-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEngagement, Retention, and Progression to Type 2 Diabetes: A Retrospective Analysis of the Cluster-Randomised "Let's Prevent Diabetes" Trial
Attendance in a lifestyle and eating educational program is associated with a reduced incidence of diabetes Gray and colleagues reveal.
Published in the journal: . PLoS Med 13(7): e32767. doi:10.1371/journal.pmed.1002078
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002078Summary
Attendance in a lifestyle and eating educational program is associated with a reduced incidence of diabetes Gray and colleagues reveal.
Introduction
The prevention of diabetes is a global health care priority. A recent study estimated that in 2015 there were 5 million people with nondiabetic hyperglycaemia (NDH, also known as “prediabetes” or at high risk of diabetes) in England, equating to around 11.4% of the population aged 16 y and over [1]. A systematic review of progression rates suggested an incidence rate of progression to type 2 diabetes mellitus (T2DM) of 35.6 per 1,000 person years [2]. With rising levels of obesity, sedentary lifestyles, and low fitness, these numbers are expected to rise over the coming decades; the International Diabetes Federation estimates that worldwide levels of NDH will increase from 318 million to 482 million by 2040 [3].
There is robust evidence that T2DM can be prevented or delayed in those with NDH. Pivotal trials conducted globally showed that lifestyle modification programmes promoting a healthy diet, weight loss, and increased physical activity could reduce the incidence of T2DM by up to 58% [4–7]. These programmes were intensive—for example, in the first year of the United States Diabetes Prevention Programme (DPP), participants received 16 1-h one-to-one counselling sessions followed by an average of eight additional contacts and two telephone consultations [4,8]. Participants were also offered supervised exercise classes. The difficulty, therefore, has been translating such programmes in a resource-limited setting, such as the National Health Service (NHS). A review of programmes that had attempted to translate these findings into a real-world setting found a lower T2DM reduction of 26% [9].
The Let’s Prevent Diabetes programme is a 6-h structured group education programme with annual 3-h refresher sessions and telephone support that aims to translate the findings of the large-scale prevention studies into a pragmatic lower-resource programme suitable for delivery in the NHS [10,11]. The programme was evaluated in a published 3-y cluster-randomised trial, in which the primary analysis showed a no reduction in T2DM (hazard ratio [HR 0.74], 95% CI 0.48–1.14, p = 0.18) and modest benefits in biomedical, lifestyle, and psychosocial outcomes in those receiving the Let’s Prevent Diabetes programme compared to those receiving standard care. The per-protocol analysis excluding those participants from the intervention arm who did not attend the initial education session also showed no reduction in T2DM incidence (HR 0.65, 95% CI 0.41–1.03, p = 0.07). When assessing the joint distribution of cost and effect differences (measured using quality-adjusted survival), the programme was estimated to be cost-effective at a willingness-to-pay threshold of £20,000 per quality-adjusted life year (QALY) gained [10].
In 2014, the NHS Five Year Forward View outlined an ambition for England to be the first country to implement a national NHS Diabetes Prevention Programme (NDPP) [12]; the programme will launch in 2016. In order to inform the design and implementation of the NDPP, this study aimed to examine the effects of engagement and retention with the Let’s Prevent Diabetes education programme on the outcome found in comparison to standard care. A further aim was to examine the characteristics of those participants who completed the entire programme (retainers) and those who failed to engage with the education at all (nonengagers).
Methods
The methodology of the Let’s Prevent Diabetes trial and the main trial results have been published previously [11,13,14]. Briefly, the Let’s Prevent Diabetes trial cluster randomised general practices to either the intervention or standard care. Participants found to have NDH via a two-stage risk score screening programme were invited to take part in the trial [14,15]. The inclusion criteria for screening were ages 40–75 y if white European or 25–75 y if South Asian. Participants were excluded if they were unable to give informed consent, pregnant, or lactating, had established diabetes or a terminal illness, or if they required an interpreter for a language other than one of the locally used South Asian languages accommodated within the trial. All those agreeing to take part received an oral glucose tolerance test (OGTT). Only participants who were identified as having NDH (impaired fasting glucose [IFG] and/or impaired glucose tolerance [IGT] WHO 1999 criteria [16]) during screening took part in the randomised controlled trial (RCT). Participants within intervention practices were invited to attend the Let’s Prevent Diabetes programme [17], which is a group-based education programme underpinned by psychological theories aimed at increasing knowledge and promoting realistic perceptions of NDH and by promoting healthy behaviours (reducing weight, following a healthy diet, and increasing physical activity). The programme involves a 6-h session at baseline followed by 3-h refresher sessions at 12 and 24 mo, which reinforced key messages. In addition, participants received a 15-min telephone call every 3 mo from health care professionals trained to offer ongoing support in behaviour change. Those who did not attend the initial session were not invited to the refresher sessions but continued to be followed up. Participants in standard care practices received an information booklet that included information on risk factors for T2DM and how dietary and lifestyle changes and increased physical activity can prevent progression to T2DM.
The primary outcome of the trial was progression to T2DM assessed over 3 y. T2DM was diagnosed according to WHO 1999 criteria/guidelines [16], and from January 2013, HbA1c was also incorporated into the diagnostic criteria [18]. Secondary outcomes included glucose, lipid levels, blood pressure, weight, waist, and body mass index (BMI). Participants also completed a questionnaire containing a number of validated questionnaires that included measures of self-reported sitting time [19], anxiety and depression [20], and quality of life [21]. Participants also wore a sealed pedometer (NL-800, New Lifestyles, Lees Summit, Missouri, US) with a 7-d memory during waking hours to provide habitual ambulatory activity (average daily step count was derived by summing total accumulated steps and dividing by days worn). Outcomes were assessed at baseline and 6, 12, 24, and 36 mo post baseline. Follow-up rates were similar across the two intervention groups [11].
Statistical Analysis
Here we present the results from a secondary analysis of the intervention arm of a completed randomised trial. This analysis was not part of the original statistical analysis plan for the trial, and a separate prospective analysis plan for the analyses presented here was not written prior to undertaking the work; therefore, these results should be viewed as hypothesis generating. The baseline characteristics of participants in the intervention arm grouped by level of attendance were compared; we compared those who attended the first education session with those who did not (these are termed engagers versus nonengagers throughout) and those who attended all education sessions with those who did not (these are termed retainers versus nonretainers throughout). This analysis differs from the per-protocol analysis published as part of the primary trial findings as that analysis restricted the intervention group to those who attended at least the first education session compared to standard care [11]. Logistic regression was used to compare groups; standard errors were adjusted for the clustering.
Progression to T2DM was analysed by education attendance level. Participants not developing T2DM were censored at the date of their last clinical appointment. Cox proportional hazards models with the intervention group as a covariable were fitted; practices were assumed to have the same frailty. Secondary outcomes at 3 y were compared between (1) retainers versus standard care and (2) retainers versus nonretainers. For the secondary outcomes assessed, participants who developed T2DM during the study had their last value from before their diagnosis carried forward for the remainder of the study. This method was used in a previous similar study [22]. Secondary outcomes were analysed using generalised estimating equation models with an exchangeable correlation structure, which adjusted for clustering [23]. We also adjusted both analyses (primary and secondary outcomes) by age, sex, deprivation score, smoking status, and BMI; both adjusted and unadjusted data are shown.
Given that 55% of the intervention participants attended the core session and at least one refresher session compared to only 29% who attended all sessions, we conducted a number of sensitivity analyses that repeated the retainer analyses using the 55% as the group of interest rather than the 29% who attended all sessions. We have defined this group as “plus min one”.
Statistical significance was set at 5% for all analyses, with 95% confidence intervals reported. Throughout, missing data were not replaced, and an available case approach was taken. All analyses were conducted using Stata version 13.
Results
In total, 880 participants were recruited from 43 general practices, 447 to intervention practices and 433 to control practices [11]. Of the participants included from practices randomised to the intervention group, 346 (77.4%) attended the initial 6-h education session, i.e., were engagers (Fig 1). One hundred and thirty participants (29.1%) attended all sessions—i.e., were retainers, with 248 attending the initial sessions plus a minimum of one refresher session (55.5%). The baseline characteristics across levels of attendance are given in S1 Table.
Fig. 1. Attendance at education sessions. 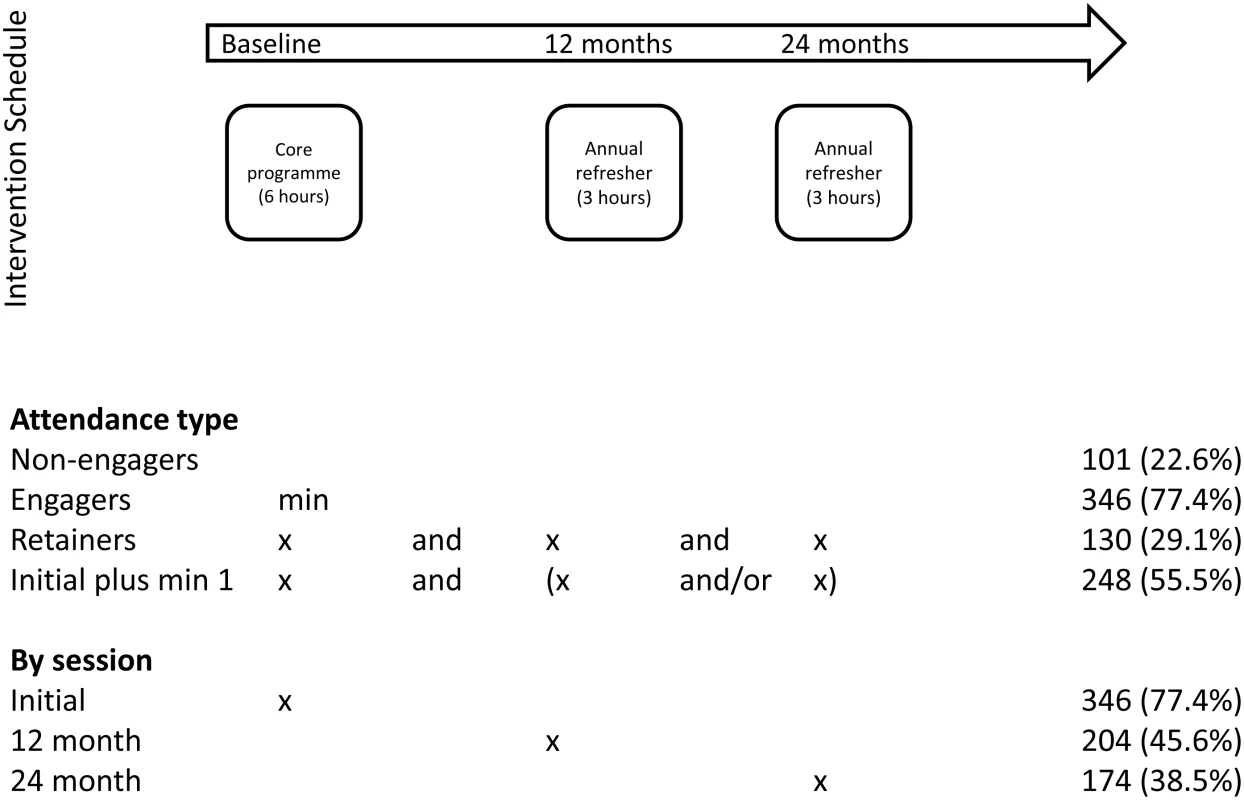
Table 1 compares the baseline characteristics of engagers versus nonengagers. Overall, engagers were older and more likely to be male and nonsmokers, be from less socioeconomically deprived areas, and have lower BMI than nonengagers. Table 2 compares retainers against nonretainers. Retainers were more likely to be older, nonsmokers, and have a lower BMI than nonretainers. Similar finding were found when assessing those who attended at least one refresher session (S2 Table).
Tab. 1. Comparison of baseline characteristics of those who engage versus nonengagers (defined as attending the first education session). 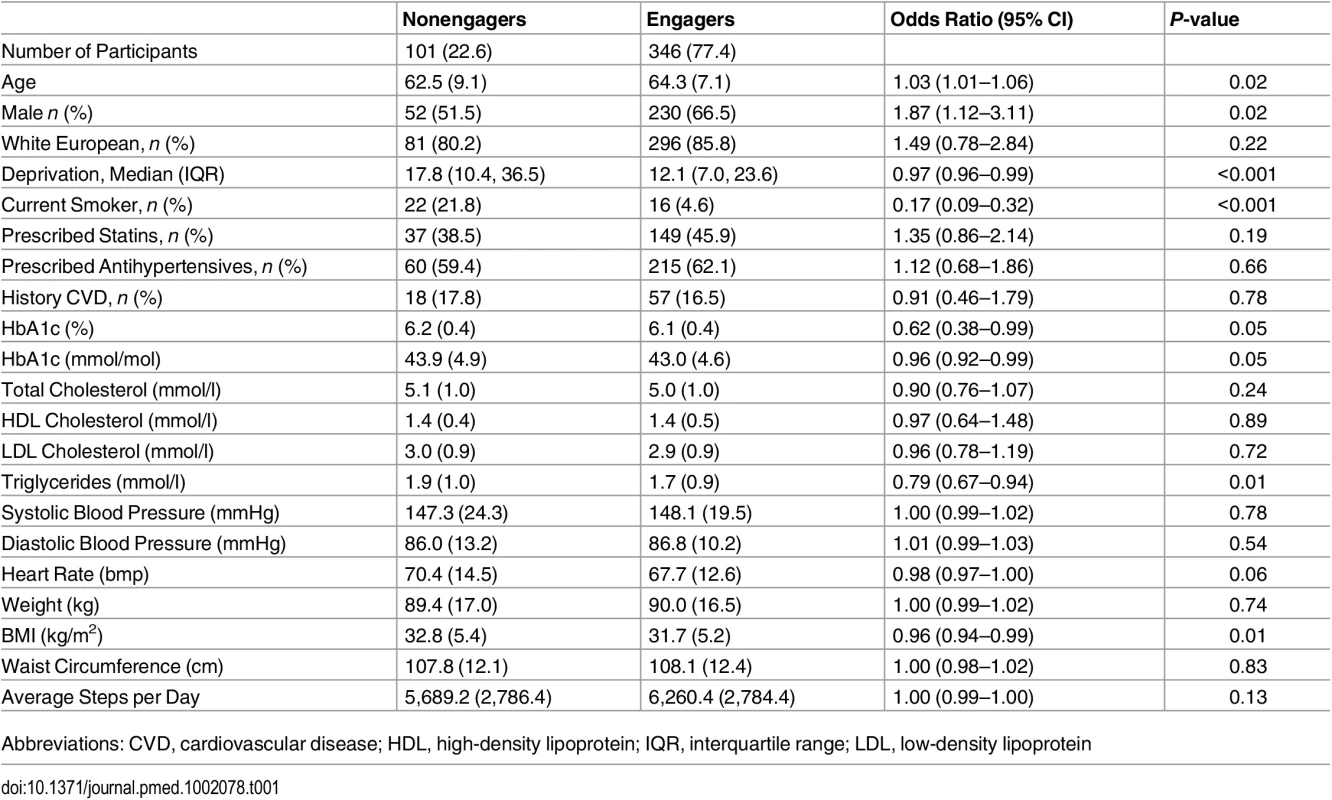
Data are given as mean (standard deviation [SD]) unless otherwise stated. The odds ratio gives the odds associated with being an engager compared to a nonengager, 95% CI adjusted for clustering. Tab. 2. Comparison of baseline characteristics of those who retain (defined as attending all education sessions) versus nonretainers. 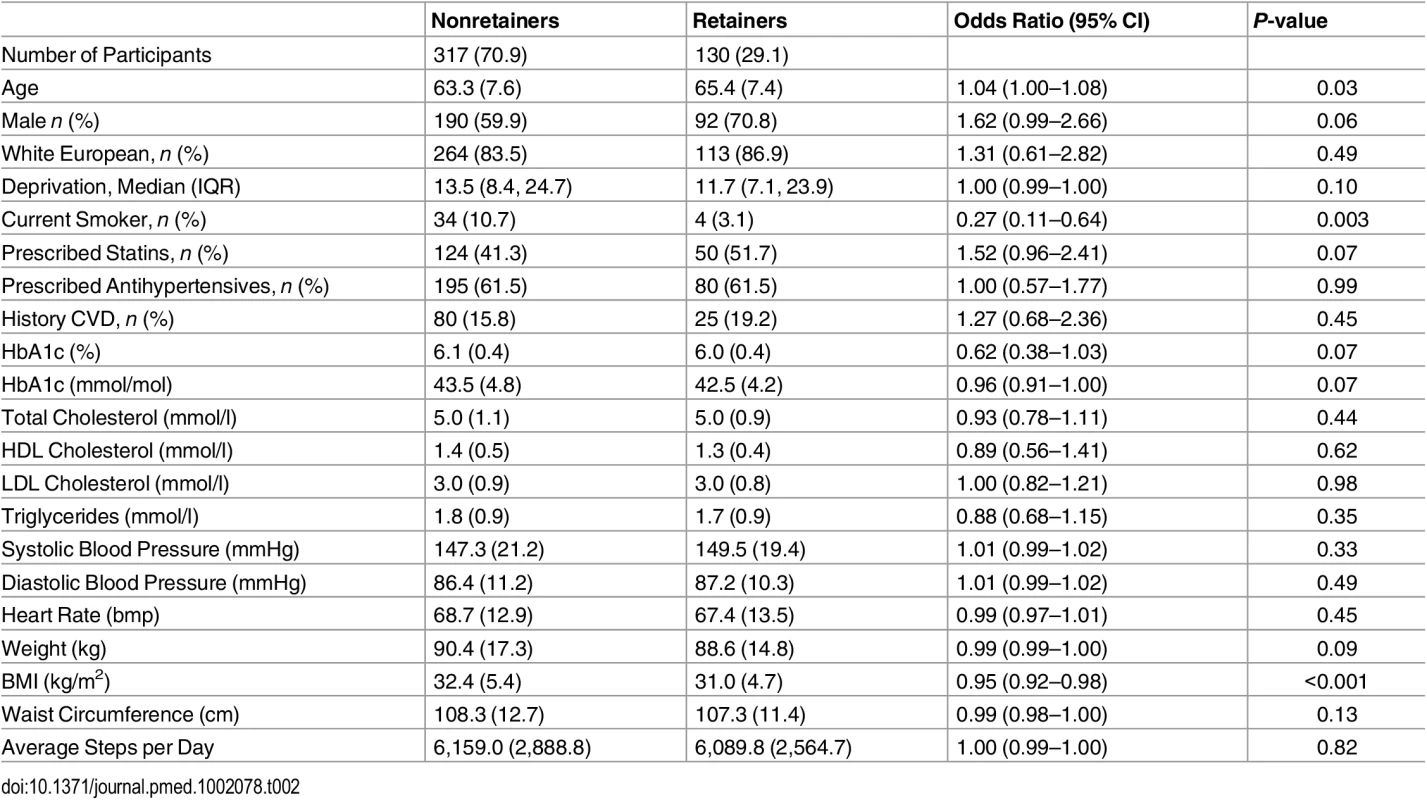
Data are given as mean (SD) unless otherwise stated. The odds ratio gives the odds associated with being a retainer compared to a nonretainer, 95% CI adjusted for clustering. The intention-to-treat (ITT) primary analysis of the Let’s Prevent Diabetes trial showed no reduction in the incidence of T2DM (Fig 2), an incidence rate of 57.60 per 1,000 person years in the intervention group compared to 63.16 per 1,000 person years in the standard care group (Table 3). When assessing the difference in incidence between the intervention and standard care group by attendance, a dose-response relationship was observed, with a greater reduction in incidence being seen with increasing retention; incidence rate in those who attended all sessions was 16.86 per 1,000 person years. A statistically significant association was observed in those who attend the initial session and then a minimum of one refresher session (HR 0.38 [95% CI 0.236–0.62]) and in retainers (HR 0.12 [95% CI 0.05–0.28]) compared to standard care. Adjusting for age, sex, deprivation score, smoking status, and BMI did not alter the interpretation of these results.
Tab. 3. Comparison of T2DM events and incidence rates across groups. 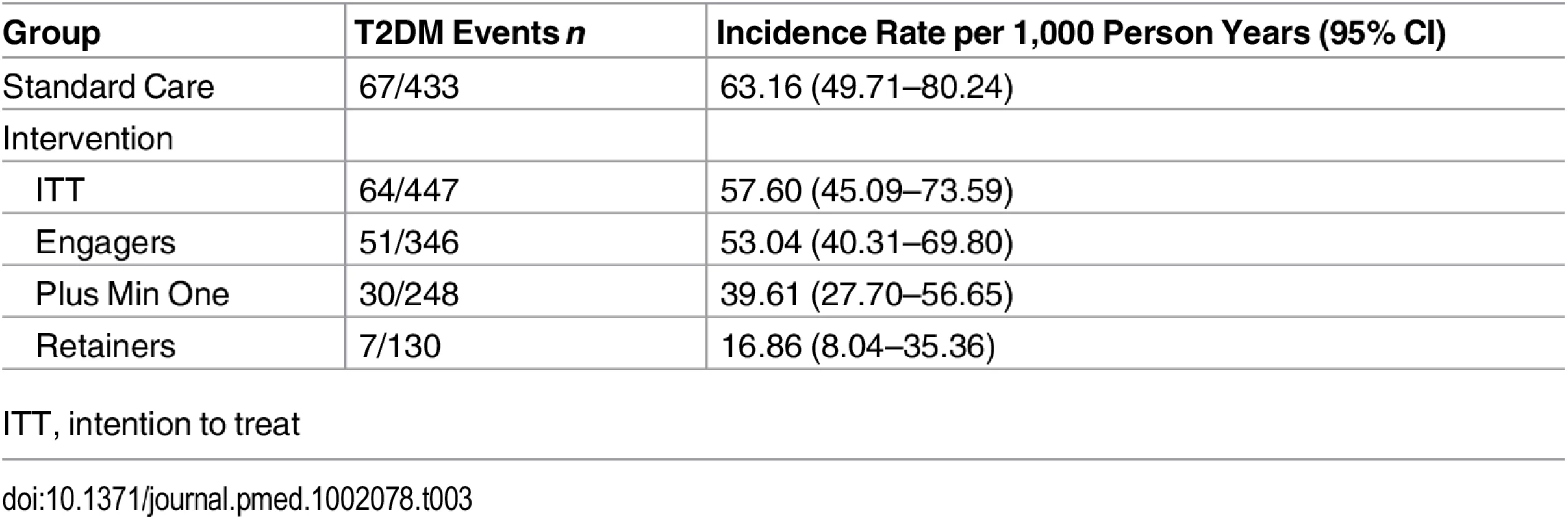
ITT, intention to treat Fig. 2. Incidence of type 2 diabetes by attendance. 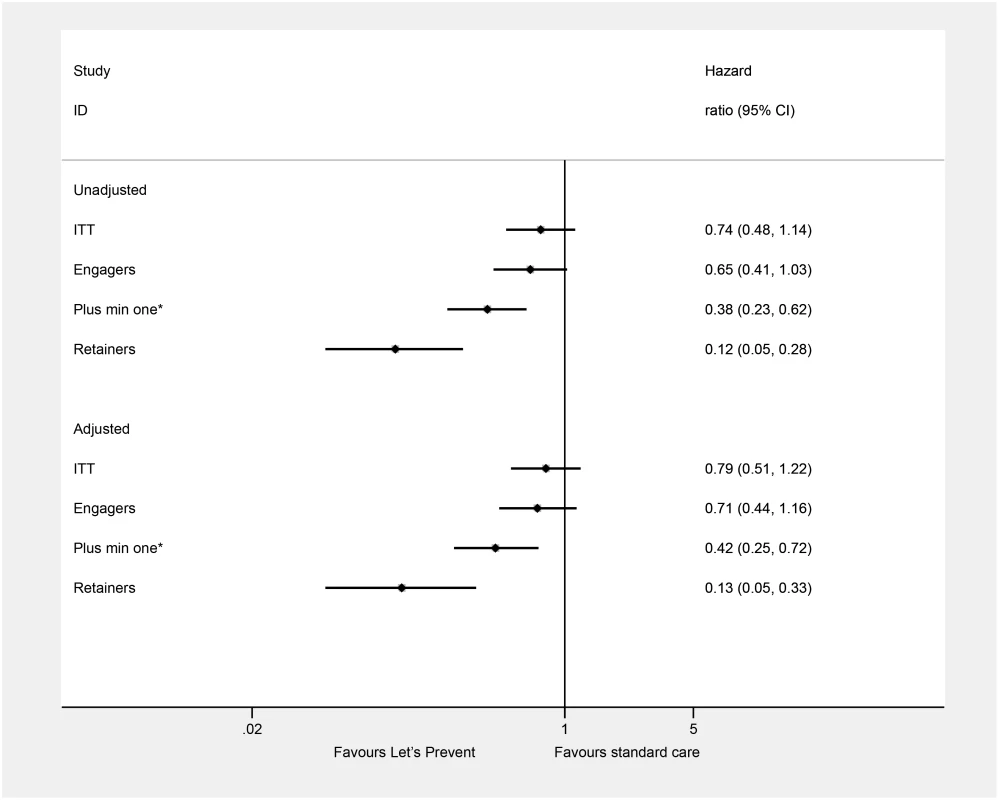
HR (95% CI) takes into account clustering, and the adjusted models include age, sex, deprivation score, smoking status, and BMI. Engagers: attended the initial education session; retainers: attended all education sessions; plus min one: attended the initial education plus a minimum of one refresher session; * these are not mutually exclusive groups. Table 4 shows two subgroup analyses for key secondary outcomes. Retaining was associated with statistically significant improvements in fasting and 2-h glucose and HbA1c compared to those receiving standard care and nonretainers. Retainers had on average HbA1c values that were 0.16% lower than those who received standard care at 3 y; this seemed to be driven by an increase in HbA1c in the standard care group. Retainers were also significantly leaner than those who received standard care and nonretainers, with lower weight, BMI, and waist circumference. Retainers were on average 1.70 kg lighter than nonretainers and 1.28 kg lighter than those in the standard care arm. Lower levels of anxiety and higher quality of life were also seen in those who retained compared to standard care. On average, those who completed the whole programme had a significantly higher step count of 925 steps per d compared to standard care. Adjusting for age, sex, deprivation score, smoking status, and BMI did not alter the interpretation of these results.
Tab. 4. Secondary outcomes in those retaining compared to standard care and retainers versus nonretainers. 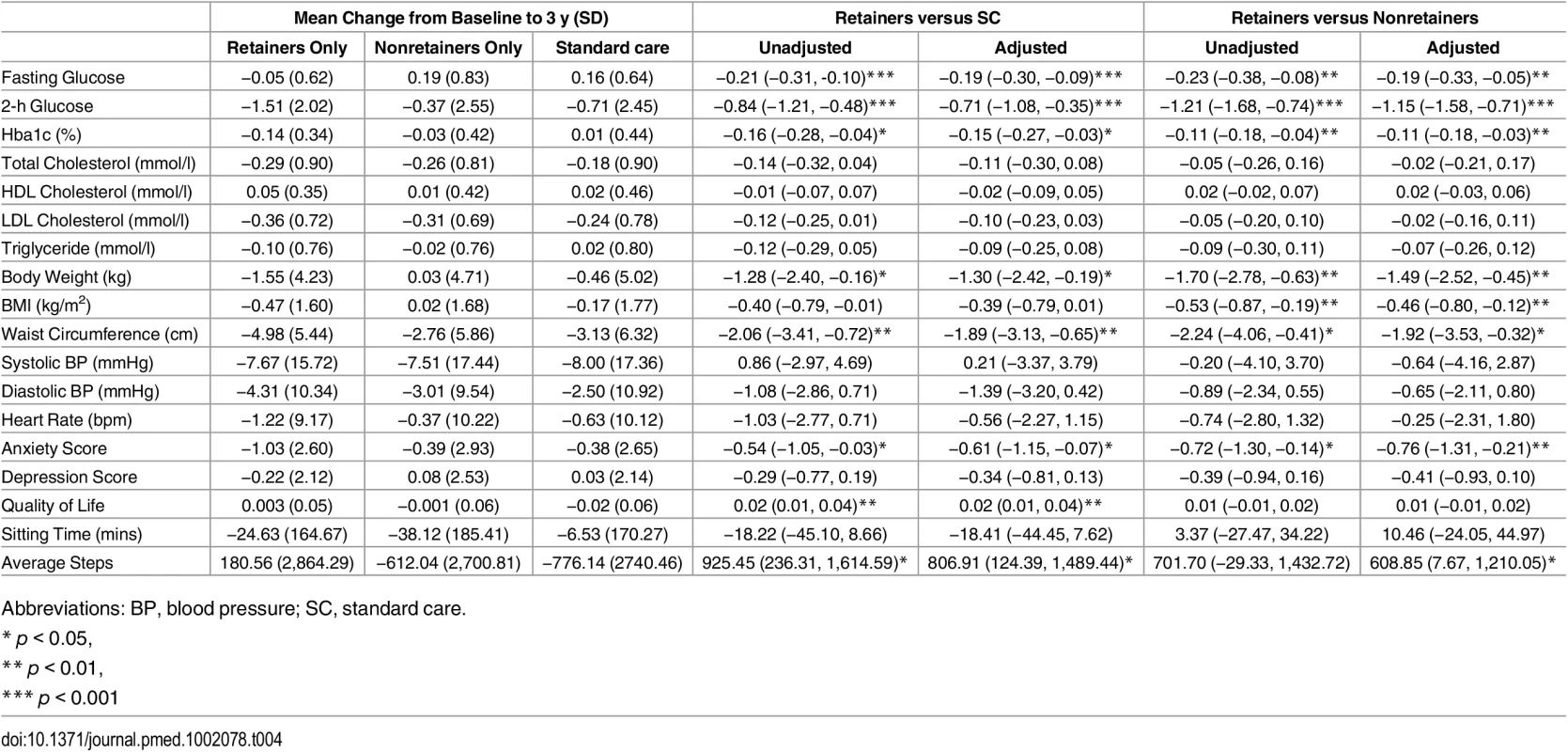
Coefficients show the mean effect of the intervention compared to standard care adjusted for baseline value and cluster. Adjusted columns are additionally adjusted for age, sex, deprivation score, smoking status, and BMI. The sensitivity analysis of those who attended at least one refresher session is shown in S3 Table. The majority of the findings are consistent with those based on participants who attended all sessions, although there were some notable differences. Comparing to standard care using this group as the comparator, no differences in change in body weight or average step count were seen. When comparing within the intervention group those who attended at least one refresher session compared to those who did not, larger differences were seen for systolic blood pressure (unadjusted −2.28 mmHg, adjusted −3.21 mmHg) than when assessing retainers. Across both comparisons, no differences in anxiety score were seen.
Discussion
Although the primary analysis of the Let’s Prevent Diabetes trial including all randomised participants (including those who never accessed the intervention) showed a no effect on the incidence of T2DM, we have shown a large difference in T2DM incidence over 3 y (88%; HR 0.12 [95% CI 0.05–0.28]) in those who attended all education sessions compared to standard care. A dose-response relationship with the incidence of T2DM being reduced as attendance increased was seen. Importantly, these relationships were independent of age, sex, smoking status, deprivation score, and BMI. Significantly improved secondary outcomes were also seen in those who retained compared to those who received standard care and those who did not complete all education sessions. Similar results were seen when assessing those who attended the core session and at least one refresher session. These results combined suggest that both engagement with a prevention programme and retaining that engagement are critical for the effectiveness of diabetes prevention programmes.
Similar findings have been shown for other non-United Kingdom prevention programmes. In the US, a study that translated the 16-session DPP for use in American Indian and Alaska native communities found a statistically significant lower incidence rate of T2DM in the two-thirds who completed all 16 sessions compared to those who did not, with approximately half the number of cases [24]. The retention rate was higher than that seen in the Let’s Prevent Diabetes trial, which may be reflective of the shorter programme duration—all 16 sessions were delivered within 6 mo. A US-based weight management programme found that both weight and diabetes incidence were reduced in a similar manner to the results shown here when comparing retainers to those who did not participate [25]. A meta-analysis of programmes modelled on the DPP showed that with every additional session attended, weight loss increased by 0.26 percentage points [26]. To date, there is no published data assessing compliance with a diabetes prevention programme and outcome in a UK population.
We have also identified similar characteristics in those who do not engage and those who do not retain. These groups were younger, more likely to be smokers, and have a higher BMI than those who do engage/retain. The younger age may be related to employment and availability to attend multiple sessions run during the working week. Prevention programmes in clinical practice should offer sessions at a variety of times and at weekends or in the workplace. Overweight and obesity are major drivers of T2DM risk; additionally, smokers may represent a less healthy cohort. Therefore, developing mechanisms to engage this higher-risk group are essential. Deprivation was also found to be important in terms of initial engagement, with those from more deprived areas being less likely to engage with the education. This is consistent with a 10-wk UK prevention programme that also reported an association between deprivation and follow-up noncompletion [27].
The NDPP will be a 9-mo programme including a minimum of 13 contacts totalling 16 hours, as informed by the evidence review of completed real-world diabetes prevention programmes and National Institute for Health and Care Excellence (NICE) recommendations [9]. The challenge will be replicating these outcomes in a real-world setting and ensuring retention in the programme for 9 mo. Previous studies have shown a dilution of effect when translating programmes that have worked in a research setting to routine clinical practice [28]. Here, we show that even in a much less resource-intensive programme, albeit over a longer duration, adherence was not achieved in the majority of participants. Unfortunately, no data were collected within the Let’s Prevent trial regarding why participants chose not to attend the education sessions. Future studies in this area should aim to collect such data to inform the development of future programmes. Here, we have identified drivers for engaging and retaining; the providers of the NDPP may want to focus some additional resources on these hard-to-engage-and-retain groups, such as those of working age and those from deprived areas. The data collected during the rollout of the NDPP could also be used to assess the minimum dose of intervention required to see reductions in risk factors in a real-world setting.
This study has limitations that should be considering when interpreting the results. These data are from a cluster-randomised trial; therefore, it could be argued that this may not be reflective of what would happen if the Let’s Prevent Diabetes programme was rolled out in the real world. Those taking part might reflect a more engaged sample than the population with NDH; indeed, only 19% of those invited to take part consented [11]. Therefore, these results should be viewed as optimistic. Additionally, this is a secondary unplanned analysis. The Let’s Prevent Diabetes trial was not powered to assess outcomes by attendance status, and the multiple testing may have increased the chance of making a type one error—although, reassuringly, the pattern of results seen here is consistent with other research in this area. The trial was powered to assess the data on an intention-to-treat basis that includes all those randomised irrespective of the intervention received [29]. It could be argued that this is more reflective of the results that could be achieved in clinical practice [30]. However, this type of efficacy analysis is useful for informing future studies and implementation policy that may assess novel methods for increasing retention to achieve such outcomes. Finally, participants also received a 15-min telephone call every 3 mo from health care professionals trained to offer ongoing support in behaviour change. This analysis has not considered the effectiveness of this additional support.
This study suggests characteristics that could be used to identify those at high risk of either not engaging or not retaining. Future studies could build on these findings to develop validated models for predicting nonengagement/nonretainment. Trials could then assess the effectiveness of providing additional incentives or communications in such groups. The rollout of the NDPP could be a platform on which to embed such studies to increase the efficiency of the programme; similar studies have been embedded in RCTs [31].
In conclusion, we have shown that attending all sessions of the Let’s Prevent Diabetes programme is associated with an 88% reduction in T2DM incidence over 3 y compared to those who received standard care in the trial. Those who do not engage or retain in the whole programme tend to be younger and of a higher T2DM risk status. Commissioners of prevention programmes need to ensure programmes are accessible to all and keep participants engaged and motivated to continue.
Supporting Information
Zdroje
1. National Cardiovascular Intelligence Network (NCVIN) (2015) NHS Diabetes Prevention Programme (NHS DPP) Non-diabetic hyperglycaemia. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/456149/Non_diabetic_hyperglycaemia.pdf
2. Morris DH, Webb D, Achana F, Srinivasan B, Gray LJ, et al. Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 2013;56(7):1489–1493. doi: 10.1007/s00125-013-2902-4 23584433
3. International Diabetes Federation (2015) IDF Diabetes Atlas—Seventh Edition.
4. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 346 : 393–403. 11832527
5. Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, et al. (2003) The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 26 : 3230–3236. 14633807
6. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, et al. (2006) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia 49 : 289–297. 16391903
7. Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, et al. (2007) Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 334 : 299. 17237299
8. Group TDPPR (2003) Costs Associated With the Primary Prevention of Type 2 Diabetes Mellitus in the Diabetes Prevention Program. Diabetes Care 26 : 36–47. 12502656
9. Ashra N SR, Carter P, DAvies M, Dunkley A, Gillies C, Greaves C, Khunti K, Sutton S, Yates T, Youssef D, Gray LJ, (2015) A systematic review of the effectiveness of lifestyle interventions for the prevention of type 2 diabetes mellitus (T2DM) in routine practice. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/456147/PHE_Evidence_Review_of_diabetes_prevention_programmes-_FINAL.pdf
10. Davies M, Gray L, Ahrabian D, Leal J, Gray A, Troughton J, et al. A community based primary prevention programme for type 2 diabetes in the UK: A cluster randomised controlled trial. World Diabetes Congress 2015. 30 November-4 December. Abstract 1021-PD. http://www.idf.org/files/abstracts/data/HtmlApp/main.html#
11. Davies MJ, Gray LJ, Troughton J, Gray A, Tuomilehto J, et al. (2016) A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let's Prevent Diabetes cluster randomised controlled trial. Preventive Medicine 84 : 48–56. doi: 10.1016/j.ypmed.2015.12.012 26740346
12. NHS (2014) Five year forward view. https://www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf.
13. Gray LJ, Khunti K, Williams S, Goldby S, Troughton J, et al. (2012) Let's Prevent Diabetes: study protocol for a cluster randomised controlled trial of an educational intervention in a multi-ethnic UK population with screen detected impaired glucose regulation. Cardiovascular Diabetology 11 : 56. 22607160
14. Gray LJ, Khunti K, Edwardson C, Goldby S, Henson J, et al. (2012) Implementation of the automated Leicester Practice Risk Score in two diabetes prevention trials provides a high yield of people with abnormal glucose tolerance. Diabetologia 55 : 3238–3244. doi: 10.1007/s00125-012-2725-8 23001376
15. Gray LJ, Davies MJ, Hiles S, Taub NA, Webb DR, et al. (2012) Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multiethnic UK community setting. Diabetologia 55 : 959–966. doi: 10.1007/s00125-011-2432-x 22231125
16. World Health Organisation (1999) Definition, Diagnosis, and Classification of Diabetes Mellitus and its Complications. Report of a WHO consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organisation.
17. Troughton J, Chatterjee S, Hill SE, Daly H, Martin Stacey L, et al. (2015) Development of a lifestyle intervention using the MRC framework for diabetes prevention in people with impaired glucose regulation. Journal of Public Health. doi: 10.1093/pubmed/fdv110
18. World Health Organisation (2011) Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Geneva.
19. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, et al. (2003) International physical activity questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise 35 : 1381–1395. 12900694
20. Zigmond AS, Snaith RP (2006) The hospital anxiety and depression scale. Acta Psychiatr Scand 67 : 361–370.
21. Sintonen H, Pekurinen M (1993) A fifteen-dimensional measure of health-related quality of life (15D) and its applications. In: Walker SR, Rosser RM, editors. Quality of Life Assessment: Key Issues in the 1990s. Dordrecht: Kluwer Academic Publishers. pp. 185–195.
22. Yates T, Davies M, Gorely T, Bull F, Khunti K (2009) Effectiveness of a pragmatic education programme aimed at promoting walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care 32 : 1404–1410. doi: 10.2337/dc09-0130 19602539
23. Hayes RJ, Moulton LH (2009) Cluster randomised trials. Florida, USA: Chapman & Hall/CRC.
24. Jiang L, Manson SM, Beals J, Henderson WG, Huang H, et al. (2013) Translating the Diabetes Prevention Program Into American Indian and Alaska Native Communities: Results from the Special Diabetes Program for Indians Diabetes Prevention demonstration project. Diabetes Care 36 : 2027–2034. doi: 10.2337/dc12-1250 23275375
25. Jackson SL, Long Q, Rhee MK, Olson DE, Tomolo AM, et al. (2015) Weight loss and incidence of diabetes with the Veterans Health Administration MOVE! lifestyle change programme: an observational study. The Lancet Diabetes & Endocrinology 3 : 173–180.
26. Ali MK, Echouffo-Tcheugui JB, Williamson DF (2012) How Effective Were Lifestyle Interventions In Real-World Settings That Were Modeled On The Diabetes Prevention Program? Health Affairs 31 : 67–75. doi: 10.1377/hlthaff.2011.1009 22232096
27. Penn L, Ryan V, White M (2013) Feasibility, acceptability and outcomes at a 12-month follow-up of a novel community-based intervention to prevent type 2 diabetes in adults at high risk: mixed methods pilot study. BMJ Open 3: e003585. doi: 10.1136/bmjopen-2013-003585 24227871
28. Cardona-Morrell M, Rychetnik L, Morrell SL, Espinel PT, Bauman A (2010) Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health 10 : 1–17.
29. Abraha I, Cherubini A, Cozzolino F, De Florio R, Luchetta ML, et al. (2015) Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study. BMJ 350.
30. Wareham NJ Mind the gap: efficacy versus effectiveness of lifestyle interventions to prevent diabetes. The Lancet Diabetes & Endocrinology 3 : 160–161.
31. Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, et al. (2014) Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open 4: e003821. doi: 10.1136/bmjopen-2013-003821 24496696
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- The Clinical and Public Health Challenges of Diabetes Prevention: A Search for Sustainable Solutions
- Screening for Dysglycemia: Connecting Supply and Demand to Slow Growth in Diabetes Incidence
- Diabetes: A Cinderella Subject We Can’t Afford to Ignore
- Prescribing Exercise and Lifestyle Training for High Risk Women in Pregnancy and Early Post-partum—Is It Worth It?
- Population Approaches to Prevention of Type 2 Diabetes
- First-Year Evaluation of Mexico’s Tax on Nonessential Energy-Dense Foods: An Observational Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
- Leveraging Genetics to Advance Type 2 Diabetes Prevention
- Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis
- Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults
- Dietary Diversity, Diet Cost, and Incidence of Type 2 Diabetes in the United Kingdom: A Prospective Cohort Study
- Detecting Dysglycemia Using the 2015 United States Preventive Services Task Force Screening Criteria: A Cohort Analysis of Community Health Center Patients
- Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial)
- Associations between Recreational and Commuter Cycling, Changes in Cycling, and Type 2 Diabetes Risk: A Cohort Study of Danish Men and Women
- Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study
- Engagement, Retention, and Progression to Type 2 Diabetes: A Retrospective Analysis of the Cluster-Randomised "Let's Prevent Diabetes" Trial
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Cycling and Diabetes Prevention: Practice-Based Evidence for Public Health Action
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



