-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIdentification of DNA-Damage DNA-Binding Protein 1 as a Conditional Essential Factor for Cytomegalovirus Replication in Interferon-γ-Stimulated Cells
The mouse cytomegaloviral (MCMV) protein pM27 represents an indispensable factor for viral fitness in vivo selectively, antagonizing signal transducer and activator of transcription 2 (STAT2)-mediated interferon signal transduction. We wished to explore by which molecular mechanism pM27 accomplishes this effect. We demonstrate that pM27 is essential and sufficient to curtail the protein half-life of STAT2 molecules. Pharmacologic inhibition of the proteasome restored STAT2 amounts, leading to poly-ubiquitin-conjugated STAT2 forms. PM27 was found in complexes with an essential host ubiquitin ligase complex adaptor protein, DNA-damage DNA-binding protein (DDB) 1. Truncation mutants of pM27 showed a strict correlation between DDB1 interaction and their ability to degrade STAT2. SiRNA-mediated knock-down of DDB1 restored STAT2 in the presence of pM27 and strongly impaired viral replication in interferon conditioned cells, thus phenocopying the growth attenuation of M27-deficient virus. In a constructive process, pM27 recruits DDB1 to exploit ubiquitin ligase complexes catalyzing the obstruction of the STAT2-dependent antiviral state of cells to permit viral replication.
Published in the journal: . PLoS Pathog 7(6): e32767. doi:10.1371/journal.ppat.1002069
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002069Summary
The mouse cytomegaloviral (MCMV) protein pM27 represents an indispensable factor for viral fitness in vivo selectively, antagonizing signal transducer and activator of transcription 2 (STAT2)-mediated interferon signal transduction. We wished to explore by which molecular mechanism pM27 accomplishes this effect. We demonstrate that pM27 is essential and sufficient to curtail the protein half-life of STAT2 molecules. Pharmacologic inhibition of the proteasome restored STAT2 amounts, leading to poly-ubiquitin-conjugated STAT2 forms. PM27 was found in complexes with an essential host ubiquitin ligase complex adaptor protein, DNA-damage DNA-binding protein (DDB) 1. Truncation mutants of pM27 showed a strict correlation between DDB1 interaction and their ability to degrade STAT2. SiRNA-mediated knock-down of DDB1 restored STAT2 in the presence of pM27 and strongly impaired viral replication in interferon conditioned cells, thus phenocopying the growth attenuation of M27-deficient virus. In a constructive process, pM27 recruits DDB1 to exploit ubiquitin ligase complexes catalyzing the obstruction of the STAT2-dependent antiviral state of cells to permit viral replication.
Introduction
Cytomegaloviruses (CMVs) constitute prototypical β-herpesviruses. 50–95% of the global adult population are infected lifelong with human CMV (HCMV). HCMV is a leading cause of disease burden of newborns in western countries due to transplacental transmission of the virus from the mother to the foetus during pregnancy [1]. HCMV infections can also cause life-threatening symptoms in immunocompromised individuals. As a result of an intimately shared evolutionary history with their hosts, CMVs are highly species-specific precluding in vivo analysis of HCMV in small animal models, hampering our understanding of HCMV pathogenesis. Infection of mice with mouse cytomegalovirus (MCMV) has been proven to be a suitable model to study CMV pathogenesis [2].
A coordinated response of interferons (IFNs) together with T - and NK-cells controls MCMV reactivation from latency in vivo [3]. Consistently, cells with deficiencies in either the IFN induction or the IFN signalling system show increased MCMV susceptibility [4]–[9] underscoring the indispensable role of both type I (IFN-α/β) as well as type II (IFN-γ) IFN for the control of CMV replication.
IFNs directly trigger immune responses by inducing antiviral effector mechanisms and indirectly by activating adaptive immune responses. Thereby, IFNs constitute a constant and selecting pressure for CMV, highlighted by the multitude of viral IFN antagonists [10]. IFNs elicit their antiviral activity by initiating specific transcriptional programs. Upon binding of type I IFNs to the cognate receptor, the Janus kinase (Jak)-signal transducer and activator of transcription (STAT) signalling cascade is activated. Jak1 and tyrosine kinase 2 initiate a phosphorylation cascade at the IFN receptor chain 2 and 1, respectively. The Janus kinases phosphorylate STAT1 and STAT2. Phosphorylated STATs dimerize due to a reciprocal SH2-phospho-Tyr-interaction. The STAT heterodimers, together with the IFN regulatory factor 9 (IRF-9), constitute the IFN stimulated gene factor 3 (ISGF3), which translocates to the nucleus, binds to IFN stimulated response elements (ISRE) of IFN-inducible genes (ISGs) and recruits the transcriptional machinery to express the respective gene.
We identified the protein pM27 as MCMV-encoded inhibitor of the Jak-STAT signalling cascade [11]. M27 is an early-late expressed gene essential for reducing STAT2 amounts upon MCMV infection. ΔM27-MCMV replication is attenuated upon IFN treatment in vitro, reproducing the observed attenuation in vivo [11], [12]. Interestingly, ΔM27-MCMV shows a remarkable growth reduction in IFN-γ-treated cells, revealing the importance of an IFNAR1-independent IFNGR1-initiated activation of STAT2 [11]. ΔM27-MCMV induces increased levels of ISGs [13] but does not induce more IFN-β mRNA [14], consistent with the notion that MCMV antagonizes IFN-β enhanceosome assembly M27-independently before an ISRE-dependent positive feed-back loop can be initiated [14].
The present study aimed to delineate the molecular mechanism and to identify host factors exploited by pM27. Here we report that pM27 exploits DNA-damage DNA-binding protein (DDB)1-dependent ubiquitin (Ub)-ligase complexes to catalyze ubiquitin-conjugation of STAT2. Ablation of host DDB1 phenocopied genetic deletion of M27 from the viral genome, demonstrating that viral fitness relies on the availability of a distinct host factor, DDB1.
Results
The pM27-dependent reduction of STAT2 occurs post-transcriptionally
Having demonstrated that pM27 is essential and sufficient to decrease STAT2 amounts and that both proteins co-precipitate [11], we intended to elucidate the mechanism of pM27. MCMV mutants expressing C-terminal HA-epitope tagged pM27 (M27-HA-MCMV) or pM28 (M28-HA-MCMV), the gene product of the M28 gene directly adjacent to M27 in the MCMV genome, were proven to be able to reduce STAT2, whereas ΔM27-MCMV and UV-inactivated virus did not decrease STAT2 amounts (Figure S1), indicating suitability of above mentioned mutants for further analysis. A quantitative experimental setup reveals a time-dependent decline of endogenous STAT2 amounts upon infection with wt-MCMV but not upon infection with ΔM27-MCMV until 24 h post infection (Figure S2). Pre-incubation with IFN-γ significantly increased levels of STAT2 but did not comprise pM27 function (Figure S3). During the early phase (24 h post infection) of MCMV replication pM27 seems to be the only MCMV-encoded protein significantly reducing STAT2 amounts (Figure S1, S2 and S5). Nevertheless, at late times of replication (≥48 h post infection) some STAT2 reduction was observed in ΔM27-MCMV infected cells, raising the possibility that additional MCMV gene products might affect STAT2 (Figure S3).
pM27 operates independent of other viral proteins since pM27-Flag expression from a recombinant vaccinia virus (VACV) vector (M27-Flag-VACV), but not wt-VACV or a control VACV, dose-dependently reduced the cellular STAT2 amount in mouse M2-10B4 cells and also in human HeLa (data not shown) and human MRC-5 cells (Figure 1A) indicating that co-factors of pM27 are evolutionary conserved (see below).
Fig. 1. pM27 is essential and sufficient to reduce the amount of human and mouse STAT2. 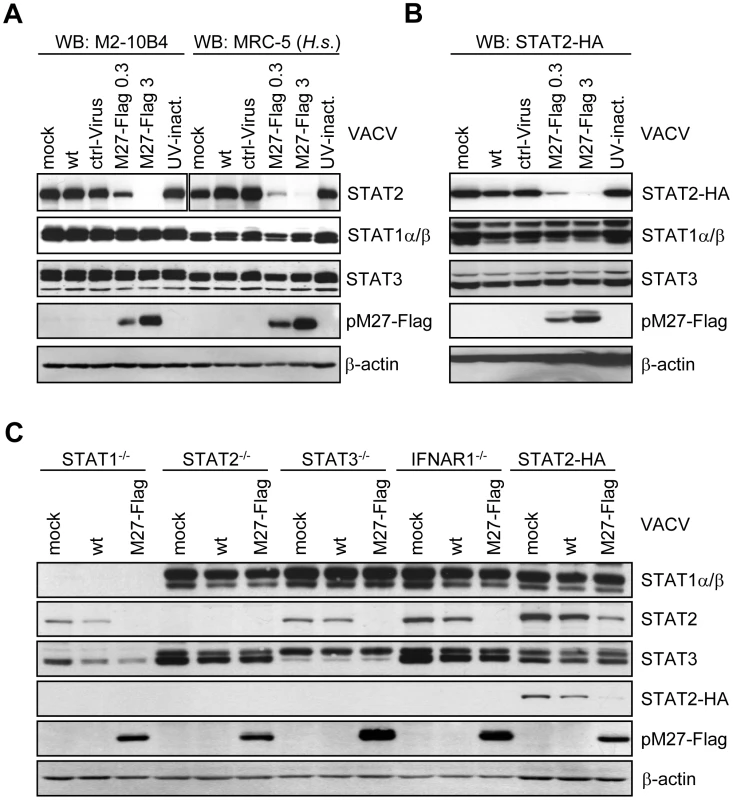
(A) Human MRC-5 and mouse M2-10B4 cells were infected (0.3 or 3 PFU/cell, 16 h) with indicated VACVs, lysed and subjected to western blotting. (B) STAT2-HA expressing cells were infected as in (B) for 24 h. Figure 1B is part of a larger experiment which is shown in Figure S6. (C) Cells of indicated genotype were infected (5 PFU/cell, 15 h) with wt-VACV or M27-Flag-VACV, lysed and analyzed by western blotting. Please note cross reactivity of the STAT3 antibody for STAT1. To elucidate the molecular mechanism of pM27, we first constructed an expression construct encompassing the coding sequence of the STAT2 gene devoid of the complete 3′-UTR driven by the constitutive active HCMV major IE promoter. Next, a stably transfected cell line ectopically expressing a C-terminal HA-epitope tagged version of STAT2 complementing STAT2-deficient mouse fibroblasts [15] was generated, designated STAT2-HA, which was permissive for MCMV. The transfectant produced HA-tagged STAT2 at high levels which could be detected either by STAT2 - or HA-specific antibodies and became tyr-phosphorylated upon type I IFN treatment, followed by translocation into the nucleus, formation of ISGF3 complexes and induction of IRF-1 expression (Figure S4), indicating a preserved responsiveness and signalling function of STAT2-HA. M27-HA-MCMV, but not ΔM27-MCMV, reduced the amount of STAT2 in STAT2-HA cells (Figure S5), indicating that neither the intrinsic STAT2 promoter nor the 3′-UTR are required for the observed reduction, in accordance with a post-transcriptional mechanism of STAT2 depletion. To confirm the reduction of STAT2-HA, STAT2-HA cells were infected with M27-Flag-VACV, resulting in a loss of STAT2-HA in a time - (Figure S6) and dose-dependent manner (Figure 1B), reproducing the data received with endogenous STAT2. Immunofluorescence staining revealed a decrease of STAT2 amounts upon transfection of M27 expression plasmids (Figure S7), formally ruling out an intracellular sequestration of STAT2 in detergent resistant compartments.
pM27 recognizes unphosphorylated and bona fide monomeric STAT2
VACV encodes a multitude of IFN antagonists [16] but does not reduce STAT2 amounts (Figure 1B) while interfering with STAT2 phosphorylation and activation [17]. The ability of VACV-expressed pM27-Flag to affect STAT2 thus suggested that STAT2 is recognized by pM27 in its unphosphorylated and bona fide monomeric state. To test this hypothesis further, STAT1-, STAT2-, STAT3 - and IFNAR1-deficient cells were infected with M27-Flag-VACV and the relative efficiency of pM27 to reduce the amounts of STAT2 was analyzed. M27-Flag-VACV, but not wt-VACV, induced the reduction of STAT2 in all cells (Figure 1C), indicating that pM27 can recognize non-phosphorylated STAT2 molecules, independent of their incorporation into ISGF3 complexes or previously described STAT3∶STAT2 heterodimers [18].
MCMV decreases cellular STAT2 amounts by reducing its protein half-life
To assess if pM27 affects the pre-existing STAT2 protein pool, STAT2 amounts were compared in presence and absence of pM27 upon administration of the protein synthesis inhibitor cycloheximide (CHX) and the transcription inhibitor actinomycin D (ActD). The pM27-dependent STAT2 reduction preceded the reduction upon blockade of de novo protein biosynthesis (Figure 2A). 5 h post MCMV infection STAT2 was hardly detectable whereas combined treatment with CHX and ActD did not significantly affect STAT2 - a finding which is consistent with the previously described long half-life of STAT2 [19]. Next, pulse-chase experiments were performed to compare the STAT2-HA half-life in mock-infected and M27-HA-MCMV-infected cells. Cells were labelled with 35S-L-Met/L-Cys and chased for the indicated time (Figure 2B) before the cells were lysed and STAT2-HA protein was precipitated. Upon infection with M27-expressing MCMV the half-life of STAT2-HA was strongly reduced when compared to mock-infected cells, which was not observed upon infection with ΔM27-MCMV either (Figure 2C). pM27 protein longevity lasted more than 9 h (Figure 2C). Altogether, the results demonstrated that STAT2 protein stability becomes strongly down-regulated by pM27. Interestingly, an additional long-lived ∼125 kDa protein emerged which was co-precipitated with pM27-HA irrespectively of STAT2 presence (Figure 2C).
Fig. 2. pM27 affects STAT2 protein levels. 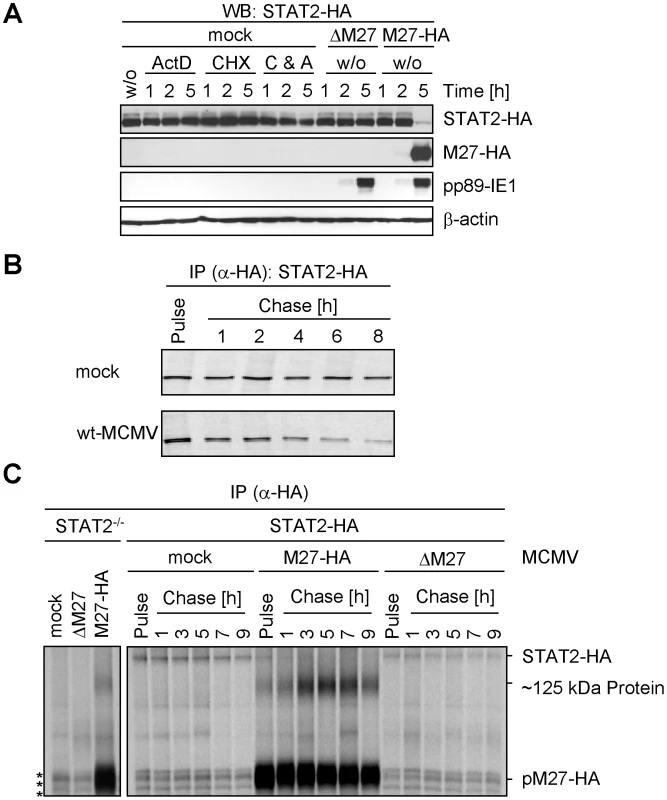
(A) STAT2-HA cells were infected with indicated MCMV mutants (30 PFU/cell). Cells were incubated with or without cycloheximide (100 µg/ml) and actinomycin D (5 µg/ml). After 1, 2 or 5 h cells were analyzed by western blotting. The inhibitors precluded expression of pp89-IE1 and pM27-HA indicating tight inhibition. (B) 35S-L-Cys/L-Met labelled STAT2-HA cells were infected (5 PFU/cell, 20 h). Cells were metabolically labelled (30 min) and subsequently chased for the indicated time. STAT2-HA was precipitated using an HA-specific antibody. Proteins were separated by 8% SDS-PAGE and visualized by autoradiography. (C) As in (B), but cells were infected (20 PFU/cell) for 90 min prior to the indicated pulse-chase regime. Asterisks indicate non-specifically precipitated proteins. pM27 induces STAT2 ubiquitination and degradation along the ubiquitin proteasome pathway
To investigate whether pM27 uses the Ub proteasome pathway, cells were treated with MG132, an inhibitor of the proteasome. STAT2-HA levels became largely restored and high-molecular weight forms of STAT2 accumulated in the presence of pM27 (Figure 3A). Exploiting the intrinsic host-shut-off mechanism of VACV, thereby blocking STAT2 neo-synthesis, we quantified STAT2-HA amounts upon infection with wt-VACV in comparison to M27-Flag-VACV in presence and absence of MG132. The STAT2-HA half-life was drastically reduced by pM27 but could be largely restored upon administration of MG132 (Figure S8). To confirm this phenotype for endogenous STAT2, NIH3T3 cells were infected with M27-Flag-VACV and treated with MG132. As shown in Figure S9, higher molecular weight forms of STAT2 could be detected by a STAT2-specific antibody. When the cells were infected with pM27-encoding VACV only for a short period precluding complete STAT2 degradation, a modification of STAT2-HA was observed in the presence of pM27-Flag and lactacystin, an inhibitor of the proteasome (Figure 3B). The modification was not seen upon expression of a non-functional truncation mutant, pM27 1–487, in untreated or in DMSO solvent-treated cells. The identity of STAT2 was further confirmed by comparison with STAT2−/− cells (Figure 3B).
Fig. 3. pM27 facilitates the proteasome to degrade STAT2. 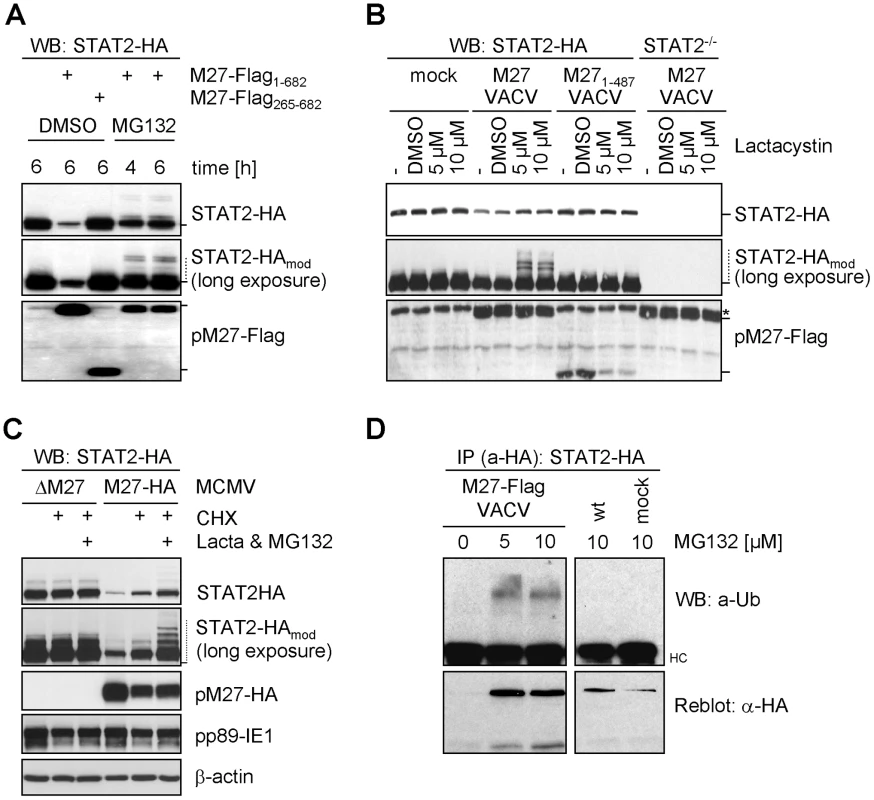
(A) STAT2-HA cells were infected with VACV (3 PFU/cell, 8 h) expressing full-length M27-Flag or a non-functional (M27-Flag265–682) truncation mutant and treated for 4 or 6 h with 10 µM MG132 or solvent DMSO and analyzed by western blotting. (B) STAT2-HA and STAT2−/− cells were infected (3 PFU/cell, 4 h) with VACV expressing pM27-Flag or a non-functional mutant (M27-Flag1–487), treated (4 h) with 5 or 10 µM lactacystin or DMSO and analyzed by western blotting. * indicates an unspecifically detected protein running close to pM27-Flag. (C) STAT2-HA cells were infected (20 PFU/cell, 2 h) with ΔM27-MCMV or M27-HA-MCMV and incubated (3 h) with CHX (50 µg/ml) alone or a combined treatment of CHX, lactacystin (10 µM) and MG132 (20 µM). (D) STAT2-HA cells were infected (10 PFU/cell) with M27-Flag-VACV or wt-VACV. 4 h post infection MG132 (10 µM) was added. 24 h post infection (M27-Flag-VACV) or 20 h post infection (wt-VACV) cells were lysed and STAT2-HA was precipitated by HA-specific antibody. Precipitated proteins were separated by SDS-PAGE and blotted. The membrane was probed with a Ub-specific and reprobed with an HA-specific antibody. It has been demonstrated that viral gene expression and genome replication of both CMV and VACV are blocked by inhibitors of the proteasome [20], [21]. To exclude that STAT2 restoration by proteasome inhibitors occurs indirectly due to reduced pM27-HA expression, CHX was co-administrated with MG132 and lactacystin to terminate protein synthesis. Under this regime pM27 amounts remain unchanged upon proteasome inhibition. Nevertheless, restoration and modification of STAT2 was still evident (Figure 3C), indicating that the proteolytic activity of the proteasome is directly required for pM27-induced STAT2 degradation.
To corroborate that the STAT2-modifying moiety is Ub, STAT2-HA cells were infected with M27-Flag-VACV before treatment with MG132. STAT2-HA was precipitated and analyzed using an Ub-specific antibody. As expected, MG132 treatment stabilized the otherwise degraded STAT2 in the presence of pM27-Flag (Figure 3D). Higher molecular weight forms of STAT2-HA were recognized by an Ub-specific antibody in the presence of pM27 and MG132. In conclusion, these results indicate that pM27 induces STAT2 ubiquitination targeting the protein for proteasomal degradation.
pM27 binds a cellular 125 kDa protein
As we did not detect sequences or motifs that are characteristical for Ub-ligases within M27 we surmised that pM27 serves an indirect function to shuttle STAT2 into the Ub-proteasome pathway. To identify potential interaction partners of pM27, a co-immunoprecipitation (IP) strategy was ensued. STAT2-HA cells were infected with M27-HA-MCMV and metabolically labelled. This allowed to follow up the fate of pM27-HA and STAT2-HA simultaneously. By comparing STAT2-HA transfectants with STAT2−/− cells, pM27-HA and STAT2-HA derived co-precipitations could be distinguished. Interestingly, antibodies recognizing pM27-HA specifically co-precipitated a ∼125 kDa protein reproducing the observation made before (compare Figure 4A with Figure 2C). The ∼125 kDa protein was visible after pM27-HA IP but not upon precipitation of pM28-HA (Figure 4B). The co-precipitated protein was also observed in NIH3T3 cells and could be freed by addition of an excess of uncoupled HA-peptides (Figure S10), confirming that it was recovered via an epitope-specific interaction of HA antibodies. Next, split IP samples were simultaneously analyzed by autoradiography upon metabolic 35S-Met/Cys-labeling and by anti-HA immunoblotting. The co-precipitated ∼125 kDa protein was visible in the autoradiography but remained undetectable in the immunoblot with HA antibodies (Figure 4B) indicating that it is not derived from pM27-HA.
Fig. 4. pM27 co-precipitates a ∼125 kDa protein. 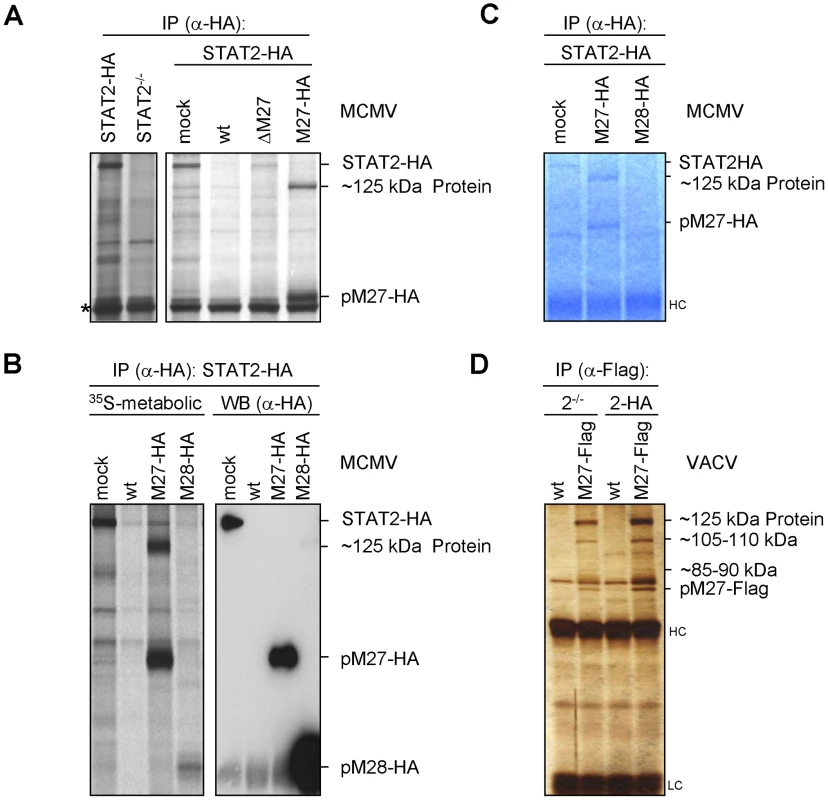
(A) STAT2-HA (2HA) and STAT2-deficient (2−/−) cells were infected (5 PFU/cell, 24 h) with wt-MCMV, ΔM27-MCMV, M27-HA-MCMV or left uninfected. Cells were starved (1 h) and then 35S-L-Met/L-Cys labelled (150 min). Lysates were subjected to IP using an HA-specific antibody. The asterisk indicates a non-specific protein. (B) As in (A), but cells were infected (10 PFU/cell, 48 h) with M27-HA-MCMV, M28-HA-MCMV, wt-MCMV or left uninfected. Lysates were split and analyzed by western blotting in parallel to autoradiography. pM28-HA forms multimers leading to the weak high-molecular weight signal in the rightmost lane. (C) As in (A), but cells were infected for 22 h. SDS-PAGE was followed by Coomassie-staining. (D) STAT2-HA or STAT2−/− cells were infected (5 PFU/cell, 20 h) with M27-Flag-VACV or wt-VACV, lysed and analyzed by HA-specific IP. Proteins were separated by SDS-PAGE and visualised by silver staining. Upon up-scaling and optimization the co-precipitated protein could be visualized by Coomassie staining of the gels (Figure 4C). The co-precipitating ∼125 kDa protein was also observed upon expression of pM27-Flag by a VACV (Figure 4D) confirming its interaction with pM27. Recovery of the ∼125 kDa protein was achieved in STAT2-HA and in STAT2−/− cells (Figure 4D), ruling out that the protein is STAT2, a degradation product of STAT2 or that STAT2 is required for its interaction with pM27. In summary, these experiments identified the ∼125 kDa protein as a novel cellular co-factor of pM27. Since pM27-Flag co-precipitated further proteins of various sizes (Figure 4D) pM27 was assumed to associate with a cellular multi-protein complex.
The ∼125 kDa interaction partner of pM27 is DDB1
The 125 kDa band was cleaved from a Coomassie-stained gel and analyzed by mass-spectrometry. Five peptides (YLAIAPPIIK, ALYYLQIHPQELR, VTLGTQPTVLR, IVVFQYSDGK and SVLLLAYKPMEGNFEEIAR) were found, all belonging to DDB1, a host 127 kDa protein, concordant with the size of the pM27 co-precipitated material. Two further replications of DDB1-pM27-co-precipitations and subsequent mass-spectrometry analysis reached a peptide coverage rate of 24.8% and 30.2% of the ∼127 kDa full length protein, respectively, unequivocally defining DDB1 as pM27-interacting protein. DDB1 is an adapter protein for the cellular Cul4A-RocA E3-Ub-ligase complex, previously shown to be an interaction partner for paramyxoviral IFN antagonists targeting STAT molecules for proteasomal degradation [22]–[25]. In cells, DDB1 fulfils a function as component of a multimeric ubiquitin-ligase complex involved in nucleotide excision repair and induces ubiquitination of the licensing factor Cdt1 upon UV irradiation [26]. Next, the pM27-DDB1 association was confirmed by immunoblotting with a DDB1-specific antibody upon pM27 immunoprecipitation (Figure 5A). Moreover, STAT2 was not required for the binding of DDB1 by pM27HA (Figure 5A). As expected, DDB1 was not retrieved upon anti-HA IP from cells infected with wt-MCMV lacking the HA epitope fused to the M27 sequence (Figure 5B). Conversely, DDB1 co-immunoprecipitation was seen with antibodies recognizing pM27-Flag expressed by VACV, irrespective of the presence of STAT2 and in the presence of MG132, confirming that the interaction occurs independently of the epitope tag and the activity of the proteasome (Figure 5C). The retrieval of pM27-HA-DDB1 complexes was pM27-dose-dependent (Figure S11) and resistant to calf intestine phosphatase (CIAP), the phosphatase inhibitor NaF, the detergent CHAPS and tolerated more than 500 mM NaCl and up to 5 mM EDTA (data not shown), reflecting a strong protein-protein interaction.
Fig. 5. pM27 co-precipitates with DDB1. 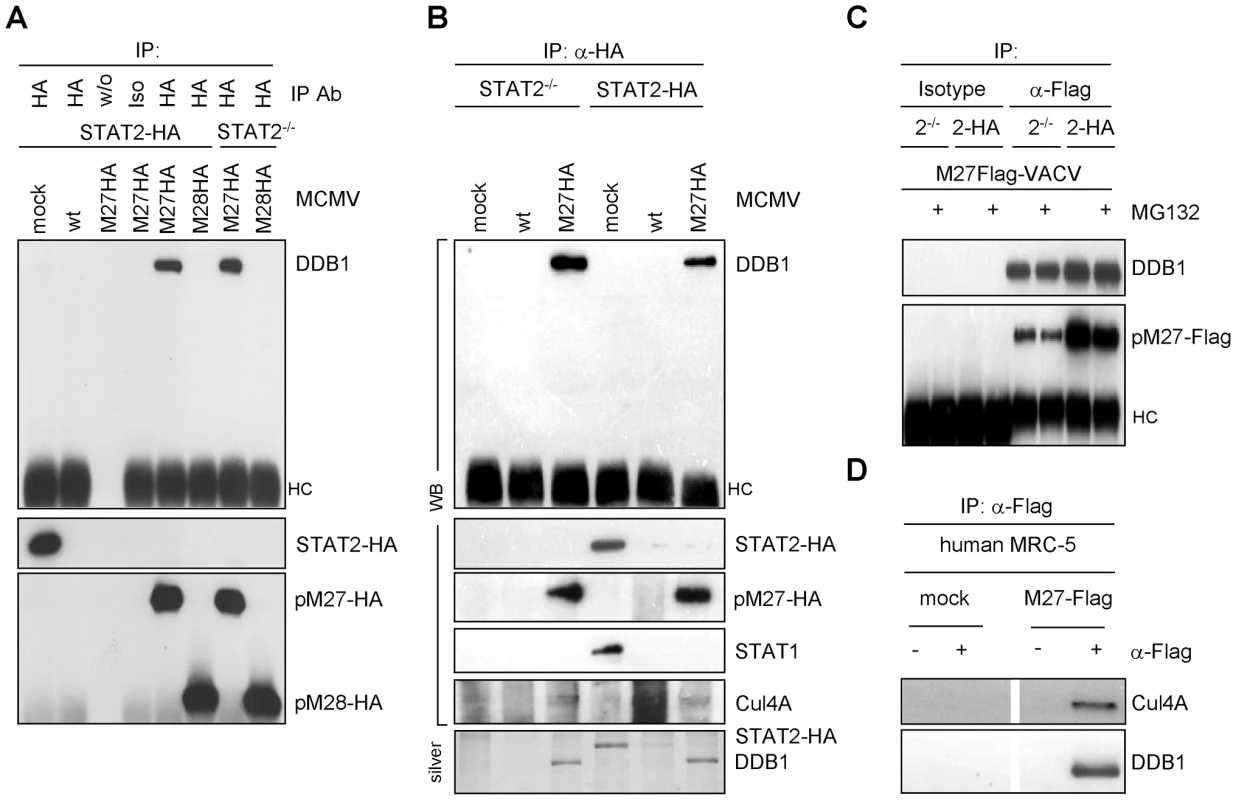
(A) STAT2-HA and STAT2−/− cells were infected (5 PFU/cell, 24 h) with indicated MCMV-mutants (wt-MCMV, M27-HA-MCMV or M28-HA-MCMV), lysed and subjected to IP using HA-specific antibody, an isotype or no antibody. Precipitated proteins were analyzed by western blotting. (B) Infection was done as in (A). Cells were lysed and used for HA-specific IP. Precipitated proteins were analyzed by western blotting and by silver staining. (C) STAT2-HA and STAT2−/− cells were infected (5 PFU/cell, 18 h) with M27-Flag-VACV, incubated with MG132 (10 µM, for the last 4 h), lysed and subjected to IP using a Flag-specific antibody or an isotype antibody. Precipitated proteins were detected by western blotting. (D) MRC-5 cells were infected (5 PFU/cell, 18 h) with M27-Flag-VACV, lysed and analyzed by IP with Flag-specific antibodies. The immune complexes were separated by SDS-PAGE and analyzed by western blotting. DDB1 is involved in UV-induced DNA damage responses, and the UV-DDB complex consists of the two separate proteins DDB1-p127 and DDB2-p48 [27]. Hamster cells induce significantly less DNA-binding UV-DDB complexes due to the complete absence of DDB2 [28]. When pM27 was expressed in Chinese hamster ovary (CHO) cells, DDB1 was readily retrieved by co-immunoprecipitation of pM27-HA but not pM28-HA (Figure S12), suggesting that the interaction of the proteins can occur independently of DDB2.
In addition to DDB1 further pM27 co-precipitated proteins were noticed (Figure 4D). Since DDB1 acts as an adapter protein for the Cul4A-RocA complex, we next analysed the co-precipitation of pM27 with the scaffold protein Cul4A which recruits the catalytic RING-finger-containing Ub-ligase RocA. A pM27-Cul4A co-precipitation was weakly visible in mouse cells by immunoprecipitation, presumably due to a poor reactivity of Cul4A antibodies to mouse Cul4A. We therefore expressed pM27 in human cells resulting in a complete STAT2 down-regulation (Figure 1A), reproducing co-precipitation of Cul4A with pM27 and DDB1 (Figure 5D). We concluded that pM27 co-precipitates DDB1 and Cul4A irrespective of the presence of STAT2 or DDB2.
Mutants of pM27-Flag define the minimal functional and the DDB1 binding domain
To define the essential domain for the interaction of pM27 with DDB1 a panel of Flag epitope tagged truncation mutants of pM27 expressing VACVs was constructed. As depicted in Figure 6A only the truncation of the first N-terminal 68 amino acids and the last C-terminal 30 amino acids were fully dispensable for the ability of pM27 to induce STAT2 degradation. All functional pM27-Flag mutants able to induce STAT2 degradation invariably co-precipitated DDB1 (Figure 6B), revealing a correlation between STAT2 degradation and their DDB1 binding capacity. Mutants lacking the N - or C-terminal 195 amino acids showed a reduced but still detectable binding to DDB1 without being able to degrade STAT2 (Figure 6B). From the fact that neither the first 195 N-terminal nor the last 195 amino acids were essential for DDB1 precipitation we conclude that the minimal DDB1-co-precipitating sequence lies within aa195–487 of pM27. To corroborate this finding, we constructed a mutant lacking the N - as well as the C-terminus (pM27-Flag-(Δ5-118)-651). This mutant was still capable to co-precipitate DDB1 upon transient transfection (data not shown) and upon expression from a recombinant VACV (Figure 6C).
Fig. 6. Truncation analysis of pM27-Flag indicates correlation between DDB1 binding and STAT2 degradation. 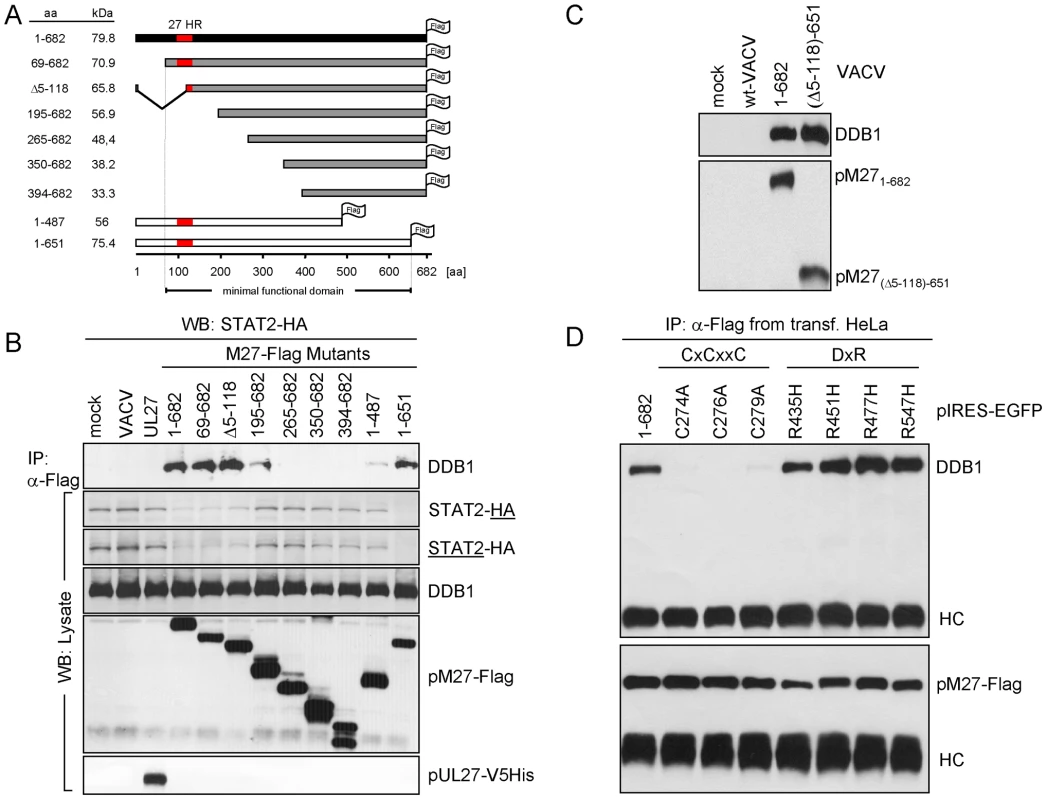
(A) The schema depicts the generated M27 truncation mutants, all constructs are Flag-epitope tagged and expressed by recombinant VACVs. ‘27HR’ indicates a conserved domain shared between homologous proteins of related cytomegaloviruses. (B) STAT2-HA cells were infected with the indicated pM27-expressing VACV (16 h; 5 PFU/cell). Proteins from cell lysates were immunoprecipitated using a Flag-specific antibody (upper panel). The precipitated proteins were detected by western blotting. A part of the lysate was acetone precipitated and used to analyse the overall protein amounts (lower panel). (C) Cells were infected with wt-VACV or a VACV expressing a pM27-Flag protein, lacking aa 5–118 at the N-terminus and 652–682 at the C-terminus. After precipitation with α-Flag antibody, proteins were analyzed by western blotting. (D) pIRES-EGFP plasmids expressing the indicated pM27-Flag mutants were transiently transfected into human HeLa cells and subjected to α-Flag immunoprecipitation and subsequent western blotting using DDB1-specific and Flag-specific antibodies. Notably, within this minimal functional domain DxR motifs and a conserved CxCxxC motif are present (Figure S13): Binding partners of DDB1 have the consensus motif WDxR, or less frequently YDxR [29], [30]. PM27 contains a WD dipeptide and four DxR sequences, one of them forming the sequence YDxR (aa544–aa547). We therefore decided to mutate these motifs. Based on the well-described abrogation of DDB1-binding due to a single mutation (R273H) in the DxR motif of DDB2, found in individuals with xeroderma pigmentosum group E ([31]), we mutated the arginine (R) to histidine (H). All four mutant proteins were fully functional in terms of DDB1 co-precipitation (Figure 6D) and in terms of STAT2-degradation (exemplarily shown for R435H –Figure S14) indicating functional redundancy of these sites or that pM27 exhibits an unusual DDB1 interaction.
SV-5 protein, a paramyxoviral DDB1-binding protein, contains two zinc binding pockets critically required for DDB binding [32], one of which with the sequence CxCxxC (aa206–211) [33]. Remarkably, a CxCxxC motif is also present in pM27 (aa274–279) raising the question if pM27 is also a Zn2+-binding protein. Intriguingly, the CxCxxC motif is conserved throughout cytomegalovirus evolution in M27 homologs with the exception of HCMV and CCMV (Figure S13). We therefore mutated individual cysteins to alanine. All three mutant proteins were impaired in their capacity to co-precipitate DDB1 (Figure 6D) upon transient transfection into HeLa cells and upon expression by recombinant VACVs (Figure S14). Consistent with the hypothesis of DDB1 requirement for pM27-mediated STAT2 degradation, the C279A mutant shows a diminished STAT2 degradation potential (Figure S14).
pM27 but not its homolog pUL27 binds human DDB1
Like MCMV, HCMV induces a down-regulation of STAT2 in infected cells, which is sensitive to inhibitors of the proteasome. This effect occurs independent of pUL27, the HCMV homolog of pM27 [34]. Consistently, pUL27 expression by VACV neither degraded STAT2-HA nor was sufficient to co-precipitate DDB1 (Figure 6B and Figure S15). In contrast, pM27 readily co-precipitated DDB1 in human cells (Figure 5D), consistent with the high degree of sequence conservation of DDB1 and the functional competence of pM27 in human cells. From this comparative analysis between HCMV and MCMV we conclude that despite the phenotypical match of STAT2 degradation via the ubiquitin-proteasome pathway the genetic and molecular basis between both viruses is remarkably different.
Knock-down of DDB1 partially restores the STAT2 amount
Recently, a floxed DDB1 allele has been cloned and recombined into the DDB1 gene locus in mice. Global Cre-mediated DDB1 excision results in embryonic lethality [35]. Additionally, conditional DDB1 gene knock-down causes a severe growth defect and apoptosis in the chicken DT40 B cell line [36]. This approach prompted us to carefully ablate DDB1 synthesis by siRNA to analyze the functional relevance of DDB1 for the pM27-dependent down-regulation of STAT2. Transfection of DDB1-specific siRNAs induced a continuous reduction of DDB1 protein amounts (Figure S16). To exclude that siRNA transfection influences the levels of STAT2 due to type I IFN induction, we performed the experiment in IFNAR1-deficient fibroblasts. As expected, infection with M27-HA-MCMV, but not ΔM27-MCMV, induced STAT2 degradation in cells treated with control siRNA. Conversely, siRNA-mediated knock-down of pM27 restored STAT2 (Figure 7A, lanes 6 & 12). Likewise, DDB1 ablation fully restored STAT2 amounts 4 h post infection (lane 4) and partially after 24 h (lane 10). Consistent results were obtained upon pM27 expression from VACV (data not shown). These findings establish that DDB1 is a prerequisite to execute effective STAT2 proteolysis by pM27.
Fig. 7. siRNA mediated knock-down of DDB1 restores STAT2 amount and phenocopies M27-deficiency. 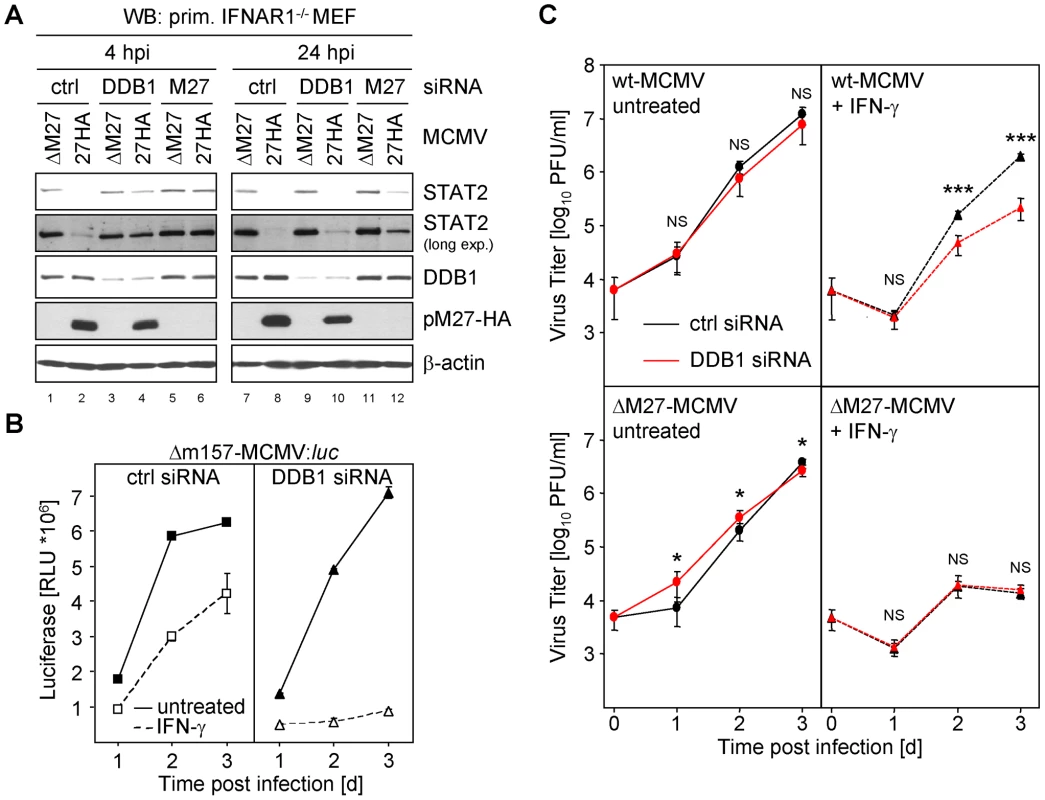
(A) Primary IFNAR1-deficient MEF cells (passage 3) were transfected with 200 nM DDB1-siRNA, M27-siRNA and an irrelevant siRNA, respectively. 24 h later cells were infected for 4 or 24 h with 10 PFU/cell M27-HA-MCMV or ΔM27-MCMV. Cells were lysed and subjected to western blotting. (B) Primary MEFs were transfected as in (B), infected (0.1 PFU/cell, 48 h) with Δm157-MCMV:luc and luciferase activity was measured. (C) As in (C) but cells were infected with wt-MCMV or ΔM27-MCMV and infectious virus progeny was determined by plaque titration. siRNA-mediated knock-down of DDB1 phenocopies M27-deficiency
M27-positive MCMVs antagonize the induction of IFN-γ-stimulated, STAT2-containing, ISRE-DNA-binding complexes (Figure S17). Consistently, replication of the ΔM27-MCMV mutant is characterized by its enormous susceptibility towards IFN-γ in vitro and in vivo [11]. To test whether DDB1 is relevant for this effect, we transfected MEF with DDB1-specific - (or control-) siRNAs 48 h prior to infection before the cells were incubated with IFN-γ24 h prior to infection. The MCMV infection was performed with a luciferase expressing mutant, Δm157-MCMV:luciferase in which the coding sequence of m157 has been replaced by the luciferase gene, and cells were harvested 1, 2 and 3 days post infection. Luciferase activity paralleled the kinetics of MCMV replication. Accordingly, luciferase activity was inhibited upon IFN-γ pretreatment of MEF (Figure 7B, left panel). While DDB1 knock-down precluded viral luciferase expression by the M27-positive Δm157-MCMV:luciferase mutant in IFN-γ preincubated cells, luciferase production was unaffected in cells which were not IFN-treated (Figure 7B). The DDB1 knock-down and reduced viral gene expression was confirmed by western blot analysis of cell lysates using DDB1 - and pp89-IE1-specific antibodies (data not shown). The experiment was repeated with wt-MCMV and the progeny virus yield was quantified by standard plaque titration. IFN-γ pre-treatment of cells, which had been treated with DDB1-specific siRNA, strongly impaired MCMV growth (Figure 7C). In clear contrast, the replication of ΔM27-MCMV was highly susceptible to IFN-γ and was not further impaired by ablation of DDB1 by siRNA (Figure 7C, lower panel). Altogether, these data indicate that DDB1 by itself is not required for MCMV replication. However, the virus requires DDB1 to overcome the STAT2-dependent antiviral capacity of IFN-γvia pM27. The phenocopy of host DDB1 depletion and viral M27-deletion provides complementary evidence for a model in which DDB1 is indispensable for pM27 subversion of the antiviral IFN-γ response.
Discussion
In this study we have elucidated the mechanism of the cytomegaloviral IFN antagonist pM27. This viral protein is essential and sufficient for the MCMV-encoded inhibition of the IFN signalling cascade by binding and degrading STAT2, a transcription factor which becomes activated in the type I and type II IFN receptor signal transduction. Replication of ΔM27-MCMV is highly attenuated in IFN-treated cells and in infected mice [11], [12] indicating that STAT2 initiates an efficient antiviral effector program unless its degradation is accomplished. pM27 is demonstrated to degrade STAT2 via the ubiquitin proteasome pathway by binding to DDB1 (Figure 8: Model of pM27 function). This host factor is per se not required for MCMV replication, but becomes conditional essential in the presence of IFN-γ. From these findings we infer a crucial role of DDB1 for MCMV replication in vivo.
Fig. 8. Current model of pM27 function. 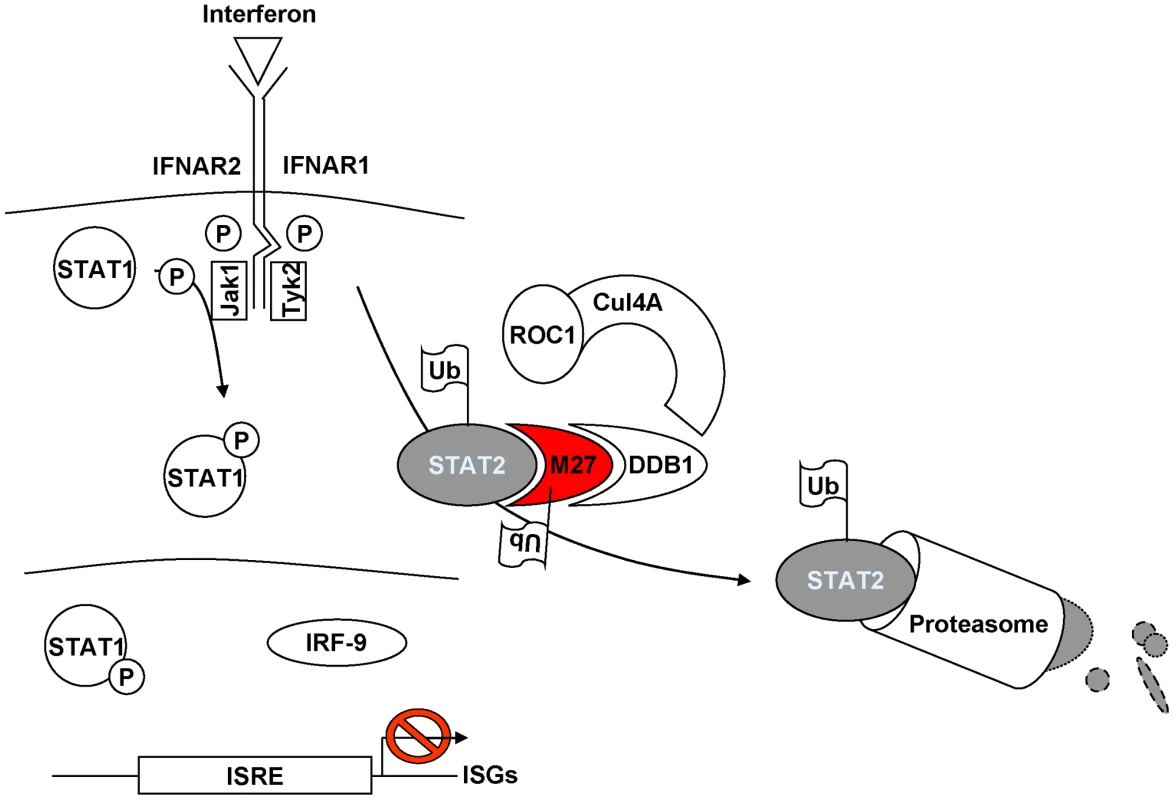
pM27 bridges STAT2 to DDB1-containing ubiquitin-ligase complexes thereby inducing poly-ubiquitination and subsequent proteasomal degradation of STAT2 to antagonize induction of interferon stimulated genes. Implications of DDB1 as a cofactor for viral replication
Recently, genome-wide siRNA-based large-scale screening approaches have been conducted to uncover host factors required for replication of certain viruses including HIV and influenza [37]–[39], representing new potential targets for antiviral therapy. Despite the fact that relevant factors were successfully identified, these attempts suffer from two common shortcomings. First, the implicit counter-selection against siRNAs which are detrimental for cell survival, i.e. a screening bias against ‘essential’ host proteins. It is tempting to speculate that those ‘essential’ proteins are exactly the host factors many viruses favour as interaction partners due to their evolutionary conservation and the inability of the host to mutate or delete the responsible genes. We feel that our results exemplify the fundamental need to pursue ‘top-down’ approaches to refine biological observation (e.g. the growth attenuation of ΔM27-MCMV upon conditioning with IFN-γ) allowing the characterization of underlying molecular mechanisms and finally the identification of (conditional) essential host factors. We were surprised to see that viral infection (presumably due to control over cell cycle progression and apoptosis), increased the ability of cells to resist knock-down of DDB1, raising the apparent question whether it might be reasonable to conduct above mentioned siRNA screens without any previous negative pre-selection. Second, our study documents that distinct host factors are not constitutively essential but become essential under certain conditions defined by the host cell environment, e.g. the IFN-induced antiviral state. It is well possible that only the simulation of conditions which are closer to infected and inflamed organs leads to additional induced essential host factors important for viral replication because they escaped the screening performed under standard cell culture conditions.
pM27 interaction with STAT2
pM27 has adopted a remarkable substrate specificity to capture its cellular target, monomeric STAT2 [11]. Several findings are fully in accord with the notion that the down-regulation of STAT2 is achieved via the ubiquitin-proteasome pathway: i) pM27 affected the half-life of STAT2, ii) STAT2 reduction was sensitive to proteasome inhibitors, iii) in the presence of proteasome inhibitors pM27 generated higher molecular weight forms of STAT2, and iv) the modification of STAT2 was shown to be conjugated Ub. Given the long protein half-life of STAT2 catalyzing its proteolytic destruction represents a direct and immediate mechanism to shut off its antiviral function. The recognition and binding of STAT2 requires a large and central domain of the pM27 protein as revealed by probing of a set of truncation mutants.
pM27 interaction with DDB1
Co-IP studies revealed the prominent binding quality of pM27 to a second host protein which was identified to be DDB1. Forming an adaptor protein of the Cul4A-RocA Ub-ligase complex, the linkage of pM27 with DDB1 generated the hypothesis that pM27 delivers STAT2 to proteasomal destruction via this factor. Two findings support the notion that DDB1 is indeed required for the loss of STAT2 in MCMV-infected cells: i) truncation mutants of pM27 induced the break-down of STAT2 only when their binding to DDB1 was fully intact; ii) siRNA-mediated knock-down of DDB-1 protected STAT2 from degradation.
Binding partners of DDB1 have the consensus motifs WDxR, or less frequently YDxR [29], [30]. pM27 contains a WD dipeptide and four DxR sequences, one of them forming the sequence YDxR (aa544–aa547). Nevertheless, single R>H mutations of the DxR motifs did not impair DDB1 co-precipitation. This might either indicate functional redundancy or that pM27 exhibits an unconventional DDB1-binding mode.
Based on experimental data obtained in the fission yeast (Schizosaccharomyces pombe), a so called DDB1-box has been defined to be present in DDB1 binding partners like WDR21 and comprising a RQLG-like motif surrounded by hydrophobic amino acids in positions −7 to −3 and +7 or +9 [40]. PM27 bears two non-identical motifs, which resemble this DDB-box within aa232–256 and aa358–377, overlapping with the domain that is required for degradation of STAT2. Future analysis will define further essential amino acids which are critical for DDB1-pM27-complex formation and might delineate the molecular requirements for recruitment and exploitation of DDB1-Cul4A-RocA complexes.
The finding that the CxCxxC motif is important for DDB1 co-precipitation suggests that pM27 harbours a coordinative Zn2+ binding pocket. Interestingly, this domain is conserved in different cytomegaloviruses (Figure S13), raising the apparent question whether the basic function of the pM27 homologs, proteasomal degradation, might also be conserved.
pM27 – not just a paramyxoviral analogue device
At the first glimpse pM27 seems to imitate paramyxoviral SV-5 V-proteins which recruit DDB1 and induce proteasomal degradation of STAT proteins. Neither pM27 nor SV-5 V-protein contain a fully conserved WDxR motif. Besides the CxCxxC motif, pM27 and the SV-5 V-protein are considerably different with regard to structure, function and substrate recognition and they do not share homologous amino acid stretches. V-proteins discriminate between human and mouse STATs and require the presence of both STAT1 and STAT2 to induce the degradation of the other [41], [42], whereas pM27 induces the selective degradation of human and mouse STAT2 as a monomer. Several biological observations further imply differences in their molecular functions. pM27 does not affect the induction of type I IFN [14] contrasting with V-proteins [43], [44]. Stable expression of pM27 was not possible (M. Trilling, unpublished observation) but was readily achieved for SV-5 V-protein [45], suggesting a different mode of interaction with DDB1 which is essential for cell survival [36]. MCMV can arrest the cell cycle of infected fibroblasts both in G1 and in G2 [46]. Since DDB1 is required especially for proliferating cells [35], an attractive hypothesis would be that MCMV can afford a blockade of DDB1 functions due to its ability to arrest the cell cycle prior to the DDB1-sensitive checkpoint. In line with this hypothesis, DDB1 knock-down did not abrogate MCMV replication in MEF by itself, but became strongly antiviral if cells were pretreated with IFN-γ. Given that DDB1 is expressed ubiquitously in all mouse tissues [35] the conditional exploitation of DDB1 by a proviral protein like pM27 appears to be a perfect strategy which combines the need for a broad cell tropism to establish ‘replication factories’ in a large variety of tissues with the defence against the permanent encounter of omnipresent IFN-γ which is produced in response to the herpesviral life style bringing sustained immune exposure.
Material and Methods
Cells and cytokines
MRC-5 (ATCC CCL-171), M2-10B4 (ATCC CRL-1972), immortal STAT2−/− - [15] and STAT1−/− mouse fibroblasts [47], crisis immortalized IFNAR1-deficient (generated from primary IFNAR1-deficient MEF [11]) and primary MEF (prepared as described [48]) were grown in Dulbecco's modified eagle medium (D-MEM) with 10% foetal bovine serum, streptomycin, penicillin and 2 mM glutamine. NIH3T3 cells were grown in 10% newborn calf serum. STAT2-HA cells were generated from STAT2−/− cells [15]. STAT2-HA [11] was subcloned into a pcDNA3.1 (Invitrogen)-derived pcDNA3.1-zeocin expression vector. Cell lines were selected under 200 µg/ml zeocin (Invitrogen). IFN-γ (#12500-1) was purchased from PBL Biomedical Laboratories, New Jersey, USA. Inhibitors of the proteasome (MG132 and lactacystin) were purchased from Boston Biochemicals, USA.
Viruses and plasmids
swt-like MCMV MW97.01, ΔM27-MCMV, M27-HA-MCMV, M27-Flag-VACV and STAT2-HA-VACV have been described [11]. M28-HA-MCMV was generated by amplifying a frt-site flanked kanar-cassette using primers containing M28-homologous sequences prolonged by an HA-epitope encoding sequence (underlined): AZ-M28-HA1: TGCGGGCTCCGTCCGGGATAGCCGAGACCTGCGTGCCCACGCTCGGGTACCCATACGATGTTCCAGATTACGCGTGACCAGTGAATTCGAGCTCGGTAC and AZ-M28-2: AGGCGAGGCGAAACTGGCGGGATAACTGCAAGAGAGGGGAAAAGCGGTCGATCCCAGCCGGACCATGATTACGCCAAGCTCC using pFRT1 as template. The PCR fragment was introduced into the MCMV-BAC by homologous recombination in E.coli. The kanar-cassette was excised from the BAC by FLP-mediated recombination. m157 was deleted accordingly by zeocin selection after replacement of the m157 coding sequence against a zeor-cassette by homologous recombination between the MCMV-BAC and a PCR amplificate generated with the primers: AZ-m157-1-CAGGAGAATCTGAACCCCGATATTTGAGAAAGTGTACCCC GATATTCAGTACCTCTTGAC CCAGTGAATTCGAGCTCGGTAC and AZ-m157-2-AGATCGTGACCATTATCACCAAGATAGTTCCCACCATAATTCCCATCGTCACTAGAGTCGGACCATGATTACGCCAAGCTCC and pFRT-Zeo as template. Afterwards zeor was replaced by the luciferase gene (derived from pTA-luc [Clontech]) by homologous recombination between the Δm157-MCMV:zeor BAC and a vector, harbouring a luciferase gene flanked by 800 nts of the MCMV genome, surrounding the m157 coding sequence. BAC-derived MCMV mutants were reconstituted in primary MEFs und correct mutagenesis was confirmed by restriction fragment pattern analysis and PCR (data not shown).
Truncation mutagenesis of pM27-Flag-VACV was performed based on the described VACV expression plasmid p7.5k131-M27-Flag. The C-terminal sequence of the M27 ORF was amplified with the Az-M27-m1_forw: 5′-CAGAAGATCGGCACGAAGTACC-3′ primer with either the MF-M27-m2_rev: 5′-CGCGCGACTAGTCTCGTTGTCGTCGTCCTCGTAG-3′ or - MF-M27-m4_rev: 5′-CGCGCGACTAGTGGAGCCCGACGAATCCTTGTC-3′. Amplificates were cleaved by BamHI and SpeI (underlined, primer intrinsic site) and cloned into p7.5k-M27Fl/SphI vector between an N-terminal fragment of M27 and an in-frame C-terminal Flag-epitope.
For N-terminal truncations, M27-intrinsic restriction sites (ApaI, SacII, PvuI, NcoI, BamHI and MscI) were used together with a vector intrinsic BglII site. After re-ligation the next ATG in frame served as start codon. The pM27-Flag-(Δ5-118)-651 mutant was constructed by replacing the C-terminal part of the Δ5-118 ‘SacII’ mutant with the truncated C-terminal sequence using an internal BamHI site. VACV mutants were selected with BrdU in tk−143 cells.
Site-directed mutagenesis of pM27-Flag was performed using the Quick Change kit (Stratagene) according to the instructions of the manufacturer using the following primers and its respective reverse complementary primers: KL-C274A: 5′-catctacgatcaactcGCGtactgtcgcgagtgtc-3′, KL-C276A: 5′-cgatcaactctgttacGCGcgcgagtgtcggatgc-3′, KL-C279A: 5′-gttactgtcgcgagGCGcggatgcgccgggg-3′, KL-R435H: 5′-gcgacgtcgacgccCACatccgcgcgggagc-3′, KL-R451H: 5′-gtcgcctccgaccccCACcaggacggcatctcg-3′, KL-R477H: 5′-caccttctcggacgagCACcccgacggctacgagg-3′ and KL-R547H: 5′-gaggatgtacgacgagCACccgctggccggcttc-3′. Mutations were confirmed by sequencing.
UV inactivation of viruses (MCMV and VACV) was done by exposing viruses for 25 min to UV light (254 nm) from a light source 10 cm afar.
Western blotting
Cells were lysed in RIPA+-buffer (50 mM Tris-HCl, 150 mM NaCl, 1% [vol/vol] IGEPAL, 1% Na-Deoxycholate [vol/vol], 0.1% [weight/vol] SDS, 1 mM DTT, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/ml leupeptin, 1 µg/ml pepstatin, 50 mM NaF, 0.1 mM Na-vanadate with Complete protease inhibitors (Roche) pH 7.5). Samples were normalized according to Bradford protein staining and equal amounts were subjected to denaturing SDS-PAGE. Gels were blotted on nitrocellulose membranes (Schleicher and Schuell) and probed with indicated antibodies. The same membrane was used and consecutively stripped with reblot solution (Calbiochem). The following commercially available antibodies were used: α-β-actin,α-Flag M-2 and α-HA from Sigma-Aldrich; α-IRF-9, α-STAT1, α-mSTAT2, α-STAT3 from Santa Cruz; α-Cul4A (Acris), α-DDB1 (Bethyl), α-pp89-IE1 (Croma101, kindly provided by Stipan Jonjić, Rijeka, Croatia), α-hSTAT2 (Upstate) and α-Ub (Dako).
Immunoprecipitation
Immunopreciptation was done as described. Briefly, cells were lysed (lysisbuffer: 0.1 mM EDTA; 200 mM NaCl; 10 mM KCl; 10 mM MgCl2; 10% [vol/vol] glycerol; 20 mM HEPES [pH 7,4]; 0.5% [vol/vol] IGEPAL; 0.1 mM PMSF; 1 mM DTT; 0.4 mM pepstatin A; 0.1 mM Na-vanadate; Complete protease inhibitor (Roche)). Lysates were spun (30 min at 4°C and 16000 g) and IP antibody was added to the supernatant. Immune complexes were precipitated with Protein-G-Sepharose (Amersham). The pellet was washed by 6–10 consecutive rounds with lysis buffer.
For metabolic labelling and pulse-chase experiments cells were starved (30 min) in L-Met-/L-Cys-free media and subsequently pulsed (90 min) with ∼10 MBq/∼106 cells EasyTag Express 35S protein labelling mix (PerkinElmer). After the pulse cells were washed 3 times with chase media (10%-FBS D-MEM supplemented with 1.5 mg/ml L-Met/L-Cys) and chased as indicated. Immune complexes were separated by SDS-PAGE. Gels were either stained by silver - or Coomassie-staining or fixed, dried and visualized by autoradiography.
siRNA Transfection
2.5–7.5 * 104 primary MEF cells were transfected with siRNA using RNAiMax transfection reagent (Invitrogen) following manufacturers instructions. The siRNAs were purchased from IBA. The following siRNAs were used for the knockdown: DDB1 (5′-[PO4] r(AACCUGUUGAUUGCCAAAAACTT)-3′), luc-siRNA (5′-[PO4] r(CUUACGCUGAGUACUUCGATT)-3′) and M27 (5′-[PO4] r(CAAUAAGCCCUUUAAUCAC)dTdT-3′).
Supporting Information
Zdroje
1. LudwigAHengelH 2009 Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro Surveill 14 26 32
2. MocarskiESShenkTPassRF 2007 Cytomegaloviruses. KnipeDKHowleyPM Field's Virology Philadelphia Lippincott, Williams & Wilkins 2701 2772
3. PolicBHengelHKrmpoticATrgovcichJPavicI 1998 Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med 188 1047 1054
4. CrozatKGeorgelPRutschmannSMannNDuX 2006 Analysis of the MCMV resistome by ENU mutagenesis. Mamm Genome 17 398 406
5. GilMPBohnEO'GuinAKRamanaCVLevineB 2001 Biologic consequences of Stat1-independent IFN signaling. Proc Natl Acad Sci U S A 98 6680 6685
6. PrestiRMPollockJLDal CantoAJO'GuinAKVirginHW 1998 Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med 188 577 588
7. PrestiRMPopkinDLConnickMPaetzoldSVirginHW 2001 Novel cell type-specific antiviral mechanism of interferon gamma action in macrophages. J Exp Med 193 483 496
8. StroblBBubicIBrunsUSteinbornRLajkoR 2005 Novel functions of tyrosine kinase 2 in the antiviral defense against murine cytomegalovirus. J Immunol 175 4000 4008
9. TabetaKGeorgelPJanssenEDuXHoebeK 2004 Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A 101 3516 3521
10. HengelHKoszinowskiUHConzelmannKK 2005 Viruses know it all: new insights into IFN networks. Trends Immunol 26 396 401
11. ZimmermannATrillingMWagnerMWilbornMBubicI 2005 A cytomegaloviral protein reveals a dual role for STAT2 in IFN-{gamma} signaling and antiviral responses. J Exp Med 201 1543 1553
12. AbenesGLeeMHaghjooETongTZhanX 2001 Murine cytomegalovirus open reading frame M27 plays an important role in growth and virulence in mice. J Virol 75 1697 1707
13. KhanSZimmermannABaslerMGroettrupMHengelH 2004 A cytomegalovirus inhibitor of gamma interferon signaling controls immunoproteasome induction. J Virol 78 1831 1842
14. LeVTTrillingMZimmermannAHengelH 2008 Mouse cytomegalovirus inhibits beta interferon (IFN-beta) gene expression and controls activation pathways of the IFN-beta enhanceosome. J Gen Virol 89 1131 1141
15. ParkCLiSChaESchindlerC 2000 Immune response in Stat2 knockout mice. Immunity 13 795 804
16. HagaIRBowieAG 2005 Evasion of innate immunity by vaccinia virus. Parasitology 130 S11 S25
17. TrillingMLeVTZimmermannALudwigHPfefferK 2009 Gamma interferon-induced interferon regulatory factor 1-dependent antiviral response inhibits vaccinia virus replication in mouse but not human fibroblasts. J Virol 83 3684 3695
18. StancatoLFDavidMCarter-SuCLarnerACPrattWB 1996 Preassociation of STAT1 with STAT2 and STAT3 in separate signalling complexes prior to cytokine stimulation. J Biol Chem 271 4134 4137
19. LeeCKBluyssenHALevyDE 1997 Regulation of interferon-alpha responsiveness by the duration of Janus kinase activity. J Biol Chem 272 21872 21877
20. KaspariMTavalaiNStammingerTZimmermannASchilfR 2008 Proteasome inhibitor MG132 blocks viral DNA replication and assembly of human cytomegalovirus. FEBS Lett 582 666 672
21. SatheshkumarPSAntonLCSanzPMossB 2009 Inhibition of the ubiquitin-proteasome system prevents vaccinia virus DNA replication and expression of intermediate and late genes. J Virol 83 2469 2479
22. AndrejevaJPooleEYoungDFGoodbournSRandallRE 2002 The p127 subunit (DDB1) of the UV-DNA damage repair binding protein is essential for the targeted degradation of STAT1 by the V protein of the paramyxovirus simian virus 5. J Virol 76 11379 11386
23. PreciousBChildsKFitzpatrick-SwallowVGoodbournSRandallRE 2005 Simian virus 5 V protein acts as an adaptor, linking DDB1 to STAT2, to facilitate the ubiquitination of STAT1. J Virol 79 13434 13441
24. UlaneCMHorvathCM 2002 Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology 304 160 166
25. UlaneCMRodriguezJJParisienJPHorvathCM 2003 STAT3 ubiquitylation and degradation by mumps virus suppress cytokine and oncogene signaling. J Virol 77 6385 6393
26. HuJMcCallCMOhtaTXiongY 2004 Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat Cell Biol 6 1003 1009
27. LiuWNicholsAFGrahamJADualanRAbbasA 2000 Nuclear transport of human DDB protein induced by ultraviolet light. J Biol Chem 275 21429 21434
28. HwangBJToeringSFranckeUChuG 1998 p48 Activates a UV-damaged-DNA binding factor and is defective in xeroderma pigmentosum group E cells that lack binding activity. Mol Cell Biol 18 4391 4399
29. AngersSLiTYiXMacCossMJMoonRT 2006 Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature 443 590 593
30. JinJAriasEEChenJHarperJWWalterJC 2006 A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell 23 709 721
31. NicholsAFItohTGrahamJALiuWYamaizumiM 2000 Human damage-specific DNA-binding protein p48. Characterization of XPE mutations and regulation following UV irradiation. J Biol Chem 275 21422 21428
32. LinGYPatersonRGRichardsonCDLambRA 1998 The V protein of the paramyxovirus SV5 interacts with damage-specific DNA binding protein. Virology 249 189 200
33. LiTChenXGarbuttKCZhouPZhengN 2006 Structure of DDB1 in complex with a paramyxovirus V protein: viral hijack of a propeller cluster in ubiquitin ligase. Cell 124 105 117
34. LeVTTrillingMWilbornMHengelHZimmermannA 2008 Human cytomegalovirus interferes with signal transducer and activator of transcription (STAT) 2 protein stability and tyrosine phosphorylation. J Gen Virol 89 2416 2426
35. CangYZhangJNicholasSABastienJLiB 2006 Deletion of DDB1 in mouse brain and lens leads to p53-dependent elimination of proliferating cells. Cell 127 929 940
36. WakasugiMMatsuuraKNagasawaAFuDShimizuH 2007 DDB1 gene disruption causes a severe growth defect and apoptosis in chicken DT40 cells. Biochem Biophys Res Commun 364 771 777
37. KarlasAMachuyNShinYPleissnerKPArtariniA 2010 Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature 463 818 822
38. KonigRZhouYEllederDDiamondTLBonamyGM 2008 Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell 135 49 60
39. KonigRStertzSZhouYInoueAHoffmannHH 2010 Human host factors required for influenza virus replication. Nature 463 813 817
40. FukumotoYDohmaeNHanaokaF 2008 Schizosaccharomyces pombe Ddb1 recruits substrate-specific adaptor proteins through a novel protein motif, the DDB-box. Mol Cell Biol 28 6746 6756
41. ParisienJPLauJFHorvathCM 2002 STAT2 acts as a host range determinant for species-specific paramyxovirus interferon antagonism and simian virus 5 replication. J Virol 76 6435 6441
42. ParisienJPLauJFRodriguezJJUlaneCMHorvathCM 2002 Selective STAT protein degradation induced by paramyxoviruses requires both STAT1 and STAT2 but is independent of alpha/beta interferon signal transduction. J Virol 76 4190 4198
43. ChildsKSAndrejevaJRandallREGoodbournS 2009 Mechanism of mda-5 Inhibition by paramyxovirus V proteins. J Virol 83 1465 1473
44. PooleEHeBLambRARandallREGoodbournS 2002 The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 303 33 46
45. AndrejevaJYoungDFGoodbournSRandallRE 2002 Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J Virol 76 2159 2167
46. WiebuschLNeuwirthAGrabenhenrichLVoigtSHagemeierC 2008 Cell cycle-independent expression of immediate-early gene 3 results in G1 and G2 arrest in murine cytomegalovirus-infected cells. J Virol 82 10188 10198
47. DurbinJEHackenmillerRSimonMCLevyDE 1996 Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84 443 450
48. BruneWHengelHKoszinowskiUH 2001 A mouse model for cytomegalovirus infection. Curr Protoc Immunol Chapter 19 Unit
49. NavarroLMowenKRodemsSWeaverBReichN 1998 Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol Cell Biol 18 3796 3802
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous SystemsČlánek The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral ReleaseČlánek Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1Článek High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The N-Terminus of the RNA Polymerase from Infectious Pancreatic Necrosis Virus Is the Determinant of Genome Attachment
- Evolutionary Analysis of Inter-Farm Transmission Dynamics in a Highly Pathogenic Avian Influenza Epidemic
- Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous Systems
- Endemic Dengue Associated with the Co-Circulation of Multiple Viral Lineages and Localized Density-Dependent Transmission
- The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral Release
- The -glycan Glycoprotein Deglycosylation Complex (Gpd) from Deglycosylates Human IgG
- The Lipid Transfer Protein CERT Interacts with the Inclusion Protein IncD and Participates to ER- Inclusion Membrane Contact Sites
- Induction of Noxa-Mediated Apoptosis by Modified Vaccinia Virus Ankara Depends on Viral Recognition by Cytosolic Helicases, Leading to IRF-3/IFN-β-Dependent Induction of Pro-Apoptotic Noxa
- Environmental Constraints Guide Migration of Malaria Parasites during Transmission
- HIV-1 Efficient Entry in Inner Foreskin Is Mediated by Elevated CCL5/RANTES that Recruits T Cells and Fuels Conjugate Formation with Langerhans Cells
- Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis
- Highly Pathogenic Avian Influenza Virus H5N1 Infects Alveolar Macrophages without Virus Production or Excessive TNF-Alpha Induction
- Uses Host Triacylglycerol to Accumulate Lipid Droplets and Acquires a Dormancy-Like Phenotype in Lipid-Loaded Macrophages
- Compensatory T Cell Responses in IRG-Deficient Mice Prevent Sustained Infections
- Detection of Inferred CCR5- and CXCR4-Using HIV-1 Variants and Evolutionary Intermediates Using Ultra-Deep Pyrosequencing
- A Dynamic Landscape for Antibody Binding Modulates Antibody-Mediated Neutralization of West Nile Virus
- HSV-2 Infection of Dendritic Cells Amplifies a Highly Susceptible HIV-1 Cell Target
- “Rational Vaccine Design” for HIV Should Take into Account the Adaptive Potential of Polyreactive Antibodies
- Impact of Endofungal Bacteria on Infection Biology, Food Safety, and Drug Development
- Infection Reduces B Lymphopoiesis in Bone Marrow and Truncates Compensatory Splenic Lymphopoiesis through Transitional B-Cell Apoptosis
- The Intrinsic Antiviral Defense to Incoming HSV-1 Genomes Includes Specific DNA Repair Proteins and Is Counteracted by the Viral Protein ICP0
- Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph
- Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1
- A Freeze Frame View of Vesicular Stomatitis Virus Transcription Defines a Minimal Length of RNA for 5′ Processing
- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Tipping the Balance: Secreted Oxalic Acid Suppresses Host Defenses by Manipulating the Host Redox Environment
- Bacteria-Induced Dscam Isoforms of the Crustacean,
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Insertion of an Esterase Gene into a Specific Locust Pathogen () Enables It to Infect Caterpillars
- A Role for TLR4 in Infection and the Recognition of Surface Layer Proteins
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
- Low CCR7-Mediated Migration of Human Monocyte Derived Dendritic Cells in Response to Human Respiratory Syncytial Virus and Human Metapneumovirus
- HIV/SIV Infection Primes Monocytes and Dendritic Cells for Apoptosis
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- Productive Parvovirus B19 Infection of Primary Human Erythroid Progenitor Cells at Hypoxia Is Regulated by STAT5A and MEK Signaling but not HIFα
- Identification of DNA-Damage DNA-Binding Protein 1 as a Conditional Essential Factor for Cytomegalovirus Replication in Interferon-γ-Stimulated Cells
- The Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon at the Level of the MAVS Adaptor Protein
- Passively Administered Pooled Human Immunoglobulins Exert IL-10 Dependent Anti-Inflammatory Effects that Protect against Fatal HSV Encephalitis
- Infection of Induces Antifungal Immune Defenses
- Merozoite Invasion Is Inhibited by Antibodies that Target the PfRh2a and b Binding Domains
- Cyclic di-GMP is Essential for the Survival of the Lyme Disease Spirochete in Ticks
- Cross-Neutralizing Antibodies to Pandemic 2009 H1N1 and Recent Seasonal H1N1 Influenza A Strains Influenced by a Mutation in Hemagglutinin Subunit 2
- Spatial Dynamics of Human-Origin H1 Influenza A Virus in North American Swine
- Coronavirus Gene 7 Counteracts Host Defenses and Modulates Virus Virulence
- Clathrin Facilitates the Morphogenesis of Retrovirus Particles
- Contribution of Intrinsic Reactivity of the HIV-1 Envelope Glycoproteins to CD4-Independent Infection and Global Inhibitor Sensitivity
- Functional Analysis of Host Factors that Mediate the Intracellular Lifestyle of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



