-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaPPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
Cerebral malaria (CM) is a severe complication of Plasmodium falciparum infection that is associated with long-term neurocognitive impairment in about a third of survivors even when optimal anti-malarial therapy is used. Since both the parasite and the host immune response to infection play a role in the development of CM, adjunctive therapies that modulate the host response, given in conjunction with anti-parasitic therapy, may improve survival and prevent neurocognitive injury. Here we examine the effects of PPARγ agonists on neurocongitive injury using a mouse model of CM. We demonstrate that PPARγ agonists, when administered with anti-malarials, protected mice from developing brain atrophy and neurocognitive impairment. This was associated with induction of anti-oxidant and neuroprotective pathways in the brains of infected mice. We also observed the same neuroprotective pathways induced in patients with falciparum malaria that received PPARγ adjunctive therapy. Our findings suggest that PPARγ agonists may be valuable in the treatment and prevention of CM-induced neurocognitive injury, and support the testing of PPARγ agonists in patients with CM.
Published in the journal: . PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1003980
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003980Summary
Cerebral malaria (CM) is a severe complication of Plasmodium falciparum infection that is associated with long-term neurocognitive impairment in about a third of survivors even when optimal anti-malarial therapy is used. Since both the parasite and the host immune response to infection play a role in the development of CM, adjunctive therapies that modulate the host response, given in conjunction with anti-parasitic therapy, may improve survival and prevent neurocognitive injury. Here we examine the effects of PPARγ agonists on neurocongitive injury using a mouse model of CM. We demonstrate that PPARγ agonists, when administered with anti-malarials, protected mice from developing brain atrophy and neurocognitive impairment. This was associated with induction of anti-oxidant and neuroprotective pathways in the brains of infected mice. We also observed the same neuroprotective pathways induced in patients with falciparum malaria that received PPARγ adjunctive therapy. Our findings suggest that PPARγ agonists may be valuable in the treatment and prevention of CM-induced neurocognitive injury, and support the testing of PPARγ agonists in patients with CM.
Introduction
Cerebral malaria (CM) is a severe complication of Plasmodium falciparum infection that is associated with high mortality rates despite potent anti-anti-malarial therapy [1]–[2]. Adjunctive therapies, aimed at modifying the pathophysiological processes of malaria infection have been pursued as a way to improve outcome in CM, albeit with limited success to date [3].
CM is characterized by seizures and coma in the presence of parasitemia and in the absence of any other recognized cause of coma. While it was long believed that the majority of children surviving CM were left neurologically intact, recent studies have challenged this assumption and provided evidence that many CM survivors have long-term cognitive and neurological deficits [4]–[6].
Both parasite and host determinants contribute to the onset and neurological outcome of CM. Host innate immune responses to infection combined with sequestration of parasitized erythrocytes (PEs) in the microvasculature of the brain, result in dysregulated inflammation, endothelial activation and dysfunction, microvascular occlusion, vascular leak, and ultimately loss of blood-brain barrier (BBB) function and integrity [7]. Sequestered PEs, perfusion abnormalities, hemorrhages, edema, local tissue hypoxia and ischemia, and focal disruptions of the BBB are common fundoscopic and autopsy findings in CM patients [8]–[9]. Oxidative stress and axonal injury in the vicinity of brain hemorrhages and in areas of vascular occlusion are observed in CM and may contribute to neurological dysfunction pre-mortem and in CM survivors [10]. In experimental models of CM (ECM), intravital microscopy studies have revealed that neurological signs in ECM are associated with dysfunction of the neuroimmunological BBB, including vascular leak from post-capillary venules [11].
Peroxisome proliferators-activated receptor gamma (PPARγ) is a member of the family of nuclear hormone receptors that function as ligand-activated transcription factors [12]. PPARγ agonists are reported to have anti-inflammatory and anti-oxidant properties [13], and may also possess neuroprotective properties mediated in part by promoting neuron repair mechanisms [14]–[15]. We have previously shown that PPARγ agonists decrease human monocyte inflammatory responses to P. falciparum PEs in vitro, and reduce systemic inflammation and improve survival in vivo in the ECM model [16]. These pre-clinical observations were extended and confirmed in a randomized double-blind placebo controlled trial in Thai patients with P. falciparum malaria. In this clinical trial adjunctive therapy with PPARγ agonists was associated with significantly faster parasite clearance time and reduced systemic inflammatory responses to infection [17]. Based on the above observations and putative role of PPARγ activation in neuroprotection, we tested the hypothesis that PPARγ agonists would enhance endothelial quiescence and BBB integrity, and activate neuroprotective pathways in mice and humans. Here we report that PPARγ agonist therapy resulted in improved survival and long-term neurocognitive performance in the ECM model and was associated with the induction of neuroprotective pathways, such as brain derived neurotrophic factor (BDNF), in both P. berghei ANKA-infected mice and humans with P. falciparum malaria.
Results
Adjunctive therapy with PPARγ agonists improves survival in ECM
We have previously shown that mice treated prophylactically with rosiglitazone are protected from developing CM and have significantly higher survival rates than control mice [16]. To model clinically relevant scenarios we designed treatment protocols in the ECM model whereby Plasmodium berghei ANKA (PbA)-infected mice were treated with a combination of artesunate (10 mg/kg), and a PPARγ agonist (rosiglitazone; 2.5 mg/kg) or saline as a control starting on day 3 post-infection, when parasitemia becomes detectable peripherally, or when mice displayed neurological symptoms and began to die from CM (between day 5 and 6 of infection) (see Figure S1 for treatment protocols). Starting treatment on day 3 post-infection resulted in 100% survival in the rosiglitazone-treated group compared to a 40% survival in the group receiving artesunate alone (Figure 1A, p = 0.0042). All untreated mice succumbed to their infection. Starting treatment at the onset of CM resulted in a 90% survival in the rosiglitazone-treated group compared to 57.5% survival in the group receiving artesunate alone (Figure 1B, p = 0.0012). Similar survival benefit was observed with pioglitazone (a PPARγ agonist belonging to the same class of drugs as rosiglitazone). Pioglitazone (20 mg/kg) adjunctive therapy commencing at the onset of CM signs resulted in an 85% survival compared to 45.5% survival in the group receiving artesunate alone (p = 0.0093, by Logrank test, n = 20/group). Parasitemia did not significantly differ between the treatment groups (Figure 1C–D). All mice that survived the CM window went on to develop hyperparasitemia.
Fig. 1. Rosiglitazone adjunctive therapy improves survival in ECM. 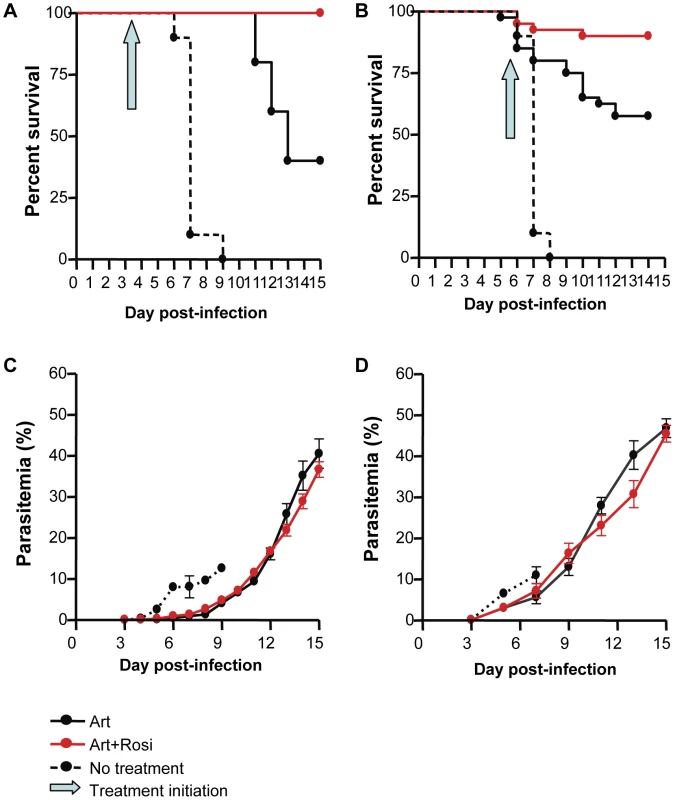
Survival curves (A and B) and parasitemia (C and D) for mice infected with PbA and receiving, no treatment (dashed line), 10 mg/kg artesunate plus saline (solid black line), or 10 mg/kg artesunate plus 2.5 mg/kg rosiglitazone (red line). The blue arrow indicates treatment initiation (day 3 post-infection for A and C, and onset of CM for B and D). In (A), p = 0.0042 for artesunate vs. artesunate + rosiglitazone; p<0.0001 for no treatment vs. artesunate + rosiglitazone, and for no treatment vs. artesunate, by Logrank test, N = 10/group. In (B), p = 0.0012 for artesunate vs. artesunate + rosiglitazone; p<0.0001 for no treatment vs. artesunate + rosiglitazone, and for no treatment vs. artesunate, by Logrank test, N = 40 for the artesunate and artesunate + rosiglitazone groups, N = 10 for the no treatment group. (C–D) Parasitemia curves did not differ significantly between artesunate and artesunate + rosiglitazone groups. Abbreviations: Art, artesunate; Rosi, rosiglitazone. PPARγ therapy reduces endothelial activation and enhances blood brain barrier integrity
Endothelial activation and dysfunction leading to loss of vascular integrity are thought to be central to the pathophysiology of CM. The angiopoietin-Tie-2 system has been identified as a key regulator of vascular integrity, and plays a role in disease progression and outcome in malaria infection [18]. The interaction between angiopoietin-1 (Ang-1) and its receptor Tie-2 promotes endothelial quiescence and inhibits vascular leakage, while Ang-2 antagonizes the Ang-1-Tie-2 interaction and promotes endothelial activation. We investigated whether rosiglitazone adjunctive therapy has an impact on angiopoietin levels in ECM. Mice receiving adjunctive rosiglitazone therapy (starting either on day 3 post-infection or at the onset of neurological signs) had significantly higher circulating levels of Ang-1 (Figure 2A and B, and Figure S2), consistent with less endothelial activation compared to mice receiving artesunate alone. Compared to uninfected mice, Ang-1 levels were significantly lower in infected mice treated with artesunate alone, while Ang-1 levels were maintained in mice that received rosiglitazone adjunctive therapy. Using real time quantitative PCR analysis of brain homogenates we observed significantly higher Ang-1 mRNA levels (Figure S3A), a trend towards lower Ang-2 mRNA levels (Figure S3B), and a significantly lower Ang-2 to Ang-1 mRNA ratio in mice treated with rosiglitazone compared to those treated with artesunate alone (Figure 2C and Figure S3C). Our data suggest that rosiglitazone adjunctive therapy induces a shift in the Ang-2 to Ang-1 balance towards a more quiescent endothelium.
Fig. 2. Rosiglitazone adjunctive therapy helps maintain peripheral angiopoietin-1 levels, and protects from blood brain barrier breakdown. 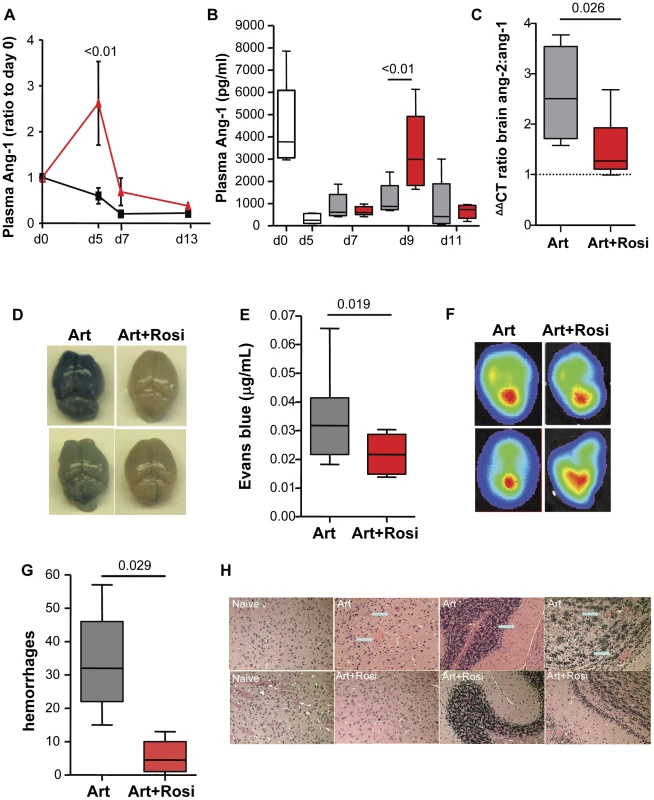
(A) Mice infected with PbA were treated with artesunate plus saline (black line) or artesunate plus rosiglitazone (red line) starting on day 3 post-infection. Blood was collected serially on day 0, 5, 7, and 13 and angiopoietin-1 (Ang-1) levels were tested. Ang-1 levels were normalised to d0 values. Data were analysed by two-way ANOVA with Bonferroni post-test, N = 6/group. (B-C) Mice infected with PbA were treated with artesunate plus saline (Art, grey bars), or artesunate plus rosiglitazone (Art+Rosi, red bars) beginning at the onset of CM signs. Plasma levels of Ang1 are shown in (B). The white bars indicate ang-1 levels prior to treatment. The ratio of ang-2 to ang-1 mRNA expression in the brain of mice on day 7 post-infection is shown in (C). The dashed line indicates median values for uninfected mice. Data shown are medians with range. Statistical comparisons by Kruskal-Wallis with Dunn's post-test in B and Mann Whitney test in C, N = 6/group, one of two independent experiments. (D–H) Mice infected with PbA or luciferase-expressing PbA (PbGFP-LUC(con)) were treated with either artesunate plus saline (Art) or artesunate plus rosiglitazone (Art+Rosi) beginning on day 3 post-infection. (D–E) On day 10 of infection parasitemia matched mice were injected with Evans blue to assess vascular leak in the brain. Photographs of representative brains from artesunate (on the left) and artesunate plus rosiglitazone (on the right) treated mice are shown in (D), and quantification of Evans blue in the brains is shown in (E). The dashed line is the median Evans blue value for uninfected mice. All comparisons by Mann Whitney test, N = 10/group. (F) Parasite accumulation was assessed by bioluminescence detection of mice injected with luciferin. Parasite accumulation was similar between groups. (G) Brain hemorrhages were assessed blindly on formalin-fixed brain sections from parasitemia-matched mice collected on day 10 post-infection. Comparisons by Mann Whitney, N = 5/group. Data shown are medians with range. (H) Representative images of H&E stained brain sections. Blue arrows indicate hemorrhages. We extended these observations to investigate whether this was also associated with enhanced BBB integrity and reduced vascular leakage during ECM. Mice receiving rosiglitazone had significantly reduced levels of vascular leak (as assessed by Evans blue) and preserved BBB function compared to mice treated with artesunate alone (Figure 2D–E), despite having similar peripheral parasitemia levels and similar parasite accumulation in the brain (Figure 2F) (as assessed by bioluminescence imaging of mice infected with luciferase expressing PbA) [19]. Compared to mice treated with artesunate alone, mice receiving rosiglitazone also had significantly fewer brain hemorrhages (Figure 2G and H), providing additional evidence of enhanced BBB integrity and neuroprotection.
To further examine whether the elevated levels of Ang-1 observed in rosiglitazone-treated mice directly contributed to improved survival and neuroprotection we infected mice that were either sufficient for Ang-1 or had one Ang-1 allele deleted (Ang-1del, the kind gift of Dr. SE Quaggin [20]) with PbA and treated them with artesunate plus either rosiglitazone or saline as a control starting on day 5.5 post-infection. Ang-1del mice produce 30-50% the Ang-1 levels of Ang-1 sufficient mice (Figure S4). Rosiglitazone significantly improved survival and reduced disease severity (as assessed by a modified rapid murine coma and behaviour scale (RMCBS) [21]) only in the Ang-1 sufficient mice, but not in the Ang-1del mice (Figure S4), supporting a direct role for Ang-1 in mediating, at least partly, the observed protective effects of rosiglitazone.
PPARγ therapy increases expression of the anti-oxidant enzymes SOD-1 and catalase in the brains of PbA-infected mice
Malaria infection is associated with release of free heme that can trigger oxidative stress and tissue injury. Oxidative stress and axonal damage are thought to contribute to neuronal injury and dysfunction in CM patients [10]. Increased oxidative stress has also been observed in the brains of mice with ECM [22]. Upregulation of endogenous anti-oxidant enzymes can help maintain redox balance and protect neurons from injury. In agreement with its putative neuroprotective role, anti-oxidant activity is decreased in patients who develop CM and in mice susceptible to ECM [22]–[23]. PbA infection was associated with a significant decrease in brain mRNA levels of the anti-oxidant enzymes SOD-1 and catalase (Figure 3A–B, day 0 vs. day 5). Compared to mice receiving artesunate alone, addition of rosiglitazone adjunctive therapy resulted in significantly increased levels of SOD-1 and catalase expression and full recovery to pre-infection levels by day 9 post-infection (Figure 3A–B). We did not observed a significant difference in HO-1 levels between treatment groups (Figure S5).
Fig. 3. Rosiglitazone adjunctive therapy increases brain expression of the anti-oxidant enzymes SOD-1 and catalase. 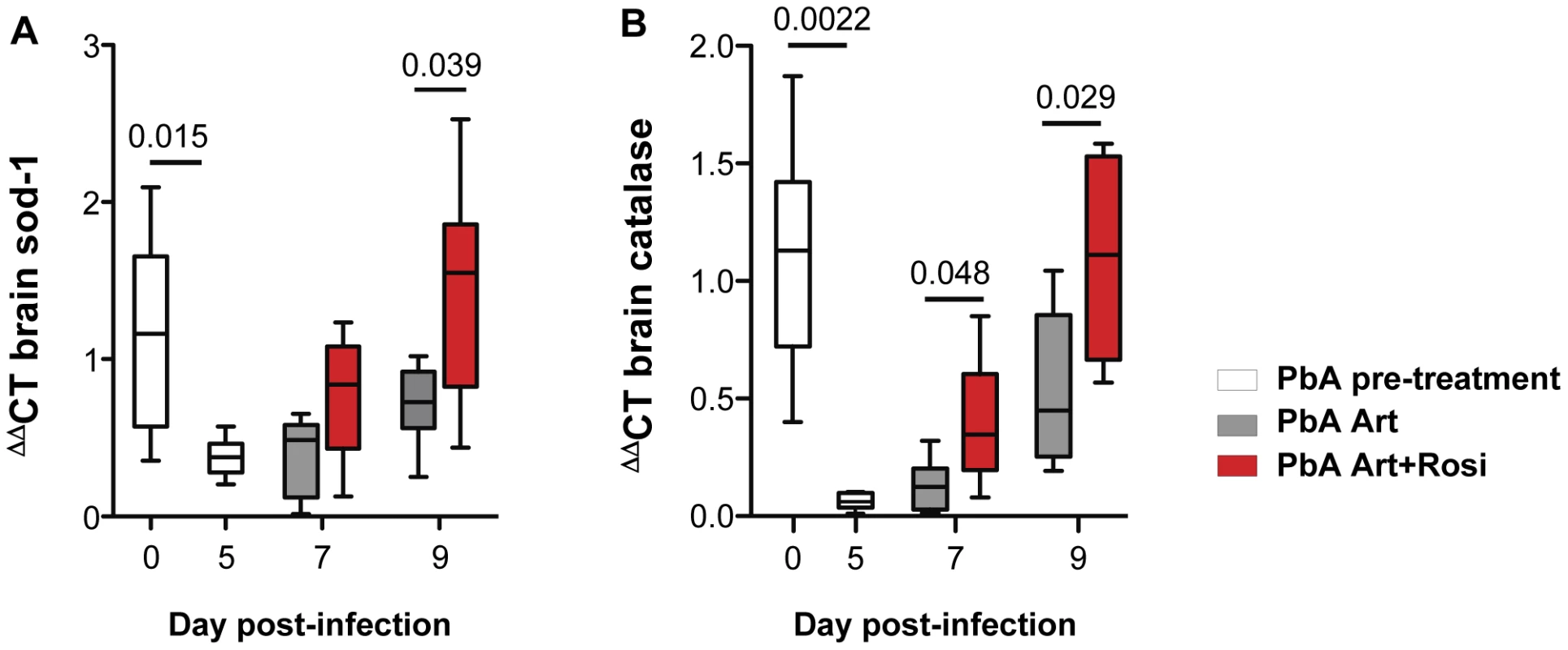
Mice infected with PbA were treated with artesunate plus saline (grey bars), or artesunate plus rosiglitazone (red bars) starting at the onset of CM signs. Expression of SOD-1 (A) and catalase (B) mRNA was assessed in brain homogenates collected from uninfected mice (day 0), infected mice prior to the initiation of therapy (day 5), and infected mice following treatment initiation (on day 7 and 9 post-infection). Data were analysed by one-way ANOVA with Bonferroni's multiple comparison test, N = 6 per group. In summary, rosiglitazone treatment resulted in higher brain expression of SOD-1 and catalase, compared to artesunate treatment alone.
PPARγ agonists increase expression of neurotrophic factors BDNF and NGF in the brains of PbA-infected mice
Neurotrophic factors, such as BDNF and nerve growth factor (NGF) play an important neuroprotective role [24]. Decreased BDNF mRNA expression in the brain has been correlated with increased CM severity in PbA-infected mice [25]. In agreement with previous observations, we observed a 55% decrease in BDNF mRNA expression in the brains of PbA-infected mice at the onset of neurological signs (Figure 4A day 0 vs. day 5). Mice receiving rosiglitazone adjunctive therapy had higher levels of BDNF compared to mice receiving artesunate alone (Figure 4A). BDNF expression was significantly higher in the rosiglitazone group on day 9 post-infection. Although NGF levels were not affected by PbA infection, rosiglitazone treatment was also associated with higher levels of NGF expression on day 11 post-infection (Figure 4B).
Fig. 4. Rosiglitazone adjunctive therapy increases expression of neurotrophic factors in the brains of malaria-infected mice. 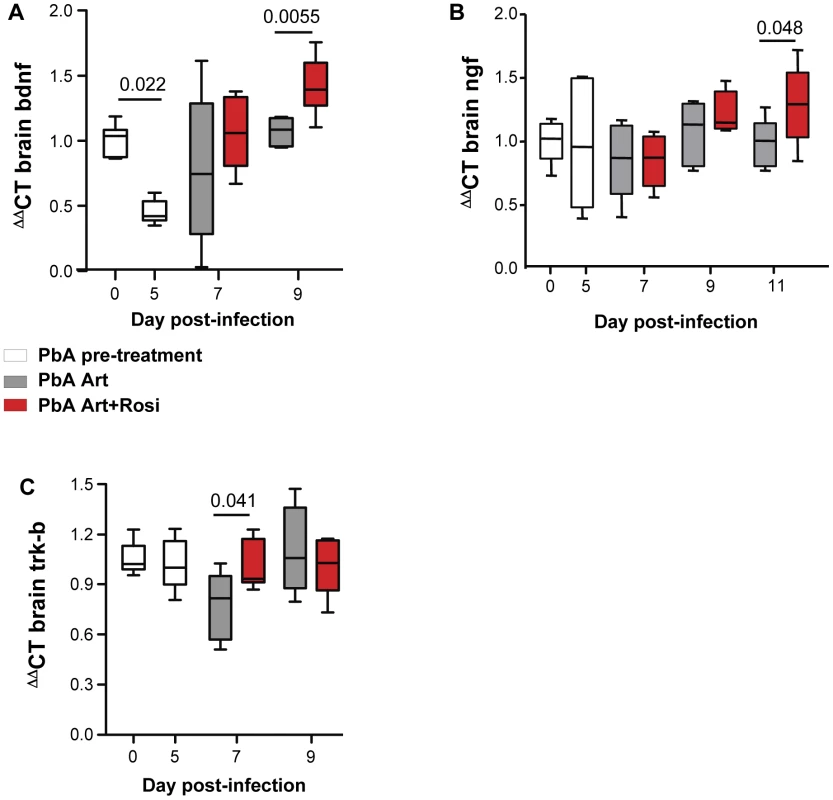
(A–C) Mice infected with PbA were treated with artesunate plus saline (grey bars), or artesunate plus rosiglitazone (red bars) starting at the onset of CM signs. Expression of BDNF (A), NGF (B), and the BDNF receptor Trk-B (C) mRNA was assessed in brain homogenates collected from uninfected mice (day 0), infected mice prior to the initiation of therapy (day 5), and infected mice following treatment initiation (on day 7 and 9 post-infection). All comparisons by one-way ANOVA with Bonferroni's multiple comparison tests. The synaptic effects of BDNF are mediated by its receptor Trk-B [26]. Trk-B expression in the brain was preserved in mice receiving rosiglitazone, while it decreased on day 7 post-infection in mice receiving artesunate alone (Figure 4C).
Thus, rosiglitazone adjunctive therapy was associated with both elevated levels of BDNF and NGF expression and maintenance of Trk-B expression in the brains of mice infected with malaria.
PPARγ adjunctive therapy protects mice from cognitive and motor impairment
Long-term neurocognitive impairment is observed in children surviving CM. Mice, drug-cured following the onset of ECM, also demonstrate neurocognitive impairment [22], [27]. Our data thus far indicated that rosiglitazone adjunctive therapy is associated with improved survival in ECM but it is unclear if this is at the cost of increased neurocognitive injury in ECM survivors. Alternatively our observations of preserved BBB integrity and induction of CNS anti-oxidant enzymes and neurotrophic factors suggest that PPARγ-treatment might confer neuroprotection in ECM. To test this latter hypothesis, we drug-cured mice at the onset of CM signs with a combination of artesunate and mefloquine, with or without rosiglitazone adjunctive therapy for a total of 7 days (see Figure S1 for dosing regimen). Mice remained parasite-free following treatment for the entire testing period. Uninfected mice that received the same drug treatment regimens were used as controls. Mice were evaluated using a battery of standardized neurocognitive tests of learning, memory, exploratory behaviour, anxiety, and motor performance, starting 2 months following the completion of curative anti-malarial therapy. Compared to infected mice cured with artesunate/mefloquine alone, we observed significant improvements in neurocognitive performance in mice that received rosiglitazone adjunctive therapy.
The novel object recognition (NOR) test is a test of non-spatial learning and memory and is based on the innate tendency of rodents to preferentially explore novel objects over familiar ones. PbA-infected mice treated with artesunate/mefloquine had significantly lower memory (preference index) scores compared to uninfected controls for the novel object following a 3-hour retention interval (Figure 5A). In contrast, PbA-infected mice that received rosiglitazone adjunctive therapy had preference index scores similar to uninfected controls, and significantly better than mice that were treated with artesunate/mefloquine alone (Figure 5A). Differences in preference scores could not be attributed to differences in mobility or total exploration time between the groups (Figure S6).
Fig. 5. Mice treated with rosiglitazone adjunctive therapy perform better in the novel object recognition and rotarod tests. 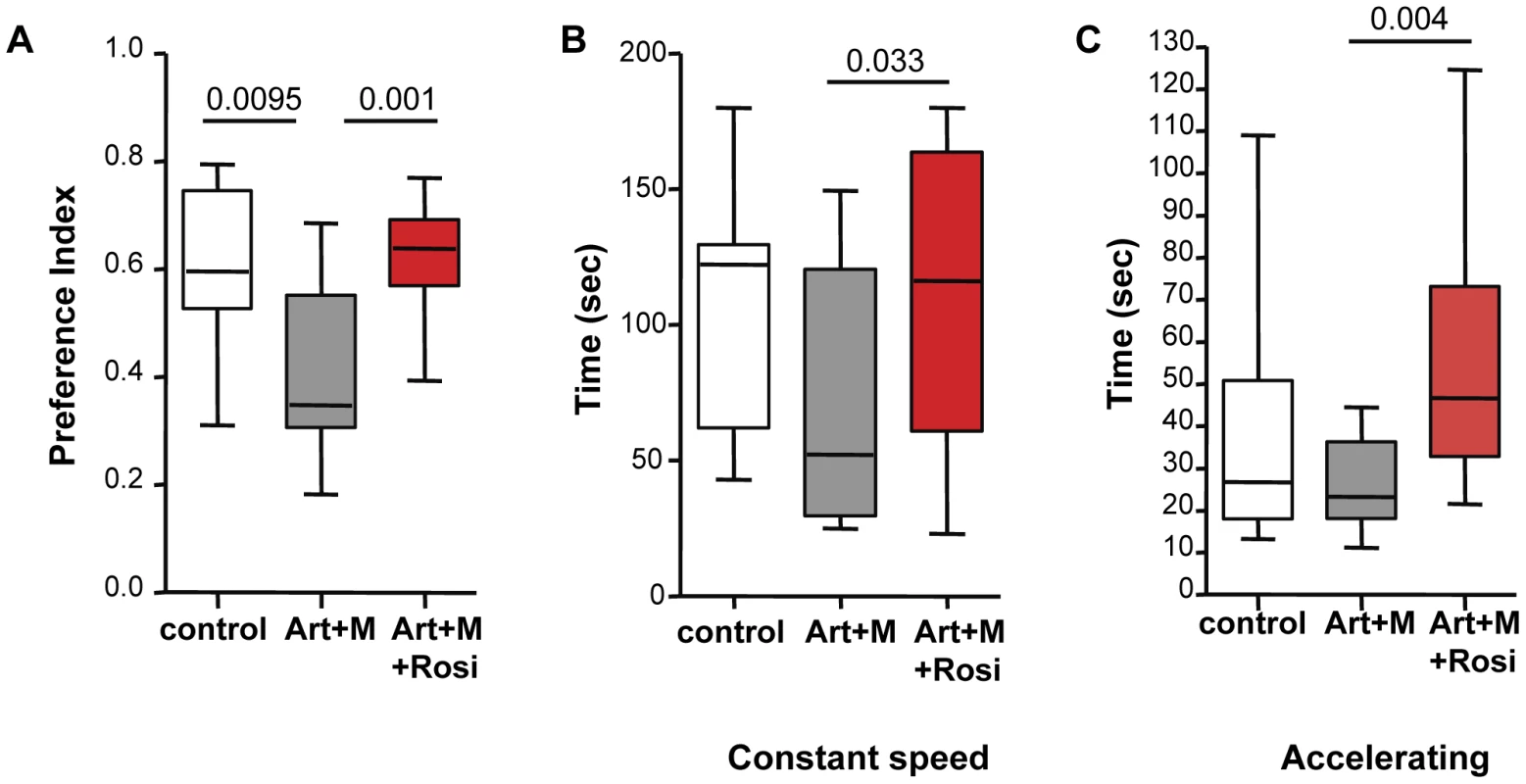
Mice infected with PbA were drug-cured at the onset of CM with artesunate/mefloquine plus saline (grey bars), or plus rosiglitazone (red bars). Drug-treated uninfected mice were used as controls (white bars). Mice remained parasite-free throughout testing. Testing was performed 2 months following completion of treatment. Data for the novel object recognition test (NOR) are shown in (A). The preference index indicates the preference for exploring the novel object over the familiar one. Data for the constant speed rotarod test are shown in (B) and for the accelerating rotarod test in (C). The average latency (measured in seconds) to fall off the rod apparatus for 5 trials is shown. All comparisons by one-way ANOVA with Boneferroni's multiple comparison tests. To assess motor performance we conducted both a constant and an accelerating speed rotarod test. In both tests PbA-infected mice that received rosiglitazone adjunctive therapy performed significantly better than PbA-infected mice treated with artesunate/mefloquine alone (Figure 5B–C), suggesting better motor coordination and balance in the rosiglitazone-treated group. In both tests the PbA-infected mice treated with artesunate/mefloquine alone showed impaired performance compared with uninfected controls, however the difference between the groups did not reach statistical significance (p = 0.063 for constant rotarod test, and p = 0.12 for accelerating rotarod test).
We did not observe any differences between groups in the open field test, which assesses exploratory and locomotor behaviour, in the tail suspension test, which assesses anxiety and depression, or in the contextual fear-conditioning test, which assesses associative learning (Figure S7).
These data suggest that CM is associated with deficits in memory and motor skills, and that rosiglitazone adjunctive therapy conferred protection to these ECM-associated neurocognitive deficits.
PPARγ therapy protects mice from PbA-induced brain atrophy
Specific brain regions can undergo structural plastic changes in response to demands or injury, such as learning a new task, or response to and recovery from a serious infection [28]. CT and MRI assessment of children during acute CM and of CM survivors with neurological sequelae have demonstrated brain structural abnormalities including focal and multifocal atrophy [29]–[30]. We next performed high-resolution MRI studies to determine if the behavioural phenotypes we observed correlated with morphological changes in the CNS. Following completion of all behavioral testing, mice were perfused and brain tissue was fixed for MRI. Volumetric analysis was performed on total brain volume and 62 distinct brain regions outlined based on a previously established mouse brain atlas [31]–[32]. We observed significant differences in 12 brain regions between mice that received anti-malarials alone and mice that received rosiglitazone adjunctive therapy (Figure 6A and Figure S8). In all cases the brain regions had a preserved (larger) volume in the rosiglitazone-treated mice. Notably, we observed significant differences in the corpus callosum, hippocampus, and thalamus (Figure 6B–D). These areas are involved in new memory formation and detection of novel events and stimuli. Atrophy in these areas may relate to the impaired responses observed in the NOR test with mice treated with anti-malarial alone. We also observed significant differences in the midbrain, the arbor vitae of the cerebellum, and the globus pallidus of the basal ganglia (Figure 6E–G). These are regions that play a critical role in motor coordination and balance, and corresponding atrophy in these areas may be related to the impaired rotarod test performance observed in mice treated with anti-malarials alone.
Fig. 6. Rosiglitazone adjunctive therapy protects mice from malaria-induced brain atrophy. 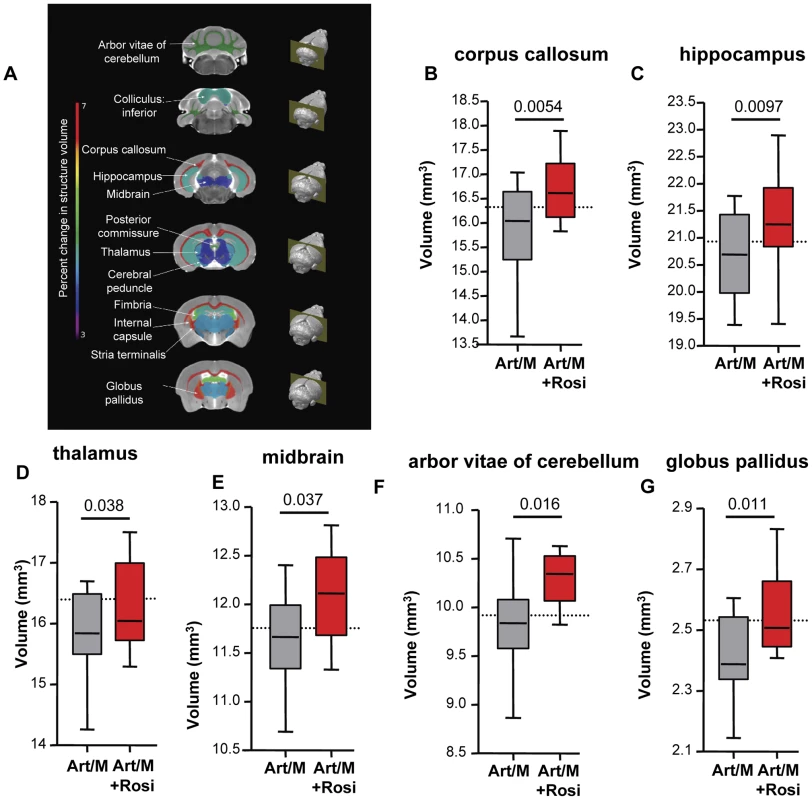
Following completion of all behavioural testing mouse brains were scanned using magnetic resonance imaging (MRI). Image registration and volumetric analysis of the brain volume of 62 distinct regions were performed. Linear regression analysis was used to identify areas that differed significantly between the mice treated with artesunate/mefloquine plus saline (grey bars), and the mice treated with artesunate/mefloquine plus rosiglitazone (red bars). (A) Identified brain regions are shown. Percentage change in brain structure volume is indicated by colour (see rainbow bar on the left). Significant differences were observed in (B) the corpus callosum, (C) the hippocampus, (D) the thalamus, (E) the midbrain, (F) the arbour vitae of the cerebellum, and (G) the globus pallidus of the basal ganglia. N = 13 for artesunate/mefloquine, N = 10 for artesunate/mefloquine + rosiglitazone. The median volume for uninfected mice is shown as a dashed line (N = 10 for uninfected control). Additional brain regions are shown in Figure S8. In summary, we observed that rosiglitazone adjunctive therapy was associated with protection from brain injury/atrophy in brain regions relevant to memory and motor functions.
Rosiglitazone adjunctive therapy in patients with P. falciparum malaria is associated with a lower Ang-2 to Ang-1 ratio and higher levels of BDNF
To extend our findings to a relevant human population we examined the levels of Ang-1, Ang-2, and BDNF in plasma banked from patients that had participated in a randomized double-blind placebo controlled trial of rosiglitazone plus atovaquone-proguanil (AP) versus AP plus placebo for the treatment of uncomplicated P. falciparum malaria acquired on the Thai-Burmese border [17]. At the time of our trial recruitment (2004–2005) AP was an accepted 1st line agent for the treatment of uncomplicated mefloquine-resistant malaria prevalent in the study area. Patients were randomly assigned to receive either placebo or rosiglitazone (4 mg twice daily) for 3 days. The primary end points of this trial were safety and tolerability of rosiglitazone administration as adjunctive therapy of uncomplicated P. falciparum malaria. Secondary endpoints were 50% and 90% parasite clearance time (PCT, the time required for patients to reduce their parasite burden by 50% and 90%). Rosiglitazone was found to be safe and well tolerated, and patients receiving rosiglitazone had significantly shorter 50% and 90% PCT, [rosiglitazone vs. placebo: 19.0 h vs. 24.6 h (p = 0.029) for 50% PCT; and 30.9 h vs. 40.4 h (p = 0.004) for 90% PCT [17]. We also detected significantly lower levels of inflammatory biomarkers IL-6 and MCP-1 in the rosiglitazone arm [17]. Patients receiving rosiglitazone had significantly higher levels of BDNF starting on day 2 post-treatment initiation (Figure 7A), and a significantly lower Ang-2 to Ang-1 ratio on day 3 post-treatment initiation compared to those receiving placebo (Figure 7B).
Fig. 7. Rosiglitazone adjunctive therapy increased BDNF and lowered the angiopoietin-2:angiopoietin-1 ratio in patient with falciparum malaria. 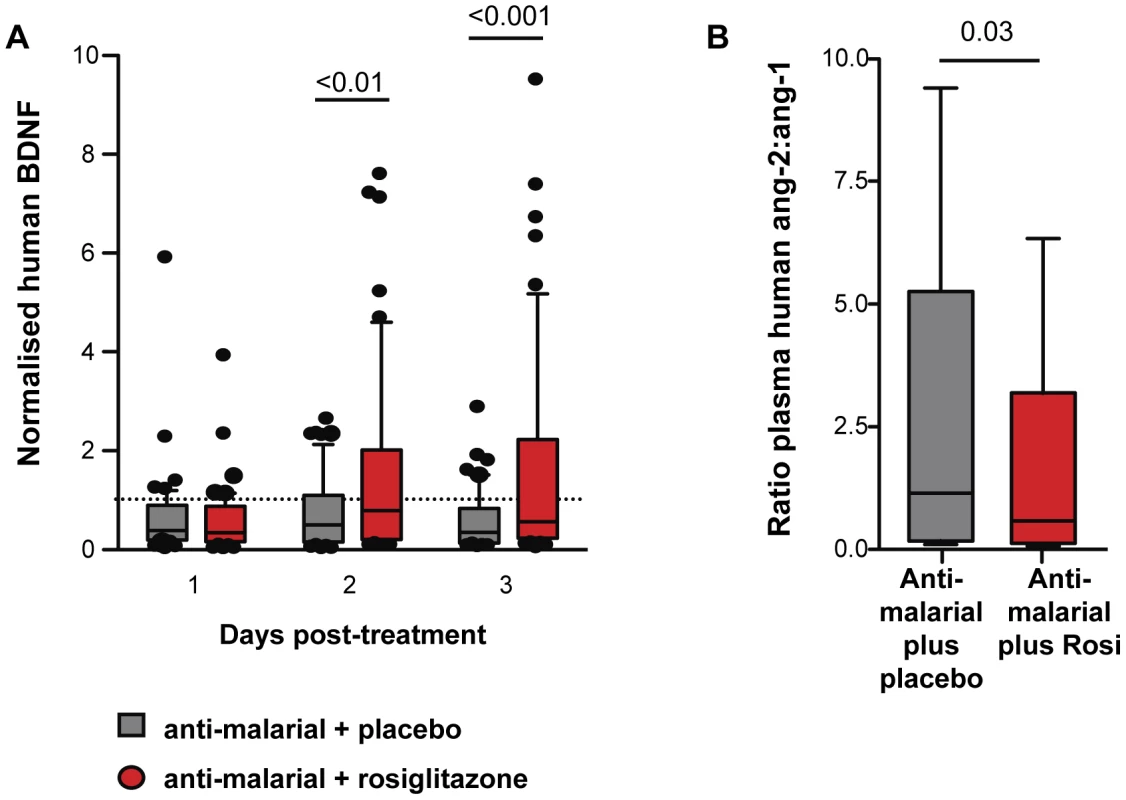
BDNF, Ang-1, and Ang-2 levels were determined in plasma collected from patients with uncomplicated malaria randomised to atovaquone proguanil plus either rosiglitazone or placebo. (A) BDNF levels were normalized to levels measured prior to treatment initiation. Normalised BDNF levels for day 1-3 post-treatment initiation are shown. Comparisons between placebo vs. rosiglitazone by Kruskal-Wallis test with Dunn's post-test, N = 52/group. Comparison between baseline vs. treatment by Wilcoxon Signed Rank test: p<0.001, p = 0.0024, and p<0.0001 for placebo baseline vs. placebo day 1, day 2, and day 3 respectively; and p<0.0001, p = 0.84, and p = 0.94 for rosiglitazone baseline vs. day 1, day 2, and day 3 respectively. (B) The ratio of Ang-2 to Ang-1 in plasma assessed 3 days post-treatment initiation. Data shown are medians with range. All comparisons by Mann Whitney test. N = 67/group. Discussion
Cerebral malaria is associated with high fatality rates and long-term neurodisabilities despite optimal anti-malarial therapy. There is a need for adjunctive therapies to reduce mortality and to improve neurocognitive outcome in survivors. Here we report that adjunctive therapy with the PPARγ agonist rosiglitazone was associated with enhanced survival, and complete protection from neurocognitive impairment and acute brain injury and subsequent brain atrophy in mice infected with PbA. Rosiglitazone improved outcome in part by reducing endothelial activation, increasing BBB integrity, and upregulating expression levels of the anti-oxidant enzymes SOD-1 and catalase, and the neurotrophic factors BDNF and NGF in the brains of infected mice. We further report that rosiglitazone adjunctive therapy was also associated with reduced endothelial activation, as evidenced by a lower Ang-2 to Ang-1 ratio, and enhanced levels of the neuroprotective factor BDNF in patients with P. falciparum malaria.
Recent studies have demonstrated epilepsy, persistent attention and memory problems, developmental delays in language and gross motor function, and attention deficit hyperactivity disorder (ADHD)-like behavior in ∼one-third of children who survive CM [4]–[6], [33]–[35]. Mice, drug-cured following the onset of ECM, also demonstrate neurocognitive deficits [27]. We observed that rosiglitazone, administered concomitantly with anti-malarial therapy beginning at the onset of ECM, completely protected mice from long-term neurocognitive impairment, specifically impairment in learning, memory, attention, and motor coordination. Mice that received rosiglitazone performed as well as uninfected mice and significantly better than infected mice that received anti-malarials alone.
High resolution MRI analysis for morphological CNS changes in mice drug-cured at the onset of ECM revealed significant volumetric differences between mice that received rosiglitazone and those that did not. The observed volumetric differences in the CNS correlated well with the behavioural phenotype observed in mice surviving ECM. Rosiglitazone treatment was associated with less atrophy in brain areas implicated in memory and spatial learning, specifically the corpus callosum, hippocampus, and thalamus, and in areas implicated in motor performance, specifically the cerebellum, basal ganglia, and midbrain. Injuries to these brain areas generate symptoms that are often seen in CM survivors, including deficits in learning, memory, and executive functions, problems with verbal fluency, impaired motor function, and ADHD-like symptoms [36]–[40]. Of note, our ECM data mirror MRI and CT findings observed in human CM. Changes in brain morphology, including changes in brain volume, abnormal T2 intensity, and diffusion-weighted MRI abnormalities, have been reported in acute CM and in the majority of CM survivors with neurological sequelae [29]–[30], [41]. Abnormal T2 signal intensity and DWI (diffusion weighted imaging) abnormalities in the basal ganglia was identified in 84% of children with CM, and over 49% of children showed such abnormalities in the thalamus, corpus callosum, and cerebellum.
Rosiglitazone therapy was associated with the induction of several potentially neuroprotective and neurorestorative pathways, including regulation of BDNF. As previously reported [25], [42], we observed reduced BDNF levels in the brains of PbA-infected mice. While anti-malarial treatment was associated with some increase in BDNF levels, mice that received rosiglitazone adjunctive therapy had significantly higher levels of BDNF and NGF expression in their brain, compared to mice receiving anti-malarials alone. Decreases in BDNF levels have been associated with alterations in neuronal maintenance and regeneration, structural abnormalities in the brain, and reduced neuronal plasticity resulting in impaired ability to adapt to crisis situations [43]. Lower BDNF levels are associated with a decline in neuroprotection, and administration of BDNF has been shown to be protective in acute brain injury and infection models [44]–[45]. Maintenance of BDNF levels in the brain is also required for normal learning and memory function [46]. Additionally, BDNF levels have been linked to motor performance [47]. The lower levels of BDNF induced by malaria infection may have contributed to the observed deficits in learning, memory, and motor performance in mice treated with anti-malarials alone, while the quicker recovery and the higher levels achieved in rosiglitazone-treated mice may have conferred protection from neurological injury.
Here we report for the first time that circulating BDNF levels also decline in P. falciparum infection. Using samples collected during a randomized double-blind placebo-controlled trial of rosiglitazone adjunctive therapy in patients with uncomplicated P. falciparum infection, we observed a decrease in circulating BDNF in both placebo and rosiglitazone arms in the first 24 hours following admission. However, while BDNF levels remained low in the placebo group, the rosiglitazone treatment group had a significant increase in circulating BDNF levels. These findings imply that even uncomplicated falciparum malaria may be associated with some degree of neurological compromise or injury, at least as suggested by a persistent decrease in BDNF. This observation is in agreement with previous reports of impaired school performance in children with a history of uncomplicated malaria [4]. Our data also demonstrate that rosiglitazone adjunctive therapy may protect against neurological compromise in P. falciparum malaria by inducing established neuroprotective pathways such as BDNF.
It is important to note that our measurements of BDNF were limited to peripheral levels and may not necessarily reflect BDNF levels in the brain. However, previous studies in animal models have shown that circulating BDNF levels correlate with CNS levels of BDNF [48]. Thus circulating BDNF may be diagnostically useful as a biomarker for CNS injury in malaria infection, although this correlation will need to be confirmed in prospective studies.
Also of note is that the anti-malarial used in this trial was atovaquone-proguanil (which remains a 1st line agent for the treatment of uncomplicated mefloquine-resistant malaria prevalent in the study site) rather than artemisinin-combination therapy (the current standard of care in Thailand). Although, the anti-malarial regimens differed between our animal model and our patient data, in both cases rosiglitazone was associated with induction of similar protective pathways indicating that its protective effects are independent of the anti-malarial used.
While PbA infection was not associated with a decline in NGF, we observed significant increases in NGF levels late in the course of treatment only in the mice that received rosiglitazone. NGF has been shown to promote neurogenesis and improve survival and differentiation of newly generated neurons in the adult brain, especially following injury [49]. Thus, the late increase in NGF levels in mice treated with rosiglitazone may have contributed to enhanced neurogenesis and recovery from CM-induced CNS injury.
Endothelial activation and loss of vascular integrity is central to the pathophysiology of CM, and vascular leakage has been shown to directly correlate with neurological disease onset in ECM [11]. Rosiglitazone adjunctive therapy contributed to the preservation of endothelial quiescence and vascular integrity in part by preventing malaria-induced alterations in the Ang-Tie2 axis. This was evidenced by the maintenance of peripheral Ang-1 levels in rosiglitazone-treated mice (Ang-1 sharply declined in infected mice), and a lower Ang-2 to Ang-1 mRNA ratio in the brain. A lower Ang-2 to Ang-1 ratio was also observed in patients with P. falciparum infection treated with rosiglitazone. Low Ang-1, elevated Ang-2, and an Ang-2 to Ang-1 ratio favouring Ang-2 have been reported in severe and cerebral malaria compared to uncomplicated malaria, and have been associated with mortality in CM [50]–[52].
Rosiglitazone adjunctive therapy was also associated with increased levels of the antioxidant enzymes SOD-1 and catalase in the brain, which correlated with protection from neurocognitive impairment. This is in agreement with previous observations showing that antioxidant adjunctive therapy was associated with reduced cognitive damage in mice [22]. Oxidative stress is thought to contribute to neuronal dysfunction in CM patients [10], and reduced anti-oxidant activity has been reported in CM patients and in ECM [23], [53]. Enhancement of anti-oxidant enzyme expression in endothelial and neuronal cells has been reported for PPARγ agonists, and is thought to contribute to the neuroprotective effects observed for PPARγ agonists in other models of CNS injury and disease [14].
Malaria immunopathogenesis is complex and therefore therapeutic interventions targeting a single pathway may not be sufficient to reduce mortality and morbidity in CM. We have demonstrated that rosiglitazone (which can exert its effects via activation of the nuclear transcription factor PPARγ and/or via PPARγ-independent mechanisms) can target multiple pathways implicated in the pathogenesis of CM including inflammation, oxidative stress, and endothelial activation, and is capable of initiating or enhancing neuroprotective mechanisms including the induction of neurotrophic factors. We provide direct evidence for the involvement of the Angiopoietin-Tie2 axis in mediating, at least in part, the observed neuroprotective effects of rosiglitazone. Future studies will be required to establish causality for the other implicated pathways, however, overall our data clearly demonstrate that rosiglitazone adjunctive therapy resulted in increased survival and protection from long-term neurocognitive impairment in ECM over what was observed with anti-malarial treatment alone. Importantly we provide evidence that rosiglitazone may also induce such putative protective mechanisms in falciparum-infected patients. Our findings are in agreement with the effects observed with PPARγ agonists in models of ischemic and hemorrhagic stroke, and CNS disease including Alzheimer's disease, multiple sclerosis, amyotrophic lateral sclerosis and Parkinson's disease [54]–[56]. PPARγ agonist use in these models and in patients was associated with reduced brain injury, attenuated neuronal loss, and improved neurological outcomes including motor and memory performance [57]–[61].
PPARγ agonists are currently approved for use in humans for the treatment of type II diabetes, which may accelerate their path to clinical evaluation and impact. Rare cardiac adverse events have been reported with long-term use of this class of drugs in elderly patients at high risk of cardiac disease; however in short-term use they have an excellent safety and tolerability profile. Rosiglitazone adjunctive therapy has already been found to be safe, well tolerated, and efficacious in adult patients with uncomplicated malaria [17], and short course therapy in a patient population that is typically at low risk of cardiac disease is unlikely to be associated with these types of adverse events.
In summary our results in an experimental model of CM and in patients with uncomplicated falciparum malaria suggest that PPARγ agonists are a promising adjunctive therapy for CM that may improve survival and prevent long-term neurocognitive impairment, and support the testing of PPARγ agonists in patients with CM.
Materials and Methods
Ethics statement
All animal experiments were approved by the University Health Network and the University of Toronto Animal Use Committees, and were performed according to the policies and guidelines of the Canadian Council on Animal Care.
Human plasma samples used in this study were collected from participants of a randomized double-blind placebo controlled trial testing the safety and efficacy of rosiglitazone adjunctive therapy in patients with P. falciparum malaria in Thailand performed during the period of December 2004 through December 2005 [17]. The study was approved by Mahidol University Research Ethics Committee, Bangkok, Thailand, and Toronto Academic Health Sciences Network Research, University Health Network. Written informed consent was obtained from all participating adult patients. For patients under the age of 18 written informed consent was obtained from their guardians. The trial was registered with ClinicalTrials.gov (identifier NCT00149383).
Murine model of experimental cerebral malaria
P. berghei ANKA (PbA) (MR4, Bethesda MD) was maintained by passage in naive mice. Female 7–8 week old C57BL/6 mice (Charles River Laboratories) were infected with 1×106 PbA parasitized erythrocytes by intraperitoneal (i.p.) injection (day 0 of infection) [16]. Mice were treated starting either on day 3 of infection, or on the onset of CM signs (day 5–6 of infection). For the day 3 experiments (Figure S1A), mice were treated with 10 mg/kg artesunate suspended in PBS i.p. on day 3, 4, 5 and 6 post-infection, and either 2.5 mg/kg rosiglitazone in 100 µl of saline (rosiglitazone group), or 100 µl of saline alone (control group) once daily by oral gavage starting on day 3 until the end of the experiment. Otherwise, mice were treated with 10 mg/kg artesunate i.p. for two days starting on the onset of CM signs, and either 2.5 mg/kg rosiglitazone or saline as a control by oral gavage daily starting at the same time as artesunate treatment and lasting until the end of the experiment (Figure S1B). Parasitemia was monitored daily by Giemsa-stained thin-blood smears.
For all drug-cure experiments (for behavioural and MRI testing) mice were treated at the onset of CM signs with 7 once daily doses of 100 mg/kg artesunate, 2 doses of 15 mg/kg mefloquine on the first and last day of treatment, and 7 once daily doses of 2.5 mg/kg rosiglitazone (Figure S1C). Parasitemia was monitored weekly. Mice remained parasite-free for the duration of behavioural testing.
Ang-1del mice on a BALB/c background were the kind gift of Dr. Susan Quaggin (Mount Sinai Hospital, Toronto, Canada) [20]. BALB/c wild-type breeding pairs were purchased from the Jackson Laboratory and bred in house. Cerebral malaria score for each mouse was assessed on multiple days by a single observer, who was blinded to genotype and treatment allocation, using a modified version of the rapid coma and behavioural score [21].
Analysis of systemic endothelial activation
Heparinized plasma was collected via saphenous vein or by cardiac puncture and frozen at −80°C. Plasma levels of Ang-1 and sICAM-1 were determined by ELISA (R&D Systems, Minneapolis, MN).
Analysis of vascular permeability
On day 10 post-infection drug-treated mice were injected i.p. with 300 µl of 2% Evans blue. 2 h post-injection mice were euthanized using isoflurane, and perfused with 20 mls of PBS. Brains were collected, photographed, and placed in formamide for 48 h to extract the Evans blue. Evans blue was quantified using a spectrophotometer at 605 nm and compared to a standard curve.
Luciferase imaging of parasite distribution
Mice were infected i.p. with 1×106 PEs of a luciferase-expressing PbA (PbGFP-LUC(con)) and treated as described above [19]. Imaging was conducted on day 10 post-infection as previously described [62].
Histological analysis
Brains were collected on day 10 post-infection from parasitemia-matched mice, following perfusion with 20 mls of PBS, were fixed in 4% formalin for 3 days, and then paraffin embedded. 4 µm sagittal slices were stained with H&E and examined blinded to the treatment group, to quantify the number of hemorrhages. The entire brain section was scanned and all hemorrhages (defined as collections of RBC observed outside of blood vessels) were recorded.
Brain quantitative real time PCR
Total RNA was isolated from snap frozen brain tissue after homogenization in TRIzol (1 mL/100 mg tissue; Invitrogen, Burlington, ON) according to manufacturer's protocol. Extracted RNA (1μg/sample) was treated with DNase I (Fermentas, Burlington, ON), and reverse transcribed to cDNA (BioRad, Mississauga, ON). cDNA was amplified in triplicate with SYBR Green master mix (Roche, Laval, QC) in the presence of 1μM of forward and reverse primers in a Light Cycler 480 (Roche, Laval, QC). Relative amounts of transcript were calculated by the comparative Ct method (2−ΔΔCT). GAPDH was used as an endogenous control. The primer sequences are shown in Table S1.
Behavioural testing
Behavioural testing began 2 months following completion of treatment, when the mice were approximately 4 months of age, and lasted approximately 3 weeks. During testing, the experimenter alternated between mice from each experimental group. Tests were performed in the following order: open field test (OFT), novel object recognition test (NOR), tail suspension test (TST), rotarod test, and contextual fear conditioning test (CFC). Methodological details for the OFT, TST, and CFC are included in the Text S1. Supplemental Methods.
For the NOR test, mice were habituated to the testing apparatus (empty clear plastic mouse cages) for 10 minutes over 6 daily sessions. On the test day, each animal was exposed for 10 minutes to a LEGO construct (LEGO Group, Billund, Denmark) and a Hot Wheels car (Mattel, Inc., El Segundo, CA, USA). The objects were previously determined to be of matched saliency in mice. All tests were video recorded suing ANYMAZE software. Time spent exploring both objects was recorded. Exploration was coded when the mouse touched an object with its forepaws or snout, bit, licked, or sniffed the objects from a distance of no more than 1.5 cm. Following exploration mice were returned to their home cage. Three hours after the initial exposure, mice were returned to the test cage and exposed for 5 minutes to one object from the original test pair and one novel object. The possible confound of mice exhibiting preference for the right or left side of the cage was addressed by counterbalancing for placements of the new object. A “preference index” (PI) was calculated as: PI = tn/(tn + tf), wherein “tn” represents time exploring a novel object or object in a novel placement area and “tf” the duration of familiar object exploration [63]. All animals with a total exploration time of less than 10 seconds in either the exploration or testing phase were removed from the analysis.
For the rotarod test, an Economex accelerating rotarod (Columbus Instruments, Columbus, OH, USA) was used to assess postural sensorimotor impairment. Mice were placed on the rod as it turned at a constant speed of 4 rpm. The latency to fall was recorded in five daily trials conducted at 30 min intervals, performed for 3 consecutive days. The daily performance score for each animal was the sum latency to fall off the apparatus across all five daily trials. To measure adaptive performance on the 4th and 5th day mice were placed on the rod starting at a constant speed of 4 rpm, with an acceleration of 0.2 rpm/s for 5 successive trials over a 30 min interval. Latency to fall was recorded for each trial. A daily performance score for each animal was obtained by summing latency over the 5 trials.
Ex vivo Magnetic Resonance Imaging
Specimen preparation for MRI was as described in [64]. Upon completion of all behavioural testing animals were anesthetized with a ketamine (150 mg/kg)/xylazine (10 mg/kg) mix and perfused transcardially with 30 mL of solution A (1×PBS +2mM Prohance (a contrast agent used for MRI) +1 µL/mL Heparin) and then with 30 mL of solution B (1×PBS +4% Paraformaldehyde +2 mM Prohance). Animals were then decapitated and skin, cartilage and lower jaw was removed. Tissue was left at 4°C for 24 h in 10 mL of solution B and then transferred into 10 mL of solution C (1×PBS +0.02% sodium azide +2 mM Prohance) for storage prior to scanning. Tissue was left in solution C for a maximum of 6 months prior to scanning. The MRI methods used here have previously been described in detail [28], [31]. An anatomical mouse brain atlas was used to define brain regions and structures and to compute volumes for each mouse brain [31]–[32]. Additional details can be found in the Text S1. Supplemental Methods.
Randomized clinical trial patient population and analysis of biomarkers
Ang-1, Ang-2, and BDNF levels were assessed by ELISA (R&D Systems, Minneapolis, MN) in plasma collected from participants of a randomized double-blind placebo controlled trial testing the safety and efficacy of rosiglitazone adjunctive therapy in patients with P. falciparum malaria in Thailand. The details of this trial are described in [17].
Statistical analysis
Survival studies were performed at least 3 times. Other experiments were repeated at least once. Statistical significance for survival studies was assessed by log-rank test. Other comparisons were assessed by Mann-Whitney test or Students t-test, or one-way or two-way ANOVA with post-hoc tests. Statistical analyses were performed using GraphPad Prism software (LaJolla, CA). Linear regression modeling was used to identify brain regions that differed significantly between treatment groups.
Supporting Information
Zdroje
1. DondorpA, NostenF, StepniewskaK, DayN, WhiteN, et al. (2005) Artesunate versus quinine for treatment of severe falciparum malaria, a randomised trial. Lancet 366 : 717–725.
2. DondorpAM, FanelloCI, HendriksenIC, GomesE, SeniA, et al. (2010) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT), an open-label, randomised trial. Lancet 376 : 1647–1657.
3. JohnCC, KutambaE, MugaruraK, OpokaRO (2010) Adjunctive therapy for cerebral malaria and other severe forms of Plasmodium falciparum malaria. Expert Rev Anti Infect Ther 8 : 997–1008.
4. JohnCC, BangiranaP, ByarugabaJ, OpokaRO, IdroR, et al. (2008) Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 122: e92–99.
5. BoivinMJ, BangiranaP, ByarugabaJ, OpokaRO, IdroR, et al. (2007) Cognitive impairment after cerebral malaria in children, a prospective study. Pediatrics 119: e360–366.
6. BirbeckG, MolyneuxME, KaplanPW, SeydelKB, ChimalizeniYF, et al. (2010) Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol 9 : 1173–1181.
7. FernandoSD, RodrigoC, RajapakseS (2010) The hidden burden of malaria, cognitive impairment following infection. Malaria J 9 : 366.
8. TaylorTE, FuWJ, CarrRA, WhittenRO, MuellerJS, et al. (2004) Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10 : 143–145.
9. WhiteVA, LewallenS, BeareN, KayiraK, CarrRA, et al. (2001) Correlation of retinal haemorrhages with brain haemorrhages in children dying of cerebral malaria in Malawi. Trans R Soc Trop Med Hyg 95 : 618–621.
10. MedanaIM, DayNP, HienTT, MaiNT, BethellD, et al. (2002) Axonal injury in cerebral malaria. Am J Pathol 160 : 655–666.
11. NacerA, MovillaA, BaerK, MikolajczakSA, KappeSH, et al. (2012) Neuroimmunological blood brain opening in experimental cerebral malaria. PloS Pathog 8: e1002982.
12. BergerJ, MollerDE (2002) The mechanism of action of PPARs. Ann Rev Med 53 : 409–435.
13. LehrkeM, LazarMA (2005) The many faces of PPARgamma. Cell 123 : 993–999.
14. KapadiaR, YiJH, VemugantiR (2008) Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci 13 : 1813–1826.
15. JinJ, AlbertzJ, GuoZ, PengQ, RudowG, et al. (2013) Neuroprotective effects of PPAR-γ agonist rosiglitazone in N171-82Q mouse model of Huntington's disease. J Neurochem 125 : 410–419.
16. SerghidesL, PatelSN, AyiK, LuZ, GowdaDC, et al. (2009) Rosiglitazone modulates the innate immune response to Plasmodium falciparum infection and improves outcome in experimental cerebral malaria. J Infect Dis 199 : 1536–1545.
17. BoggildAK, KrudsoodS, PatelSN, SerghidesL, TangpukdeeN, et al. (2009) Use of peroxisome proliferator-activated receptor gamma agonists as adjunctive treatment for Plasmodium falciparum malaria, a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 49 : 841–849.
18. KimH, HigginsS, LilesWC, KainKC (2011) Endothelial activation and dysregulation in malaria, a potential target for novel therapeutics. Curr Opion Hematol 18 : 177–185.
19. Franke-FayardB, WatersAP, JanseCJ (2006) Real-time in vivo imaging of transgenic bioluminescent blood stages of rodent malaria parasites in mice. Nat Protoc 1 : 476–485.
20. JeanssonM, GawlikA, AndersonG, LiC, KerjaschkiD, et al. (2011) Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121 : 2278–2289.
21. CarrollRW, WainwrightMS, KimKY, KidambiT, GomezND, et al. (2010) A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS One 5: e13124.
22. ReisPA, ComimCM, HermaniF, SilvaB, BarichelloT, et al. (2010) Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy. PLoS Pathog 24: e1000963.
23. PabonA, CarmonaJ, BurgosLC, BlairS (2003) Oxidative stress in patients with non-complicated malaria. Clin Biochem 36 : 71–78.
24. SkaperSD (2012) The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol 846 : 1–12.
25. LinaresM, Marin-GarciaP, Perez-BenaventeS, Sanchez-NogueiroJ, PuyetA, et al. (2013) Brain-derived neurotrophic factor and the course of experimental cerebral malaria. Brain Res 1490 : 210–224.
26. Huang EJ, Reichardt LF (2003) Trk receptors, roles in neuronal signal transduction. Annu Rev Biochem 72, 609–642.
27. DaiM, ReznikSE, SprayDC, WeissLM, TanowitzHB, et al. (2010) Persistent cognitive and motor deficits after successful antimalarial treatment in murine cerebral malaria. Microbes Infect 12 : 1198–1207.
28. LerchJP, YiuAP, Martinez-CanabalA, PekarT, BohbotVD, et al. (2011) Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage 54 : 2086–2095.
29. PotchenMJ, BirbeckGL, DemarcoJK, KampondeniSD, BeareN, et al. (2010) Neuroimaging findings in children with retinopathy-confirmed cerebral malaria. Eur J Radiol 74 : 262–268.
30. KampondeniSD, PotchenMJ, BeareNA, SeydelKB, GloverSJ, et al. (2013) MRI findings in a cohort of brain injured survivors of pediatric cerebral malaria. Am J Trop Med Hyg 88 : 542–546.
31. LerchJP, SledJG, HenkelmanRM (2011) MRI phenotyping of genetically altered mice. Methods Mol Biol 711 : 349–361.
32. DorrAE, LerchJP, SpringS, KabaniN, HenkelmanRM (2008) High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 42 : 60–69.
33. BoivinMJ, GladstoneMJ, VokhiwaM, BirbeckGL, MagenJG, et al. (2011) Developmental outcomes in Malawian children with retinopathy-positive cerebral malaria. Trop Med Int Health 16 : 263–271.
34. KiharaM, CarterJA, HoldingPA, Vargha-KhademF, ScottRC, et al. (2009) Impaired everyday memory associated with encephalopathy of severe malaria, the role of seizures and hippocampal damage. Malaria J 8 : 273.
35. CarterJA, Mung'ala-OderaV, NevilleBG, MuriraB, MturiN, et al. (2005) Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry 76 : 476–481.
36. MasiG, BrovedaniP (2011) The hippocampus, neurotrophic factors and depression, possible implications for the pharmacotherapy of depression. CNS Drugs 25 : 913–931.
37. Van der Werf YD, Witter MP, Uylings HB, Jolles J (2000) Neuropsychology of infarctions in the thalamus, a review. Neuropsychologia 38, 613–627.
38. PozzilliC, BastianelloS, PadovaniA, PassafiumeD, MillefioriniE, et al. (1991) Anterior corpus callosum atrophy and verbal fluency in multiple sclerosis. Cortex 27 : 441–445.
39. HinesM, ChiuL, McAdamsLA, BentlerPM, LipcamonJ (1992) Cognition and the corpus callosum, verbal fluency, visuospatial ability, and language lateralization related to midsagittal surface areas of callosal subregions. Behav Neurosci 106 : 3–14.
40. AggletonJP, O'MaraSM, VannSD, WrightNF, TsanovM, et al. (2010) Hippocampal-anterior thalamic pathways for memory, uncovering a network of direct and indirect actions. Eur J Neurosci 31 : 2292–2307.
41. PotchenMJ, KampondeniSD, SeydelKB, BirbeckGL, HammondCA, et al. (2012) Acute brain MRI findings in 120 Malawian children with cerebral malaria, new insights into an ancient disease. AJNR Am J Neuroradiol 33 : 1740–1746.
42. ComimCM, ReisPA, FrutusoVS, FriesGR, FragaDB, et al. (2012) Effects of experimental cerebral malaria in memory, brain-derived neurotrophic factor and acetylcholinesterase activity in the hippocampus of survivor mice. Neurosci Lett 523 : 104–107.
43. Gomez-Palacio-SchjetnanA, EscobarML (2013) Neurotrophins and synaptic plasticity. Curr Top Behav Neurosci 15 : 117–136.
44. ShiQ, ZhangP, ZhangJ, ChenX, LuH, et al. (2009) Adenovirus-mediated brain-derived neurotrophic factor expression regulated by hypoxia response element protects brain from injury of transient middle cerebral artery occlusion in mice. Neurosci Lett 465 : 220–225.
45. BarichelloT, BelarminoEJr, ComimCM, CiprianoAL, GenerosoJS, et al. (2010) Correlation between behavioral deficits and decreased brain-derived neurotrophic factor in neonatal meningitis. J Neuroimmunol 223 : 73–76.
46. BekinschteinP, CammarotaM, KatcheC, SlipczukL, RossatoJI, et al. (2008) BDNF is essential to promote persistence of long-term memory storage. Proc. Natl Acad Sci USA 105 : 2711–2716.
47. CanalsJM, PinedaJR, Torres-PerazaJF, BoschM, Martin-IbanezR, et al. (2004) Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J Neurosci 24 : 7727–7739.
48. KleinAB, WilliamsonR, SantiniMA, ClemmensenC, EttrupA, et al. (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol 14 : 347–353.
49. SofroniewMV, HoweCL, MobleyWC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24 : 1217–1281.
50. LovegroveFE, TangpukdeeN, OpokaRO, LaffertyEI, RajwansN, et al. (2009) Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 4: e4912.
51. ConroyAL, GloverSJ, HawkesM, ErdmanLK, SeydelKB, et al. (2012) Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria, a retrospective case-control study. Crit Care Med 40 : 952–959.
52. YeoTW, LampahDA, GitawatiR, TjitraE, KenangalemE, et al. (2008) Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A 105 : 17097–17102.
53. NarsariaN, MohantyC, DasBK, MishraSP, PrasadR (2012) Oxidative stress in children with severe malaria. J Trop Pediatr 58 : 147–150.
54. DrewPD, XuJ, RackeMK (2008) PPAR-gamma, therapeutic potential for multiple sclerosis. PPAR Res 2008 : 627463.
55. VemugantiR (2008) Therapeutic potential of PPARγ activation in stroke. PPAR Res 2008 : 461981.
56. BrightJJ, KanakasabaiS, ChearwaeW, ChakrabortyS (2008) PPAR regulation of inflammation signaling in CNS diseases. PPAR Res 2008 : 658520.
57. Mandrekar-ColucciS, KarloJC, LandrethGE (2012) Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer's disease. J Neurosci 32 : 10117–10128.
58. EscribanoL, SimónAM, Pérez-MediavillaA, Salazar-ColochoP, RíoJD, et al. (2009) Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer's disease mouse model. Biochem Biophys Res Commun 379 : 406–410.
59. SchintuN, FrauL, IbbaM, CaboniP, GarauA, et al. (2009) PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. Eur J Neurosci 29 : 954–963.
60. WatsonGS, CholertonBA, RegerMA, BakerLD, PlymateSR, et al. (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone, a preliminary study. Am J Geriatr Psychiatry 13 : 950–958.
61. KaiserCC, ShuklaDK, StebbinsGT, SkiasDD, JefferyDR, et al. (2009) A pilot test of pioglitazone as an add-on in patients with relapsing remitting multiple sclerosis. J Neuroimmunol 211 : 124–130.
62. SerghidesL, KimH, LuZ, KainDC, MillerC, et al. (2011) Inhaled nitric oxide reduces endothelial activation and parasite accumulation in the brain, and enhances survival in experimental cerebral malaria. PLoS One 6: e27714.
63. FrancisBM, KimJ, BarakatME, FraenkiS, YucelYH, et al. (2012) Object recognition memory and BDNF expression are reduced in young TgCRND8 mice. Neurobiol Aging 33 : 555–563.
64. CahillLS, LaliberteCL, EllegoodJ, SpringS, GleaveJA, et al. (2012) Preparation of fixed mouse brains for MRI. Neuroimage 60 : 933–939.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek Oral Bacteria and CancerČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



