-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBacteria-Induced Dscam Isoforms of the Crustacean,
The Down syndrome cell adhesion molecule, also known as Dscam, is a member of the immunoglobulin super family. Dscam plays an essential function in neuronal wiring and appears to be involved in innate immune reactions in insects. The deduced amino acid sequence of Dscam in the crustacean Pacifastacus leniusculus (PlDscam), encodes 9(Ig)-4(FNIII)-(Ig)-2(FNIII)-TM and it has variable regions in the N-terminal half of Ig2 and Ig3 and the complete Ig7 and in the transmembrane domain. The cytoplasmic tail can generate multiple isoforms. PlDscam can generate more than 22,000 different unique isoforms. Bacteria and LPS injection enhanced the expression of PlDscam, but no response in expression occurred after a white spot syndrome virus (WSSV) infection or injection with peptidoglycans. Furthermore, PlDscam silencing did not have any effect on the replication of the WSSV. Bacterial specific isoforms of PlDscam were shown to have a specific binding property to each tested bacteria, E. coli or S. aureus. The bacteria specific isoforms of PlDscam were shown to be associated with bacterial clearance and phagocytosis in crayfish.
Published in the journal: . PLoS Pathog 7(6): e32767. doi:10.1371/journal.ppat.1002062
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002062Summary
The Down syndrome cell adhesion molecule, also known as Dscam, is a member of the immunoglobulin super family. Dscam plays an essential function in neuronal wiring and appears to be involved in innate immune reactions in insects. The deduced amino acid sequence of Dscam in the crustacean Pacifastacus leniusculus (PlDscam), encodes 9(Ig)-4(FNIII)-(Ig)-2(FNIII)-TM and it has variable regions in the N-terminal half of Ig2 and Ig3 and the complete Ig7 and in the transmembrane domain. The cytoplasmic tail can generate multiple isoforms. PlDscam can generate more than 22,000 different unique isoforms. Bacteria and LPS injection enhanced the expression of PlDscam, but no response in expression occurred after a white spot syndrome virus (WSSV) infection or injection with peptidoglycans. Furthermore, PlDscam silencing did not have any effect on the replication of the WSSV. Bacterial specific isoforms of PlDscam were shown to have a specific binding property to each tested bacteria, E. coli or S. aureus. The bacteria specific isoforms of PlDscam were shown to be associated with bacterial clearance and phagocytosis in crayfish.
Introduction
The immunoglobulin super family (IgSF) is composed of proteins that contain at least one immunoglobulin domain [1]. Several members of IgSF are expressed on the cell surface and there serve as receptors for diverse ligands, and contribute to a variety of cellular activities [2]. In vertebrates, many IgSF members play essential roles as immune molecules (also known as antibodies) by recognizing non-self entities and then promoting their elimination [3]. Although it appears as if all invertebrates lack true antibodies, diversified IgSF molecules have been shown to be involved in immune defense of several invertebrates [4], [5]. However, this does not imply that the diversification of IgSF in invertebrates have any relation to the antibody diversification in vertebrates [5]. Recently, one IgSF member, the Down syndrome cell adhesion molecule gene or Dscam, that can generate hypervariable isoforms through alternative splicing was shown to act as an opsonin and to enhance phagocytosis in insects [4], [6].
Dscam was first detected on the human chromosome 21q22, a region associated with Down Syndrome [7]. Then, orthologues of Dscam were identified in various species and the typical domain structure of the Dscam gene is highly conserved. The Dscam molecules are widely expressed in the nervous system and play an essential role in neural circuit formation [8], [9]. Moreover, transcripts of fly Dscam was detected in fat body cells and hemocytes, which both are important components of the insect immune system [4], [10]. The Dscam in hemocytes of Drosophila melanogaster and Anopheles gambiae can bind to Escherichia coli and potentially acts as both a phagocytic receptor and as an opsonin. In the mosquito, Dscam can generate pathogen-specific spliced forms upon immune challenge [6]. These findings provide some evidences that Dscam may have functions not only in neuronal wiring, but also in innate immunity in insects.
In the present study, the full-length cDNA and variable regions of P. leniusculus Dscam (PlDscam) were identified and characterized. We also present results showing that different isoforms of PlDscam can be induced by immune challenge, that they can bind bacteria and that they are important in bacterial clearance.
Results
Isolation and characterization of the PlDscam
A large open reading frame of PlDscam (6,009 bp) was identified that encodes a polypeptide of 2,002 aa (Figure 1A). The closest sequence matching that of PlDscam was Dscam of L. vannamei (identity = 85%). Domain homology analysis using SMART showed that the deduced amino acid sequence contains a signal peptide at amino acids 1–24, ten tandem repeated immunoglobulin domains (Ig), six fibronectin type III domains (FNIII) and a transmembrane domain (TM). The domain organization of PlDscam is 9(Ig)-4(FNIII)-(Ig)-2(FNIII)-TM (Figure 1B). It also has a conserved cell attachment sequence (Arg-Gly-Asp: RGD motif) between Ig6 and Ig7 (Figure 1A). The sequence in the 3′ UTR contains a polyadenylation signal (AATAA) and is located at 55 bp upstream of the poly A tail (Figure 1C).
Fig. 1. Identification and characterization of Dscam in P. leniusculus. 
A) Amino acid sequence of PlDscam; double underlining represents signal peptide, while the Immunoglobulin (Ig) and Fibronectin type III (FNIII) domains are indicated by light and dark gray shading, respectively. A conserved RGD motif between Ig6 and Ig7 is indicated by single underlining. In addition, the single transmembrane domain is shown in a box. B) Schematic diagram showing the domain organization of the PlDscam protein. C) 3′ UTR containing the polyadenylation signal (AATAA) is indicated by double underlining and a stop codon is bold and underlined. Expression of PlDscam transcript diversity
To identify the variable regions of PlDscam, several regions of the PlDscam were amplified using different primer pairs and the location of each pair of primers is shown in Figure S1. Fifty clones of each of the six amplified regions were selected and sequenced, translated and aligned using ClustalW. Alternatively spliced mRNA segments of the PlDscam were detected in the N-terminal of Ig2 and Ig3, in the entire length of the Ig7 domain, in the transmembrane domain and in the cytoplasmic tail (Figure 2B–E and Figure S1A). In total 12, 29, 32 and 2 alternative spliced forms of the exons encoding Ig2, Ig3, Ig7 and the transmembrane domains, respectively were detected and therefore at least 22,000 different unique isoforms could in theory be generated (Figure 2A–2E). The other domains were highly conserved, especially Ig8-FNIII4. The cytoplasmic tail of PlDscam contains some highly conserved motifs similar to the corresponding area in Dscams of other species (Figure S1B).
Fig. 2. High diversity regions of Dscam in freshwater crayfish. 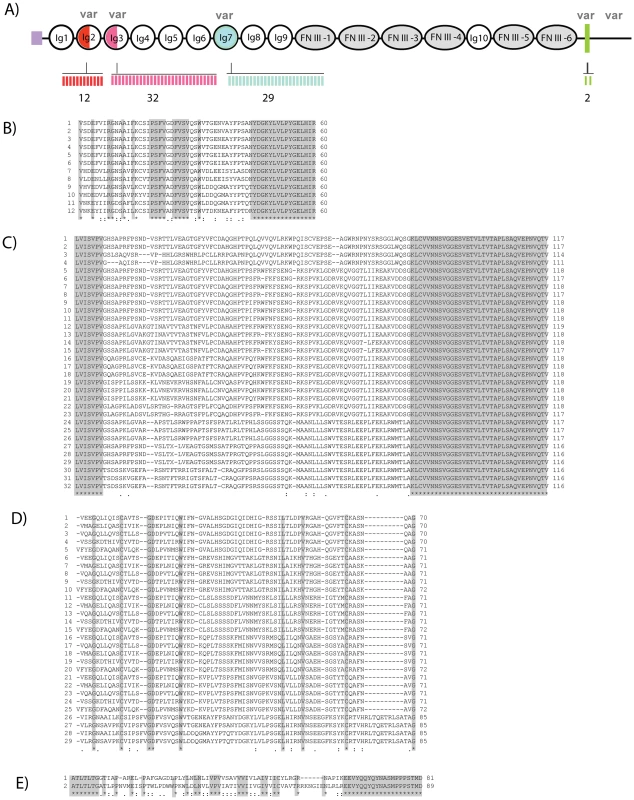
A) Diagram showing the locations of putative alternatively spliced exons of PlDscam. The variable regions are indicated at the N-terminal parts of Ig2 (red) and Ig3 (pink) and complete Ig7 (blue) and transmembrane (green) domains in crayfish. The number of alternative exons is presented by bars under the diagram. B–E) Multiple amino acid sequence alignment of PlDscam variable regions of Ig2(B), Ig3(C), Ig7(D) and transmembrane domain (E) aligned by ClustalW, Dark shading indicates the boundary of each domain. Tissue expression and phylogenetic analysis of PlDscam
In crayfish, PlDscam was abundantly expressed in the heart, moderately expressed in the testis, the hematopoietic tissue (HPT), brain and nerve, whereas low expression was detected in the stomach, gill, muscle and hemocytes. The PlDscam was not detected in hepatopancreas or in intestine (Figure 3A).
Fig. 3. Tissue distribution and phylogenetic analysis. 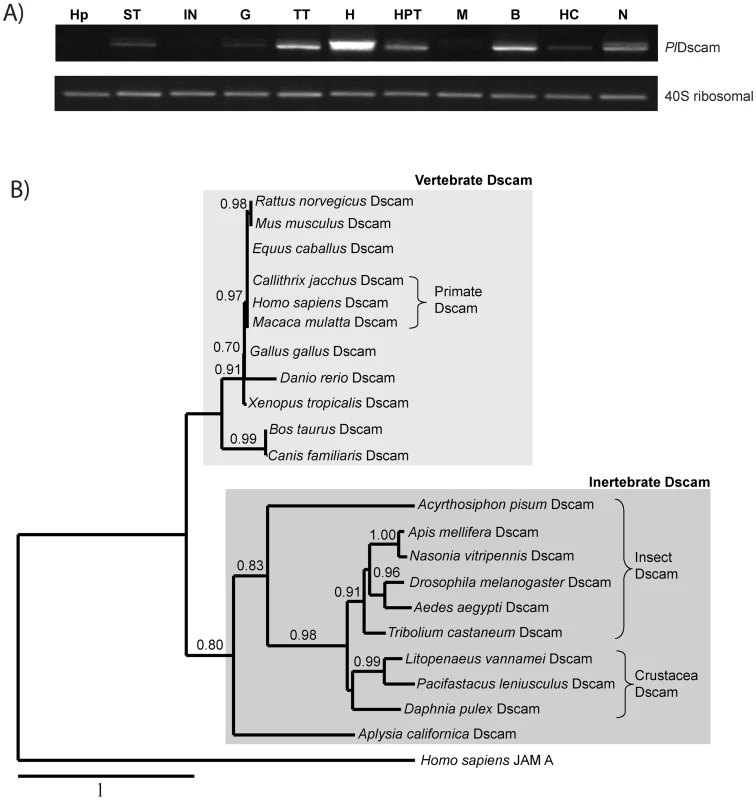
A) The expression of PlDscam was studied in various tissues, such as HP = Hepatopancreas, ST = Stomach, IN = Intestine, G = Gill, TT = Testis, H = Heart, M = Muscle, B = Brain, HC = Hemocyte and N = abdominal nerve. A 40S ribosomal gene was used as an internal control. B) A phylogram based on multiple alignments between PlDscam and other Dscam proteins; Drosophila melanogaster (AAF71926), Apis mellifera (AAT96374), Homo sapiens (AF217525), Mus musculus (NP_112451), Danio rerio (AAT36313), Daphnia pulex (ACC65888), Xenopus tropicalis (NP_001136135), Gallus gallus (XP_416734), Bos taurus (XP_002693048), Canis familiaris (XP_546506) Rattus norvegicus (AAL57167), Macaca mulatta (XP_002803170), Callithrix jacchus (XP_002761477), Tribolium castaneum (NP_001107841), Nasonia vitripennis (XP_001599258), Acyrthosiphon pisum (XP_001949262), Equus caballus (XP_001491675), Aedes aegypti (EAT37388), Aplysia californica (ABS30432) and Litopenaeus vannamei (ACZ26466). A human junctional adhesion molecule A or JAMA was used as outgroup (NP_058642). A phylogenetic analysis clearly separated the Dscam proteins of vertebrates and invertebrates in two different groups and PlDscam clustered with other members of invertebrate Dscams (Figure 3B).
LPS and bacteria injection induce PlDscam transcription
To investigate whether immune challenge induces higher expression of PlDscam in crayfish hemocytes, LPS, peptidoglycan (PG), E. coli, S. aureus or WSSV was used as immune elicitors. The results of quantitative RT-PCR revealed that the PlDscam mRNA expression was significantly induced by LPS-, E. coli- and S. aureus at 6 h to 24 h post injection compared to controls (Figure 4A, 4C, 4D). In contrast, the expression profile of PlDscam in PG - and WSSV - injected animals was not changed (Figure 4B and 4E).
Fig. 4. Expression profiles of PlDscam in response to immune challenge. 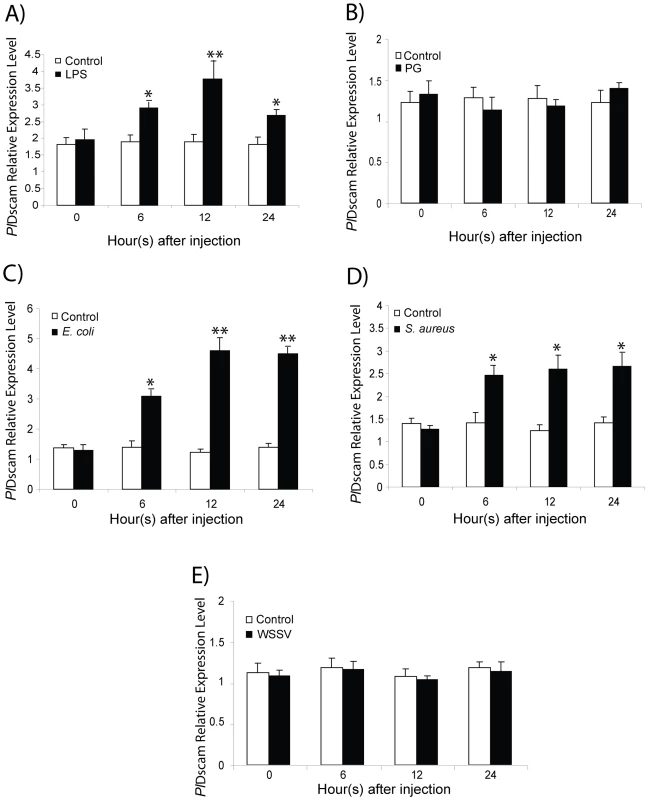
PlDscam expression was significantly higher than the control from 6 h post-injection with LPS (A), E. coli (C) and S. aureus (D). In contrast, PG (B) and WSSV (E)-injection did not have any effect on the PlDscam expression level. The asterisk indicates that the expression levels are significantly different (*P<0.05, **P<0.01). Clustering of PlDscam isoforms in hemocytes during a bacterial infection
In order to characterize the major isoforms of PlDscam in hemocytes of normal and bacteria injected crayfish, PlDscam cDNA fragments encompassing the signal peptide to Ig3 from each group were amplified and cloned (Figure 5A). A total of 50 clones of each cDNA fragment were sequenced. Ten isoforms were detected in all groups, i.e. normal, E. coli injected and S. aureus injected crayfish. These isoforms were named as Normal isoform (N) (Table S2 and Table S3). Furthermore ten abundant isoforms were found only in the E. coli or S. aureus injected group and were named E. coli induced isoform (E) or S. aureus induced isoform (S), respectively (Table S2 and Table S3). All isoforms were subjected to multiple sequence alignment using clustalW. The similarity of each PlDscam isoform was then clustered by the maximum likelihood (ML) and Bayesian inference (BI) methods. As shown in Figure 5B, the clustering tree contained two major branches. The E. coli induced isoforms were separated into one branch, whereas the other branch consisted of two sub-branches of normal isoform and S. aureus induced isoform (Figure 5B).
Fig. 5. Bacteria-induced PlDscam isoforms. 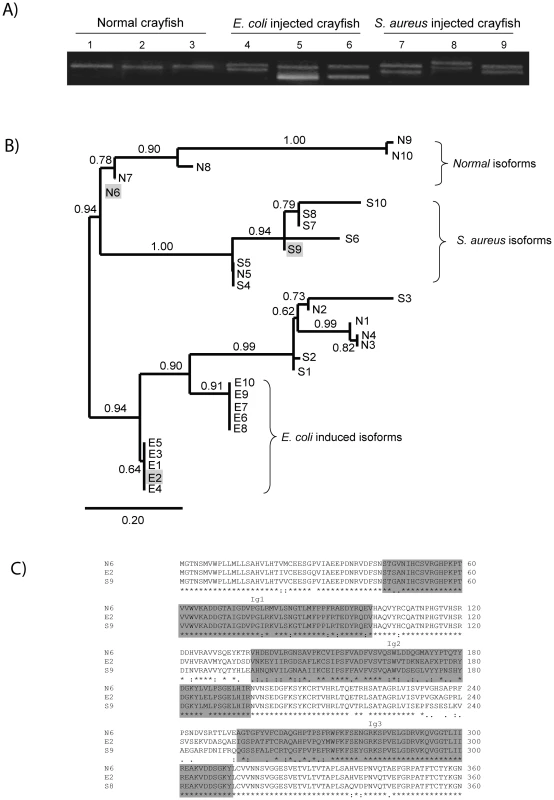
A) Different isoforms of PlDscam were amplified from hemocyte cDNA of normal crayfish, at 12 h post injection with E. coli or S. aureus. The PCR products were detected on 1.2% agarose gel. The PCR-products from normal crayfish (N) are shown in lanes 1–3, while PCR-products from E. coli (E) and S. aureus (S) injected animals are shown in lanes 4–6 and 7–9 respectively. B) Clustering of all PlDscam isoforms is based on the similarity of their amino acid sequence. The gray shaded boxes represent the chosen isoforms. C) Multiple amino acid sequence alignment of the isoforms E2, N6 and S9 aligned by ClustalW. Dark shading indicates the boundary of each domain. The E isoforms of PlDscam isoforms had a VNKEYIIRGDSA(F/I)LKCSIPSFVA(D/N) motif and a EIGSPATFTCRAQAHPVPQY motif present at the N-terminal of the Ig2 and Ig3 domains, respectively. As shown in Figure 5B and 5C the isoforms N6, E2 and S9 are different, and therefore we used these isoforms as representative alternative spliced forms of normal, E. coli - and S. aureus-induced isoforms for a bacterial binding assay.
Binding of rPlDscam to E. coli and S. aureus
Recombinant proteins covering the Ig1-Ig3 domains of the PlDscam isoforms of N6, E2 and S9 were produced and tested whether these domains have any putative function in binding to E. coli or S. aureus. These proteins were expressed in bacterial systems and the size of all soluble recombinant proteins was ∼67 kDa (Figure 6A). All recombinant proteins were fused with GST at the N-terminus and contained the Ig1-Ig3 domains. We used these rPlDscams in bacterial binding assays to reveal whether different isoforms are capable of direct binding to bacteria. The GST protein was used as a non-specific binding control in these experiments. The in vitro bacterial binding assays showed that all isoforms of PlDscam had different binding ability to the two tested bacteria. The rPlDscam of isoform E2 clearly bound to E. coli and had significantly higher binding than the N6 and S9 isoforms. In contrast, binding of rPlDscam S9 to S. aureus was significantly higher (P<0.05) than that of N6 and E2 (Figure 6B).
Fig. 6. Bacteria-induced PlDscam isoforms have different binding ability to bacteria. 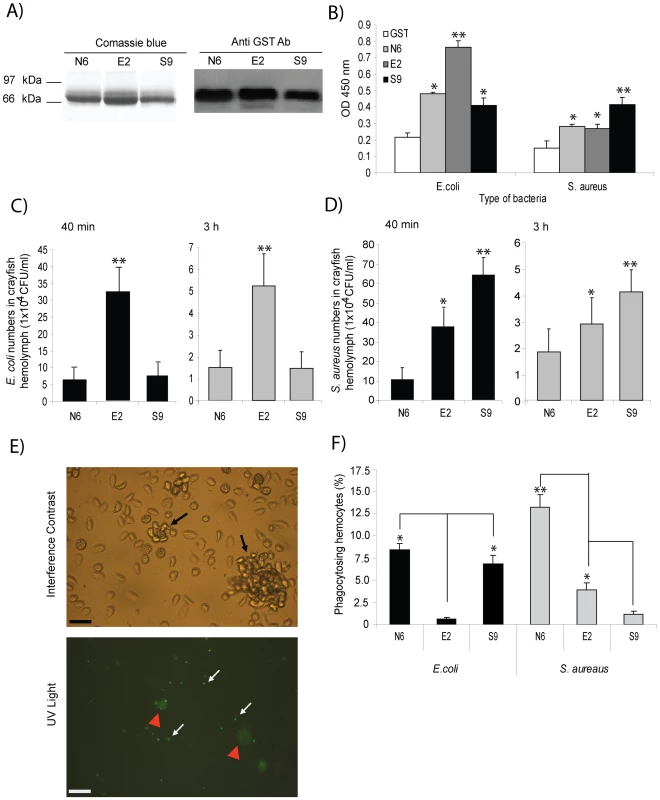
A) Different PlDscam isoforms were amplified from N6, E2 and S9 subclones and recombinant proteins were produced. The recombinant isoforms were subjected to 12% SDS and were confirmed by western blotting. Isoforms from non-injected animals in lanes 1 and 4; E. coli- induced isoform in lanes 2 and 5; and S. aureus-induced isoform in lanes 3 and 6. B) In vitro binding assays of rPlDscam isoforms of E. coli (left) and S. aureus (right), respectively. Binding of bacteria to different rPlDscam isoforms, consisting of N6 = isoforms from non-induced animal, E2 = E. coli induced isoforms, S9 = S. aureus induced isoforms or GST = control recombinant protein was determined using ELISA by measuring the absorbance of samples at 450 nm (OD450). C and D) The effect of bacteria induced isoforms on the clearance process in vivo was tested by pre-incubation of the bacteria E. coli (C) and S. aureus (D) with each recombinant isoform prior to injection into crayfish. The bacterial numbers in crayfish were determined as CFU/ml of crayfish in the hemolymph at 40 min (black) and 3 h (grey) after the bacterial challenge. This experiment was repeated three times and data represent means of these experiments. E) Phagocytosis and nodule formation of FITC-conjugated heat-killed bacteria by crayfish hemocytes. Black arrows show nodule formation under normal light microscopy. White arrows show the ingested FITC-conjugated heat-killed bacteria by crayfish hemocytes under UV light microscope. Red arrows show nodules that contain several ingested bacterial particles under UV light microscope. F) The percentage of crayfish hemocytes ingesting FITC-conjugated heat-killed E. coli (left) and S. aureus (right) that had been pre-incubated with each isoform of the recombinant proteins and the phagocytic hemocytes was determined at 1.5 h after challenge. Significant difference compared to control is marked by * = P<0.05 and ** = P<0.01. The effect of specific bacteria induced isoforms on bacterial clearance and phagocytosis
Recombinant proteins of isoforms E2 and S9 that specifically interacted with E. coli and S. aureus, respectively, were found to interfere with bacterial clearance and phagocytosis in crayfish. Pre-incubation of E. coli with E2 followed by injection into the animals did increase the number of bacteria in circulation (Figure 6C). Similarly with S. aureus, the number of bacteria was the highest in the S9 isoform of rPlDscam pre-incubated group (Figure 6D). This result clearly indicates that the specific rPlDscam could interfere with bacterial binding to the hemocytes. These results were in agreement with the phagocytosis assay, since if E. coli and S. aureus were coated with the E2 and S9 isoforms respectively, this resulted in a significant decrease in the phagocytic activity (Figure 6F).
In vitro effect of PlDscam gene silencing on WSSV replication
The role of PlDscam during a WSSV infection was investigated using PlDscam RNAi to suppress the PlDscam expression. The PlDscam gene was completely knocked down in an HPTcell culture (Figure 7A) whereas the 40S ribosomal gene was unaffected. However, PlDscam silencing did not have any effect on WSSV replication as shown with no changes in transcription level of WSSV structural protein transcript VP28 between control and PlDscam silenced groups (Figure 7B and 7C). Moreover, this result also indicates that the expression of PlDscam was not affected by WSSV infection. This agrees with our previous experiment where we injected WSSV to live crayfish and the transcript level of Dscams was not affected (Figure 4E).
Fig. 7. Effect of PlDscam silencing on viral replication of WSSV in vitro. 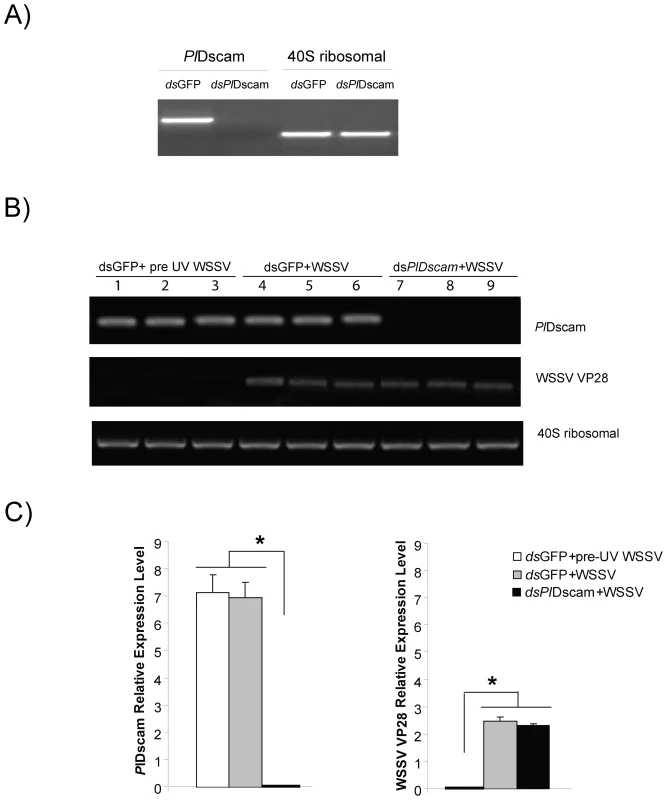
A) Silencing of PlDscam using dsRNA in cultured HPT cells was confirmed by RT-PCR before WSSV inoculation. B) WSSV VP28 expression in PlDscam silenced animals (lane 7–9) and the control (lane 4–6). UV-killed WSSV was served as control (lane 1–3), analyzed by semi-quantitative RT-PCR. C) Statistical analysis after comparing different band intensity of the results shown in (B). Significant differences are indicated by asterisks (P<0.05). Discussion
The typical domain structure of Dscam with an extracellular domain, a single transmembrane domain and a C-terminal cytoplasmic tail, is highly conserved within arthropods and vertebrates [7], [11]. This domain architecture was also found in PlDscam. Diversity in Dscam is generated through alternative splicing and variable alternative exons were found in the N-terminal half of Ig2 and Ig3, in the entire Ig7 domain and in the complete transmembrane domain [12]. These four variable domains are highly conserved within arthropods, including the PlDscam. It is noticeable that the alternative splicing of these exons clearly contributes to separate Dscam of vertebrates from invertebrates in our phylogenetic analysis [11].
The Dscam was initially identified for its essential roles in neuronal wiring, so its transcript is present in high quantity in neuronal organs [13]. The PlDscam was also detected in the neural system of crayfish. However, recently a putative role of Dscam in host defense was shown in D. melanogaster and A. gambiae [4], [6]. Both D. melanogaster and A. gambiae Dscams were required for host resistance and phagocytosis of bacteria [14]. Dscams of the mosquito respond to pathogen infection by generating specific isoforms and these pathogen-specific isoforms of Dscam can bind directly to pathogens [15]. The response of IgSF molecules to pathogens does not only generate specific isoforms, but also an increase in the number of these isoforms, such as is the case with the fibrinogen-related proteins (FREPs) in snails [5]. FREP production is enhanced following parasitic invasion, and these proteins can bind to parasitic invaders or their products. In crayfish challenge with both Gram-negative and Gram-positive bacteria induced higher transcription of PlDscam. A high transcription of PlDscam was obtained after LPS injection, whereas PG or viral injection of WSSV had no such effect. Most Gram-positive bacterial cell walls or cell membranes contain several components, including PG, lipoteichoic acid (LTA), and lipoproteins [16]. The high transcription of PlDscam achieved as a response to S. aureus injection but no response to PG may indicate that the PlDscam respond to other components of the S. aureus cell wall, such as LTA which has similar physiochemical properties to LPS from Gram-negative bacteria [17].
Previous results from D. melanogaster and L. vannamei showed that the hemocytes of immune challenged animals exhibited higher variability of the Ig2 and Ig3 domains but only a few Ig7 variants compared to normal animal [4], [18]. This is the reason why we studied bacteria specific induced isoforms and produced recombinant proteins covering only the Ig1-Ig3 region. Interestingly, PlDscam isoforms of E. coli injected crayfish mainly encoded “VNKEYIIRGDSA(F/I)LKCSIPSFVA(D/N)” and “EIGSPATFTCRAQAHPVPQY” motifs at the N-terminal part of the Ig2 and Ig3 domains, respectively. This implies that these two motifs might be important parts of the specific PlDscam isoforms in E. coli-infected crayfish. This is consistent with results from our bacterial binding assay, which showed that the recombinant proteins containing these two motifs (E2), could bind to E. coli better than the other isoforms (N6 and S9). In addition, binding of S. aureus-induced isoforms (S9) to S. aureus was also higher than E2 and N6. These results indicate that the pathogen induced isoforms of PlDscam have a specific binding property to each type of challenged bacteria. This specific interaction may be associated with some immune defense reaction as shown with mosquito Dscam [6]. To address this question, recombinant proteins (N6, E2 and S9) were pre-incubated with bacteria or FITC conjugated heat killed bacteria to study bacteria clearance and phagocytosis in vivo. When, the bacteria-induced PlDscam isoforms were coated on E. coli or S. aureus and then injected into live animals, this resulted in lowered clearing rates of bacteria in the hemolymph. This implies that the specific PlDscam fragments covered the binding sites of the bacteria so they could not bind to the membrane bound PlDscam on the hemocytes and hence the bacterial number increases since the clearance of bacteria by phagocytes is inhibited.
In the case of mosquito, AgDscam is not only a determinant of resistance to bacteria but also affects the resistance towards the malaria parasite Plasmodium [6]. This implies that Dscam might be involved in other host pathogens reactions in crayfish. The Dscam belongs to a subfamily of the Immunoglobulin super family (IgSF). Indeed, many members of IgSF proteins have been reported to interact with and promote entry of numerous virus, including for example the junctional adhesion molecule A (JAM A), that could bind with and facilitate entry of reovirus [19], [20]. So, it is possible that PlDscam could bind to virus like the other members of IgSF and maybe facilitate entry of virus into crayfish hemocytes. WSSV is a virulent pathogen that causes death in many species of crustaceans such as crayfish [21] and therefore we tested a possible relationship between PlDscam and this important arthropod virus. We performed experiments to reveal whether WSSV challenge could increase transcription of PlDscam and whether PlDscam RNAi had any effect on WSSV replication. However, we could not detect any increase in PlDscam mRNA expression after WSSV infection, and more important, if the PlDscam gene was completely silenced this could not affect WSSV infection or replication.
Materials and Methods
Crayfish
Healthy intermolt freshwater crayfish (P. leniusculus) were obtained from Lake Hjälmaren, Sweden and maintained in aerated tap water at 10°C.
Cloning of full length PlDscam cDNA
Total RNA (at least 1 µg) was extracted from the heart and converted into cDNA using ThermoScript (Invitrogen). The degenerate primers were designed from the conserved region of insect Dscam including Drosophila, Apis, Aedes and Tribolium (DSCAM-e5 F: 5′-AARCAYMGIYTIACIGGIGARAC-3′; DSCAM-e7 R: 5′-GTI ARIACIGTYTCIACI SWYTC-3′). The resulting PCR product was purified and cloned into a TOPO vector (Invitrogen) and sequenced. A partial sequence was used for the further step. Gene specific primers for Rapid amplification of cDNA ends (RACE) technique (Table S1) were designed from the partial sequence and 5′ or 3′ RACE-PCR was performed with a SMART universal primer A mix (SMARTer RACE cDNA Amplification Kit user manual, Clontech). Thermal cycling was as follows: 25 cycles of 94°C 30 s, 68°C 30 s, and 72°C 3 min. The 5′ and 3′ RACE PCR products were cloned into TOP10 vector and sequenced.
PlDscam sequence analysis and phylogenetic analysis
The nucleotide sequence of PlDscam was compared to others in Genbank using BlastX. Multiple sequence alignment was done by ClustalW (http://www.ebi.ac.uk/Tools/clustalw/index.html). The deduced amino acid domain was predicted with SMART (http://smart.embl-heidelberg.de/). A phylogenetic tree representing the relationship between PlDscam and other proteins was analyzed by the maximum likelihood (ML) and Bayesian inference (BI) methods. A PhyML program (under the Whelan and Goldman (WAG) and gamma model with four categories) was used in ML analysis [22]. For the BI method, we used MrBayes program [23] with CAT model (3,000 cycles, first 1,000 cycles removed as burn-in, and the analysis was repeated three times with identical results). Internal blanch support values was from analysis of 1,000 ML bootstrap replicates. This evolutionary phylogram was based on the conserved region of Dscam from Ig8 to FNIII4, whereas phylogram for clustering of all PlDscam isoforms was based on the similarity of their amino acid sequence and were used with the same methods as described above.
Tissue distribution of PlDscam mRNA
RNA from various tissues, including hepatopancreas, stomach, intestine, heart, hematopoietic tissue (Hpt), muscle, brain, hemocytes and nerves, was extracted following the instruction of GenElute Mammalian Total RNA Miniprep kit (Sigma) followed by treatment with RNase-Free DNase I (Ambion, Austin, TX). Complementary DNA was synthesized using ThermoScript. PlDscam gene specific primers (GSP-PlDscam-F, 5′ - TGGGAAGTGATGCCAGGTTAGA-3′; GSP-PlDscam-R, 5′-TTGAATCAGCAGACATAACCAAAGC-3′) were designed from full-length cDNA of PlDscam and its PCR product covered the conserved Dscam region (from Ig8 to FNIII3). A 40S ribosomal gene was used as internal control in all PCR experiments and its specific primers (40S-F, 5′-CCAGGACCCCCAAACTTCTTAG-3′; 40S-R, 5′-GAAAACTGCCACAGCCGTTG-3′) were designed from P. leniusculus Lamda Zap Express library Hpt cDNA (Genbank accession no. CF542417). PCR conditions were as follows: 94°C 2 min, followed by 30 cycles of 94°C 20 s, 58°C 20 s, and 72°C 1 min for the PlDscam gene and 25 cycles for 40S ribosomal gene. The PCR products were analyzed on 1.2% agarose gel stained with ethidium bromide.
Identification of alternatively expressed regions in PlDscam
Due to the large size of full length of PlDscam, it was necessary to use several pairs of primers for identification of the different variable regions. PCR of each region was performed with gene specific primers (Table S1) and thermal cycling was as follows: 94°C 2 min, followed by 30 cycles of 94°C 20 s, 60°C 20 s, and 72°C 1.30 min. The PCR products from four different tissues, such as heart, brain, hemocytes and HPT, were cloned into TOP10 vector and 25 individual clones from each tissue were sequenced.
Immune challenge, sample collection and PlDscam transcription analysis
E. coli and S. aureus were cultured in LB broth at 37°C until OD 600 was ca 0.5. The bacteria were washed three times with 0.85% NaCl by centrifugation at 900 g for 10 min at room temperature. The pellets were resuspended in sterile 0.85% NaCl and adjusted to an approximate concentration of 2×108 CFU/ml. Two groups of three crayfish were injected in the base of the forth walking leg with 100 µl of E. coli and S. aureus, respectively (approximately 2×107 CFU/crayfish). The control group was injected with 100 µl of 0.85% NaCl.
WSSV was purified with a method described by Xie et al.[24]. The purified virus was resuspended in sterile crayfish saline buffer (CFS: 0.2 M NaCl, 5.4 mM KCl, 10 µM CaCl2, and 10 mM MgCl2, 2 mM NaHCO3, pH 6.8) at a concentration of 2×107 copies/ml. One hundred microliter of WSSV (equivalent to 2×106 copies) or CFS (as control group) was injected as previously described. The experimental setup was made in triplicates.
Two groups of three crayfish received injections with 100 µl lipopolysaccharides (LPS: Sigma, from E. coli) or peptidoglycan (PG: Sigma, from S. aureus) in a sterile CFS with a concentration of 0.2 mg/ml. One hundred microliter of CFS was used for the controls.
Hemolymph of crayfish from all experiments was collected at 0, 6, 12 and 24 h post injection and the hemocytes were separately isolated for RNA extraction. The transcript levels of PlDscam were detected by quantitative RT-PCR using the QuantiTect SYBR green PCR kit (QIAGEN). The expression of PlDscam was normalized to the expression of the mRNA encoding the crayfish ribosomal protein gene (R40s) for each sample. The primers used are shown in Table S1. The qPCR reactions contained 5 µl of 1∶10 diluted cDNA template, 1× QuantiTect SYBR Green PCR master mix (QIAGEN) and 5 µM forward and reverse primers in a 25-µl reaction volume. The following amplification profile was used: 95°C for 15 min, followed by 45 cycles of 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s. All qPCR reactions were performed in duplicate. The hemocytes from a least three crayfish were used for each time point.
Transcription analysis of PlDscam isoforms in response to E. coli and S. aureus
The variable region, from the signal peptide to the Ig3 domain region, was amplified from hemocyte cDNA templates from groups of crayfish injected with E. coli or S. aureus, respectively, and the control group at 12 h post injection with F1 and R2 primers (Table S1). The PCR products from each of these groups were subcloned into TOP10 vector and fifty colonies of each group were sampled and subsequently sequenced. Multiple sequence alignment was done by ClustalW and clustering tree of the different isoforms was constructed as described above.
Recombinant protein of the PlDscam isoforms
The ORFs without the signal peptide encoding different PlDscam isoforms were amplified from the original templates N4, E1 and S7 with Dscam-expression-BamHI-Forward (5′-TTTTGGATCCAACCCGACAACCGTGTGGACTTCA-3′) and Dscam-expression-XhoI-Reverse (5′ - TTTCTCGAGCCAGGTAACAGACTTGACGGGGTTG-3′) primers. The resulting insert was cloned into pGEX-4T-1(GE healthcare) at BamHI and XhoI and transformed into BL21 E. coli. Single colonies were grown in LB medium containing 100 µg/ml ampicillin to OD600 = 0.6 and induced with 1 µM IPTG for 5 h at 37°C. The protein was expressed as a fusion product with a glutathione S-transferase part at the N-terminus of rPlDscam. After purifying this GST-fusion protein on a GST-trap FF column (GE healthcare), the presence of the recombinant protein was confirmed by western blot. The protein samples were subjected to 12% SDS-PAGE and then transferred electrophoretically to PDVF membranes. The membrane was blocked by immersion in 10% skimmed milk in TBST for 1 h and washed three times in 1 x TBST (10 mM Tris-HCl, pH 7.5, containing 150 mM NaCl and 0.1% Tween 20). The membrane was then incubated with 1 : 2,000 dilution of a primary antibody for GST (Sigma) in TBST for 1 h. Then, the previous washing procedure was repeated before the membrane was incubated with anti-mouse-IgG peroxidase-linked species-specific whole antibody from sheep (GE Healthcare) at 1 : 3,000 in 1xTBST for 1 h and washed with TBST for 3×10 min. For detection, the ECL Western blotting reagent kit (Amersham Biosciences) was used according to the manufacturer's instructions.
Bacterial binding assay
A bacterial binding assay was modified from the method described by Yu et al.[25] Briefly, E. coli or S. aureus was prepared as previously described. An aliquot 100 µl of the bacteria was immobilized on 96 well plates at approximately 108 CFU/well by incubating for 30 min at room temperature and then shifted to incubation at 4°C overnight. The bacteria were then blocked with 3% (w/v) BSA in TBS buffer at room temperature for 1 hour. To access the binding of the proteins to the bacteria, 20 µg of each PlDscam isoform (with GST fusion tag) or GST diluted in 1% BSA in TBS were added to the wells and incubated at room temperature for 2 h. Following three careful washes with TBS, the bound PlDscam protein was detected with the GST antibody (1∶2,000) followed by rabbit antimouse HRP-conjugated secondary antibody (1∶3000). After adding 100 µl tetramethylbenzidine or TMB substrate (Sigma) for 20 min and 100 µl of stop solution (0.5 M sulfuric acid), the absorbance of the resulting color was measured at 450 nm. All binding assays were performed in triplicates and experiment was repeated three times.
Bacteria clearance assay
E. coli and S. aureus were washed six times in 0.9% NaCl at 1,200× g for 10 min and then incubated with 10 µg recombinant proteins of each PlDscam isoforms for 1 h at 4°C, followed by washing six times with 0.9% NaCl. One hundred microliter of E. coli or S. aureus at concentrations of 6×108 and 3×109 CFU/ml, respectively, in CFS were injected into crayfish. The bacteria count was carried out in hemolymph collected 40 min and 3 h after the bacterial injection. The homonym was serial diluted serially and was then dotted onto LB agar (10 µl for each dot) and then incubated at 37°C overnight followed by counting the bacterial colony forming units (CFU).
Phagocytosis assay
Both, heat killed E. coli and S. aureus were conjugated with fluoresce in isothiocyanate (FITC) using a method previously described by Hed [26]. Briefly, heat-killed E. coli and S. aureus were washed six times in 0.9% NaCl at 1,200× g for 10 min and then incubated at concentration of 109 particles/ml in 0.1 M Na2CO3 containing 0.1 mg/ml FITC (Sigma), pH 9.5 for 30 min at 37°C, followed by washing six times with 0.9% NaCl as above. The FITC-conjugated heat-killed bacteria were incubated with 10 µg recombinant proteins of each PlDscam isoforms for 1 h at 4°C. The bacteria were washed five times as described above and resuspended at a concentration of 108 particles/ml in CFS. Two hundred microliter of FITC-conjugated heat-killed bacteria coated with recombinant PlDscam isoform (4×106 particles/ml in CFS) was injected into the animals via the base of the fourth walking leg. Hemocytes were bled for phagocytosis detection at 1.5 h post FITC-conjugated heat-killed bacteria injection. The fluorescence of FITC-conjugated heat-killed bacteria particles was quenched by adding 20 µl of 0.04% trypan blue (Pfaltz & Bauer, Waterbury, CT). The ingested bacteria were easily detected under the UV light microscope due to their fluorescence. The percentage of phagocytosing cells was determined by counting 10 individual fields and dividing the number of cells with ingested fluorescent bacteria with the total number of counted cells. Each experiment was performed in triplicates.
Crayfish Hpt cell culture and maintenance
The hematopoietic tissue was dissected according to Söderhäll et al.[27]. The Hpt was washed with CPBS (crayfish phosphate buffered saline: 10 mM Na2HPO4, 10 mM KH2PO4, 150 mM NaCl, 10 µM CaCl2, and 10 µM MnCl2, pH 6.8) and incubated in 600 µl of 0.1% collagenase (type I and IV) (Sigma) in CPBS at room temperature for 45 min to separate the Hpt cells. The separated cells were washed twice with CPBS by spinning down at 800× g for 5 min at room temperature. The cell pellet was re-suspended in modified L-15 medium and subsequently cells were seeded at a density of 2.5×106 cells/150 µl in 96-well plates. The Hpt cells were supplemented with partially purified plasma as a source of astakine [28] after 1 h of attachment at room temperature and the culture plates were incubated at 16°C, and 1/3 of the medium was changed at 48 h intervals.
Generation of dsRNA
dsRNA of PlDscam was designed from the conserved region during Ig8-FNIII3. Gene specific primers for PlDscam and GFP was incorporated with a T7 promoter sequence (italic letters) at 5′ ends (DscamRNAi-F, 5′ - TAATACGACTCACTATAGGGGCATCAAGCTGAGTGGACAA-3′; DscamRNAi-R, 5′ - TAATACGACTCACTATAGGG GAAGCCAGGTAGGGGAAATC -3′ and GFP 63+, TAATACGACTCACTATAGGGCGACGTAAACGGCCACAAGT; GFP 719-, TAATACGACTCACTATAGGGTTCTTGTACAGCTCGTCCATG) and used to amplify PCR products as template for dsRNA synthesis. A GFP transcript was amplified with the pd2EGFP-1 vector (Clontech) as template and used as control. The amplified products were then purified using GenElute Gel extraction kit (Sigma) followed by in vitro transcription using the MegaScript kit (Ambion). The dsRNA was purified with the Trizol LS reagent (Invitrogen).
RNAi in vitro study
The Hpt cells were divided into three groups with four replicates in each group. The Hpt cells received different treatments as follows: group 1: GFP dsRNA plus UV-killed WSSV, group 2: GFP dsRNA plus WSSV and group 3: PlDscam dsRNA plus WSSV. The dsRNA transfection and WSSV infection into Hpt cell cultures were performed as described by Liu et al.[29]. Briefly, 4 µl of dsRNA (250 ng/µl) was mixed with 3 µl of histone H2A (1 mg/ml) and with 20 µl of modified L15 and added to one well of 1-day-old Hpt cell cultures. The cells were then incubated at 16°C. At day 3, one replicate of group 2 and 3 were subjected to RNA extraction to determine RNAi efficiencies. For remaining replicates, the medium was replaced with 150 µl of L15 medium together with 5 µl of WSSV stock suspension and 5 µl crude astakine preparation and were further incubated for 36 h at 20°C followed by isolation of RNA. Total HPT RNA was extracted to determine PlDscam and WSSV VP28 transcripts by semi-quantitative RT-PCR. PCR was performed with three oligonucleotide primers (WSSV VP28 (Genbank accession no. AF502435):-F: 5′-TCACTCTTTCGGTCGTGTCG-3′ and -R: 5′ - CCACACACAAAGGTGCCAAC-3′; previous primer for 40s ribosomal and PlDscam gene). The PCR conditions were as follows: 94°C 2 min, followed by 30 cycles of 94°C 20 s, 60°C 20 s, and 72°C 30 s for PlDscam and VP28, while 25 cycles for 40S ribosomal gene.
Statistic analysis
The relative expression levels of different time groups were examined by One-way ANOVA followed by Duncan's new multiple range test and Tukey test. Differences were considered statistically significant at P<0.05. Results are expressed as the mean ± SE.
Supporting Information
Zdroje
1. GarverLSXiZDimopoulosG 2008 Immunoglobulin superfamily members play an important role in the mosquito immune system. Dev Comp Immunol 32 519 531
2. DermodyTSKirchnerEGuglielmiKMStehleT 2009 Immunoglobulin superfamily virus receptors and the evolution of adaptive immunity. PLoS Pathog 5 e1000481
3. HutterHVogelBEPlenefischJDNorrisCRProencaRB 2000 Conservation and novelty in the evolution of cell adhesion and extracellular matrix genes. Science 287 989 994
4. WatsonFLPuttmann-HolgadoRThomasFLamarDLHughesM 2005 Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 309 1874 1878
5. ZhangSMAdemaCMKeplerTBLokerES 2004 Diversification of Ig superfamily genes in an invertebrate. Science 305 251 254
6. DongYTaylorHEDimopoulosG 2006 AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol 4 e229
7. YamakawaKHuotYKHaendeltMAHubertRChenXN 1998 DSCAM: a novel member of the immunoglobulin superfamily maps in a Down syndrome region and is involved in the development of the nervous system. Hum Mol Genet 7 227 237
8. AgarwalaKLGaneshSTsutsumiYSuzukiTAmanoK 2001 Cloning and functional characterization of DSCAML1, a novel DSCAM-like cell adhesion molecule that mediates homophilic intercellular adhesion. Biochem Biophys Res Commun 285 760 772
9. HattoriDMillardSSWojtowiczWMZipurskySL 2008 Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol 24 597 620
10. BoehmT 2007 Two in one: dual function of an invertebrate antigen receptor. Nat Immunol 8 1031 1033
11. SchmuckerDChenB 2009 Dscam and DSCAM: complex genes in simple animals, complex animals yet simple genes. Genes Dev 23 147 156
12. WojtowiczWMFlanaganJJMillardSSZipurskySLClemensJC 2004 Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell 118 619 633
13. SchmuckerDClemensJCShuHWorbyCAXiaoJ 2000 Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell 101 671 684
14. StuartLMEzekowitzRA 2008 Phagocytosis and comparative innate immunity: learning on the fly. Nat Rev Immunol 8 131 141
15. WilliamsMJ 2007 Drosophila hemopoiesis and cellular immunity. J Immunol 178 4711 4716
16. BeveridgeTJ 1981 Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol 72 229 317
17. TakeuchiOHoshinoKKawaiTSanjoHTakadaH 1999 Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11 443 451
18. ChouPHChangHSChenITLinHYChenYM 2009 The putative invertebrate adaptive immune protein Litopenaeus vannamei Dscam (LvDscam) is the first reported Dscam to lack a transmembrane domain and cytoplasmic tail. Dev Comp Immunol 33 1258 1267
19. BartonESForrestJCConnollyJLChappellJDLiuY 2001 Junction adhesion molecule is a receptor for reovirus. Cell 104 441 451
20. ProtaAECampbellJASchellingPForrestJCWatsonMJ 2003 Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc Natl Acad Sci U S A 100 5366 5371
21. WatthanasurorotAJiravanichpaisalPSöderhällISöderhällK 2010 A gC1qR prevents white spot syndrome virus replication in the freshwater crayfish Pacifastacus leniusculus. J Virol 84 10844 10851
22. GuindonSGascuelO 2003 A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52 696 704
23. HuelsenbeckJPRonquistF 2001 MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17 754 755
24. XieXLiHXuLYangF 2005 A simple and efficient method for purification of intact white spot syndrome virus (WSSV) viral particles. Virus Res 108 63 67
25. YuSLoweAW 2009 The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli Type 1 fimbriae. BMC Gastroenterol 9 58
26. HedJ 1986 Methods for distinguishing ingested from adhering particles. Methods Enzymol 132 198 204
27. SöderhällIBangyeekhunEMayoSSöderhällK 2003 Hemocyte production and maturation in an invertebrate animal; proliferation and gene expression in hematopoietic stem cells of Pacifastacus leniusculus. Dev Comp Immunol 27 661 672
28. SöderhällIKimYAJiravanichpaisalPLeeSYSöderhällK 2005 An ancient role for a prokineticin domain in invertebrate hematopoiesis. J Immunol 174 6153 6160
29. LiuHJiravanichpaisalPSöderhällICereniusLSöderhällK 2006 Antilipopolysaccharide factor interferes with white spot syndrome virus replication in vitro and in vivo in the crayfish Pacifastacus leniusculus. J Virol 80 10365 10371
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous SystemsČlánek The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral ReleaseČlánek Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1Článek High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The N-Terminus of the RNA Polymerase from Infectious Pancreatic Necrosis Virus Is the Determinant of Genome Attachment
- Evolutionary Analysis of Inter-Farm Transmission Dynamics in a Highly Pathogenic Avian Influenza Epidemic
- Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous Systems
- Endemic Dengue Associated with the Co-Circulation of Multiple Viral Lineages and Localized Density-Dependent Transmission
- The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral Release
- The -glycan Glycoprotein Deglycosylation Complex (Gpd) from Deglycosylates Human IgG
- The Lipid Transfer Protein CERT Interacts with the Inclusion Protein IncD and Participates to ER- Inclusion Membrane Contact Sites
- Induction of Noxa-Mediated Apoptosis by Modified Vaccinia Virus Ankara Depends on Viral Recognition by Cytosolic Helicases, Leading to IRF-3/IFN-β-Dependent Induction of Pro-Apoptotic Noxa
- Environmental Constraints Guide Migration of Malaria Parasites during Transmission
- HIV-1 Efficient Entry in Inner Foreskin Is Mediated by Elevated CCL5/RANTES that Recruits T Cells and Fuels Conjugate Formation with Langerhans Cells
- Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis
- Highly Pathogenic Avian Influenza Virus H5N1 Infects Alveolar Macrophages without Virus Production or Excessive TNF-Alpha Induction
- Uses Host Triacylglycerol to Accumulate Lipid Droplets and Acquires a Dormancy-Like Phenotype in Lipid-Loaded Macrophages
- Compensatory T Cell Responses in IRG-Deficient Mice Prevent Sustained Infections
- Detection of Inferred CCR5- and CXCR4-Using HIV-1 Variants and Evolutionary Intermediates Using Ultra-Deep Pyrosequencing
- A Dynamic Landscape for Antibody Binding Modulates Antibody-Mediated Neutralization of West Nile Virus
- HSV-2 Infection of Dendritic Cells Amplifies a Highly Susceptible HIV-1 Cell Target
- “Rational Vaccine Design” for HIV Should Take into Account the Adaptive Potential of Polyreactive Antibodies
- Impact of Endofungal Bacteria on Infection Biology, Food Safety, and Drug Development
- Infection Reduces B Lymphopoiesis in Bone Marrow and Truncates Compensatory Splenic Lymphopoiesis through Transitional B-Cell Apoptosis
- The Intrinsic Antiviral Defense to Incoming HSV-1 Genomes Includes Specific DNA Repair Proteins and Is Counteracted by the Viral Protein ICP0
- Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph
- Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1
- A Freeze Frame View of Vesicular Stomatitis Virus Transcription Defines a Minimal Length of RNA for 5′ Processing
- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Tipping the Balance: Secreted Oxalic Acid Suppresses Host Defenses by Manipulating the Host Redox Environment
- Bacteria-Induced Dscam Isoforms of the Crustacean,
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Insertion of an Esterase Gene into a Specific Locust Pathogen () Enables It to Infect Caterpillars
- A Role for TLR4 in Infection and the Recognition of Surface Layer Proteins
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
- Low CCR7-Mediated Migration of Human Monocyte Derived Dendritic Cells in Response to Human Respiratory Syncytial Virus and Human Metapneumovirus
- HIV/SIV Infection Primes Monocytes and Dendritic Cells for Apoptosis
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- Productive Parvovirus B19 Infection of Primary Human Erythroid Progenitor Cells at Hypoxia Is Regulated by STAT5A and MEK Signaling but not HIFα
- Identification of DNA-Damage DNA-Binding Protein 1 as a Conditional Essential Factor for Cytomegalovirus Replication in Interferon-γ-Stimulated Cells
- The Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon at the Level of the MAVS Adaptor Protein
- Passively Administered Pooled Human Immunoglobulins Exert IL-10 Dependent Anti-Inflammatory Effects that Protect against Fatal HSV Encephalitis
- Infection of Induces Antifungal Immune Defenses
- Merozoite Invasion Is Inhibited by Antibodies that Target the PfRh2a and b Binding Domains
- Cyclic di-GMP is Essential for the Survival of the Lyme Disease Spirochete in Ticks
- Cross-Neutralizing Antibodies to Pandemic 2009 H1N1 and Recent Seasonal H1N1 Influenza A Strains Influenced by a Mutation in Hemagglutinin Subunit 2
- Spatial Dynamics of Human-Origin H1 Influenza A Virus in North American Swine
- Coronavirus Gene 7 Counteracts Host Defenses and Modulates Virus Virulence
- Clathrin Facilitates the Morphogenesis of Retrovirus Particles
- Contribution of Intrinsic Reactivity of the HIV-1 Envelope Glycoproteins to CD4-Independent Infection and Global Inhibitor Sensitivity
- Functional Analysis of Host Factors that Mediate the Intracellular Lifestyle of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



