-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFunctional Analysis of Host Factors that Mediate the Intracellular Lifestyle of
Cryptococcus neoformans (Cn), the major causative agent of human fungal meningoencephalitis, replicates within phagolysosomes of infected host cells. Despite more than a half-century of investigation into host-Cn interactions, host factors that mediate infection by this fungal pathogen remain obscure. Here, we describe the development of a system that employs Drosophila S2 cells and RNA interference (RNAi) to define and characterize Cn host factors. The system recapitulated salient aspects of fungal interactions with mammalian cells, including phagocytosis, intracellular trafficking, replication, cell-to-cell spread and escape of the pathogen from host cells. Fifty-seven evolutionarily conserved host factors were identified using this system, including 29 factors that had not been previously implicated in mediating fungal pathogenesis. Subsequent analysis indicated that Cn exploits host actin cytoskeletal elements, cell surface signaling molecules, and vesicle-mediated transport proteins to establish a replicative niche. Several host molecules known to be associated with autophagy (Atg), including Atg2, Atg5, Atg9 and Pi3K59F (a class III PI3-kinase) were also uncovered in our screen. Small interfering RNA (siRNA) mediated depletion of these autophagy proteins in murine RAW264.7 macrophages demonstrated their requirement during Cn infection, thereby validating findings obtained using the Drosophila S2 cell system. Immunofluorescence confocal microscopy analyses demonstrated that Atg5, LC3, Atg9a were recruited to the vicinity of Cn containing vacuoles (CnCvs) in the early stages of Cn infection. Pharmacological inhibition of autophagy and/or PI3-kinase activity further demonstrated a requirement for autophagy associated host proteins in supporting infection of mammalian cells by Cn. Finally, systematic trafficking studies indicated that CnCVs associated with Atg proteins, including Atg5, Atg9a and LC3, during trafficking to a terminal intracellular compartment that was decorated with the lysosomal markers LAMP-1 and cathepsin D. Our findings validate the utility of the Drosophila S2 cell system as a functional genomic platform for identifying and characterizing host factors that mediate fungal intracellular replication. Our results also support a model in which host Atg proteins mediate Cn intracellular trafficking and replication.
Published in the journal: . PLoS Pathog 7(6): e32767. doi:10.1371/journal.ppat.1002078
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002078Summary
Cryptococcus neoformans (Cn), the major causative agent of human fungal meningoencephalitis, replicates within phagolysosomes of infected host cells. Despite more than a half-century of investigation into host-Cn interactions, host factors that mediate infection by this fungal pathogen remain obscure. Here, we describe the development of a system that employs Drosophila S2 cells and RNA interference (RNAi) to define and characterize Cn host factors. The system recapitulated salient aspects of fungal interactions with mammalian cells, including phagocytosis, intracellular trafficking, replication, cell-to-cell spread and escape of the pathogen from host cells. Fifty-seven evolutionarily conserved host factors were identified using this system, including 29 factors that had not been previously implicated in mediating fungal pathogenesis. Subsequent analysis indicated that Cn exploits host actin cytoskeletal elements, cell surface signaling molecules, and vesicle-mediated transport proteins to establish a replicative niche. Several host molecules known to be associated with autophagy (Atg), including Atg2, Atg5, Atg9 and Pi3K59F (a class III PI3-kinase) were also uncovered in our screen. Small interfering RNA (siRNA) mediated depletion of these autophagy proteins in murine RAW264.7 macrophages demonstrated their requirement during Cn infection, thereby validating findings obtained using the Drosophila S2 cell system. Immunofluorescence confocal microscopy analyses demonstrated that Atg5, LC3, Atg9a were recruited to the vicinity of Cn containing vacuoles (CnCvs) in the early stages of Cn infection. Pharmacological inhibition of autophagy and/or PI3-kinase activity further demonstrated a requirement for autophagy associated host proteins in supporting infection of mammalian cells by Cn. Finally, systematic trafficking studies indicated that CnCVs associated with Atg proteins, including Atg5, Atg9a and LC3, during trafficking to a terminal intracellular compartment that was decorated with the lysosomal markers LAMP-1 and cathepsin D. Our findings validate the utility of the Drosophila S2 cell system as a functional genomic platform for identifying and characterizing host factors that mediate fungal intracellular replication. Our results also support a model in which host Atg proteins mediate Cn intracellular trafficking and replication.
Introduction
Over the past half-century, Cryptococcus neoformans (Cn), an opportunistic encapsulated yeast, has emerged as the major causative agent of fungal meningoencephalitis in humans and animals worldwide [1], [2]. The pathogen is found in a variety of environmental niches, including soil, vegetation, and avian excreta. Initial infection in the lungs results from the inhalation of fungal cells from the environment [1], [2]. The most common clinical form of systemic Cryptococcus infection in humans is meningoencephalitis.
An elucidation of the molecular mechanisms mediating the interaction of Cn with host cells will further the development of measures to control cryptococcosis [3]. Several Cn features, such as polysaccharide capsule synthesis [4], [5], melanin production [6], [7], and growth at host physiological temperatures [7] are associated with virulence. In addition, several signaling pathways, including the cAMP-PKA pathway, three MAP kinase pathways (involving Cpk1, Hog1 and Mpk1), the Ras specific pathway, and the Ca2+-calcineurin pathway modulate Cn morphological differentiation, virulence, and stress responses [8]. Numerous Cn virulence factors have also been characterized [9].
Insights into host cell functions that mediate infection have also been garnered. For example, host toll-like receptors (TLRs) help recognize fungal pathogens such as Candida albicans, Aspergillus fumigatus and Cn [10]. Recognition of pathogen-associated molecular patterns (PAMPs) by TLRs, either alone or with other TLR or non-TLR receptors, induces signals responsible for activation of the host innate immune response [10]. Recent studies have demonstrated that MyD88, but neither TLR2 nor TLR4, plays a central role in host defense against Cn [11], [12]. A variety of host cells, such as T helper type 1 (Th1) [13], B cells [14], [15], gamma-delta antigen receptor-bearing T (γ δ T) cells [15], dendritic cells [16], alveolar macrophages [17] and bronchial epithelial cells [18] are known to be involved in coordinating the host immune response to Cn infection. Cell-mediated host immunity to infection is characterized by immune cell activation and secretion of cytokines, including gamma interferon (IFN-γ), IL-12, IL-18, IL-23 and tumor necrosis factor alpha (TNF-α) [1]. Despite these insights, the molecular mechanisms mediating host cell invasion, intracellular replication and escape of Cn from host cells remain obscure.
The fate of internalized Cn cells is cell type-dependent. However, common patterns of intracellular trafficking are observed. After entry into host cells, Cn resides in early endosomal antigen 1 (EEA1)-positive compartments [16], and also interacts with membranes containing the endosome/lysosome markers CD63 [19] and lysosomal-associated membrane protein 1 (LAMP-1) [16], [20], [21]. The pathogen is killed in the lysosomes of dendritic cells [16]. However, in macrophages, Cn cells can survive, replicate, and extrude from phagosome/phagolysosome compartments without inducing host cell death [21], [22]. This extrusion of replicative Cn cells may constitute a mechanism for disseminating Cn among host cells or tissues while limiting detection by host surveillance systems [21], [23]. Despite these observations, the subcellular pathways that Cn uses to enter, replicate and escape from host cells remain obscure.
Recent studies have indicated that activation of the host cell autophagy pathway can control the replication of intracellular pathogens [24], [25], [26]. Autophagy is a highly conserved process in which cellular materials, including proteins and organelles, are engulfed into an autophagosome, a specialized double membrane bounded compartment that delivers material to lysosomes for degradation [27]. Nutrient starvation, endoplasmic reticulum stress, and pathogen attack and/or subversion can activate the autophagy machinery. Autophagosome biogenesis requires the activity of autophagy-specific proteins, including Atg5, an ubiquitin-like protein. Atg5 regulates the conversion of microtubule-associated protein 1 light chain 3 (LC3-I) to a lipidated form LC3-II, which localizes to the autophagic membrane [28], [29]. Membrane-bound LC3-II interacts with the adaptor molecules p62 and NBR1, which capture cytoplasmic cargo earmarked for autophagic degradation [26]. Therefore, the amount of LC3-II within a cell reflects the amount of newly formed autophagic membrane and/or the number of autophagosomes.
Previous studies have demonstrated that host autophagy may be involved in inhibiting the growth of intracellular pathogens, including intracellular bacteria [30], [31], [32] and parasites [32], [33]. However, some microorganisms, including bacteria such as Porphyromonas gingivalis, Brucella abortus, and Coxiella burnetii [34], and viruses [35], [36], [37] may subvert host autophagic processes to promote their intracellular survival and replication.
We exploited Drosophila melanogaster S2 cells and RNAi technology to identify host factors that mediate the phagocytosis, intracellular replication and escape of Cn from host cells. Several features of the Drosophila cell system made it an attractive choice for these studies. First, the macrophage-like Drosophila S2 cell system has proven useful for elucidating evolutionarily conserved components of innate immunity [38], [39]. Second, RNAi strategies can be used to efficiently deplete proteins in these cells. Third, S2 cells have been successfully employed to identify host factors for several microbial pathogens, including Listeria monocytogenes [38], [40], Brucella melitensis [41] and the fungal pathogen C. albicans [39]. Importantly, findings in insect cell models have been validated in their mammalian cell counterparts [41], [42], [43], [44]. Thus, the insect cell system has proven useful for resolving physiologically relevant host factors in evolutionarily divergent organisms. Finally, some Cryptococcus species have been shown to reside in a variety of environmental niches, including in association with insects [45], [46]. Moreover, both the caterpillar Galleria mellonella [47] and D. melanogaster [48] have been previously used as model hosts to study fungal virulence and host defense. Therefore, Drosophila cells constitute a host system from which biologically relevant information can be extracted.
Here, we demonstrate that Drosophila S2 cells share striking similarities with their mammalian host cell counterparts in supporting the uptake, intracellular replication, cell-to-cell dissemination and escape of Cn cells. We also demonstrate that Cn interacts with host cell membranes containing the autophagosome marker LC3, the endosome/lysosome marker LAMP-1, and the lysosome marker cathepsin D in Drosophila S2 cells, murine J774.A1 and RAW264.7 macrophages and mouse embryonic fibroblasts (MEFs). Finally, we demonstrate that several autophagy-associated proteins mediate Cn infection in these systems, thereby implicating the autophagy pathway in supporting the intracellular lifestyle of the pathogen. These data provide the first functional genomic analysis of Cn host factors and provide new insights into mechanisms mediating this host-pathogen interaction.
Results
Drosophila S2 cells support Cn infection
Time-lapse microscopy of live cells provides a compelling approach for visualizing dynamic interactions between host and pathogen cells. Moreover, this approach has proven useful for demonstrating extrusion of Cn from host cells [21], [22]. We employed time-lapse microscopy to determine whether Drosophila S2 cells can support infection by Cn. An analysis of time-lapse images revealed several important aspects of this interaction. First, S2 cells efficiently internalized Cn from the surrounding medium (Figure 1A, yellow arrows; Supplemental Videos 1 and 2 at: http://www.youtube.com/user/deFigueiredoLab). Second, internalized Cn cells were observed to replicate within S2 cells (Figure 1A, wide light-green arrows; Figure 1B, yellow arrows; Supplemental Video 1) with a doubling time of ∼2 to 3 hrs. Third, Cn cells were observed to extrude from host cells, either in groups or individually (Figure 1A, red arrows and Supplemental Video 1 and 2). Cn extrusion across the plasma membrane was rapid (<2 min) and was not accompanied by host cell lysis (Figure 1A, Supplemental Videos 1 and 2). Finally, Cn cell-to-cell dissemination by means of re-uptake of escaped Cn cells into new host cells (Figure 1A, yellow arrows and Supplemental Videos 1 and 2), direct cell-to-cell movement (Figure 1B, red arrows) and division of infected host cells (Figure 1A, white arrows and Supplemental Video 2 in yellow frame) was also observed. Although the viability of host cells was not compromised following extrusion, after long-term infection in cell culture (>24 hrs), host cells lysed and intracellular Cn were released (Figure 1A, t = 27∶36∶03). Taken together, our time-lapse microscopy analyses indicated that key aspects of host-Cn cell interactions (i.e., pathogen uptake, replication, cell-to-cell dissemination, extrusion, and host cell lysis) were shared by the Drosophila S2 host cell model and mammalian macrophages.
Fig. 1. Live cell images indicate that Drosophila S2 cells support the phagocytosis, intracellular replication, cell-to-cell dissemination and escape of Cryptococcus neoformans (Cn). 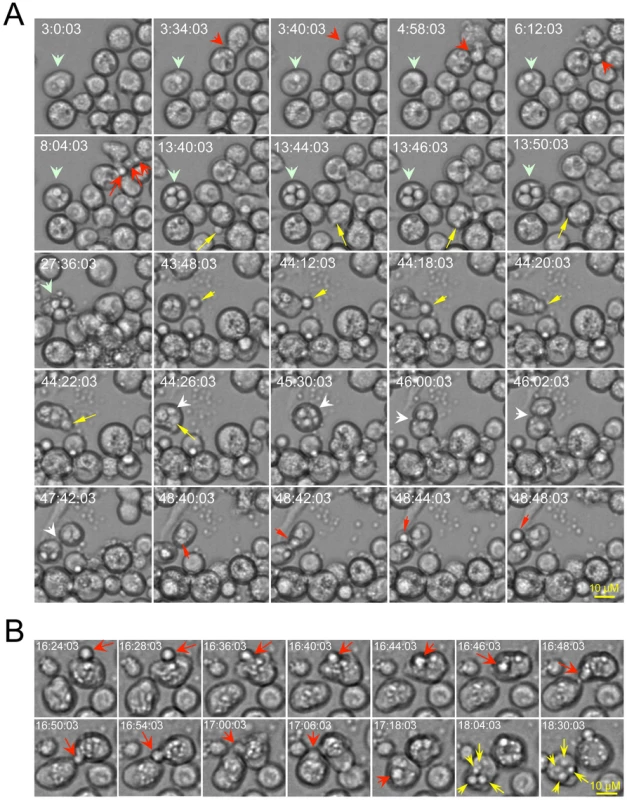
Live cell images were selected from a representative time-lapse movie (n = 3). Time-lapse images were taken every 2 min. Frames are labeled according to the start of the imaging process, which was initiated approximately 3.5 hrs post-infection. Before the imaging was initiated, the infected cells were washed three times with 1×PBS. Fresh serum-free medium supplemented with 20 µg/ml fluconazole was then added to the cells to inhibit the replication of the extracellular (but not intracellular) population of Cn cells. A. Drosophila S2 cells support Cn infection. Yellow arrows: Phagocytosis of Cn cells by S2 cells. Light green arrows: Cn replication precedes host cell lysis. Red arrows: Examples of Cn extrusion from host cells. White arrow: Cn cell-to-cell dissemination by host cell division. B. Cn direct cell-to-cell spread (red arrows) and intracellular replication (yellow arrows) in S2 cells. To determine whether S2 cells constitute an evolutionarily conserved system in which to interrogate Cn interactions with host cells, we employed fluorescence microscopy to visualize eGFP or dsRed-expressing Cn cells (AI132-GFP and AI100-dsRed, respectively) during a time course of infection of S2 or J774.A1 cells. First, we demonstrated that GFP or dsRed expression in Cn cells did not affect pathogen interactions with host cells (Figure S1 A). Next, we employed fluorescence microscopy to analyze the replication of these Cn cells within host cells. For these experiments, we used Alexa 488-conjugated phalloidin or rhodamine-phalloidin to resolve the host cell actin cytoskeleton and thereby demarcate the region occupied by host cells. We then quantified the number of intracellular Cn cells (Figure S1B, upper panel), and found that their number increased over a time course of infection in both S2 and J774.A1 cells (Figure 2, A1 and B1). Interestingly, we also observed Cn cells that appeared to be escaping from S2 cells (Figure S1B, lower panel). These data supported results obtained from our live cell time-lapse microscopy studies (Figure 1 and Supplemental Videos 1 and 2) and encouraged us to develop a system that can differentiate between Cn uptake, replication, and escape in the context of a functional genomic host factor screening experiment.
Fig. 2. Drosophila S2 cells and murine J774.A1 macrophages support similar patterns of Cn phagocytosis and intracellular replication. 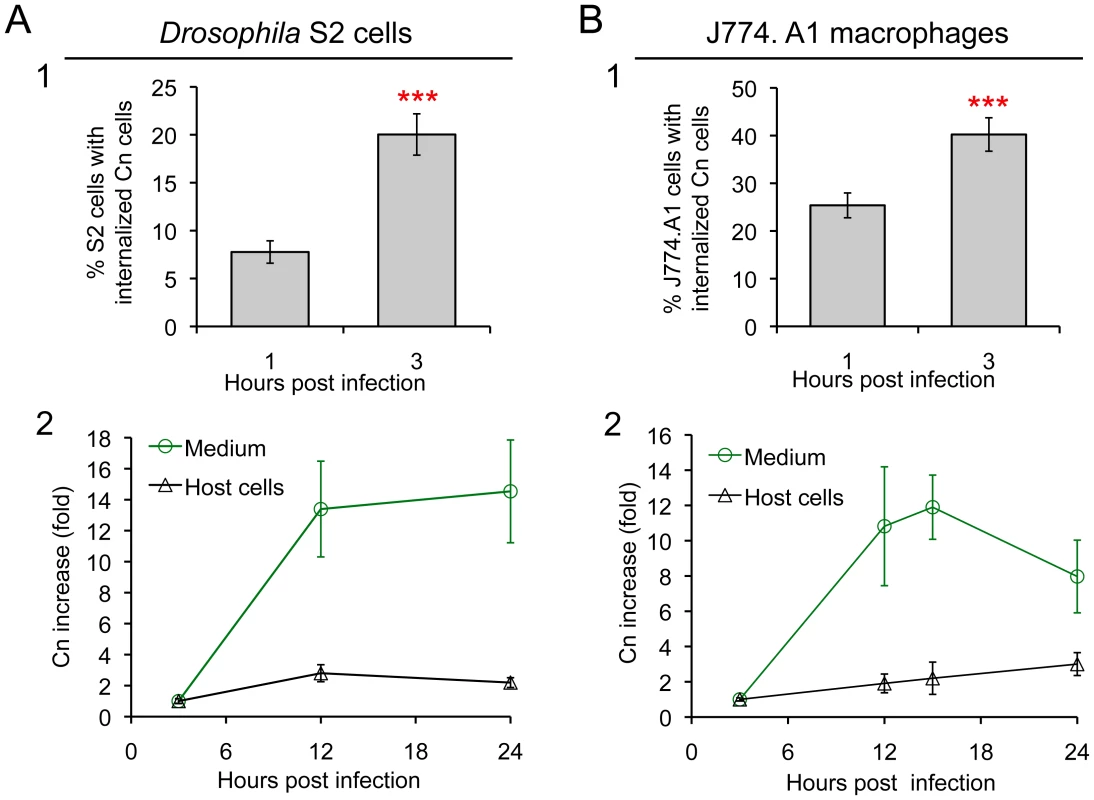
A1 and B1. Host cell phagocytosis of Cn cells. Infected S2 and J774.A1 cells (on coverslips in 24-well plates) were extensively washed after coincubation with Cn (AI100-dsRed) cells for 1 or 3 hrs. The infected cells were fixed, stained with rhodamine-phalloidin and then analyzed by fluorescence microscopy. The numbers of Cn within host cells were quantified, and the percentages of host cells that had been phagocytosed at the indicated time points post-infection were determined. For each well in each experiment, at least 500 cells were analyzed. A2 and B2. Host cells support Cn intracellular replication and escape from infected cells. At 3 h.p.i., Cn (AI100-dsRed) infected host cells were extensively washed. Fresh medium supplemented with 20 µg/ml fluconazole was added to each well containing infected host cells. The infected cells were then continuously incubated at 28°C (S2 cells) or 37°C, 5% CO2 (J774.A1 macrophages). At the indicated time points, media were collected from each well to determine the number of Cn cells that had escaped. The number of CFUs recovered from each sample was then quantified, and the fold-increase of Cn cells (compared to the initial amount recovered at 3 h.p.i.) was calculated and plotted as a function of time. In parallel, at various times post-infection, the corresponding infected S2 and J774.A1 cells were lysed and the number of CFUs contained therein determined after plating and growth on solid medium. The fold-increase of Cn cells was then calculated and plotted (black line) as above. Data represent means ± standard deviation (SD) from at least three independent experiments in which triplicate wells were analyzed. Fluconazole protection assay differentiates Cn escape and intracellular replication
Antibiotic protection assays have proven useful for identifying and characterizing interactions between intracellular bacterial pathogens and host cells. Despite the power of these assays, similar systems have not been developed for studying interactions between intracellular fungal pathogens and host cells. We therefore developed such an assay to monitor the intracellular replication and escape of Cn from host cells. Fluconazole is a widely used fungistatic agent that inhibits the growth and proliferation of Cn [49]. To test whether fluconazole differentially affects the intracellular or extracellular populations of Cn cells, we infected Drosophila S2 and assorted mammalian cell lines with Cn and then treated the infected cells with various concentrations of fluconazole. We then monitored the growth of the intracellular population by analyzing the number of colony forming units (CFUs) within host cells after lysis and plating onto solid medium. The extracellular populations were also measured by plating media collected from the culture. Finally, the viability of the host cells was assessed in trypan blue exclusion experiments [41]. Fluconazole (0 to 40 µg/ml) had no effect on the proliferation/viability of host cells in culture as demonstrated previously [50] and shown in Figure S2A. However, the replication of Cn cells in fluconazole-treated (20 µg/ml) fresh or conditioned medium (CM) was inhibited (Figure S2B). After 15 hrs, the number of Cn cells decreased in fresh or conditioned mammalian tissue culture medium (Dulbecco's Modified Eagle's Medium: DMEM) at 37°C under 5% CO2 (Figure S2B, red line), indicating that prolonged treatment of Cn with fluconazole in DMEM was toxic to Cn cells. However, the number of Cn cells in Drosophila fresh or conditioned medium remained at a similar level during 24 hrs of incubation at 28°C in ambient air (Figure S2B, green line). When fluconazole was added to Cn infected S2 or J774.A1 cells, levels of intracellular replication were similar during the first 15 hrs of infection (Figure 2, A2 and B2, black lines).
We were encouraged by these findings and exploited the fluconazole protection approach to evaluate the escape of Cn cells from infected host cells (Figure S2C). When culture media was collected over a 15 hr time period from Cn-infected S2 or J774.A1 cells that had been treated with fluconazole, the number of Cn cells in the media increased dramatically (Figure 2, A2 and B2, green line). The fluconazole containing culture medium did not support the replication of Cn (Figure S2B), which was consistent with the fungistatic nature of this compound. Moreover, fungal cells retained viability in this medium [49]. The dramatic increase of Cn cells in fluconazole-containing culture media could therefore be regarded as derived from a population of Cn cells that had replicated in and escaped from host cells. Therefore, our CFU analysis provided a means of quantifying the number of Cn cells that escaped from host cells over time. The number of Cn cells recovered from the extracellular media of infected J774.A1 cell cultures decreased after 15 hrs (Figure 2, B2, green line), consistent with our observation that prolonged incubation of Cn in fluconazole-containing DMEM impaired Cn viability (Figure S2B, red line). Hence, experiments that exploited fluconazole to measure the amount Cn escape from mammalian cells were not conducted for more than 15 hrs. In contrast, a loss of Cn viability was not observed in the population of escaped cells that were recovered from the supernatants of S2 cell cultures (Figure 2, A2, green line), again consistent with our observation that Cn viability remained at a similar level during 24 hrs of incubation in S2 cell culture supernatants that contained fluconazole (Figure S2B, green line). Taken together, our data indicated that S2 and J774.A1 cells displayed striking similarities in supporting infection by Cn (Figure 1 and Figure 2) and that fluconazole protection assays could be used to assess the intracellular replication and escape of Cn from both host cell types.
Cn traffics similarly in Drosophila S2 and murine J774.A1 cells
We hypothesized that the intracellular trafficking of Cn in S2 and J774.A1 cells share similarities. To test this hypothesis, we used immunofluorescence microscopy assays to compare the intracellular trafficking patterns of AI100-dsRed in these cell types. Antibodies that recognize evolutionarily conserved Drosophila and murine proteins that reside in assorted subcellular compartments were used for these experiments (Figure S3A, and [51]). These antibodies were shown to display minimal cross-reactivity with the pathogen in immunofluorescence microscopy experiments (Figure S3B).
The movement of Cn from the host cell plasma membrane to its replicative niche involves several sequential steps [16], [19], [20], [21]. Upon entry, Cn cells were localized within a vacuole that contained the early endosome marker EEA1 (Figure 3, A3 and B3). The maturing Cn-containing vacuole (CnCV) could also be sequentially stained with antibodies directed against the late endosome marker mannose-6-phosphate receptor (M6PR), the late endosome/lysosome marker LAMP-1, and the lysosome marker cathepsin D (Figure 3 and data not shown). The pathogen was also observed to be tightly associated with membranes that contained the ER marker calreticulin (Figure 3, A12 and B12). However, Cn interactions with the Golgi marker Grasp65 were not observed at the indicated time point (Figure 3, A15 and B15). Similar findings were obtained in both S2 and mammalian cell lines (Figure 3). Quantitative analysis of these marker protein-positive CnCVs indicated that the host endocytic pathway was involved in the phagocytosis and intracellular trafficking of the pathogen (Figure S4). Therefore, these data indicated the route of intracellular trafficking of Cn in S2 cells, and also demonstrated that Cn intracellular trafficking and replication in S2 and mammalian cells share strikingly similarities.
Fig. 3. Cryptococcus neoformans intracellular trafficking in Drosophila S2. 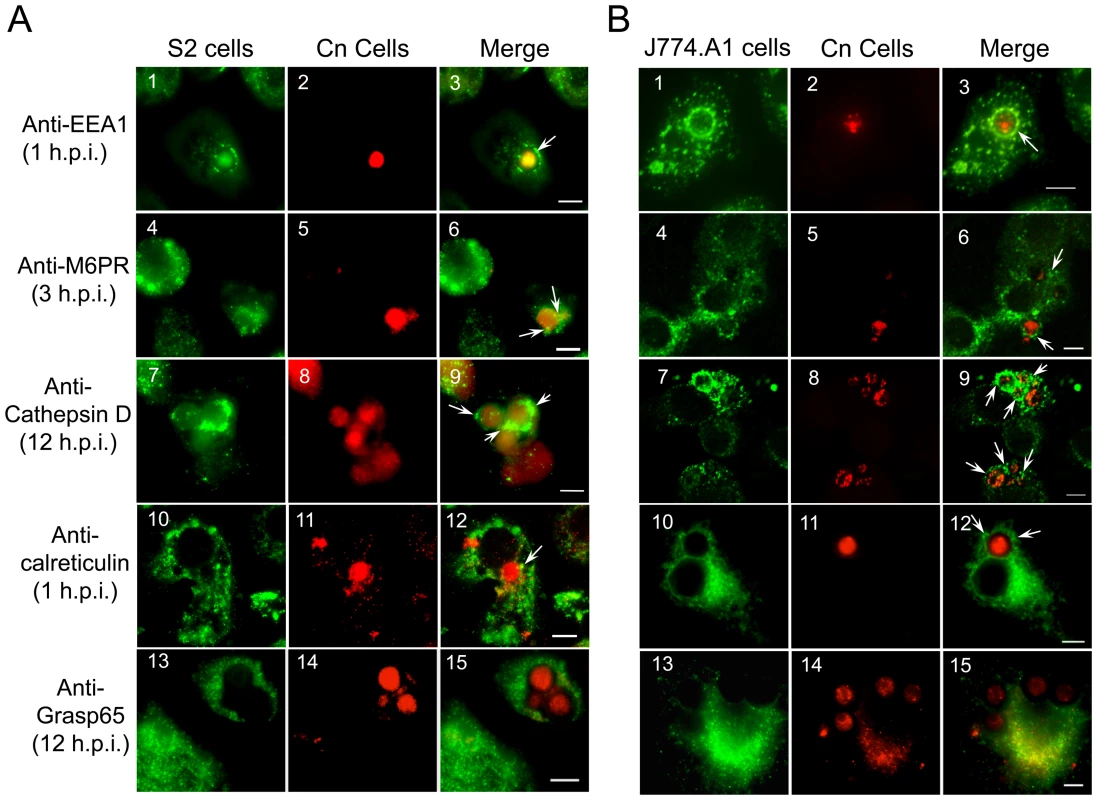
(A) and J774.A1 (B) cells. Host cells were seeded on 12-mm coverslips, placed on the bottom of 24-well plates and then infected with AI100-dsRed. The infected cells were washed three times with 1×PBS at 3 h.p.i., and fresh medium supplemented with 20 µg/ml fluconazole was added to each well containing infected host cells. The infected cells were then continuously incubated at 28°C (S2 cells) or 37°C, 5% CO2 (J774.A1 cells). At the indicated time points, the infected cells were fixed and stained for analysis by immunofluorescence microscopy using antibodies directed against the indicated proteins. In Panels A and B, the localizations of the early endosome marker EEA1 (1), late endosome marker M6PR (4), lysosome marker cathepsin D (7), endoplasmic reticulum (ER) marker calreticulin (10) and Golgi marker Grasp65 (13) in host cells are shown in green. Internalized or replicative Cn cells are shown in red (2, 5, 8, 11, 14). Cn cells in vacuoles that are surrounded by EEA1 (3), decorated with antibodies directed against the late endosome marker M6PR (6) or the lysosome marker cathepsin D (9), or surrounded by calreticulin (12) are shown in the merged images (white arrows). Tight association between Cn and Grasp65 was not observed at the indicated time point (15). Representative images from a single representative experiment are shown. At least three independent experiments were performed and each experiment gave similar results. Scale bars indicate 5 µM. Cn wild-type and mutant strains display similar infection profiles in both S2 and mammalian cell systems
To further examine whether Drosophila S2 cells can serve as a model host cell system for studying host-Cn interactions, the infection phenotypes of several serotypes and mutant variants of Cn (Table S1) were compared in J774.A1 and S2 cells. These mutant strains were selected for study because they were derived from the wild-type (WT) H99, displayed varying degrees of virulence in murine models of cryptococcosis, and possessed defects in distinct genetic pathways (Table S1). When the tested Cn strains were used to infect mammalian macrophages and S2 cells, similar patterns of pathogen uptake, replication and escape were observed (Figure 4 and Figure S5). For example, strain XL1601 displayed similar uptake, replication, and escape efficiencies in both cell systems (Figure 4). The number of XL1601 CFUs that were recovered from inside host cells was similar to that of the AI100-dsRed control (Figure 4, A3 and B3). However, the size of the corresponding extracellular population was larger than the AI100-dsRed control population (Figure 4, A2 and B2), consistent with the possibility that the enhanced capability of this mutant to escape from host cells contributes to its enhanced virulence in animal models of cryptococcosis [52]. The acapsular mutant cap59 displayed ∼6-fold higher rates of phagocytosis than capsular strains (Figure 4, A1 and B1), consistent with previous observations [53]. These results indicated that WT and mutant strains of Cn displayed similar infection profiles in both mammalian and S2 cell systems, thereby supporting the conclusion that the S2 cell system provides a useful model host cell system.
Fig. 4. Cryptococcus neoformans mutants behave similarly in Drosophila S2 cells. 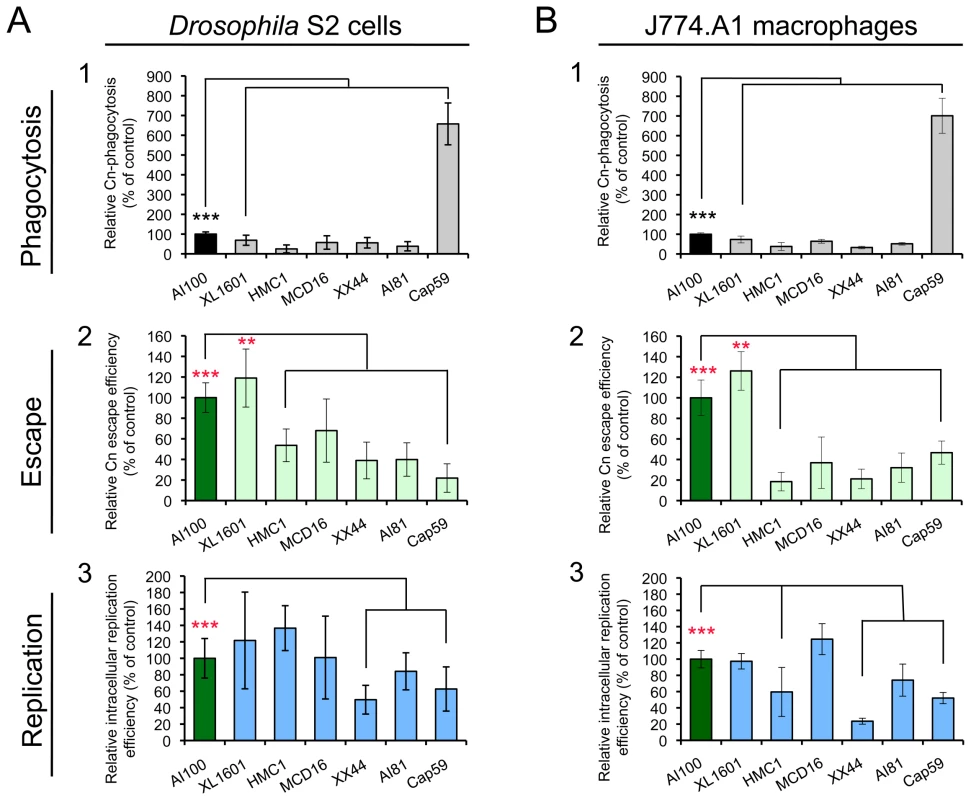
(A) and murine J774.A1 macrophages (B). Host cells were infected with the following Cn strains: AI100-dsRed, XL1601, HMCA1, MCD16, XX44, AI81 and Cap59. The infected cells were then extensively washed at 3 h.p.i. and then fresh medium supplemented with 20 µg/ml fluconazole was added to each well. The infected cells were continuously incubated at 28°C (S2 cells) or at 37°C, 5% CO2 (J774.A1 cells). At 3 and 15 h.p.i., the number of Cn cells present in the supernatant or within host cells was determined as described in the Materials and Methods section. Cn mutants display similar internalization patterns (A1 and B1), levels of Cn escape (A2 and B2) and relative intracellular replication efficiencies (A3 and B3) in S2 (A, left panel) and J774.A1 (B, right panel) cells. Data from the control (AI100-dsRed) were normalized to 100%. Data represent the means ± SD from at least three independent experiments. **, *** indicates significance at P<0.01 and P<0.001, respectively. Cn infection profiles were similar in drug-treated Drosophila S2 and J774.A1 cells
If Drosophila S2 cells are to provide a model system for elucidating evolutionarily conserved host molecules, then they must share Cn infection phenotypes with mammalian cells when host cell functions are disrupted. With this idea in mind, we examined whether Cn (AI100-dsRed) interacted similarly with S2 and J7774.A1 cells that had been pre-treated (1 hr) and then continuously incubated with a variety of compounds that disrupt host cell functions (Table S2). Fluconazole was added to the media immediately after infection so that the intracellular replication and escape of the pathogen from host cells could be independently monitored using fluconazole protection assays. We found that the tested compounds neither inhibited fungal growth in culture media (Figure S6, A and B) nor compromised the viability/growth of host cells under the examined conditions (data not shown and [41]). We also showed that pretreating (3 hrs) Cn with these compounds at the indicated concentrations before host cell infection did not impair the entry, intracellular replication and escape of the pathogen from host cells (Figure S6C).
We examined whether compound-treated S2 and J774.A1 cells supported similar patterns of pathogen uptake and replication. Treatment of either cell type with cytochalasin D (CytD), which disrupts actin cytoskeleton polymerization, dramatically reduced the amount of Cn phagocytosis. However, this compound did not inhibit the escape of replicative Cn cells from host cells (Figure 5, A2 and B2). When host cells were treated with LY294002 or 3-Methyladenine (3-MA), which disrupts class III PI3-kinase activity [54], [55], the entry, intracellular replication and escape of Cn from host cells were significantly reduced (Figure 5). Finally, bafilomycin A1 (BAF), an autophagy inhibitor that disrupts vacuolar H+-ATPase activity and endolysosomal acidification [56], significantly inhibited intracellular replication of internalized Cn cells. However, this compound had limited effect on the escape of Cn cells from mammalian macrophages (Figure 5). These data indicated that the infection profiles of Cn were similar in Drosophila S2 and J774.A1 cells that had been treated with compounds that disrupt host cell functions.
Fig. 5. Effect of selected compounds on Cryptococcus neoformans infection. 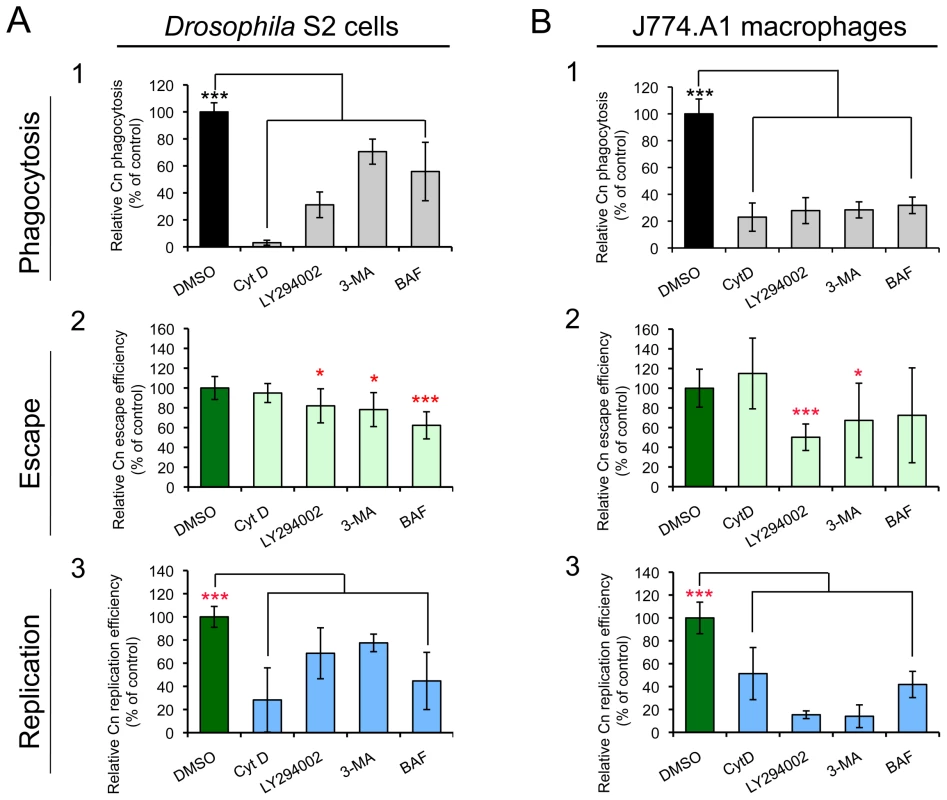
Drosophila S2 cells (A) and murine J774.A1 macrophages (B) were coincubated with the indicated compounds for 1 hr at the concentrations shown in Table S2. Next, Cn cells were added to the compound-treated host cells and incubated (in the presence of compound) for 3 hrs. The infected host cells were then extensively washed to remove the extracellular population of Cn cells, and new media containing fresh compounds and fluconazole (20 µg/ml) were added. Phagocytosis of Cn cells by host cells (A1 and B1) was determined at 3 h.p.i. by lysing the infected cells, plating the cell lysate on YPD solid medium, and determining the CFUs present after 24 hr incubation at 37°C. Finally, at 12 h.p.i., the escape (A2 and B2) or intracellular replication (A3 and B3) efficiencies of Cn cells were measured separately as described in the Materials and Methods section. *, **, *** indicates significance at P<0.05, 0.01 and 0.001, respectively, compared with the DMSO treated control. CytD, 3-MA and BAF indicate that host cells were treated with cytochalasin D, 3-Methyladenine and bafilomycin A1, respectively. Data represent the means ± standard deviations from at least three independent experiments. RNAi screen identifies host factors that mediate Cn infection
Encouraged by our findings that Cn infection of S2 cells recapitulates salient aspects of the corresponding infection of mammalian cells, we examined whether the S2 cell system and RNAi technology could be combined to identify host factors that mediate host-Cn interactions. We used a novel RNAi screening approach (Figure S7) to perform a pilot screen for these host factors. Cn is known to replicate within a poorly characterized phagosome-derived compartment [21], [22], [57], suggesting that host factors involved in membrane remodeling, trafficking, or phagosome biogenesis and maturation may be critical to the intracellular lifestyle of the pathogen. For our screen, we pre-selected 410 dsRNAs that targeted the knockdown of genes that (1) had been annotated to be associated with these events (http://flybase.org/) and (2) were available in Release 1.0 of the Drosophila RNAi Library (Open Biosystems, Huntsville, AL, USA). After two rounds of screening, sixty-two dsRNAs that significantly altered Cn infection of S2 cells were identified. Among the 62 candidates, 57 high priority hits (Table S3) were identified after three rounds of screening. A hit was defined as a dsRNA treatment in which the relative infection index (RIF, see the Materials and Methods) differed by two fold of the standard deviation (SD) from the untreated control. We also screened 96 dsRNAs that were randomly picked from one of the 76 source plates in the Drosophila RNAi library. Because the manufacturer randomly arrayed dsRNAs into source plates, this strategy for selecting dsRNAs introduced no bias in gene function into this analysis. Six of the randomly selected dsRNAs impaired host cell growth. However, after two rounds of screening, dsRNA hits were not uncovered in this experiment. Therefore, we estimate the hit frequency in the whole Drosophila genome to be less than 1%, and note that our preselected set of dsRNAs was significantly enriched in dsRNAs that target Cn host cell functions.
We analyzed our high priority hits in several ways. First, we classified hits into functional classes, including vesicle-mediated transport proteins, cytoskeletal proteins, and vacuolar proton-transport factors (Figure 6A and Table S3), according to the gene ontology system of biological and molecular function, cellular component, or protein domains as reported in FlyBase (www.flybase.org). Interestingly, of the 57 high priority hits, twenty-eight hits (49.1% of the total hits recovered) had been previously shown to mediate infection of S2 cells by fungal or intracellular bacterial pathogens, including Brucella melitensis [41], Chlamydia caviae [43], Chlamydia trachomatis [44], Listeria monocytogenes [38], [40] or Mycobacterium fortuitum [40], [42] (Figure 6B and Table S3). Among the 28 hits, 11 genes (19.3% of the hits) are known to be required for phagocytosis of C. albicans [39], a fungal pathogen that causes oral and genital infections in humans and is also associated with morbidity and mortality in immunocompromised patients (Figure 6B and Table S3). Several host factors previously known to function in phagocytosis [39], such as Rac1, actins, and PI3-kinase (class III) and COPI coatomers, were identified as high priority hits (Table S3). Consistent with these findings, pharmacological disruption of the functions of these host factors with CytD or LY294002 reduced host cell phagocytosis of Cn cells (Figure 5, A1 and B1). Finally, we identified 29 genes (50.9% of the high priority hits) that had not been previously reported to be involved in mediating intracellular Cn infection (Table S3). Therefore, our screening strategy was sufficiently robust to uncover suspected host factors as well as factors with novel functions.
Fig. 6. Identification of host factors regulating Cryptococcus neoformans infection. 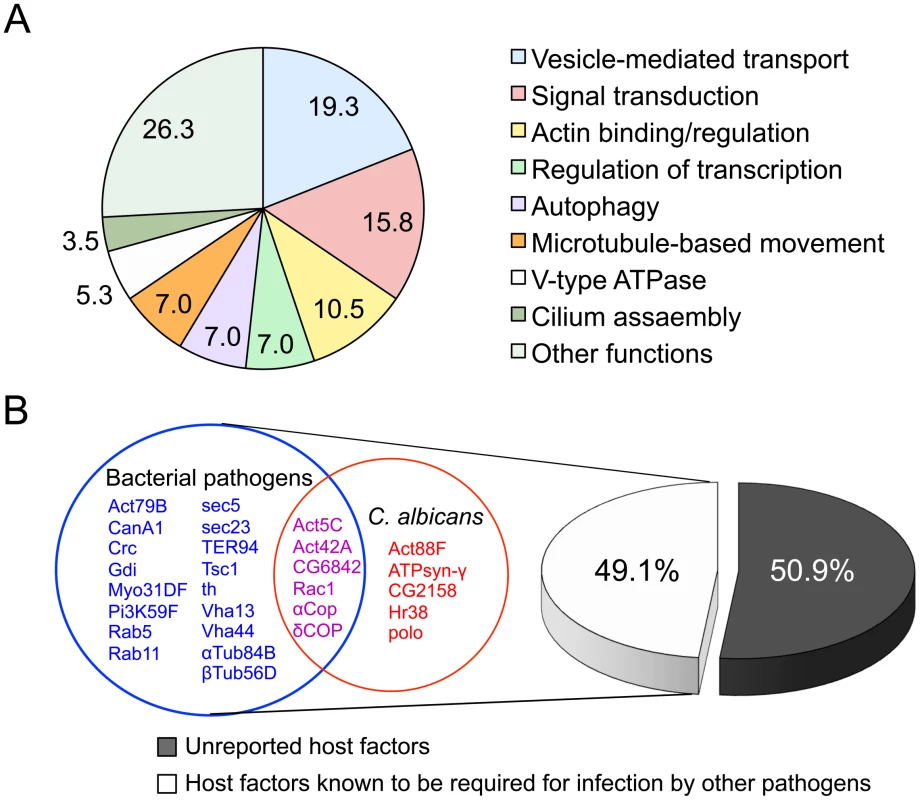
A. Fifty-seven host factors that, when depleted, significantly altered Cn infection phenotypes were identified in an RNAi screen using Drosophila S2 host cells. The 57 host factors were analyzed using established gene ontology categories (http://flybase.org/). The results were plotted in a pie chart with the percentage of genes in each category indicated. B. Comparison of the 57 identified host factors with those that were reported to mediate bacterial or fungal pathogen infection of host cells. Among the 57 hits, twenty-nine (50.9%) had not been previously reported to function as host factors for intracellular fungal pathogens. Shared and unique host factors were determined by analyzing published data from screens performed in S2 cells infected with the following pathogens: Brucella melitensis [41], Chlamydia caviae [43], Chlamydia trachomatis [44], Listeria monocytogenes [38], [40], Mycobacterium fortuitum [40], [42] and Candida albicans [39]. The host factors that are shared with other human pathogens (49.1%) are listed in red (C. albicans), magenta (C. albicans and bacterial pathogens) and blue (bacterial pathogens). Each host factor mediates Cn phagocytosis and/or intracellular replication. A role for host cell autophagy proteins in mediating Cn infection
Our screen uncovered several autophagy (Atg) genes, including Atg2 (CG1241), Atg5 (CG1642) and Atg9 (CG3615), which play conserved roles in autophagosome biogenesis [58]. However, the engagement of host cell Atg proteins by Cn had not been previously reported. To determine whether elements of the host cell autophagy pathway regulate Cn interactions with macrophages, we exploited murine RAW264.7 macrophages as a host cell system. RAW264.7 cells provided an established model for interrogating Cn interactions with mammalian macrophages [9]. RNAi mediated gene knockdown can also be readily achieved in these cells, which was advantageous for our studies. We thus treated RAW264.7 cells with siRNAs to knock down the expression of the murine orthologs of several Drosophila Atg genes that were uncovered in our S2 cell screen. The targeted mammalian genes included Atg2a, Atg5, Atg9a, Atg12 and LC3. After validating gene knockdown by immunoblotting analysis (Figure S8), we infected the siRNA treated cells with Cn (H99), and then quantified the phagocytosis, replication, and escape of the pathogen using our fluconazole protection assay. When host cells that had been depleted of the indicated Atg proteins were infected with Cn, the uptake and/or replication of the pathogen were significantly decreased compared to controls that had been treated with scrambled siRNAs (Figure 7). These data supported findings obtained from our RNAi studies using the S2 cell system (Table S3) that indicated the engagement of host cell autophagy proteins in supporting Cn infection.
Fig. 7. Host Atg proteins mediate Cryptococcus neoformans infection of host cells. 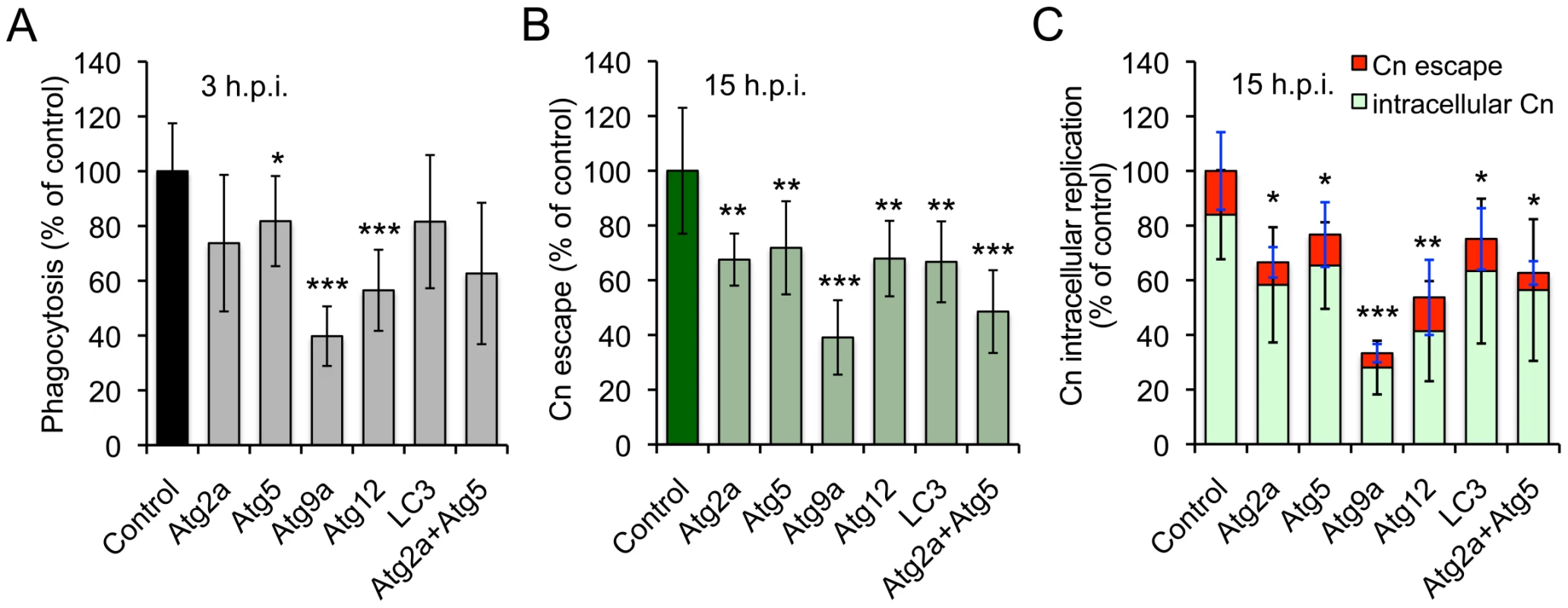
Murine RAW264.7 macrophages were transfected with the indicated siRNAs (10 nM). Two days post-transfection, the host cells were infected with Cn (H99) for 3 hrs. The infected cells were extensively washed to remove the extracellular population of Cn cells, and fresh medium supplemented with 20 µg/ml fluconazole was added. At the indicated time points post infection, the supernatant and lysate from infected cells were collected separately. The materials were then plated, and the number of CFUs per well was determined. The resultant CFUs were used to calculate the relative phagocytosis (A), Cn escape (B) and intracellular replication (C) as described in the Materials and Methods. Data represent the means ± SD from at least four independent experiments with triplicate wells examined for each treatment. *, **, *** indicates significance at p<0.05, 0.01 and 0.001, respectively. Host Atg proteins are recruited to Cn-containing vacuoles during infection
Our siRNA experiments in RAW264.7 cells suggested that Atg2, Atg5 and Atg9 support Cn infection of both Drosophila S2 and mammalian cell systems. To further investigate the roles of Atg proteins in supporting pathogen infection, we employed confocal immunofluorescence microscopy to analyze the interaction of assorted Atg proteins with Cn cells during a time course of Cn infection of mammalian host cells. Upon internalization, Cn was observed in close apposition to membranes that were decorated with LC3, Atg9a and Atg5 (Figure 8, A–D), suggesting that these autophagy elements are recruited to the vicinity of CnCVs. Interestingly, the intimate interactions between Cn and these Atg proteins during the early stages of Cn infection were observed in several mammalian host cell types, including J774.A1, MEF and RAW264.7 cells (Figure 8, A–D and data not shown). Quantitative analysis revealed that the recruitment of Atg proteins to the vicinity of CnCVs was reduced after 3 h.p.i. (Figure 8, A and B, white arrows and data not shown). These microscopy experiments therefore supported findings from our siRNA experiments.
Fig. 8. Cryptococcus neoformans interactions with Atg proteins during infection. 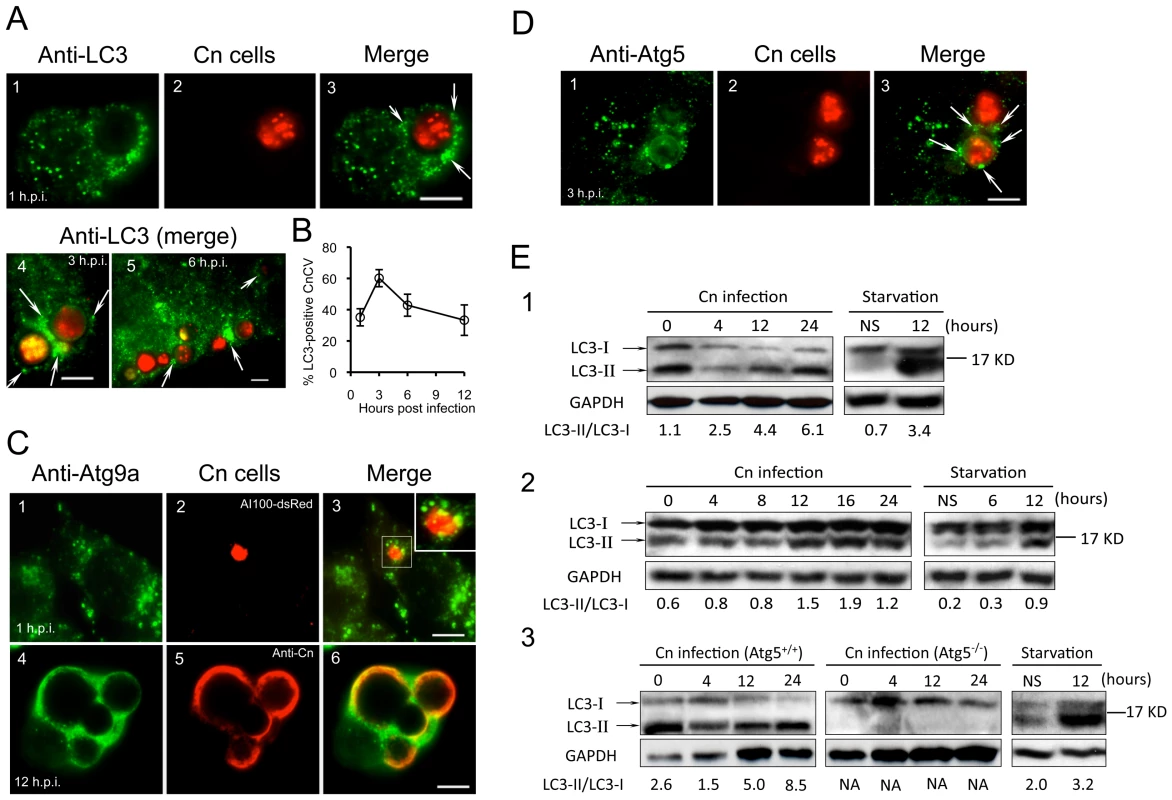
Murine J774.A1 or RAW264.7 cells were seeded on 12-mm coverslips placed on the bottom of 24-well plates and then infected with Cn (AI100-dsRed) for 1 or 3 hr. At 3 h.p.i., the infected cells were wash three times and then continuously incubated in fresh medium supplemented with 20 µg/ml fluconazole. At the indicated time points, the infected cells were fixed and prepared for immunofluorescence microscopy using antibodies directed against mouse proteins LC3 (A, J774.A1 cells) or Atg9a (C, RAW264.7 cells. Inset in C3: a magnified section shows Atg9a in close apposition to CnCvs during an early stage of infection) and Atg5 (D, J774.A1 cells). Regions of colocalization appear yellow in the images (C3 and C6). CnCVs surrounded by host proteins are indicated by white arrows. Scale bars indicate 5 µM. Images were taken from a representative experiment of at least three independent experiments. B. Quantification of Cn cells that resided in host LC3-positive compartments during a time course of infection. At each time point, the percent of total cells analyzed in which LC3 was tightly associated with CnCvs was calculated and plotted as a function of time. Data represent the means ± SD from at least three independent experiments. For each experiment, at least 300 internalized or replicative Cn cells were analyzed. E. Induction of LC3-I to LC3-II processing by Cn infection. Murine J774.A1 (E1), RAW264.7 (E2) macrophages or MEFs (E3) were infected with Cn strain AI100-dsRed or cultured in serum free DMEM (starvation) for 12 hr. At the indicated time points, Cn infected and serum starved cell samples were collected, and subjected to immunoblotting analysis using antibodies directed against mouse LC3. NS = non-starved. Representative images were taken from a single experiment. Three independent experiments gave similar results. LC3-I conversion to LC3-II during Cn infection
To further investigate the possibility that the activation of proteins in the autophagosome biogenesis pathway supports Cn infection, we examined the conversion of host LC3-I to LC3-II as an indicator of these events. As expected, overnight nutritional limitation of host cells induced the conversion of LC3-I to LC3-II, resulting in an increased ratio of LC3-II/LC3-I (Figure 8E); however, this conversion was not significantly increased over a 24 hr time course of incubation in the absence of Cn infection (Figure 8E and data not shown). Immunoblotting results indicated that the level of LC3-II, and the ratio of LC3-II/LC3-I increased during Cn infection of WT J774.A1, RAW264.7 and Atg5+/+ MEF cells (Figure 8E). However, the conversion of LC3-I to LC3-II was not detected in Atg5−/− MEFs at all of the tested time points (Figure 8E3). This latter finding was consistent with our observations that Cn cells did not reside in LC3-positive phagosomes/vacuoles in the Atg5−/− MEFs (data not shown). Taken together, our data demonstrated that Cn infection of host cells involves interactions with LC3-positive phagosome membranes.
Discussion
Yeast or basidiospore forms of Cn typically use the host respiratory system to gain entry into the host. Cn then rapidly disseminates to extrapulmonary tissues, including the brain, which accounts for the meningoencephalitis that is typically associated with cryptococcosis. Macrophages constitute important cellular targets of infection. Cn infects and replicates within alveolar macrophages [17], [59], and can also exploit macrophages to gain entry into the central nervous system via a Trojan horse mechanism in which these immune cells ferry ingested Cn cells across the blood brain barrier [60], [61]. Understanding host mechanisms that support the survival and replication of the pathogen within macrophages is therefore important to understanding pathogen dissemination and disease progression.
Non-vertebrate models have emerged as tractable experimental systems for illuminating host-pathogen interactions [62], [63]. The current study indicates that the combination of macrophage-like Drosophila S2 cells and RNAi technology provide an appealing system for elucidating the roles of host factors in mediating the host-Cn interaction. Cn infection of S2 cells recapitulated salient aspects of mammalian macrophage cell infection. First, Cn infection of S2 and mammalian cells shared similar infection phenomena, including phagocytosis, intracellular replication, cell-to-cell dissemination, extrusion and host cell lysis profiles. Moreover, the intracellular trafficking and replication of Cn in murine macrophages and Drosophila S2 cells shared striking similarities, suggesting that the fungal pathogen subverts conserved host factors to secure an intracellular replicative niche and to disseminate. Second, Cn strains from different genetic backgrounds and mutants with altered virulence in mouse models of infection displayed similar patterns of uptake and replication in S2 and J774.A1 cells. Third, similar patterns of Cn phagocytosis, intracellular replication, and escape were observed in host cells that had been treated with structurally diverse compounds that target specific host cell functions. These data suggested that both host cell systems share common molecular requirements to support these processes. Fourth, results from an RNAi screening experiment demonstrated that host factors previously known to be required for the phagocytosis of various bacterial and fungal pathogens [39] are also required for Cn infection of host cells (Table S3 and Figure 6B). These data support the idea that evolutionarily conserved host functions support pathogen interactions with S2 and mammalian cells. Finally, Cn cells have been isolated from insects such as cockroaches and beetles [45], [46], which is consistent with the possibility that aspects of the observed interactions with S2 cells may recapitulate pathogen interactions with insects in the natural environment. It has been postulated that the pathogenic strategies of Cn in mammals evolved from interactions between Cn and its natural environmental predators [64]. Our data support the conclusion that S2 cells provide a useful model system for elucidating host-Cn interactions.
Autophagy is a catabolic process in which cytosolic constituents are sequestered from the rest of the cytoplasm by the autophagosome [27], which delivers the sequestered constituents to lysosomes for degradation. Autophagy is a tightly regulated process that is essential for survival, differentiation, development and homeostasis [25], [26]. Several forms of autophagy have been identified that differ with respect to their physiological functions and the mode of cargo delivery to the lysosome [25]. Atg5 is essential for most forms of mammalian autophagy [65], [66], [67]. This protein mediates the conversion of LC3-I to LC3-II, which drives autophagosome biogenesis, and indicates activation of the autophagy pathway. Atg5 also directs the localization of LC3-II to autophagosomes [28], [29]. Atg9a, the only transmembrane Atg protein in mammals, is proposed to mediate membrane transport to generate autophagosomes [68]. The Atg18-Atg2 complex is involved in the transport of Atg9 to autophagic membranes [69], [70]. Finally, an alternative, Atg5/Atg7-independent macroautophagy pathway has been described in which conversion of LC3-I to LC3-II does not occur [71], [72]. Therefore, autophagy can be engaged without recruiting the full complement of Atg proteins that have been annotated to be associated with this process.
Recent studies indicate that autophagy may be an important pathogen control mechanism [24], [25], [26]. Atg5 plays roles in inhibiting the growth of pathogens, such as L. monocytogenes [30], [31], [32] and T. gondii [32], [33]. In Drosophila, autophagy helps control L. monocytogenes infection of primary hemocytes [31]. In contrast to the potential protective role for autophagy against bacterial and parasitic infection, other studies suggest that several bacteria and parasites may subvert host autophagic processes to establish a successful infection; these pathogens include Staphylococcus aureus [73], Legionella pneumophila [74], B. melitensis [41], C. burnetii [75], Helicobacter pylori [76], [77], Trypanosoma cruzi [78] and T. gondii [79]. In addition, some pathogens, such as the HSV-1 virus, are known to exploit elegant mechanisms for inhibiting signaling processes that induce autophagy [80], [81]. T. gondii infection induces host cell autophagy via a mechanism that depends on host Atg5. In fact, the growth of this parasite is defective in Atg5-deficent cells [79]. Therefore, in addition to their roles in the canonical autophagy pathway, host Atg proteins may also contribute to modulating the level of replication of viral or intracellular pathogens.
In this study, we demonstrated that Cn exploits host cell autophagy proteins to secure an intracellular replicative niche. First, we showed that replicative Cn cells reside in CnCVs that are tightly associated with the autophagosome marker LC3 and lysosomal marker LC3. However, similar LC3 localization was not observed in pathogen-infected Atg5−/− MEFs. Second, we demonstrated that compounds that are known to disrupt the activities of proteins that regulate the host autophagy pathway (BAF, LY294002 and 3-MA) inhibit Cn uptake and replication. In an early stage of autophagosome formation, the Beclin 1-hVps34 (class III PI3-kinase) complex supplies phosphatidylinositol-3-phosphate (PI[3]P) to the pre-autophagosome membrane, a process that is essential for autophagosome membrane formation [82]. 3-MA and LY294002 are inhibitors of class III PI3-kinase and also block autophagy [54], [55]. Our data demonstrate that host cells treated with 3-MA or LY294002 display reduced levels of Cn infection, and implicate PI3-kinase activity in supporting the intracellular lifestyle of the pathogen. Finally, we showed that RNAi-mediated depletion of host Atg proteins, including Atg2a, Atg5, Atg9a, reduced Cn infection of host cells. RNAi-mediated depletion of Pi3K59F (class III PI3-kinase) also failed to support Cn infection in S2 cells. Taken together, our data demonstrate that Cn exploits host proteins that regulate the host autophagy machinery to facilitate intracellular replication. Interestingly, Cn employs autophagy as a survival mechanism and virulence-associated trait. Cn strains lacking the Vps34 PI3-kinase and (vps34Delta) and depletion of Atg8 markedly attenuated virulence in a mouse model of infection [83]. Therefore, autophagy pathways in both the host and pathogen play important roles in supporting the interaction between Cn and host cells.
Based on our findings, we propose a model in which key elements of the autophagy pathway, including Atg5-Atg12, Atg9a and LC3, coordinate with other host factors identified in our RNAi screen to regulate the sequential intracellular replication and escape of Cn from host cells (Figure 9). The following step-wise events are envisioned. First, phagocytosis of Cn is mediated by several factors, including host actins and class III PI3-kinase activities. Upon entry into host cells, Cn traffics to EEA1-positive CnCVs, which then traffic along the endocytic pathway, interact with membranes containing late endosome markers and autophagosomal markers (LC3), and fuse with lysosomes to become late CnCVs that are decorated with markers associated with endosome/lysosome (LAMP-1) and lysosome (cathepsin D) proteins. These compartments are permissive for Cn intracellular replication. Finally, Cn cells can escape from CnCvs at any stage of the maturation process (Figure 9).
Fig. 9. Proposed model depicting Cn subversion of Atg proteins for intracellular replication and dissemination. 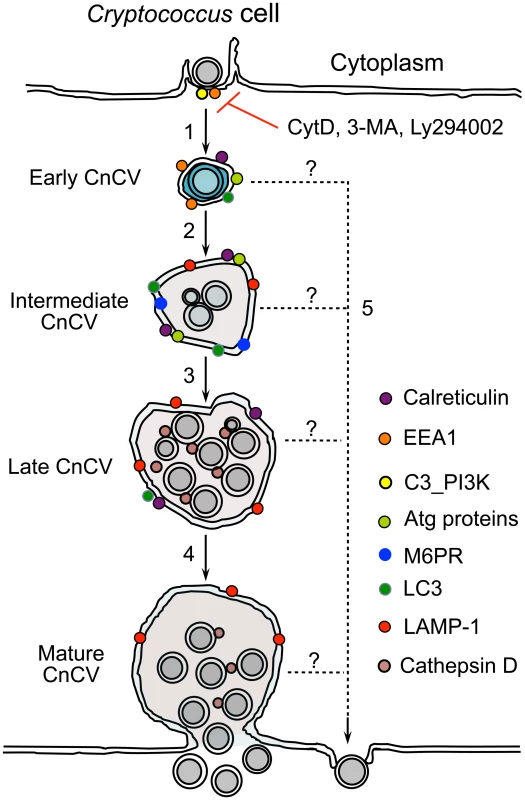
Host cell phagocytosis of Cn cells is class III PI3-kinase (C3_PI3K) and actin-dependent. This process is blocked by the PI3-kinase inhibitors 3-MA, LY294002 and the actin polymerization inhibitor cytochalasin D (CytD) (1). Internalized Cn resides in a Cn containing vacuole (CnCv). Early CnCvs interact with components of the endocytic pathway and sequentially obtain early endosome (EEA1), late endosome (M6PR) and lysosome (LAMP-1 and cathepsin D) markers (2). Infection by Cn may also activate the host cell autophagy pathway. Atg proteins such as Atg5, Atg9a and LC3 are recruited to the vicinity of CnCVs. Activated host cell Atg proteins promote the maturation of early CnCVs to form intermediate CnCVs (2). These compartments support Cn replication (3). Finally, late CnCVs may fuse with the plasma membrane, via an undefined mechanism, which results in the escape of replicative Cn cells from infected host cells (4). Extrusion in the absence of Cn replication and/or CnCv maturation may also occur (5). Intracellular and extracellular populations of Cn each contribute to disease progression in the infected host. For example, enlarged Cn cells that resist phagocytosis have been reported [84], [85], [86], [87]. Strains that harbor mutations in signaling pathways of the pathogen that support giant cell formation display reduced virulence [85]. These observations are consistent with the conclusion that this extracellular population of Cn cells is important for the infection and dissemination of the pathogen. On the other hand, several lines of evidence support the conclusion that macrophage invasion and replication constitutes an important to disease progression in humans and whole animal model systems. For example, animals depleted of macrophages by clodronate treatment display reduced pathogen load in a variety of organs after infection with Cn [88]. Moreover, Cryptococcus gatti strains with enhanced abilities for intracellular replication display enhanced virulence in humans and murine models of infection [89]. The proposed model of intracellular trafficking dynamics put forth in this report will thus illuminate an important aspect of fungal pathogenesis.
Materials and Methods
Cryptococcus neoformans strains
The C. neoformans strains used in this study are listed in Table S1. Yeast forms of Cn cells were grown on YPD (Difco) agar plates and maintained on the plates for no more than one week prior to experimentation. For infection, 2.4 ml of YPD broth was inoculated with a loop of Cn cells taken from a freshly streaked YPD plate. Cultures were then grown overnight with shaking at 37°C.
Cell culture
Drosophila S2 cells were maintained at 25°C in Insectagro DS2 serum free medium (DS2-SFM, Cellagro) with or without 10% fetal bovine serum (FBS). Murine J774.A1 and RAW264.7 macrophages and Atg5-deficient (Atg5−/−) MEFs and the corresponding control (Atg5+/+) [65] were routinely incubated at 37°C in a 5% CO2 atmosphere in DMEM supplemented with 10% FBS. Cells were seeded in 24 or 48-well plates and cultured overnight before infection. For CFU assays, 1.0×106 S2 and 2.5×105 mammalian cells were seeded in each well of a 24-well plate; for a 48-well plate, 5.0×105 S2 and 1.2×105 mammalian cells were seeded; for fluorescence microscopy assays, 6.0×105 S2 and 1.0×105 mammalian cells were seeded on 12-mm glass coverslips (Fisherbrand) that were placed on the bottom of 24-well plates. The seeded S2 and mammalian cells were cultured overnight before infection.
Cryptococcus neoformans infection
rosophila S2 and mammalian cells were infected with Cn cells at a multiplicity of infection (MOI) of 5 and 10, respectively, unless otherwise indicated. Infected cells were then incubated at 25°C (S2 cells) or 37°C in a 5% CO2 atmosphere (mammalian cells). One or 3 h.p.i., culture media was removed, and the infected cells were extensively (6 to 8 times) washed with 1×phosphate buffered saline (PBS) (S2 and J774.A1cells) or a mixture of 1×PBS and DMEM with 10% FBS (V∶V = 1∶1) (RAW264.7 cells and MEFs). Fresh media, supplemented with fluconazole (20 µg/ml), was added to each well containing infected host cells and the infected cells were continuously incubated in this antifungal agent for various lengths of time at the indicated conditions. At the indicated time points, culture medium (containing escaped Cn cells) and the corresponding Cn infected host cells were collected separately to measure the number of CFUs. Fluconazole inhibition of Cn replication in S2 and mammalian cell media was also measured by inoculating Cn in fresh or conditioned medium containing 20 µg/ml fluconazole and performing CFU assays. Cn intracellular replication or escape efficiencies for experiments (in which infection was initiated at starting time point t0 and proceeded to time point tn) were calculated as described in the Mathematical Formulae section (see below). If no CFUs were recovered from the culture medium at t0, then we could not calculate escape efficiency. In this case, the net increases in the number of CFUs recovered from the media for the experimental treatments were compared to the corresponding net increase of the control at tn.
Cryptococcus neoformans CFU/fluconazole protection assays
We used fluconazole protection assays to measure the number of intracellular Cn and also the number of Cn that had escaped from these same host cells. To measure the intracellular population of Cn cells, we infected host cells for 3 hrs, washed these cells extensively (6–8 times), and then added fresh medium containing fluconazole (20 µg/ml). The cells were then continuously incubated for various lengths of time. At the indicated time points, the media was removed. The cells were then lysed by the addition of 0.5% Tween-20 in sterile water (100 µl) and incubation at room temperature (S2 cells) or at 37°C (mammalian cells) for ∼10 min. Additional 100 µl of YPD was added to the resultant whole cell lysates (which contained the intracellular Cn population). Finally, 5 µl of cell lysates were plated onto solid YPD medium after serial dilution. CFUs were counted after 24 hrs of incubation at ∼30°C. To measure the escaped Cn cells, the removed media were transferred to 96-well plates (if total volume <300 µl) or 1.5 ml microcentrifuge tubes (if total volume >300 µl) and centrifuged at 1200×g for 12 min. The media with fluconazole were then removed and the Cn cells were re-suspended in YPD broth. The Cn suspension was serially diluted in YPD broth or 1×PBS buffer, and 5 µl of the diluted Cn suspension was plated onto solid YPD agar plates to determine CFUs as above. To measure internalized Cn cells, after extensive wash, the infected cells were lysed and performed CFU assay as described above. To measure the number of Cn cells in the culture media at the starting time point (t0, 3 h.p.i.), after extensive wash (6∼8 times), fresh media (200 µl) without fluconazole or 1×PBS were added to the wells containing infected host cells. The supernatants (which contained the extracellular Cn population) were transferred to 96-well plates immediately after the plates containing infected cells were briefly shaken. After serial dilution, the supernatants were then performed CFU assay as described above.
Viability of host cells
Host cells were coincubated with or without pharmacological compounds at the indicated concentration (Table S2) for various lengths of time. Quantification of the viability of treated S2 and mammalian cells as well as the total cell number was performed as previously described [41].
Fluorescence Microscopy and Immunofluorescence Microscopy Assay (IFMA)
To visualize intracellular populations of Cn cells, host cells (e.g., S2 or J774.A1 cells) were seeded on 12-mm coverslips that had been placed on the bottom of wells of 24-well plates. Next, these cells were infected with Cn strain AI100-dsRed or AI132-GFP. At 1 or 3 h.p.i., the infected host cells were extensively washed and then continuously incubated in fresh medium supplemented with 20 µg/ml fluconazole. At different time points post infection, the coverslips with infected host cells were fixed and stained with Alexa 488-conjugated phalloidin (for AI100-dsRed infected cells) or rhodamine phalloidin (for AI132-GFP infected cells) to resolve the host cell actin cytoskeleton. The infected cells were then visualized by confocal fluorescence microscopy. Differential interference contrast (DIC) and fluorescence images were used to analyze phagocytosis of Cn cells by host cells. To elucidate Cryptococcus intracellular trafficking and interactions with host proteins, Drosophila S2, J774.A1 and RAW264.7 cells that were seeded onto 12-mm coverslips on the bottom of 24-well plates were infected with AI100-dsRed for various lengths of time. To analyze infections of less than 3 hrs, the infected cells were washed three times with 1×PBS or the mixture of PBS and DMEM with 10% FBS, and then the infected cells were fixed before IFMA. Otherwise, at 3 h.p.i., the infected cells were washed three times with 1×PBS or a mixture of PBS and DMEM. In experiments in which the cross-reactivity of extracellular Cn cells with the indicated antibodies was assessed, the Cn infected host cells were washed only once. Next, fresh media supplemented with 20 µg/ml fluconazole were added to the wells containing Cn infected host cells. At various time points post infection, the infected cells were fixed and processed for IFMA as previously described [41] with the following modification: before incubation with antibody, the infected cells were blocked with 10% non-fat dry milk in PBSTT buffer (0.05% Triton X 100+0.05% Tween 20 in 1×PBS) for ∼2 hrs at room temperature, and then the cell samples were incubated with antibody in PBSTT with 5% non-fat dry milk. The primary antibodies used were as follows: goat-anti-human EEA1; rabbit anti-human M6PR; rabbit anti-human LAMP-1; rabbit anti-human cathepsin D; rabbit anti-mouse LC3; rabbit anti-Calreticulin; purified Grasp65 from rabbit; mouse anti-human Atg5; mouse anti-Cn (Meridian Life Science, Inc., USA). Samples were stained with Alexa Fluor 488-conjugated and/or Alexa Fluor 594-conjugated secondary antibody (Molecular Probes, 1∶1000). Acquisition of confocal images, image processing and analyses were performed as previously described [41].
Live cell imaging
J774.A1 or Drosophila S2 cells were seeded in 6 - or 24-well plate with glass bottom. The cells were overnight cultured before Cn (AI100-dsRed) infection (MOI = 3). At 3 h.p.i., Cn infected host were washed 3 times with 1×PBS. Fresh media with 20 µg/ml fluconazole were added to the infected cells, and the cells were then incubated in the small environmental chamber (28°C for S2 cells and 37°C, 5% CO2 for J774.A1 macrophages) mounted on the laser confocal microscopy (Eclipse Ti, Nikon). Live cell images were automatically taken every 2 min for 24 (J774.A1) and 48 (S2 cells) hrs with NIS elements AR 3.0 software (Nikon), which was initiated after phagocytosing. Images were processed with NIS elements AR 3.0 software.
Drug treatments
In 48 well plates, Drosophila S2 and murine macrophage J774.A1 cells were coincubated with assorted pharmacological compounds including CytD, LY294002, 3 - 3-MA and BAF at the indicated concentrations (Table S2). Cells were treated with these drugs 1 hr before and during infection with Cn strain AI100-dsRed. The treated cells were incubated at 25°C (S2 cells) or at 37°C with 5% CO2 (J774.A1 macrophages). At 3 h.p.i., the cells were extensively washed with 1×PBS. Fresh media supplemented with the same concentration of the drugs and 20 µg/ml fluconazole were then added to the infected cells. Fluconazole was used to inhibit the replication of extracellular Cn cells, if any. To evaluate phagocytosis of Cn cells, the infected cells (3 h.p.i.) were lysed with 0.5% Tween 20 after extensive washing with 1×PBS. The cell lysate was used to perform CFU assays as described above. In addition, the media were separately collected for CFU assays which determined the initial number of Cn cells present in the media at the starting time point (t0). At 12 h.p.i., the media and extracts derived from infected cells were also analyzed using CFU assays as described above. To evaluate the effects of drugs on Cn pathogenicity, the Cn cells were pre-treated with the indicated drugs for 3 hrs and then the drugs were washed out. These cells were then used to infect normal host cells. Phagocytosis, intracellular replication and escape of the drug pre-treated Cn cells were analyzed as described above.
Generation of dsRNAs
Generation of dsRNAs that target the knockdown in the expression of Drosophila proteins were performed as previously described [41]. The dsRNA products were stored at −80°C until use.
RNAi screen for host factors mediating Cn infection
As shown in Figure S7, dsRNAs were added to 48-well plates at a final concentration of 15 µg/ml. dsRNAs were tested in duplicate in independent plates. S2 cells were then seeded in the plates at a density of 5.0×105 cells/well in 150 µl DS2-SFM medium. dsRNA-treated cells were incubated at 25°C for 1 day; 150 µl of fresh medium with 10% FBS was then added to each well to allow the cells to recover from dsRNA treatment. The dsRNA-treated cells were continuously incubated at 25°C for an additional three days to allow the knockdown of target gene expression. The efficiency of dsRNA mediated gene knock down was checked as previously described [41]. The treated cells (in 100 µl) together with 100 µl of fresh DS2-SFM medium were added into 48 well plates, and the treated cells were incubated overnight at 25°C before Cn infection. Cn strain H99 or AI100-dsRed was used to infect the dsRNA-treated S2 cells at an MOI of 5. At 3 h.p.i., the infected cells were washed 6∼8 times with 1×PBS and then 200 µl of fresh DS2-SFM medium supplemented with 30 µg/ml fluconazole was added to inhibit the replication of extracellular Cn cells, if any. To determine the number of Cn cells present in the medium at the starting time point (3 h.p.i., t0), the media were transferred to U-bottom 96-well plates (MicroTEST). Cn cells were centrifuged (1,200×g, 12 min) and resuspended in 200 µl of YPD broth. CFU assays were performed on the recovered Cn cells. To evaluate the effects of dsRNA-mediated knockdown of target genes in S2 cells on the phagocytosis of Cn cells, the infected cells were extensively washed and then lysed with 100 µl of 0.5% Tween 20 in sterile water for 15 min. 100 µl of YPD broth was added to the cell lysates. The material was thoroughly mixed and the cell lysate was transferred to a 96-well plate. CFU assays were performed as described above. To assess the effects of dsRNA treatment on Cn intracellular replication and escape at 24 h.p.i, media and cell lysates were first transferred to U-bottom 96-well plates. CFU assays were then performed on this material. Five µl of diluted cell lysate and Cn cell suspension were separately plated onto single well OmniTray (Nunc) plates containing YPD solid medium. CFUs were read after 24 hr of incubation at 30°C, and the infection index and RIF (see the Mathematical Formulae section) were calculated. dsRNAs that displayed a RIF that differed by more than 2-fold of SD from the untreated control in the screen were picked out for next round of screen. Candidates identified after two rounds of screening were selected for re-testing in triplicate.
siRNA mediated depletion of target proteins in murine RAW264.7 macrophages
2.0×104 RAW264.7 macrophages were seeded in each well of a 48-well plate one day before siRNA transfection. siRNA treatment was performed using Lipofectamine RNAiMAX or Lipofectamine 2000 transfection reagent (Invitrogen) in accordance with the manufacturer's instructions. All the siRNA constructs, including the scrambled siRNA and siRNAs targeting Atg2a, Atg5, Atg9a, Atg12 and LC3 were purchased from Santa Cruz Biotech., Inc. (Santa Cruz, CA, USA) or US Sigma-Aldrich. After two days of transfection, the medium was replaced with fresh DMED containing 10% FBS at least 2 hr before Cn (strain H99) infection. The growth and viability of siRNA-treated cells was monitored using the trypan blue exclusion assay [41].
Immunoblotting analysis
At the indicated time points, siRNA transfected RAW264.7 macrophages or Cn infected host cells were washed three times with cold 1×PBS. The washed cells were then lysed without disrupting the intact Cn cells. ∼15 µg of the recovered protein extracts were separated by 12% SDS-PAGE, transferred onto polyvinylidene difluoride membranes (PVDM), and blotted with the indicated antibodies. The blots were detected using an enhanced chemiluminescence kit (Pierce, Rockford, IL). Blots were stripped and reprobed for the protein level of GAPDH (Glyceraldehyde 3-phosphate dehydrogenase, a constitutive housekeeping gene) as a loading control. Primary antibodies, including anti-LC3, anti-Atg5, anti-Atg12 and anti - GAPDH were purchased from Santa Cruz Biotech., Inc. Anti-Atg9a was purchased from Thermo Scientific. Densitometry of blots was performed using the ImageJ (http://rsbweb.nih.gov/ij/) software package. For each blot, the value of Blot area×Mean gray value×Integrated density (BaMI) was calculated. The ratio of blot LC3-II/LC3-I at an indicated time points were calculated as shown in the Mathematical Formulae section (see below).
Mathematical formulae
Statistical analysis
All quantitative data were derived from results obtained from triplicate experiments that were independently performed at least three times. To easily compare results from independent experiments, the data from controls were normalized as 1 or 100%. The significance of the data was assessed using the Student's t-test.
Accession numbers
The NCBI (http://www.ncbi.nlm.nih.gov/) database accession numbers for the mouse genes and gene products discussed in this paper are Atg2a: 329015, Atg5: 11793, Atg9a: 245860, Atg12: 67526 and LC3β: 67443.
Supporting Information
Zdroje
1. BlancoJLGarciaME 2008 Immune response to fungal infections. Vet Immunol Immunopathol 125 47 70
2. LinXHeitmanJ 2006 The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol 60 69 105
3. TournuHSerneelsJVan DijckP 2005 Fungal pathogens research: novel and improved molecular approaches for the discovery of antifungal drug targets. Curr Drug Targets 6 909 922
4. FromtlingRAShadomyHJJacobsonES 1982 Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia 79 23 29
5. ChangYCKwon-ChungKJ 1994 Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol 14 4912 4919
6. WangYAisenPCasadevallA 1996 Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect Immun 64 2420 2424
7. Kwon-ChungKJPolacheckIPopkinTJ 1982 Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol 150 1414 1421
8. MaHMayRC 2009 Virulence in Cryptococcus species. Adv Appl Microbiol 67 131 190
9. LiuOWChunCDChowEDChenCMadhaniHD 2008 Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 135 174 188
10. NeteaMGFerwerdaGvan der GraafCAVan der MeerJWKullbergBJ 2006 Recognition of fungal pathogens by toll-like receptors. Curr Pharm Des 12 4195 4201
11. NakamuraKMiyagiKKoguchiYKinjoYUezuK 2006 Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol Med Microbiol 47 148 154
12. SiegemundSAlberG 2008 Cryptococcus neoformans activates bone marrow-derived conventional dendritic cells rather than plasmacytoid dendritic cells and down-regulates macrophages. FEMS Immunol Med Microbiol 52 417 427
13. VecchiarelliA 2000 Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol 38 407 417
14. RiveraJZaragozaOCasadevallA 2005 Antibody-mediated protection against Cryptococcus neoformans pulmonary infection is dependent on B cells. Infect Immun 73 1141 1150
15. KawakamiK 2004 Regulation by innate immune T lymphocytes in the host defense against pulmonary infection with Cryptococcus neoformans. Jpn J Infect Dis 57 137 145
16. WozniakKLLevitzSM 2008 Cryptococcus neoformans enters the endolysosomal pathway of dendritic cells and is killed by lysosomal components. Infect Immun 76 4764 4771
17. OsterholzerJJMilamJEChenGHToewsGBHuffnagleGB 2009 Role of dendritic cells and alveolar macrophages in regulating early host defense against pulmonary infection with Cryptococcus neoformans. Infect Immun 77 3749 3758
18. GuillotLCarrollSFBadawyMQureshiST 2008 Cryptococcus neoformans induces IL-8 secretion and CXCL1 expression by human bronchial epithelial cells. Respir Res 9 9
19. Artavanis-TsakonasKLoveJCPloeghHLVyasJM 2006 Recruitment of CD63 to Cryptococcus neoformans phagosomes requires acidification. Proc Natl Acad Sci U S A 103 15945 15950
20. LevitzSMNongSHSeetooKFHarrisonTSSpeizerRA 1999 Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect Immun 67 885 890
21. AlvarezMCasadevallA 2006 Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol 16 2161 2165
22. MaHCroudaceJELammasDAMayRC 2006 Expulsion of live pathogenic yeast by macrophages. Curr Biol 16 2156 2160
23. MaHCroudaceJELammasDAMayRC 2007 Direct cell-to-cell spread of a pathogenic yeast. BMC Immunol 8 15
24. LevineBDereticV 2007 Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol 7 767 777
25. LevineBKroemerG 2008 Autophagy in the pathogenesis of disease. Cell 132 27 42
26. DereticVLevineB 2009 Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5 527 549
27. YorimitsuTKlionskyDJ 2007 Endoplasmic reticulum stress: a new pathway to induce autophagy. Autophagy 3 160 162
28. KabeyaYMizushimaNUenoTYamamotoAKirisakoT 2000 LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19 5720 5728
29. MizushimaNOhsumiYYoshimoriT 2002 Autophagosome formation in mammalian cells. Cell Struct Funct 27 421 429
30. PyBFLipinskiMMYuanJ 2007 Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy 3 117 125
31. YanoTMitaSOhmoriHOshimaYFujimotoY 2008 Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat Immunol 9 908 916
32. ZhaoZFuxBGoodwinMDunayIRStrongD 2008 Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 4 458 469
33. Konen-WaismanSHowardJC 2007 Cell-autonomous immunity to Toxoplasma gondii in mouse and man. Microbes Infect 9 1652 1661
34. SchmidDMunzC 2007 Innate and adaptive immunity through autophagy. Immunity 27 11 21
35. KirkegaardKTaylorMPJacksonWT 2004 Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol 2 301 314
36. JacksonWTGiddingsTHJrTaylorMPMulinyaweSRabinovitchM 2005 Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol 3 e156
37. WilemanT 2006 Aggresomes and autophagy generate sites for virus replication. Science 312 875 878
38. ChengLWVialaJPStuurmanNWiedemannUValeRD 2005 Use of RNA interference in Drosophila S2 cells to identify host pathways controlling compartmentalization of an intracellular pathogen. Proc Natl Acad Sci U S A 102 13646 13651
39. Stroschein-StevensonSLFoleyEO'FarrellPHJohnsonAD 2006 Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol 4 e4
40. AgaisseHBurrackLSPhilipsJARubinEJPerrimonN 2005 Genome-wide RNAi screen for host factors required for intracellular bacterial infection. Science 309 1248 1251
41. QinQMPeiJAnconaVShawBDFichtTA 2008 RNAi screen of endoplasmic reticulum-associated host factors reveals a role for IRE1alpha in supporting Brucella replication. PLoS Pathog 4 e1000110
42. PhilipsJARubinEJPerrimonN 2005 Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science 309 1251 1253
43. DerreIPypaertMDautry-VarsatAAgaisseH 2007 RNAi screen in Drosophila cells reveals the involvement of the Tom complex in Chlamydia infection. PLoS Pathog 3 1446 1458
44. ElwellCACeesayAKimJHKalmanDEngelJN 2008 RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog 4 e1000021
45. ErginCIlkitMKaftanogluO 2004 Detection of Cryptococcus neoformans var. grubii in honeybee (Apis mellifera) colonies. Mycoses 47 431 434
46. Baroni FdeAPaulaCRSilvaEGVianiFCRiveraIN 2006 Cryptococcus neoformans strains isolated from church towers in Rio de Janeiro City, RJ, Brazil. Rev Inst Med Trop Sao Paulo 48 71 75
47. MylonakisEMorenoREl KhouryJBIdnurmAHeitmanJ 2005 Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 73 3842 3850
48. ApidianakisYRahmeLGHeitmanJAusubelFMCalderwoodSB 2004 Challenge of Drosophila melanogaster with Cryptococcus neoformans and role of the innate immune response. Eukaryot Cell 3 413 419
49. BurgessDSHastingsRW 2000 A comparison of dynamic characteristics of fluconazole, itraconazole, and amphotericin B against Cryptococcus neoformans using time-kill methodology. Diagn Microbiol Infect Dis 38 87 93
50. ZhaiBZhouHYangLZhangJJungK 2010 Polymyxin B, in combination with fluconazole, exerts a potent fungicidal effect. J Antimicrob Chemother 65 931 938
51. LungOBlissardGW 2005 A cellular Drosophila melanogaster protein with similarity to baculovirus F envelope fusion proteins. J Virol 79 7979 7989
52. LinXJacksonJCFeretzakiMXueCHeitmanJ 2010 Transcription factors Mat2 and Znf2 operate cellular circuits orchestrating opposite - and same-sex mating in Cryptococcus neoformans. PLoS Genet 6 e1000953
53. Geunes-BoyerSOliverTNJanbonGLodgeJKHeitmanJ 2009 Surfactant protein D increases phagocytosis of hypocapsular Cryptococcus neoformans by murine macrophages and enhances fungal survival. Infect Immun 77 2783 2794
54. PetiotAOgier-DenisEBlommaartEFMeijerAJCodognoP 2000 Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem 275 992 998
55. BlommaartEFKrauseUSchellensJPVreeling-SindelarovaHMeijerAJ 1997 The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 243 240 246
56. YamamotoATagawaYYoshimoriTMoriyamaYMasakiR 1998 Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23 33 42
57. TuckerSCCasadevallA 2002 Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci U S A 99 3165 3170
58. KnechtEAguadoCCarcelJEstebanIEsteveJM 2009 Intracellular protein degradation in mammalian cells: recent developments. Cell Mol Life Sci 66 2427 2443
59. BottsMRHullCM 2010 Dueling in the lung: how Cryptococcus spores race the host for survival. Curr Opin Microbiol 13 437 442
60. CasadevallA 2010 Cryptococci at the brain gate: break and enter or use a Trojan horse? J Clin Invest 120 1389 1392
61. CharlierCNielsenKDaouSBrigitteMChretienF 2009 Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun 77 120 127
62. ChamilosGLionakisMSLewisREKontoyiannisDP 2007 Role of mini-host models in the study of medically important fungi. Lancet Infect Dis 7 42 55
63. MylonakisECasadevallAAusubelFM 2007 Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog 3 e101
64. CasadevallA 2005 Host as the variable: model hosts approach the immunological asymptote. Infect Immun 73 3829 3832
65. KumaAHatanoMMatsuiMYamamotoANakayaH 2004 The role of autophagy during the early neonatal starvation period. Nature 432 1032 1036
66. KomatsuMWaguriSUenoTIwataJMurataS 2005 Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169 425 434
67. MizushimaNYamamotoAHatanoMKobayashiYKabeyaY 2001 Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol 152 657 668
68. SaitohTFujitaNHayashiTTakaharaKSatohT 2009 Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A 106 20842 20846
69. ReggioriFTuckerKAStromhaugPEKlionskyDJ 2004 The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev Cell 6 79 90
70. ObaraKSekitoTNiimiKOhsumiY 2008 The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J Biol Chem 283 23972 23980
71. NishidaYArakawaSFujitaniKYamaguchiHMizutaT 2009 Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461 654 658
72. ShimizuSKonishiANishidaYMizutaTNishinaH 2010 Involvement of JNK in the regulation of autophagic cell death. Oncogene 29 2070 2082
73. SchnaithAKashkarHLeggioSAAddicksKKronkeM 2007 Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J Biol Chem 282 2695 2706
74. SwansonMSIsbergRR 1995 Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun 63 3609 3620
75. RomanoPSGutierrezMGBeronWRabinovitchMColomboMI 2007 The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell Microbiol 9 891 909
76. WangYHWuJJLeiHY 2009 The autophagic induction in Helicobacter pylori-infected macrophage. Exp Biol Med (Maywood) 234 171 180
77. WangYHWuJJLeiHY 2009 When Helicobacter pylori invades and replicates in the cells. Autophagy 5 540 542
78. RomanoPSArboitMAVazquezCLColomboMI 2009 The autophagic pathway is a key component in the lysosomal dependent entry of Trypanosoma cruzi into the host cell. Autophagy 5 6 18
79. WangYWeissLMOrlofskyA 2009 Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth. J Biol Chem 284 1694 1701
80. OrvedahlAAlexanderDTalloczyZSunQWeiY 2007 HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1 23 35
81. OrvedahlALevineB 2008 Autophagy and viral neurovirulence. Cell Microbiol 10 1747 1756
82. AxeELWalkerSAManifavaMChandraPRoderickHL 2008 Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182 685 701
83. HuGHachamMWatermanSRPanepintoJShinS 2008 PI3K signaling of autophagy is required for starvation tolerance and virulenceof Cryptococcus neoformans. J Clin Invest 118 1186 1197
84. KronstadJWAttarianRCadieuxBChoiJD'SouzaCA 2011 Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol 9 193 203
85. ZaragozaOGarcia-RodasRNosanchukJDCuenca-EstrellaMRodriguez-TudelaJL 2010 Fungal cell gigantism during mammalian infection. PLoS Pathog 6 e1000945
86. FeldmesserMKressYCasadevallA 2001 Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 147 2355 2365
87. CruickshankJGCavillRJelbertM 1973 Cryptococcus neoformans of unusual morphology. Appl Microbiol 25 309 312
88. KechichianTBSheaJDel PoetaM 2007 Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun 75 4792 4798
89. MaHHagenFStekelDJJohnstonSASionovE 2009 The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci U S A 106 12980 12985
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous SystemsČlánek The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral ReleaseČlánek Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1Článek High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The N-Terminus of the RNA Polymerase from Infectious Pancreatic Necrosis Virus Is the Determinant of Genome Attachment
- Evolutionary Analysis of Inter-Farm Transmission Dynamics in a Highly Pathogenic Avian Influenza Epidemic
- Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous Systems
- Endemic Dengue Associated with the Co-Circulation of Multiple Viral Lineages and Localized Density-Dependent Transmission
- The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral Release
- The -glycan Glycoprotein Deglycosylation Complex (Gpd) from Deglycosylates Human IgG
- The Lipid Transfer Protein CERT Interacts with the Inclusion Protein IncD and Participates to ER- Inclusion Membrane Contact Sites
- Induction of Noxa-Mediated Apoptosis by Modified Vaccinia Virus Ankara Depends on Viral Recognition by Cytosolic Helicases, Leading to IRF-3/IFN-β-Dependent Induction of Pro-Apoptotic Noxa
- Environmental Constraints Guide Migration of Malaria Parasites during Transmission
- HIV-1 Efficient Entry in Inner Foreskin Is Mediated by Elevated CCL5/RANTES that Recruits T Cells and Fuels Conjugate Formation with Langerhans Cells
- Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis
- Highly Pathogenic Avian Influenza Virus H5N1 Infects Alveolar Macrophages without Virus Production or Excessive TNF-Alpha Induction
- Uses Host Triacylglycerol to Accumulate Lipid Droplets and Acquires a Dormancy-Like Phenotype in Lipid-Loaded Macrophages
- Compensatory T Cell Responses in IRG-Deficient Mice Prevent Sustained Infections
- Detection of Inferred CCR5- and CXCR4-Using HIV-1 Variants and Evolutionary Intermediates Using Ultra-Deep Pyrosequencing
- A Dynamic Landscape for Antibody Binding Modulates Antibody-Mediated Neutralization of West Nile Virus
- HSV-2 Infection of Dendritic Cells Amplifies a Highly Susceptible HIV-1 Cell Target
- “Rational Vaccine Design” for HIV Should Take into Account the Adaptive Potential of Polyreactive Antibodies
- Impact of Endofungal Bacteria on Infection Biology, Food Safety, and Drug Development
- Infection Reduces B Lymphopoiesis in Bone Marrow and Truncates Compensatory Splenic Lymphopoiesis through Transitional B-Cell Apoptosis
- The Intrinsic Antiviral Defense to Incoming HSV-1 Genomes Includes Specific DNA Repair Proteins and Is Counteracted by the Viral Protein ICP0
- Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph
- Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1
- A Freeze Frame View of Vesicular Stomatitis Virus Transcription Defines a Minimal Length of RNA for 5′ Processing
- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Tipping the Balance: Secreted Oxalic Acid Suppresses Host Defenses by Manipulating the Host Redox Environment
- Bacteria-Induced Dscam Isoforms of the Crustacean,
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Insertion of an Esterase Gene into a Specific Locust Pathogen () Enables It to Infect Caterpillars
- A Role for TLR4 in Infection and the Recognition of Surface Layer Proteins
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
- Low CCR7-Mediated Migration of Human Monocyte Derived Dendritic Cells in Response to Human Respiratory Syncytial Virus and Human Metapneumovirus
- HIV/SIV Infection Primes Monocytes and Dendritic Cells for Apoptosis
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- Productive Parvovirus B19 Infection of Primary Human Erythroid Progenitor Cells at Hypoxia Is Regulated by STAT5A and MEK Signaling but not HIFα
- Identification of DNA-Damage DNA-Binding Protein 1 as a Conditional Essential Factor for Cytomegalovirus Replication in Interferon-γ-Stimulated Cells
- The Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon at the Level of the MAVS Adaptor Protein
- Passively Administered Pooled Human Immunoglobulins Exert IL-10 Dependent Anti-Inflammatory Effects that Protect against Fatal HSV Encephalitis
- Infection of Induces Antifungal Immune Defenses
- Merozoite Invasion Is Inhibited by Antibodies that Target the PfRh2a and b Binding Domains
- Cyclic di-GMP is Essential for the Survival of the Lyme Disease Spirochete in Ticks
- Cross-Neutralizing Antibodies to Pandemic 2009 H1N1 and Recent Seasonal H1N1 Influenza A Strains Influenced by a Mutation in Hemagglutinin Subunit 2
- Spatial Dynamics of Human-Origin H1 Influenza A Virus in North American Swine
- Coronavirus Gene 7 Counteracts Host Defenses and Modulates Virus Virulence
- Clathrin Facilitates the Morphogenesis of Retrovirus Particles
- Contribution of Intrinsic Reactivity of the HIV-1 Envelope Glycoproteins to CD4-Independent Infection and Global Inhibitor Sensitivity
- Functional Analysis of Host Factors that Mediate the Intracellular Lifestyle of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



