-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaConflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
article has not abstract
Published in the journal: . PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1003910
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003910Summary
article has not abstract
Introduction
Strife concerning the accessibility of essential trace elements, such as transition metals, represents an important aspect of the dynamic interaction between a pathogenic fungus and its mammalian host. The host defends itself against infection by sequestering these essential micronutrients away from the invading pathogen via a phenomenon termed “nutritional immunity” [1]. In turn, the fungus employs an array of tactics (scavenging and storage) to hoard micronutrients and support growth when these resources are scarce. In addition, micronutrient limitation triggers the expression of virulence determinants that can aggravate disease [2]–[4].
Half of all enzymes and a third of all proteins require metals for their functionality [5], yet many transition metals are insoluble and potentially toxic at neutral pH and in an oxygenated, aqueous milieu. For example, iron and copper are redox active and capable of generating free radicals via Fenton chemistry under these conditions [6]. Furthermore, toxic excess of a non-native metal can displace the native metals from metalloproteins or inhibit the function of non-metalloproteins. Therefore, the levels of iron, copper, and zinc and their partitioning within the body must be tightly regulated to maintain cellular homeostasis whilst avoiding cellular damage.
In the mammalian host, iron is the most abundant transition metal (3–4 g/adult human), followed by zinc (1.4–2.3 g/adult human), with copper trailing behind (∼100 mg/adult human) [7]. These metals have roles in signalling, immune responses, physiology, and development [6], and both metal deficiency and overload are detrimental to the host. Iron deficiency, the most common dietary deficiency in the world, weakens the immune system, whilst copper deficiency renders the host more susceptible to infection [5], [8]. On the other hand, iron overload is a significant risk factor for some fungal diseases, especially for mucormycosis [9]. Furthermore, individuals with acute myeloid leukemia, characterised by increased serum iron, have a higher risk of Candida and Aspergillus infections [10]. Iron overload also exacerbates meningoencephalitis in a mouse model of cryptococcosis [11], [12]. Conversely, fungal pathogens depend on effective metal acquisition mechanisms for full virulence [3], [13]–[16].
The Many Faces of Nutritional Immunity
The mammalian host exploits both “constitutive” and “inducible” nutritional immunity to sequester transition metals away from microbial pathogens. The host regulates its interchangeable metal pools by sequestering metals via protein carriers or by partitioning them between intracellular stores [1], [9]. This essentially imposes constitutive nutritional immunity by depleting the extracellular milieu of essential metals and creating a micronutrient-limiting environment for the pathogen. For example, the estimated serum concentration of free ferric iron is 10−24 M, many orders of magnitude lower than that expected based on its solubility (10−9 M) [17]. Likewise, the intracellular “free pool” of copper is less than one copper atom per cell [18]. Pathological disruption of this constitutively low metal ion environment is one factor that predisposes patients to fungal infection [10], [18].
Upon infection, the host imposes “inducible” nutritional immunity by readjusting its global micronutrient homeostasis to further limit microbial access to endogenous metal ion pools. This response is achieved both on systemic and local levels. Systemic metal readjustments are observed, such as the hypoferraemia associated with disseminated infections. In this case, regulation is achieved via the secreted hepatic hormone hepcidin, which itself is responsive to various regulators, such as IL-6 [19]. Indeed, disseminated C. albicans infections are accompanied by increases in hepcidin and decreases in the degree of iron saturation of serum transferrin [20]. At the local level, inducible mechanisms include the secretion by macrophages and neutrophils of siderocalins (such as lipocalin-2), which bind siderophores [1] (vide infra). The neutrophil copper-binding protein calgranulin C might also contribute to nutritional immunity [21].
Most data on nutritional immunity relate to bacterial infections. However, there is evidence for localised, induced nutritional immunity during systemic fungal disease. During disseminated candidiasis, infiltrating neutrophils appear to seal off the developing fungal lesions from the rest of the tissue to create a metal-deprived environment [22]. Macrophages parasitized by Histoplasma capsulatum are reprogrammed to exclude zinc and, probably, iron [23]. Neutrophil extracellular traps (NETs), which can control fungal growth via either cidal (C. albicans, Cryptococcus neoformans) or static mechanisms (Aspergillus fumigatus), are decorated with proteins such as the zinc-scavenging calprotectin [24]. Furthermore, the release of the polymorphonuclear leukocyte protein lactoferrin at sites of infection inhibits the growth of C. albicans and A. fumigatus, presumably by inducing localized iron deprivation [25].
Metal deprivation is not the only means by which the host manipulates micronutrient levels to contain an infection. A contrasting mechanism, involving the “poisoning” of microbes with excess metal ions, is gaining attention in the literature [16]. An elegant example is the case of copper poisoning during Cr. neoformans infection, where copper redistribution in alveolar macrophages is countered by expression of fungal metallothioneins sequestering the toxic excess of metal [16].
Overcoming Host Nutritional Defences—Many Tricks up the Fungal Sleeve
Fungi exploit many mechanisms to scavenge metal ions from different substrates, adjusting their metal acquisition machinery as required [26]. The molecular mechanics that underlie metal sensing remain unclear [5], and the regulatory networks that control metal acquisition systems are complex, responding not just to micronutrient availability but also to environmental cues such as carbon source, hypoxia, and pH. These are reviewed elsewhere (e.g., [2], [3], [14]). Here, we summarise the fungal micronutrient acquisition pathways themselves and discuss how they respond to nutritional immunity.
Multiple acquisition pathways
Low and high affinity fungal transporters have been characterised for copper and zinc, but little is known about the acquisition of these metals in vivo. Notable exceptions include the Pra1/Zrt1-based zinc uptake system in C. albicans [27] and copper acquisition by Cr. neoformans via CTR1 and CTR4 [16], [28]. In contrast, iron acquisition is relatively well researched.
Complex iron homeostasis within the host involves a plethora of storage proteins (e.g., ferritin), carrier proteins (e.g., transferrin, lactoferrin), and the machinery for senescent red blood cell recycling [6]. The continual transfer of iron between various body compartments provides potential targets for fungal interference. To access iron, fungi employ: (1) a reductive iron acquisition (RIA) pathway, comprising a permease and a multicopper oxidase for high-affinity ferric import; (2) siderophore transporters for endogenous or exogenous siderophores; (3) secreted and cell surface reductants; and (4) the endocytic pathway for haem internalisation, in conjunction with intracellular haem oxygenase-1 (Hmx1) for haem iron extraction (Figure 1). Not all of these pathways are present in all fungi. For example, A. fumigatus mainly exploits endogenous siderophores for iron scavenging and also possesses the RIA system (FtrA/FetC), but only the former pathway is required for virulence [29]. C. albicans utilizes RIA (Ftr1/Fet3 and additional Ftr1/ferroxidase-homologue combinations), xenosiderophore transport (Sit1 transporter), and haem acquisition (Rbt5 and other receptors) [30]. Cr. neoformans relies on RIA (Cft1/Cfo1), a xenosiderophore transporter (Sit1), secreted and extracellular reductants (3-hydroxyanthranilic acid, melanin), and haem acquisition (Cig1) [11]. H. capsulatum employs RIA (Ftr1/Fet3), produces and internalises siderophores, secretes gamma-glutamyltransferase and glutathione-dependent ferric reductase, and utilizes haem (Figure 1) [15], [21].
Fig. 1. Fungal iron scavenging competes with mammalian nutritional immunity. 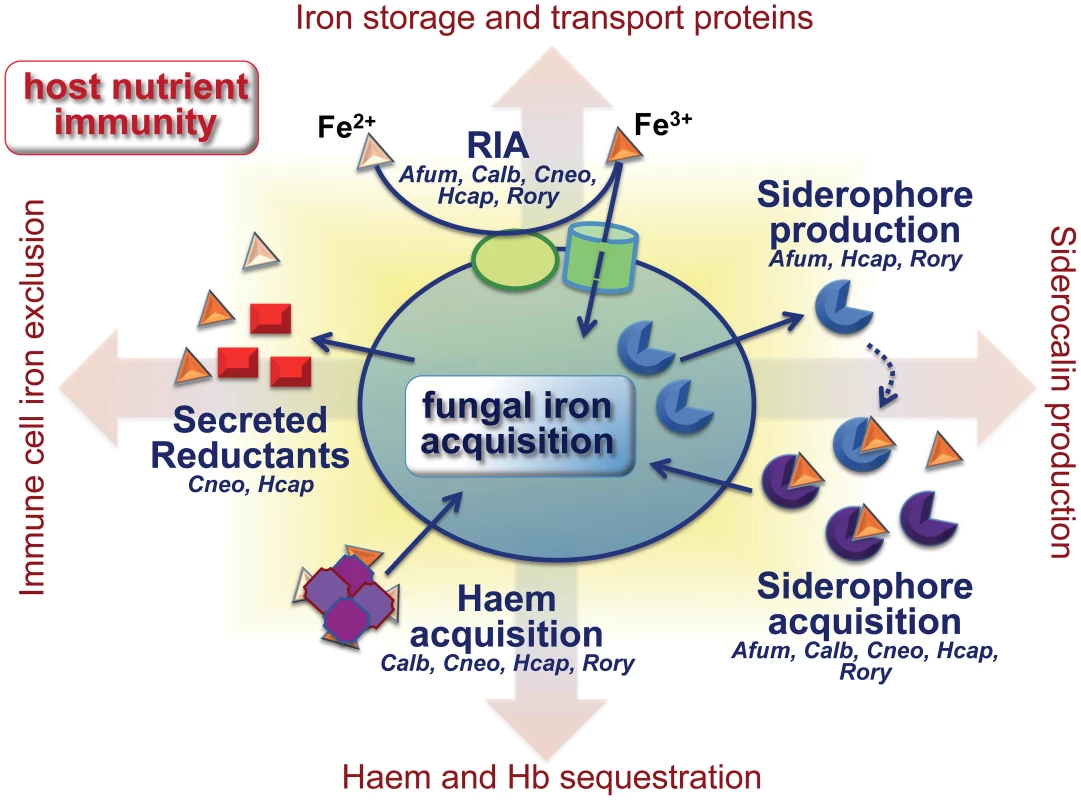
Pathogenic fungi employ different combinations of evolutionarily related strategies to acquire iron in the limiting microenvironments of the host. The figure focuses on those fungal species for which data are currently available. See text for details. Hb: haemoglobin, RIA: reductive iron acquisition, Afum: A. fumigatus, Calb: C. albicans, Cneo: Cr. neoformans, Hcap: H. capsulatum, Rory: R. oryzae. Certain substrates require specialised surface receptors. For example, C. albicans uses the protein Als3 for ferritin iron and the Rbt5 receptor (and other CFEM-type receptors) for haemoglobin and haem [30]. Als3 also acts as an adhesin and invasin. It is expressed during hyphal development, promoting the adhesion and host endocytosis of C. albicans cells. This suggests interesting interconnections between micronutrient availability and acquisition and other types of fungus–host interaction during disease progression. Haem is also readily utilised by Cr. neoformans via an uptake pathway that involves the secreted haemophore Cig1 [31]. In Rhizopus oryzae, the ferric reductase Ftr1 has been implicated in haem iron acquisition [32]. No specific uptake systems for haem, ferritin, or transferrin have been described for A. fumigatus, although, like H. capsulatum, this fungus uses secreted siderophores to chelate ferric iron from transferrin [15], [33].
The various iron acquisition mechanisms seem to become prominent at different stages of infection. Accordingly, C. albicans requires Sit1 for epithelial escape, RIA for the establishment of disseminated bloodstream infections, and Hmx1 for sustaining deep-seated organ infections [13], [30]. In contrast, H. capsulatum requires RIA during early infection stages and siderophore biosynthesis later in the infection process [34].
Scavenging
Siderophores are small organic molecules with ferric iron binding affinities of ∼1030 M at neutral pH. Other than the carboxylate siderophore rhizoferrin found in zygomycetes (R. oryzae), all other known fungal siderophores are hydroxamates [21]. In H. capsulatum and A. fumigatus, the SID1/SIDA gene is required for the first dedicated step in siderophore biosynthesis. This gene plays a role in the later stages of H. capsulatum infection and is required for A. fumigatus virulence [15], [29], [34]. Furthermore, A. fumigatus produces secreted siderophores, as well as intracellular siderophores for hyphal and conidial iron storage [14]. Although C. albicans and Cr. neoformans do not produce their own siderophores, they can exploit hydroxamate-type xenosiderophores produced by other microorganisms [11], [30].
It is important to emphasise that iron is not the only transition metal limiting to the fungus within the host environment. This is evident from the discovery that fungi also take advantage of induced secretory mechanisms to obtain non-ferric metal ions. For example, C. albicans possesses a dedicated zinc scavenging system, comprised of the secreted Pra1 “zincophore” and the Zrt1 transporter [27]. Additionally, methanobactin-like compounds, i.e., secreted scavengers of copper, have been discovered in Saccharomyces cerevisiae [35], [36]. However, the prevalence of such molecules among fungal pathogens of mammals remains unknown.
Storage and conservation
In fungi, the intracellular storage of transition metals is not limited to vacuoles [7]. A. fumigatus produces intracellular siderophores for hyphal and conidial iron storage, and both intra - and extracellular siderophores are required for full virulence [14]. Ferritin-like genes have been identified in A. fumigatus and R. oryzae, but not in basidiomycetous genomes [26]. Copper hyperaccumulation by Cr. neoformans suggests the existence of copper storage mechanisms in this pathogen, which might promote fungal survival inside the macrophages (see above) [16], [37].
When micronutrients are scarce, some fungi fine-tune their metabolism towards micronutrient conservation. For example, Cr. gattii and C. albicans respond to zinc restriction by down-regulating zinc-dependent alcohol dehydrogenases to increase zinc availability for other metalloproteins [3], [38]. Similarly, in A. fumigatus, C. albicans, and Cr. neoformans, iron-conserving mechanisms operate via the transcription factor HapX to down-regulate genes in iron-consuming pathways, such as amino acid metabolism, tricarboxylic acid cycle, respiration, and haem biosynthesis [26].
Autophagy, a major pathway for the bulk degradation of cytosolic components during nutrient starvation, might represent a non-conventional mechanism for recycling and conserving essential micronutrients to sustain hyphal growth in A. fumigatus, although its role in vivo is unverified. Other fungi, such as C. albicans, also undergo autophagy, but unlike in A. fumigatus, this phenomenon is not suppressed by zinc supplementation [39].
Impact on virulence factors
As already stated, low metal concentrations induce the expression of fungal metal acquisition genes, which themselves are indispensable for host colonisation, dissemination, and virulence for many fungal pathogens [3], [4], [13]–[16]. Furthermore, pathogenic fungi can activate other virulence determinants in response to micronutrient limitation. For example, iron depletion induces capsule formation in Cryptococci, which allows the pathogens to escape recognition by the immune system [4], [40]. As another example, iron deprivation of A. fumigatus results in the expression of the major surface allergen ribotoxin AspF1, which could potentiate allergic bronchopulmonary aspergillosis [2]. Thus, in the act of depriving fungal pathogens of nutrients to limit their growth, the host can trigger virulence factors that may exacerbate fungal disease.
Perspectives
The metal tug of war between pathogen and host is a complex and dynamic process, with each party striving to procure and retain essential micronutrients. The great complexity of this interaction is only now being realised. It is becoming apparent that the host responds to infection by redirecting micronutrients via niche - and perhaps infection-stage–specific mechanisms to various ends, e.g., micronutrient limitation versus micronutrient poisoning. On the other hand, the apparent redundancy of some fungal metal acquisition mechanisms needs to be explored, and their essentiality to the different stages of pathogenesis remains to be established. Also, before we can start drawing global comparisons of metal homeostasis in fungal and bacterial infectious agents, more information is required about micronutrient warfare in fungal pathogens outside the small handful of established model organisms. The ultimate challenge will be to integrate the responses of host and pathogen into a holistic model that describes how the host modulates micronutrient homeostasis during infection and how the pathogen responds to these changes, spatially and temporally. New technologies, such as live-animal imaging of fungal gene expression [16], MALDI (matrix-assisted laser desorption/ionization) mass spectrometry imaging of proteins, and 2-D element mapping directly from biological specimens [22], are beginning to illuminate the pathogen–host tug-of-war over micronutrients. We are confident that the future holds many exciting discoveries in this field.
Zdroje
1. WangL, CherayilBJ (2009) Ironing out the wrinkles in the host defence: interactions between iron homeostasis and innate immunity. J Innate Immun 1 : 455–464.
2. SchrettlM, BeckmannN, VargaJ, HeinekampT, JacobsenID, et al. (2010) HapX-mediated adaptation to iron starvation is crucial for virulence of Aspergillus fumigatus. PLOS Pathog 6: e1001124 doi:10.1371/journal.ppat.1001124
3. Schneider RdeO, Fogaça NdeS, KmetzschL, SchrankA, VainsteinMH, et al. (2012) Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii. PLOS ONE 7: e43773 doi:10.1371/journal.pone.0043773
4. KronstadJW, HuG, JungWH (2013) An encapsulation of iron homeostasis and virulence in Cryptococcus neoformans. Trends Microbiol 21 : 457–465.
5. WaldronKJ, RutherfordJC, FordD, RobinsonNJ (2009) Metalloproteins and metal sensing. Nature 460 : 823–830.
6. EhrensbergerKM, BirdAJ (2011) Hammering out details: regulating metal levels in eukaryotes. Trends Biochem Sci 36 : 524–531.
7. BleackleyMR, MacgillivrayRT (2011) Transition metal homeostasis: from yeast to human disease. Biometals 24 : 785–809.
8. SamanovicMI, DingC, ThieleDJ, DarwinKH (2012) Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe 11 : 106–115.
9. IbrahimAS (2011) Host cell invasion in mucormycosis: role of iron. Curr Opin Microbiol 14 : 406–411.
10. Iglesias-OsmaC, Gonzalez-VillaronL, San MiguelJF, CaballeroMD, VazquezL, et al. (1995) Iron metabolism and fungal infections in patients with haematological malignancies. J Clin Pathol 48 : 223–225.
11. JungWH, KronstadJW (2008) Iron and fungal pathogenesis: a case study with Cryptococcus neoformans. Cell Microbiol 10 : 277–284.
12. BarluzziR, SaleppicoS, NocentiniA, BoelaertJR, NegliaR, et al. (2002) Iron overload exacerbates experimental meningoencephalitis by Cryptococcus neoformans. J Neuroimmunol 132 : 140–146.
13. RamananN, WangY (2000) A high-affinity iron permease essential for Candida albicans virulence. Science 288 : 1062–1064.
14. SchrettlM, BignellE, KraglC, SabihaY, LossO, et al. (2007) Distinct roles for intra - and extracellular siderophores during Aspergillus fumigatus infection. PLOS Pathog 3: e128 doi:10.1371/journal.ppat.0030128
15. HiltyJ, George SmulianA, NewmanSL (2011) Histoplasma capsulatum utilizes siderophores for intracellular iron acquisition in macrophages. Med Mycol 49 : 633–642.
16. DingC, FestaRA, ChenYL, EspartA, PalaciosÒ, et al. (2013) Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe 13 : 265–276.
17. RaymondKN, DertzEA, KimSS (2003) Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A 100 : 3584–3588.
18. RaeTD, SchmidtPJ, PufahlRA, CulottaVC, O'HalloranTV (1999) Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284 : 805–808.
19. DrakesmithH, PrenticeAM (2012) Hepcidin and the iron-infection axis. Science 338 : 768–772.
20. ArmitageAE, EddowesLA, GileadiU, ColeS, SpottiswoodeN, et al. (2011) Hepcidin regulation by innate immune and infectious stimuli. Blood 118 : 4129–4139.
21. SilvaMG, SchrankA, BailãoEF, BailãoAM, BorgesCL, et al. (2011) The homeostasis of iron, copper, and zinc in Paracoccidioides brasiliensis, Cryptococcus neoformans var. Grubii, and Cryptococcus gattii: a comparative analysis. Front Microbiol 2 : 49 doi:10.3389/fmicb.2011.00049
22. PotrykusJ, SteadD, MacCallumDM, UrgastDS, RaabA, et al. (2013) Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events. PLOS Pathog 9: e1003676 doi:10.1371/journal.ppat.1003676
23. WintersMS, ChanQ, CarusoJA, DeepeGSJr (2010) Metallomic analysis of macrophages infected with Histoplasma capsulatum reveals a fundamental role for zinc in host defenses. J Infect Dis 202 : 1136–1145.
24. UrbanCF, ErmertD, SchmidM, Abu-AbedU, GoosmannC, et al. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLOS Pathog 5: e1000639 doi:10.1371/journal.ppat.1000639
25. ZaremberKA, SuguiJA, ChangYC, Kwon-ChungKJ, GallinJI (2007) Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J Immunol 178 : 6367–6373.
26. CanessaP, LarrondoLF (2012) Environmental responses and the control of iron homeostasis in fungal systems. Appl Microbiol Biotechnol 97 : 939–955.
27. CitiuloF, JacobsenID, MiramónP, SchildL, BrunkeS, et al. (2012) Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLOS Pathog 8: e1002777 doi:10.1371/journal.ppat.1002777
28. WatermanSR, ParkYD, RajaM, QiuJ, HammoudDA, et al. (2012) Role of CTR4 in the virulence of Cryptococcus neoformans. MBio 3: e00285–12 doi:10.1128/mBio.00285-12
29. HissenAH, WanAN, WarwasML, PintoLJ, MooreMM (2005) The Aspergillus fumigatus siderophore biosynthetic gene SIDA, encoding 1-ornithine N5 - oxygenase, is required for virulence. Infect Immun 73 : 5493–5503.
30. AlmeidaRS, WilsonD, HubeB (2009) Candida albicans iron acquisition within the host. FEMS Yeast Res 9 : 1000–1012.
31. CadieuxB, LianT, HuG, WangJ, BiondoC, et al. (2013) The mannoprotein Cig1 supports iron acquisition from heme and virulence in the pathogenic fungus Cryptococcus neoformans. J Infect Dis 207 : 1339–1347.
32. IbrahimAS, GebremariamT, LinL, LuoG, HusseinyMI, et al. (2010) The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol Microbiol 77 : 587–604.
33. SeifertM, NairzM, SchrollA, SchrettlM, HaasH, et al. (2008) Effects of the Aspergillus fumigatus siderophore systems on the regulation of macrophage immune effector pathways and iron homeostasis. Immunobiology 213 : 767–778.
34. HwangLH, MayfieldJA, RineJ, SilA (2008) Histoplasma requires SID1, a member of an iron-regulated siderophore gene cluster, for host colonization. PLOS Pathog 4: e1000044 doi:10.1371/journal.ppat.1000044
35. CobinePA, PierrelF, BestwickML, WingeDR (2006) Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J Biol Chem 281 : 36552–36559.
36. BalasubramanianR, RosenzweigAC (2008) Copper methanobactin: a molecule whose time has come. Curr Opin Chem Biol 12 : 245–249.
37. RajaMR, WatermanSR, QiuJ, BleherR, WilliamsonPR, et al. (2013) A copper hyperaccumulation phenotype correlates with pathogenesis in Cryptococcus neoformans. Metallomics 5 : 363–371.
38. NobileCJ, NettJE, HerndayAD, HomannOR, DeneaultJS, et al. (2009) Biofilm matrix regulation by Candida albicans Zap1. PLOS Biol 7: e1000133 doi:10.1371/journal.pbio.1000133
39. RichieDL, FullerKK, FortwendelJ, MileyMD, McCarthyJW, et al. (2007) Unexpected link between metal ion deficiency and autophagy in Aspergillus fumigatus. Eukaryot Cell 6 : 2437–2447.
40. CrestaniJ, CarvalhoPC, HanX, SeixasA, BroettoL, et al. (2012) Proteomic profiling of the influence of iron availability on Cryptococcus gattii. J Proteome Res 11 : 189–205.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek Oral Bacteria and CancerČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



