-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaMycobacteriophages: Windows into Tuberculosis
article has not abstract
Published in the journal: . PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1003953
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003953Summary
article has not abstract
What Are Mycobacteriophages?
Mycobacteriophages are viruses that infect mycobacterial hosts, such as Mycobacterium tuberculosis and Mycobacterium smegmatis [1]. Because the discovery and genomic characterization of mycobacteriophages has been the focus of integrated research and education programs, including the Phage Hunters Integrating Research and Education (PHIRE) and the Howard Hughes Medical Institute Science Education Alliance Phage Hunters Advancing Genomics and Evolutionary Science (HHMI SEA-PHAGES), thousands of phages have been isolated using a single host strain, M. smegmatis mc2155, over 500 of which have been completely sequenced [2]–[5]. These are mostly from environmental samples, but mycobacteriophages have also been isolated from stool samples of tuberculosis patients [6], although these have yet to be genomically analyzed. Clearly, these mycobacteriophages represent only a tiny piece of the overall phage population, which is predicted to include 1031 particles, making them the majority of all life-forms in the biosphere [7].
Mycobacteriophages display a remarkable genetic diversity (Figure 1). About 30 distinct types (called clusters, or singletons if they have no relatives) that share little or no nucleotide sequence similarity have been identified. Many of the clusters span sufficient diversity that the genomes warrant division into subclusters (Figure 1). However, the genomes are characteristically mosaic in their architecture [8], which is readily evident from the shared genes revealed by amino acid sequence comparisons [9]. There is also considerable range in overall guanine plus cytosine content (GC%), from 50.3% to 70%, with an average of 64% (M. smegmatis is 67.3%). Thus, phage GC% does not necessarily match that of its host, and the consequent mismatch of codon usage profiles does not appear to be detrimental. Because new mycobacteriophages lacking extensive DNA similarity with the extant collection are still being discovered, and as there are at least seven singletons for which no relatives have been isolated, we clearly have yet to saturate the diversity of this particular population. The pace of discovery is rapid, so the profile of diversity is constantly changing.
Fig. 1. Diversity of mycobacteriophages. 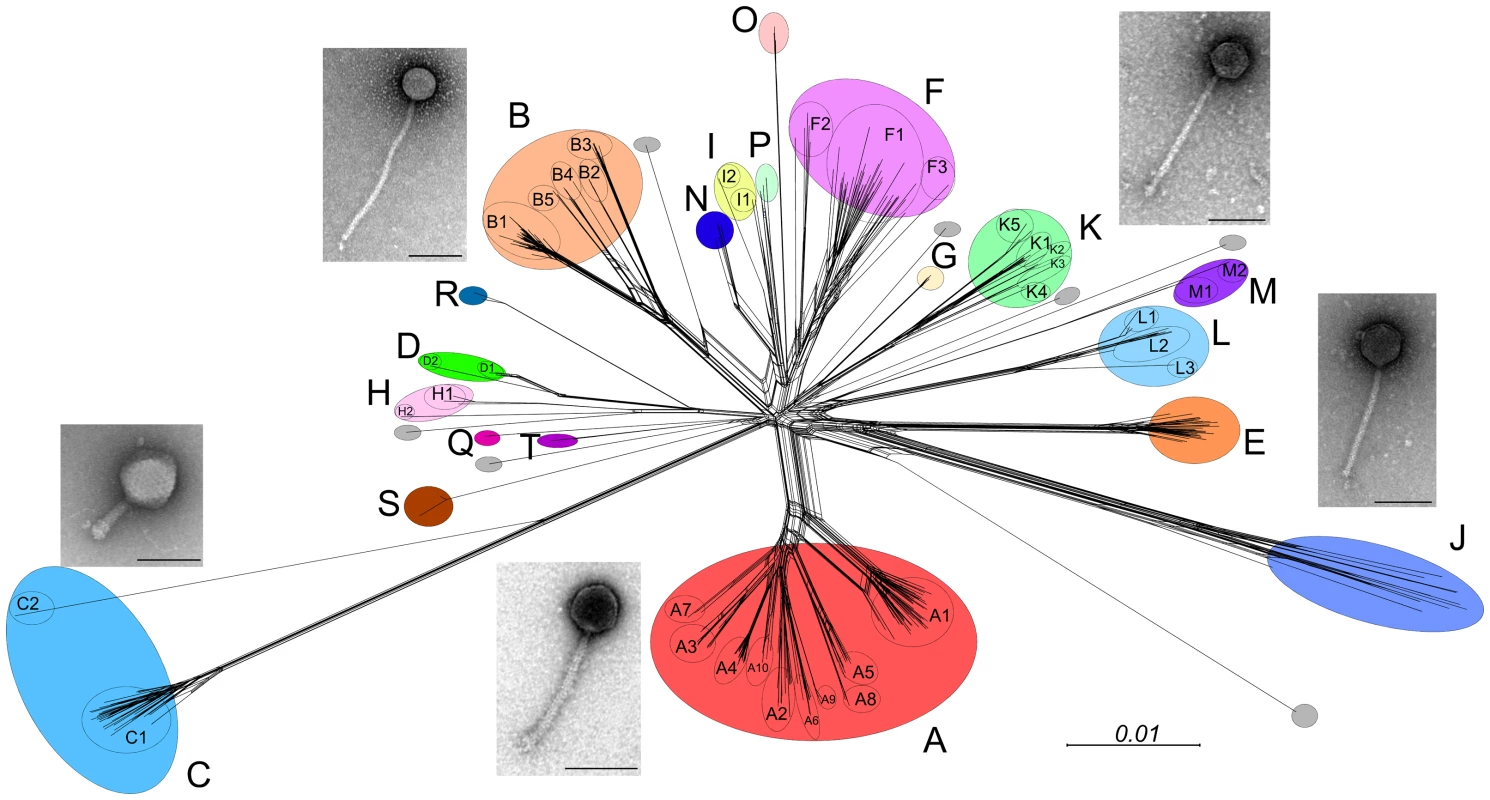
Sequenced genomes for 471 mycobacteriophages were compared according to their shared gene contents, and the relationships are displayed using Splitstree [24]. The genomes are clustered according to overall nucleotide sequence similarity, and the clusters (A, B, C…) correlate closely with their gene content. Colored circles encompass Clusters A–T as indicated, and grey circles represent singleton genomes that have no close relatives. Ten of the clusters are divided into subclusters (e.g., A1, A2, A3….) and are shown as circles within each cluster. Micrographs show the two morphotypes observed, typified by the myoviral Cluster C phages and the siphoviruses (all others) that primarily differ in tail length (scale bars, 100 nm). With the exception of one singleton (DS6A), all of the phages infect M. smegmatis mc2155. DS6A, the Cluster K phages and a subset of Cluster A phages also infect M. tuberculosis. The collection of >50,000 genes can be sorted into >3,900 groups (phamilies) according to their shared amino acid sequences. Most of these phamilies (∼75%) do not have homologues outside of the mycobacteriophages and are of unknown function. Extrapolation to the broader population suggests that phages have the largest repertoire of unexplored sequences in nature. Genetic studies with mycobacteriophage Giles show that 45% of the genes are nonessential for lytic growth, raising questions as to how and why they were acquired [10].
What Is the Basis of Viral Diversity?
If the diversity of phages of M. smegmatis mc2155 is this great, then the diversity of the phage population as a whole must be massive, as indicated from metagenomic studies [11]. Host range analysis shows that not all of these mycobacteriophages infect other strains of M. smegmatis, and only phages in Cluster K and in certain subclusters of Cluster A efficiently infect M. tuberculosis (Figure 1) [12]. However, mutants can be readily isolated from some phages that expand their host range to infect these other strains [12]. Thus, the observed phage diversity can be explained by assuming that wherever broad and diverse ranges of hosts are present, the phages can rapidly dance across the microbial landscape, using the hosts as “stepping-stones.” Migration across this landscape requires that the “stepping-stones” be spaced sufficiently close (genetically) to enable a host-range transition jump with either very few mutations or a gene acquisition event. The diversity of the mycobacteriophages therefore reflects the specific evolutionary pathways that they have pursued, sometimes occupying portions of the landscape dominated by strains other than M. smegmatis and probably outside of the genus Mycobacterium. For example, the lower GC% phages may have predominantly infected the lower GC% Corynebacteria and represent merely accidental tourists in the Mycobacterium locale. In this model, genes that were acquired because they were needed to grow in some recently visited host may not be needed in a current host but have yet to be selected against. Some of the nonessential Giles genes may thus correspond to these “legacy” genes. The host preference of the phages is presumably determined in part by the use of specific surface receptors, although few have been identified or characterized.
How Can Mycobacteriophages Facilitate Tuberculosis Genetics?
M. tuberculosis is challenging to grow because of its slow growth rate (24-hour doubling time) and its pathogenicity. It was intractable to genetic manipulation until breakthroughs in the late 1980s that took advantage of mycobacteriophages to bootstrap methods for transfection and transformation, electroporation, plasmid vectors, and selectable markers [13]–[15]. Furthermore, phages have continued to be key players in developing a more facile genetic system. For example, several applications rely specifically on the ability of phages to inject their DNA into essentially every cell within a mycobacterial population, making phages ideal for transposon delivery and preparation of complex transposon libraries [16], gene replacement using specialized transduction [17], and tuberculosis (TB) diagnosis by inclusion of a reporter gene [18], [19]. But the component parts of the phages also have tremendous utility and often work in M. tuberculosis even if the phage doesn't actually infect it. Examples include integration-proficient plasmid vectors and recombineering strategies, although there are numerous other potential applications that have yet to be exploited. The overall diversity of the phages massively fuels these approaches, providing a toolkit of over 50,000 genes that can be exploited.
Do Mycobacteriophages Influence Mycobacterial Physiology?
It is well established that phage-encoded toxins contribute to the virulence of a variety of bacterial pathogens, including Escherichia coli, Salmonella sp., Coynebacterium diphtheria, and Vibrio cholera. M. tuberculosis, however, clearly differs from these in its pathogenesis, and there is no evidence for phage-encoded toxins. Most M. tuberculosis strains carry one or both of two small (∼10 kbp) prophage-like elements φRv1 and φRv2, but it seems unlikely they contribute to virulence. Some mycobacteriophages (in Cluster D) do encode a vegetative insecticidal protein (VIP2)-like insect toxin genes that could confer virulence to a bacterial host, although it is unclear what that host might be, or what it might infect; they do not infect M. tuberculosis. We note that several mycobacterial strains, such as M. cannetti, M. marinum, M. abscessus, and M. ulcerans, carry seemingly intact prophages, which could influence their biology.
Expression of phage-encoded proteins is only one way that phages can influence their hosts. An alternative route is by integration of the phage genome into a host gene that is required for some physiological process. Phage integration is typically site-specific—involving integrase-mediated recombination between phage and bacterial attachment sites (attP and attB, respectively)—and two distinct types of enzymes are used. Tyrosine-integrases are the most common and typically mediate integration into a host tRNA gene (the well-studied phage lambda integrase is a notable exception). Because such phages carry the 3′ half of the tRNA gene at attP, tRNA functionality is maintained following prophage establishment. In contrast, phages using a serine-integrase typically use an attB site located within a host's protein-coding genes, which is interrupted by the integration event. These attB loci are small and cannot be readily predicted bioinformatically, and relatively few have been identified experimentally. We predict there are six to 12 different attB sites within M. smegmatis and M. tuberculosis, and integration into those that are mediated by a serine-integrase could potentially alter host physiology.
For mycobacteriophage Bxb1, the consequences of integration for host physiology are well established [20]. Bxb1 uses a serine-integrase to integrate into an attB site located within the groEL1 gene of M. smegmatis [21]. GroEL1 serves as a dedicated chaperone for regulation of mycolic acid biosynthesis and is required for the formation of mature biofilms. Thus, lysogens of Bxb1 are defective in forming biofilms, perhaps providing a selective advantage as cheaters within a broader population of non-lysogenic biofilms [22]. This phenotype would be easy to miss unless searching for it specifically, and we predict that many other phages that use serine-integrases also influence host physiology.
Do Mycobacteriophages Have Therapeutic Potential?
The increased prevalence of antibiotic resistance in bacterial pathogens has spurred renewed interest in the therapeutic use of bacteriophages. Although phages have been extensively used therapeutically in former Soviet Union countries, they have yet to find widespread use in either the United States or in Europe. Phage preparations have been approved for use against E. coli and Listeria meat contamination, and trials are in progress for control of several human infections. Skin afflictions and burns seem to be especially attractive targets.
What about phage therapy for tuberculosis? Antibiotic resistance is certainly a growing and worrisome development, particularly with the emergence of extensively drug-resistant (XDR) and totally drug-resistant (TDR) strains, both of which are especially difficult to control. Delivery of phages to the lungs should be relatively simple, although there is considerable doubt as to whether they would effectively reach their bacterial hosts, which may be intracellular and within granulomas. An intriguing suggestion for addressing the access question is to use infected surrogate mycobacterial cells for the delivery [23]. Unfortunately, relatively few efficient phage killers of M. tuberculosis are available, and because phage resistance is to be expected, a suite of three to six phages that efficiently kill M. tuberculosis and elicit different resistance mechanisms in the host are needed. Because only a subset of those phages isolated on M. smegmatis also infect M. tuberculosis, isolation of additional phages known to infect M. tuberculosis is desirable.
In spite of these concerns, there is considerable potential to use phages prophylactically by interfering specifically with TB transmission. For example, if a patient was diagnosed with tuberculosis, family members and coworkers could aspirate phages into the upper respiratory tract, where the phages could infect and kill M. tuberculosis cells as they are breathed in and before they establish an infection. As transmission typically involves small numbers of cells, it ought to be possible to deliver a sufficient amount of phage particles while minimizing phage-resistance. Safety is not expected to be a concern, and there should be few impediments to evaluating mycobacteriophages prophylaxis.
Zdroje
1. HatfullGF (2012) The secret lives of mycobacteriophages. Adv Virus Res 82 : 179–288.
2. HanauerDI, Jacobs-SeraD, PedullaML, CresawnSG, HendrixRW, et al. (2006) Inquiry learning. Teaching scientific inquiry. Science 314 : 1880–1881.
3. HatfullGF, PedullaML, Jacobs-SeraD, CichonPM, FoleyA, et al. (2006) Exploring the mycobacteriophage metaproteome: phage genomics as an educational platform. PLOS Genet 2: e92 doi:10.1371/journal.pgen.0020092.
4. HatfullGF (2012) Complete Genome Sequences of 138 Mycobacteriophages. J Virol 86 : 2382–2384.
5. HatfullGF (2013) Science Education Alliance Phage Hunters Advancing Genomics and Evolutionary Science [SEA-PHAGES] Program, KwaZulu-Natal Research Institute for Tuberculosis and HIV [K-RITH] Micobacterial Genomics Course, University of California—Los Angeles Research Immersion Laboratory in Virology, Phage Hunters Integrating Research and Education [PHIRE] Program (2013) Complete Genome Sequences of 63 Mycobacteriophages. Genome Announc 1: e00847–13.
6. CaterJC, RedmondWB (1963) Mycobacterial phages isolated from stool specimens of patients with pulmonary disease. Am Rev Respir Dis 87 : 726–729.
7. HendrixRW (2002) Bacteriophages: evolution of the majority. Theor Popul Biol 61 : 471–480.
8. PedullaML, FordME, HoutzJM, KarthikeyanT, WadsworthC, et al. (2003) Origins of highly mosaic mycobacteriophage genomes. Cell 113 : 171–182.
9. CresawnSG, BogelM, DayN, Jacobs-SeraD, HendrixRW, et al. (2011) Phamerator: a bioinformatic tool for comparative bacteriophage genomics. BMC Bioinformatics 12 : 395.
10. DedrickRM, MarinelliLJ, NewtonGL, PoglianoK, PoglianoJ, et al. (2013) Functional requirements for bacteriophage growth: gene essentiality and expression in mycobacteriophage Giles. Mol Microbiol 88 : 577–589.
11. AnglyFE, FeltsB, BreitbartM, SalamonP, EdwardsRA, et al. (2006) The marine viromes of four oceanic regions. PLOS Biol 4: e368 doi:10.1371/journal.pbio.0040368.
12. Jacobs-SeraD, MarinelliLJ, BowmanC, BroussardGW, Guerrero BustamanteC, et al. (2012) On the nature of mycobacteriophage diversity and host preference. Virology 434 : 187–201.
13. JacobsWRJr, TuckmanM, BloomBR (1987) Introduction of foreign DNA into mycobacteria using a shuttle phasmid. Nature 327 : 532–535.
14. SnapperSB, LugosiL, JekkelA, MeltonRE, KieserT, et al. (1988) Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc Natl Acad Sci U S A 85 : 6987–6991.
15. SnapperSB, MeltonRE, MustafaS, KieserT, JacobsWRJr (1990) Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol 4 : 1911–1919.
16. SassettiCM, BoydDH, RubinEJ (2003) Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48 : 77–84.
17. BardarovS, BardarovSJr, PavelkaMSJr, SambandamurthyV, LarsenM, et al. (2002) Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148 : 3007–3017.
18. JacobsWRJr, BarlettaRG, UdaniR, ChanJ, KalkutG, et al. (1993) Rapid assessment of drug susceptibilities of Mycobacterium tuberculosis by means of luciferase reporter phages. Science 260 : 819–822.
19. PiuriM, JacobsWRJr, HatfullGF (2009) Fluoromycobacteriophages for rapid, specific, and sensitive antibiotic susceptibility testing of Mycobacterium tuberculosis. PLOS ONE 4: e4870 doi:10.1371/journal.pone.0004870.
20. OjhaA, AnandM, BhattA, KremerL, JacobsWRJr, et al. (2005) GroEL1: a dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell 123 : 861–873.
21. KimAI, GhoshP, AaronMA, BibbLA, JainS, et al. (2003) Mycobacteriophage Bxb1 integrates into the Mycobacterium smegmatis groEL1 gene. Mol Microbiol 50 : 463–473.
22. ZambranoMM, KolterR (2005) Mycobacterial biofilms: a greasy way to hold it together. Cell 123 : 762–764.
23. BroxmeyerL, SosnowskaD, MiltnerE, ChaconO, WagnerD, et al. (2002) Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: a model for phage therapy of intracellular bacterial pathogens. J Infect Dis 186 : 1155–1160.
24. HusonDH (1998) SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14 : 68–73.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek Oral Bacteria and CancerČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



