 : 604–605. 16425144
22. Salomon JA, Hogan DR (2008) Evaluating the impact of antiretroviral therapy on HIV transmission. AIDS 22 (Suppl 1): S149–S159. doi: 10.1097/01.aids.0000327636.82542.87 18664947
23. Xiridou M, Geskus R, de Wit J, Coutinho R, Kretzschmar M (2004) Primary HIV infection as source of HIV transmission within steady and casual partnerships among homosexual men. AIDS 18 : 1311–1320. 15362664
24. Jacquez JA, Koopman JS, Simon CP, Longini IM (1994) Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr 7 : 1169–1184. 7932084
25. Pinkerton SD, Abramson PR (1996) Implications of increased infectivity in early-stage HIV infection: application of a Bernoulli-process model of HIV transmission. Eval Rev 20 : 516–540. 10183259
26. Koopman JS, Jacquez JA, Welch GW, Simon CP, Foxman B, et al. (1997) The role of early HIV infection in the spread of HIV through populations. J Acquir Immune Defic Syndr Hum Retrovirol 14 : 249–258. 9117458
27. Kretzschmar M, Dietz K (1998) The effect of pair formation and variable infectivity on the spread of an infection without recovery. Math Biosci 148 : 83–113. 9597826
28. Pinkerton SD (2007) How many sexually-acquired HIV infections in the USA are due to acute-phase HIV transmission? AIDS 21 : 1625–1629. 17630558
29. Prabhu VS, Hutchinson AB, Farnham PG, Sansom SL (2009) Sexually acquired HIV infections in the United States due to acute-phase HIV transmission: an update. AIDS 23 : 1792–1794. doi: 10.1097/QAD.0b013e32832e7d04 19684485
30. Branson BM (2010) The future of HIV testing. J Acquir Immune Defic Syndr 55 (Suppl 2): S102–S105. doi: 10.1097/QAI.0b013e3181fbca44 21406978
31. R Development Core Team (2014) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
32. Robb ML (2012) Viral dynamics and immune response in acute infection and their impact on viral set-point [abstract]. PL02.02. AIDS Vaccine 2012; 9–12 September 2012; Boston, Massachusetts, US. Available: http://www.aidsvaxwebcasts.org/console/player/19054?mediaType=audio&. Accessed 16 May 2014.
33. Bellan SE, Fiorella KJ, Melesse DY, Getz WM, Williams BG, et al. (2013) Extra-couple HIV transmission in sub-Saharan Africa: a mathematical modelling study of survey data. Lancet 6736 : 1–9. doi: 10.1109/EMBC.2013.6611102 24111289
34. (2000) Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet 355 : 1131–1137. 10791375
35. Grabowski MK, Lessler J, Nalugoda F, Reynolds SJ, Ssekubugu R, et al. (2014) Introduction of HIV into stable heterosexual couples in Rakai, Uganda before and after ART [abstract]. Conference on Retroviruses and Opportunistic Infections 2014; 3–6 Mar 2014; Boston, MA, US.
36. Porter L, Hao L, Bishai D, Serwadda D, Wawer MJ, et al. (2004) HIV status and union dissolution in sub-Saharan Africa: the case of Rakai, Uganda. Demography 41 : 465–482. 15461010
37. Toni T, Welch D, Strelkowa N, Ipsen A, Stumpf MP (2009) Approximate Bayesian computation scheme for parameter inference and model selection in dynamical systems. J R Soc Interface 6 : 187–202. 19205079
38. Eaton JW, Johnson LF, Salomon JA, Barnighausen T, Bendavid E, et al. (2012) HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 9: e1001245. doi: 10.1371/journal.pmed.1001245 22802730
39. Kretzschmar ME, Schim van der Loeff MF, Birrell PJ, De Angelis D, Coutinho RA (2013) Prospects of elimination of HIV with test-and-treat strategy. Proc Natl Acad Sci U S A 110 : 15538–15543. doi: 10.1073/pnas.1301801110 24009342
40. Nolan S, Wood E (2014) End of the debate about antiretroviral treatment initiation. Lancet Infect Dis 14 : 258–259. doi: 10.1016/S1473-3099(13)70329-3 24602843
41. Owen SM (2012) Testing for acute HIV infection: implications for treatment as prevention. Curr Opin HIV AIDS 7 : 125–130. doi: 10.1097/COH.0b013e3283506613 22314506
42. Cori A, Ayles H, Beyers N, Schaap A, Floyd S, et al. (2014) HPTN 071 (PopART): a cluster-randomized trial of the population impact of an HIV combination prevention intervention including universal testing and treatment: mathematical model. PLoS ONE 9: e84511. doi: 10.1371/journal.pone.0084511 24454728
43. Bellan SE, Pulliam JRC, Scott JC, Dushoff J (2012) How to make epidemiological training infectious. PLoS Biol 10: e1001295. doi: 10.1371/journal.pbio.1001295 22509129
44. Williams BG, Granich R, Dye C (2011) Role of acute infection in HIV transmission. Lancet 378 : 1913–1915. doi: 10.1016/S0140-6736(11)61833-3 22137836
45. Nagelkerke NJD, Arora P, Jha P, Williams B, McKinnon L, et al. (2014) The rise and fall of HIV in high-prevalence countries: a challenge for mathematical modeling. PLoS Comput Biol 10: e1003459. doi: 10.1371/journal.pcbi.1003459 24626088
46. Bello G, Simwaka B, Ndhlovu T, Salaniponi F, Hallett TB (2011) Evidence for changes in behaviour leading to reductions in HIV prevalence in urban Malawi. Sex Transm Infect 87 : 296–300. doi: 10.1136/sti.2010.043786 21429896
47. Alam SJ, Zhang X, Romero-Severson EO, Henry C, Zhong L, et al. (2013) Detectable signals of episodic risk effects on acute HIV transmission: strategies for analyzing transmission systems using genetic data. Epidemics 5 : 44–55. doi: 10.1016/j.epidem.2012.11.003 23438430
48. Brenner BG, Roger M, Routy J-P, Moisi D, Ntemgwa M, et al. (2007) High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis 195 : 951–959. 17330784
49. Volz EM, Ionides E, Romero-Severson EO, Brandt M-G, Mokotoff E, et al. (2013) HIV-1 transmission during early infection in men who have sex with men: a phylodynamic analysis. PLoS Med 10: e1001568. doi: 10.1371/journal.pmed.1001568 24339751
50. Romero-Severson E, Skar H, Bulla I, Albert J, Leitner T (2014) Timing and order of transmission events is not directly reflected in a pathogen phylogeny. Mol Biol Evol 31 : 2472–2482. doi: 10.1093/molbev/msu179 24874208
51. Robinson K, Fyson N, Cohen T, Fraser C, Colijn C (2013) How the dynamics and structure of sexual contact networks shape pathogen phylogenies. PLoS Comput Biol 9: e1003105. doi: 10.1371/journal.pcbi.1003105 23818840
52. Grabowski MK, Redd AD (2014) Molecular tools for studying HIV transmission in sexual networks. Curr Opin HIV AIDS 9 : 126–133. doi: 10.1097/COH.0000000000000040 24384502
53. Rasmussen DA, Volz EM, Koelle K (2014) phylodynamic inference for structured epidemiological models. PLoS Comput Biol 10: e1003570. doi: 10.1371/journal.pcbi.1003570 24743590
54. Barnabas R, Webb E (2011) The role of coinfections in HIV epidemic trajectory and positive prevention: a systematic review and meta-analysis. AIDS 25 : 1559–1573. doi: 10.1097/QAD.0b013e3283491e3e 21633287
55. Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, et al. (2014) Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis: 281–290. doi: 10.1016/S1473-3099(13)70692-3 24602844
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 3- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Nutraceutikum Armolipid Plus podle klinických důkazů zlepšuje lipidový profil − metaanalýza
- Snižuje terapie betablokátory kardiovaskulární benefit aerobního cvičení u pacientů s arteriální hypertenzí?
-
Všechny články tohoto čísla
- Testing and Treating the Missing Millions with Tuberculosis
- UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age
- Association between Traffic-Related Air Pollution in Schools and Cognitive Development in Primary School Children: A Prospective Cohort Study
- Broad Blockade Antibody Responses in Human Volunteers after Immunization with a Multivalent Norovirus VLP Candidate Vaccine: Immunological Analyses from a Phase I Clinical Trial
- Strengthening the Detection of and Early Response to Public Health Emergencies: Lessons from the West African Ebola Epidemic
- HIV Treatment-As-Prevention Research: Authors’ Reply
- Role of Acute HIV Infection in Driving HIV Transmission: Implications for HIV Treatment as Prevention
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- HIV Treatment-As-Prevention Research: Taking the Right Road at the Crossroads
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
- A Public Health Approach to Hepatitis C Control in Low- and Middle-Income Countries
- Development and Validation of a Risk Score for Chronic Kidney Disease in HIV Infection Using Prospective Cohort Data from the D:A:D Study
- Reassessment of HIV-1 Acute Phase Infectivity: Accounting for Heterogeneity and Study Design with Simulated Cohorts
- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





